Abstract
Members of the genus Malassezia, lipophilic yeasts, are considered to be one of the exacerbating factors in atopic dermatitis (AD). We examined variation in cutaneous colonization by Malassezia species in AD patients and compared it with variation in healthy subjects. Samples were collected by applying transparent dressings to the skin lesions of AD patients. DNA was extracted directly from the dressings and amplified in a specific nested PCR assay. Malassezia-specific DNA was detected in all samples obtained from 32 AD patients. In particular, Malassezia globosa and M. restricta were detected in approximately 90% of the AD patients and M. furfur and M. sympodialis were detected in approximately 40% of the cases. The detection rate was not dependent on the type of skin lesion. In healthy subjects, Malassezia DNA was detected in 78% of the samples, among which M. globosa, M. restricta, and M. sympodialis were detected at frequencies ranging from 44 to 61%, with M. furfur at 11%. The diversity of Malassezia species found in AD patients was greater (2.7 species detected in each individual) than that found in healthy subjects (1.8 species per individual). Our results suggest that M. furfur, M. globosa, M. restricta, and M. sympodialis are common inhabitants of the skin of both AD patients and healthy subjects, while the skin microflora of AD patients shows more diversity than that of healthy subjects. To our knowledge, this is the first report of the use of a nested PCR as an alternative to fungal culture for analysis of the distribution of cutaneous Malassezia spp.
Members of the genus Malassezia, lipophilic yeasts, colonize the skin of the head, neck, and shoulders of humans and are one of the causative factors in pityriasis versicolor and seborrheic dermatitis (3). Malassezia species are also considered to be one of the factors that exacerbate atopic dermatitis (AD), based on the finding that AD patients (but not healthy subjects) have specific serum immunoglobulin E (IgE) antibodies against Malassezia spp. (9, 22, 23). Application of topical antimycotic agents to AD patients decreases Malassezia colonization and the severity of eczematous lesions (2), suggesting that Malassezia species play a role in AD. In addition, several candidate Malassezia antigens have been implicated in the pathogenesis of AD (10, 11, 16, 17, 19, 24).
The taxonomy of the genus Malassezia was recently revised, primarily by using rRNA gene sequences, into seven species: M. furfur, M. globosa, M. obtusa, M. restricta, M. pachydermatis, M. slooffiae, and M. sympodialis (4, 5, 6). M. globosa, M. obtusa, M. restricta, and M. slooffiae were formerly designated M. furfur. The frequency of isolation of each species and its correlation with the clinical manifestations of AD have not been well investigated. Studies examining colonization by Malassezia spp. may aid in the understanding of the mechanism of AD and the development of an effective treatment. Due to the difficulties inherent in culturing Malassezia spp., we analyzed the cutaneous Malassezia microflora directly from the skin lesions of AD patients by using a nested PCR.
MATERIALS AND METHODS
Subjects.
Thirty-two AD outpatients at Juntendo University Hospital were included in this study. As a comparison group of healthy subjects, 18 students at Meiji Pharmaceutical University who were negative for anti-Malassezia-specific IgE antibody were also included.
Sample collection.
Malassezia samples were collected by applying OpSite transparent dressings (3 by 7 cm; Smith and Nephew Medical Ltd., Hull, United Kingdom) to the skin of AD patients and healthy subjects. Samples were collected from skin lesions (erosive, erythematous, and lichenoid) on the scalps, backs, and napes of AD patients. Patients had been treated intermittently by topical application of medium- to high-strength steroid ointment in a petrolatum base. Samples were collected from the scalps and napes of healthy subjects.
DNA extraction.
The collected OpSite dressing was placed in 1.5 ml of lysing solution (100 mM Tris-HCl [pH 8.0], 30 mM EDTA [pH 8.0], 0.5% sodium dodecyl sulfate) and incubated for 15 min at 100°C. The OpSite dressing was then removed from the tube, and the suspension was extracted with phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol). Subsequently, the samples were extracted with chloroform-isoamyl alcohol (24:1, vol/vol) and the DNA was precipitated with 2-propanol, using Ethatimate (Nippon Gene, Toyama, Japan) as a precipitation activator. The DNA pellet was resuspended in 50 μl of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8.0]). An unused OpSite dressing was used as a negative control.
Detection of Malassezia DNA by nested PCR.
