Abstract
Background
Sanitation workers are essential to global public health and societal wellbeing. However, the health risks and outcomes associated with exposure to occupational risk factors among sanitation workers are neither well understood nor well quantified. We undertook a systematic review to (1) identify occupational risk factors among sanitation workers and (2) assess the effect of occupational exposure to human fecal sludge and wastewater on selected health outcomes among these workers.
Methods
We searched four databases (i.e., PubMED, MEDLINE, EMBASE, and LILACS) for eligible studies from inception through to January 01, 2020. The included population was workers ≥15 years engaged, formally or informally, in installing, operating, servicing, cleaning or emptying a sanitation technology at any step of the sanitation chain. The included comparator was workers in other occupations or the general population. Eligible outcomes were: mortality (any or all causes), gastroenteritis, occupational injuries, respiratory diseases, musculoskeletal disorders, and mental and social health conditions. Risk of bias was assessed separately on exposure assessment and health outcome using a modified Liverpool Quality Assessment Tool (LQAT). We pooled sufficiently homogenous studies using inverse variance meta-analysis with random effects.
Results
A total of 65 studies (9 cohort studies, 56 cross-sectional studies) met the inclusion criteria. One quarter of studies (n = 15) were from middle-income countries. Few studies assessed occupational risk factor exposures directly; most assigned exposure via proxy of occupation of sanitation worker. We judged nearly all studies to have “high risk of bias” in exposure and outcome assessment. Despite these limitations, the consistency of the overall evidence suggests that sanitation workers are at increased risk of gastroenteritis and respiratory conditions, and may be at increased risk of musculoskeletal disorders and mental/social health conditions. The pooled odds ratio for hepatitis A--the only outcome deemed suitable for meta-analysis--was 2.09 (95% Predicted Interval: 1.39–3.00, 12 studies). There was conflicting evidence from studies of increased risk of mortality; only one study reported on injuries.
Conclusion
Despite a large number of studies, there is limited evidence to date of the health risks faced by sanitation workers, particularly among groups that may be at particular risk-- women, informal workers and those living in low-income countries. Nevertheless, the research to date provides suggestive evidence of elevated occupational risk among sanitation workers across a range of health condition. More research is needed to improve the current bodies of evidence for all included health outcomes to be able to quantify disease burden among this occupational group.
Keywords: Sanitation workers, Occupational exposures, Occupational health, Systematic review
1. Introduction
Sanitation workers are individuals responsible for cleaning, maintaining, operating, or emptying a sanitation technology at any step of the sanitation chain (World Bank Group and World H, 2019). They are essential in maintaining safe sanitation services in homes, schools, hospitals, and other settings and protecting public health (WHO, 2020) but face many health risks in doing so, including from exposure to a wide range of biological and chemical agents (Heldal et al., 2019; Tschopp et al., 2009). Additionally, they may be at risk of injury from heavy labor, poor and prolonged postures and positions and confined spaces, as well as psychosocial stress (Wang et al., 2019; Smith et al., 1998). These risks are exacerbated under conditions of poverty, illness, poor nutrition, poor housing, child labor, migration, drug and alcohol abuse, discrimination, social stigma and societal neglect (World Bank Group and World H, 2019; Rangamani et al., 2015). In many low- and middle-income countries (LMICs), sanitation workers are more vulnerable due to unregulated or unenforced environmental and labor protections, and lack of occupational health and safety measures (WHO, 2020; Comaru and Werna, 2013).
While the definition of sanitation workers encompasses a vast line of services, the number of sanitation workers globally is difficult to estimate because they often have multiple jobs or are categorized with other sectors (e.g., solid waste and healthcare facility management) (World Bank Group and World H, 2019). Furthermore, many sanitation workers, especially in low-income areas, are informally employed and therefore difficult to localise (WHO, 2020). Combined with the lack of clarity about the health risks, this uncertainty about the number of persons exposed makes it difficult to estimate the burden of disease associated with occupational exposure to sanitation work.
This systematic review aimed to identify and describe occupational health risks among sanitation workers. It was designed to inform future efforts on identifying research priorities on the topic, and possibly quantifying the sanitation workforce and estimating the burden of disease faced by this occupational group for selected health outcomes. Such evidence could inform the production of burden of diseased estimates for the global comparative risk assessment (Ezzati et al., 2004), for example through the World Health Organization (WHO) and International Labour Organization (ILO) Joint Estimates of the Work-related Burden of Disease and Injury (Pega et al., 2021a, 2021b).
2. Methods
The methods used for this review were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Moher et al., 2009; The 17 Goals). The review was completed in accordance with a pre-specified protocol and registered with PROSPERO (CRD42020184401) and the protocol is available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020184401).
2.1. Population, exposure, comparison
Populations consisted of persons of working age (≥15 years); child laborers were excluded from the systematic review because they may present a different profile of exposures and health outcomes. Workers were included regardless of the type of employment contract held, with both those employed by a company and self-employed contractors included, as well as workers without a formal employment contract but compensated in another way. Exposure consisted of sanitation work, defined as installing, operating, servicing, cleaning or emptying a sanitation technology at any step of the sanitation chain. The included comparator was workers in other occupations or the general population.
2.2. Outcomes
The review extends to a range of health outcomes, grouped generally an accordance with clinical conventions. The following definitions were used for this purpose:
-
•
All-cause mortality: fatality associated with the specified occupational exposure.
-
•
Gastrointestinal conditions: Self-reported, clinically confirmed or other recorded morbidity associated with gastroenteritis, including acute, persistent, inflammation of the stomach and intestines, self-reported, clinically confirmed or other recorded morbidity or mortality associated with diarrhea, including acute, persistent, bloody and watery diarrhea and dysentery. This included the WHO definition of diarrhea, which is three or more loose stools in 24 h. In studies that did not use such definitions, we used the case definition from the study. Diarrhea and vomiting are usual symptoms of gastrointestinal infections caused by bacterial, viral, or parasitic infections. This also includes infection with pathogens associated with any of the foregoing conditions.
-
•
Injuries: physical or mental harm caused by accident or disease associated with the occupational exposure. Includes self-reported, clinically confirmed or other recorded morbidity or mortality associated with injuries, including but not limited to punctures, wounds, cuts, or blunt force trauma
-
•
Respiratory conditions: Self-reported, clinically confirmed or other recorded morbidity associated with respiratory conditions, including influenza like symptoms, chronic obstructive pulmonary disease, upper airway inflation, coughing, phlegm production, wheezing, shortness of breath, nasal congestion, sore throat, headache; self-reported, clinically confirmed or other recorded morbidity or mortality associated with asthma, caused by inhalation of sewer gas (toxic and non-toxic), inhalation of gases from decayed household or industrial waste, inhalation of hydrogen sulfide, ammonia, methane, carbon dioxide, sulfur dioxide, nitrous oxides, or fumes from chlorine bleaches, industrial solvents, or gasoline (Athanasiou et al., 2010).
-
•
Musculoskeletal disorders: Self-reported, clinically confirmed or other recorded morbidity or mortality associated with musculoskeletal disorders; join pain, osteoarthritis, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis; bone damage, osteoporosis, osteopenia, associated fragility fractures, traumatic fractures; muscle pain such as sarcopenia; spine conditions such as back and neck pain; other multi-body area conditions such as widespread pain disorders, inflammatory disease, connective tissue disease such as vasculitis (Musculoskeletal condition, 2020).
-
•
Mental and social health conditions: Self-reported, clinically confirmed or other recorded morbidity or mortality associated with mental and/or social health, including inability to cope with stress, rapid social change, stressful work conditions, gender discrimination, social exclusion, unhealthy lifestyles, physically ill-health, human rights violations, and socioeconomic stressors or pressures (Mental health: strengthen, 2020).
2.3. Study eligibility
We included randomized controlled trials (RCTs) that are individually-or cluster-RCT and the following non-randomized controlled studies (NRS): quasi-RCTs, non-RCTs, controlled before-and-after studies, interrupted-time-series studies, historically controlled studies, case-control studies, cohort studies and cross-sectional studies that include a comparison group (see definitions in Supplementary Material 1). We excluded studies that report only qualitative data. Studies that were published in English, French, and Spanish were eligible for inclusion.
2.4. Search strategy, study screening and data extraction, synthesis
We systematically searched four databases - PubMed, MEDLINE, Embase, and LILACS from inception to January 2020. The search strategy and search terms are included in Supplementary Material 2. Results from the four databases were managed and duplicates were removed in the reference management software, Zotero. Titles and abstracts returned by the search were screened by ML using Microsoft Excel and full copies of titles and abstracts were obtained for further examination of potentially relevant studies. Data was extracted using a prescribed extraction form developed in a Microsoft Excel spreadsheet.
We meta-analyzed the studies reporting hepatitis A infection where the measure of effect was similar across the studies. We also present forest plots, developed using R programing (version 4.0.2), that show results on certain gastrointestinal and respiratory endpoints; however, we present no pooled analysis of those results because of substantial heterogeneity in study designs, settings, populations, case definitions, exposure assessment and case definitions. Results for other outcomes are tabulated and described narratively due to this lack of comparability among studies.
