Abstract
We retrospectively compared the long-term evolution of IgG anti-spike (S) and anti-nucleocapsid (N) levels (Abbott immunoassays) in 116 non-severe and 115 severe SARS-CoV-2 infected patients from 2 university hospitals up to 365 days post positive RT-PCR. IgG anti-S and anti-N antibody levels decayed exponentially up to 365 days after a peak 0 to 59 days after positive RT-PCR. Peak antibody level/cut-off ratio 0 to 59 days after positive RT-PCR was more than 70 for anti-S compared to less than 6 for anti-N (P < 0.01). Anti-S and anti-N were significantly higher in severe compared to non-severe patients up to 180 to 239 days and 300 to 365 days, respectively (P < 0.05). Despite similar half-lives, the estimated time to 50% seronegativity was more than 2 years for anti-S compared to less than 1 year for anti-N in non-severe and severe COVID-19 patients, due to the significantly higher peak antibody level/cut-off ratio for anti-S compared to anti-N.
Keywords: SARS-CoV-2, COVID-19 testing, COVID-19, Serological Testing, Immunoassay, Spike, Nucleocapsid, IgG
Abbreviations: anti-N, anti-nucleocapsid; anti-S, anti-spike; CI, 95% confidence interval; COVID-19, Coronavirus Disease 19; HCW, healthcare workers; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2
1. Introduction
Most individuals produce specific antibodies directed against the spike (S) and the nucleocapsid (N) protein after an infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with seroconversion rates of 85-100% in the first weeks after infection [1], [2], [3], [4], [5]. Anti-N IgG antibodies become detectable several days earlier than anti-S antibodies, both with automated immunoassays and lateral flow immunoassays [6,7]. Seroconversion for IgG occurs approximately 2 weeks after onset of symptoms and, similar to SARS-CoV-1, around the same time as IgM and IgA [8], [9], [10]. Despite some early reports describing rapid seroreversion within 3 months [11,12], recent studies on long-term antibody kinetics demonstrated that many infected individuals still show detectable anti-S antibodies for up to 12 to 14 months after infection [13], [14], [15], [16], [17]. The half-life and time to seronegativity of anti-SARS-CoV-2 antibodies depends on antibody class and the antigenic target, with IgG remaining positive for a longer period than IgM and IgA [2,4,9,18] and anti-S antibodies persisting for a longer period than anti-N [4,15,[19], [20], [21], [22], [23], [24]]. IgG levels seem to peak around 2 to 8 weeks after infection, followed by an initial fast waning and subsequent slowing of antibody decay after 4 to 6 months [16,23,25,26]. Note that the observed antibody kinetics are assay-dependent, as several studies have shown longer seropositivity and even increases in signal over time using the competitive total Ig Roche Elecsys anti-N and anti-S immunoassays, while antibody levels typically decline using non-competitive IgG immunoassays [19,[27], [28], [29], [30]]. The signal of competitive total Ig immunoassays is impacted by antibody avidity and relative abundance of the different immunoglobulin classes, impacting long-term kinetics [19,[27], [28], [29], [30]].
The presence and amount of IgG anti-SARS-CoV-2 antibodies, particularly anti-S, has been shown to correlate with neutralizing antibody titers [31,32] and protection from (re)infection [22,[33], [34], [35]]. Individuals who had a more severe COVID-19 episode seem to produce higher peak antibody levels and remain seropositive for longer, which might influence protection from reinfection [16,24,32,[36], [37], [38]]. Several studies have estimated the protection against repeat infection around 80% to 95% for at least 6 to 10 months in seropositive individuals after primary SARS-CoV-2 infection [39], [40], [41], [42]. If reinfection does occur, it's severity is usually milder compared to a primary infection [43]. The available SARS-CoV-2 vaccines induce a strong initial humoral immune response [44], with subsequent waning of antibody levels and protective efficacy in the months following vaccination [45], [46], [47]. Vaccinated individuals with a history of prior COVID-19 (“hybrid immunity”) show higher antibody levels and longer lasting protection following vaccination, compared to vaccinated immune naive individuals [44,48]. Individuals with hybrid immunity are better protected against highly immune-evading variants such as the recently reported Omicron variant [49]. Characterization of the long-term humoral immune response after natural infection is of importance to understand and develop strategies against the ongoing COVID-19 pandemic [15,16].
In this study, we report the long-term kinetics of anti-S and anti-N levels in non-severe and severe COVID-19 patients up to 12 months after first positive reverse transcriptase polymerase chain reaction (RT-PCR), and estimate half-life and time to seronegativity of IgG anti-S and IgG anti-N.
2. Materials & methods
2.1. Study design
This retrospective study was performed at the University Hospitals Leuven and Ghent University Hospital (Belgium) after approval by the local ethics committees from both hospitals (S63897 and BC07662, respectively).
The long-term kinetics of anti-S and anti-N were determined in 882 residual samples from 231 adult patients who were positive for SARS-CoV-2 with RT-PCR on nasopharyngeal swabs between March 9th and June 12th 2020, before the introduction of SARS-CoV-2 vaccines. The circulating strains at the time precede the emergence of the variants of concern and thus correspond to “wild type” strains. Samples were collected 0 to 365 days after positive RT-PCR. Most of the samples up to 240 days post positive RT-PCR were included in a previous study describing the evolution of IgG anti-N levels up to 8 months after infection [36]. Samples from four individuals taken after occurrence of a suspected reinfection (based on a ≥3-fold rise in anti-N and anti-S antibody titers) were excluded, as well as samples taken after vaccination.
