Abstract
Skeletal muscle tissue explants have been cultured and studied for nearly 100 years. These cultures, which retain complex tissue structure in an environment suited to precision manipulation and measurement, have led to seminal discoveries of the extrinsic and intrinsic mechanisms regulating contractility, metabolism and regeneration. This review discusses the two primary models of muscle explant: isolated myofiber and intact muscle. It reviews the unique challenges and capabilities of each, with a focus on impactful current and future novel applications.
Keywords: Isolated myofiber, Intact muscle, In-vitro culture, Ex-vivo stimulation, Satellite cell
Introduction
The culture of isolated mammalian cells has dramatically changed the landscape of medicine; advancing our understanding of tissue regeneration and driving the discovery of drugs and vaccines to fight disease. However, as we continue to devise new ways to harness the potential of cells in-vivo we are becoming increasingly aware of the limitations of culture in-vitro. Specifically, despite remarkable advances in micro- and nano-engineering, we are still unable to fully recapitulate the structural and cellular complexity of most in-vivo tissues – a complexity that influences numerous cellular functions. Advances in genetic engineering and imaging technology have enabled select studies to overcome this limitation by moving experiments in-vivo, but many cells and processes remain inaccessible in-vivo, necessitating in-vitro experimentation. The culture of excised tissues and organs (i.e. “explants”) provides an attractive middle-ground for experimentation where important features of tissue complexity can be maintained in an environment compatible with manipulation and visualization. Thus, explant culture has dramatically expanded from the study of embryonic development initiated in the 1920s [1,2] to the study of mature cellular processes in numerous mammalian tissues from the brain to the gut.
Though the term “skeletal muscle explant” is rarely used, isolated portions of muscle have been cultured to examine cellular function since the 1930s [3,4]. These “explants” are of two main types: 1) isolated intact muscle fibers (myofibers) used predominately to study progenitor (satellite) cell dynamics and 2) isolated intact muscles used predominately to study myofiber contractility and signaling. Like other explant models, these improve on traditional cell culture systems by maintaining complex in-vivo cell-cell and cell-matrix interactions. However, unlike many explant models, both muscle preparations are delicate and challenging to prepare. They require myofibers to remain intact and undamaged from tendon to tendon and be sufficiently thin to enable oxygen and nutrients to reach the explant core. Thus, mammalian preparations are limited to a few specific muscles of the mouse or rat and are not compatible with biopsy or minced tissue. Fortunately, a number of groups have diligently refined these models over the past 80 years and explant culture of muscle has provided critical mechanistic insights into the early events in development and regeneration as well as intracellular mechanical, electrical and biochemical signaling pathways.
This review will focus on the unique attributes of skeletal muscle explant culture and the applications of existing models. It will predominately cover rodent models, which are the most commonly used today, with some brief discussion of the history of amphibian models and the future of human models. It will also focus on extended culture experiments, as the totality of experiments using excised muscle extend beyond the scope of this review. We will finally cover recent work broadening the utility of these models and discuss some advances in tissue engineering that are helping to define the critical features of muscle survival in-vitro.
The structural complexity of skeletal muscle
No review of skeletal muscle would be complete without a discussion of hierarchical cellular organization (Fig 1). This is because muscle force generation, passive loadbearing and metabolic function are all highly dependent on the alignment and connectivity of various cells and structures. In fact, myofibers are so dependent on their complex extracellular environment that genetic mutation to seemingly minor components of the extracellular matrix (e.g. perlecan [5]), various non-myogenic cell populations (e.g. fibroblasts [6]), and proteins that mediate connectivity between myofibers and their supporting matrix (e.g. dystrophin [7]) can cause dramatic dysfunction.
Figure 1.
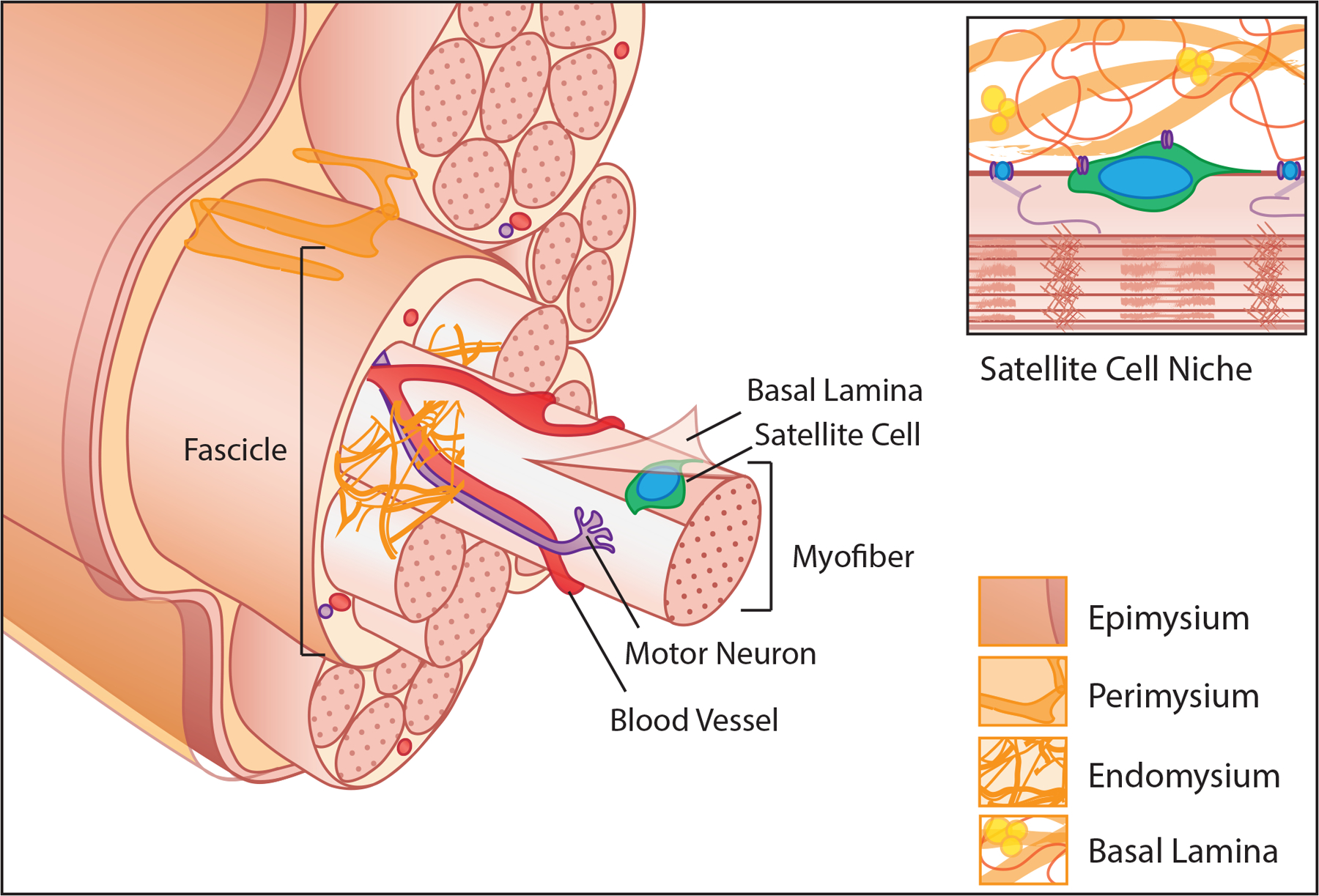
An exploded view of hierarchical structural organization of muscle tissue. Long cylindrical myofibers packed with myofibrils are encapsulated in the basal lamina with satellite cells lying between the muscle fiber membrane and basal lamina. Motor neurons and capillary networks interact with each myofiber to transmit signals and supply nutrients. Layers of interstitial ECM that store soluble growth factors and cytokines and guide cellular patterning surround myofibers (endomysium), bundles of myofibers termed fascicles (perimysium), and the whole muscle (epimysium). Inset, the basal lamina and myofiber provide direct mechanical and biochemical cues to the muscle satellite cell that are integral to maintenance of quiescence and self-renewal.