2.5. Risk of bias and analysis
Risk of bias was assessed separately for exposure assessment and health outcomes using a modified Liverpool Quality Assessment Tool (LQAT), an adaptation of the Newcastle-Ottawa Scale, which has been used previously (Pope et al., 2010; Freeman et al., 2017 etc.). This was an attractive choice since it is adaptable to various studies designs and considers the risk of bias in both exposure and outcome measures. Based on the information provided in the study, each exposure and outcome reported in articles were scored between 0 (highest risk of bias) and 1 (lowest risk of bias). Assessment of the exposure methods included scores regarding exposure assessment, response rate bias, and selection bias. Additionally, allocation bias was assessed for intervention studies and follow-up bias was assessed for longitudinal studies. Exposure assessment asked how the exposure was assessed (i.e., personal recall or laboratory/physician assessment) and, if applicable, how often were the measurements taken (i.e., grab samples or longitudinal samples). Selection bias assessed the methods for selection of sanitation facility/plant, sanitation workers, and comparison group/control. Assessment of the outcome methods included scores regarding outcome assessment, response rate bias, and selection bias. Additionally, allocation bias and bias in ascertainment were assessed for intervention studies and follow-up bias was assessed for longitudinal studies. Similar to the exposure assessment, the outcome was scored based on how the outcome was assessed (i.e., personal recall, fieldworker assessment, or laboratory/physician assessment).
3. Results
3.1. Study selection
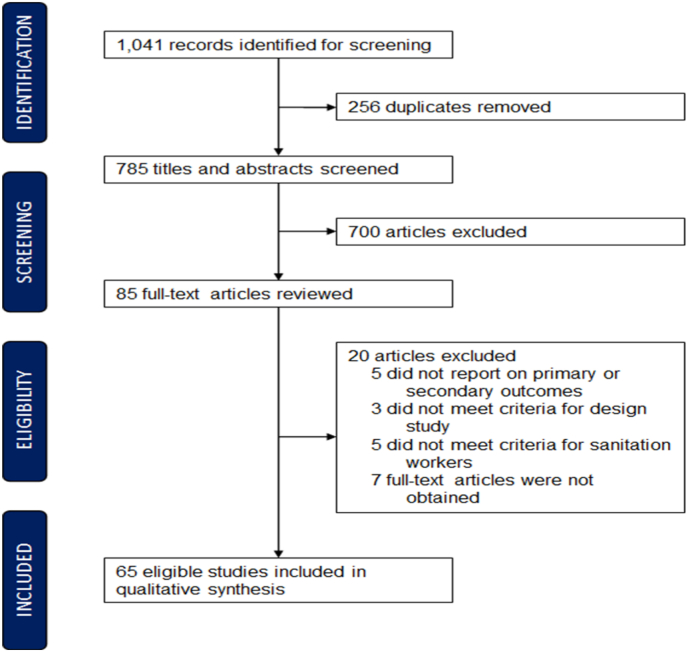
A total of 1041 studies were identified from the four databases, and 256 duplicates were removed (Fig. 1). The titles and abstracts of the remaining 785 studies were screened, of which 700 were excluded. As a result of the full-text review of the remaining 85 studies, 20 studies were excluded, of which, five studies did not report on primary or secondary outcomes, three did not meet the eligibility criteria for study design, five did not meet the criteria for sanitation workers, and seven full-text articles could not be obtained. In total, 65 studies were included in the review. Twelve of the 65 studies reported on two or more outcomes, so that the overall number of associations included in this analysis exceeds the number of studies.
Fig. 1.
PRISMA flow diagram summarizing study selection.
3.2. Study overview
Eligible studies included in the review are listed in Table 1, with more details presented in the Supplemental Material (Table S1). Table 2 gives a summary of studies by date of publication, study design, gender and outcome. Studies were largely conducted in high-income settings (78%) with the remaining in middle-income settings (22%). None were from low-income settings. The majority of studies (62%) were published between 2000 and 2019. A total of 56 studies (86%) used a cross-sectional study design, with the balance employing a quasi-experimental or cohort design. Nine studies (14%) provided gender disaggregated data. Twenty-two (34%) studies focused solely on men, and two (3%) focused solely on women (Table 2).
Table 1.
Studies that met inclusion criteria with associated primary or secondary health outcome, country of study, study design, and detailed health outcome description.
| Health Outcome | Reference | Country | Study Design | Detailed Health Outcome |
|---|---|---|---|---|
| Gastroenteritis | Arvanitidou et al., 1998 | Greece | Cross-Sectional | Hepatitis B Virus Infection |
| Arvanitidou et al., 2004 | Greece | Cross-Sectional | Hepatitis A and B Infections | |
| Bonanni et al., 2000 | Italy | Cross-Sectional | Hepatitis A | |
| Brugha et al., 1998 | United Kingdom | Cross-Sectional | Anti-HAV Positivity | |
| Cadilhac and Roudot-Thoraval, 1996 | France | Cross-Sectional | HAV Seropositivity | |
| Divizia, 2008 | Italy | Cross-Sectional | Antibody Presence for Hepatitis A, Coxsackievirus, Echovirus Types 1 & 9 | |
| El-Esnawy, 2000 | Egypt | Cross-Sectional | Hepatitis E | |
| Friis, 1998 | Sweden | Cross-Sectional | Dyspepsia, IBS, Nausea, Diarrhea, Stomach Pain, Peptic Ulcer | |
| Fuhrimann et al, 2016 | Uganda | Cross-Sectional | Intestinal Parasites; Helminths; Soil-Transmitted Helminths; Intestinal Protozoa; Self-Reported Signs & Symptoms | |
| Fuhrimann et al, 2016 | Vietnam | Cross-Sectional | Intestinal Parasitic Infection; Self-Reported Diarrhea, Skin Problems & Eye Problems | |
| Hammouda et al., 1992 | Egypt | Cross-Sectional | Intestinal Parasitic Infection | |
| Heng et al., 1994 | Singapore | Cross-Sectional | Hepatitis A | |
| Jeggli et al., 2004 | Switzerland | Prospective Cohort | Hepatitis E, Heliobacter pylori, & other GI Symptoms | |
| Jeyakumar et al., 2008 | India | Cross-Sectional | Leptospirosis; Other Health Problems | |
| Lerman, 1999 | Israel | Retrospective Cohort | Hepatitis A | |
| Levin et al., 2000 | Israel | Cross-Sectional | Seropositivity to Hepatitis A | |
| Lundholm and Rylander, 1983 | Sweden | Cross-Sectional | White Blood Cell Counts; Serum Immunoglobulins; Urinary Fibrinogen Degradation Production Concentrations; GI/Work-Related Symptoms | |
| Poole and Shakespeare 1993 | United Kingdom | Cross-Sectional | Seropositivity to Hepatitis A | |
| Scarlett-Kranz et al., 1987 | United States | Cross-Sectional | Diarrhea; Headache; Blurred Vision; Presence/Absence of Urinary Mutagens | |
| Schlosser et al., 1999 | France | Retrospective Cohort | Intestinal Parasite Carriage - whipworm, Giardia lamblia, Entamoeba coli, and Endolimax nanus. | |
| Schoniger-Hekele et al, 2007 | Austria | Cross-Sectional | Tropheryma whipplei | |
| Sharma et al., 2006 | India | Cross-Sectional | Leptospirosis | |
| Skinhoj et al., 1981 | Denmark | Cross-Sectional | Antibodies Against Hepatitis A and B Viruses and Leptospirosis | |
| Toseva et al., 2018 | Bulgaria | Cross-Sectional | Anti-HAV Antibodies | |
| Tschopp et al., 2009 | Switzerland | Prospective Cohort | Hepatitis E | |
| Vaidya et al., 2003 | India | Cross-Sectional | Anti-HEV-IgG Antibodies | |
| Van Hooste et al., 2010 | Belgium | Cross-Sectional | Helicobacter pylori Infections; GI Symptoms | |
| Venczel et al., 2003 | United States | Cross-Sectional | Hepatitis A | |
| Mortality | Friis et al., 1993 | Sweden | Retrospective Cohort | Risk of Cancer - Brain, Stomach, Kidneys, Lungs |
| Friis et al., 1999 a | Sweden | Retrospective Cohort | Cancer | |
| Lafleur and Vena, 1991 | United States | Retrospective Cohort | Standardized Moratality Ratios for Cancers Reported | |
| Nasterlack et al., 2009 | Germany | Retrospective Cohort | Cancer | |
| Wild et al., 2006 | France | Prospective Cohort | Standardized Moratality Ratios for Cancers Reported | |
| Respiratory | Bener et al., 1998 | United Arab Emirates | Cross-Sectional | Chronic Symptoms: Chronic cough or phlegm, chronic bronchitis, occupational asthma, allergic rhinitis, nasal catarrh, sinusitis dyspneoa, chest tightness, breathlessness, and pulmonary function impairment. Acute Symptoms: Throat irritation/dryness, secretion, nose irritation/drynes, eye irritation, and skin disorders. |
| Chandra and Arora, 2019 | India | Cross-Sectional | Pulmonary Tuberculosis; COPD; Bronchial Asthma; Hypertension, Ischaemic Heart Disease, Diabetes mellitus, Metabolic Syndrome and Congestive Heart Failure | |
| Cyprowski et al., 2015 | Poland | Cross-Sectional | Lung Function | |
| Cyprowski, 2019 | Poland | Cross-Sectional | Pro-Inflammatory Mediators; Interleukin Concentrations | |
| Dzaman et al., 2009 | Poland | Cross-Sectional | Taste Perception Threshold | |
| Fahim, 2012 | Egypt | Cross-Sectional | Bronchial Hyper-Responsiveness (BHR) | |
| Friis et al., 1999 b | Sweden | Cross-Sectional | Respiratory Symptoms | |
| Heldal et al., 2010 | Norway | Cross-Sectional | Lung Function & General Health Symptoms | |
| Heldal, 2013 | Norway | Cross-Sectional | Serum-Levels of Pneumoproteins - CC16, SP-A & SP-D | |
| Heldal et al., 2019 | Norway | Cross-Sectional | Inflammatory Effects; Lung Function | |
| Mattsby, 1978 | Sweden | Cross-Sectional | Fever, Eye Irritation, Diarrhea, Fatigue; IgG Antibodies | |
