Abstract
BACKGROUND:
The benefits of moderate and vigorous physical activity (MVPA) for breast cancer survivors are well established. Yet, most are insufficiently active. Fit2Thrive used Multiphase Optimization Strategy (MOST) methodology to determine the effect of five intervention components on MVPA in this population.
METHODS:
Participants [n=269;Mage=52.5 (SD=9.9)] received a core intervention (Fit2Thrive self-monitoring app and Fitbit) and were randomly assigned to five intervention components set to on/off in a full factorial experiment: support calls, deluxe app, buddy, online gym, and text messages. The intervention was delivered over 12 weeks with a 12-week follow-up. MVPA was measured via accelerometry at baseline (T1), 12 (T2) and 24 (T3) weeks. Main effects and interaction effects at each time point were examined for all components.
RESULTS:
Trial retention was high; 93% had valid accelerometer data at T2 or T3. Across all conditions, there were significant increases in MVPA (+53.6 min/week;p<0.001) and the proportion of survivors meeting MVPA guidelines (+22.3%, p<0.001) at T2 that were maintained but attenuated at T3 (+24.6 min/week, p<0.001; +12.6% meeting guidelines, p<0.001). No individual components significantly improved MVPA although, increases were greater for the “on” versus “off” level for support calls, buddy, and text messages at T2 and T3.
CONCLUSIONS:
The Fit2Thrive core intervention (self-monitoring app and Fitbit) is promising for increasing MVPA in breast cancer survivors, but the components provided no additional increases in MVPA. Future research should evaluate the core intervention in a randomized trial and determine what components optimize MVPA behaviors in breast cancer survivors.
Keywords: Physical Activity, Breast Cancer Survivors, Behavior Change, Intervention, Digital Health
Precis:
Systematically testing five technology-supported physical activity promotion intervention components alongside the core Fit2Thrive intervention (Fit2Thrive self-monitoring app and Fitbit) resulted in an increase in physical activity at post-intervention and at 12-week follow-up in breast cancer survivors. However, increases were not significantly greater with any component turned “on” versus “off” indicating the Fit2Thrive core is a promising scalable strategy for increasing MVPA.
INTRODUCTION
Increased moderate to vigorous intensity physical activity (MVPA) is associated with fewer treatment-related side effects, higher quality of life (QOL), increased survival and reduced recurrence and mortality among breast cancer survivors1–4. However, up to 90% do not meet MVPA guidelines (i.e. 150 minutes/week)5, 6. Although professionally-led supervised MVPA programs are efficacious for increasing MVPA and improving health outcomes in this population, scalability is low due to expense and limited trained professionals.7, 8 On-site appointments, travel, and schedule limitations also increase access barriers for patients.9, 10 Remotely-delivered, technology-supported interventions may be a low burden, scalable, strategy to increase MVPA. Recent reviews11, 12 conclude distance-based and technology-supported MVPA interventions are feasible and acceptable among cancer survivors, but most studies have small sample sizes and few use wearables (i.e. Fitbit) or smartphone applications (apps), pervasive technologies that are viewed as acceptable to survivors.13–15 A recent review found only three randomized trials evaluating interventions that used wearables to promote MVPA in BCS, and all of these interventions demonstrated increases in MVPA.16 A more recent randomized controlled trial (RCT) evaluating an intervention that used wearables combined with a goal-setting session and behavioral counseling calls in breast cancer survivors demonstrated a significant increase in MVPA over 12 weeks favoring the intervention group.17 Findings with regard to smartphone apps are more mixed.12 This can be attributed, at least in part, to the use of commercially-available MVPA apps which were concluded to be unsuitable for cancer survivors in a recent review.18
While existing data are promising, interventions largely test multicomponent treatment packages making it impossible to determine what intervention components meaningfully increase MVPA. This information is necessary to increase intervention effectiveness and efficiency to drive decision making about what features to implement given resource constraints to translate MVPA promotion research to practice. The Multiphase Optimization Strategy (MOST) is an innovative framework that uses highly efficient experiments to systematically evaluate discrete effects of intervention components.19, 20 MOST is based on the resource management and continual optimization engineering principles.21 The overall goal is to develop interventions that maximize public health impact using available resources.21 The factorial experiment, one research design used in the MOST framework, allows researchers to “see inside the black box” of bundled, multi-component behavioral interventions to simultaneously test which individual intervention components or component levels, independently or combined, meaningfully contribute to the desired outcome using fewer resources than multiple randomized trials.22 That information guides decision making to assemble an optimized treatment package to achieve desired outcomes within resource constraints. To our knowledge, no studies have used a MOST approach to test which intervention components are most effective for increasing MVPA among cancer survivors.
The primary purpose of Fit2Thrive was to rigorously and systematically identify which of five potential technology-supported intervention components contribute to increased MVPA among breast cancer survivors over a 24-week period.
METHODS
Study Design
Fit2Thrive utilized a full factorial experimental design (see Table 1) to estimate each component’s effect and interactions among components. Participants were randomly assigned to 1 of 32 experimental conditions representing all possible (25) combinations of the 5 intervention components to be tested. The present study focuses on the primary outcome, post-intervention 12-week MVPA and effect maintenance at 24-week follow-up. The study protocol was previously published.23
Table 1.