2.2. Clinical classification and inclusion criteria
Patients were classified after a review of the patient records as described previously [50]: (1) mild (n = 41): mild clinical symptoms without manifestation of pneumonia on imaging; (2) moderate (n = 75): fever, respiratory symptoms, and with radiological findings of pneumonia; (3) severe (n = 68): meeting any one of the following criteria: respiratory distress, hypoxia (SpO2≤93%), or abnormal blood gas analysis: (PaO2<60 mm Hg, PaCO2>50 mm Hg); (4) critical (n = 47): meeting any one of the following criteria: respiratory failure requiring mechanical ventilation, shock, organ failure that requires ICU care or death of the patient due to COVID-19. Mild and moderate patients were considered together as “non-severe patients” and severe and critical patients as “severe patients.”
The inclusion criterion was at least 2 available residual samples (range 2–12 samples/patient) including at least one sample collected 30 days or later after the first positive RT-PCR. This criterion ensured that a longitudinal evolution of antibody levels could be determined for each patient. In Fig. 3 we included the results of a previously published study in 118 health care workers (HCW) with an asymptomatic or mild SARS-CoV-2 infection (Ethics committee aproval S64152) [21] and a group of 13 asymptomatic individuals (39 samples) who tested positive with RT-PCR and for whom we had at least 1 sample 60 days after positive RT-PCR (Ethics committee approval S63897 and BC07662).
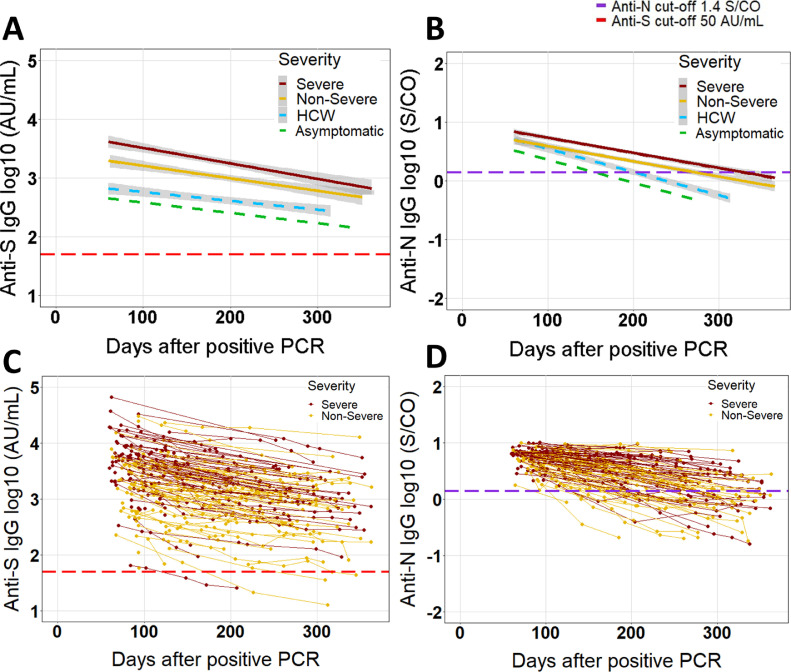
Fig. 3.
Longitudinal evolution of log10 IgG anti-S and anti-N antibody levels (log10) in patients who tested positive at least once ≥60 days after RT-PCR. (A−B) simple linear regression (antibody level ∼ days after positive RT-PCR) with 95% confidence band (grey zone). No confidence band is shown for the asymptomatic patients given the small size. For comparison, the dashed lightblue line with 95% confidence band shows the results of simple linear regression in health care workers (HCW) from a previous study [21]. (C−D) results of individual non-severe and severe COVID-19 patients are shown as dots connected by lines. Dashed red and violet lines represent the manufacturer's cut-offs for positivity for anti-S (50 AU/mL) and anti-N (1.4 S/CO). Analysis performed with 589 samples from 214 patients for anti-S and 538 samples from 188 patients for anti-N.
Samples were included regardless whether or not a patient became seronegative. Severely immunocompromised patients (hematological cancer, recent organ transplantation) and patients receiving the B-cell suppressing agents rituximab or azathioprine were excluded.
2.3. Antibody measurement
Antibodies were measured on Abbott Architect (Abbott, Lake Forest Illinois) with the chemiluminiscence SARS-CoV-2 IgG (anti-N) and IgG II Quant (anti-S) assays using the manufacturer's cut-offs for positivity of 1.4 S/CO and 50 AU/mL, respectively. The specificity of the IgG anti-S assay was determined in 110 left-over samples collected before January 2020 that were previously used to evaluate the specificity of the Abbott IgG anti-N assay [36]. Three of the original 113 samples were excluded because of insufficient volume. The tested samples were serum or lithium heparin plasma, both appropriate sample types according to the manufacturer's instructions. Samples above the extended measuring range of 80.000 AU/mL for anti-S were further diluted 1:2 using Abbott multi-assay manual diluent. The units of the quantitative Abbott anti-S assay (AU/mL) which uses a 6-point calibration curve can converted to WHO units (BAU/mL) by multypling with a factor of 0.142 according to the manufacturer. This is not possible for the semi-quantitative Abbott anti-N assay (S/CO) which only uses a 2-point calibration curve.
