Abstract
Background:
A geriatric assessment (GA) intervention improves communication about aging-related concerns, but its effect on communication in patients with varying levels of frailty is unknown.
Methods:
This was secondary analysis of a nationwide trial of patients aged ≥70 years with incurable cancer and impairment on ≥1 GA domain (NCT02107443; PI: Mohile). Practice sites were randomized to either the GA-intervention or usual-care. Frailty was assessed using a deficit accumulation index (DAI; range 0-1), and patients were stratified as robust (0-<0.2), pre-frail (0.2-<0.35), or frail (≥0.35). The clinic visit after the GA-intervention was audio-recorded, transcribed, and coded to evaluate the number and quality of conversations about aging-related concerns. Linear mixed models examined differences in the number and quality of conversations within and between arms. All P values are 2-sided.
Results:
Patients (n=541) were classified as robust (27%), pre-frail (42%), or frail (31%). In usual-care, frail patients (versus robust) engaged in more aging-related conversations (adjusted mean difference=1.73; 95% Confidence Interval (CI)=0.59-2.87), of higher quality (difference=1.12; 95%CI=0.24-2.0), and more discussions about evidence-based recommendations (difference=0.71; 95%CI=0.04-1.38); all p≤0.01. Similarly, in GA-intervention, frail patients (versus robust) engaged in more aging-related conversations (difference=2.49; 95%CI=1.51-3.47), of higher quality (difference=1.31; 95%CI=0.56-2.06), and more discussions about evidence-based recommendations (difference=0.87; 95%CI=0.32-1.42); all p≤0.01. Furthermore, the GA-intervention significantly improved the number and quality of conversations in all patients; robust, pre-frail and frail (all p≤0.01).
Conclusion:
Patients with higher degrees of frailty and those exposed to the GA-intervention had more and higher quality conversations about aging-related concerns with oncologists.
Keywords: frailty, communication, geriatric assessment, satisfaction with communication, older adults with cancer
Lay Summary:
A geriatric assessment (GA) intervention improves communication about aging-related concerns, but its effect on communication in patients with varying levels of frailty is unknown. We conducted secondary analysis of a nationwide trial of patients aged ≥70 years with incurable cancer and ≥1 GA domain impairment. Patients were stratified as robust, pre-frail, or frail. The number and quality of conversations about aging-related concerns that occurred during the clinic visit after the GA-intervention were determined. We found that patients with higher degrees of frailty and those in the GA-intervention arm had more and higher quality conversations about aging-related concerns with oncologists.
Precis:
A geriatric assessment (GA) intervention improves communication about aging-related concerns, but its effect on communication in patients with varying levels of frailty is unknown. We found that patients with higher degrees of frailty and those in the GA-intervention arm had more and higher quality conversations about aging-related concerns with oncologists.
Background
Older adults constitute a heterogeneous population, such that individuals of the same chronological age can have markedly different biological ages, resulting in varied clinical outcomes.1 This diversity in biological age has been attributed to frailty, described by a state of accelerated accumulation of deficits, with the quantity of deficits accumulated associated with increasing frailty.2 In the context of cancer, frailty is particularly important. Forty to fifty percent of older adults are characterized as either pre-frail or frail, and this status confers increased risk of morbidity and mortality from cancer treatments.3–8 The geriatric assessment (GA) is a validated multidisciplinary evaluation of the functional, psychosocial, physical, and cognitive abilities of older adults, as well as their comorbidities and medication use.9–12 It captures domains not commonly measured by routine oncology assessments,13 and effectively measures the frailty status of older adults with cancer.14 Implementing the GA along with targeted management to address specific impairments has been shown to reduce cancer treatment toxicities, improve quality of life, and improve communication about aging-related concerns.15–18 Accordingly, an American Society of Clinical Oncology (ASCO) geriatric oncology guideline recommends that all older adults with cancer undergo a GA prior to starting chemotherapy.14
Patient-centered communication between health care teams and their patients is an important aspect of providing high quality care to older adults with cancer. Patient-centered communication has been shown to improve quality of life and satisfaction with care.19 Patients who report effective clinician-patient communication also report higher satisfaction with care, increased likelihood of following treatment plans, and greater ease in making end-of-life decisions.20–23 A qualitative study of frail older adults found that patients’ perceived good communication with their healthcare teams as a major factor influencing their engagement with medical decisions.24 Furthermore, physician-patient discordances have been identified in perceptions of quality communication and/or care that may interfere with providing quality care, including health communication.25 However, several interventions, using tailored communication guides and training for patients and oncologists, have been shown to improve patient-centered communication.26, 27
We have recently shown that a GA-intervention improved the number and quality of communication about aging-related concerns between older patients with advanced cancer and their oncologists.15 However, the effects of the GA-intervention on communication in patients with varying levels of frailty are unknown. Thus, we aimed to assess: 1) the associations of patients’ frailty status on the number and quality of conversations about aging-related concerns between patients and their oncologists in each study arm and 2) the moderating effect of the GA-intervention on patient-oncologist communication at varying levels of patients’ frailty. We hypothesized that, in older patients with advanced cancer there is an association between patients’ frailty and patient-oncologist communication; furthermore, we hypothesized that a GA-intervention would improve this communication in older patients with advanced cancer.