Nested PCR was conducted by using two sets of primers as shown in Table 1. The species-specific primers were derived from the internal transcribed spacer region of the rRNA gene (13). Internal transcribed spacer sequences were obtained from GenBank (accession numbers AB019329 to AB019350). Extracted DNA (20 μl) from each sample was added to 30 μl of the PCR master mixture, which consisted of 5 μl of 10× PCR buffer (100 mM Tris-HCl [pH 8.3], 500 mM KCl, 15 mM MgCl2; Takara Inc., Shiga, Japan), 4 μl of 200 μM deoxynucleoside triphosphates (an equimolar mixture of dATP, dCTP, dGTP, and dTTP; Takara), 30 pmol of each primer, and 2.5 U of Ex Taq DNA polymerase (Takara). PCR was performed in a thermocycler (model 9700; Applied Biosystems, Foster City, Calif.) with an initial denaturation of 94°C for 3 min, followed by 30 cycles of 30 s at 94°C, 1 min at 57°C, and 50 s at 72°C and a final extension at 72°C for 10 min. In the nested PCR step, 1 μl of the first amplification product was added to a new reaction mixture with the same composition as the first. The PCR procedure consisted of an initial denaturation of 94°C for 3 min, followed by 30 cycles of 30 s at 94°C, 1 min at 62°C, and 40 s at 72°C and a final extension at 72°C for 10 min. The PCR products were cloned by using a TA Cloning Kit (Invitrogen Corp., Carlsbad, Calif.) and sequenced with an ABI PRISM Cycle Sequencing Kit (Applied Biosystems) in accordance with the manufacturer's instructions.
TABLE 1.
Oligonucleotides used in the nested PCR
| Test, species, and primers used | Sequence (5′→3′) | DNA fragment size (bp) |
|---|---|---|
| 1st PCR for all 7 Malassezia species | ||
| ITSIF-N | GGATCATTAGTGATTGCCTTTATA | |
| ITS4-R | TCCTCCGCTTATTGATATG | |
| 2nd PCR for each Malassezia species | ||
| M. furfur | 230 | |
| M.f-F | CTACTCGCGTACAACGTCTCTG | |
| 5.8S-R | TTCGCTGCGTTCTTCATCGA | |
| M. globosa | 270 | |
| M.gl-F | CAATAAGTGTGTCTCTGCGG | |
| 5.8S-R | TTCGCTGCGTTCTTCATCGA | |
| M. obtusa | 180 | |
| M. ob-F | ACCCGTGTGCACACTGTTGAG | |
| 5.8S-R | TTCGCTGCGTTCTTCATCGA | |
| M. pachydermatis | 220 | |
| M.pa-F | CTGCCATACGGATGCGCAAG | |
| 5.8S-R | TTCGCTGCGTTCTTCATCGA | |
| M. restricta | 320 | |
| M.rt-F | CTTGGTTGGACCGTCACTGG | |
| M.rt-R | AGGCGGATGCAAAGTGTCTC | |
| M. slooffiae | 230 | |
| M.sl-F | ACGCACGCTAACACAACGTG | |
| 5.8S-R | TTCGCTGCGTTCTTCATCGA | |
| M. sympodialis | 190 | |
| M.sy-F | CGGACGCAAACACGTCTCTG | |
| 5.8S-R | TTCGCTGCGTTCTTCATCGA |
Specificity of the PCR assay.
Table 2 shows the strains used to investigate the specificity of the PCR assay. For basidiomycetous yeasts, DNA was extracted by the method of Makimura et al. (12). For ascomycetous yeasts, a DNA extraction kit (Nucleon MiY; Amersham International plc, Little Chalfont, United Kingdom) was used.
TABLE 2.