2.4. SARS-CoV-2 RT-PCR
Due to supply limitations 4 different PCR platforms were used in the University Hospitals Leuven: Hologic Panther Fusion system (Hologic, Marlborough, Massachusetts), Hologic Panther Aptima SARS-CoV-2 assay, Xpert Xpress SARSCoV-2 (Cepheid, Sunnyville, California), and an in-house method complying with the WHO guidelines [51].
In Ghent University Hospital, only one PCR platform was used: an in-house PCR for E-gene (FAM) using primers described by Corman et al [51]. Nucleic acid extraction was performed automatically using NucliSENS Easymag (Biomérieux, Marcy l'Etoile, France). Reverse transcription and amplification was performed using the Qiagen One Step RT-PCR Kit (Qiagen, Hilden, Germany) with the Dia ControlRNA (Diagenode, Liège, Belgium) (Cy5) as internal control. PCR was performed using a CFX96 real-time cycler and results were analysed with CFX software (Bio-Rad, Hercules, California). Positivity was determined based on a cycle threshold (Ct) value below 42, a threshold determined after internal validation.
2.5. Data analysis
The percentage of patients who seroconverted and the percentage of patients who were seropositive in a given time window were compared using Fisher's exact test, and 95% confidence intervals were calculated using the modified Wald method method with Graphpad QuickCalcs (www.graphpad.com/quickcalcs/). Antibody levels and estimated median time to seronegativity were compared using a nonparametric rank sum test (Mann–Whitney–Wilcoxon) with R Studio (v1.3.1093). A P-value < 0.05 was considered significant.
Data were divided in 30-day time windows for beeswarm plots (Fig. 2) to visualize antibody levels of each sample and in 60-day time windows to statistically compare antibody levels between non-severe and severe COVID-19 cases (Supplementary Fig. 1). Only one sample per patient was included per time window. The sample with the highest antibody level was included for the 0 to 29 days, 30 to 59 days and 0 to 59 days time windows and the last sample for the later time windows. Anti-S and anti-N levels were correlated per 60 day window using all available results of the non-severe and severe COVID-19 patients included in this study (Fig. 1 ).
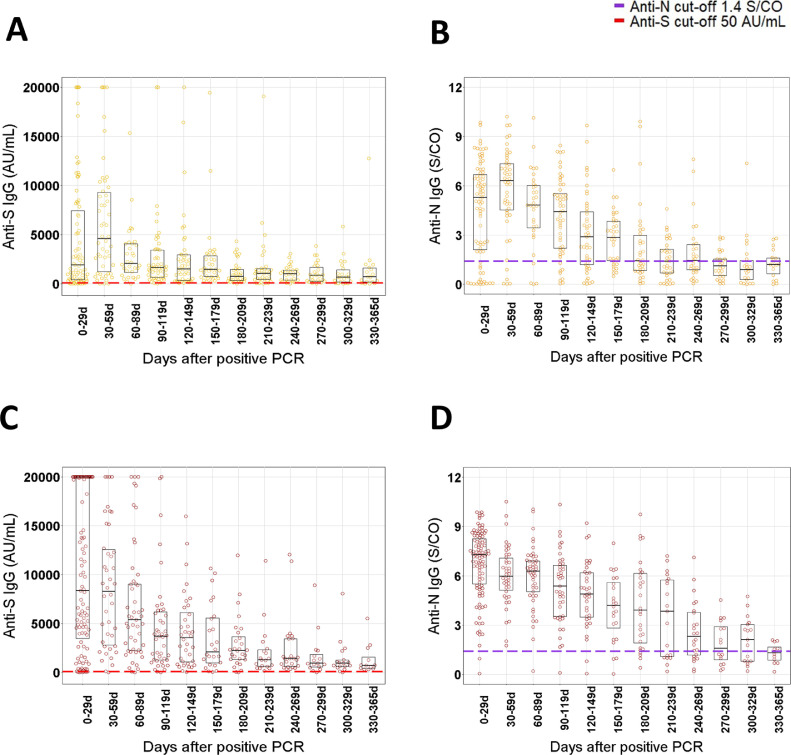
Fig. 2.
Beeswarm plots of the IgG anti-S and IgG anti-N results per 30 day time window after positive RT-PCR in non-severe (A−B, yellow) and severe (C−D, red) COVID-19 patients. Boxplots show the P25, median and P75 of antibody levels per 30 day time window. Dashed red and violet lines represent the manufacturer's cut-offs for positivity for anti-S (50 AU/mL) and anti-N (1.4 S/CO). Results for IgG anti-S higher than 20.000 AU/mL are shown as 20.000 AU/mL. Number of patients per 30 day window (severe/non-severe): 0 to 29d: n = 103/83, 30 to 59d: n = 42/50, 60 to 89d: n = 49/32, 90 to 119d: n = 43/52, 120 to 149d: n = 36/45, 150 to 179d: n = 24/34, 180 to 209d: n = 28/34, 210 to 239d: n = 19/33, 240 to 269d: n = 24, 270 to 299d: n = 16/29, 300 to 329d: n = 17/22, 330 to 365d: n = 12/18. d = day.
