Abstract
Background
Asian women with breast cancer are often studied in aggregate, belying significant intra-group diversity. We sought to examine differences in breast cancer characteristics and outcomes among Asian women.
Methods
Asian, non-Hispanic Black, Hispanic, and non-Hispanic White women≥18y diagnosed with breast cancer 1990-2016 were identified in the SEER18 database. Asian patients were sub-classified as Chinese, Japanese, Korean, Filipino, Vietnamese, South Asian (Asian Indian or Pakistani), Southeast Asian (SEA; Cambodian, Laotian, Hmong, or Thai) or Other Asian. Unadjusted overall (OS) and cancer-specific survival (CSS) were estimated using the Kaplan-Meier method. Cox proportional hazards models were used to estimate adjusted OS and CSS.
Results
910,415 women were included: Asian=63,405, Black=92,226, Hispanic=84,451, White=670,333. Asian women had higher rates of HER2+ disease than White women (18.7% vs 13.8%) and the highest 10y unadjusted OS and CSS among all racial/ethnic groups (all p<0.001). SEA women had the highest rates of stage IV disease at presentation, while Japanese women had the lowest (5.9% vs 2.7%, p<0.001). Japanese women had the highest 10y unadjusted CSS (89.4%, 95% CI 88.7-90.1%) of any distinct Asian group, while SEA women had the worst (78%, 95% CI 74.1-81.3%, p<0.001). After adjustment, SEA women had the worst OS of any Asian group and were the only Asian group without improved OS compared to White women (reference, p=0.08).
Conclusion
Breast cancer characteristics and outcomes vary significantly among Asian women. Future research should consider disaggregation by country or region of origin to identify subgroups at risk for worse outcomes than aggregated data may suggest.
Keywords: Asian, Breast Neoplasms, Health Care Disparities, Racial Disparities, Ethnic Disparities, Health Outcomes
Precis:
Studying breast cancer among Asian women grouped as a single entity can lead to inaccurate generalizations regarding cancer-specific mortality and distribution of tumor subtypes. Future research should disaggregate these populations to better understand, treat, and counsel Asian patients with breast cancer.
Lay summary:
Asian women with breast cancer are frequently studied as a single entity. However, Asian ethnic groups differ greatly by country of origin, genetic ancestry, disease frequency, socioeconomic status, patterns of immigration, as well as dietary and cultural practices. We found that women of different Asian ethnicities vary significantly with regard to cancer characteristics, such as mortality and tumor subtype. Future research should disaggregate these populations to better understand, treat, and counsel Asian patients with breast cancer.
Introduction
An estimated 23 million people in the United States (US) identify as Asian.1 Within the Asian population, cancer is the leading cause of death2, and breast cancer is the most commonly diagnosed cancer among Asian women.3
Racial differences in breast cancer diagnosis, prognosis, and survival are well-documented.4–10 However, these studies typically aggregate women from geographically diverse countries in East Asia, Southeast Asia, and the Indian subcontinent into a single “Asian” racial group. However, Asians in America vary greatly with regards to country of origin, genetic ancestry, disease frequency, socioeconomic status, patterns of immigration, as well as dietary and cultural practices. Thus, this generalization likely masks important epidemiological and sociocultural differences between groups that may also contribute to disparate outcomes among Asian patients. For example, Korean women in the US demonstrate lower rates of mammography participation when compared to Vietnamese and Chinese women.3
While many studies focus on breast cancer within select Asian populations, such as Chinese or South Asian, there is a paucity of research for many other Asian ethnicities, such as Hmong, Cambodian, or Laotian.11–15 Even fewer studies compare disease characteristics and outcomes between Asian ethnic subgroups. Furthermore, some of these published findings are somewhat contradictory. For example, it remains unclear whether or not menopause occurs at an earlier age in people living in or originating from some parts of Asia as compared to what is observed in Western countries. Understanding if menopause occurs earlier in certain Asian populations is relevant for breast cancer research given the complex association between menopausal status at diagnosis and long-term outcome.16, 17
In light of the ancestral, cultural, and socioeconomic heterogeneity of this population, we sought to examine characteristics and outcomes after breast cancer diagnosis among distinct Asian subgroups living in the US and classified according to self-reported country or region of origin. We further explored differences among the Asian subgroups and also between Asians and members of other racial/ethnic groups in the US and compared findings between aggregated and disaggregated populations. We hypothesized that analysis of Asian subgroups would reveal differences otherwise lost in racial aggregation and that such knowledge can better personalize care for patients and inform efforts to improve equity in breast cancer care.
Methods
Adult female breast cancer patients were selected from the 1975-2016 Surveillance, Epidemiology, and End Results (SEER) 18 database (released November 2018; Figure 1).18 Patients with missing or unknown survival data, estrogen receptor (ER) status, or progesterone receptor (PR) status; and those diagnosed before 1990 were excluded. Those with International Classification of Disease (ICD)-O-3 histologies not included in the World Health Organization (WHO) classification and those with self-reported race/ethnicity other than Asian, Hispanic, Non-Hispanic Black (NHB), or Non-Hispanic White (NHW) were also excluded. Patients reporting Asian descent were classified as Chinese, Japanese, Korean, Filipino, Vietnamese, South Asian (Asian Indian or Pakistani), Southeast (SE) Asian (Cambodian/Kampuchean, Laotian, Hmong, or Thai), or Other Asian. Although patients may have selected more than one race, SEER only reports one racial categorization per patient, with non-White race prioritized for individuals listing White race and another race. HER2 status was only available for patients diagnosed in or after 2010, while insurance status was only available for patients diagnosed in or after 2007, thus analyses using these variables only include patients diagnosed in or after 2010 and 2007, respectively. Tumor subtypes were categorized as: (1) HER2+ (HER2+, ER+/ER−/ER borderline, and PR+/PR−/PR borderline), (2) hormone-receptor positive [HR+]/HER2− (estrogen-receptor positive [ER+] and/or progesterone-receptor positive [PR+] and HER2−/HER2 borderline), and (3) triple-negative breast cancer (TNBC; estrogen-receptor negative [ER−]/ estrogen receptor [ER] borderline, progesterone-receptor negative [PR−]/progesterone [PR] borderline, and HER2−/HER2 borderline). Only patients diagnosed 2010 and after were included in analyses for which tumor subtype was relevant. SEER does not differentiate between clinical and pathological American Joint Commission on Cancer (AJCC) stage, thus we categorized extent of disease into 3 groups: (1) non-metastatic with no nodal involvement (M0/X and N0), (2) non-metastatic with nodal involvement (M0/X and N1-3), and (3) metastatic disease (stage IV, M1 disease and any N stage). M0/X patients with NX or missing N-stage were assigned a missing value for stage.
Figure 1. Women diagnosed with breast cancer, Surveillance, Epidemiology, and End Results (SEER) 18 database, 1975-2016.
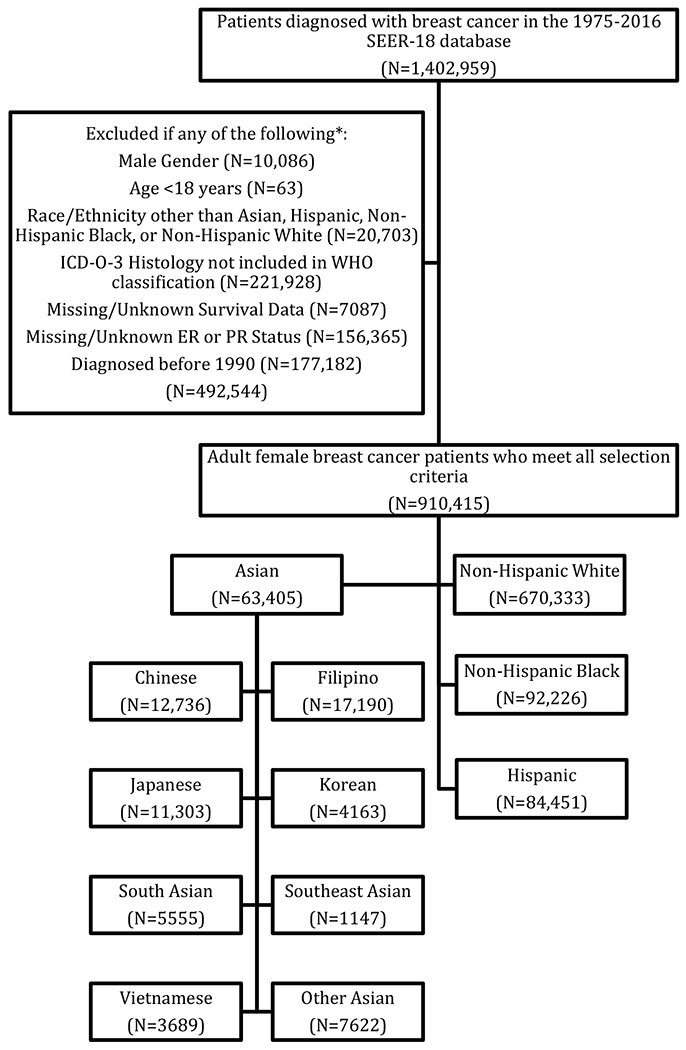
*Patients may have been excluded for more than one of the listed criteria; therefore, the total number of excluded patients is less than the sum of all exclusion criteria sample sizes.
Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. Chi-square tests and analysis of variance (ANOVA) were used to test for differences in categorical and continuous variables, respectively, for two comparisons: (1) Asian vs. Hispanic vs. NHB vs. NHW patients and (2) Chinese vs. Japanese vs. Korean vs. Filipino vs. Vietnamese vs. South Asian vs. SE Asian vs. Other Asian vs. Hispanic vs. NHB vs. NHW patients.
Overall survival (OS) was defined as the time from diagnosis to death from any cause, and patients who did not die were censored at the time of last follow-up. Cancer-specific survival (CSS) was defined as the time from diagnosis to death due to breast cancer, and patients who did not die or who died from other causes were censored at time of last follow up. Patients who had other primary cancers before their breast cancer diagnosis or had unknown cause of death were excluded from all CSS analyses. Unadjusted OS and CSS were estimated using the Kaplan-Meier (KM) method, and log-rank tests were used to compare unadjusted OS and CSS between groups. Follow-up was estimated using the reverse KM method. Cox Proportional Hazards models were used to estimate the association of Asian vs. Hispanic vs. NHB vs. NHW race/ethnicity and Chinese vs. Japanese vs. Korean vs. Filipino vs. Vietnamese vs. South Asian vs. SE Asian vs. Other Asian vs. Hispanic vs. NHB vs. NHW race/ethnicity with OS and CSS after adjustment for available covariates including age, year of diagnosis, marital status, residential location, stage, grade, ER status, PR status, treatment with chemotherapy, treatment with radiation therapy, and surgery type. Additional models were conducted including one limited to patients diagnosed in or after 2010 and that included tumor subtype as a covariate in place of ER/PR. Subgroup analyses including those with tumor subtype were further stratified by pre- and post-menopausal status, defined by proxy as age <50 vs ≥50 years, respectively.19
Only patients with complete data were included in each analysis, and effective sample sizes are included for all tables and figures. No adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary NC). Our study was deemed exempt by our institutional review board given our use of de-identified data.
Results
Demographics
The final cohort consisted of 910,415 women diagnosed with breast cancer from 1990 to 2016 (Figure 1, Table 1; for complete list of variables, see Supplemental Table 1): 73.6% NHW (n=670,333), 10.1% NHB (n=92,226), 9.3% Hispanic (n=84,451), 7.0% Asian (n=63,405). Among Asian women, the largest ethnic subgroup was Filipino, which comprised 27.1% (n=17,190) of Asian women, followed by Chinese (20.1%; n=12,736), Japanese (17.8%; n=11,303), Other Asian (12.0%; n=7,622), South Asian (8.8%; n=5,555), Korean (6.6%; n=4,163), Vietnamese (5.8%; n=3,689) and SE Asian (1.8%; n=1,147).
Table 1.
Women diagnosed with breast cancer, Surveillance, Epidemiology, and End Results (SEER) 18 database, 1990-2016
| All Patients (N=910,415) | Chinese (N=12,736) | Filipino (N=17,190) | Japanese (N=11,303) | Korean (N=4163) | South Asian (N=5555) | Southeast Asian (N=1147) | Vietnamese (N=3689) | Other Asian (N=7622) | All Asian (N=63,405) | Hispanic (N=84,451) | Non-Hispanic Black (N=92,226) | Non-Hispanic White (N=670,333) | P1 | P2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | <0.001 | <0.001 | |||||||||||||
| <50 | 202,233 (22.2%) | 4192 (32.9%) | 4715 (27.4%) | 2271 (20.1%) | 1622 (39%) | 2138 (38.5%) | 465 (40.5%) | 1438 (39%) | 2748 (36.1%) | 19,589 (30.9%) | 28,858 (34.2%) | 26,110 (28.3%) | 127,676 (19%) | ||
| ≥50 | 708,182 (77.8%) | 8544 (67.1%) | 12,475 (72.6%) | 9032 (79.9%) | 2541 (61%) | 3417 (61.5%) | 682 (59.5%) | 2251 (61%) | 4874 (63.9%) | 43,816 (69.1%) | 55,593 (65.8%) | 66,116 (71.7%) | 542,657 (81%) | ||
| Age (y) Median (IQR) | 61 (51 - 72) | 56 (47 - 67) | 57 (49 - 66) | 64 (52 - 74) | 53 (46 - 63) | 54 (45 - 64) | 53 (44 - 61) | 53 (45 - 62) | 54 (46 - 64) | 57 (47 - 67) | 55 (46 – 66) | 58 (48 - 68) | 63 (52 - 73) | <0.001 | <0.001 |
| Insurance Status3 | <0.001 | <0.001 | |||||||||||||
| Insured | 430,167 (85.2%) | 6309 (82.7%) | 8720 (82.6%) | 4971 (94.5%) | 1853 (70.5%) | 3099 (77.2%) | 488 (63.6%) | 1608 (65.8%) | 4783 (86.5%) | 31,831 (82%) | 37,108 (68.1%) | 42,209 (75%) | 319,019 (89.7%) | ||
| Any Medicaid | 56,458 (11.2%) | 1151 (15.1%) | 1540 (14.6%) | 141 (2.7%) | 621 (23.6%) | 668 (16.6%) | 253 (33%) | 769 (31.5%) | 556 (10.1%) | 5699 (14.7%) | 14,132 (25.9%) | 11,231 (19.9%) | 25,396 (7.1%) | ||
| Uninsured | 8097 (1.6%) | 87 (1.1%) | 153 (1.4%) | 29 (0.6%) | 110 (4.2%) | 162 (4%) | 18 (2.3%) | 39 (1.6%) | 90 (1.6%) | 688 (1.8%) | 2036 (3.7%) | 1775 (3.2%) | 3598 (1%) | ||
| Marital Status | <0.001 | <0.001 | |||||||||||||
| Married or Domestic Partner | 500,567 (55%) | 8642 (67.9%) | 10,922 (63.5%) | 6812 (60.3%) | 2882 (69.2%) | 4206 (75.7%) | 687 (59.9%) | 2401 (65.1%) | 5104 (67%) | 41,656 (65.7%) | 46,047 (54.5%) | 32,369 (35.1%) | 380,495 (56.8%) | ||
| Divorced or Separated | 103,619 (11.4%) | 726 (5.7%) | 1359 (7.9%) | 871 (7.7%) | 365 (8.8%) | 222 (4%) | 110 (9.6%) | 246 (6.7%) | 526 (6.9%) | 4425 (7%) | 10,369 (12.3%) | 15,178 (16.5%) | 73,647 (11%) | ||