| Melbostad, 1994 | Norway | Cross-Sectional | Eye Irritation, Nose Irritation, Tiredness, Nausea, etc. | |
| Nethercott and Holness, 1988 | Canada | Cross-Sectional | Lung Function & General Health Symptoms | |
| Rylander, 1999 | Sweden | Cross-Sectional | Lung Function & General Health Symptoms | |
| Shadab et al., 2014 | India | Cross-Sectional | Pulmonary Function; Oxidative Stress | |
| Singh and Ladusingh, 2017 | India | Cross-Sectional | Chronic Bronchitis | |
| Thorn and Beijer, 2004 | Sweden | Cross-Sectional | Work-Related Symptoms; Inflammatory Markers; Lung Function | |
| Yu et al., 1988 | China | Cross-Sectional | Pulmonary Tuberculosis | |
| Zuskin et al., 1993 | Croatia | Cross-Sectional | Respiratory Symptoms & Ventilatory Capacity | |
| Mental and Social Health Conditions | Giri et al., 2011 | India | Cross-Sectional | Occupational Stress |
| Smith et al., 1998 | Australia | Quasi-Experimental | Work satisfaction, circadian malaise, muscular complaints, minor infections, sleep quality, home life, social life, work life, etc. | |
| Musculoskeletal Disorders | Friedrich et al., 2000 | Austria | Cross-Sectional | Neck pain, upper back pain and low back pain |
| Silva et al., 2016 | Portugal | Cross-Sectional | Back Pain | |
| Taha et al., 2018 | Egypt | Cross-Sectional | Bone Health; Osteoprotegerin (OPG) Levels | |
| Wang et al., 2019 | Taiwan | Cross-Sectional | Neck, Shoulder, Elbow, Wrist/Hand, Upper/Lower Back, Hip/Thigh, Knee, Ankle/Feet Pain | |
| Respiratory and Mental and Social Health Conditions | Rieger et al., 2018 | Germany | Cross-Sectional | Total Cell Count, Proportion of Neutrophils, Total Protein, IL-8, IL-6, IL1B TNF-a, and sCD14 Self-reported stress, health status, work satisfaction |
| Gastroenteritis and Respiratory | Douwes et al., 2001 | Netherlands | Cross-Sectional | Flu-like, Respiratory, Throat, Eye & Nose, and GI Symptoms |
| Krajewski et al., 2004 | Poland | Cross-Sectional | General Health Symptoms | |
| Smit et al., 2005 | Netherlands | Cross-Sectional | Respiratory Symptoms & Gastrointestinal Symptoms | |
| Thorn et al., 2002 | Sweden | Cross-Sectional | Work-Related Symptoms | |
| Gastroenteritis, Respiratory, and Mental and Social Conditions | Lee et al., 2007 | United States | Cross-Sectional | Health symptoms were categorized into four groups: respiratory, GI, ocular and skin irritations, and neurology. Job performance and psychological symptoms such as depression and the symptom of concentration or memory difficulties |
Table 2.
Study overview by publication date, study design, gender related reporting, and health outcome stratified by country type.
| Descriptors | Country Type, n (%) |
|||||
|---|---|---|---|---|---|---|
| Middle Income | High Income | Total | ||||
| Study Publication Date (by Decade) | ||||||
| 1970–1979 | 0 | (0.0%) | 1 | (2%) | 1 | (2%) |
| 1980–1989 | 1 | (7%) | 5 | (10%) | 6 | (9%) |
| 1990–1999 | 1 | (7%) | 17 | (34%) | 18 | (28%) |
| 2000–2009 | 4 | (27%) | 18 | (36%) | 22 | (34%) |
| 2010–2019 | 9 | (60%) | 9 | (18%) | 18 | (28%) |
| Study Design | ||||||
| Cross-Sectional | 15 | (100%) | 41 | (82%) | 56 | (86%) |
| Prospective Cohort | 0 | (0%) | 3 | (6%) | 3 | (5%) |
| Retrospective Cohort | 0 | (0%) | 5 | (10%) | 5 | (8%) |
| Quasi Experimental | 0 | (0%) | 1 | (2%) | 1 | (2%) |
| Gender Related Reporting | ||||||
| Aggregated | 3 | (20%) | 19 | (38%) | 22 | (34%) |
| Disaggregated | 4 | (27%) | 5 | (10%) | 9 | (14%) |
| Female Only | 1 | (7%) | 1 | (2%) | 2 | (3%) |
| Male Only | 5 | (33%) | 17 | (34%) | 22 | (34%) |
| Not Specified | 2 | (13%) | 8 | (16%) | 10 | (15%) |
| Primary/Secondary Outcome | ||||||
| Mortality | 0 | (0%) | 5 | (10%) | 5 | (8%) |
| Gastroenteritis | 7 | (47%) | 20 | (40%) | 27 | (42%) |
| Injuries | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Respiratory Conditions | 5 | (33%) | 10 | (20%) | 15 | (23%) |
| Musculoskeletal Disorders | 1 | (7%) | 3 | (6%) | 4 | (6%) |
| Mental & Social Health Conditions | 1 | (7%) | 1 | (2%) | 2 | (3%) |
| Two or More Outcomesa | 1 | (7%) | 11 | (22%) | 12 | (18%) |
a, b Friis et al., 1999 reported on mortality and respiratory health and is therefore reported twice; ‘a’ denotes reporting of mortality results and ‘b’ denotes reporting of respiratory results. denotes reporting of respiratory results.
There were 12 articles that reported on at least gastroenteritis and respiratory conditions. Four studies reported only on those two outcomes. One study also reported on injuries; four also reported on musculoskeletal disorders, two also reported on mental and social health outcomes. One study also reported on injuries and musculoskeletal disorders.
Eligible studies provided a range of sanitation work. Given that sanitation work at any step of the sanitation chain was included, sanitation workers encompassed cleaning staff, sewage treatment plant workers, wastewater treatment plant workers, etc. Some studies focused on a subset of sanitation workers and are therefore summarized using the appropriate terminology (e.g. ‘sewage worker’ instead of ‘sanitation worker’ when sewage workers were specifically studied).
3.3. Risk of bias
Most studies included in the review present a high risk of bias on both exposure (60 studies, 92% of all studies) and outcome assessment (50 studies, 77%) (Supplemental Material 3). Selection bias was common; most studies did not specify the inclusion/exclusion criteria and selection method for the workplace sanitation workers, and comparators. Exposure assessment relied principally on personal recall with the remaining studies split between no exposure assessment and laboratory and/or physician assessment. For outcome assessment, laboratory confirmation and/or physician diagnoses were used in most studies (57%); other studies relied on personal recall (31%) or fieldworker/health worker assessment (13%). The majority of the laboratory and/or physician assessments consisted of analyses of biosamples (i.e. blood, stool, or urine) or chest x-rays.
3.4. Mortality
Mortality was reported in five studies. Several of these studies focused on cancer due to potential exposure to carcinogenic or mutagenic agents (Friis et al., 1993; Lafleur and Vena, 1991; Nasterlack et al., 2009; Wild et al., 2006). Wild et al. were concerned with the chemicals released upon decomposition of feces in aerobic and anaerobic conditions.19. Two studies reported standardized incidence ratios and three studies reported standardized mortality ratios (SMRs). The three studies reported the three possible effects: exposure to sanitation was found to decrease (Friis et al., 1993), increase (Wild et al., 1996) or have no effect (Laffleur and Vena, 1991) on SMRs.
The standardized incidence ratios were focused only on cancers, while the SMRs reported on several causes of death (e.g. cancers, infectious diseases, respiratory diseases, etc.). Friis et al., 1999a and Nasterlack et al. both reported standardized incidence ratios among municipal male sewage treatment plant workers in Sweden and municipal male wastewater treatment plant workers in Germany, respectively (Nasterlack et al., 2009; Friis et al., 1999). Both Friis et al., 1999a and Nasterlack et al. reported that overall there was not a significant increase in the risk of cancer (Nasterlack et al., 2009; Friis et al., 1999). Additionally, Friis et al., 1993 and Lafleur & Vena reported that was not a significant increase in total mortality (Friis et al., 1993; Lafleur and Vena, 1991). Though Wild et al. concluded an increased total mortality among sewage workers in Paris, the increase was attributed to excessive alcohol consumption (Wild et al., 2006). The SMR from all alcohol-related diseases was reported as 1.65 (95% CI: 1.37–1.97), and the researchers followed up with managers and occupational physicians to confirm that alcoholism and poor personal hygiene were common among sewage workers in Paris. Furthermore, Wild et al. reported that suicide was in excess (SMR: 2.90; 95% CI: 1.66–4.71) (Wild et al., 2006). With respect to the exposure assessment, all studies reporting on mortality had a medium or high risk of bias. However, with respect to the outcome assessment, only Friis et al., 1999 had a high risk of bias, while all other studies had a low risk of bias (Friis et al., 1993; Laffleur and Vena, 1991; Nasterlack et al., 2009; Wild et al., 1996).
