Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic caused disruptions in treatment for cancer. Less is known about its impact on new cancer diagnoses, where delays could cause worsening long‐term outcomes. This study quantifies decreases in encounters related to prostate, lung, bladder and colorectal cancers, procedures that facilitate their diagnosis, and new diagnoses of those cancers in the COVID era compared to pre‐COVID era.
Methods
All encounters at Veterans' Affairs facilities nationwide from 2016 through 2020 were reviewed. The authors quantified trends in new diagnoses of cancer and in procedures facilitating their diagnosis, from January 1, 2018 onward. Using 2018 to 2019 as baseline, reductions in procedures and new cancer diagnoses in 2020 were estimated. Calculated absolute and percentage differences in annual volume and observed‐to‐expected volume ratios were calculated. Heat maps and funnel plots of volume changes were generated.
Results
From 2018 through 2020, there were 4.1 million cancer‐related encounters, 3.9 million relevant procedures, and 251,647 new cancers diagnosed. Compared to the annual averages in 2018 through 2019, colonoscopies in 2020 decreased by 45% whereas prostate biopsies, chest computed tomography scans, and cystoscopies decreased by 29%, 10%, and 21%, respectively. New cancer diagnoses decreased by 13% to 23%. These drops varied by state and continued to accumulate despite reductions in pandemic‐related restrictions.
Conclusion
The authors identified substantial reductions in procedures used to diagnose cancer and subsequent reductions in new diagnoses of cancer across the United States because of the COVID‐19 pandemic. A nomogram is provided to identify and resolve these unmet health care needs and avoid worse long‐term cancer outcomes.
Lay Summary
The disruptions due to the COVID‐19 pandemic have led to substantial reductions in new cancers being diagnosed.
This study quantifies those reductions in a national health care system and offers a method for understanding the backlog of cases and the resources needed to resolve them.
Keywords: bladder cancer, cancer diagnosis, cancer screening, colorectal cancer, coronavirus disease 2019 (COVID‐19), lung cancer, prostate cancer
Short abstract
The disruptions due to the COVID‐19 pandemic have led to substantial reductions in procedures that could diagnose new cancers and subsequent reductions in new diagnoses of cancer in medical facilities across the United States. To avoid the potential for worse long‐term cancer outcomes due to delayed diagnoses, health systems must quantify their deficits and find ways to clear the backlog of undiagnosed cases.
Introduction
To slow coronavirus disease 2019 (COVID‐19) transmission, safeguard patients, and preserve health care resources, a nationwide moratorium on elective clinical activities was instituted in March, 2020. 1 , 2 The restrictions had immediate and anticipated impacts on treatment of patients with known cancer. 3 , 4 However, restrictions also included clinical procedures such as colonoscopies, prostate biopsies, computed tomography (CT), and cystoscopies that offer an opportunity to detect suspected or unexpected cancer. The moratorium‐related decrease in access to care may have delayed identification of new cancers, leading to worse long‐term outcomes. 5 , 6 , 7 , 8 , 9 Current research examining this impact on screening and diagnosis of cancer has demonstrated significant decreases but has been limited to the early pandemic, without providing follow‐up on how these encounters rebounded once restrictions were lifted. 3 , 10 , 11 , 12 , 13
Despite a gradual lifting of restrictions starting May, 2020, recurrent COVID‐19 outbreaks, new variants, regional variations in COVID transmission rates, institutional caution, and patient fears about COVID‐19 exposure can be anticipated to keep health care visits and procedures well below 2019 levels across the United States (US). 3 , 14 The accumulated backlog of procedures and resultant undiagnosed cancers due to the pandemic have not been determined at a national or regional level. There are few nationwide databases that provide near real‐time, state‐by‐state data that can be used to assess this backlog. Without these data, an informed plan to allocate resources for recovery cannot be formulated.
We reviewed nationwide data from the Veterans Health Administration (VA), the largest health care system in the United States, for the 4 most common cancers in that population: 1) prostate, 2) lung, 3) bladder, and 4) colorectal. 15 The VA health system provides a large population that spans the entire United States and includes broad racial, ethnic, and geographic diversity—an important cross‐section of US health care. Given the standardized approach to care at VA facilities, changes in cancer management patterns are more reliably attributable to external pressures. Our analysis has four aims: 1) quantify the temporal trends in cancer‐related clinical encounters, procedures that may facilitate the diagnosis of new cancers, and new cancers diagnosed in 2020 compared to baseline years (2018‐2019), 2) estimate the accumulated backlog of unperformed procedures and undiagnosed cancers accrued in 2020, 3) identify geographic variation in deficits across the United States, and 4) develop a tool to calculate additional capacity and time required to recover from these deficits.