Fit2Thrive Experimental Conditions
| Exp Condition | Core | Support Calls | App Type | Buddy | Online Gym | Text Messages |
|---|---|---|---|---|---|---|
| 1 | Yes | No | Standard | No | No | No |
| 2 | Yes | No | Standard | No | No | Yes |
| 3 | Yes | No | Standard | No | Yes | No |
| 4 | Yes | No | Standard | No | Yes | Yes |
| 5 | Yes | No | Standard | Yes | No | No |
| 6 | Yes | No | Standard | Yes | No | Yes |
| 7 | Yes | No | Standard | Yes | Yes | No |
| 8 | Yes | No | Standard | Yes | Yes | Yes |
| 9 | Yes | No | Deluxe | No | No | No |
| 10 | Yes | No | Deluxe | No | No | Yes |
| 11 | Yes | No | Deluxe | No | Yes | No |
| 12 | Yes | No | Deluxe | No | Yes | Yes |
| 13 | Yes | No | Deluxe | Yes | No | No |
| 14 | Yes | No | Deluxe | Yes | No | Yes |
| 15 | Yes | No | Deluxe | Yes | Yes | No |
| 16 | Yes | No | Deluxe | Yes | Yes | Yes |
| 17 | Yes | Yes | Standard | No | No | No |
| 18 | Yes | Yes | Standard | No | No | Yes |
| 19 | Yes | Yes | Standard | No | Yes | No |
| 20 | Yes | Yes | Standard | No | Yes | Yes |
| 21 | Yes | Yes | Standard | Yes | No | No |
| 22 | Yes | Yes | Standard | Yes | No | Yes |
| 23 | Yes | Yes | Standard | Yes | Yes | No |
| 24 | Yes | Yes | Standard | Yes | Yes | Yes |
| 25 | Yes | Yes | Deluxe | No | No | No |
| 26 | Yes | Yes | Deluxe | No | No | Yes |
| 27 | Yes | Yes | Deluxe | No | Yes | No |
| 28 | Yes | Yes | Deluxe | No | Yes | Yes |
| 29 | Yes | Yes | Deluxe | Yes | No | No |
| 30 | Yes | Yes | Deluxe | Yes | No | Yes |
| 31 | Yes | Yes | Deluxe | Yes | Yes | No |
| 32 | Yes | Yes | Deluxe | Yes | Yes | Yes |
Procedures
Participants
Participants were recruited via an email blast sent to the Love Research Army© listserv, an initiative to connect researchers with individuals interested in participating in breast cancer research. Inclusion criteria were: female, age ≥18 years; diagnosed with stage I-III breast cancer ≤5 years ago; ≥3 months post-primary treatment (i.e. surgery, chemotherapy, radiation therapy); capable of MVPA participation without exacerbating pre-existing condition(s); able to read, write, and speak English; own a smartphone; have access to a computer with internet; and self-report <60 minutes of weekly MVPA. Participants were required to live anywhere in the United States or Puerto Rico. Women also indicated willingness to receive telephone support calls and find a buddy to participate with them.
Those who responded to the email blast were emailed a secure link to the online screening survey. Eligible women were emailed a study overview and informed consent copy and completed a call to review study procedures, and confirm eligibility and interest. Following this call, they were sent a link to the online informed consent form. Participants were also required to pass the Physical Activity Readiness Questionnaire (PAR-Q)24 or obtain physician consent. All study procedures were approved by the Institutional Review Board.
Figure 1 displays a Consolidated Standards of Reporting Trials (CONSORT) diagram showing participant flow through the study.
Figure 1.
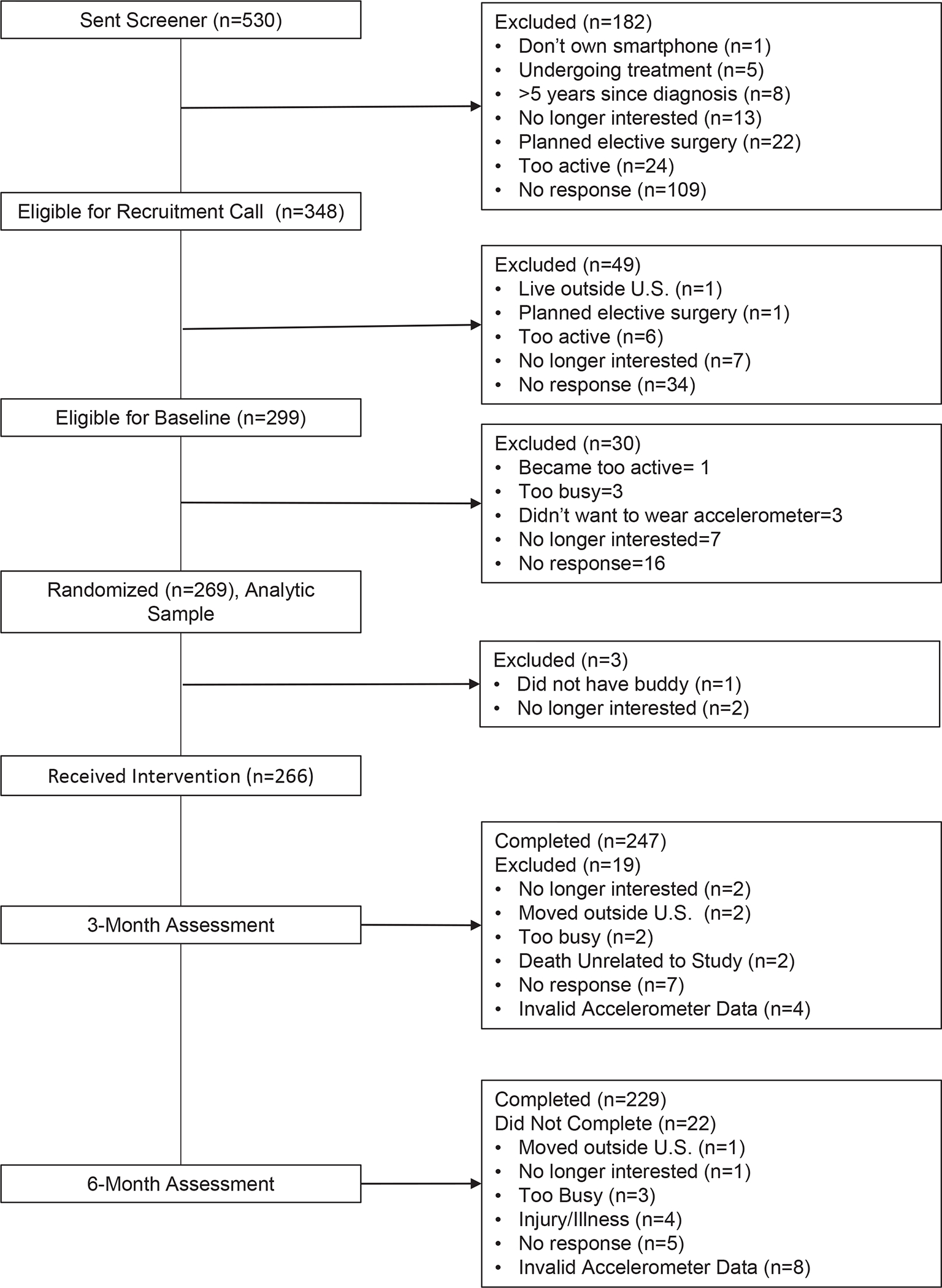
Consolidated Standards of Reporting Trials (CONSORT) diagram depicting participant flow through the study
Randomization
Following baseline assessment completion, participants were randomized to have each component turned “on” or “off” in one of the 32 conditions (see Table 1) using computer-generated randomly permuted blocks. Women were made aware of each intervention component to which they were assigned but were unaware these components were assigned by a study randomization procedure
Intervention