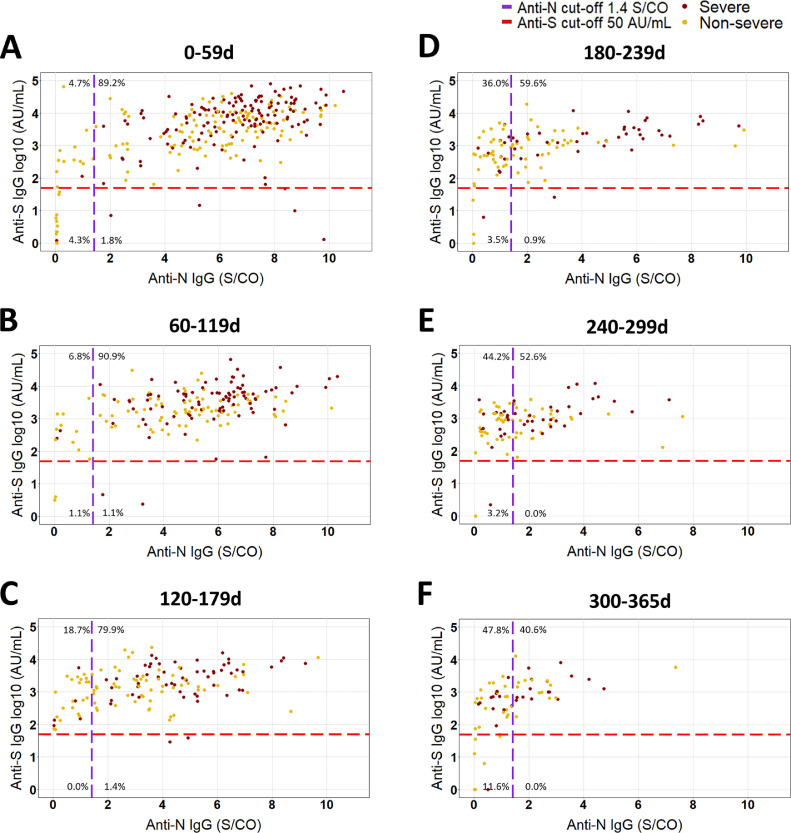
Fig. 1.
Correlation between anti-S (log10) and anti-N (linear) antibody levels per 60 day time window after positive RT-PCR in non-severe and severe COVID-19 patients. (A) 0 to 59d (n = 278 samples, 231 patients), (B) 60 to 119d (176 samples, 152 patients), (C) 120 to 179d (139 samples, 110 patients), (D) 180 to 239d (114 samples, 83 patients), (D) 240 to 299d (95 samples, 77 patients), (F) 300 to 365d (69 samples, 56 patients). The proportion of samples in each quadrant is depicted as a percentage of total samples per window. All available results of non-severe and severe COVID-19 patients included in this study are shown.
Computed half-life (the number of days that elapse between halving of the antibody levels) and estimated median time to seronegativity (the number of days it takes for the antibody levels to drop below the cut-off in 50% of the population) were calculated by performing a simple linear regression using R studio with the log10 of the antibody titer and days post positive RT-PCR, corresponding to a one phase exponential decay of the antibody levels. Multiple regression analysis was performed with the log10 of the antibody titer as the dependent variable and days post positive RT-PCR, severity (non-severe or severe), gender (man or women) and age (continuous variable in years) as covariates. Only samples after day 59 fom patients who tested positive for anti-N (n = 188 patients, 538 samples) or anti-S (n = 214 patients, 589 samples) 60 days or more after RT-PCR were included for regression analyses. The slopes of the regression lines (used to calculate the half-life) were statistically compared between groups using ANCOVA.
3. Results
3.1. Patient population and positivity rate
The patient characteristics are described in Table 1 . The median age did not differ between non-severe and severe patients, but the proportion of men was significantly higher in severe than in non-severe patients (P < 0.01, Table 1). Time between onset of symptoms and first positive RT-PCR was 5.7 days and 7.4 days in non-severe and severe patients (P < 0.01). The specificity of the antibody assays (determined in prepandemic samples) was 99.1% [95% confidence interval (CI): 94.7–100] for IgG anti-N and 99.1% [CI: 94.5–100] for IgG anti-S.
Table 1.
Patient characteristics and long-term kinetics.