Myofibers are one of the largest cells in the body both in volume and in length (up to 0.5 mm3 and 300 mm in humans, respectively). They are multinucleated with a highly organized cytoskeleton that supports a packed arrangement of sarcomeres (the contractile unit of muscle). Myofibers are also tightly packed within a muscle, with the combined effect maximizing force generation of a given tissue volume by maximizing the number of aligned sarcomeres contracting in concert. The packing of fibers is guided by layers of extracellular matrix (ECM), reviewed in [8]. Each myofiber is ensheathed by a basal lamina and embedded in an endomysial “mesh like” ECM composed primarily of fibrillar collagen types I and III. Fibers are further arranged into tightly packed groups called fascicles by a perimysial “cable-like” ECM and are ensheathed at the muscle boundary by an epimysial “sheet-like” ECM. The ECM functions to transmit forces between fibers and to tendons, localize soluble growth factors and glycoproteins and guide the ancillary cells and structures that support contraction. These include the terminal axons of motor neurons which initiate contraction, capillary beds that deliver oxygen and metabolites and satellite cells which are the predominant resident progenitor cell for repair and regeneration [9].
Satellite cells reside in a unique niche between the basal lamina and the sarcolemma of a muscle fiber (Fig 1 inset). In homeostasis, they exist in a quiescent, undifferentiated state. However, disruptions in their microenvironment cause satellite cells to activate, proliferate, differentiate into myoblasts, migrate to sites of injury and fuse to repair or regenerate damaged fibers, reviewed in [10]. The ability of satellite cells to maintain quiescence in absence of injury, respond to injury signals and to replenish the population of quiescent, undifferentiated progenitors upon injury resolution is critical to the sustained potential for muscle growth and regeneration [9,11]. These behaviors are guided both by the satellite cell’s contact with the myofiber membrane and the basal lamina, through which it senses changes in composition, stiffness and topography, reviewed in [12].
The vast majority of in-vitro muscle studies are investigations using satellite cells, or their immortalized counterparts (C2C12, L6, etc). Satellite cells are relatively easy to isolate through enzymatic digestion and, when placed in culture, rapidly activate, proliferate and differentiate. By comparison, mature myofibers are extremely large and fragile cells that are not motile and do not proliferate. They are highly dependent on their unique in-vivo environment and do not adapt well to traditional culture techniques. As such, nearly all myofiber explant models study satellite cell dynamics (illustrated in Fig 2), specifically the regulation of quiescence, transition to activation and renewal of the satellite cell pool – processes which are highly dependent on an intact basal lamina niche [13]. The study of myofibers is relegated primarily to intact muscle explants where the integration between fibers is maintained (illustrated in Fig 3). Both explant models originated in the frog, but were adapted to mice and rats in the 1970s, where they are most commonly used today. This review will focus on these rodent models.
Figure 2.
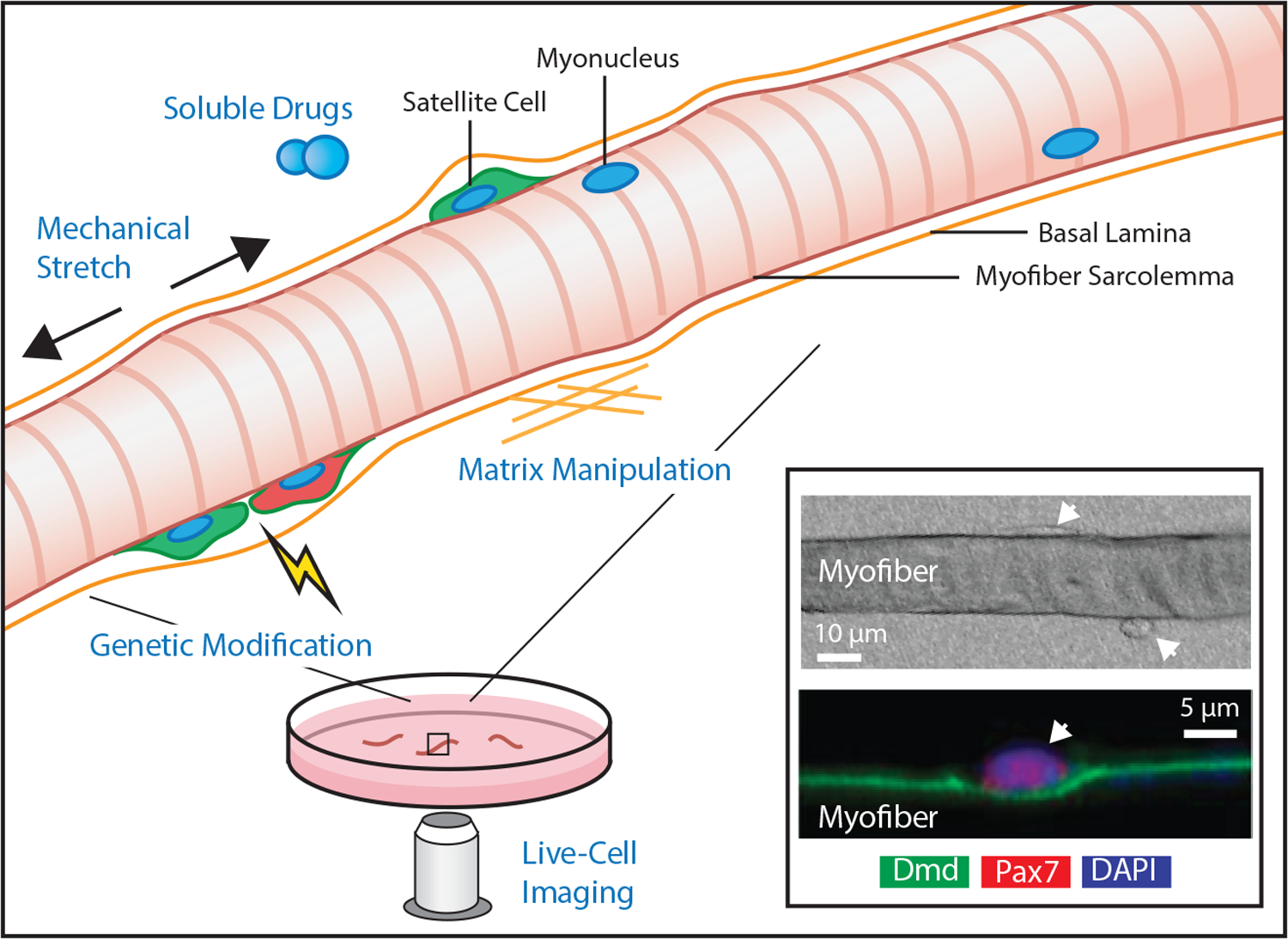
Graphical illustration of myofiber explant. Striated multi-nucleated myofibers are freed from surrounding muscle tissue with the basal lamina intact. The myofiber explant culture conditions enable precise control of mechanical stretch, soluble factors, interstitial matrix mimics, and genetic manipulations while also allowing direct high-resolution imaging. Inset, Example light microscopy image of a myofiber 48 hours after isolation with associated attached and extravasating satellite cells (upper panel, top and bottom arrows respectively) and fluorescent microscopy image depicting nuclear staining positive for Pax7, the canonical satellite cell marker (lower panel, arrrow). The cell resides in direct contact with the myofiber sarcolemma, labeled with dystrophin (Dmd), included with permission from [65]).
Figure 3.