3.5. Gastrointestinal conditions
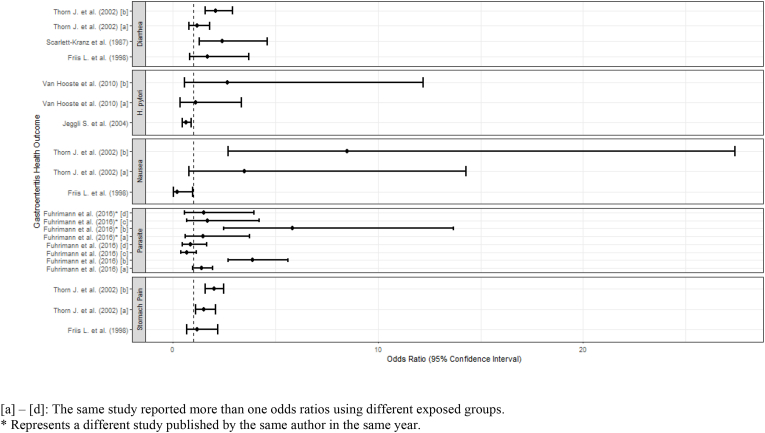
Gastrointestinal conditions were the most frequently reported outcomes (39 studies), including 27 studies reporting on gastrointestinal conditions only and 12 studies that reported on gastrointestinal and other health outcomes (Douwes et al., 2001; Fuhrimann et al., 2016a; Krajewski et al., 2004; Lee et al., 2007; Nethercott and Holness, 1988; Rieger et al., 2018; Rylander, 1999; Scarlett-Kranz et al., 1987; Schira et al., 1987; Smit et al., 2005; Thorn and Beijer, 2004; Thorn et al., 2002). Gastrointestinal conditions included symptoms of gastroenteritis (diarrhea, nausea or stomach pain) or presence of infectious agents in stool. Results are summarized in forest plots (Fig. 2). However, due to substantial heterogeneity in effect estimates, meta-analysis was not appropriate and no pooled estimate of effect is shown.
Fig. 2.
Forest plot of odds ratios of selected gastrointestinal conditions. [a] – [d]: The same study reported more than one odds ratios using different exposed groups. * Represents a different study published by the same author in the same year.
Five studies reported on bacteria-related gastroenteritis (Leptospira, Tropheryma whipplei, Helicobacter pylori, and gram-negative bacteria) (Sharma et al., 2006; Jeyakumar et al., 2008; Schöniger-Hekele et al., 2007; Van Hooste et al., 2010; Lundholm and Rylander, 1983). Sharma et al. and Jeyakumar et al. found sewage workers to be frequently exposed and prone to leptospiral infections (Sharma et al., 2006; Jeyakumar et al., 2008). Schoniger-Hekele et al. found T. whipplei in the stool of workers exposed to wastewater at a significantly higher rate compared to the unexposed population in the study, and Lundholm et al. suggests sewage treatment plant workers have specific, work-related symptoms due to acute effects of gram-negative bacteria (Schöniger-Hekele et al., 2007; Lundholm and Rylander, 1983). However, in a study that reports on H. pylori, Van Hooste et al. found no significant associations between infection status and gastrointestinal symptoms, occupational exposures in different tasks, or with hygiene practices (Van Hooste et al., 2010).
Four studies reported on intestinal parasitic infections (Fuhrimann et al., 2016a, 2016b; Hammouda et al., 1992; Schlosser et al., 1999). Hammouda et al. found sewage workers were at a higher risk of developing intestinal parasitic infections, specifically ascariasis and amoebiasis (Hammouda et al., 1992). A nail examination also revealed sewage workers were at a higher risk of infection by Enterobius vermicularis (pinworm). Schlosser et al. reported significantly higher odds ratios for sewage workers with intestinal parasitic infections as compared to the control population in some but not in other years (Fuhrimann et al., 2016b). The authors attributed this difference to an increase in compliance with hygiene rules and standards.
Three studies assessed occupational exposures to hepatitis B virus (HBV) (Arvanitidou et al., 1998, 2004; Skinhoj et al., 1981). Skinhoj et al. did not find a clear increase in association between exposure to sewage and infection with HBV, while Arvanitidou et al., 1998 found worker exposure to sewage independently associated with positivity for HBV infection (p < 0.001). (Arvanitidou et al., 1998; Skinhoj et al., 1981).
Four studies assessed occupational exposures to hepatitis E virus (HEV) (Tschopp et al., 2009; El-Esnawy, 2000; Vaidya et al., 2003; Jeggli et al., 2004). El-Esnawy et al. detected HEV Immunoglobulin G (IgG) in 205 workers from various treatment plants with various positions, of which 1.9% workers were with recent infection within the past year, 52.1% workers were infected more than one year earlier, 25.3% workers had a possible past infection, and 20.4% workers were negative for HEV IgG (El-Esnawy, 2000). Vaidya et al. found IgG-anti-HEV positivity to be higher among sewage treatment staff members (57%) as compared to controls (19%) (Vaidya et al., 2003). There was also a significant rise in anti-HEV positivity among staff who had been working longer than five years (p < 0.05), and a multivariate regression analysis identified contact with sewage as the independent variable associated with anti-HEV positivity. However, both Jeggli et al. and Tschopp et al. found no clear link between occupational exposure to sewage and H. pylori or HEV (Tschopp et al., 2009; Jeggli et al., 2004). Both of these studies were conducted in Switzerland, and Tschopp et al. noted the sewage workers included in the study were trained, provided with personal protective equipment, and working in a region with good sanitation (Tschopp et al., 2009; Jeggli et al., 2004).
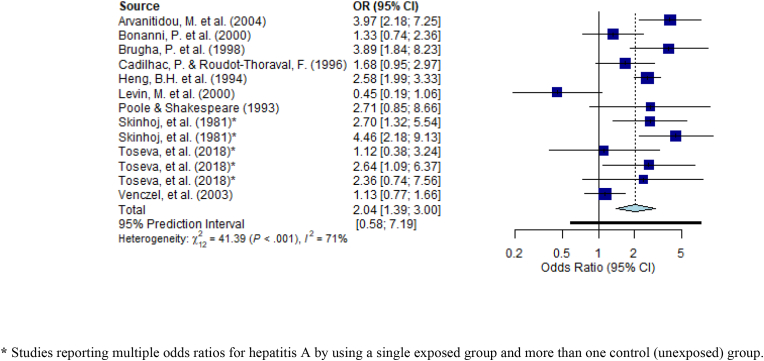
Among the enteric viruses, 12 studies reported on hepatitis A. Nine of those studies (reporting a total of 13 comparisons) were deemed suitable for meta-analysis based on similar study design (cross-sectional), outcome (infection or seropositivity of hepatitis A through blood samples and subsequent laboratory analyses) and measure of effect (odds ratios). Results are displayed in Fig. 3. While there is some inconsistency, evidence suggests that sanitation workers are at increased odds of hepatitis A infection. The pooled odds ratio was 2.09 (95% Predicted Interval: 1.39–3.00). When limited to the 10 comparisons from three studies (Heng et al., 1994; Toseva et al., 2018; Skinhoj et al., 1981) deemed to be of lower risk of bias, the pooled odds ratio increased to 2.62 (PI: 1.76–3.89).
Fig. 3.
Odds ratios for studies on hepatitis A infection/seropositivity among sanitation workers. * Studies reporting multiple odds ratios for hepatitis A by using a single exposed group and more than one control (unexposed) group.
Arvanitidou et al., 2004 concluded that HAV infections were significantly associated with occupational exposure to sewage, and therefore, supported the institution of a vaccination policy (Arvanitidou et al., 2004). While this was concluded for wastewater treatment plant workers, additional studies supported the same conclusion of risk among both sewage and wastewater treatment plant workers (Heng et al., 1994; Poole and Shakespeare, 1993; Venczel et al., 2003). However, Bonanni et al. and Levin et al. reported on sewage treatment plant workers and concluded that HAV infections were not significantly higher (Bonanni et al., 2000; Levin et al., 2000). Brugha et al. found that managers of sewage treatment workers considered sewage workers, flushers and fitters to be at high risk of occupational exposure; sewage workers were those that enter sewers to unblock pipes and carry out maintenance work, and flushers and fitters were those that clean inlet screens and sewage pumps (Brugha et al., 1998). According to the workers’ independent reporting of occupational exposure to raw sewage, exposure to raw sewage most of the time was a significant factor for HAV infection (Fig. 3). (Brugha et al., 1998) However, exposure to raw sewage some of the time was not a significant factor for HAV infection (OR: 1.14, 95% CI: 0.50, 2.59) (Brugha et al., 1998). The conclusions of Cadilhac and Roudot-Thoraval, supported this where sewage workers occupationally exposed to sewage was an independent significant factor for HAV seropositivity (Fig. 3). (Cadilhac and Roudot-Thorava, 1996) Similar to Brugha et al. Toseva et al. also investigated three different groups of workers within the wastewater treatment plant (WWTP), including operators, support staff (electricians, fitters), and other workers exposed to biological agents (distributors, drivers, launderers, etc.) (Toseva et al., 2018). There was a significant increase in the risk of HAV when comparing operators and support staff; however, there was a significant increase when comparing operators and others exposed (OR: 2.914; 95% CI: 1.149, 7.393) and support staff and others exposed (OR: 4.29; 95% CI: 1.075, 17.167). (Toseva et al., 2018).