Intervention components were chosen based on our prior needs assessment and interviews with breast cancer survivors25 and the smartphone app was designed in conjunction with breast cancer survivors.26 Intervention components were developed to target Social Cognitive Theory (SCT) constructs27 identified as useful targets for MVPA interventions in cancer survivors28, 29 including self-efficacy, outcome expectations, social support, and goal-setting. Every participant, regardless of randomization, received the core intervention including a Fitbit Zip and a custom-designed Fit2Thrive app available for iOS and Android. The Fitbit and Fit2Thrive app were chosen as the core intervention to provide the essential competencies needed to safely increase physical activity. Participants were instructed to wear the Fitbit during all waking hours. Fit2Thrive app features included: a) prescription to gradually increase MVPA to ≥150 minutes/week; b) written information about MVPA benefits, safe MVPA adoption, and SCT-based behavioral strategies for increasing MVPA (i.e. goal-setting, realistic outcome expectations, building self-efficacy, and adding facilitators including social support and healthy rewards); c) automatic syncing and integration with the Fitbit via Bluetooth and d) activity self-monitoring (MVPA minutes, steps and distance) via manual entry or Fitbit.
Intervention Components
In addition to the core intervention, all participants were randomized to have 0 to 5 components listed below turned “on.” Components were completely independent from one another. Each component was designed with a primary SCT target mediator as the hypothesized mechanism for increasing MVPA. Full details of the conceptual model and rationale for choosing each component are published elsewhere23.
Telephone support calls:
Telephone coaching and support calls have been associated with increased activity among cancer survivors 30. Participants assigned to “on” received six bi-weekly 10- to-15 minute support calls from their assigned coach. These calls were primarily designed to increase self-efficacy, but they also discussed behavior change techniques targeting other SCT constructs, MVPA and self-monitoring adherence, and strategies for overcoming challenges.
Deluxe app:
Participants assigned to deluxe app “on” received additional Fit2Thrive app features designed to target more specific goal-setting and planning which has been associated with increased physical activity participation28 and adherence31:
Goal-Setting/Planning Tool: Could schedule activities for specific days and times with reminders.
Self-Challenges: Weekly challenges (i.e., 8,000 steps/day) available for enrollment. Harder challenges were “unlocked” as less demanding challenges were completed.
Fit News: Included two weekly posts: Fit Study (lay summary of MVPA and breast cancer research) and Fit Survivor Spotlight (success stories of survivors who increased MVPA).
Fitbit buddy:
This component was designed to primarily target social support because higher levels of social support are associated with increased MVPA among cancer survivors.32–35
Participants assigned to “on” chose an individual from their personal life (i.e. friend, colleague, spouse) to be their “Fitbit Buddy.” Buddies were mailed a Fitbit Zip and training materials on how to support the study participant. The participant and buddy were both sent 6 bi-weekly emails with ideas for facilitating social support for MVPA.
Online gym:
Participants assigned to online gym “on” were provided access to stream a commercially available DVD and publicly available online workout videos embedded into a study website. Weekly schedules for suggested use were emailed.
Text messages/push notifications:
Participants assigned to “on” received 1 to 5 messages per day as automated push notifications. Messages were motivational, targeted SCT constructs and provided tailored progress feedback.23 Participants assigned to “off” only received messages reminding them to track MVPA on days no data were transmitted.
Follow-up
During the 12-week follow-up period, participants had access to all the intervention materials they were assigned. To create a seamless app experience during the 24-week study, all Fit2Thirve app functionality for the core app or components being tested within the app (i.e. deluxe app and text messages) was maintained. All staff contact embedded in components ceased during the follow-up period (i.e. no coaching calls or buddy and online gym emails).