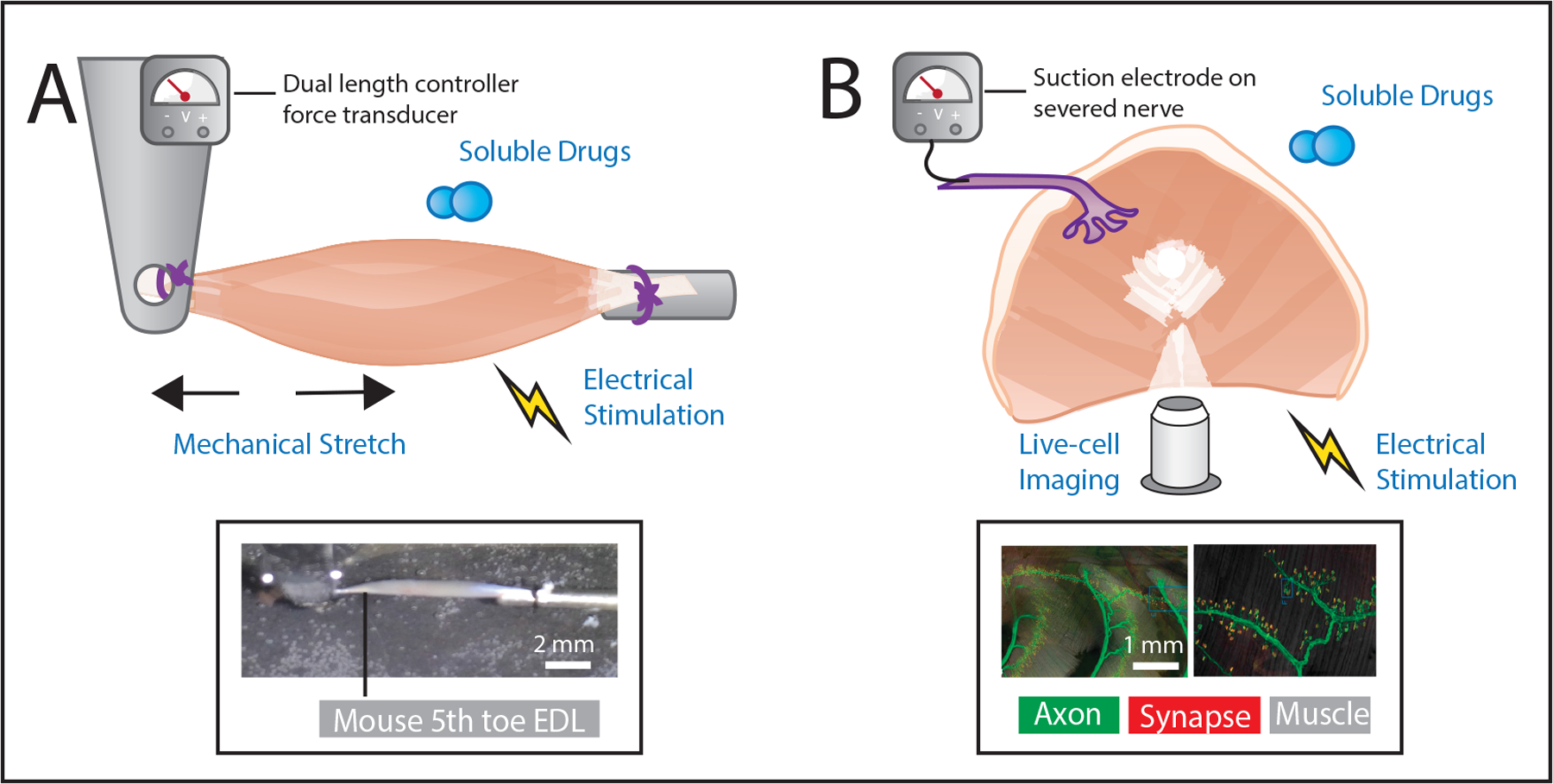
Graphical illustration of two common whole muscle explant preparations: hindlimb preparations for mechanical manipulation and measurement and ribcage associated muscles for live-cell imaging and direct neural recordings. A, Isolated hindlimb muscles are prepared in a bath, where soluble factors may be manipulated, with attachments near the myotendinous junction. Mechanical stretch of the muscle can be precisely delivered with length controller, electrical stimulation of the muscle through current delivered via electrodes in the bath, and the resulting forces recorded by a force transducer. Inset, Example image of a mouse 5th toe EDL muscle prepared for mechanical testing as described. B, Depiction of a diaphragm whole muscle explant that includes the associated nerve. While control of length and potentially force recording may be sacrificed due to the complex architecture, it enables inclusion of neuronal stimulation of the muscle as well as recording of muscle afferent signals through an electrode placed on the severed nerve. The thinness of the diaphragm muscle also makes it well suited for live imaging. Inset, Example images of the triangularis sterni muscle explants and its innervation using genetic labeling of thyl with YFP to mark axons (green) and bungarotoxin used to label the motor endplate (red). Left, shows multisegmental innervation of the muscle and right, shows high resolution imaging of an endplate band, included with permission from [58].
A brief history of skeletal muscle explant
The observation of the incredible ability of muscle to regenerate in-vivo when tissue architecture is preserved [14] has driven a large and fruitful field of in-vitro muscle research. Early studies culturing muscle pieces from adult rats and humans for several weeks noted a plethora of cells emigrating from the explant [15]. The initial abundant outgrowth was noted primarily as fibroblasts followed by myoblast migration from day 3–10 [16]. The myoblasts generated from these explants were capable of early differentiation, characterized by expression of myogenic transcription factors and fusion with each other to form multinucleated syncytia called myotubes. Notably, however, these myotube precursors did not mature into structures as ordered as myofibers. While the source of these myoblasts was originally suspected to be budding off of myonuclei from the fiber, it wasn’t until the discovery of a cell population beneath the basal lamina of a myofiber that satellite cells were understood as the predominant muscle stem cell [17]. As explants taken from biopsies and dissections that disrupt the muscle integrity activate satellite cells, these cultures were largely used to model regenerative processes following a severe injury.
In order to study the satellite cell in its niche, explants were required in which the quiescent state is maintained for extended periods of time. Methods were designed to carefully isolate single fibers and their associated satellite cells from flexor digitorum brevis (FDB) muscles of rats using collagenase to free the fibers and associated basal lamina [18]. These myofibers were able to maintain satellite cells in a non-proliferative state while retaining viability for up to 4 days [18]. Myofiber explants were used to demonstrate that extrinsic signals (chick embryo extract and isolated fibroblast growth factor) and intrinsic signals (extract of injured adult muscle) are able to induce activation and proliferation of satellite cells while maintaining a connection to the cultured myofiber [18–20]. Additionally, the usefulness of maintaining a connection between the satellite cell and the sarcolemma was demonstrated by adhering satellite cells directly to collagen coated substrates and damaged myofibers where cells became more responsive to mitogens [20]. These studies highlighted the impact of a healthy myofiber in maintaining quiescence of satellite cells and defined some of the major niche cues involved in activation and proliferation in response to injury.
The delicacy of single fibers separated from the supportive scaffold of ECM in myofiber explant culture lowers throughput and increases variability. Thus, many of the foundations of muscle physiology, including contraction mechanics, metabolism, and mechanotransduction, have been conducted on whole muscle explants. These models exploit the broad heterogeneity in muscle architecture by selecting small intact muscles to be representative of the tissue rather than an excised portion (as is typical for explant culture). These muscles retain undamaged fibers in their native niche and are thin enough to allow nutrient diffusion from the culture media to reach the core. The distinct advantage to testing these muscles in-vitro was the ability to isolate muscle intrinsic behavior by precisely controlling stimulation, loading and metabolic substrates.