3.6. Respiratory conditions
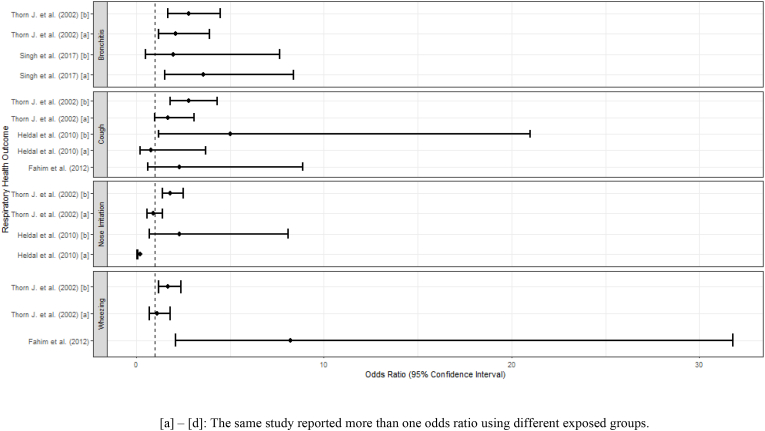
Respiratory conditions were the second most common outcome, with a total of 27 studies reporting on a variety of respiratory endpoints. Several of these studies reported on acute and/or chronic symptoms, including bronchitis, cough, nose irritation or wheezing. These are presented in forest plots (Fig. 4). However, due to substantial heterogeneity in the studies, meta-analysis was not appropriate and no pooled estimate of effect is shown. Other studies reported on asthma, chest X-rays and pulmonary function, bronchial hyperresponsiveness (BHR), chronic obstructive pulmonary disorder (COPD), and general lung function, and/or pulmonary tuberculosis.
Fig. 4.
Forest plot of odds ratios of selected respiratory symptoms. [a] – [d]: The same study reported more than one odds ratio using different exposed groups.
Most the studies reported sanitation workers to be at elevated risk of respiratory infections or other adverse conditions. Fahim et al. reported on BHR and found a statistically significant difference in the prevalence of dyspnea and clinical chest findings for sanitary/cleaning women workers associated with passive smoking in the workplace (Fahim and El-Prince, 2012). Chandra et al. examined chronic morbidity profiles for illnesses among sewage workers in India; these illnesses included pulmonary tuberculosis, COPD, bronchial asthma, hypertension, ischemic heart disease, diabetes mellitus, metabolic syndrome, and congestive heart failure (Chandra and Arora, 2019). In the study, sewage workers were found to have an adverse chronic morbidity profile for both tuberculosis and non-communicable diseases (Chandra and Arora, 2019). Exposure to sewage working places was associated with a high prevalence of respiratory symptoms, lower spirometric lung function, and high amino acid concentrations (Bener et al., 1998). Dzaman et al. investigated the effects of biological and chemical agents on the functioning of taste and smell among sewage treatment plant workers (Dzaman et al., 2009). Results indicated these workers, as well as landfill workers, are at a higher risk of smell and taste dysfunctions (Dzaman et al., 2009). Heldal et al., 2010 assessed the associations between dust, endotoxins, and bacterial exposure and health effects in sewage workers (Heldal et al., 2010). The prevalence of nose irritation, cough, headache, one or more respiratory symptoms, and one or more work-related symptoms were generally highest among sewage workers handling dry sludge (Heldal et al., 2010). Additionally, levels of C-reactive protein, which is linked to lung function, among sewage workers were higher than in control groups, however, this was not significant after adjusting for age and smoking. Furthermore, Heldal et al., 2019 found even moderate levels of endotoxin and hydrogen sulfide exposure resulted in impacts on lung function and low-grade systemic inflammation among wastewater workers (Heldal et al., 2019). In Nethercott et al. those working near sewage sludge that was frequently boiled and dried reported an intermittent, acute illness that was described by a cough, fever, and sore throat (Nethercott and Holness, 1988). Reduced lung function was also noted among those frequently near dried sludge incineration (Nethercott and Holness, 1988). A link between PBC levels and related symptoms and clinical findings was not established. Sewage workers were linked to oxidative stress and impaired lung functions (Shadab et al., 2014).
Singh et al. reported a higher prevalence of chronic bronchitis for sewage workers as compared to waste collectors and drivers (Singh and Ladusingh, 2017). Zuskin et al. aimed to estimate the prevalence of respiratory symptoms and the ventilatory capacity in a group of sewage workers (Zuskin et al., 1993). The authors found closed channel and drainage workers had a higher prevalence of chronic respiratory symptoms as compared to the control group. Additionally, baseline ventilatory capacity significantly decreased as compared to predicted values among sewage workers. The authors suggested this indicated obstructive changes in smaller airways. Sewage workers in Friis et al., 1999b) reported adult asthma at a significantly higher rate compared to the control group (Friis et al., 1999). Douwes et al. investigated the correlation between exposure to endotoxins and 29 different symptoms among sewage treatment workers using a factor analysis (Douwes et al., 2001). Respiratory symptoms included cough, cough with phlegm, wheezing in the chest, dyspnea, shortness of breath, and chest tightness. Both higher and lower airway symptoms were positively associated with working with sewage; however, the association was not significant. Similarly, Thorn et al. reported on respiratory symptoms with the addition of breathlessness, chronic bronchitis, and toxic pneumonitis. Thorn et al. reported an increased risk for airway symptoms among sewage workers. Lee et al., 2007 examined the relationship between exposure to endotoxins as well as hydrogen sulfide during and health symptoms among wastewater treatment plant workers (Lee et al., 2007). The study found statistically higher odds of respiratory symptoms among wastewater treatment workers in comparison to water treatment workers. Rieger et al., 2018 also found airway inflammatory markers were consistently significantly higher among workers than the control population (Rieger et al., 2018; Thorn et al., 2002). In contrast, upper respiratory symptoms were not found to be associated with endotoxin exposure status among wastewater treatment workers in Smit et al. (2005) Exposure status was determined via self-report.
However, not all studies found evidence for increased respiratory damage or associated health risks among sanitation workers. Cyprowski et al., 2015 assessed the effects of inhaled endotoxins on respiratory airflow among various types of workers, and found relatively low levels of endotoxins among sewage treatment plant workers (Cyprowski et al., 2015). Additionally, Yu et al. aimed to estimate the risk ratio and risk difference for tuberculosis among various sanitation workers (i.e., auxiliary, garbage clean-up, feces clean-up, and staff), however, the authors were not able to establish a significant relationship between type of worker and tuberculosis (Yu et al., 1988).
3.7. Musculoskeletal disorders
Musculoskeletal disorders were reported in four studies (Rangamani et al., 2015; Friedrich et al., 2000; Silva et al., 2016; Taha et al., 2018). The studies mainly focus the working postures of sanitation workers and assessed the effects of bent working positions and heavy lifting on back, elbow, neck, and shoulder pain experienced by the sanitation workers. Additionally, one article assessed the correlation between cadmium exposure and symptoms of bony aches, joint pain, and bone fractures (Taha et al., 2018). Overall, the studies provide some evidence of increased musculoskeletal disorders associated with sanitation work. Different assessments or questionnaires were used to assess back and neck pain. Based on the Nordic Questionnaire, Friedrich et al. described high 12-month prevalence of neck pain (52.4%), lower back pain (72.8%), and upper back pain (54.8%) among sewage workers in Austria (Friedrich et al., 2000). Wang et al. focused on cleaning workers in Taiwan, but also reported a high 12-month prevalence of neck pain (54.7%) and lower back pain (57.9%), in addition to elbow and shoulder pain (60.9% and 63.9%, respectively), due to the cleaning tools available and postures required (Wang et al., 2019). Wang et al. recommended proper training to ensure correct working postures that limit musculoskeletal strain and proper tools relative to the height of the cleaning workers (Wang et al., 2019). In addition to the physical demands of sanitation workers causing musculoskeletal disorders, Taha et al. discussed the occupational hazard of cadmium and its effect on bone/musculoskeletal health (Taha et al., 2018). Taha et al. concluded significant differences in the presence of bony aches (OR: 31.7; 95% CI: 6.7, 150.6) and joint pain (OR: 3.68, 95% CI: 1.43, 9.47) among cadmium-exposed workers in a sewage plant versus non-cadmium exposed workers (Taha et al., 2018).