Methods
Participants and Methods:
We conducted an exploratory analysis utilizing data of older patients with incurable cancer who participated in a nationwide cluster randomized controlled trial that evaluated the effect of a GA-intervention on communication about aging-related concerns between patients, caregivers, and oncologists (University of Rochester Cancer Center [URCC] 13070; ClinicalTrials.gov identifier NCT02107443; PI: Mohile).15 The parent study was conducted within the URCC National Cancer Institute Community Oncology Research Program (NCORP), and 31 community oncology practice sites participated in the study between October 2014 and April 2017; 541 patients were recruited from 30 of these sites.15 Practice sites were randomized to either usual-care (17 sites) or GA-intervention (13 sites). Patients were aged ≥70, had a diagnosis of advanced solid tumor or lymphoma, were considering or receiving cancer treatment, and had an impairment in at least one GA domain (excluding polypharmacy; definitions of GA domains have been reported previously).15, 28–32 Patients in both arms underwent the GA. Only patients and oncologists in the GA-intervention arm received a summary of the GA plus a list of GA-guided recommendations to address specific impairments (i.e., GA-interventions). Institutional review boards at the URCC NCORP Research Base and each of the NCORP Community Affiliates approved the study. All participants provided informed consent. This analysis was not pre-planned at the initiation of the parent study.
Measures
Frailty:
At baseline, patients completed the GA. Frailty was calculated using a deficit accumulation index (DAI). The DAI is a single variable that measures the effect of multisystem physiological changes and it is known to be predictive of adverse health outcomes and mortality. Stratifying older adults with cancer based on the DAI using variables from the GA, has been shown to assist clinicians in predicting future adverse outcomes.33 The DAI was developed following the standard procedures for creating a deficit accumulation frailty index.7, 34 The DAI was calculated from 50 individual items as described and validated in older adults with cancer by Cohen et al.33 These items included: marital status, instrumental activities of daily living, activities of daily living, performance status, fall history, number of regularly taken medications, comorbidity, cognition, nutrition, level of social activity and social support, level of physical activity, depression, anxiety, and basic laboratory values.33 Items were coded and scored following the methodology used and validated in older adults with cancer by Cohen et al.33 For items with binary answers, patients received a score of zero if the abnormal value was absent and one if the abnormal item was present. For items with graded responses, patients received a score of zero if the condition was absent, one if the condition was intermediate, and two if the condition was the most adverse. Scores of the individual items were summed and the DAI was calculated as the ratio of the actual deficit score to the potential deficit score with final scores ranging from zero to one and higher scores indicating more deficits and therefore greater frailty.33 Patients were then stratified into three groups based on their DAI scores: robust (0-<0.2), pre-frail (0.2-<0.35), and frail (≥0.35), using previously described and validated cut-offs.33
Number and Quality of Conversations about Aging-Related Concerns
In both the usual-care and GA-intervention arms, an oncology clinic visit within four weeks of completing the GA was audio-recorded and transcribed. The audio-recording occurred after patients and oncologists in the GA-intervention arm received the GA-guided intervention. The content analysis methodology of the audio-recorded visits has been previously reported.15 Conversations were quantified into the number of conversations about aging-related concerns and categorized into different groups that were a priori developed to evaluate the quality of conversations;15, 35 i.e. the number of aging-related concerns that were acknowledged (concerns further explored without implementing any care processes) and addressed (concerns appropriately addressed via evidence-based management; e.g. referral to physical therapy for falls or recommendation for the use of a pill box for medication management).15, 36 The more frequently that aging-related concerns were acknowledged and addressed, the higher the quality of the conversation.15
Statistical Analyses
Descriptive statistics were used to examine sociodemographic factors, clinical information, and the number and quality of conversations between patients and oncologists. Chi-square tests and analysis of variance compared demographic and clinical factors as well as the number and quality of conversations about aging-related concerns among robust, pre-frail, and frail patients. The statistical analysis plan for the parent study including the sample size calculation, was previously published.15 To account for the cluster-randomized study design, separate linear mixed models (LMMs) were conducted to examine the difference in the number and quality of patient-oncologist conversations within arm (robust, pre-frail, and frail patients) and between arms (usual-care versus GA-intervention for each frailty group).15, 37 Models included the study arm and frailty status as fixed effects and practice sites as a random effect independent of residual error; estimation was performed using restricted maximum likelihood. To examine interaction effects, an interaction term between 3 levels of frailty and the study arm was added to the model. Within and between arm comparisons were obtained using the SAS procedure PROC MIXED and LSMESTIMATE statement. All analyses were conducted with SAS v.9.4 (SAS Institute Inc., Cary, NC) and JMP Pro 15 (SAS Institute Inc., Cary, NC). All P values were from 2-sided tests, and the results were deemed statistically significant at P <0.05.