| Non-severe patients | Severe patients | P value | |
|---|---|---|---|
| Median age in y (range) | 60 (23−91) | 62 (28−92) | P = 0.34 |
| Male/Female (% men) | 55/61 (47.4%) | 85/30 (73.9%) | P < 0.01 |
| Days between symptom onset and RT-PCR+ (range) | 5.7 (0−22) | 7.4 (0−31) | P < 0.01 |
| Median samples per patient (range) | 3 (2−12) | 3 (2−11) | P = 0.29 |
| Anti-S | |||
| Linear regression | |||
| Patients positive after day 59 (samples) | 106 (322) | 108 (267) | |
| Computed half-life in days [95%CI] | 141.6 [105.7−214.6] | 113.7 [88.9−157.4] | P = 0.86 |
| Time to 50% seronegativity in days [95%CI] | 809.6 [604.2−1226.7] | 785.9 [615.1−1088.1] | P = 0.14 |
| Linear regression | |||
| Patients positive after day 59 (samples) | 85 (275) | 103 (263) | |
| Computed half-life in days [95%CI] | 115.9 [99.2−139.2] | 116.3 [101.8−135.8] | P = 0.18 |
| Time to 50% seronegativityin days [95%CI] | 273.1 [233.8−328.1] | 327.3 [286.3−382.0] | P = 0.09 |
Of the 116 non-severe patients, 87.9% [CI: 80.6–92.8] seroconverted for anti-N compared to 96.6% [CI: 91.2–98.9] for anti-S (P < 0.01). Of the 115 severe patients, all patients seroconverted for anti-N (100% [CI: 96.1–100]) and 114 of the 115 patients for anti-S (99.1% [CI: 94.8–100]) (P = 1.0).
For IgG anti-S, the percentage of seropositive patients remained relatively stable after day 59, with 81.8% [65.2–91.8] of non-severe and 95.7% [CI: 77.3–100] of severe patients still showing IgG anti-S antibodies above the cut-off up to 300 to 365 days after positive RT-PCR (P = 0.09 and 1.0 respectively vs 0–59 days) (see Table 2 ). In contrast, anti-N seropositivity decreased significantly after day 59 (see Table 2), with a higher fraction of severe patients testing positive in every 60 day window compared to non-severe patients (Table 2). Only 56.5% [CI: 36.8–74.4] of severe patients and 30.3% [CI: 17.3–47.5] of non-severe patients were positive for IgG anti-N 300 to 365 days after positive RT-PCR (both P < 0.01 vs. 0-59 days). The fraction of patients testing positive for IgG anti-S was significantly higher than for anti-N in non-severe patients in each time window after day 59 and in the 3 time windows after day 179 in severe patients (see Table 2) Fig. 1. shows the correlation of the quantitative results of anti-S and anti-N of all the included samples in each 60-day time window.
Table 2.
Percentage seropositive patients per time window (days post positive RT-PCR).
| Abbott anti-S |
Abbott anti-N |
P valuee | |||||
|---|---|---|---|---|---|---|---|
| Non-severe patients | Negative | Positive | % positive [CI] | Negative | Positive | % positive [CI] | |
| 0−59 d | 8 | 102 | 92.7% [86.1−96.5] | 18 | 92 | 83.6% [75.5−89.5] | P = 0.06 |
| 60−119 d | 2 | 68 | 97.1% [89.6−99.8] | 11 | 59 | 84.3% [73.8−91.2] | P < 0.05 |
| 120−179 d | 0 | 58 | 100% [92.6−100] | 18 | 40 | 69.0%c [56.1−79.4] | P < 0.01 |
| 180−239 d | 3 | 47 | 94.0% [83.2−98.6] | 27 | 23 | 46.0%d [33.0−59.6] | P < 0.01 |
| 240−299 d | 2 | 39 | 95.1% [83.0−99.5] | 23 | 18 | 43.9%d [29.9−59.0] | P < 0.01 |
| 300−365 d | 6 | 27 | 81.8% [65.2−91.8] | 23 | 10 | 30.3%d [17.3−47.5] | P < 0.01 |
| Severe Patients | Negative | Positive | % positive [CI] | Negative | Positive | % positive [CI] | |
| 0−59 d | 5 | 108 | 95.6% [89.8−98.4] | 1 | 112 | 99.1%b [94.6−100] | P = 0.21 |
| 60−119 d | 1 | 81 | 98.8% [92.8−100] | 1 | 81 | 98.8%b [92.8−100] | P = 1.0 |
| 120−179 d | 1 | 51 | 98.1% [88.9-100] | 4 | 48 | 92.3%b,c [81.3−97.5] | P = 0.36 |
| 180−239 d | 2 | 31 | 93.9% [79.4−99.3] | 9 | 24 | 72.7%a,d [55.6−85.1] | P < 0.05 |
| 240−299 d | 1 | 33 | 97.1% [83.8−100] | 12 | 22 | 64.6%d [48.9−78.6] | P < 0.01 |
| 300−365 d | 1 | 22 | 95.7% [77.3−100] | 10 | 13 | 56.5%d [36.8−74.4] | P < 0.01 |
CI = 95% confidence interval.
P < 0.05 vs non-severe,
P < 0.01 vs non-severe,
P < 0.05 vs day 0 to 59,
P < 0.01 vs day 0 to 59,
Anti-S vs anti-N.
3.2. Long-term antibody kinetics
The median antibody levels for anti-S and anti-N were the highest in the 0 to 59 day window in both severe and non-severe patients, followed by an exponential decline (Fig. 2, Fig. 3 and Fig. 2, Fig. 3 , Table 2). In the 0 to 59 day window after positive RT-PCR, the median peak antibody level/cut-off ratio was more than 70 for anti-S compared to less than 6 for anti-N in severe as well as in non-severe patients (P < 0.01 each). After this peak, a waning of antibody levels was observed up to 365 days after positive RT-PCR (Fig. 3). Antibody levels were significantly higher in severe compared to non-severe patients in each 60 day time window up to 300 to 365 days for anti-N and up to 180 to 239 days for anti-S (Supplementary Fig. 1). Anti-S antibody levels remained higher in severe compared to non-severe COVID-19 patients 240 to 299 and 300 to 365 days after positive RT-PCR, but the difference was no longer statistically significant (P = 0.06 and P = 0.13, respectively).