Materials and Methods
Data Source
This time‐series study examines data from Computerized Patient Record System, the VA's electronic medical record. The information is stored in the Department of Veterans Affairs Informatics and Computing Infrastructure (VINCI), one of the only nationwide, real‐time, patient‐level data sets. Data from >9 million veterans at 1244 VA medical facilities offers a unique opportunity to examine the temporal and geographic variation in cancer care across the United States, providing administrative, demographic, and clinical information on all inpatient and outpatient visits. The Institutional Review Board of the University of Maryland School of Medicine and Baltimore VA Medical Center Research and Development Committee approved this study.
Study Population
All patients with a clinical encounter in a US‐based VA facility from January 1, 2018, through December 31, 2020, were evaluated. We included all patients with an encounter for our cancers of interest, a procedure that might identify 1 of these cancers, or a new diagnosis of our cancers of interest. Encounters were searched for International Classification of Diseases‐10 (ICD‐10, see Supporting Table 1) or Current Procedural Terminology (CPT, see Supporting Table 2) codes related to the 4 most common cancers in the VA population (prostate, lung, bladder, and colorectal), or CPT codes for diagnostic and screening procedures related to the identification of these cancers: prostate biopsies, chest CT scans (classified as screening or diagnostic), cystoscopies, colonoscopies, sigmoidoscopies, and fecal occult blood tests (FOBTs). 3 , 10 Patients were considered to have a new cancer diagnosis if they had no encounter with a diagnosis code for the same cancer over a period of 2 years before the new diagnosis. To ensure this, we reviewed all encounters from January 1, 2016, to December 31, 2017. Patients were included regardless of age, gender, race, or geographic location, and we did not exclude patients based on death or disenrollment.
Outcomes
Outcomes of interest included 1) cancer‐related health care encounters, 2) cancer‐related diagnostic or screening procedures, and 3) new cancer diagnoses. Cancer‐related encounters were defined as any health care encounter (inpatient or outpatient) with an associated ICD‐10 diagnosis code for our 4 cancers of interest. Cancer‐related diagnostic and screening procedures included prostate biopsies, chest CT scans (screening and diagnostic), cystoscopies, colonoscopies, sigmoidoscopies, and FOBTs.
Statistical Analyses
Outcome measures were grouped by the month and year of the patient encounter. Using pre‐COVID baseline data from January 1, 2018, to December 31, 2019, a monthly baseline, accounting for seasonal variation, was calculated. We then calculated monthly encounters from January 1, 2020, to December 31, 2020, as a percentage of baseline and as a raw deficit. In addition to monthly deficits, the annual deficit for 2020 for each outcome was calculated as a raw number and as a percentage of the baseline annual volume. Annual deficits were calculated for each state to examine geographic variation.
A heat map of the United States for each diagnostic and screening procedure was generated to examine geographic variation by state. Observed‐to‐expected (O/E) ratios were calculated for the annual number performed for each diagnostic and screening procedure in each state, with the observed being the number of procedures performed in 2020 and the expected being the average annual number of procedures from 2018 through 2019. Funnel plots compared O/E ratios in each state by expected procedure numbers.
To compute the duration and magnitude of the increase in relevant procedures required to recover from the current backlog, we created a nomogram that can be used to compute 1) the number of months needed to clear the backlog, given the current backlog (expressed as percentage of baseline monthly procedures) and state (or institutional) capacity to increase monthly procedures (expressed as a percentage), and 2) the monthly capacity above baseline needed to clear the backlog, given the current backlog and the number of months over which a state (or institution) wants to clear the backlog. Statistical analyses were generated with R (v. 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
From January 1, 2018, to December 31, 2020, there were 4.1 million cancer‐related encounters, 3.9 million relevant diagnostic and screening procedures, and 251,647 patients with new diagnoses for cancer in the VA. New cancer diagnoses included prostate (n = 139,960 patients), lung (n = 51,224), colorectal (n = 27,697), and bladder (n = 32,766). Patients undergoing our diagnostic or screening procedures for the 4 cancers of interest had a median age of 67 years and were predominantly male (92%), with 22% Black and 6.1% Latino patients. Compared to patients undergoing these procedures in 2018 through 2019, 2020 patients had similar ages, although fewer in the 60‐ to 69‐year‐old range (29% vs 36%; P < .001) (Table 1). Although statistically significant, the differences in demographic characteristics were not clinically important.