Results
Description of Sample: Demographics, Frailty and Conversations about Aging-Related Concerns
All 541 patients in the primary study were included (Figure 1);15 27% were classified as robust, 42% as pre-frail, and 31% as frail. Patients’ demographics and clinical variables stratified by frailty status are shown in Table 1. There were no significant differences across the frailty strata except for gender (Table 1). There was also no significant difference in the mean frailty score of patients across study arms; usual-care=0.31; SD=0.16 versus GA-intervention=0.30; SD=0.15; p=0.71 (Figure 2).
Figure 1:
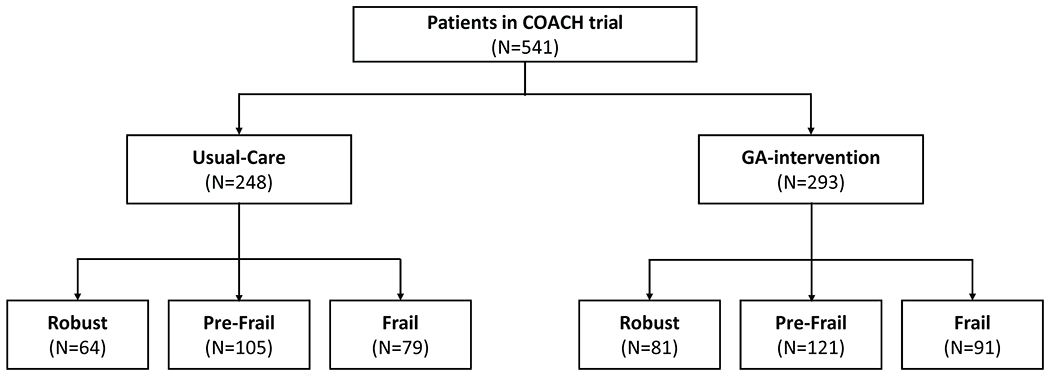
CONSORT flow diagram
Table 1:
Distribution of Demographic and Clinical Variables
| Variables | All Participants N=541 | Robust N=145 | Pre-Frail N=226 | Frail N=170 | P-value |
|---|---|---|---|---|---|
| Age, years: N(%) | |||||
| Mean [Range] | 76.6 [70-96] | 76.1 [70-93] | 76.3 [70-92] | 77.3 [70-96] | 0.50 |
| 70-79 | 401 (74.3) | 109 (75.7) | 173 (76.5) | 119 (70.0) | |
| 80-89 | 127 (23.5) | 32 (22.2) | 47 (20.8) | 48 (28.2) | |
| ≥90 | 12 (2.2) | 3 (2.1) | 6 (2.7) | 3 (1.8) | |
|
| |||||
| Gender: N(%) | |||||
| Male | 276 (51.1) | 87 (60.4) | 115 (50.9) | 74 (43.5) | 0.01 |
| Female | 264 (48.9) | 57 (39.6) | 111 (49.1) | 96 (56.5) | |
|
| |||||
| Race: N(%) | |||||
| White | 482 (89.3) | 131 (91.0) | 204 (90.3) | 147 (86.5) | 0.37 |
| Non-white | 58 (10.7) | 13 (9.0) | 22 (9.7) | 23 (13.5) | |
|
| |||||
| Education: N(%) | |||||
| High school or below | 261 (48.3) | 58 (40.3) | 116 (51.3) | 87 (51.2) | 0.20 |
| Some college or above | 279 (51.7) | 86 (59.7) | 110 (48.7) | 83 (48.8) | |
|
| |||||
| Cancer Type: N(%) | |||||
| Gastrointestinal | 138 (22.6) | 30 (20.7) | 68 (30.1) | 40 (23.7) | 0.16 |
| Lung | 140 (25.9) | 34 (23.4) | 58 (25.7) | 48 (28.4) | |
| Other | 262 (48.5) | 81 (55.9) | 100 (44.2) | 81 (47.9) | |