Data Collection
Following informed consent completion, participants were mailed an assessment packet including an ActiGraph GT3X-BT accelerometer (ActiGraph, Pensacola, Florida) on an elasticized waistband, accelerometer instructions, wear log, and postage-paid return envelope. Participants were emailed a personalized REDCap link to a questionnaire battery. Participants received completion reminders until the accelerometer was returned. The same procedures were followed at 12- and 24-weeks.
Measures
Outcomes
MVPA, the primary outcome, was measured using the ActiGraph GT3X-BT, a valid and reliable physical activity measure.36, 37 Participants were instructed to wear the activity monitor on the non-dominant hip during all waking hours (except when bathing or swimming) for seven consecutive days. Data were downloaded and processed in ActiLife 6.0 using 60-second epochs. Non-wear time was defined as intervals of ≥90 minutes of zero vertical axis activity counts allowing for 2 minutes of interruption (>0 counts/minute) with 30-minute upstream and downstream screening for artifactual movements.38 Wear time ≥10 hours was required for a day to be considered valid.39 Average time spent in sedentary, light, moderate, and vigorous activity were calculated using established cut points.40 Moderate and vigorous activity were summed to obtain MVPA volume. Wear days for calculations were determined by matching monitor days with log date entries. For participants without logs, days were chosen by examining accelerometry data for scheduled days with typical wearing patterns. Weekly MVPA minutes were totaled for persons with ≥7 valid days and estimated as 7 times the average daily total for >3 and <7 valid monitoring days. A dichotomous outcome for meeting guidelines (i.e. ≥150 minutes/week) was calculated at each time point. The optimization criteria to consider a main effect or interaction effect of any component on MVPA was set at p=0.05.
Covariates
Covariates including demographics [age, body weight and height (to calculate body mass index), and race/ethnicity] and breast cancer history [diagnosis date (to calculate time since diagnosis) and disease stage] were self-reported at baseline.
Statistical Power
We based the statistical power for the study on the change in MVPA at 12 and 24 weeks. An intraclass correlation (ICC) of 0.633, a standard deviation of 17.82 min/day of MVPA and an attrition rate of up to 15% at the 24 week time point was assumed based on our prior studies.41 There is greater than 80% power to detect a main effect as small as 6 minutes per day under a two-tailed hypothesis test with a sample size of 269 using these assumptions.
Statistical Analysis
Data were analyzed on an intent-to-treat basis. To examine intervention component effects on mean MVPA over time, weekly MPVA minutes at baseline, 12-, and 24-weeks were modeled using a generalized linear mixed model with a gamma distribution and log link, and a compound symmetric residual error variance-covariance matrix. To make comparisons between components in minutes/week rather than log minutes/week, we computed predicted means by component and their difference at 12- and 24-weeks. For the meeting guidelines (dichotomous) analyses, we used a mixed-effects logistic regression model.
Effect coding was used for the five experimental components with “off” levels coded as −1 and “on” levels coded as 1. Models included indicator variables for 12- and 24-weeks to represent the overall change relative to baseline at 12- and 24-weeks. All models adjusted for wear time by including average weekly wear hours centered at the baseline mean as a covariate. To examine whether baseline MVPA influences component effects, we conducted post-hoc moderation analyses by including an indicator variable for whether a participant had ≥60 minutes/week of baseline accelerometer-assessed MVPA and its interaction with all other predictors in the primary analysis.
Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC)42 using the GLIMMIX procedure. Predicted MVPA means were calculated using the SAS macro %NLEstimate.43
RESULTS
A total of 269 participants were randomized (Fig 1). Of these, 247 (91.8%) and 229 (85.1%) had valid 12- and 24-week MVPA assessments, respectively. One participant was excluded from regression analyses because she had implausible MVPA values at 12- and 24-weeks. Baseline characteristics, MVPA minutes, or wear time did not significantly differ by experimental component level randomization (Table 2). Participants were on average 52.5 (SD=9.9) years old with a 28.9 body mass index (BMI). Most were White (87%), non-Hispanic (94%) and had early stage disease (I and II=81%). On average, participants were 3.0 (SD=2.4) years since diagnosis and wore the accelerometer 14.4 (SD=1.3) hours/day. No adverse events were reported during the Fit2Thrive trial.
Table 2.