Multiple regression revealed that days post positive RT-PCR and severity were significant predictors of the antibody levels for both anti-S and anti-N. In addition, age but not gender was a significant predictor for anti-S levels, while the opposite was true for anti-N levels. A multiple regression analysis with only days post positive RT-PCR and gender or age (<62 or ≥62 years old, the median age of the severe patient cohort) as covariates confirmed the above-mentioned associations (Supplementary Fig. 2). Results of these multiple regression analyses should, however, be interpreted with caution as the covariates are not independent (e.g. men have a higher risk of severe COVID-19 (see Table 1)).
3.3. Computed half-life and estimated time tot 50% seronegativity
The computed mean half-life between day 60 and 365 after positive PCR (based on a one-phase exponential decay model) for anti-S was 141.6 days [CI: 105.7–214.6] in non-severe and 113.7 days [CI: 89.0–157.4] in severe patients (P = 0.86), with an estimated median time to seronegativity at 809.6 and 785.9 days, respectively (Table 1, P = 0.14). For anti-N, the computed mean half-life was 115.9 days [CI: 99.2–139.2] and 116.3 days [CI: 101.8–135.8] in non-severe compared to severe patients (P = 0.18), with an estimated 50% of patients becoming seronegative at 273.1 and 327.3 days, respectively (Table 1, P = 0.09).
4. Discussion
We studied the dynamics of SARS-CoV-2 IgG anti-S and anti-N antibody levels in non-severe and severe COVID-19 patients up to 365 days after first positive RT-PCR. Significantly more non-severe patients seroconverted for anti-S than for anti-N and anti-N seropositivity declined more rapidly compared to anti-S. This difference in generation and persistence of anti-N versus anti-S antibodies is in line with other studies across different populations and analytical platforms [4,15,[19], [20], [21], [22], [23], [24]]. The fact that most individuals remained seropositive for anti-S up to a year after primary infection confirms other studies with a similar follow-up duration [[13], [14], [15], [16], [17],38]. IgG anti-S and anti-N levels, as well as anti-N seroconversion rate, were significantly higher in patients who had severe COVID-19 compared to non-severe patients, in line with previous studies [16,21,24,32,[36], [37], [38]].
In the seropositive patients of our study, anti-S and anti-N antibody levels decreased exponentially following an initial peak 0-59 days after positive PCR without reaching an apparent plateau at the end of follow-up. A one phase exponential decay during the first months after infection has been described by several reports [4,22] although some authors have suggested that antibody levels after infection display a bi-or even triphasic decay, with slowing of decay 4 to 6 months after infection as long-lived plasma cells and B-memory cells arise [13,17,25,52]. While we could not demonstrate a better fit of a polynomial regression line between antibody levels and days after infection (corresponding to a bi or triphasic antibody decay), this might be attributable to the relatively small number of patients in our study.
Decay of antibody levels led to an estimated median time to seronegativity for anti-S of more than 2 years, compared to less than one year for anti-N despite a similar half-life. Using the Abbott assays, this is attributable to a higher peak antibody level of anti-S compared to anti-N, compared to their respective cut-off values. Knowlegde of the IgG anti-N kinetics is valuable in determining prior infection status in vaccinated individuals. Anti-S antibodies cannot be used for this purpose as these are induced by the vaccines themselves. As assays detecting total Ig anti-N (such as the Roche Elecsys) remain positive for a longer period, these could be more useful for serosurveillance purposes [19,27,28].
The presence of detectable anti-S antibodies translates into residual immunity, as multiple studies have shown protection from reinfection in COVID-19 convalescent individuals for up to a year after primary infection [39], [40], [41], [42]. For estimation of protective immunity on an individual level, quantitative IgG anti-S assays (such as the one from Abbott) might be more useful than total Ig assays given the good correlation of IgG anti-S levels with neutralizing antibody titers and the correlation of neutralizing antibody titers with protection [44,53]. Neutralizing antibody levels also seem to decline exponentially over time in most individuals, similar to the kinetics of quantitative IgG anti-S levels [28,54].
There is not yet a defined antibody “threshold” which identifies individuals with (in)adequate protection against infection and disease. It seems that a lower level of (neutralizing) antibodies is required for protection against severe disease compared to the level required to protect against mild disease or asymptomatic infection [53]. It should be noted that protection is also dependent on other immunological mechanisms such as memory T-cells and B-cells capable of a potent secondary immune response on re-exposure [8,13,55]. Variants of concern further complicate the relation between anti-S antibody levels and protection, as mutations in the spike protein can confer (partial) immune evasion and therefore higher antibody levels are required for protection against such variants [49,53].
The strength of this study is the well characterized cohort including both non-severe and severe COVID-19 patients sampled at multiple time points up to 365 days post positive PCR. Most published studies investigated the immune response in health care workers or plasma donors, who are typically younger than the average hospitalized COVID-19 patient, or patients with relatively mild disease.