TABLE 1.
Demographic Characteristics of Patients Undergoing Screening or Diagnostic Procedures for Cancer in 2018 Through 2020
| Variable | Year of Procedure | P | |
|---|---|---|---|
| 2018‐2019 | 2020 | ||
| No. (%) | 1,618,014 (80.1) | 400,729 (19.9) | |
| Age, median (IQR), y | 67 (59‐72) | 66 (56‐72) | <.001 |
| Age (category), y, No. (%) | <.001 | ||
| <40 | 49,727 (3.1) | 19,289 (4.8) | |
| 40‐49 | 66,806 (4.1) | 23,398 (5.8) | |
| 50‐59 | 320,447 (19.8) | 87,451 (21.8) | |
| 60‐69 | 578,090 (35.7) | 117,444 (29.3) | |
| ≥70 | 602,944 (37.3) | 153,147 (38.2) | |
| Sex, No. (%) | <.001 | ||
| Female | 120,946 (7.5) | 36,399 (9.1) | |
| Male | 1,497,068 (92.5) | 364,330 (90.9) | |
| Race, No. (%) | <.001 | ||
| White | 1,160,450 (77.1) | 276,782 (74.6) | |
| Black | 323,822 (21.5) | 84,555 (22.8) | |
| Other | 20,312 (1.4) | 9905 (2.7) | |
| Ethnicity, Latino, No. (%) | 93,555 (6) | 25,037 (6.5) | <.001 |
| Location, No. (%) | <.001 | ||
| Inpatient | 10,510 (0.6) | 3346 (0.8) | |
| Outpatient | 1,607,504 (99.4) | 397,383 (99.2) | |
| Procedure, No. (%) | <.001 | ||
| Prostate biopsy | 31,242 (1.9) | 8670 (2.2) | |
| Chest CT scan | 579,748 (35.8) | 138,139 (34.5) | |
| Cystoscopy | 83,437 (5.2) | 22,058 (5.5) | |
| Colonoscopy | 923,587 (57.1) | 231,862 (57.9) | |
Abbreviations: CT, computed tomography; IQR, interquartile range.
Numbers represent frequency (%) unless otherwise specified. Other race represents Asian, American Indian, Pacific Islander, or unknown.
Cancer‐related encounters dropped precipitously during April and May of 2020 compared to the same months in 2018 and 2019, with encounters for prostate cancer falling from an average of 63,496 in April 2018 through 2019 to 46,938 in April 2020 (26% decrease) (Fig. 1A). Encounters rebounded to near baseline levels by the summer. In April, 2020, encounters for cancers of the lung, bladder, and colorectum decreased by 10%, 27%, and 19% from baseline, respectively. In addition, prostate biopsies, chest CT scans, cystoscopies, colonoscopies, and FOBT decreased by 80%, 64%, 74%, 93%, and 54%, respectively, from baseline volumes in April 2018 and 2019 (Fig. 1B). Unlike cancer‐related encounters, which rebounded after a few months, the procedures continued to remain below baseline throughout 2020. Colonoscopy had the largest decrease, resulting in an estimated deficit of 24,871 unperformed procedures in April and 5840 in December (Fig. 1C). The decrease in procedures during 2020 without a rebound sufficient to make up for the deficits during the moratorium resulted in an estimated total annual deficit of 133,231 colonoscopies (45% of annual baseline colonoscopy volume), 7838 prostate biopsies (29%), 62,793 chest CT scans (12%), 20,680 cystoscopies (21%), and 49,334 FOBTs (13%) (Fig. 1D). Screening chest CT scans had a similar initial deficit but rebounded so no annual deficit existed for 2020.
Figure 1.