Baseline participant characteristics by component level
| Experimental Component | Age M(SD) | BMI M(SD) | Time since diagnosis M(SD) | Disease stage n(%) | Race n(%) | Ethnicity n(%) | Weekly MVPA min M(SD) | Daily wear hours M(SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| I | II | III | UK | White | AA | Other | Not Hispanic | Hispanic | ||||||
|
|
||||||||||||||
| Overall | 52.5 (9.9) | 28.9 (6.4) | 3.0 (2.4) | 105 (39.0) | 113 (42.0) | 43 (16.0) | 8 (3.0) | 234 (87) | 16 (5.9) | 19 (7.1) | 251 (93.3) | 18 (6.7) | 98.0 (85.1) | 14.4 (1.3) |
|
| ||||||||||||||
| Support Calls | ||||||||||||||
| Off | 51.5 (9.4) | 29.3 (7.1) | 3.1 (2.7) | 54 (39.7) | 52 (38.2) | 26 (19.1) | 4 (2.9) | 119 (87.5) | 9 (6.6) | 8 (5.9) | 127 (93.4) | 9 (6.6) | 95.3 (80.6) | 14.3 (1.3) |
| On | 53.6 (10.4) | 28.5 (5.5) | 2.9 (2.0) | 51 (38.3) | 61 (45.9) | 17 (12.8) | 4 (3.0) | 115 (86.5) | 7 (5.3) | 11 (8.3) | 124 (93.2) | 9 (6.8) | 100.9 (89.6) | 14.5 (1.4) |
| Deluxe App | ||||||||||||||
| Off | 54.0 (9.6) | 28.3 (6.0) | 3.11 (2.6) | 54 (40.9) | 60 (45.5) | 15 (11.4) | 3 (2.3) | 114 (86.4) | 4 (3.0) | 14 (10.6) | 120 (90.9) | 12 (9.1) | 90.0 (77.6) | 14.5(1.3) |
| On | 51.1 (10.1) | 29.6 (6.7) | 2.90 (2.2) | 51 (37.2) | 53 (38.7) | 28 (20.4) | 5 (3.6) | 120 (87.6) | 12 (8.8) | 5 (3.6) | 131 (95.6) | 6 (4.4) | 105.9 (91.4) | 14.4(1.3) |
| Buddy | ||||||||||||||
| Off | 52.7 (9.7) | 29.2 (6.7) | 2.92 (2.2) | 57 (42.5) | 56 (41.8) | 18 (13.4) | 3 (2.2) | 118 (88.1) | 5 (3.7) | 11 (8.2) | 127 (94.8) | 7 (5.2) | 96.0 (80.0) | 14.5 (1.3) |
| On | 52.3 (10.2) | 28.7 (6.1) | 3.09 (2.6) | 48 (35.6) | 57 (42.2) | 25 (18.5) | 5 (3.7) | 116 (85.9) | 11 (8.1) | 8 (5.9) | 124 (91.9) | 11 (8.1) | 100.1 (90.2) | 14.4 (1.3) |
| Online Gym | ||||||||||||||
| Off | 52.4 (9.2) | 29.1(5.9) | 2.91 (2.3) | 54 (39.4) | 59 (43.1) | 22 (16.1) | 2 (1.5) | 123 (89.8) | 6 (4.4) | 8 (5.8) | 128 (93.4) | 9 (6.6) | 93.3 (82.1) | 14.5 (1.5) |
| On | 52.6 (10.6) | 28.7 (6.8) | 3.09 (2.5) | 51 (38.6) | 54 (40.9) | 21 (15.9) | 6 (4.5) | 111 (84.1) | 10 (7.6) | 11 (8.3) | 123 (93.2) | 9 (6.8) | 103.07 (88.2) | 14.4 (1.1) |
| Text Messages | ||||||||||||||
| Off | 53.0 (9.9) | 28.6 (6.3) | 2.9 (2.4) | 51 (38.1) | 57 (42.5) | 22 (16.4) | 4 (3.0) | 113 (84.3) | 8 (6.0) | 13 (9.7) | 124 (92.5) | 10 (7.5) | 97.66 (83.3) | 14.5 (1.3) |
| On | 52.0 (10.0) | 29.2 (6.5) | 3.1 (2.4) | 54 (40.0) | 56 (41.5) | 21 (15.6) | 4 (3.0) | 121 (89.6) | 8 (5.9) | 6 (4.4) | 127 (94.1) | 8 (5.9) | 98.4 (87.2) | 14.4 (1.4) |
|
| ||||||||||||||
| Test | F=1.9; df=5 p=0.10 | F=1.04; df=5;p=0.39 | F=0.3; df=5; p=0.90 | ꭓ2=12.5;df=15;p=0.64 | ꭓ2=17.3;df=10;p=0.07 | ꭓ2=3.7;df=5;p=0.60 | F=0.7; df=5;p=0.60 | F= 0.6;df=5;p=0.69 | ||||||
Omnibus tests for distribution across levels of factors (age/BMI/MVPA/time since diagnosis: ANOVA; disease stage/race: nominal regression; ethnicity: logistic regression. n=269 except age: n=267; BMI: n=266; MVPA minutes: n=267; wear hours: n=267).
M=mean; SD=standard deviation; df=degree of freedom; MVPA=moderate to vigorous physical activity; BMI=body mass index; UK=unknown; AA=African American
The main effects model demonstrated MVPA increased by an average of 53.6 (95% CI=40.7–66.4;p<0.001) minutes/week from 97.7 at baseline to 151.2 at 12-weeks. MVPA improvements were from baseline were maintained but attenuated at 24-weeks (122.3 minutes/week). The magnitude was attenuated to 24.6 (95% CI=13.5–35.8;p<0.001) minutes/week. Participants meeting MVPA guidelines increased 22.3% from baseline (20.0%) to 12-weeks (42.3%;p<0.001); improvement from baseline were maintained but attenuated at 24-weeks (32.7%;p=0.002).
Table 3 summarizes weekly mean MVPA changes from baseline to 12- and 24-weeks by experimental component level (on v. off) and differences in changes by component level. All two-way interactions between each component and time (conceptually equivalent to component main effects) were statistically insignificant. MVPA minutes improved more for the “on” versus “off” levels for support calls, buddy, and text messages ranging from an increase of 3.8 to 12.3 additional minutes per week for “on” v. “off”. MVPA improvements were smaller for the deluxe app (Mdiff= −19.2) and online gym (Mdiff= −8.9) “on” versus “off” at 12-weeks. Patterns were consistent at 24-weeks; improvements were smaller for all components with positive differences while the negative difference was smaller for the deluxe app but larger for the online gym.
Table 3.