3.8. Mental and social health outcomes
Mental health and social conditions were also reported in a total of four studies; they also provide some evidence of adverse effects associated with sanitation work. The studies ranged in the mental and social health conditions reported, but provide some evidence of adverse outcomes associated with sanitation occupations. Giri et al. focused on occupational stress experienced by sanitation workers through semi-structured interviews and Occupational Stress Index (OSI) questionnaire, while the questionnaire utilized by Smith et al. included circadian malaise and perceived home, social, and work life satisfaction (Smith et al., 1998; Giri et al., 2011). Based on responses to 46 items, the OSI questionnaire placed sewage workers in a low, moderate, or high occupational stress category; 66.67% of sewage workers were reported to have moderate to high occupational stress (Giri et al., 2011). Intrinsic impoverishment, powerlessness, strenuous working conditions, and unprofitability were large contributors to high occupational stress (Giri et al., 2011). The study also addressed alcohol addiction; 66% of workers with low stress, 65% of workers with moderate stress, and 80% of workers with high stress responded that they were addicted to alcohol (Giri et al., 2011).
3.9. Injuries
Only one study reported on injuries. Schira et al. reported on cutaneous symptoms, such as skin lesions, along with its outcomes on gastroenteritis and respiratory conditions (Schira et al., 1987).
4. Discussion
To our knowledge, this is the first systematic review of occupational and work-related health outcomes among sanitation workers, compared with workers in other occupations. We identified a large number of studies that met the review's inclusion criteria, representing a potentially large body of evidence. They covered a large range of outcomes, especially gastrointestinal and respiratory. They were conducted in multiple countries, though surprising, none in low-income countries. Most were conducted in the last two decades, perhaps showing an increased interest in this subject area.
The quality of the evidence, however, is not matched by the quantity. All were observational studies, and the vast majority were cross-sectional. Most studies did not specify criteria for selecting sanitation facilities/plants and/or sanitation workers, raising the potential for selection bias. Additionally, exposure assessments were most often not assessed, or were based on personal recall or reported occupation; studies that did not assess the exposure relied on the certainty that sanitation workers were exposed based on their occupation. This raises important issues of recall and measurement bias. The substantial heterogeneity among study populations, settings and methods limited the potential for pooling results across studies.
Nevertheless, taken as a whole, the evidence does suggest an increased risk of adverse health across a range of outcomes. This is especially true in the case of hepatitis A infections, where a pooled estimate from 13 studies showed a doubling of odds associated with sanitation work, and even higher odds when limited to studies with lower risk of bias. There was also largely consistent evidence of elevated odds of adverse gastrointestinal and respiratory conditions associated with sanitation work, the most common outcomes reported. While some of the studies relied on reported symptoms that may be subject to bias, others focused on clinical evidence of infection (the presence of pathogens or serological response). Comparatively few studies reported on adverse musculoskeletal and mental/social impacts, but these again consistently found elevated odds among sanitation workers. There was inconsistent evidence on mortality.
This review also highlights the gaps in research characterizing the health risks of sanitation workers in four main areas: low-income countries, among women and those under informal employment. Perhaps most striking is the fact that none of the studies that met the review's inclusion criteria were conducted in low-income countries. This, despite widespread reports of the large numbers of sanitation workers in such settings and the special risks that they are likely to face due to the lack of legal protections, access to personal protective equipment and other safegards (WHO, 2020; Comaru and Werna, 2013). Studies may also be biased in favor of male subjects, as reports from certain countries in the Indian sub-continent indicate that women make up at least half of the urban sanitation workforce and may undertake work that could put them at higher risk (Srivastava). Finally, we identified no studies that describe the working conditions or the burden of disease faced by sanitation workers who are informally employed, another clear gap in the current research.
National and global infectious disease outbreaks, like the COVID-19 pandemic, may exacerbate the risks among sanitation workers, specifically those in LMICs. Patwary et al. pointed out many of the inequalities and occupational hazards faced by sanitation workers, including irregular pay and worsening working conditions during the pandemic (Patwary et al., 2021). During the lockdowns of the COVID-19 crisis, sanitation workers continued work and experienced increased workloads (Patwary et al., 2021). While there is little if any evidence to date of actual transmission of SARS Cov2 via waste, the presence of the viruses in fecal sludge and sewage has the potential of placing sanitation workers at greater risk (Medema et al., 2020; Pan et al., 2020; Peccia et al., 2020). One-third of sanitation workers in Nepal were not supplied with crucial protective gear, and 40% of workers in four South Asian countries were not provided with hand-washing facilities, two resources that are necessary for protection against COVID-19 a wide range of other infectious diseases (Sanitation workers left e, 2020). While facing increased health risks and occupational hazards during a global pandemic, many in these regions did not receive additional income (e.g. stimulus packages) or access to health insurance (In India and 90% sanitation, 2021). Patwary et al. underscored the need for policies that protect sanitation workers, specifically in LMICs, on a national and international level. This includes providing PPE, trainings, health insurance, and medical care, as well as adequate policies, standards, regulations (Patwary et al., 2021).
This review has important limitations, many of which are common in systematic reviews of environmental health. First among these is the heterogeneity among the studies' setting, population, study design, case definitions, exposure assessment, and outcome assessments that limit the potential for synthesizing studies using meta-analysis or meta-regression. As a result, this review is mainly descriptive. We did not independently assess publication bias, though that has been shown to be present in other reviews of sanitation interventions (Freeman et al., 2017). While we employed a robust search strategy, terms used to describe sanitation workers may vary across geographical regions, countries, languages, and cultures and some studies may have not been identified. Furthermore, many articles did not define their studies’ sanitation worker population of interest or may not have specified that their sanitation workers were exposed to human fecal sludge or wastewater which inadvertently excluded them from the review. The initial review of study eligibility was undertaken by a single reviewer, not independently by two reviewers, due to resource limitations. We also note the limitations of conducting the risk of bias in the context of observational studies (Dekkers et al., 2019; Mueller et al., 2018). Finally, we note the challenges of conducting systematic reviews of observational studies of occupational and environmental health exposures (Arroyave et al., 2021).
Regarding use of this evidence for informing official global norms and standards, this systematic review demonstrates that more and better primary studies from a more diverse set of regions and countries is required to arrive at a body of evidence that would allow producers of official statistics to consider quantifying the work-related burden of disease and injury among sanitation workers within the framework global comparative risk assessment (Ezzati et al., 2004). Thereafter, for such quantification of health loss among these workers, systematic reviews and meta-analyses conducted specifically for such estimation within a framework of global health risk assessment (such as (Li et al., 2020) and (Descatha et al., 2020) as the basis for (Pega et al., 2021b)) are also needed (Pega et al., 2021b; Li et al., 2020; Descatha et al., 2020).
5. Conclusion
This review provides suggestive evidence of elevated occupational risk among sanitation workers across a range of health conditions. The extent to which existing research can form a reasonable basis for policy or even estimates of the burden of disease is very limited due to the gaps in research and scientific rigor. Nevertheless, this review demonstrates a clear need for further investigation and quantification of the health risks faced by sanitation workers, as compared to workers in other occupations, particularly in low-income countries, and of the effectiveness of governmental policies and other efforts to mitigate these risks.
Funding
This work was supported by a grant from the World Health Organization (Grant No. WSH/PHE 2019/974207). Hemali Oza was supported in part by a grant from the National Institute of Environmental Health Sciences, USA (5T32ES12870-17). Frank Pega was funded by staff salary from the World Health Organization.