Mammalian preparations were originally used to study glucose uptake in response to insulin or oxygen [21]. Later, methods were adapted from frog studies to permit measurement of contractility including maximum isometric force and rates of force development [22], fatiguability [23] and eccentric contraction induced damage [24]. Isolated muscle also permitted the study of how those forces feed back into muscle regulatory pathways, a key component of muscle adaptation to altered loading. For example, the mechanosensitive MAPK pathway was shown to be intrinsic to muscle and not meditated systemically by stimulation of rat epitrochlearis muscle explants [25]. These explant models and testing methodologies have been refined through the past decades and are now widely used. The totality of investigations that use explants (either single fiber or whole muscle) are far too expansive detail in a single review. This review will focus on longer term cultures that allow enhanced visualization of the time course of cellular events, unique perturbations of muscle that explore cellular functions, and those that leverage the complex nature of muscle beyond the myofiber.
Current methods: technical details and experimental applications
For the purposes of this review, the two main paradigms of skeletal muscle explant cultures will be discussed separately. Both strategies are designed to preserve features of the muscle structure which support in-vivo cellular physiology, but they are tailored for investigations of different cell populations and processes on different time and size scales. Isolation and culture methodologies as well as experimental considerations also differ between the two. Detailed protocols for these strategies have been published elsewhere so this section will provide an overview, some technical notes and references for further reading.
Isolated myofiber culture considerations
The culture of isolated myofibers is a powerful tool in the study of niche regulation of satellite cell quiescence, activation and proliferation. Though in-vivo studies have provided critical insights into satellite cell behavior in the native niche, they are unable to access the dynamics of individual cells in real-time, and instead infer behavior from population measures at set endpoints [26,27]. On the other hand, culture of satellite cells isolated from enzymatically digested muscle enables precision manipulation of cellular genetics and biochemical signaling in an environment compatible with live-cell imaging [28]. However, removal of the satellite cell from its niche causes rapid activation, re-entry into the cell cycle and myogenic differentiation [29] and thus this method is not compatible with the study of quiescence, activation or self-renewal. The isolation of intact myofibers provides an attractive alternative, where the satellite cell’s intimate connections to the fiber sarcolemma and the basal lamina remain intact, yet this niche is isolated in a system compatible with transient manipulation and visualization (Fig 2).
However, the isolation of intact and undamaged myofibers is technically challenging. In fact, successful isolation and culture of myofibers has only been reported from three small muscles of rodents: the FDB [18], extensor digitorum longus (EDL) [30] and soleus [30]. Reports suggest that the isolation protocol could be adapted to other muscles (e.g. the tibialis anterior) [30], however larger muscles without distinct tendons are likely to suffer from increased myofiber damage and low yield. To isolate myofibers, muscles are carefully dissected with attached origin and insertion tendons to ensure myofibers are not damaged. Dissected muscles are then placed into a collagenase-based digestive solution until endo/peri/epi-mysial collagen connections sever sufficiently to allow fibers to separate. Separation can be visualized by pipetting the muscle through a large bore pipet under a dissecting microscope. Isolation continues with sequential incubation and gentle pipetting until myofibers fully separate (see [31] for video demonstration). Some myofibers will be damaged during isolation, but those that remain intact can be visualized as long, translucent fibers with uniform diameter. Patient and gentle pipetting is the key to successful isolation. Any damage to the myofiber will induce hypercontraction and satellite cells will respond by activating and migrating away from the damaged niche. See Pasut et al. [31] for representative images of undamaged and hypercontracted fibers. Successfully isolated intact myofibers retain their basal lamina and associated quiescent satellite cells [18]. Following separation, undamaged myofibers can be isolated by gravity sedimentation (FDL) or transferred by pipet (EDL and soleus) to a pre-prepared dish for culture.
The culture conditions selected will depend on the experimental objectives. Fibers can be cultured in either a floating configuration in horse serum coated dishes or a substrate-adherent configuration in matrix (typically Matrigel) coated dishes. In the floating configuration satellite cells are not subject to foreign forces or matrix components from the substrate which influence behavior, but in the adherent configuration substrate stiffness and composition can be manipulated. The media composition must also be tailored to the investigation as satellite cells are sensitive to serum concentrations. Generally, to maintain quiescence, myofibers should be cultured in a low-serum media (typically 10%) and to study activation, proliferation and differentiation culture media should be switched to high-serum (typically 20–30%). Specific media formulations vary from study to study and can be tailored to investigate the role of specific components (e.g. growth factors, cytokines, chemical inhibitors) on specific processes. Culture times will also depend on experimental objectives. Myofibers cultured in the floating condition can only be maintained for 3–4 days before they begin to hypercontract [31]. Adherent cultures can be maintained for up to 3–4 weeks in a low serum media [32], but in a high serum media, satellite cells will rapidly (within 12–24 hours) activate, proliferate and migrate away from the myofiber [30] and within a week will fuse to form new myotubes [18].
Satellite cells residing on myofiber explants can be manipulated using nearly every in-vitro tool within experimental constraints. Table 1 provides references to published protocols which include detailed extraction and culture instructions compatible with specific downstream applications. Generally speaking, cells can be transfected with oligonucleotides, plasmids or viruses to alter expression of target genes [31], treated with soluble labels to assay proliferation [33] and exposed to drugs and compounds to promote or inhibit target functions [34]. These techniques are particularly powerful when combined with live-cell imaging where the dynamics of cellular activation or division can be tracked [28]. When live-cell imaging is combined with expression of a fluorescent reporter, either by in-vitro transfection or isolation from reporter mouse lines, the temporal dynamics of gene expression can be mapped onto real-time cellular behavior. This has led to characterization of the transcriptional and functional heterogeneity of the satellite cell pool [35] polarity in cell division events [36], temporal regulation of early activation by soluble signals [37], niche regulation of motility [28], maintenance of self-renewal capacity [38,39] and fiber type specificity [40]. Modified myofiber explants can also be transplanted back into naïve recipient mice to examine the relative role of systemic vs. local niche factors in growth and regeneration [38].
Table 1.
A survey of published protocols for isolated myofiber preparation, culture and testing. This list focuses on unique downstream applications and methods and is not comprehensive. FDB: flexor digitorum brevis, EDL: extensor digitorum longus.
| Reference | Muscle | Applications | Features |
|---|---|---|---|
| Bischoff et al. [18] | FDB | Creatine kinase assay, DNA synthesis measurement, electron microscopy, fixation and immunostaining | First publication of isolated myofiber culture |
| Rosenblatt et al. [30] | EDL & Soleus | Fixation and immunostaining | Details fiber isolation from Soleus muscle |
| Ravenscroft et al. [34] | FDB | Intracellular calcium flux, adenoviral transduction, fixation and immunostaining | Describes bulk processing in a 96-well format |
| Anderson et al. [80] | FDB | Electron microscopy and in-situ hybridization | Also provides protocol for isolating fibers from zebrafish |
| Keire et al. [81] | FDB & EDL | Fixation and immunostaining | Directly compares FDB and EDL isolation protocols |
| Pasut et al. [31] | EDL | Oligonucleotide or plasmid transient transfection, viral infection, live imaging, fixation and immunostaining | Video demonstration of myofiber isolation protocol |
| Vogler et al. [33] | EDL | Transplantation of myofiber-associated satellite cells, transfection, CFDA-SE self-renewal assay, EdU proliferation assay, AraC treatment, fixation and immunostaining | Detailed protocol with tips for both isolation and applications |
| Gallot et al. [82] | EDL | Fixation and immunostaining | Bulk processing in a 6-well format |
| Brun et al. [83] | EDL | Fixation and immunostaining | Details floating fiber culture technique |
Though the vast majority of studies utilizing isolated myofiber culture focus on the satellite cell, it is worth noting that the myofibers themselves do remain viable in culture for a period of time. In their initial publications, Bekoff and Betz noted rapid changes in the cultured myofiber characteristic of denervation, including changes at the neuromuscular junction, sarcoplasmic sprouting and the beginning stages of myofiber fragmentation [41]. However, more recently, serum-free culture has been shown to mediate these changes and maintain calcium transients in response to field stimulation for more than a week [32,34]. This could enable high throughput screening of drugs and compounds in a mature muscle culture system [34] or extended studies of myofiber mechanotransduction where loads can be precisely delivered and intracellular responses tracked in real-time [42]. However, it should be noted that not all mature myofiber processes can be adequately recapitulated in adherent culture. Like satellite cells, myofibers are dependent on their complex in-vivo niche for mature function. For one, myofibers are so tuned to changes in innervation that removal of the motor neuron will rapidly initiate denervation-related pathways even in serum free culture [34]. Furthermore, maintenance of passive loading is essential to mitigate atrophic breakdown of sarcomere structures [43]. In-vivo loads are distributed between fibers in 3 dimensions through complex ECM structures that cannot be replicated on a flat culture surface. These limitations have led to the refinement of isolated intact muscle explant model systems which retain connections between fibers and tendons as well as the potential to retain and study neuromuscular transmissions in-vitro.