| Single | 119,463 (13.1%) | 1578 (12.4%) | 2273 (13.2%) | 1509 (13.4%) | 464 (11.1%) | 334 (6%) | 199 (17.3%) | 615 (16.7%) | 987 (12.9%) | 7959 (12.6%) | 14,914 (17.7%) | 25,425 (27.6%) | 71,165 (10.6%) | ||
| Widowed | 149,668 (16.4%) | 1403 (11%) | 2044 (11.9%) | 1844 (16.3%) | 336 (8.1%) | 613 (11%) | 106 (9.2%) | 284 (7.7%) | 658 (8.6%) | 7288 (11.5%) | 9227 (10.9%) | 14,714 (16%) | 118,439 (17.7%) | ||
| Location Type | <0.001 | <0.001 | |||||||||||||
| Metropolitan | 815,871 (89.6%) | 12,273 (96.4%) | 16,276 (94.7%) | 8854 (78.3%) | 4018 (96.5%) | 5499 (99%) | 1120 (97.6%) | 3659 (99.2%) | 7517 (98.6%) | 59,216 (93.4%) | 80,972 (95.9%) | 86,277 (93.5%) | 589,406 (87.9%) | ||
| Urban | 79,594 (8.7%) | 75 (0.6%) | 362 (2.1%) | 545 (4.8%) | 36 (0.9%) | 46 (0.8%) | 21 (1.8%) | 22 (0.6%) | 90 (1.2%) | 1197 (1.9%) | 3110 (3.7%) | 5435 (5.9%) | 69,852 (10.4%) | ||
| Rural | 10,125 (1.1%) | 0 (0%) | 2 (0%) | 3 (0%) | 1 (0%) | 5 (0.1%) | 2 (0.2%) | 0 (0%) | 9 (0.1%) | 22 (0%) | 139 (0.2%) | 478 (0.5%) | 9486 (1.4%) | ||
| Year of Diagnosis – Median (IQR) | 2008 (2002 - 2012) | 2008 (2002 - 2013) | 2009 (2003 - 2013) | 2006 (1999 - 2011) | 2009 (2004 - 2013) | 2011 (2006 - 2014) | 2010 (2005 - 2013) | 2010 (2005 – 2013) | 2011 (2006 – 2014) | 2009 (2003 - 2013) | 2009 (2004 - 2013) | 2009 (2003 - 2013) | 2007 (2001 - 2012) | <0.001 | <0.001 |
| Stage | <0.001 | <0.001 | |||||||||||||
| No Nodal Involvement | 589,881 (64.8%) | 8544 (67.1%) | 10,986 (63.9%) | 8255 (73%) | 2628 (63.1%) | 3300 (59.4%) | 689 (60.1%) | 2356 (63.9%) | 5185 (68%) | 41,943 (66.2%) | 49,701 (58.9%) | 51,991 (56.4%) | 44,6246 (66.6%) | ||
| Nodal Involvement | 260,822 (28.6%) | 3593 (28.2%) | 5189 (30.2%) | 2516 (22.3%) | 1340 (32.2%) | 1869 (33.6%) | 366 (31.9%) | 1165 (31.6%) | 2108 (27.7%) | 18,146 (28.6%) | 29,356 (34.8%) | 31,769 (34.4%) | 181,551 (27.1%) | ||
| Metastatic Disease | 38,917 (4.3%) | 406 (3.2%) | 756 (4.4%) | 302 (2.7%) | 125 (3%) | 292 (5.3%) | 68 (5.9%) | 134 (3.6%) | 202 (2.7%) | 2285 (3.6%) | 3839 (4.5%) | 6305 (6.8%) | 26,488 (4%) | ||
| Grade | <0.001 | <0.001 | |||||||||||||
| 1 | 177,994 (19.6%) | 2276 (17.9%) | 2634 (15.3%) | 2753 (24.4%) | 653 (15.7%) | 848 (15.3%) | 152 (13.3%) | 536 (14.5%) | 1494 (19.6%) | 11,346 (17.9%) | 13,713 (16.2%) | 11,108 (12%) | 141,827 (21.2%) | ||
| 2 | 357,599 (39.3%) | 5231 (41.1%) | 7044 (41%) | 4785 (42.3%) | 1533 (36.8%) | 2131 (38.4%) | 396 (34.5%) | 1473 (39.9%) | 3209 (42.1%) | 25,802 (40.7%) | 31,980 (37.9%) | 30,205 (32.8%) | 269,612 (40.2%) | ||
| 3 | 306,842 (33.7%) | 4379 (34.4%) | 6457 (37.6%) | 3029 (26.8%) | 1733 (41.6%) | 2242 (40.4%) | 543 (47.3%) | 1478 (40.1%) | 2534 (33.2%) | 22,395 (35.3%) | 33,543 (39.7%) | 43,509 (47.2%) | 207,395 (30.9%) | ||
| Breast Surgery | <0.001 | <0.001 | |||||||||||||
| Lumpectomy | 422,665 (46.4%) | 5393 (42.3%) | 6573 (38.2%) | 5102 (45.1%) | 1801 (43.3%) | 2558 (46%) | 460 (40.1%) | 1472 (39.9%) | 3474 (45.6%) | 26,833 (42.3%) | 38,081 (45.1%) | 40,144 (43.5%) | 317,607 (47.4%) | ||
| Mastectomy | 325,898 (35.8%) | 5293 (41.6%) | 8011 (46.6%) | 3575 (31.6%) | 1772 (42.6%) | 2368 (42.6%) | 540 (47.1%) | 1815 (49.2%) | 3374 (44.3%) | 26,748 (42.2%) | 33,801 (40%) | 34,829 (37.8%) | 230,520 (34.4%) | ||
| No Surgery | 51,159 (5.6%) | 585 (4.6%) | 941 (5.5%) | 366 (3.2%) | 261 (6.3%) | 392 (7.1%) | 86 (7.5%) | 169 (4.6%) | 479 (6.3%) | 3279 (5.2%) | 6001 (7.1%) | 8647 (9.4%) | 33,232 (5%) | ||
| Other or NOS | 640 (0.1%) | 8 (0.1%) | 7 (0%) | 1 (0%) | 1 (0%) | 8 (0.1%) | 0 (0%) | 1 (0%) | 3 (0%) | 29 (0%) | 68 (0.1%) | 83 (0.1%) | 460 (0.1%) | ||
| Treatment with Radiation | <0.001 | <0.001 | |||||||||||||
| No or Unknown | 449,507 (49.4%) | 6516 (51.2%) | 9208 (53.6%) | 5215 (46.1%) | 2116 (50.8%) | 2534 (45.6%) | 616 (53.7%) | 2014 (54.6%) | 4032 (52.9%) | 32,251 (50.9%) | 43,132 (51.1%) | 46,514 (50.4%) | 327,610 (48.9%) | ||
| Yes | 439,722 (48.3%) | 6013 (47.2%) | 7544 (43.9%) | 5893 (52.1%) | 1931 (46.4%) | 2867 (51.6%) | 485 (42.3%) | 1595 (43.2%) | 3358 (44.1%) | 29,686 (46.8%) | 38,306 (45.4%) | 42,725 (46.3%) | 329,005 (49.1%) | ||
| Treatment with Chemotherapy | <0.001 | <0.001 | |||||||||||||
| No or Unknown | 551,516 (60.6%) | 7333 (57.6%) | 9008 (52.4%) | 7589 (67.1%) | 2177 (52.3%) | 2664 (48%) | 527 (45.9%) | 1852 (50.2%) | 4368 (57.3%) | 35,518 (56%) | 43,007 (50.9%) | 45,594 (49.4%) | 427,397 (63.8%) | ||
| Yes | 358,899 (39.4%) | 5403 (42.4%) | 8182 (47.6%) | 3714 (32.9%) | 1986 (47.7%) | 2891 (52%) | 620 (54.1%) | 1837 (49.8%) | 3254 (42.7%) | 27,887 (44%) | 41,444 (49.1%) | 46,632 (50.6%) | 242,936 (36.2%) | ||
| # LNs Examined – Median (IQR) | 4 (1 - 12) | 4 (2 - 12) | 4 (2 - 13) | 4 (1 - 14) | 4 (2 - 13) | 4 (2 - 12) | 5 (2 - 12) | 4 (2 - 12) | 3 (1 - 9) | 4 (2 - 12) | 4 (2 - 13) | 5 (1 - 13) | 4 (1 - 12) | <0.001 | <0.001 |
| # Positive LNs – Median (IQR) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 0) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | 0 (0 - 1) | <0.001 | <0.001 |
Data presented as N (%) unless otherwise specified. Percentages may not add up to 100 due to rounding or missing values.
IQR, interquartile range. NOS, not otherwise specified. ER, estrogen receptor. PR, progesterone receptor. HER2, human epidermal growth factor receptor 2. HR, hormone receptor. TNBC, triple-negative breast cancer. LN, lymph node.