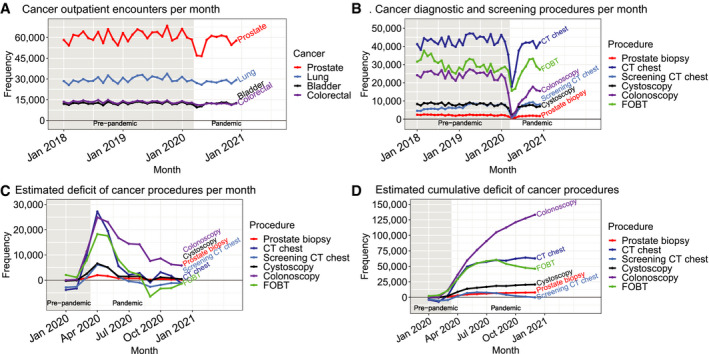
Temporal trends in (A) cancer encounters and (B) diagnostic and screening procedures in the VA health system from 2018 to 2020. The estimated (C) monthly and (D) cumulative deficit in procedures based on 2018 to 2019, seasonally adjusted baseline is presented. The gray, shaded region indicates the pre‐pandemic era, defined as before March 11, 2020. CT indicates computed tomography; FOBT, fecal occult blood test; VA, Veterans Health Administration.
The annual procedural deficits generated during 2020 varied by state, although no consistent patterns were detected by geographic region (Fig. 2) or state size (Fig. 3). Two‐thirds of states had deficits >25% of their annual baseline prostate biopsy volume. For chest CT scans, 44% of states had minimal deficits (<10%) and 10% had >25% deficits. For cystoscopies, 36% of states had deficits of >25%. Colonoscopies had the largest deficits; 29% of states had >50% deficits and only 1 state (Connecticut) had a <20% decrease. The funnel plots demonstrated that decreased procedure performance occurred in both large and small states.
Figure 2.
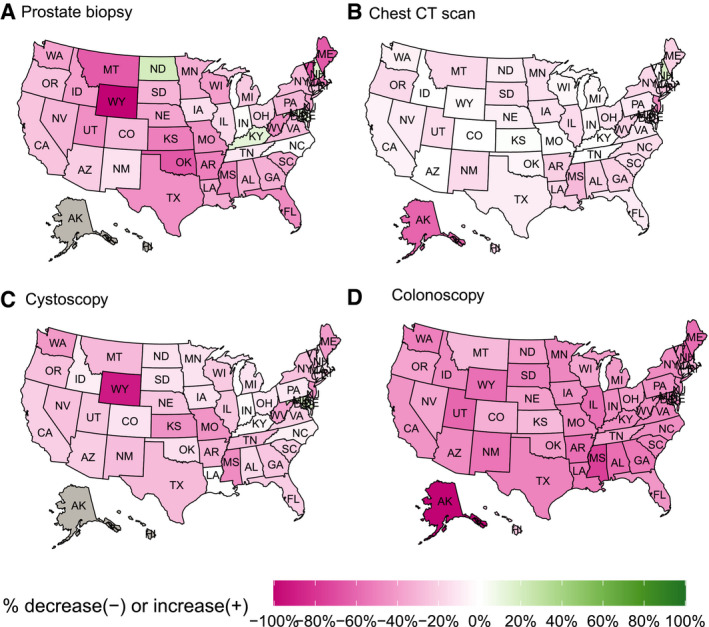
Heatmap of the change in the number of diagnostic and screening procedures performed for cancer in 2020 compared to 2018 through 2019 baseline in each state for (A) prostate biopsy, (B) chest CT scan, (C) cystoscopy, and (D) colonoscopy. Green states represent a relative increase in procedures performed in 2020, and pink indicates a relative decrease in procedures performed. Gray indicates no data for that state. CT indicates computed tomography (includes both screening and diagnostic).
Figure 3.
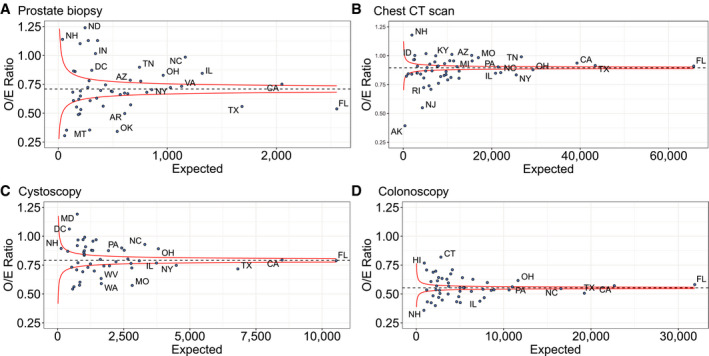
Funnel plot of observed to expected number of diagnostic and screening procedures performed for cancer in 2020 by expected number of procedures (based on 2018‐2019 data) per state for (A) prostate biopsy, (B) chest CT scan, (C) cystoscopy, and (D) colonoscopy. CT indicates computed tomography (includes both screening and diagnostic). Selected states labeled. Red lines represent 95% confidence intervals.