Specificity of primers for seven Malassezia species
| Species and strain(s): | Specificity of oligonucleotide primersb for:
|
||||||
|---|---|---|---|---|---|---|---|
| M. furfur | M. globosa | M. obtusa | M. pachydermatis | M. restricta | M. slooffiae | M. sympodialis | |
| Malassezia furfur | |||||||
| CBS 1878a | + | − | + | − | − | − | − |
| CBS 7981 | + | − | + | − | − | − | − |
| Isolate MF1 | + | − | + | − | − | − | − |
| Isolate MF2 | + | − | + | − | − | − | − |
| Isolate MF3 | + | − | + | − | − | − | − |
| Malassezia globosa | |||||||
| CBS 7966 | − | + | − | − | − | − | − |
| CBS 7990 | − | + | − | − | − | − | − |
| Isolate MG1 | − | + | − | − | − | − | − |
| Isolate MG2 | − | + | − | − | − | − | − |
| Isolate MG3 | − | + | − | − | − | − | − |
| Malassezia obtusa | |||||||
| CBS7876 | + | − | + | − | − | − | − |
| Malassezia pachydermatis | |||||||
| CBS 1879 | − | − | − | + | − | − | − |
| CBS 7044 | − | − | − | + | − | − | − |
| Malassezia restricta | |||||||
| CBS 7991 | − | − | − | − | + | − | − |
| CBS 7877 | − | − | − | − | + | − | − |
| Isolate MR1 | − | − | − | − | + | − | − |
| Isolate MR2 | − | − | − | − | + | − | − |
| Isolate MR3 | − | − | − | − | + | − | − |
| Malassezia slooffiae | |||||||
| CBS 7956 | − | − | − | − | − | + | − |
| CBS 7972 | − | − | − | − | − | + | − |
| Malassezia sympodialis | |||||||
| CBS 7222 | − | − | − | − | − | − | + |
| CBS 7977 | − | − | − | − | − | − | + |
| Isolate MS1 | − | − | − | − | − | − | + |
| Isolate MS2 | − | − | − | − | − | − | + |
| Isolate MS3 | − | − | − | − | − | − | + |
| Candida albicans CBS 562 | − | − | − | − | − | − | − |
| Candida glabrata CBS 160 | − | − | − | − | − | − | − |
| Candida guilliermondii CBS 566 | − | − | − | − | − | − | − |
| Candida parapsilosis CBS 604 | − | − | − | − | − | − | − |
| Cryptococcus albidus CBS 142 | − | − | − | − | − | − | − |
| Rhodotorula mucilaginosa CBS 17 | − | − | − | − | − | − | − |
| Trichosporon asahii CBS 2479 | − | − | − | − | − | − | − |
| Staphylococcus aureus | |||||||
| Isolate SA1 | − | − | − | − | − | − | − |
| Isolate SA2 | − | − | − | − | − | − | − |
Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
+, product obtained; −, no product obtained.
Anti-Malassezia IgE antibody detection.
Specific IgE antibodies in the sera of AD patients and healthy controls were assayed by using AlaSTAT (Nippon DPC, Tokyo, Japan). Reactivity was defined in terms of antibody titers (14) as positive (more than 0.35 IU/ml) or negative (less than 0.35 IU/ml).
Statistical analysis.
Fisher's exact probability test was applied to analyze the differences in Malassezia DNA detection between AD patients and healthy subjects.
RESULTS
Sensitivity and specificity of the nested PCR assay.
The sensitivity of the PCR assay using two pairs of nested oligonucleotides was examined by using purified Malassezia DNAs from cultures. The limit of detection of purified DNA by the nested PCR assay was 1 fg. The species specificity of the nested PCR assay is shown in Table 2. Primers for the differentiation M. furfur from M. obtusa could not be prepared, since the M. furfur primer also amplified M. obtusa DNA. Therefore, all of the PCR products amplified by the primers for M. furfur and M. obtusa were cloned and the DNA was sequenced. The primers for the other four Malassezia species were species specific and amplified DNA only from the target Malassezia species. The primers did not amplify the DNA of other clinically relevant pathogenic yeast species or from Staphylococcus aureus, a common skin-colonizing bacterium.
Detection of Malassezia DNA.
Table 3 shows the rates of Malassezia DNA detection in samples from AD patients and healthy subjects. We collected 58 samples from skin lesions (erosive, erythematous, and lichenoid) of 32 AD patients. DNAs of M. restricta and M. globosa were detected in 61.5 to 86.7% and 73.3 to 80.8%, respectively, of the samples taken from the three different types of skin lesion, with DNAs of M. sympodialis and M. furfur detected less frequently (26.9 to 35.3% and 23.1 to 47.1%, respectively). M. slooffiae DNA was found in less than 6.7% of the samples, and neither M. obtusa nor M. pachydermatis DNA was detected in any of the 58 samples from AD patients. The extent of Malassezia flora appeared to be independent of the type of skin lesion in which it was detected. Overall, Malassezia DNA was present in 86.7 to 96.2% of the samples from each type of skin eruption. M. restricta and M. globosa were the most common species detected in AD patients (87.5 and 93.8%, respectively), followed by M. sympodialis and M. furfur (40.6%). In healthy subjects, the DNAs of M. restricta, M. globosa, and M. sympodialis were detected in 61.1, 44.4, and 50.0%, respectively, and that of M. furfur was detected in 11.1%, while M. slooffiae, M. obtusa, and M. pachydermatis DNAs were not detected. Altogether, Malassezia DNA was detected in 77.8% of the samples from healthy subjects. A comparison of the DNA detection frequencies revealed that Malassezia DNA sequences, especially those of M. restricta, M. globosa, and M. furfur, were present at a significantly higher frequency in samples from AD patients than in those from healthy subjects (P < 0.05). In contrast, M. sympodialis was detected at the same rate in both AD patients and healthy subjects (P > 0.05).