Intact muscle culture considerations
Explants of intact mammalian muscle have also been utilized since the 1970s to examine contractile performance [22], mechanotransduction [44], metabolic flux [45], neuromuscular junction development [46] and passive mechanics [47]. These preparations have not traditionally been referred to as “cultures” as they are not typically kept in-vitro for more than a few hours. However, they otherwise meet definitions of explant culture: they are removed from the body, supported by a culture media, manipulated mechanically and/or biochemically and used to readout resulting changes in cellular function.
The limited viability of isolated muscles in culture is primarily driven by the loss of vasculature which supports the high metabolic demand of contracting myofibers in-vivo. Without supporting vasculature, most muscles contracting in-vitro will develop an hypoxic core resulting from high oxygen utilization rates in the interior exceeding diffusion rates from the exterior media [48]. To minimize these effects and improve diffusion of other media components (e.g. metabolites, drugs), the majority of explant studies utilize very thin muscles. For most murine assays, the EDL and soleus are the gold standard [22,49], though the FDL [50], diaphragm [51,52] or triangularis sterni [53] are also used. The EDL and soleus are muscles of the hindlimb with uniform fiber orientation and distinct origin and insertion tendons that are easy to locate and dissect intact. The EDL is a multi-bellied muscle and frequently only the 5th toe belly will be tested to eliminate architectural differences between bellies [54]. The composition of the EDL is predominately fast fibers, while the soleus is predominately slow fibers and thus both are frequently tested to evaluate fiber-type specificity in performance or signaling. These preparations have been reported to be kept viable in properly buffered solutions with high levels of dissolved oxygen for 12–16 hours [55,56]. However, most protocols complete testing within an hour, as this is generally sufficient to characterize contractile performance, early signaling events in mechanotransduction and short-term metabolic responses, such as insulin-stimulated glucose uptake.
The first key to successful muscle explant testing is careful dissection. Damage to myofibers will immediately alter their force generation capacity, metabolic profile and response to extrinsic cues. As a major benefit to ex-vivo testing is precision manipulation and measurement, this damage “noise” can be highly detrimental. Muscles with distinct tendons are ideal for isolation, but it is possible to test myofiber bundles or strips from larger muscles with more complex architecture [57]. However, it is much more difficult to generate intact preparations. The second key is providing a temperature controlled, buffered and oxygenated media. The high metabolic activity of muscle (especially electrically stimulated muscle) will rapidly deplete dissolved oxygen and alter the pH of standard culture media. Maintenance of dissolved oxygen is typically achieved by bubbling an oxygen line through a reservoir. Maintenance of pH is typically achieved by including bicarbonate in the media. Media is maintained either at 37°C with a heated water jacket around the bath or at room temperature to improve the stability of contraction. A number of standard media formulations exist and are detailed in the references in Table 2.
Table 2.
A survey of published protocols for intact muscle preparation, culture and testing. This list focuses on unique downstream applications and methods and is not comprehensive. FDB: flexor digitorum brevis, EDL: extensor digitorum longus.
| Reference | Muscle | Applications | Features |
|---|---|---|---|
| Wilkinson et al. [84] | EDL | Muscle-nerve co-culture, electrical stimulation, passive stretching, recording of muscle spindle afferrents | Describes recording sensory information from attached nerve |
| Kerschensteiner et al. [58] | Triangularis Sterni | Muscle-nerve co-culture, timelapse microscopy, fixation and immunostaining | Details neuronal degeneration in culture |
| Hanson et al. [46] | Diaphragm | Muscle-xenonerve co-culture, electrical stimulation, drug treatment, fixation and immunostaining | Describes xenonerve culture |
| Clark et al. [85] | Lumbrical | Muscle-nerve co-culture, neurotoxin delivery, electrical stimulation, fixation and immunostaining | Protocol for isolating multiple preparations from a single mouse |
| Hansen et al. [86] | Epitrochlearis, FDB & Soleus | Electrical stimulation, insulin stimulation, glucose uptake, hexokinase activity | An in-vitro 2-deoxyglucose assay for muscle |
| Park et al. [55] | EDL & Soleus | Electrical stimulation, force-frequency relationship, fatigue, mechanic alternans | Video demonstration of muscle dissection and mounting |
| Moorwood et al. [51] | EDL and Diaphragm strips | Electrical stimulation, eccentric contraction testing | Video demonstration of muscle dissection and mounting |
Whole muscle explants are used widely for a variety of measurements. Electrically stimulated contractile testing is the most common application (see [55] and [51] for video demonstrations). In this testing, tendons are attached to an apparatus which contains a motor arm and a force transducer, enabling precise control of muscle length and accurate measurement of force output. Electrodes stimulate contraction through the media, directly depolarizing myofiber membranes. This testing is useful because it isolates muscle intrinsic capacity from cardiovascular and neural components of in-vivo performance. EDL and soleus muscles are ideal for this testing due to their distinct origin and insertion tendons and fusiform myofiber architecture. Other common applications with these muscles include passive stretch paradigms to study mechanotransduction signaling [44] and addition or subtraction of metabolites from the media to examine metabolic regulation [45]. The diaphragm and triangularis sterni muscles are several-fold thinner than the EDL or soleus and provide excellent specimens for live cell imaging [58]. These muscles insert into the rib cage and are typically dissected with the associated ribs and frequently with the associated nerve terminus. Though contraction can be elicited and measured in these preparations [46], they are most frequently used to study the nerve-muscle interface as the innervating nerves are superficial and easy to track, allowing full reconstruction of motor units. Dissection of the various explant muscles and additional details of their applications are provided in the references in Table 2.
Recent advances and future directions for myofiber explants
Traditionally, myofiber explant models have been used to visualize the satellite cell in its niche and to map early activation and proliferation events. However, recently, new tools have been applied to myofiber explant that expand its utility to include models of membrane repair, direct visualization of calcium handling, in-niche small molecule screens and live-cell tracking of satellite cell migration.
Precision high power laser irradiation can be used to disrupt the cultured myofiber membrane in a highly localized manner [59]. Bansal et al. used this model to demonstrate that membrane disruption is quickly resealed with dysferlin localizing to the repaired region. In cultured myofibers of dysferlin null mice, a model of limb girdle muscular dystrophy, the repair process was defective; establishing that dysferlin is vital for sarcolemma repair [59]. The myofiber laser wound assay was also used to elegantly investigate additional components of the calcium dependent membrane repair machinery, including MG53 [60] and annexin A6 [61] which play critical roles in membrane resealing. Fascinatingly, dysferlin has also been shown to stabilize calcium transients [62] using the myofiber culture system with calcium sensitive dyes to locally visualize calcium release. This application of myofiber culture has been particularly powerful in identifying intrinsic [63] and extrinsic [64] regulators of excitation-contraction coupling.