New diagnoses of cancer showed a similar temporal trend to that seen for diagnostic and screening procedures, with a dramatic decrease from March through June, 2020 (Fig. 4A). When examined as percentage of diagnoses normalized by 2018 to 2019 baseline data, the pattern of decreased diagnoses is consistent across prostate, lung, bladder, and colorectal cancers (Fig. 4B). Prostate cancer diagnoses fell >50% by May, 2020, resulting in 2304 fewer cancers diagnosed in May alone (Fig. 4C). Similar proportional decreases were seen in new diagnoses of colorectal, lung, and bladder cancer. Although new diagnoses of cancer began to increase in June 2020, they did not reach their pre‐pandemic baseline, and no rebound above baseline was seen. As a result, the deficit of new cancer diagnoses continued to accumulate throughout 2020 (Fig. 4D). An estimated 11,362 fewer prostate cancers (23% of annual baseline), 2365 fewer lung cancers (13%), 2130 fewer bladder cancers (18%), and 1979 fewer colorectal cancers (20%) were diagnosed in 2020 compared to baseline years 2018 through 2019.
Figure 4.

Temporal trends in new cancer diagnoses in the VA health system from 2018 to 2020 are presented as (A) monthly cases and (B) percentage of seasonally adjusted baseline. The estimated (C) monthly and (D) cumulative number of undiagnosed cancers in 2020. The gray, shaded region indicates the pre‐pandemic era, defined as before March 11, 2020. VA indicates the Veterans Health Administration.
To help states (or institutions) plan efforts to clear the backlog of missed procedures, we designed a nomogram displaying the relationship between 3 factors: 1) case deficit as a percent of monthly baseline, 2) percent increase in monthly volume to clear backlog of cases, and 3) number of months needed to clear the backlog (Fig. 5). Knowing any 2 values allows the user to calculate the third value by constructing a straight line passing through the 2 known inputs. For example, if colonoscopies dropped to 50% of expected volume over a 6‐month period during the pandemic, a backlog of unperformed colonoscopies equal to 300% of average monthly volume would exist (50% × 6). If resources allow an institution to increase their colonoscopy rates to 25% above their monthly average; then connecting 25 (percent increase in monthly volume to clear cases) and 300 (case deficit as percent of monthly baseline) would find that 12 months are required to clear the backlog. Supporting Figure 1 presents this nomogram using absolute numbers.
Figure 5.
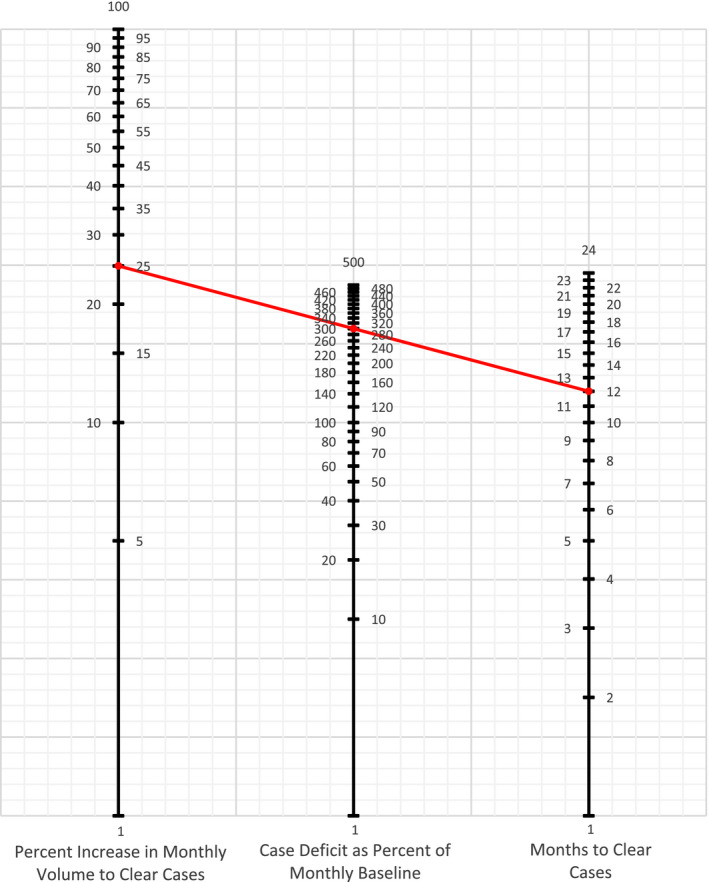
Nomogram to calculate the relationship between unperformed cases, potential monthly capacity above baseline, and months needed to clear all unperformed cases. Formula: Monthly percent increase in volume to clear unperformed cases = unperformed cases as percent of monthly baseline/months to clear unperformed cases. Example (red line): From April 1, 2020, to October 1, 2020 (6 months), we performed 50% of our expected monthly volume of colonoscopies. We have a backlog of 300% of our monthly volume: 6 × 50 = 300%. We can perform up to 125% of our expected monthly volume of colonoscopies, an additional 25%. We connect the 25 on the far‐left line with 300 on the middle line using a straight edge to calculate that it will take us 12 months to clear these unperformed cases. Alternatively, we calculate that we have an unperformed case load of 300% of our monthly volume. We want to clear these cases within 1 year or 12 months. We connect the 12 on the far‐right line with 300 on the middle line using a straight edge to calculate that it will take an increased volume of 25% to clear these cases in the desired time period.