TABLE 3.
Detection of Malassezia DNA in AD patients and healthy subjects
| Subjects | No. of patients | No. of samples | Detection of DNA (%) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Malassezia spp. | M. restricta | M. globosa | M. sympodialis | M. furfur | M. slooffiae | M. obtusa | M. pachydermatis | |||
| AD patients | 32 | 100a | 87.5 | 93.8 | 40.6 | 40.6 | 6.3 | 0 | 0 | |
| Types of lesions: | ||||||||||
| Erosive | 15 | 17 | 88.2b | 82.4 | 70.6 | 35.3 | 47.1 | 5.9 | 0 | 0 |
| Erythematous | 24 | 26 | 96.2b | 61.5 | 80.8 | 26.9 | 23.1 | 0 | 0 | 0 |
| Lichenoid | 13 | 15 | 86.7b | 86.7 | 73.3 | 33.3 | 26.7 | 6.7 | 0 | 0 |
| Total | 58 | 91.4b | 74.1 | 75.9 | 31.0 | 31.0 | 3.4 | 0 | 0 | |
| Healthy persons | 18 | 27 | 77.8a | 61.1 | 44.4 | 50.0 | 11.1 | 0 | 0 | 0 |
| 81.5b | 59.3 | 48.1 | 40.7 | 3.7 | 0 | 0 | 0 | |||
Value is a percentage of the number of patients.
Value is a percentage of the number of samples.
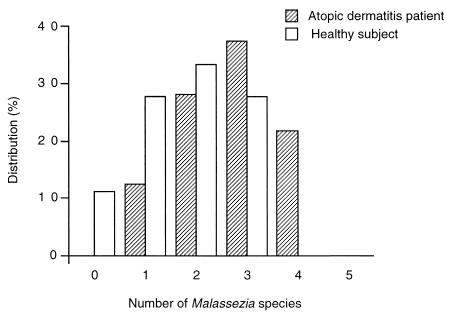
Diversity of Malassezia species among individuals.
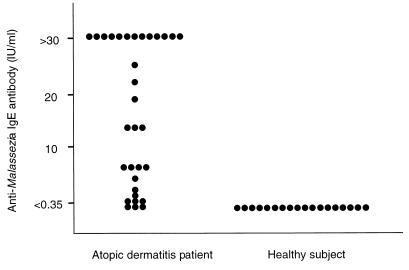
The number of Malassezia species detected in each individual is shown in Fig. 1. Samples from AD patients contained, on average, 2.7 ± 0.9 species, compared with an average of 1.8 ± 1.0 species in samples from healthy subjects. Additionally, AD patients showed increased sensitization to Malassezia antigens (Fig. 2). Anti-Malassezia IgE antibody was detected (>0.35 IU/ml) in 29 (91%) of the 32 AD patients, while none of the healthy subjects showed a positive reaction (P < 0.001). This corroborates previous observations of specific IgE antibody in AD patients and healthy controls (7, 14, 18, 20, 23).
FIG. 1.
The number of Malassezia species detected in AD patients and healthy subjects.
FIG. 2.
Anti-Malassezia IgE antibody in 32 AD patients and in 18 healthy controls.