About a decade of elegant studies using myofiber explants have described substantial transcriptional and functional heterogeneity in the satellite cell pool, including the polarity and symmetry of division events [35,36,65]. Fascinatingly, symmetric divisions which produce two uncommitted daughter cells are predominately a planar polarity where each daughter retains contact with both the myofiber membrane and basal lamina while asymmetric divisions with one committed daughter were a predominately vertical polarity with the committed cell on the basal lamina side. The soluble factor Wnt7a was shown to be an important factor regulating the polarity of satellite cell division and absence of Wnt7a reduced the quiescent satellite cell pool and future regenerative potential [36]. The versatility of the myofiber explant system has recently been exploited to identify additional soluble compounds that could impact polarity of satellite cell division and improve regenerative potential [66]. Through screening 640 pharmacological compounds the epidermal growth factor receptor (EGFR) was identified as a key pathway in regulating polarity and EGF was effectively used to restore asymmetric division of satellite cells from dystrophic myofiber cultures.
One of the major advantages of the myofiber explant culture model is the ability to visualize individual satellite cell behaviors in real time. Live-cell myofiber imaging shows that satellite cells are highly motile, with much of the motility dependent on interactions with the basal lamina through the laminin binding integrin α7β1 [28]. Live cell imaging over 2 days also reveals many interactions between satellite cells, with daughter cells remaining associated for long periods, especially after asymmetric division [67], however more distantly related cells that crossed paths also developed extended associations. This suggests caution in supposing a common progenitor from doublet myoblasts from single time point myofiber cultures. Another note of caution in these studies is that though isolated myofibers maintain many of the features of the in-vivo niche, they lack the endomysial connection to neighboring fibers which may spatially restrict both migration and vertical division. Models that provide further structure to the satellite cell niche such as embedding myofibers in a collagen gel [67] or moving to intact muscle explant models will be instrumental in deciphering the role of satellite cells in vivo.
Recent advances and future directions for intact muscle explants
The majority of intact muscle explant testing is fundamentally unchanged from its inception in the 1930s. Commercial manufacture of highly sensitive instrumentation has improved the accuracy and accessibility of testing, but the novelty of most current studies lies in the mouse and not in the testing methodology. However, there is a burgeoning renewed interest in the role of tissue cross-talk in physiology - be it chemical, mechanical or electrical – and muscle explant culture provides a unique platform to probe the physiological connections between nerve, muscle, tendon and bone. Several groups have recently leveraged muscle explant models to mechanistically explore the nerve-muscle interface.
The established diaphragm and triangularis sterni explant systems are frequently cultured with a portion of the nerve to study synaptic development and function. As a part of their methodology publication, Hanson et al. [46] used this system to define intrinsic and extrinsic cues that regulate neuromuscular junction development. By including and excluding the nerve in two culture conditions, they were able to demonstrate that release of neurotransmitters from the cultured nerve act in concert with excitation-contraction coupling within the muscle to stabilize acetylcholine receptor clusters. Essentially, that signals from both tissues are required to properly develop their connection. Nerve-muscle explants are not limited to the muscles associated with the ribcage, which have a highly specialized function and composition, but can be adapted to the muscles of the hindlimb as well. Woo et al. [56] and Lin et al. [68] recently used an explant cultures of the EDL and soleus, respectively, with a portion of the respective branch of the sciatic nerve to measure stretch-evoked neuronal activity. Precision stretch of the EDL or soleus was delivered through a standard dual force and length controller while afferent firing of individual units was detected with an electrode on the cut nerve. Using these preps, these groups defined the role of two ion channels, Asic3 and Piezo2, in proprioceptive mechanotransduction. Essentially, mechanical stretch is converted to electrical signals by these channels in sensory neurons and relayed to the brain to control positional awareness and motor coordination.
It is easy to imagine adapting muscle explant models to study the effects of muscle loading on tendon and bone, or vice versa, as these tissues remain with the explant in many preparations. However, the only study we are aware of with this approach severed muscle fibers in the preparation, compromising their biochemical and mechanical signaling [69]. Further methodological refinement may be required to expand the impact of these multi-tissue explant cultures. Specifically, longer survival of isolated muscles in culture will enable exploration of slower targets of cross-talk, such as collagen remodeling by tenocytes, matrix turnover in bone and cytoskeletal or metabolic remodeling in muscle.
Prolonging explant culture: lessons from tissue engineering
A major benefit of muscle explant culture is that it enables the study of mature structural and functional features that are absent or immature in “muscle” grown in a dish from myogenic precursors. However, myogenic cell culture models are much more extensively used because processes can be visualized and manipulated over a much longer period of time – they are simply developmental rather than mature processes. Interestingly, there is substantial overlap in the challenges that face both cell and explant culture models as they attempt to move to long-term study of mature muscle function in a dish. Strategies undertaken in the field of tissue engineering have not only moved engineered constructs closer to in-vivo muscle, but have provided valuable insight into critical components of myofiber maturation and survival in-vitro.
Much of the last 20 years of fabricating tissue engineered skeletal muscle has focused on adjusting the matrix composition for optimal cellular survival and 3-D mechanical properties. The critical role of the ECM in muscle development and function cannot be overstated, but interestingly refining the matrix composition alone has not achieved full maturation of myotubes [70]. Inclusion of a heterogeneous muscle-resident cell population improves myofiber area, sarcomere definition and engraftment of satellite cells over pure myoblast culture [71], highlighting the important role of supporting cells such as fibroblasts in muscle development and function. Further application of dynamic strain [71] and electrical stimulation [72] to these constructs increases size and force generating capacity suggesting that physiological loading synergizes with signals from supporting cells to support a mature and complex in-vivo tissue niche.
However, even with these additions, diameter and specific force production of myofibers in these constructs remains about 4-fold below mature explants [72]. Co-culture of engineered muscle constructs with in-vitro differentiated motor neurons can generate functional neuromuscular junctions and improve contractile force generation [73,74]. These neurons survive in culture for weeks, if not longer, without the degeneration that plagues severed nerves in existing explant co-culture models. Extrapolating this model, culture of muscle explants with in-vitro generated motor neurons could sustain contractile activity and stave off the effects of denervation. However, the need to form new neuromuscular junctions remains a significant challenge. This is another area of interest for tissue engineering as in-vitro engineered constructs must ultimately form new neuromuscular junctions when transplanted in-vivo. Treatment of constructs with neuronal paracrine factors, such as agrin, has been shown to accelerate implant innervation in-vivo [75]. This is thought to be driven by changes to acetylcholine clustering on the myofiber which prime it for innervation. Similar treatment may prime explants for re-innervation and ultimately, stable long-term culture.
On the flip side, explant culture is also advancing the field of muscle tissue engineering: the better we understand the complexities of the in-vivo niche, the better we can replicate it in-vitro with engineering tools. The complex composition and structure of the matrix, the heterogeneous resident cell populations that are still being characterized and the soluble mileu which includes endocrine and paracrine factors from yet undefined sources make perfect replication a significant challenge. It is likely that an imperfect construct that can be implanted and remodeled by the host into functional muscle is the most tangible goal for tissue engineering. However, it is possible that improvements to engineered constructs could also make them a valuable replacement or complement to explants with capacity to replicate long-term behaviors.
Translating explant models for clinical relevance
Muscle explant models have contributed to the fundamental knowledge of muscle contraction and regeneration on which we base our understanding of human muscle function. For example, our basic understanding of the principles of mechanotransduction have guided muscle strengthening protocols to consider variables such as load and strain to maximize hypertrophy [76] and our understanding of the role of satellite cells in muscle regeneration and disease has led to satellite cell targeted therapies for muscular dystrophy [77]. The unique contribution of explant models lies in their ability to assess complex cell-matrix interactions that can either not be measured in-vivo or replicated in-vitro. These interactions are poised to be critical targets of regenerative therapies in muscle as matrix pathology is increasingly recognized to impede cell function in disease [78,79].