Discussion
There was widespread recognition of the disruptions in cancer care during the early period of the COVID‐19 pandemic. Patients with known cancers are easily identified, and reductions in treatment activities are readily quantified. Accurately identifying the backlog of missed screening or diagnostic procedures and the resulting undiagnosed cancers is challenging. Examining data from the VA health system, we quantified decreases in cancer encounters, diagnostic and screening procedures, and new diagnoses for the 4 most common cancers in VA patients. We found a dramatic decrease in all 3 metrics in the early months of the pandemic with no evidence of a subsequent rebound by the end of 2020 that would be sufficient to make‐up for lost procedures or diagnoses. Screening chest CT scans were the exception, with a rebound that resulted in no annual deficit for 2020. For other procedures, decreases of up to 45% of typical annual volumes have accumulated and continue to accrue, although at a slower pace. There were large geographic variations in the decreased diagnostic activities for cancers across states and across different cancers, although no specific patterns were identified. Finally, we developed a nomogram for institutions, health systems, or states to estimate the time and resources required to work through the backlog in diagnostic activities accrued during 2020.
With the moratorium on nonemergency clinical encounters throughout much of the United States, decreases in treatment and clinical activities that impacted identification of new cancers were seen during the peak of the pandemic. 16 The first half of 2020 saw decreased resections for colon and breast cancer, screening, and biopsies in the Medicare population. 3 , 12 , 13 Data also suggest that new diagnoses of cancer decreased during the early stages of the pandemic. 10 Although these reports were concerning, they reflected activities at the height of the pandemic. Our report examines the entirety of 2020 and estimates the accumulated deficit in new cancer diagnoses and in procedures that could facilitate the diagnosis of new cancers. By computing the cumulative decrease, we identified that through the end of 2020, we have yet to enter a “catching‐up” period, where we are clearing the backlog in diagnoses and procedures. Screening chest CT scans represent the exception to this observation, rebounding in the second half of 2020, so that no overall deficit existed by the end of 2020. Rather than focusing only on procedures that were performed specifically as a tool for cancer surveillance, we considered all diagnostic procedures in which a cancer could be diagnosed incidentally, as part of a screening program, or as part of testing based on clinical concerns, to be a “lost opportunity” to diagnose a new cancer.
There may be several reasons why the deficits have persisted even as states and institutions have relaxed COVID‐related restrictions. The variations in state‐level trends may reflect variations in statewide restrictions, institutional response to local outbreaks, or differences in perceived risk of COVID‐19 by patients and institutions. 17 Unemployment and the financial hardships from the pandemic may have restricted health care access, although not as significant a problem in the VA population as in employer‐based insurance. Perceived risks of invasive versus noninvasive procedures may explain why CT scans recovered to near baseline levels by the end of 2020 and accumulated smaller backlogs than colonoscopies. Even if the risks of COVID‐19 are minimized through vaccination and herd immunity, patients who have fallen out of the routine of their typical care may encounter barriers to re‐entry that are difficult to overcome. For these reasons, health systems will need to identify areas where patients are not following up on their routine cancer care or screening and find ways to reassure and re‐engage them.