P-value for Chinese vs. Filipino vs. Japanese vs. Korean vs. South Asian vs. Southeast Asian vs. Vietnamese vs. Other Asian vs. Hispanic vs. Non-Hispanic Black vs. Non-Hispanic White
P-value for Asian vs. Hispanic vs. Non-Hispanic Black vs. Non-Hispanic White
Insurance Type reported for patients diagnosed 2007 and after
Overall median age at diagnosis was 61 years, and Asian women were younger than NHW women (median 57 vs 63 years; p<0.001, Table 1). Japanese women were the oldest among Asians (median 64 years) and Vietnamese, Southeast Asian, and Korean women were the youngest (median 53 years for all, Table 1). More NHW women were insured compared to Asian women (89.7% vs 82%; p<0.001), though Japanese women had the highest proportion of insured patients (94.5%) of any racial/ethnic group (p<0.001, Table 1). SE Asian women had the highest proportion of patients insured with Medicaid (33.3%) while Korean women had the highest proportion of uninsured individuals overall (4.2%) (Table 1).
Disease and treatment characteristics
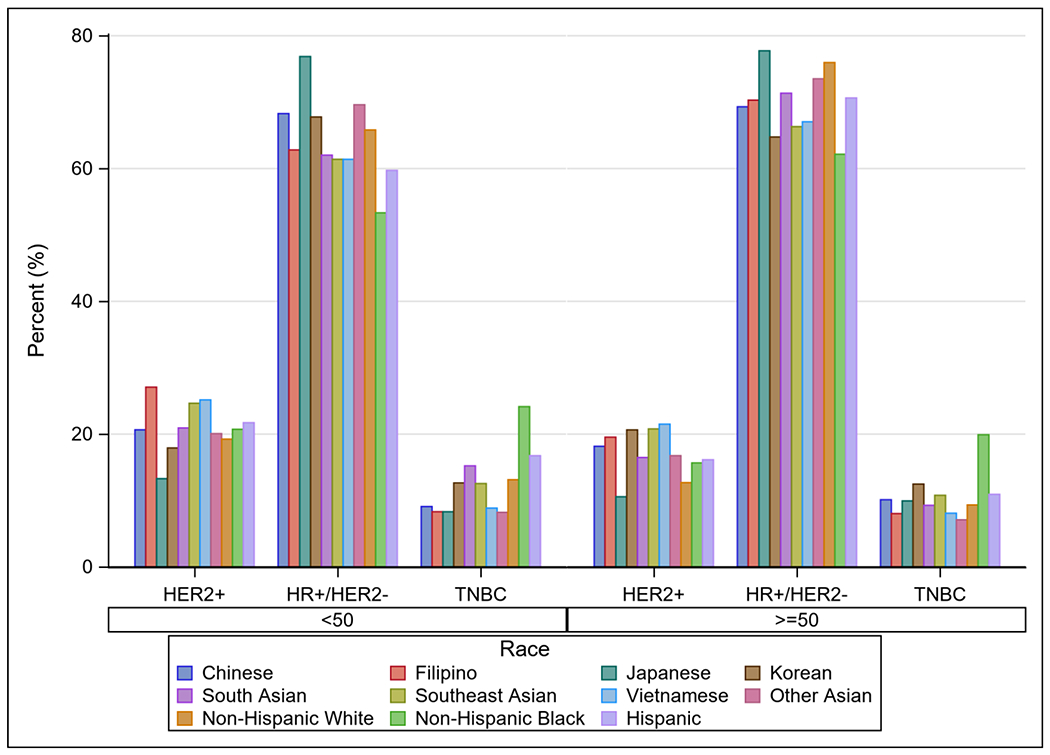
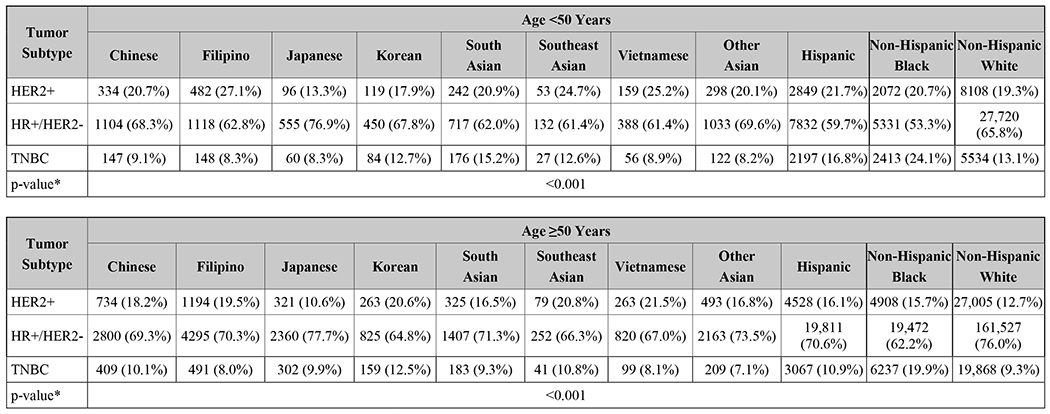
NHW women were slightly more likely to be diagnosed with no nodal involvement at diagnosis when compared to Asians overall (66.6% vs 66.2%, p<0.001), but Japanese women actually had the highest rates of non-metastatic, node-negative disease of all races and countries of origin (73%, p<0.001, Table 1). NHB women had the highest rates of advanced disease of all comparison groups (6.8%), and SE Asian women had the highest rates among Asian groups (5.9%, p<0.001, Table 1). Asian women were more likely to have HER2+ disease than NHW women (18.7% vs 13.8%, p<0.001, Table 1). Among women <50 years old, Filipino women had the highest rates of HER2+ disease (27.1%), and among women ≥50 years old, Vietnamese women had the highest rates (21.5%; both p<0.001, Figures 2A and 2B). Notably, regardless of age, Japanese women had the lowest rates of HER2+ disease of all Asian groups and also had a lower rate than NHW women (both p<0.001, Figures 2A and 2B).
Figure 2.
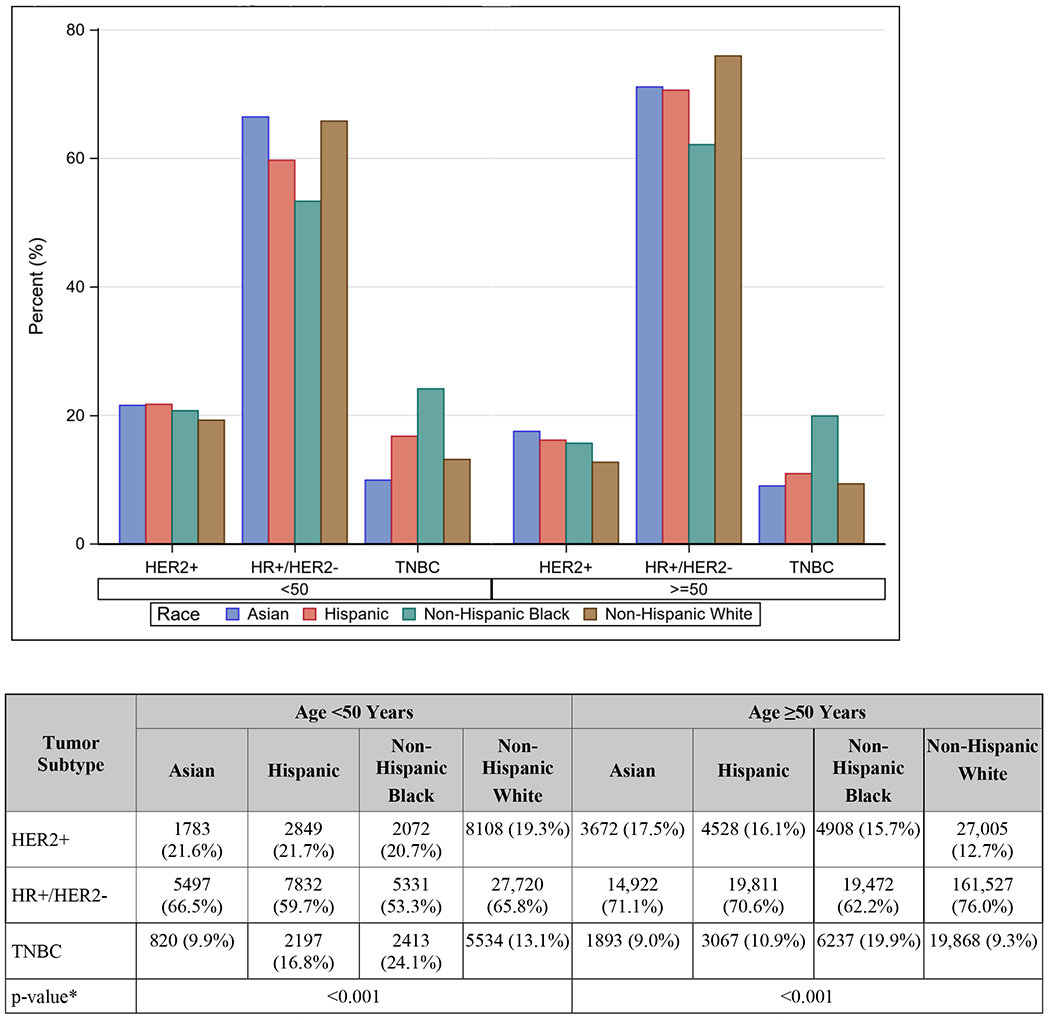
A. Tumor subtype by age and race/ethnicity
HER2, human epidermal growth factor receptor 2. HR, hormone receptor, TNBC, triple-negative breast cancer.