However, at present, explant experiments and discoveries are limited to the animal model realm. The fundamental challenge of utilizing the explant models discussed here to make direct measures in human muscle is the need to retain intact and undamaged myofibers. Due to the length of human myofibers (up to 300 mm) this is nearly impossible to achieve by standard biopsy. Theoretically, small parallel-fibered muscles could be isolated from larger, donated specimens (e.g. amputated limbs) and dissociated into individual myofibers for culture. To our knowledge, this has only been done for the isolation of satellite cells and not for sustained culture of myofibers. Thus, alternative models must be relied upon to test the translational potential of discoveries from rodent explant studies.
Discussion
Skeletal muscle explants, both myofiber and intact muscle, have enabled some of the seminal discoveries in the field of muscle physiology. They were the platform for early experiments defining key muscle structure-function relationships (e.g. lenth-tension and force-velocity) and were one of the preparations where satellite cells were first identified and cultured. Explant models have evolved with advancing technology to include genetic modification, specialized microscopy and precision engineering to define mechanisms underlying force generation and transmission, mechanotransduction, metabolic regulation and the intrinsic and extrinsic regulation of regeneration. These discoveries have not only expanded our fundamental understanding of muscle tissue, but have spurred development of targeted physical, pharmacological and genetic therapies for muscle aging, injury and disease. The majority of these studies could not have been conducted in-vivo due to complex environmental factors that limit manipulation and/or measurement, nor could they be conducted in traditional myogenic cell culture where myofiber maturation is limited and key features of excitation-contraction coupling and ECM integration do not develop. However, it is important to note that while explant culture makes some critical features of the in-vivo niche accessible to experimentation, it leaves others behind (e.g. circulating factors, tissue cross-talk, physical constraints) and is thus is a complement to, rather than replacement for, the study of in-vivo biology. As advances in experimental tools continue to push the frontier of research, in-vivo and in-vitro studies may meet in the middle, with cellular processes accessible in-vivo and sustainable in-vitro, generating a continuum of biological investigation.
References:
- 1.Strangeways TSP, Fell HB. Experimental studies on the differentiation of embryonic tissues growing in vivo and in vitro.—II. The development of the isolated early embryonic eye of the fowl when cultivated in vitro. Proc. Roy. Soc. London B; 1926. [Google Scholar]
- 2.Fell HB, Robison R. The growth, development and phosphatase activity of embryonic avian femora and limb-buds cultivated in vitro. Biochem J 1929;23(4):767–784.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill AV. The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society B; 1938. p. 136:195. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey RW, Street SF. The isometric length‐tension diagram of isolated skeletal muscle fibers of the frog. Journal of Cellular and Comparative Physiology; 1940. p. 11:34. [Google Scholar]
- 5.Zoeller JJ, McQuillan A, Whitelock J, et al. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol 2008. Apr;181(2):381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy MM, Lawson JA, Mathew SJ, et al. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011. Sep;138(17):3625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worton R Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science 1995. Nov;270(5237):755–6. [DOI] [PubMed] [Google Scholar]
- 8.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011. Sep;44(3):318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011. Sep;138(17):3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumont NA, Bentzinger CF, Sincennes MC, et al. Satellite Cells and Skeletal Muscle Regeneration. Compr Physiol 2015. Jul;5(3):1027–59. [DOI] [PubMed] [Google Scholar]
- 11.Fry CS, Lee JD, Jackson JR, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 2014. Apr;28(4):1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res 2015. Feb;56(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013. Jan;93(1):23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark WE. An experimental study of the regeneration of mammalian striped muscle. J Anat 1946. Jan;80(Pt 1):24–36.4. [PubMed] [Google Scholar]
- 15.POGOGEFF IA, MURRAY MR. Form and behavior of adult mammalian skeletal muscle in vitro. Anat Rec 1946. Jul;95:321–35. [DOI] [PubMed] [Google Scholar]
- 16.Godman G On the regeneration and redifferentiation of mammalian striated muscle. Journal of Morphology; 1957. p. 27–81. [Google Scholar]
- 17.MAURO A Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 1961. Feb;9:493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bischoff R Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 1986. May;115(1):129–39. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff R Analysis of muscle regeneration using single myofibers in culture. Med Sci Sports Exerc 1989. Oct;21(5 Suppl):S164–72. [PubMed] [Google Scholar]
- 20.Bischoff R Interaction between satellite cells and skeletal muscle fibers. Development 1990. Aug;109(4):943–52. [DOI] [PubMed] [Google Scholar]
- 21.Chaudry IH, Gould MK. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta 1969. May;177(3):527–36. [DOI] [PubMed] [Google Scholar]
- 22.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 1988. Oct;404:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang SJ, Bruton JD, Katz A, et al. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol 2006. Apr;572(Pt 2):551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren GL, Lowe DA, Hayes DA, et al. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol 1993. Aug;468:487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryder JW, Fahlman R, Wallberg-Henriksson H, et al. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J Biol Chem 2000. Jan;275(2):1457–62. [DOI] [PubMed] [Google Scholar]
- 26.McGeachie JK, Grounds MD, Partridge TA, et al. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci 1993. Nov;119(2):169–79. [DOI] [PubMed] [Google Scholar]
- 27.Sousa-Victor P, Gutarra S, García-Prat L, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014. Feb;506(7488):316–21. [DOI] [PubMed] [Google Scholar]
- 28.Siegel AL, Atchison K, Fisher KE, et al. 3D timelapse analysis of muscle satellite cell motility. Stem Cells 2009. Oct;27(10):2527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert PM, Havenstrite KL, Magnusson KE, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010. Aug;329(5995):1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenblatt JD, Lunt AI, Parry DJ, et al. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 1995. Nov;31(10):773–9. [DOI] [PubMed] [Google Scholar]
- 31.Pasut A, Jones AE, Rudnicki MA. Isolation and culture of individual myofibers and their satellite cells from adult skeletal muscle. J Vis Exp 2013. Mar(73):e50074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown LD, Schneider MF. Delayed dedifferentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol Anim 2002 2002. Jul-Aug;38(7):411–22. [DOI] [PubMed] [Google Scholar]
- 33.Vogler TO, Gadek KE, Cadwallader AB, et al. Isolation, Culture, Functional Assays, and Immunofluorescence of Myofiber-Associated Satellite Cells. Methods Mol Biol 2016;1460:141–62. [DOI] [PubMed] [Google Scholar]
- 34.Ravenscroft G, Nowak KJ, Jackaman C, et al. Dissociated flexor digitorum brevis myofiber culture system--a more mature muscle culture system. Cell Motil Cytoskeleton 2007. Oct;64(10):727–38. [DOI] [PubMed] [Google Scholar]
- 35.Kuang S, Kuroda K, Le Grand F, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007. Jun;129(5):999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Grand F, Jones AE, Seale V, et al. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 2009. Jun;4(6):535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson J, Pilipowicz O. Activation of muscle satellite cells in single-fiber cultures. Nitric Oxide 2002. Aug;7(1):36–41. [DOI] [PubMed] [Google Scholar]