Changes in MVPA by experimental components
| Experimental Component | Week | Mean Change (min/week) | On v. Off Difference in Mean Change (95% CI) | p-value | |
|---|---|---|---|---|---|
| On | Off | ||||
| Support Calls | 12 | 57.9 (9.4) | 49.3 (9.0) | 8.6(−16.9,34.0) | 0.51 |
| 24 | 28.5 (8.0) | 20.9 (7.8) | 7.5(−13.8,28.8) | 0.49 | |
| Deluxe App | 12 | 44.3 (8.6) | 63.5 (9.9) | −19.2(−44.8,6.4) | 0.14 |
| 24 | 15.8 (7.4) | 34.2 (8.4) | −18.5(−39.9,3.0) | 0.09 | |
| Buddy | 12 | 55.5 (9.2) | 51.7 (9.2) | 3.8(−21.6,29.3) | 0.77 |
| 24 | 25.9 (7.9) | 23.4 (7.8) | 2.5(−18.7, 23.8) | 0.82 | |
| Online Gym | 12 | 49.2 (9.0) | 58.1 (9.5) | −8.9(−34.4,16.6) | 0.49 |
| 24 | 15.1 (7.3) | 35.0 (8.5) | −19.9(−41.3,1.6) | 0.07 | |
| Text Messages | 12 | 59.8 (9.6) | 47.6 (8.9) | 12.3(−13.3,37.8) | 0.34 |
| 24 | 25.3 (7.9) | 24.0 (7.8) | 1.3(−20.0,22.6) | 0.90 | |
Note: Values adjusted for accelerometer wear time
For individuals with <60 minutes of MVPA at baseline (See Table 4), baseline- to 12 week MVPA minutes improved significantly more for the “on” versus “off” levels for support calls (86.9 v. 58.8) and text messages (86.1 v. 59.4) and significantly less for the deluxe app (56.9 v. 89.5). Main effects for support calls and text messages remained significant at 24-weeks. No other effects differed by baseline activity level.
Table 4.
Changes in MVPA by experimental components stratified by baseline MVPA
| Baseline MVPA level | Experimental Component | Week | Mean Change (min/wk) | On v. Off Difference in Mean Change (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| On | Off | |||||
| <60 mins (n=113) | Support Calls | 12 | 86.9 (9.8) | 58.8 (8.3) | 28.1 (3.0, 53.2) | 0.03 |
| 24 | 58.6 (7.7) | 30.1 (6.5) | 28.5 (8.7, 48.4) | <0.01 | ||
| Deluxe App | 12 | 56.9 (7.8) | 89.5 (10.4) | −32.6 (−58.0, −7.3) | 0.01 | |
| 24 | 37.5 (6.7) | 49.1 (7.5) | −11.6 (−31.2, 7.9) | 0.24 | ||
| Buddy | 12 | 72.5 (9.1) | 71.4 (8.9) | 1.1 (−23.7, 25.9) | 0.93 | |
| 24 | 39.8 (7.1) | 46.5 (7.1) | −6.7 (−26.1, 12.8) | 0.50 | ||
| Online Gym | 12 | 64.8 (8.9) | 79.5 (9.1) | −14.7 (−39.6, 10.2) | 0.25 | |
| 24 | 34.8 (6.8) | 52.4 (7.4) | −17.6 (−37.1, 2.0) | 0.07 | ||
| Text Messages | 12 | 86.1 (10.3) | 59.4 (7.9) | 26.7 (1.3, 52.1) | 0.04 | |
| 24 | 54.4 (8.2) | 33.2 (6.2) | 21.2 (1.3, 41.1) | 0.04 | ||
| ≥60 mins (n=153) | Support Calls | 12 | 34.5 (13.6) | 36.3 (13.2) | −1.8(−37.1,33.5) | 0.92 |
| 24 | 5.4 (12.0) | 8.5 (11.6) | −3.1(−33.4,27.1) | 0.83 | ||
| Deluxe App | 12 | 32.7 (12.9) | 38.2 (13.9) | −5.5(−40.8,29.9) | 0.76 | |
| 24 | −2.6 (11.2) | 17.1 (12.6) | −19.7(−50.1,10.7) | 0.21 | ||
| Buddy | 12 | 40.9 (13.3) | 30.1 (13.4) | 10.8(−24.5,46.2) | 0.55 | |
| 24 | 11.4 (11.7) | 2.6 (11.9) | 8.8(−21.4,38.9) | 0.57 | ||
| Online Gym | 12 | 36.1 (13.0) | 34.8 (13.8) | 1.3(−34.1,36.7) | 0.94 | |
| 24 | −3.1 (10.8) | 17.7 (12.9) | −20.8(−51.4,9.8) | 0.18 | ||
| Text Messages | 12 | 36.8 (13.4) | 34.1 (13.3) | 2.7(−32.6,38.0) | 0.88 | |
| 24 | 1.4 (11.4) | 12.6 (12.2) | −11.2(−41.5,19.1) | 0.47 | ||
Note: Bolded values indicate significant at p<0.05. Values are adjusted for wear time.
DISCUSSION
Fit2Thrive addressed potential barriers to MVPA intervention dissemination and implementation for breast cancer survivors by using the MOST framework to examine which of five technology-supported intervention components significantly contributed to increased MVPA. Overall, MVPA increased by ~1 hour/week at 12-weeks. Increases in MVPA were maintained, but attenuated at 24-week follow-up. While MVPA increased for the “on” and “off” levels of all components tested; no differences were statistically significant. MVPA increased more for the “on” level of support calls, buddy and text messages and less for the deluxe app and online gym at 12- and 24-weeks. For highly inactive participants, MVPA increases were significantly greater for the “on” level of support calls and notifications at 12- and 24-weeks and significantly smaller for the “on” level of the deluxe app at 12 weeks. Collectively, findings indicate the core intervention may result in significant changes in MVPA among breast cancer survivors, but component efficacy may vary for highly inactive survivors.