Data from Brazil suggest that short‐term decreases in cancer care lead to increased rates of cancer‐related deaths. 6 Although increased cancer‐specific deaths have not been demonstrated in the United States, early reports from New York City found a short‐term increase in non‐COVID‐related mortality during their early pandemic surge. 5 Modeling of pre‐pandemic data from the United Kingdom suggests that a delay in the identification and care for colorectal cancer could lead to increases in deaths by up to 20%. 8 Conversely, extrapolating data from the US‐based National Cancer Database, investigators concluded that patients with many cancers, including breast and prostate, could sustain a delay in resection of 3 to 6 months without significant increase in mortality, particularly if chemotherapy or endocrine therapy was being used. 9 , 18 , 19 We identified a population whose cancer would be undiagnosed and would therefore not be eligible for neoadjuvant therapy. In addition, the pandemic has impacted the ability of centers to deliver neoadjuvant therapy. Our data indicate that backlogs continued to accumulate through the end of 2020, suggesting that the delay in diagnosing new cancer may be longer than 6 months. Some of these cancers may become symptomatic, prompting a workup and diagnosis; however, others may progress without symptoms, becoming unresectable or metastasizing before detection. Other cancers may be indolent; for example, a majority of prostate cancers are slow growing and will not significantly benefit from early detection. We are unaware of any organized strategy to quantify, identify, and clear the deficit of unperformed procedures and undiagnosed cancers. Our results provide the first steps in this effort.
Given the decrease in diagnostic procedures, we can anticipate health care consequences including undiagnosed cancers, upstaging at subsequent diagnosis, increased intensity and complexity of treatment, and increased mortality. Our results indicate that an urgent, informed, and concerted response is needed. Along with providing a blueprint for computing the accumulated deficit within a specific environment, our study provides a tool, a nomogram to guide institutions and health systems to conceptualize the potential increased capacity and time needed to address the unmet needs. Health systems, including the VA, must develop plans to increase capacity to quickly alleviate accumulated demand. Outreach through primary care providers with access to patients who have been lost to follow‐up must be instituted. Alternatively, health systems may increase screening intervals, particularly for low‐risk individuals, or focus outreach efforts to patients with increased risk factors. Efforts should be deliberate and evidence‐based.
Future studies will need to examine the consequences of delayed cancer diagnoses. Increased incidence of unresectable disease, inability to achieve R0 resection, advanced stage at diagnosis, metastatic disease, need for adjuvant therapies, and mortality may indicate that diagnostic delays are impacting patient outcomes. These outcomes may take years to become evident, and our future goals involve identifying these outcomes and developing a surveillance system to monitor for increases. In addition, a more granular examination of state variation will be needed to understand how some states continued providing screening and diagnostic procedures at close to baseline case volumes whereas others had dramatic decreases of 50% or more.
Our study has several limitations. The VA population is older and predominantly male, making assessment of diseases like breast cancer difficult, although we found no evidence in the published literature to suggest that the pandemic affected health care for women differently than men. The patient population dictated our selection of the four target cancers, and it is possible that other cancers may offer different results. Although the results are from an administrative database, it is reasonable to anticipate that there was no selection bias with respect to clinical encounters for different cancers. The VA's Care in the Community (CITC) program allows veterans to seek care outside of the VA system, and these data would not be included in our analysis. Given evidence that clinical encounters and diagnostic procedures decreased across all health care in the United States, 3 , 10 it seems unlikely that the decrease in cancer care at VA institutions was being made up by outside centers. VA centers do not have the same financial pressures as other institutions, and these results may not be generalizable to all populations. CPT codes could not reliably differentiate screening and diagnostic colonoscopies, although all colonoscopies represent an opportunity to identify a cancer. The VINCI data involves administrative data, where miscoding of procedural data is low but exists. 20
In conclusion, health systems across the United States have suffered a major disruption in cancer care from COVID‐19. Although the extent and impact of the pandemic on immediate treatment of known cancers is predictable and has been reported on, we found a major reduction in diagnostic procedures that are used to identify new cancers and a consequent reduction in diagnosis of new cancers. The deficits vary by geographic location and by cancer type. We also provide the means to facilitate a recovery plan to resolve these unmet health care needs and avoid the potential for worse long‐term cancer outcomes.
Funding Support
This work was supported by a grant from the Department of Veterans Affairs (HSRD C19‐20‐407).