|
| |||||
| Cancer Stage: N(%) | |||||
| III | 47 (8.7) | 14 (9.7) | 19 (8.4) | 14 (8.3) | 0.99 |
| IV | 480 (88.7) | 128 (88.3) | 201 (88.9) | 151 (89.3) | |
| Other | 13 (2.4) | 3 (2.0) | 6 (2.7) | 4 (2.4) | |
|
| |||||
| Communication: Mean [Range] | |||||
| Number of Conversations | 6.3 [0-18] | 5.2 [0-15] | 6.2 [0-16] | 7.3 [0-18] | <0.01 |
| Number of Concerns Acknowledged | 3.6 [0-16] | 3.0 [0-11] | 3.5 [0-12] | 4.1 [0-16] | <0.01 |
| Number of Concerns Addressed | 2.3 [0.12] | 2.0 [0-7] | 2.2 [0-11] | 2.6 [0-12] | 0.04 |
Note: 1 participant did not provide any demographic data
Figure 2:
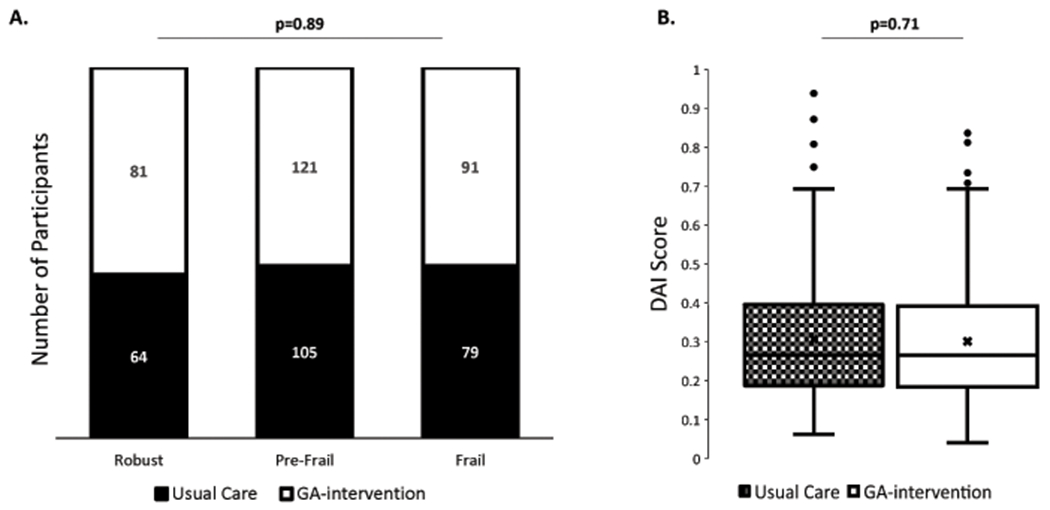
Distribution of Deficit Accumulation Index scores. (A) Proportion of robust, pre-frail, and frail participants in usual-care (black) and GA-intervention (white) arms. (B) Distribution of DAI scores in all patients in usual-care (black hash) and GA-intervention (white).
In all patients, irrespective of arm, as frailty scores increased (robust<pre-frail<frail), there was a linear increase in the average number of conversations per patient about aging-related concerns (5.2; SD=3.5 vs 6.2; SD=4.0 vs 7.3; SD=4.2; p<0.001), as well as in the number of concerns that were acknowledged (3.0; SD=2.5 vs 3.5; SD=2.7 vs 4.1; SD=3.0; p<0.001) and addressed (2.0; SD=2.2 vs 2.2; SD=2.3 vs 2.6; SD=2.6 p=0.040) (Table 1).
Number of Conversations about Aging-Related Concerns
In usual-care, an average of 1.73 (95% CI=0.59-2.87; p=0.003) more conversations per patient about aging-related concerns occurred in frail compared to robust patients (Table 2 and Figure 3). In the GA-intervention, an average of 1.31 (95% CI=0.37-2.25; p=0.007) more conversations about aging-related concerns occurred in pre-frail compared to robust patients and 2.49 (95% CI=1.51-3.47; p<0.001) more conversations occurred in frail compared to robust patients (Table 2 and Figure 3).