Failure to observe increased MVPA with additional intervention components indicates many breast cancer survivors can significantly increase MVPA with a low-cost, low-touch intervention. Accelerometer-measured MVPA changes in Fit2Thrive are smaller but comparable to RCTs of more intensive, multicomponent MVPA promotion interventions in this population: BEAT Cancer44 (tapered on-site to home-based intervention with 12 supervised exercise, 3 exercise counseling and 6 discussion groups sessions; MΔ=+68 min/week) and ACTIVATE17 (activity tracker, in-person goals session, 5 calls; MΔ=+66.5 min/week). Additionally, effects were maintained, although attenuated at 24-week follow-up. However, attenuations were smaller for Fit2Thrive than BEAT Cancer (−29 v. −40 minute/week) which may be due to continued access to the Fitbit and Fit2Thrive app during follow-up. Additional work is needed to understand how best to maintain breast cancer survivors’ MVPA post-intervention and increase long-term engagement in technology-supported interventions. The MOST framework is useful for exploring these questions as unique maintenance “booster” components’ effects (i.e. support calls, text messages) could be tested.
Although no statistically significant differences were observed between the “on” and “off” levels of the intervention components, it is important to note that the some of the point estimates for the differences between the “on” and “off” levels could be clinically meaningful. However, few data exist to define clinically meaningful thresholds for change in MVPA in cancer survivors. One study found that a change of ~1.0 MET-hour/week of activity is associated with a 2% reduction in cancer mortality among inactive survivors.45 None of the difference point estimates achieved that threshold, but smaller changes in MVPA may result in clinically meaningful changes in other outcomes. Future work should define what differences in MVPA are clinically meaningful.
For highly inactive individuals (i.e. <60 minutes/week) support calls and text messages “on” may significantly increase MVPA initiation and maintenance in addition to the core. The MVPA differences between “on” and “off” levels for both components are ~1.0 MET-hour/week. Thus, the MVPA increases for these components may be clinically meaningful.45 However, smaller MVPA improvements in the “deluxe app “on” group at 12-weeks indicates giving highly inactive survivors more components may not always increase MVPA. Additional app features, information or components may cause information overload or fatigue. Future work should consider whether tailoring components to baseline MVPA is necessary.
Based on the mixed results for component effects observed in our study, future work should explore factors that may influence component efficacy, especially in highly inactive individuals. It is also important to note that the confidence intervals around the point estimates for the differences between the “on” and “off” levels of components were wide indicating there was variability in response to the different components. Thus, intervention response to components likely varies across other factors (i.e. symptoms, built environment, age) and time. Future work should explore what factors predict variability in response to different components to determine whether certain components are more effective for subgroups or behavioral phenotypes to improve intervention tailoring and allocate resources to those who may be most responsive. Finally, future studies should explore whether adaptive interventions that provide individuals with specific components when needed (i.e. when MVPA declines) are more efficient and effective than “static” interventions. Further understanding what works for whom and under what conditions would help optimize interventions and conserve resources to enhance dissemination and implementation.
This study is not without limitations. First, intervention components tested were low intensity to increase scalability which could limit their potency for increasing MVPA. Second, we intended to conduct secondary analyses to examine which component combinations achieved maximum MVPA increases for ≤$550 per person. These analyses were not conducted because no main effects were significant and the core intervention costs are below this threshold at ~$363.01 per participant (includes Fitbit, app development, study packet mailing and technical assistance).23 Consistent with the continual optimization principle, future research is warranted to examine acceptability and engagement with the components tested and determine whether modifying tested components in response to patients’ preferences or alternative components increase MVPA while maintaining intervention efficiency and convenience.46 Third, our sample was recruited from a registry and predominately non-Hispanic, White, middle-class, early stage breast cancer survivors. Additionally, although we excluded individuals self-reporting baseline MVPA >60 minutes, over half (56.9%) exceeded this threshold for accelerometer-assessed MVPA (M=97.9 min/week) which is consistent with other behavior change trials in breast cancer survivors (BEAT Cancer M=178 min/week; ACTIVATE M=104.7 min/week). Results may not generalize to more diverse, less motivated, more inactive subpopulations. Finally, there was no formal comparison group for our “core” intervention. A RCT to test the optimized Fit2Thrive “core” intervention versus a control is warranted.
Fit2Thrive is the first study to demonstrate how a factorial experiment can be used to systematically test which intervention components meaningfully contribute to MVPA among cancer survivors. Overall, we found none of the added intervention components under consideration (i.e. support calls, deluxe app, buddy, online gym, text messages) resulted in statistically significant MVPA increases or maintenance. However, overall MVPA significantly increased at 12-weeks and effects were maintained, albeit attenuated, at 24-weeks. This indicates the low-resource “core” intervention (Fitbit + standard Fit2Thrive app) may be sufficient to increase MVPA in many breast cancer survivors. Post-hoc analyses revealed support calls and text messages significantly improved MVPA in the “on” v. “off” conditions for highly inactive participants. Future work should test the core intervention against a control to determine its efficacy for increasing MVPA and improving health and disease outcomes, and further evaluate support calls and text messages in highly inactive survivors. Research should also further examine how to prevent or reduce attenuation of MVPA gains across time. Finally, if efficacious, future work should examine how to disseminate and implement Fit2Thrive to increase MVPA in cancer survivors to, ultimately, improve health and disease outcomes.
Acknowledgments
This work was funded by K07CA196840 and R21CA219028 and the Robert H. Lurie Comprehensive Cancer Center (SP). LAG is supported by T32CA193193.
Footnotes
The authors have no conflict of interests to report.