DISCUSSION
Since the Malassezia taxonomy was revised, only a few studies have examined human Malassezia microflora, although M. furfur has long been thought to be a major component of the microflora of human skin. Leeming et al. (8) analyzed the occurrence of Malassezia species in 20 patients with pityriasis versicolor. They reported that Malassezia species were isolated from the skin surfaces of the foreheads (95%) and backs (100%) of these patients. Three species were detected, M. restricta, M. globosa, and M. sympodialis. The predominant species colonizing each site varied; e.g., M. restricta (95%) was isolated mainly from the forehead whereas M. sympodialis (95%) was predominant in samples taken from other surface areas of the head. Aspiroz et al. (1) isolated 120 Malassezia strains from the chest, back, and scalp areas of 38 healthy subjects. These strains were divided into three main Malassezia species, namely, M. restricta, M. globosa, and M. sympodialis. These two studies suggest that patients with pityriasis versicolor and healthy subjects have three Malassezia species on their skin in common. Our results confirmed that these three species predominate on the skin of healthy subjects. Although neither Leeming et al. (8) nor Aspiroz et al. (1) described the isolation of strains of M. furfur from human skin, we commonly found this species on the skin of both AD patients and healthy subjects. Recently, Nakabayashi et al. (15) compared Malassezia species by using colonies collected from the skin of patients with AD, seborrheic dermatitis, or pityriasis versicolor and normal subjects. In 13 (46%) of the 28 AD patients, Malassezia species were isolated from skin lesions, including M. furfur (21%), M. globosa (14%), M. sympodialis (7%), and M. slooffiae (4%). M. restricta was not isolated from the AD patients but was isolated from 0 to 2% of the patients with seborrheic dermatitis, 0% of the patients with pityriasis versicolor, and 1% of the normal subjects. These results are very different from ours. The cause of the discrepancy is most likely the fact that isolation of a pure, single colony of a Malassezia species uncontaminated by other yeasts is sometimes difficult. In addition, the efficiency of culturing of Malassezia strains may depend on the isolation medium employed. Indeed, Aspiroz et al. (1) pointed out that Malassezia recovery differed, depending on whether Dixon's or Leeming and Notman agar medium was used. Considering these facts, a molecular analysis-based nonculture method appears to be the most reliable and appropriate method by which to analyze in situ Malassezia distribution, since the isolation media and procedures used do not influence such a method.
In conclusion, we have determined that although Malassezia species are common on the skin of both AD patients and healthy subjects, the production of antibodies differs significantly between AD patients and healthy subjects. We feel, as do Terui et al. (21), that this is due to the disrupted barrier function of the skin surface and the effects of scratching-induced sensitization to the organisms.
ACKNOWLEDGMENTS
This study was supported in part by a Grant for the Promotion of the Advancement of Education and Research in Graduate Schools from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We are grateful to Hiroshi Kato for kindly measuring the anti-Malassezia IgE antibodies of healthy subjects. We thank Haruka Yamadaya and Kaori Takebayashi for technical assistance.
REFERENCES
- 1.Aspiroz C, Moreno L A, Rezusta A, Rubio C. Differentiation of three biotypes of Malassezia species on human normal skin: correspondence with M. globosa, M. sympodialis and M. restricta. Mycopathologia. 1999;145:69–74. doi: 10.1023/a:1007017917230. [DOI] [PubMed] [Google Scholar]
- 2.Back O, Scheynius A, Johansson S G. Ketoconazole in atopic dermatitis: therapeutic response is correlated with decrease in serum IgE. Arch Dermatol Res. 1995;287:448–451. doi: 10.1007/BF00373427. [DOI] [PubMed] [Google Scholar]
- 3.Guého E, Boekhout T, Ashbee H R, Guillot J, van Belkum A, Faergemann J. The role of Malassezia species in the ecology of human skin and as pathogens. Med Mycol. 1998;36(Suppl. 1):220–229. [PubMed] [Google Scholar]
- 4.Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie van Leeuwenhoek. 1996;69:337–355. doi: 10.1007/BF00399623. [DOI] [PubMed] [Google Scholar]
- 5.Guillot J, Guého E. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie van Leeuwenhoek. 1995;67:297–314. doi: 10.1007/BF00873693. [DOI] [PubMed] [Google Scholar]