Table 2:
Adjusted Mean Difference in the Number and Quality of Conversations about Aging-Related Concerns in Pre-Frail and Frail vs Robust Patients and Usual-Care vs GA-Intervention Patients
| Adjusted Mean Difference in Pre-Frail and Frail compared to Robust Patients |
Adjusted Mean Difference in GA-Intervention compared to Usual-Care in Robust, Pre-Frail, and Frail Patients | pValue of Interaction (frailty status*study arm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Usual-Care | GA-Intervention | |||||||
|
| ||||||||
| Difference (95% CI) | pValue | Difference (95% CI) | pValue | Difference (95% CI) | pValue | |||
| # Conversations | Robust | ref | - | ref | - | 3.27 (1.68 to 4.86) | 0.0002 | |
| Pre-Frail | 0.83 (−0.21 to 1.87) | 0.1197 | 1.31 (0.37 to 2.25) | 0.0067 | 3.75 (2.32 to 5.18) | 0.0001 | 0.6111 | |
| Frail | 1.73 (0.59 to 2.87) | 0.0031 | 2.49 (1.51 to 3.47) | <0.0001 | 4.03 (2.50 to 5.56) | 0.0001 | ||
|
| ||||||||
| # Concerns Acknowledged | Robust | ref | - | ref | - | 2.08 (1.04 to 3.12) | 0.0002 | |
| Pre-Frail | 0.79 (−0.03 to 1.61) | 0.0624 | 0.58 (−0.13 to 1.29) | 0.1077 | 1.87 (0.97 to 2.77) | 0.0002 | 0.7397 | |
| Frail | 1.12 (0.24 to 2.00) | 0.0145 | 1.31 (0.56 to 2.06) | 0.0006 | 2.27 (1.29 to 3.25) | <0.0001 | ||
|
| ||||||||
| # Concerns Addressed | Robust | ref | - | ref | - | 2.03 (0.87 to 3.19) | 0.0013 | |
| Pre-Frail | 0.2 (−0.41 to 0.81) | 0.5232 | 0.3 (−0.23 to 0.83) | 0.2634 | 2.13 (1.05 to 3.21) | 0.0005 | 0.9403 | |
| Frail | 0.71 (0.04 to 1.38) | 0.0382 | 0.87 (0.32 to 1.42) | 0.0021 | 2.19 (1.07 to 3.31) | 0.0005 | ||
Geriatric Assessment (GA), Confidence Intervals (CI)
Models included the study arm, frailty status, and interaction term between frailty status and study arm as a fixed effect and practice site as a random effect independent of residual error
Figure 3:
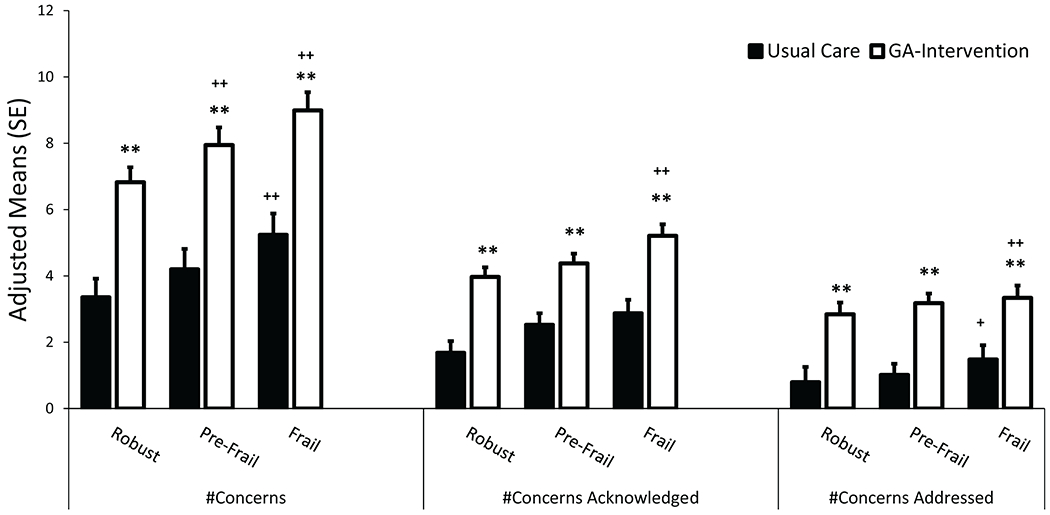
The effect of the GA-intervention on the number of conversations about aging-related concerns between oncologists and patients in the GA-intervention arm (white) compared to the usual-care arm (black) in robust, pre-frail, and frail patients. (asterisk: comparing usual-care to GA-intervention: **p<0.01, plus sign: comparing pre-frail or frail to robust: +p<0.05, ++p<0.01)
As frailty scores increased, so too did the adjusted mean difference in the number of conversations per patient about aging-related concerns in the GA-intervention compared to the usual-care arm (Table 2 and Figure 3). In robust patients, an average of 3.27 (95% CI=1.68-4.86; p<0.001) more conversations about aging-related concerns occurred in the GA-intervention compared to the usual-care arm. In pre-frail patients, there were on average 3.75 (95% CI=2.32-5.18; p<0.001) more conversations. In frail patients, there were on average 4.03 (95% CI=2.50-5.56; p<0.001) more conversations. However, the interaction between frailty status and the study arm was not statistically significant (p=0.61).