Disclaimer
The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2021.113907.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- The 17 Goals | Sustain. Dev. https://sdgs.un.org/goals
- Arroyave W.D., Mehta S.S., Guha N., et al. Challenges and recommendations on the conduct of systematic reviews of observational epidemiologic studies in environmental and occupational health. J. Expo. Sci. Environ. Epidemiol. 2021;31:21–30. doi: 10.1038/s41370-020-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitidou M., Constantinidis T.C., Doutsos J., Mandraveli K., Katsouyannopoulos V. Occupational hepatitis B virus infection in sewage workers. Med. Lav. 1998;89:437–444. [PubMed] [Google Scholar]
- Arvanitidou M., Mamassi P., Vayona A. Epidemiological evidence for vaccinating wastewater treatment plant workers against hepatitis A and hepatitis B virus. Eur. J. Epidemiol. 2004;19:259–262. doi: 10.1023/b:ejep.0000020444.64546.3b. [DOI] [PubMed] [Google Scholar]
- Athanasiou M., Makrynos G., Dounias G. Respiratory health of municipal solid waste workers. Occup. Med. 2010;60:618–623. doi: 10.1093/occmed/kqq127. [DOI] [PubMed] [Google Scholar]
- Bener A., Lestringant G.G., Dogan M., et al. Respiratory symptoms and skin disorders in sewage workers. J. Environ. Sci. Health - Part A Toxic/Hazard. Subst. Environ. Eng. 1998;33:1657–1674. [Google Scholar]
- Bonanni P., Comodo N., Pasqui R., et al. Prevalence of hepatitis A virus infection in sewage plant workers of Central Italy: is indication for vaccination justified? Vaccine. 2000;19:844–849. doi: 10.1016/s0264-410x(00)00227-9. [DOI] [PubMed] [Google Scholar]
- Brugha R., Heptonstall J., Farrington P., Andren S., Perry K., Parry J. Risk of hepatitis A infection in sewage workers. Occup. Environ. Med. 1998;55:567–569. doi: 10.1136/oem.55.8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadilhac P., Roudot-Thorava F. Seroprevalence of hepatitis A virus infection among sewage workers in the parisian area, France. Eur. J. Epidemiol. 1996;12:237–240. doi: 10.1007/BF00145411. [DOI] [PubMed] [Google Scholar]
- Chandra K., Arora V.K. Tuberculosis and other chronic morbidity profile of sewage workers of Delhi. Indian J. Tubercul. 2019;66:144–149. doi: 10.1016/j.ijtb.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Comaru F., Werna E., International Labor Office. Sectoral Activities Department . ILO; Geneva: 2013. The Health of Workers in Selected Sectors of the Urban Economy: Challenges and Perspectives. [Google Scholar]
- Cyprowski M., Sobala W., Buczyńska A., Szadkowska-Stańczyk I. Endotoxin exposure and changes in short-term pulmonary function among sewage workers. Int. J. Occup. Med. Environ. Health. 2015;28:803–811. doi: 10.13075/ijomeh.1896.00460. [DOI] [PubMed] [Google Scholar]
- Dekkers O.M., Vandenbroucke J.P., Cevallos M., Renehan A.G., Altman D.G., Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descatha A., Sembajwe G., Pega F., Ujita Y., Baer M., Boccuni F., Di Tecco C., Duret C., Evanoff B.A., Gagliardi D., Godderis L., Kang S.K., Kim B.J., Li J., Magnusson Hanson L.L., Marinaccio A., Ozguler A., Pachito D., Pell J., Pico F., Ronchetti M., Roquelaure Y., Rugulies R., Schouteden M., Siegrist J., Tsutsumi A., Iavivoli S. The effect of exposure to long working hours on stroke: a systematic review and meta-analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ. Int. 2020;142:105746. doi: 10.1016/j.envint.2020.105746. [DOI] [PubMed] [Google Scholar]
- Douwes J., Mannetje A., Heederik D. Work-related symptoms in sewage treatment workers. Ann. Agric. Environ. Med. 2001;8:39–45. [PubMed] [Google Scholar]
- Dzaman K., Wojdas A., Rapiejko P., Jurkiewicz D. Taste and smell perception among sewage treatment and landfill workers. Int. J. Occup. Med. Environ. Health. 2009;22:227–234. doi: 10.2478/v10001-009-0025-4. [DOI] [PubMed] [Google Scholar]
- El-Esnawy N.A. Examination for hepatitis E virus in wastewater treatment plants and workers by nested RT-PCR and ELISA. J. Egypt. Publ. Health Assoc. 2000;75:219–231. [PubMed] [Google Scholar]
- Ezzati M., Lopex A.D., Rodgers A., Murray C.J.L. World Health Organization; Geneva, Switzerland: 2004. Comparative Quantification of Health Risks: Global and Regional Burdedn of Disease Attributable to Selected Major Risk Factors. [Google Scholar]
- Fahim A.E., El-Prince M. Passive smoking, pulmonary function and bronchial hyper-responsiveness among indoor sanitary workers. Ind. Health. 2012;50:516–520. doi: 10.2486/indhealth.2012-0003. [DOI] [PubMed] [Google Scholar]
- Freeman M.C., Garn J.V., Sclar G.D., Boisson S., Medlicott K., Alexander K.T., Penakalapati G., Anderson D., Mahtani A.G., Grimes J.E.T., Rehfuess E.A., Clasen T.F. The impact of sanitation on infectious disease and nutritional status: a systematic review and meta-analysis. Int. J. Hyg Environ. Health. 2017 Aug;220(6):928–949. doi: 10.1016/j.ijheh.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Cermak T., Heiller I. Spinal troubles in sewage workers: epidemiological data and work disability due to low back pain. Int. Arch. Occup. Environ. Health. 2000;73:245–254. doi: 10.1007/s004200050424. [DOI] [PubMed] [Google Scholar]
- Friis L., Edling C., Hagmar L. Mortality and incidence of cancer among sewage workers: a retrospective cohort study. Br. J. Ind. Med. 1993;50:653–657. doi: 10.1136/oem.50.7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis L., Mikoczy Z., Hagmar L., Edling C. Cancer incidence in a cohort of Swedish sewage workers: extended follow up. Occup. Environ. Med. 1999;56:672–673. doi: 10.1136/oem.56.10.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrimann S., Winkler M.S., Pham-Duc P., et al. Intestinal parasite infections and associated risk factors in communities exposed to wastewater in urban and peri-urban transition zones in Hanoi, Vietnam. Parasites Vectors. 2016;9:537. doi: 10.1186/s13071-016-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrimann S., Winkler M.S., Kabatereine N.B., et al. Risk of intestinal parasitic infections in people with different exposures to wastewater and fecal sludge in kampala, Uganda: a cross-sectional study. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri P.A., Kasbe A.M., Aras R.Y. Occupational stress among sewage workers. Biomedicine. 2011;31:372–377. [Google Scholar]
- Hammouda N.A., el-Gebali W.M., Razek M.K. Intestinal parasitic infection among sewage workers in Alexandria, Egypt. J. Egypt. Soc. Parasitol. 1992;22:299–303. [PubMed] [Google Scholar]
- Heldal K.K., Madsø L., Huser P.O., Eduard W. Exposure, symptoms and airway inflammation among sewage workers. Ann. Agric. Environ. Med. 2010;17:263–268. [PubMed] [Google Scholar]
- Heldal K.K., Austigard Å.D., Svendsen K.H., et al. Endotoxin and hydrogen sulphide exposure and effects on the airways among waste water workers in sewage treatment plants and sewer net system. Ann Work Expo Health. 2019;63:437–447. doi: 10.1093/annweh/wxz020. [DOI] [PubMed] [Google Scholar]
- Heng B.H., Goh K.T., Doraisingham S., Quek G.H. Prevalence of hepatitis A virus infection among sewage workers in Singapore. Epidemiol. Infect. 1994;113:121–128. doi: 10.1017/s0950268800051530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In India, 90% sanitation workers don't have health insurance even amid the coronavirus crisis. https://scroll.in/article/969017/in-india-90-sanitation-workers-dont-have-health-insurance-even-amid-the-coronavirus-crisis (accessed March 2, 2021).
- Jeggli S., Steiner D., Joller H., Tschopp A., Steffen R., Hotz P., Hepatitis E. Helicobacter pylori, and gastrointestinal symptoms in workers exposed to waste water. Occup. Environ. Med. 2004;61:622–627. doi: 10.1136/oem.2003.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar N., Ranjithsingh A.J.A., Shankar S.K., Beula G.H. Epidemiological study on the seroprevalence of leptospirosis in Salem and Namakkal Districts, the major centre for livestock in Tamilnadu. Asian J. Microbiol. Biotechnol. Environ. Sci. 2008;10:239–244. [Google Scholar]
- Krajewski J.A., Cyprowski M., Szymczak W., Gruchała J. Health complaints from workplace exposure to bioaerosols: a questionnaire study in sewage workers. Ann. Agric. Environ. Med. 2004;11:199–204. [PubMed] [Google Scholar]
- Lafleur J., Vena J.E. Retrospective cohort mortality study of cancer among sewage plant workers. Am. J. Ind. Med. 1991;19:75–86. doi: 10.1002/ajim.4700190110. [DOI] [PubMed] [Google Scholar]
- Lee J.A., Thorne P.S., Reynolds S.J., O'Shaughnessy P.T. Monitoring risks in association with exposure levels among wastewater treatment plant workers. J. Occup. Environ. Med. 2007;49:1235–1248. doi: 10.1097/JOM.0b013e3181568b40. [DOI] [PubMed] [Google Scholar]
- Levin M., Froom P., Trajber I., Lahat N., Askenazi S., Lerman Y. Risk of hepatitis A virus infection among sewage workers in Israel. Arch. Environ. Health. 2000;55:7–10. doi: 10.1080/00039890009603378. [DOI] [PubMed] [Google Scholar]
- Li J., Pega F., Ujita Y., Brisson C., Clays E., Descatha A., Ferrario M.M., Godderis L., Iavicoli S., Landbergis P.A., Metzendorf M.I., Morgan R.L., Pachito D.V., Pikhart H., Richter B., Roncaioli M., Rugulies R., Schnall P.L., Sembajwe G., Trudel X., Tsutsumi A., Woodruff T.J., Siegrist J. The effect of exposure to long working hours on ischaemic heart disease: a systematic review and meta-analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ. Int. 2020;142:105739. doi: 10.1016/j.envint.2020.105739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm M., Rylander R. Work related symptoms among sewage workers. Br. J. Ind. Med. 1983;40:325–329. doi: 10.1136/oem.40.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. published online May 20. [DOI] [PubMed] [Google Scholar]