Conflict of Interest Disclosures
Brajesh K. Lal received grants from the Department of Veterans Affairs (RRD RX000995 and CSRD CX001621) and the National Institutes of Health (NS080168, NS097876, and AG000513). John D. Sorkin received grants from the National Institutes of Health (AG028747 and DK072488) and the Baltimore VA Medical Centre GRECC. Nikhil K. Prasad received a grant from the National Institutes of Health (T32 AG00262). The other authors made no disclosures.
Author Contributions
Brian R. Englum: Conceptualization, formal analysis, writing–original draft, and writing–review, and editing. Nikhil K. Prasad: Concept, data acquisition, and writing–review and editing. Rachel E. Lake: Formal analysis and writing–review and editing. Minerva Mayorga‐Carlin: Oversight of analysis and writing–review and editing. Douglas J. Turner: Conceptualization and writing–review and editing. Tariq Siddiqui: Data acquisition and writing–review and editing. John D. Sorkin: Conceptualization, data acquisition, oversight of analysis, and writing–review and editing. Brajesh K. Lal: Conceptualization, data acquisition, interpretation of data, and writing–review and editing.
Supporting information
Supplementary Material
References
- 1. Non‐emergent, elective medical services, and treatment recommendations. Centers for Medicare & Medicaid Services. Accessed September 17, 2020. https://www.cms.gov/files/document/cms‐non‐emergent‐elective‐medical‐recommendations.pdf [Google Scholar]
- 2. COVID‐19: guidance for triage of non‐emergent surgical procedures. American College of Surgeons. Accessed October 8, 2020. https://www.facs.org/COVID‐19/clinical‐guidance/triage [Google Scholar]
- 3. Patt D, Gordan L, Diaz M, et al. Impact of COVID‐19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID‐19 pandemic on cancer care. Nat Cancer. 2020;1:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. COVID Response Team, New York City Department of Health and Mental Hygiene . Preliminary estimate of excess mortality during the COVID‐19 outbreak‐New York City, March 11‐May 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:603‐605. [DOI] [PubMed] [Google Scholar]
- 6. Fonseca GA, Normando PG, Loureiro LVM, et al. Reduction in the number of procedures and hospitalizations and increase in cancer mortality during the COVID‐19 pandemic in Brazil. JCO Glob Oncol. 2021;7:4‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu SJ, George EL, Maggio PM, Hawn M, Nazerali R. The consequences of delaying elective surgery: surgical perspective. Ann Surg. 2020;272:e79‐e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modeling study. Lancet Oncol. 2020;21:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turaga KK, Girotra S. Are we harming cancer patients by delaying their cancer surgery during the COVID‐19 pandemic? Ann Surg. Published online June 2, 2020. doi: 10.1097/SLA.0000000000003967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID‐19) pandemic. JAMA Netw Open. 2020;3:e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller MJ, Xu L, Qin J, et al. Impact of COVID‐19 on cervical cancer screening rates among women aged 21‐65 years in a large integrated health care system‐Southern California, January 1‐September 30, 2019, and January 1‐September 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filipe MD, van Deukeren D, Kip M, et al. Effect of the COVID‐19 pandemic on surgical breast cancer care in the Netherlands: a multicenter retrospective cohort study. Clin Breast Cancer. 2020;20:454‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Haren RM, Delman AM, Turner KM, et al. Impact of the COVID‐19 pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232:600‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prasad NK, Englum BR, Turner DJ, et al. A nation‐wide review of elective surgery and COVID‐surge capacity. J Surg Res. 2021;267:211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177:693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. COVID‐19: Executive orders by state on dental, medical, and surgical procedures. American College of Surgeons. Accessed October 8, 2020. https://www.facs.org/covid‐19/legislative‐regulatory/executive‐orders [Google Scholar]
- 17. Hoehn RS, Zureikat AH. Cancer disparities in the COVID‐19 era. J Surg Oncol. 2020;122:371‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dee EC, Mahal BA, Arega MA, et al. Relative timing of radiotherapy and androgen deprivation for prostate cancer and implications for treatment during the COVID‐19 pandemic. JAMA Oncol. 2020;6:1630‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minami CA, Kantor O, Weiss A, Nakhlis F, King TA, Mittendorf EA. Association between time to operation and pathologic stage in ductal carcinoma in situ and early‐stage hormone receptor‐positive breast cancer. J Am Coll Surg. 2020;231:434‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DS, Stitt A, Wang X, et al. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22‐e26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