Quality of Conversations about Aging-Related Concerns
We next assessed whether patients’ frailty status was associated with the quality of conversations, as viewed through the number of concerns acknowledged and addressed by patients’ oncologists (Table 2 and Figure 3). In usual-care settings, there were on average 1.12 (95% CI=0.24-2.00; p=0.015) more conversations about aging-related concerns that were acknowledged and 0.71 (95% CI=0.04-1.38; p=0.038) conversations that were addressed by oncologists in frail compared to robust patients. In the GA-intervention arm, there were on average 1.31 (95% CI=0.56-2.06; p<0.001) more conversations that were acknowledged and 0.87 (95% CI=0.32-1.42; p=0.002) conversations that were addressed in frail compared to robust patients.
We further assessed the adjusted mean difference in the number of conversations per patient about aging-related concerns in the GA-intervention compared to the usual-care arm in each frailty category (Table 2 and Figure 3). In robust patients, there were on average 2.08 (95% CI=1.04-3.12; p<0.001) more conversations that were acknowledged and 2.03 (95% CI=0.87-3.19; p=0.001) more conversations that were addressed in patients who received the GA-intervention compared to usual-care. In pre-frail patients, there were on average 1.87 (95% CI=0.97-2.77; p<0.001) more conversations that were acknowledged and 2.13 (95% CI=1.05-3.21; p=0.005) more conversations that were addressed. In frail patients, there were on average 2.27 (95% CI=1.29-3.25; p<0.001) more conversations acknowledged and 2.19 (95% CI=1.07-3.31; p<0.001) more conversations that were addressed. The interaction term between frailty status and the study arm was not statistically significant neither for the number of conversations acknowledged (p=0.71) nor addressed (p=0.94).
Discussion
In this study, we found a linear relationship between frailty and communication; moreover, a GA-intervention improved communication about aging-related concerns in robust, pre-frail, and frail patients. Classifying patients based on their level of frailty using the DAI, has been found to be helpful in stratifying patients based on their risk of future adverse health outcomes.38 In this population of older adults with advanced cancer, we showed that 27% of patients were classified as robust, 42% as pre-frail, and 31% as frail. This balance across all three frailty categories allowed for the adequate evaluation of patients’ frailty status on patient-oncologist conversations about aging-related concerns. The prevalence of frailty reported here is consistent with findings from a systematic review of older adults with cancer; which reported the median prevalence of robust, pre-frail, and frail were 32% (range 11%–78%), 42% (range 6%–86%), and 43% (range 13%–79%), respectively.8
In usual-care settings, we showed that patients and oncologists had more conversations about aging-related concerns with patients who were categorized as frail compared to robust. We also showed that frail patients had better quality conversations with their oncologists. This finding indicates that frail patients and their oncologists were more likely to have aging-related conversations regarding areas such as functional status and/or nutritional status, and it was more likely for oncologists to adequately address these concerns with a referral to a physical therapist and/or nutritionist. Surprisingly, in usual care, there were no significant difference in the number of conversations about aging-related concerns in pre-frail compared to robust patients. Furthermore, the majority of concerns in both arms were not addressed during the clinic visit. Due to the influence that frailty can have on treatment decisions and prognosis,2–7 oncologists should have more frequent discussions about aging-related concerns with pre-frail and frail older patients with advanced cancer; such discussions would likely lead to better clinical outcomes. The GA can aid oncologists in identifying pre-frail individuals who might benefit from these aging-related conversations.
We found a positive linear trend relationship in the number and quality of conversations about aging-related concerns of patients and their oncologists by frailty category in the GA-intervention arm. Patients with the highest level of frailty had the most discussions about aging-related concerns, and these conversations were acknowledged and addressed more by oncologists in frail versus robust patients. The presence of frailty increases the risk of poor cancer treatment outcomes3–5 and should be considered during shared decision-making processes. A GA-intervention could facilitate these discussions in pre-frail and frail patients. Oncologists’ acknowledgement of an aging-related concern provides a communication opportunity for future discussions and an open forum in which patient-oncologist rapport and trust can be built, yielding a greater likelihood that the aging-related concern will be appropriately addressed. This process creates a greater opportunity for oncologists to intervene and address aging-related concerns that are commonly missed during clinical encounters. Future work should evaluate the effect of enhanced communication quality on the implementation of frailty-specific GA-interventions and improved health outcomes for pre-frail and frail older patients with advanced cancer.