*p-value for difference in distribution of tumor subtype by race/ethnicity within each age group.
B. Tumor subtype by age and race/ethnicity including Asian subgroups
HER2, human epidermal growth factor receptor 2. HR, hormone receptor. TNBC, triple-negative breast cancer.
*p-value for difference in distribution of tumor subtype by race/ethnicity within each age group.
Asian women underwent mastectomy more frequently than NHW women (42.2% vs 34.4%; p<0.001, Table 1). Among Asian ethnicities, however, there was significant variation in surgical treatment: Japanese women had the lowest rates of mastectomy (31.6%) while Vietnamese women had the highest (49.2%; p<0.001, Table 1). NHW women had higher rates of lumpectomy and radiation receipt than Asian women but lower rates of chemotherapy (all p<0.001, Table 1).
Overall and Cancer-specific Survival
Asian women as a combined group had the best 10-year unadjusted OS and CSS of all racial/ethnic groups (both log-rank p<0.001, Figure 3A and 3B). Among Asian women, SE Asian women had the worst 10-year unadjusted OS (70%, 95% CI 66.1–73.5%), and Japanese women had the second-worst (75.4%, 95% CI 74.5-76.3%), but the latter were also the oldest, with the highest proportion of patients ≥50 (Supplemental Figures). In contrast, Japanese women had the highest 10-year unadjusted CSS (89.4%, 95% CI 88.7-90.1%) of any Asian subgroup, while SE Asian women had the worst (78%, 95% CI 74.1-81.3%, log-rank p<0.001, Figure 3).
Figure 3.
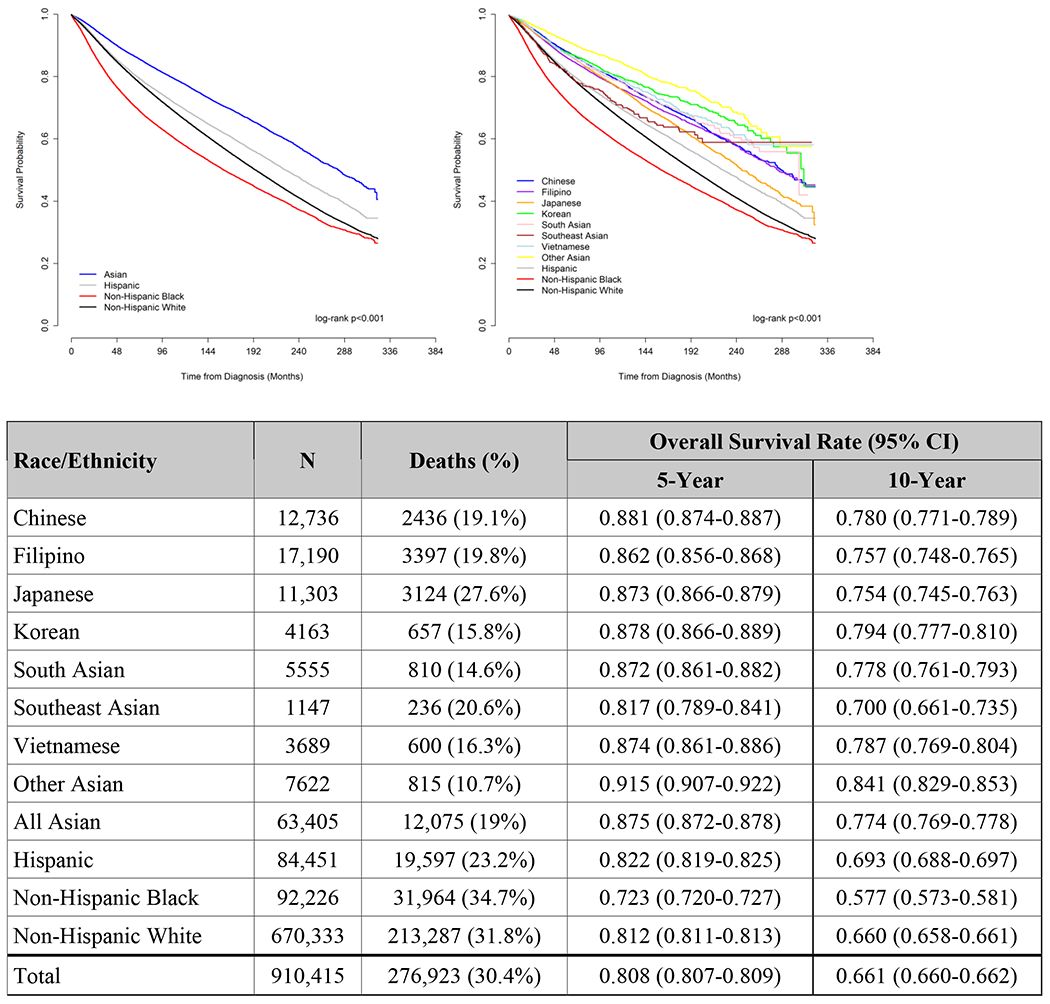
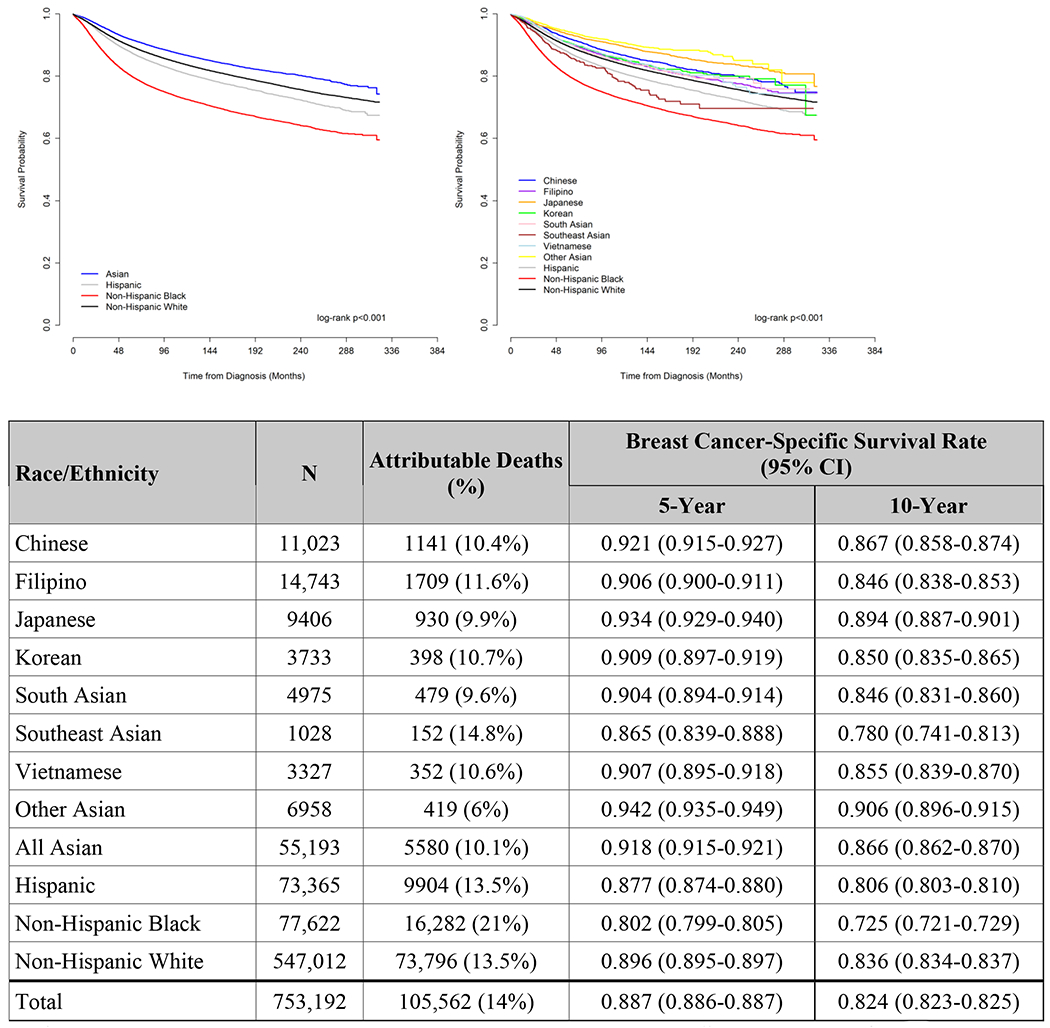
A. Unadjusted Overall Survival, Women diagnosed with Breast Cancer, Surveillance, Epidemiology, and End Results (SEER) 18 database, 1990-2016
CI, confidence interval.