- 38.Bernet JD, Doles JD, Hall JK, et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med 2014. Mar;20(3):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troy A, Cadwallader AB, Fedorov Y, et al. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38α/β MAPK. Cell Stem Cell 2012. Oct;11(4):541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenblatt JD, Parry DJ, Partridge TA. Phenotype of adult mouse muscle myoblasts reflects their fiber type of origin. Differentiation 1996. Mar;60(1):39–45. [DOI] [PubMed] [Google Scholar]
- 41.Bekoff A, Betz WJ. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol 1977. Sep;271(1):25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomero J, Pye D, Kabayo T, et al. Effect of passive stretch on intracellular nitric oxide and superoxide activities in single skeletal muscle fibres: influence of ageing. Free Radic Res 2012. Jan;46(1):30–40. [DOI] [PubMed] [Google Scholar]
- 43.Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol 1977. Jan;264(1):267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem 2006. Apr;97(6):1207–16. [DOI] [PubMed] [Google Scholar]
- 45.Young ME, Radda GK, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J 1997. Feb;322 ( Pt 1):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson MG, Niswander LA. An explant muscle model to examine the refinement of the synaptic landscape. J Neurosci Methods 2014. Dec;238:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol 2014. May;306(10):C889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leijendekker WJ, Elzinga G. Metabolic recovery of mouse extensor digitorum longus and soleus muscle. Pflugers Arch 1990. Apr;416(1–2):22–7. [DOI] [PubMed] [Google Scholar]
- 49.Close R Force: velocity properties of mouse muscles. Nature 1965. May;206(985):718–9. [DOI] [PubMed] [Google Scholar]
- 50.Cifelli C, Bourassa F, Gariépy L, et al. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol 2007. Jul;582(Pt 2):843–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moorwood C, Liu M, Tian Z, et al. Isometric and eccentric force generation assessment of skeletal muscles isolated from murine models of muscular dystrophies. J Vis Exp 2013. Jan(71):e50036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolbeck RC, Nosek TM. Fatigue of rapid and slow onset in isolated perfused rat and mouse diaphragms. J Appl Physiol (1985) 1994. Oct;77(4):1991–8. [DOI] [PubMed] [Google Scholar]
- 53.McArdle JJ, Angaut-Petit D, Mallart A, et al. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods 1981. Aug;4(2):109–15. [DOI] [PubMed] [Google Scholar]
- 54.Chleboun GS, Patel TJ, Lieber RL. Skeletal muscle architecture and fiber-type distribution with the multiple bellies of the mouse extensor digitorum longus muscle. Acta Anat (Basel) 1997;159(2–3):147–55. [DOI] [PubMed] [Google Scholar]
- 55.Park KH, Brotto L, Lehoang O, et al. Ex vivo assessment of contractility, fatigability and alternans in isolated skeletal muscles. J Vis Exp 2012. Nov(69):e4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo SH, Lukacs V, de Nooij JC, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 2015. Dec;18(12):1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barton ER, Wang BJ, Brisson BK, et al. Diaphragm displays early and progressive functional deficits in dysferlin-deficient mice. Muscle Nerve 2010. Jul;42(1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerschensteiner M, Reuter MS, Lichtman JW, et al. Ex vivo imaging of motor axon dynamics in murine triangularis sterni explants. Nat Protoc 2008;3(10):1645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003. May;423(6936):168–72. [DOI] [PubMed] [Google Scholar]
- 60.Cai C, Masumiya H, Weisleder N, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 2009. Jan;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swaggart KA, Demonbreun AR, Vo AH, et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc Natl Acad Sci U S A 2014. Apr;111(16):6004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lukyanenko V, Muriel JM, Bloch RJ. Coupling of excitation to Ca. J Physiol 2017. 08;595(15):5191–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prosser BL, Hernández-Ochoa EO, Lovering RM, et al. S100A1 promotes action potential-initiated calcium release flux and force production in skeletal muscle. Am J Physiol Cell Physiol 2010. Nov;299(5):C891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernández-Ochoa EO, Robison P, Contreras M, et al. Elevated extracellular glucose and uncontrolled type 1 diabetes enhance NFAT5 signaling and disrupt the transverse tubular network in mouse skeletal muscle. Exp Biol Med (Maywood) 2012. Sep;237(9):1068–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumont NA, Wang YX, von Maltzahn J, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 2015. Dec;21(12):1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang YX, Feige P, Brun CE, et al. EGFR-Aurka Signaling Rescues Polarity and Regeneration Defects in Dystrophin-Deficient Muscle Stem Cells by Increasing Asymmetric Divisions. Cell Stem Cell 2019. Mar;24(3):419–432.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegel AL, Kuhlmann PK, Cornelison DD. Muscle satellite cell proliferation and association: new insights from myofiber time-lapse imaging. Skelet Muscle 2011. Feb;1(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin SH, Cheng YR, Banks RW, et al. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun 2016. 05;7:11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Connizzo BK, Grodzinsky AJ. Release of pro-inflammatory cytokines from muscle and bone causes tenocyte death in a novel rotator cuff in vitro explant culture model. Connect Tissue Res 2018. Sep;59(5):423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwee BJ, Mooney DJ. Biomaterials for skeletal muscle tissue engineering. Curr Opin Biotechnol 2017. 10;47:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Juhas M, Bursac N. Roles of adherent myogenic cells and dynamic culture in engineered muscle function and maintenance of satellite cells. Biomaterials 2014. Nov;35(35):9438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khodabukus A, Madden L, Prabhu NK, et al. Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle. Biomaterials 2019. Apr;198:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Afshar Bakooshli M, Lippmann ES, Mulcahy B, et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife 2019. May;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morimoto Y, Kato-Negishi M, Onoe H, et al. Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials 2013. Dec;34(37):9413–9. [DOI] [PubMed] [Google Scholar]
- 75.Ko IK, Lee BK, Lee SJ, et al. The effect of in vitro formation of acetylcholine receptor (AChR) clusters in engineered muscle fibers on subsequent innervation of constructs in vivo. Biomaterials 2013. Apr;34(13):3246–55. [DOI] [PubMed] [Google Scholar]
- 76.Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med 2007;37(3):225–64. [DOI] [PubMed] [Google Scholar]
- 77.Negroni E, Bigot A, Butler-Browne GS, et al. Cellular Therapies for Muscular Dystrophies: Frustrations and Clinical Successes. Hum Gene Ther 2016. Feb;27(2):117–26. [DOI] [PubMed] [Google Scholar]
- 78.Meyer GA, Ward SR. Developmental Biology and Regenerative Medicine: Addressing the Vexing Problem of Persistent Muscle Atrophy in the Chronically Torn Human Rotator Cuff. Phys Ther 2016. May;96(5):722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li EW, McKee-Muir OC, Gilbert PM. Cellular Biomechanics in Skeletal Muscle Regeneration. Curr Top Dev Biol 2018;126:125–176. [DOI] [PubMed] [Google Scholar]
- 80.Anderson JE, Wozniak AC, Mizunoya W. Single muscle-fiber isolation and culture for cellular, molecular, pharmacological, and evolutionary studies. Methods Mol Biol 2012;798:85–102. [DOI] [PubMed] [Google Scholar]
- 81.Keire P, Shearer A, Shefer G, et al. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol 2013;946:431–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallot YS, Hindi SM, Mann AK, et al. Isolation, Culture, and Staining of Single Myofibers. Bio Protoc 2016. Oct;6(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brun CE, Wang YX, Rudnicki MA. Single EDL Myofiber Isolation for Analyses of Quiescent and Activated Muscle Stem Cells. Methods Mol Biol 2018;1686:149–159. [DOI] [PubMed] [Google Scholar]
- 84.Wilkinson KA, Kloefkorn HE, Hochman S. Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One 2012;7(6):e39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark AW, Bandyopadhyay S, DasGupta BR. The plantar nerves-lumbrical muscles: a useful nerve-muscle preparation for assaying the effects of botulinum neurotoxin. J Neurosci Methods 1987. Apr;19(4):285–95. [DOI] [PubMed] [Google Scholar]
- 86.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol (1985) 1994. Feb;76(2):979–85. [DOI] [PubMed] [Google Scholar]