Finally, we showed that the GA-intervention increased the number and quality of conversations across all frailty levels. Effective patient-centered communication has been shown to be a critical element in patients’ navigation of their cancer journey22 and to contribute to better patient outcomes.21 Given the importance of effective communication, an ASCO consensus guideline outlined recommendations for oncologists to develop core communication skills, effectively involve family members in discussions, and discuss clinical care decisions.39 Furthermore, a recent scoping review found that some of the barriers to communication in primary care settings, reported by health care providers, included the lack of communication skills training and lack of structured communication formats and guides.40 The results from our study show that providing oncologists with a GA-intervention, which consists of a summary of the results of impairments identified by the GA as well as specific recommendations based on patients’ needs, can facilitate frailty conversations and thus have the potential to aid physicians in overcoming a communication barrier. Moreover, we have previously shown that the GA-intervention is feasible to conduct in busy community oncology clinics and does not require specialized training.15 The GA-intervention can help oncologists identify pre-frail and frail patients and facilitate patient-centered conversations about support services that will improve quality of life and address aging-related concerns. Future studies to gauge patients’ understanding of aging-related conversations could demonstrate the role that the number and quality of these conversations might serve in promoting patient-centered communication in older adults with advanced cancer. Goals of care, patient values and preferences and any support services that will improve quality of life, for example, might be explored. Future work should also evaluate whether certain conversations about specific GA domain impairments were better facilitated by the GA-intervention compared to usual-care. Qualitative studies have shown that the term ‘frailty’ has a negative connotation for many older adults who characterized frailty as a terminal outcome.40 The GA-intervention can assist oncologists in having discussions with patients without specifically using the term frailty, by providing a holistic view of any impairments (e.g. psychological, physical, nutritional, etc.) that patients might have, where conversations will be centered on specific actions that the patients can take to mitigate some of these impairments and in so doing improve their frailty status.
It is worth noting that, in all models tested, the interaction term between frailty status and the study arm was not statistically significant; all p>0.6. The effects of the intervention and frailty were additive. While there were more conversations observed in the GA-Intervention arm, in both arms increasing frailty was associated with more and higher quality conversations about aging-related concerns at a similar rate.
Our study had several important strengths. First, the sample included 541patients over the age of 70 with at least one aging-related deficit ─ patients who are traditionally excluded from clinical trials. Secondly, this study recruited patients from community oncology sites within the United States. The fact that these are the sites that typically see the majority of patients with cancer, improves the generalizability of our findings. Thirdly, this study combined a mixture of quantitative and qualitative content analyses of clinical encounters between patients and oncologists that allowed for in-depth investigation of relationships between frailty and communication in usual-care and GA-intervention. Fourthly, there was an even distribution of patients across all frailty categories, allowing for the adequate comparison of outcomes within each frailty group. However, this study also has limitations. The study sample consisted of a predominantly white patient population and limited racial diversity, which may lessen the generalizability of our findings. The number and quality of the conversations about aging-related concerns were only assessed at a single time-point; thus we were unable to assess longer-term aging-related communication outcomes.
Conclusions:
Overall, patients who had higher degrees of frailty had more and higher quality conversations about aging-related concerns with their oncologists. Furthermore, the GA-intervention ─ summary and list of recommendations provided to oncologists and patients ─ increased the number and quality of these conversations across all frailty categories. The GA can help oncologists identify pre-frail and frail patients with advanced cancer and the GA-intervention can facilitate effective oncologist-patient communication, which can in turn improve the health and well-being of patients through improved trust, motivation, patient-oncologist relationships, compliance, and self-care skills.41 Future work should investigate whether improved patient-oncologist communication leads to improved clinical outcomes for pre-frail and frail older adults with advanced cancer.
Acknowledgments:
We acknowledge Dr. Susan Rosenthal for her editorial assistance. We would also like to graciously thank all the SCOREboard members for their valuable contributions that resulted in the profound success of the COACH trial. These data were presented at the International Society of Geriatric Oncology annual conference in 2018.
Funding:
This work was supported by the National Institutes of Health, University of Rochester Clinical Trial Science Award (KL2 TR001999); a Patient-Centered Outcomes Research Institute (4634); the National Cancer Institute (R01 CA177592, UG1 CA189961, T32 CA102618, K99 CA237744); and the National Institute on Aging (R33 AG059206-01, K24 AG056589). The investigators were independent in the conduct and analysis of the results.
Role of the funding source:
Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or Methodology Committee.
Footnotes
Disclosures: The authors report no conflicts of interest, financial or otherwise.