B. Unadjusted Cancer-Specific Survival, Women diagnosed with Breast Cancer, Surveillance, Epidemiology, and End Results (SEER) 18 database, 1990-2016
Patients with unknown cause of death or other primary cancers before this diagnosis were excluded from this analysis
CI, confidence interval.
After adjustment, SE Asian women had the worst OS (largest hazard ratio) of any Asian group, although they were not statistically worse than Japanese women based on overlapping confidence intervals; they were also the only Asian group without significantly improved OS compared to NHW women (reference, HR 0.88, 95% CI 0.76-1.02, p=0.08, Table 2). Similarly, all Asian groups, except for SE Asians, had better adjusted CSS than NHW women (all p≤0.001, Table 2). (For OS and CSS comparisons with all Asians in one category, see Supplemental Table 2).
Table 2.
Adjusted overall and cancer-specific survival by race/ethnicity, women diagnosed with breast cancer, Surveillance, Epidemiology, and End Results (SEER) 18 database, 1990-20161
| Overall Survival (N=695,698) | Cancer-Specific Survival (N=575,567) | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | Overall p-value | HR (95% CI) | p-value | Overall p-value | |
| Race/Ethnicity | <0.001 | <0.001 | ||||
| Non-Hispanic White | REF | REF | ||||
| Chinese | 0.693 (0.658-0.729) | <0.001 | 0.814 (0.756-0.877) | <0.001 | ||
| Filipino | 0.699 (0.670-0.729) | <0.001 | 0.825 (0.778-0.876) | <0.001 | ||
| Japanese | 0.807 (0.768-0.849) | <0.001 | 0.762 (0.695-0.835) | <0.001 | ||
| Korean | 0.617 (0.561-0.678) | <0.001 | 0.838 (0.744-0.943) | 0.003 | ||
| South Asian | 0.657 (0.605-0.712) | <0.001 | 0.796 (0.716-0.884) | <0.001 | ||
| Southeast Asian | 0.877 (0.755-1.018) | 0.08 | 1.045 (0.867-1.260) | 0.65 | ||
| Vietnamese | 0.667 (0.607-0.733) | <0.001 | 0.810 (0.715-0.916) | <0.001 | ||
| Other Asian | 0.505 (0.466-0.548) | <0.001 | 0.610 (0.545-0.682) | <0.001 | ||
| Hispanic | 0.938 (0.921-0.955) | <0.001 | 1.057 (1.031-1.085) | <0.001 | ||
| Non-Hispanic Black | 1.182 (1.164-1.200) | <0.001 | 1.305 (1.277-1.334) | <0.001 | ||
| Age (Years) | <0.001 | <0.001 | ||||
| <50 | REF | REF | ||||
| ≥50 | 1.787 (1.762-1.814) | <0.001 | 1.182 (1.161-1.204) | <0.001 | ||
| Year of Diagnosis | 0.986 (0.985-0.987) | <0.001 | <0.001 | 0.979 (0.977-0.981) | <0.001 | <0.001 |
| Marital Status | <0.001 | <0.001 | ||||
| Married or Domestic Partner | REF | REF | ||||
| Divorced or Separated | 1.274 (1.255-1.294) | <0.001 | 1.225 (1.196-1.254) | <0.001 | ||
| Single | 1.312 (1.293-1.332) | <0.001 | 1.273 (1.246-1.301) | <0.001 | ||
| Widowed | 2.328 (2.301-2.356) | <0.001 | 1.690 (1.655-1.727) | <0.001 | ||
| Location Type | <0.001 | <0.001 | ||||
| Metropolitan | REF | REF | ||||
| Urban | 1.116 (1.098-1.134) | <0.001 | 1.128 (1.100-1.158) | <0.001 | ||
| Rural | 1.173 (1.126-1.223) | <0.001 | 1.239 (1.160-1.323) | <0.001 | ||
| Stage | <0.001 | <0.001 | ||||
| No Nodal Involvement | REF | REF | ||||
| Nodal Involvement | 1.800 (1.779-1.820) | <0.001 | 3.012 (2.955-3.070) | <0.001 | ||
| Metastatic Disease | 6.012 (5.893-6.133) | <0.001 | 13.607 (13.238-13.987) | <0.001 | ||
HR, hazard ratio. CI, confidence interval. ER, estrogen receptor. PR, progesterone receptor. NOS, not otherwise specified.
Also adjusted for Tumor Grade, ER/PR status, Type of Breast Surgery, Treatment with Chemotherapy/Radiation (not shown)
Discussion
Asian women in the US constitute a diverse and growing population. In our retrospective study of 910,415 women, we demonstrate differences among women with breast cancer from different regions of Asia with regards to sociodemographic factors, tumor characteristics (and concomitant therapeutic eligibility), as well as survival.
In the literature, Asian women consistently demonstrate lower mortality rates than NHW women,20–23 but when analyzed by region of origin, some subgroups had mortality rates that were higher than other Asian groups and comparable to NHW women.21, 23–25 In our study, being Asian was associated with better adjusted OS and CSS both in aggregate, as well as when disaggregated into specific Asian ethnicities compared to other racial/ethnic groups in the US with one notable exception: SE Asian women fared worse than women from other Asian subgroups and fared similarly to NHW women. This finding contrasts with a study by Parise et al.,21 in which SE Asian women were actually found to have better CSS, and South Asian, Chinese, and Korean women had no significant difference in CSS compared to NHW women. However, their study combined Vietnamese women into the SE Asian group, which may account for this difference in observed outcomes. In contrast, our findings are similar to Yi et al., who found Asian groups were similar in CSS with the exception of Japanese women who fared better than other Asian groups and NHW women.26 The observation that SE Asian women have worse survival outcomes might be explained in part by higher rates of TNBC disease among SE Asian women compared to most other Asian ethnicities. While the adjusted survival estimates do account for marital status, stage at diagnosis, grade, breast surgery, and ER/PR status, we were unable to adjust for HER2 status due to limited reporting of this variable prior to 2010. (Supplemental Table 1). Additionally, SE Asian women had the highest rate of Medicaid insurance compared to other Asian groups. Again, insurance status was only available for a limited set of diagnosis years, so could not be included in the adjusted modeling; however, this finding may suggest that social factors play a role in the survival disparity seen among SE Asian women.
Notably, there are also significant differences among Asian women with regards to screening participation. A review of 2009 data from the California Health Interview Survey demonstrated that of the 5 ethnic groups (Chinese, Filipino, Japanese, Korean, and Vietnamese) included in the study, Filipino women had the highest rates of screening both in their lifetime and more recently while Korean women had the lowest rates of screening and were also the least likely to be insured,27 a finding that was also corroborated in the work we report here. Nevertheless, despite these disparities, even among Korean women, over three-quarters of respondents had had a mammogram in her lifetime, a rate that is much higher than those observed in most non-Asian ethnic groups. In addition, Filipino and Korean women in our study did not have appreciably different rates of advanced disease at diagnosis (Table 1). Accordingly, it is not clear to what extent differences in rates of screening mammography among different Asian groups might contribute to differential rates of late-stage diagnosis, recurrence, and cancer-related death.