There are also a few limitations to our study. First, we did not measure neutralizing antibodies which provide more direct correlation with protective immunity. We also did not asses other components of adaptive immunity such as memory B and T-cells. While IgG anti-S and anti-N antibody levels continued to decrease up to one year post positive PCR, we cannot rule out that antibodies might reach a plateau in some patients after more than 1 year. Finally, there was a difference is sex ratio for the non-severe and severe COVID-19 patients with more severe disease in men. While this difference is in line with other studies, it might (slightly) influence antibody kinetics [56,57].
5. Conclusions
SARS-CoV-2 IgG anti-S and anti-N antibody levels decreased exponentially after 59 days up to 365 days after positive RT-PCR. IgG anti-S antibodies were positive in 81.8% of non-severe COVID-19 and 95.7% of severe COVID-19 patients 300 to 365 days after positive PCR compared to only 30.3% of non-severe and 56.5% of severe patients for IgG anti-N. Antibody levels were significantly higher in severe compared to non-severe patients up to 240 days for anti-S and up to 365 days for anti-N. Estimated median time to seronegativity was more than 2 years for anti-S compared to less than 1 year for anti-N despite similar half-lives, due to the higher peak antibody level/cut-off ratio for anti-S compared to anti-N.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author's contributions
PV conceived the study. JVE, MO and SVDD conducted the experiments. PV and JVE analyzed the data and drafted the manuscript. All authors critically reviewed the manuscript.
Declaration of competing interests
Dr. Vermeersch reports personal fees from Roche, outside the submitted work. Dr. Lagrou reports personal fees and nonfinancial support from Pfizer, personal fees and nonfinancial support from MSD, personal fees from SMB Laboratoires, personal fees from Gilead, and personal fees from FUJIFILM Wako, outside the submitted work. The other authors state no conflicts of interests.
Acknowledgments
P. Vermeersch is a senior clinical investigator of the FWO-Vlaanderen. We thank Aline Somers, Kaat D'hooghe, Gitte Op de Beeck, Eveline Nys, Joyce Hanssens, Karla Hendrickx, Katrin De Neve, Lore Hendrickx, Siel Briké and Insafe Aouragh for their technical assistance in the storage, retrieval and analysis of the left-over patient samples.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.diagmicrobio.2022.115659.
Appendix. Supplementary materials
References
- 1.Anand SP, Prévost J, Nayrac M, Beaudoin-Bussières G, Benlarbi M, Gasser R, et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Reports Med. 2021;2 doi: 10.1016/J.XCRM.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherina N, Piralla A, Du L, Wan H, Kumagai-Braesch M, Andréll J, et al. Persistence of SARS-CoV-2 specific B- and T-cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med. 2021;2:281–295. doi: 10.1016/j.medj.2021.02.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.L'Huillier AG, Meyer B, Andrey DO, Arm-Vernez I, Baggio S, Didierlaurent A, et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dan JM, Mateus J, Kato Y, Hastie KM, Faliti CE, Ramirez SI, et al. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. Science (80-) 2020;371:eabf4063. doi: 10.1101/2020.11.15.383323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/S41586-021-03207-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Elslande J, Houben E, Depypere M, Brackenier A, Desmet S, André E, et al. Diagnostic performance of 7 rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect. 2020;26:1082–1087. doi: 10.1016/J.CMI.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Elslande J, Decru B, Jonckheere S, Van Wijngaerden E, Houben E, Vandecandelaere P, et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect. 2020;26:1557.e1–1557.e7. doi: 10.1016/j.cmi.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/nejmc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021:1–10. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobaño C, Ramírez-Morros A, Alonso S, Vidal-Alaball J, Ruiz-Olalla G, Vidal M, et al. Persistence and baseline determinants of seropositivity and reinfection rates in health care workers up to 12.5 months after COVID-19. BMC Med. 2021;19:1–6. doi: 10.1186/S12916-021-02032-2/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masiá M, Fernández-González M, Telenti G, Agulló V, García JA, Padilla S, et al. Durable antibody response one year after hospitalization for COVID-19: a longitudinal cohort study. J Autoimmun. 2021;123 doi: 10.1016/J.JAUT.2021.102703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallais F, Gantner P, Bruel T, Velay A, Planas D, Wendling MJ, et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine. 2021;71 doi: 10.1016/J.EBIOM.2021.103561/ATTACHMENT/32CCED3D-A1F0-49CA-B63D-9AC6789706B2/MMC2.DOCX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosati M, Terpos E, Ntanasis-Stathopoulos I, Agarwal M, Bear J, Burns R, et al. Sequential analysis of binding and neutralizing antibody in COVID-19 convalescent patients at 14 months after SARS-CoV-2 infection. Front Immunol. 2021;12 doi: 10.3389/FIMMU.2021.793953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng F, Wu M, Wang J, Li J, Hu G, Wang L. Over 1-year duration and age difference of SARS-CoV-2 antibodies in convalescent COVID-19 patients. J Med Virol. 2021;93:6506–6511. doi: 10.1002/JMV.27152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. J Infect Dis. 2020;223:389–398. doi: 10.1101/2020.08.05.20169128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolotin S, Tran V, Osman S, Brown KA, Buchan SA, Joh E, et al. SARS-CoV-2 seroprevalence survey estimates are affected by anti-nucleocapsid antibody decline. J Infect Dis. 2021 doi: 10.1093/infdis/jiaa796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Elslande J, Gruwier L, Godderis L, Vermeersch P. Estimated half-life of SARS-CoV-2 anti-spike antibodies more than double the half-life of anti-nucleocapsid antibodies in healthcare workers. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumley SF, Wei J, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, et al. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpos E, Stellas D, Rosati M, Sergentanis TN, Hu X, Politou M, et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chansaenroj J, Yorsaeng R, Posuwan N, Puenpa J, Wanlapakorn N, Sudhinaraset N, et al. Long-term specific IgG response to SARS-CoV-2 nucleocapsid protein in recovered COVID-19 patients. Sci Rep. 2021;11 doi: 10.1038/S41598-021-02659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021:1–5. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 26.Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Reports Med. 2021;2 doi: 10.1016/J.XCRM.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favresse J, Eucher C, Elsen M, Gillot C, Van Eeckhoudt S, Dogné JM, et al. Persistence of anti-sars-cov-2 antibodies depends on the analytical kit: a report for up to 10 months after infection. Microorganisms. 2021;9:1–13. doi: 10.3390/microorganisms9030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Germanio C, Simmons G, Kelly K, Martinelli R, Darst O, Azimpouran M, et al. SARS-CoV-2 antibody persistence in COVID-19 convalescent plasma donors: Dependency on assay format and applicability to serosurveillance. Transfusion. 2021 doi: 10.1111/trf.16555. trf.16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kral S, Banfi C, Niedrist T, Sareban N, Guelly C, Kriegl L, et al. Long-lasting immune response to a mild course of PCR-confirmed SARS-CoV-2 infection: A cohort study. J Infect. 2021;83:607–635. doi: 10.1016/J.JINF.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Saez J, Zaballa ME, Yerly S, DO Andrey, Meyer B, Eckerle I, et al. Persistence of anti-SARS-CoV-2 antibodies: immunoassay heterogeneity and implications for serosurveillance. Clin Microbiol Infect. 2021;27:1695.e7–1695.e12. doi: 10.1016/J.CMI.2021.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jääskeläinen AJ, Kuivanen S, Kekäläinen E, Ahava MJ, Loginov R, Kallio-Kokko H, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang ML, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/nejmoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuler CF, Gherasim C, O'Shea K, Manthei DM, Chen J, Zettel C, et al. Mild SARS-CoV-2 Illness is not associated with reinfections and provides persistent spike, nucleocapsid, and virus-neutralizing antibodies. Microbiol Spectr. 2021;9 doi: 10.1128/SPECTRUM.00087-21/SUPPL_FILE/SPECTRUM00087-21_SUPP_1_SEQ4.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Elslande J, Oyaert M, Ailliet S, Van Ranst M, Lorent N, Vande Weygaerde Y, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136 doi: 10.1016/j.jcv.2021.104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peghin M, De Martino M, Fabris M, Palese A, Visintini E, Graziano E, et al. The fall in antibody response to SARS-CoV-2: a longitudinal study of asymptomatic to critically Ill patients up to 10 months after recovery. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.01138-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choe PG, Kang CK, Kim KH, Yi J, Kim ES, Park SW, et al. Persistence of neutralizing antibody response up to 1 year after asymptomatic or symptomatic SARS-CoV-2 infection. J Infect Dis. 2021;224:1097–1099. doi: 10.1093/INFDIS/JIAB339. [DOI] [PubMed] [Google Scholar]
- 39.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/s0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima N, Klausner JD. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:12–14. doi: 10.1016/S1473-3099(21)00676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilz S, Chakeri A, Ioannidis JPA, Richter L, Theiler-Schwetz V, Trummer C, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51 doi: 10.1111/ECI.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021;385:2487–2489. doi: 10.1056/NEJMC2108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021:1–4. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMOA2109072/SUPPL_FILE/NEJMOA2109072_DATA-SHARING.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMOA2114228/SUPPL_FILE/NEJMOA2114228_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizrahi B, Lotan R, Kalkstein N, Peretz A, Perez G, Ben-Tov A, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun. 2021;12:1–5. doi: 10.1038/s41467-021-26672-3. 2021 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/JAMA.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt F, Muecksch F, Weisblum Y, Silva J Da, Bednarski E, Cho A, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. 2021. 10.1056/NEJMC2119641. [DOI] [PMC free article] [PubMed]
- 50.Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, et al. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Achiron A, Gurevich M, Falb R, Dreyer-Alster S, Sonis P, Mandel M. SARS-CoV-2 antibody dynamics and B-cell memory response over time in COVID-19 convalescent subjects. Clin Microbiol Infect. 2021;1 doi: 10.1016/j.cmi.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 54.Lau EHY, Tsang OTY, Hui DSC, Kwan MYW, hung Chan W, Chiu SS, et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat Commun. 2021;12:1–7. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lustig Y, Phd I, Mandelboim M, Mendelson E, Sackler) ;, Lustig Y, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers 2021. 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed]
- 57.Vassilaki N, Gargalionis AN, Bletsa A, Papamichalopoulos N, Kontou E, Gkika M, et al. Impact of age and sex on antibody response following the second dose of COVID-19 BNT162b2 mRNA vaccine in Greek healthcare workers. Microorg. 2021;9:1725. doi: 10.3390/MICROORGANISMS9081725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.