- Mental health: strengthening our response. https://www.who.int/news-room/fact-sheets/detail/mental-health-strengthening-our-response; Moher et al., 2009 Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- Mueller M., D'Addario M., Egger M., et al. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med. Res. Methodol. 2018;18:44. doi: 10.1186/s12874-018-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musculoskeletal conditions. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions
- Nasterlack M., Messerer P., Pallapies D., Ott M.G., Zober A. Cancer incidence in the wastewater treatment plant of a large chemical company. Int. Arch. Occup. Environ. Health. 2009;82:851–856. doi: 10.1007/s00420-009-0397-6. [DOI] [PubMed] [Google Scholar]
- Nethercott J.R., Holness D.L. Health status of a group of sewage treatment workers in Toronto, Canada. Am. Ind. Hyg. Assoc. J. 1988;49:346–350. doi: 10.1080/15298668891379873. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwary M.M., Hossain M.R., Shuvo F.K., Ashraf S., Sultana R., Alam M.A. Annals of Work Exposures and Health; 2021. Protecting Sanitation Workers in Low-Middle Income Countries amid COVID-19; p. wxaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., et al. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. Epidemiology. 2020 doi: 10.1101/2020.05.19.20105999. [DOI] [Google Scholar]
- Pega F., Momen N.C., Ujita Y., Driscoll T., Whaley P. Systematic reviews and meta-analyses for the WHO/ILO joint estimates of the work-related burden of disease and injury. Environ. Int. 2021;155:106605. doi: 10.1016/j.envint.2021.106605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pega F., Nafradi B., Momen N.C., Ujita Y., Streicher K.N., Pruss-Ustun A.M., Technical Advisory, Descatha A., Driscoll T., Fischer F.M., Godderis L., Kiiver H.M., Li J., Magnusson Hanson L.L., Rugulies R., Sorensen K., Woodruff T.J. Global, regional, and national burdens of ischemic heart disease and stroke attributable to exposure to long working hours for 194 countries, 2000-2016: a systematic analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ. Int. 2021;154:106595. doi: 10.1016/j.envint.2021.106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C.J.M., Shakespeare A.T. Should sewage workers and carers for people with learning disabilities be vaccinated for hepatitis A? Br. Med. J. 1993;306:1102. doi: 10.1136/bmj.306.6885.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangamani S., Bheemappa K., Gaitonde R. Health issues of sanitation workers in a town in Karnataka: findings from a lay health-monitoring study. Natl. Med. J. India. 2015;28:4. [PubMed] [Google Scholar]
- Rieger M.A., Liebers V., Nübling M., et al. Adaptation to occupational exposure to moderate endotoxin concentrations: a study in sewage treatment plants in Germany. Adv. Exp. Med. Biol. 2018;1116:89–109. doi: 10.1007/5584_2018_261. [DOI] [PubMed] [Google Scholar]
- Rylander R. Health effects among workers in sewage treatment plants. Occup. Environ. Med. 1999;56:354–357. doi: 10.1136/oem.56.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanitation workers left exposed to COVID-19. Devex. 2020 https://www.devex.com/news/sponsored/sanitation-workers-left-exposed-to-covid-19-97985 published online Sept 3. (accessed March 2, 2021) [Google Scholar]
- Scarlett-Kranz J.M., Babish J.G., Strickland D., Lisk D.J. Health among municipal sewage and water treatment workers. Toxicol. Ind. Health. 1987;3:311–319. doi: 10.1177/074823378700300303. [DOI] [PubMed] [Google Scholar]
- Schira J.C., Snella M.C., Chapon J.L. [Demonstration of gram-negative bacteria and endotoxins in the air surrounding a sewage treatment plant: effect of contaminated aerosols on the health status of the staff] Schweiz. Med. Wochenschr. 1987;117:354–358. [PubMed] [Google Scholar]
- Schlosser O., Grall D., Laurenceau M.-N. Intestinal parasite carriage in workers exposed to sewage. Eur. J. Epidemiol. 1999;15:261–265. doi: 10.1023/a:1007535426462. [DOI] [PubMed] [Google Scholar]
- Schöniger-Hekele M., Petermann D., Weber B., Müller C. Tropheryma whipplei in the environment: survey of sewage plant influxes and sewage plant workers. Appl. Environ. Microbiol. 2007;73:2033–2035. doi: 10.1128/AEM.02335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadab M., Agrawal D.K., Aslam M., Islam N., Ahmad Z. Occupational health hazards among sewage workers: oxidative stress and deranged lung functions. J. Clin. Diagn. Res. 2014;8 doi: 10.7860/JCDR/2014/5925.4291. BC11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Vijayachari P., Sugunan A.P., Natarajaseenivasan K., Sehgal S.C. Seroprevalence of leptospirosis among high-risk population of Andaman Islands, India. Am. J. Trop. Med. Hyg. 2006;74:278–283. [PubMed] [Google Scholar]
- Silva C., Barros C., Cunha L., Carnide F., Santos M. Prevalence of back pain problems in relation to occupational group. Int. J. Ind. Ergon. 2016;52:52–58. [Google Scholar]
- Singh M., Ladusingh L. Factors associated with chronic bronchitis among municipal sanitary workers in Varanasi, India. Asian J Epidemiol. 2017;10:101–107. [Google Scholar]
- Skinhoj P., Hollinger F.B., Hovind-Hougen K., Lous P. Infectious liver diseases in three groups of Copenhagen workers: correlation of hepatitis A infection to sewage exposure. Arch. Environ. Health. 1981;36:139–143. doi: 10.1080/00039896.1981.10667618. [DOI] [PubMed] [Google Scholar]
- Smit L.A.M., Spaan S., Heederik D. Endotoxin exposure and symptoms in wastewater treatment workers. Am. J. Ind. Med. 2005;48:30–39. doi: 10.1002/ajim.20176. [DOI] [PubMed] [Google Scholar]
- Smith P.A., Wright B.M., Mackey R.W., Milsop H.W., Yates S.C. Change from slowly rotating 8-hour shifts to rapidly rotating 8-hour and 12-hour shifts using participative shift roster design. Scand. J. Work. Environ. Health. 1998;24:55–61. [PubMed] [Google Scholar]
- Srivastava K. Sanitation Worker Safety and Livelihoods in India: A Blueprint for Action. ; : 127.
- Taha M.M., Mahdy-Abdallah H., Shahy E.M., Ibrahim K.S., Elserougy S. Impact of occupational cadmium exposure on bone in sewage workers. Int. J. Occup. Environ. Health. 2018;24:101–108. doi: 10.1080/10773525.2018.1518745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn J., Beijer L. Work-related symptoms and inflammation among sewage plant operatives. Int. J. Occup. Environ. Health. 2004;10:84–89. doi: 10.1179/oeh.2004.10.1.84. [DOI] [PubMed] [Google Scholar]
- Thorn J., Beijer L., Rylander R. Work related symptoms among sewage workers: a nationwide survey in Sweden. Occup. Environ. Med. 2002;59:562–566. doi: 10.1136/oem.59.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toseva E.I., Atanasova M.V., Turnovska T.H. Seroprevalence of anti-HAV total antibodies among workers in wastewater treatment plants. Int. J. Occup. Med. Environ. Health. 2018;31:307–315. doi: 10.13075/ijomeh.1896.01161. [DOI] [PubMed] [Google Scholar]
- Tschopp A., Joller H., Jeggli S., et al. Hepatitis E, Helicobacter pylori and peptic ulcers in workers exposed to sewage: a prospective cohort study. Occup. Environ. Med. 2009;66:45–50. doi: 10.1136/oem.2007.038166. [DOI] [PubMed] [Google Scholar]
- Vaidya S.R., Tilekar B.N., Walimbe A.M., Arankalle V.A. Increased risk of hepatitis E in sewage workers from India. J. Occup. Environ. Med. 2003;45:1167–1170. doi: 10.1097/01.jom.0000088874.43855.2f. [DOI] [PubMed] [Google Scholar]
- Van Hooste W., Charlier A.-M., Rotsaert P., et al. Work-related Helicobacter pylori infection among sewage workers in municipal wastewater treatment plants in Belgium. Occup. Environ. Med. 2010;67:91–97. doi: 10.1136/oem.2008.040436. [DOI] [PubMed] [Google Scholar]
- Venczel L., Brown S., Frumkin H., Simmonds-Diaz J., Deitchman S., Bell B.P. Prevalence of hepatitis A virus infection among sewage workers in Georgia. Am. J. Ind. Med. 2003;43:172–178. doi: 10.1002/ajim.10174. [DOI] [PubMed] [Google Scholar]
- Wang M.-H., Chen Y.-L., Chiou W.-K. Using the OVAKO working posture analysis system in cleaning occupations. Work. 2019;64:613–621. doi: 10.3233/WOR-193022. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization. [World Health Organization. Guidelines on sanitation and health; Geneva: 2020. Health, Safety and Dignity of Sanitation Workers: an Initial Assessment. 2018. [Google Scholar]
- Wild P., Ambroise D., Benbrik E., Tiberguent A., Massin N. Mortality among Paris sewage workers. Occup. Environ. Med. 2006;63:168–172. doi: 10.1136/oem.2005.022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank Group. World Health Organization, International Labor Organization. WaterAid. Health . 2019. Safety and Dignity of Sanitation Workers; p. 61. 2019. [Google Scholar]
- Yu G.-P., Hsieh C.-C., Peng J. Risk factors associated with the prevalence of pulmonary tuberculosis among sanitary workers in Shanghai. Tubercle. 1988;69:105–112. doi: 10.1016/0041-3879(88)90072-4. [DOI] [PubMed] [Google Scholar]
- Zuskin E., Mustajbegovic J., Schachter E.N. Respiratory function in sewage workers. Am. J. Ind. Med. 1993;23:751–761. doi: 10.1002/ajim.4700230509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.