Racial differences in breast cancer molecular subtypes are well-documented in the literature.5, 28–30 Black women are more likely to be diagnosed with TNBC29 and Asian women have higher rates of HER2+ tumors when compared to other races.11, 12, 21, 31 Our study is consistent with these findings when evaluating Asians as a single entity and when menopausal status is not stratified. However, these trends in subtype become more complex when analyzed by country/region of origin and when menopausal status is taken into account. Among pre-menopausal women, several distinct groups had higher rates of HER2+ disease compared to NHW women (19.3%), including Chinese, Filipino, South Asian, SE Asian, and Vietnamese women, with the greatest rates being among Filipino (27.1%), SE Asian (24.7%), and Vietnamese (25.2%) women. Among post-menopausal women, all but Japanese women had higher rates than NHW women (12.7%), with the greatest rates being among Korean (20.6%), SE Asian (20.8%), and Vietnamese (21.5%) women. These rates are nearly double those of Japanese women, who had the lowest rates of HER2+ disease in either age category. A study of the California Cancer Registry from 2011 yielded slightly different results, showing Korean, Filipino, Vietnamese, and Chinese women with higher HER2+ rates than NHW women, but this study did not account for menopausal status.31 Thus, the oft cited observation that HER2 overexpression is more common among Asian women belies the differences amongst different Asian subgroups when sufficiently granular categories are used. This distinction between Asian subgroups extends to rates of TNBC as well. Among pre-menopausal women, rates ranged from 15.2% among South Asians to 8.3% in Japanese and Filipino women. Likewise, there was variation in TNBC rates among post-menopausal women, from the highest among Koreans (12.5%) to the lowest among Filipino (8.0%), Vietnamese (8.1%), and Other Asians (7.1%).
The granular categories in our study enabled comparisons between specific Asian subgroups and other non-Asian racial/ethnic groups. For example, of all the Asian groups, SE Asians were most similar to NHB in terms of later stage at diagnosis and higher-grade disease. Though this observation does not seem to be accounted for by oncologic characteristics in our study, such as prevalence of TNBC disease, these disease characteristics could, in part, be explained by the lower SES more commonly observed in these two groups relative to their comparison groups.32, 33 Low SES has been found in the literature to contribute to worse health outcomes via factors including residential segregation, decreased access to healthcare, increased financial pressure, and epigenetic modifications as a result of adversity.34–37 The socioeconomic commonalities between these two groups are often overlooked, but it may be worthwhile to observe them in parallel and see how lessons learned in improving treatment receipt and outcomes in one group might potentially be applied in the other.
Japanese women were more similar to NHW women than other Asian groups along multiple dimensions associated with better survival: older median age at diagnosis, higher rates of health insurance, less urban geographic location, earlier disease stage at presentation, more frequent HR+ tumor subtype, lower rates of mastectomy, higher rates of adjuvant radiation therapy, and lower rates of chemotherapy. These trends may reflect the potential impact of immigration patterns on screening practices, health outcomes, and differing values placed on breast conservation between Asian cultures, with less acculturated Asian groups more often opting for mastectomy. Of all Asian-American groups, Japanese-Americans have one of the longest histories in the US, which is reflected in Japanese-Americans having the lowest rate of foreign-born residents compared to other Asian groups (27%, compared to the average 59% for All Asians).38 The difference in breast cancer risk among immigrant Asian-Americans compared to U.S.-born Asian-Americans suggests an association between immigration pattern and Japanese-Americans’ trends differing from those of Asians overall, meriting further study in datasets that collect immigration data.39, 40 This phenomenon among Japanese-Americans contrasts with trends observed among Latinx individuals in the US, among whom subsequent generations often have worse health outcomes than first-generation, foreign-born individuals.41 The differences between these two groups and amongst Asian-Americans illustrate the disparate effects of immigration, acculturation, and systemic bias on health outcomes of different groups and the importance of recognizing these differential effects in both research and clinical care.
Limitations
Racial data is mainly self-reported but as SEER only reports one racial/ethnic group per patient, the SEER-assigned racial designations in our cohort may not accurately reflect the identities of multiracial people in our cohort. Likewise, use of a composite “Hispanic” racial/ethnic category belies the diversity of this group, as we have previously described.42 Although we included seven regionally distinct Asian groups in our study, some ethnicities were still aggregated for the purposes of statistical analysis and due to small sample size. Data for certain social determinants of health (e.g., immigration status) and clinical characteristics (e.g., body mass index) were not available to incorporate into our analysis. Additionally, SEER does not distinguish between receiving no treatment and receiving unknown systemic or radiation therapy, thus, we were unable to determine if a patient truly did not undergo treatment or if treatment status was unknown. Menopausal status, though commonly operationalized in research as <50 and ≥50 years old, does not capture potentially different average onset of menopause across regions and ethnicities. One study from 2005 found that northern Indian women had an onset of menopause that was 3 years earlier than women in the West, but other studies have not found significant differences in menopause onset between Eastern and Western populations.23,24 43,44 Based on the relatively low level of missingness of covariates and the uninformative nature of that missingness, we assumed the unavailable data were missing at random, and complete case analysis was used for all adjusted modeling. Although this approach is appropriate when data are missing completely at random, it may have introduced some bias into the hazard ratio and confidence interval estimates. Finally, HER2 status and insurance information only became readily available in 2010 and 2007, respectively, so only a subset of the cohort is analyzable along those dimensions.
Conclusion
In summary, our findings demonstrate that studying breast cancer among Asian women grouped as a single entity can lead to inaccurate generalizations regarding tumor-subtype distribution and long-term outcomes. Future research should be devoted to disaggregating these populations to better understand, treat, and counsel Asian patients with breast cancer, as well as to better understand the mechanisms by which acculturation in the US as well as systemic racism may impact breast cancer incidence and outcome.
Supplementary Material
Funding:
This work is supported by National Institutes of Health (NIH) Award Number 1K08CA241390 (PI: Fayanju), the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan) and the Duke Global Health Institute. The content is solely the authors’ responsibility and does not represent the official views of the NIH.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest.
Previous presentation: Portions of this work were presented at the 2021 Society of Surgical Oncology Meeting.
Social Media and Promotion
Though commonly studied in aggregate, Asian women w/ breast cancer vary in mortality + distribution of tumor subtypes when stratified by country/region of origin.