REFERENCES
- 1.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. [DOI] [PubMed] [Google Scholar]
- 2.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. [DOI] [PubMed] [Google Scholar]
- 4.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arem H, Mama SK, Duan X, Rowland JH, Bellizzi KM, Ehlers DK. Prevalence of healthy behaviors among cancer survivors in the United States: how far have we come? Cancer Epidemiol Biomarkers Prev. 2020;29:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS One.2017;12:e0182554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncologica. 2013;52:195–215. [DOI] [PubMed] [Google Scholar]
- 8.Phillips SM, Alfano CM, Perna FM, Glasgow RE. Accelerating translation of physical activity and cancer survivorship research into practice: recommendations for a more integrated and collaborative approach. Cancer Epidemiol Biomarkers Prev. 2014;23:687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardcastle SJ, Maxwell-Smith C, Kamarova S, Lamb S, Millar L, Cohen PA. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Suppor Care Cancer. 2018;26: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 10.Blaney J, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey J. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire–survey. Psychooncology. 2013;22:186–194. [DOI] [PubMed] [Google Scholar]
- 11.Groen WG, van Harten WH, Vallance JK. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013–2018): We still haven’t found what we’re looking for. Cancer Treat Rev. 2018;69: 188–203. [DOI] [PubMed] [Google Scholar]
- 12.Dorri S, Asadi F, Olfatbakhsh A, Kazemi A. A systematic review of electronic health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer. 2020:1–22. [DOI] [PubMed] [Google Scholar]
- 13.Phillips SM, Conroy DE, Keadle SK, et al. Breast cancer survivors’ preferences for technology-supported exercise interventions. Support Care Cancer. 2017;25:3243–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen NH, Hadgraft NT, Moore MM, et al. A qualitative evaluation of breast cancer survivors’ acceptance of and preferences for consumer wearable technology activity trackers. Support Care Cancer. 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 15.Pew Research Center. U.S. Smartphone Use in 2015.
- 16.Coughlin SS, Caplan LS, Stone R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: a review of health intervention studies. J Cancer Surviv. 2020;14:386–392. [DOI] [PubMed] [Google Scholar]
- 17.Lynch BM, Nguyen NH, Moore MM, et al. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer. 2019;125: 2846–2855. [DOI] [PubMed] [Google Scholar]
- 18.Martin Payo R, Harris J, Armes J. Prescribing fitness apps for people with cancer: a preliminary assessment of content and quality of commercially available apps. J Cancer Surviv. 2019;13: 397–405. [DOI] [PubMed] [Google Scholar]
- 19.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32:S112–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav. 2016;20:197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins LM. Optimization of behavioral, biobehavioral, and biomedical interventions: The multiphase optimization strategy (MOST). Springer, 2018. [Google Scholar]
- 22.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14:202–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips SM, Collins LM, Penedo FJ, et al. Optimization of a technology-supported physical activity intervention for breast cancer survivors: Fit2Thrive study protocol. Contemp Clin Trials. 2018;66:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 25.Phillips SM, Conroy DE, Keadle SK, et al. Breast cancer survivors’ preferences for technology-supported exercise interventions. Suppor Care Cancer. 2017:25:3243–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch WA, Solk P, Auster-Gussman L, et al. User-centered development of a smartphone application (Fit2Thrive) to promote physical activity in breast cancer survivors. Transl Behav Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandura A. The explanatory and predictive scope of self-efficacy theory. J Soc Clin Psychol. 1986;4:359–373. [Google Scholar]
- 28.Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2012;22:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2015;9:305–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goode AD, Lawler SP, Brakenridge CL, Reeves MM, Eakin EG. Telephone, print, and Web-based interventions for physical activity, diet, and weight control among cancer survivors: a systematic review. J Cancer Surviv. 2015;9:660–682. [DOI] [PubMed] [Google Scholar]
- 31.McEwan D, Harden SM, Zumbo BD, et al. The effectiveness of multi-component goal setting interventions for changing physical activity behaviour: a systematic review and meta-analysis. Health Psychol Rev. 2016;10:67–88. [DOI] [PubMed] [Google Scholar]
- 32.Gilliam MB, Madan-Swain A, Whelan K, Tucker DC, Demark-Wahnefried W, Schwebel DC. Cognitive influences as mediators of family and peer support for pediatric cancer survivors’ physical activity. Psychooncology. 2013;22: 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su JA, Yeh DC, Chang CC, et al. Depression and family support in breast cancer patients. Neuropsychiatr Dis Treat. 2017;13:2389–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber FD. Social support and physical activity engagement by cancer survivors. Clin J Oncol Nursi. 2012;16:E84–E98. [DOI] [PubMed] [Google Scholar]
- 35.Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015;149: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bassett DR. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471. [DOI] [PubMed] [Google Scholar]
- 37.Tudor-Locke C, Ainsworth B, Thompson R, Matthews C. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exercise. 2002;34:2045–2041. [DOI] [PubMed] [Google Scholar]
- 38.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. [DOI] [PubMed] [Google Scholar]
- 40.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. [DOI] [PubMed] [Google Scholar]
- 41.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. Journal of Educational and Behavioral Statistics. 1999;24:70–93. [Google Scholar]
- 42.SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary NSII. [Google Scholar]
- 43.SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary NSII. Available from URL: https://support.sas.com/kb/58/775.html. [Google Scholar]
- 44.Rogers LQ, Courneya KS, Anton PM, et al. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015;149:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Wei S, Shi Y, et al. The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50:339–345. [DOI] [PubMed] [Google Scholar]
- 46.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41:208–226. [DOI] [PMC free article] [PubMed] [Google Scholar]