- 6.Guillot J, Guého E, Chermette R. Confirmation of the nomenclatural status of Malassezia pachydermatis. Antonie van Leeuwenhoek. 1995;67:173–176. doi: 10.1007/BF00871211. [DOI] [PubMed] [Google Scholar]
- 7.Kim T Y, Jang I G, Park Y M, Kim H O, Kim C W. Head and neck dermatitis: the role of Malassezia furfur, topical steroid use and environmental factors in its causation. Clin Exp Dermatol. 1999;24:226–231. doi: 10.1046/j.1365-2230.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 8.Leeming J P, Sansom J E, Burton J L. Susceptibility of Malassezia furfur subgroups to terbinafine. Br J Dermatol. 1997;137:764–767. [PubMed] [Google Scholar]
- 9.Leung D Y. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–318. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 10.Lindborg M, Magnusson C G, Zargari A, Schmidt M, Scheynius A, Crameri R, Whitley P. Selective cloning of allergens from the skin colonizing yeast Malassezia furfur by phage surface display technology. J Investig Dermatol. 1999;113:156–161. doi: 10.1046/j.1523-1747.1999.00661.x. [DOI] [PubMed] [Google Scholar]
- 11.Lintu P, Savolainen J, Kalimo K. IgE antibodies to protein and mannan antigens of Pityrosporum ovale in atopic dermatitis patients. Clin Exp Allergy. 1997;27:87–95. [PubMed] [Google Scholar]
- 12.Makimura K, Murayama Y S, Yamaguchi H. Detection of a wide range of medically important fungal species by polymerase chain reaction (PCR) J Med Microbiol. 1994;40:358–364. doi: 10.1099/00222615-40-5-358. [DOI] [PubMed] [Google Scholar]
- 13.Makimura K, Tamura Y, Kudo M, Uchida K, Saito H, Yamaguchi H. Species identification and strain typing of Malassezia species stock strains and clinical isolates based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Med Microbiol. 2000;49:29–35. doi: 10.1099/0022-1317-49-1-29. [DOI] [PubMed] [Google Scholar]
- 14.Mukai H, Kaneko S, Saito N, Nagase A, Arai S, Hiramatsu M, Kato H. Clinical significant of Malassezia furfur specific IgE antibody in atopic dermatitis. Jpn J Allergol. 1997;46:26–33. [PubMed] [Google Scholar]
- 15.Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000;38:337–341. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- 16.Onishi Y, Kuroda M, Yasueda H, Saito A, Sono-Koyama E, Tunasawa S, Hashida-Okado T, Yagihara T, Uchida K, Yamaguchi H, Akiyama K, Kato I, Takesako K. Two- dimensional electrophoresis of Malassezia allergens for atopic dermatitis and isolation of Mal f 4 homologs with mitochondrial malate dehydrogenase. Eur J Biochem. 1999;261:148–154. doi: 10.1046/j.1432-1327.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 17.Rasool O, Zargari A, Almqvist J, Eshaghi H, Whitley P, Scheynius A. Cloning, characterization and expression of complete coding sequences of three IgE binding Malassezia furfur allergens, Mal f 7, Mal f 8 and Mal f 9. Eur J Biochem. 2000;267:4355–4361. doi: 10.1046/j.1432-1327.2000.01475.x. [DOI] [PubMed] [Google Scholar]
- 18.Scalabrin D M, Bavbek S, Perzanowski M S, Wilson B B, Platts-Mills T A, Wheatley L M. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol. 1999;104:1273–1279. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Zargari A, Holt P, Lindbom L, Hellman U, Whitley P, van der Ploeg I, Harfast B, Scheynius A. The complete cDNA sequence and expression of the first major allergenic protein of Malassezia furfur, Mal f 1. Eur J Biochem. 1997;246:181–185. doi: 10.1111/j.1432-1033.1997.00181.x. [DOI] [PubMed] [Google Scholar]
- 20.Tengvall Linder M, Johansson C, Scheynius A, Wahlgren C. Positive atopy patch test reactions to Pityrosporum orbiculare in atopic dermatitis patients. Clin Exp Allergy. 2000;30:122–131. doi: 10.1046/j.1365-2222.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- 21.Terui T, Kudo K, Tagami H. Cutaneous immune and inflammatory reactions to Malassezia furfur. Jpn J Med Mycol. 1999;40:63–67. doi: 10.3314/jjmm.40.63. [DOI] [PubMed] [Google Scholar]
- 22.Werfel T, Kapp A. Environmental and other major provocation factors in atopic dermatitis. Allergy. 1998;53:731–739. doi: 10.1111/j.1398-9995.1998.tb03968.x. [DOI] [PubMed] [Google Scholar]
- 23.Wessels M W, Doekes G, Van Ieperen-Van Kijk A G, Koers W J, Young E. IgE antibodies to Pityrosporum ovale in atopic dermatitis. Br J Dermatol. 1991;125:227–232. doi: 10.1111/j.1365-2133.1991.tb14745.x. [DOI] [PubMed] [Google Scholar]
- 24.Yasueda H, Hashida-Okado T, Saito A, Uchida K, Kuroda M, Onishi Y, Takahashi K, Yamaguchi H, Takesako K, Akiyama K. Identification and cloning of two novel allergens from the lipophilic yeast Malassezia furfur. Biochem Biophys Res Commun. 1998;248:240–244. doi: 10.1006/bbrc.1998.8944. [DOI] [PubMed] [Google Scholar]