Twitter Handles: @DrLolaFayanju, @JenniferPlichta, @DrShelleyHwang, @GreenupRachel, @DukeBladegirl, @mom2phd, @DukeCancer, @DukeSurgery, @DukeForge, @Duke_pophealth, @alicelingyu1, @PennCancer, @PennSurgery, @PC3Innovation
References
- 1.American Community Survey 1-Year Estimates. Accessed 2020 October 20. https://data.census.gov/cedsci/table?q=asian&hidePreview=false&tid=ACSDT1Y2018.B02001&t=Asian&vintage=2018
- 2.Asian Americans and cancer. Mich Med Accessed 10/28/2020. https://www.rogelcancercenter.org/living-with-cancer/survivorship/advocacy/asian-americans-and-cancer
- 3.Shon EJ, Townsend AL. Predictors of never having a mammogram among Chinese, Vietnamese, and Korean immigrant women in the U.S. PLoS One. 2019;14(11):e0224505. doi: 10.1371/journal.pone.0224505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dania V, Liu Y, Ademuyiwa F, Weber JD, Colditz GA. Associations of race and ethnicity with risk of developing invasive breast cancer after lobular carcinoma in situ. Breast Cancer Res. Nov 14 2019;21(1):120. doi: 10.1186/s13058-019-1219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, West R, Weber JD, Colditz GA. Race and risk of subsequent aggressive breast cancer following ductal carcinoma in situ. Cancer. Sep 15 2019;125(18):3225–3233. doi: 10.1002/cncr.32200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. Jan 10 2012;30(2):142–50. doi: 10.1200/JCO.2011.36.8399 [DOI] [PubMed] [Google Scholar]
- 7.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. Jan-Feb 2014;64(1):52–62. doi: 10.3322/caac.21203 [DOI] [PubMed] [Google Scholar]
- 8.Ren JX, Gong Y, Ling H, Hu X, Shao ZM. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res Treat. Jan 2019;173(1):225–237. doi: 10.1007/s10549-018-4956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzoi MA, Schwartsmann G, de Azevedo SJ, Geib G, Zaffaroni F, Liedke PER. Differences in Breast Cancer Stage at Diagnosis by Ethnicity, Insurance Status, and Family Income in Young Women in the USA. J Racial Ethn Health Disparities. Oct 2019;6(5):909–916. doi: 10.1007/s40615-019-00591-y [DOI] [PubMed] [Google Scholar]
- 10.Sakhuja S, Deveaux A, Wilson LE, et al. Patterns of de-novo metastasis and breast cancer-specific mortality by race and molecular subtype in the SEER population-based dataset. Breast Cancer Res Treat. Apr 2021;186(2):509–518. doi: 10.1007/s10549-020-06007-4 [DOI] [PubMed] [Google Scholar]
- 11.Su Y, Zheng Y, Zheng W, et al. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer. Jul 12 2011;11:292. doi: 10.1186/1471-2407-11-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li E, Guida JL, Tian Y, et al. Associations between mammographic density and tumor characteristics in Chinese women with breast cancer. Breast Cancer Res Treat. Sep 2019;177(2):527–536. doi: 10.1007/s10549-019-05325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakarala M, Rozek L, Cote M, Liyanage S, Brenner DE. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.--a SEER analysis. BMC Cancer. May 11 2010;10:191. doi: 10.1186/1471-2407-10-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milbury K, Kavanagh A, Meng Z, et al. Depressive symptoms and positive affect in Chinese and United States breast cancer survivors: a cross-cultural comparison. Support Care Cancer. Jul 2017;25(7):2103–2109. doi: 10.1007/s00520-017-3612-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao M, Khan AJ, Moran MS, et al. Clinicopathologic presentation of Asian-Indian American (AIA) women with stage 0, I & II breast cancer. J Immigr Minor Health. Feb 2011;13(1):42–8. doi: 10.1007/s10903-010-9359-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. Oct 2010;13(5):419–28. doi: 10.3109/13697137.2010.507886 [DOI] [PubMed] [Google Scholar]
- 17.Lao C, Elwood M, Kuper-Hommel M, Campbell I, Lawrenson R. Impact of menopausal status on risk of metastatic recurrence of breast cancer. Menopause. 2021;28(10):1085–1092. doi: 10.1097/gme.0000000000001817 [DOI] [PubMed] [Google Scholar]
- 18.Data from: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975-2016 varying) - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. 2018. [Google Scholar]
- 19.Katuwal S, Jousilahti P, Pukkala E. Causes of death among women with breast cancer: A follow-up study of 50 481 women with breast cancer in Finland. Int J Cancer. Apr 22 2021;doi: 10.1002/ijc.33607 [DOI] [PubMed] [Google Scholar]
- 20.Solanki PA, Ko NY, Qato DM, Calip GS. Risk of cancer-specific, cardiovascular, and all-cause mortality among Asian and Pacific Islander breast cancer survivors in the United States, 1991-2011. Springerplus. 2016;5:82. doi: 10.1186/s40064-016-1726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parise C, Caggiano V. Breast Cancer Mortality among Asian-American Women in California: Variation according to Ethnicity and Tumor Subtype. J Breast Cancer. Jun 2016;19(2):112–21. doi: 10.4048/jbc.2016.19.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torre LA, Sauer AM, Chen MS Jr., Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA Cancer J Clin. May 2016;66(3):182–202. doi: 10.3322/caac.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Zhang C, Wang Q, Li Z, Lin J, Wang H. Differences in Stage of Cancer at Diagnosis, Treatment, and Survival by Race and Ethnicity Among Leading Cancer Types. JAMA Netw Open. Apr 1 2020;3(4):e202950. doi: 10.1001/jamanetworkopen.2020.2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinh QD, Nguyen PL, Leow JJ, et al. Cancer-specific mortality of Asian Americans diagnosed with cancer: a nationwide population-based assessment. J Natl Cancer Inst. Jun 2015;107(6):djv054. doi: 10.1093/jnci/djv054 [DOI] [PubMed] [Google Scholar]
- 25.Printz C Cancer mortality rates vary among specific Asian American ethnic groups. Cancer. Jan 1 2017;123(1):11. doi: 10.1002/cncr.30486 [DOI] [PubMed] [Google Scholar]
- 26.Yi M, Liu P, Li X, et al. Comparative analysis of clinicopathologic features, treatment, and survival of Asian women with a breast cancer diagnosis residing in the United States. Cancer. Sep 1 2012;118(17):4117–25. doi: 10.1002/cncr.27399 [DOI] [PubMed] [Google Scholar]
- 27.Ryu SY, Crespi CM, Maxwell AE. What Factors Explain Disparities in Mammography Rates Among Asian-American Immigrant Women? A Population-Based Study in California. Women’s Health Issues. 2013;23(6):e403–e410. doi: 10.1016/j.whi.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihemelandu CU, Leffall LD Jr., Dewitty RL, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res. Nov 2007;143(1):109–18. doi: 10.1016/j.jss.2007.03.085 [DOI] [PubMed] [Google Scholar]
- 29.Kong X, Liu Z, Cheng R, et al. Variation in Breast Cancer Subtype Incidence and Distribution by Race/Ethnicity in the United States From 2010 to 2015. JAMA Netw Open. Oct 1 2020;3(10):e2020303. doi: 10.1001/jamanetworkopen.2020.20303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parise CA, Caggiano V. Differences in clinicopatholgic characteristics and risk of mortality between the triple positive and ER+/PR+/HER2− breast cancer subtypes. Cancer Causes Control. May 2019;30(5):417–424. doi: 10.1007/s10552-019-01152-8 [DOI] [PubMed] [Google Scholar]
- 31.Telli ML, Chang ET, Kurian AW, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. Jun 2011;127(2):471–8. doi: 10.1007/s10549-010-1173-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook WK, Tseng W, Tam C, John I, Lui C. Ethnic-group socioeconomic status as an indicator of community-level disadvantage: A study of overweight/obesity in Asian American adolescents. Soc Sci Med. Jul 2017;184:15–22. doi: 10.1016/j.socscimed.2017.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. Apr 2016;35(4):407–11. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. Sep-Oct 2001;116(5):404–16. doi: 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartz JR, Hariri AR, Williamson DE. An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Mol Psychiatry. Feb 2017;22(2):209–214. doi: 10.1038/mp.2016.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans N 3rd, Grenda T, Alvarez NH, Okusanya OT. Narrative review of socioeconomic and racial disparities in the treatment of early stage lung cancer. J Thorac Dis. Jun 2021;13(6):3758–3763. doi: 10.21037/jtd-20-3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons RL, Lei MK, Beach SR, et al. Economic hardship and biological weathering: The epigenetics of aging in a U.S. sample of black women. Soc Sci Med. Feb 2016;150:192–200. doi: 10.1016/j.socscimed.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez G, FRadford J. 2015, Foreign-Born Population in the United States Statistical Portrait. May 3, 2017. Accessed October 27th, 2020. https://www.pewresearch.org/hispanic/2017/05/03/2015-statistical-information-on-immigrants-in-united-states/
- 39.Morey BN, Gee GC, von Ehrenstein OS, et al. Higher Breast Cancer Risk Among Immigrant Asian American Women Than Among US-Born Asian American Women. Prev Chronic Dis. Feb 14 2019;16:E20. doi: 10.5888/pcd16.180221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. May 2010;100(5):861–9. doi: 10.2105/AJPH.2009.176651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balcazar AJ, Grineski SE, Collins TW. The Hispanic health paradox across generations: the relationship of child generational status and citizenship with health outcomes. Public Health. Jun 2015;129(6):691–7. doi: 10.1016/j.puhe.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champion CD, Thomas SM, Plichta JK, et al. Disparities at the Intersection of Race and Ethnicity: Examining Trends and Outcomes in Hispanic Women With Breast Cancer. JCO Oncol Pract. Oct 7 2020:OP2000381. doi: 10.1200/OP.20.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kriplani A, Banerjee K. An overview of age of onset of menopause in northern India. Maturitas. Nov-Dec 2005;52(3-4):199–204. doi: 10.1016/j.maturitas.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 44.Boulet MJ, Oddens BJ, Lehert P, Vemer HM, Visser A. Climacteric and menopause in seven south-east Asian countries. Maturitas. Sep-Oct 2008;61(1-2):34–53. doi: 10.1016/j.maturitas.2008.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