References:
- 1.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev. 2019;180: 107–116. [DOI] [PubMed] [Google Scholar]
- 3.Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67: 362–377. [DOI] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;164: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer. 2019;27: 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56: M146–156. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353: 205–206. [DOI] [PubMed] [Google Scholar]
- 8.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 9.Hamaker ME, Te Molder M, Thielen N, van Munster BC, Schiphorst AH, van Huis LH. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol. 2018;9: 430–440. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez Torres C, Hsu T. Comprehensive Geriatric Assessment in the Older Adult with Cancer: A Review. Eur Urol Focus. 2017;3: 330–339. [DOI] [PubMed] [Google Scholar]
- 11.Corre R, Greillier L, Le Caer H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol. 2016;34: 1476–1483. [DOI] [PubMed] [Google Scholar]
- 12.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol. 2016;7: 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20: 494–502. [DOI] [PubMed] [Google Scholar]
- 14.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36: 2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohile SG, Epstein RM, Hurria A, et al. Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program. JAMA Oncology. 2019: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohile SG, Mohamed MR, Culakova E, et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). Journal of Clinical Oncology. 2020;38. [Google Scholar]
- 17.Li DN, Sun CL, Kim H, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. Journal of Clinical Oncology. 2020;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soo WK, King M, Pope A, Parente P, Darzins P, Davis ID. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. Journal of Clinical Oncology. 2020;38. [DOI] [PubMed] [Google Scholar]
- 19.Epstein RM, Street RL. Patient-centered communication in cancer care: Promoting Healing and Reducing Suffering. Bethesda, MD. , 2007. [Google Scholar]
- 20.Ha JF, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010;10: 38–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein R, Street RL. Patient-centered communication in cancer care : promoting healing and reducing suffering. Bethsda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute, 2007. [Google Scholar]
- 22.Fallowfield L, Jenkins V. Effective communication skills are the key to good cancer care. European Journal of Cancer. 1999;35: 1592–1597. [DOI] [PubMed] [Google Scholar]
- 23.Back AL. Patient-Clinician Communication Issues in Palliative Care for Patients With Advanced Cancer. J Clin Oncol. 2020;38: 866–876. [DOI] [PubMed] [Google Scholar]
- 24.Ekdahl AW, Andersson L, Friedrichsen M. “They do what they think is the best for me.” Frail elderly patients’ preferences for participation in their care during hospitalization. Patient Educ Couns. 2010;80: 233–240. [DOI] [PubMed] [Google Scholar]
- 25.Coran JJ, Koropeckyj-Cox T, Arnold CL. Are physicians and patients in agreement? Exploring dyadic concordance. Health Educ Behav. 2013;40: 603–611. [DOI] [PubMed] [Google Scholar]
- 26.Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol. 2017;3: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paladino J, Bernacki R, Neville BA, et al. Evaluating an Intervention to Improve Communication Between Oncology Clinicians and Patients With Life-Limiting Cancer: A Cluster Randomized Clinical Trial of the Serious Illness Care Program. JAMA Oncology. 2019;5: 801–809. [DOI] [PubMed] [Google Scholar]
- 28.Loh KP, Mohile SG, Lund JL, et al. Beliefs About Advanced Cancer Curability in Older Patients, Their Caregivers, and Oncologists. Oncologist. 2019;24: E292–E302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kehoe LA, Xu H, Duberstein P, et al. Quality of Life of Caregivers of Older Patients with Advanced Cancer. J Am Geriatr Soc. 2019;67: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh KP, Mohamed MR, Kadambi S, et al. Caregiver-Oncologist Prognostic Concordance, Caregiver Mastery, and Caregiver Psychological Health and Quality of Life. Oncologist. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh KP, Mohile SG, Epstein RM, et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer. 2019;125: 2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandya C, Magnuson A, Flannery M, et al. Association Between Symptom Burden and Physical Function in Older Patients with Cancer. Journal of the American Geriatrics Society. 2019;67: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122: 3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowenstein LM, Volk RJ, Street R, et al. Communication about geriatric assessment domains in advanced cancer settings: “Missed opportunities”. Journal of Geriatric Oncology. 2019;10: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsdale E, Lemelman T, Loh KP, et al. Geriatric assessment-driven polypharmacy discussions between oncologists, older patients, and their caregivers. J Geriatr Oncol. 2018;9: 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown H, Prescott R. Applied Mixed Models in Medicine, 3rd Edition. Applied Mixed Models in Medicine, 3rd Edition. 2015: 1–+. [Google Scholar]
- 38.Burn R, Hubbard RE, Scrase RJ, et al. A frailty index derived from a standardized comprehensive geriatric assessment predicts mortality and aged residential care admission. BMC Geriatr. 2018;18: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilligan T, Coyle N, Frankel RM, et al. Patient-clinician communication: American Society of Clinical Oncology Consensus Guideline. Journal of Clinical Oncology. 2017;35: 3618–3632. [DOI] [PubMed] [Google Scholar]
- 40.Lawless MT, Archibald MM, Ambagtsheer RC, Kitson AL. Factors influencing communication about frailty in primary care: A scoping review. Patient Education and Counseling. 2020;103: 436–450. [DOI] [PubMed] [Google Scholar]
- 41.Street RL, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Education and Counseling. 2009;74: 295–301. [DOI] [PubMed] [Google Scholar]


