Abstract
Objectives:
Delirium is common among patients with acute stroke and associated with worse outcomes. However, it is unclear which stroke locations or types are most associated with delirium.
Materials and Methods:
We systematically reviewed studies of patients with acute stroke that reported stroke locations and types by delirium status. We included papers in any language, through a combined search from January 2010 to June 2021. Case studies with less than 20 patients, case-control studies, and randomized controlled trials were excluded. MEDLINE, EMBASE, PsycINFO, CINAHL, and Alois databases were searched. Pooled relative risks were calculated using bivariate random effects models or network metaanalysis. Methodological quality was assessed across 8 factors.
Results:
31 patient samples representing 8,329 patients were included. Delirium was more common in patients with supratentorial lesions than infratentorial (RR [Relative Risk] 2.01, CI [Confidence Interval] 1.49-2.72); anterior circulation lesions than posterior (RR 1.41, CI 1.13-1.78); and cortical lesions than subcortical (RR 1.54, CI 1.25-1.89). Stroke side was not associated with delirium (right vs. left: RR 0.99, CI 0.77-1.28). Delirium was more common in patients with hemorrhagic strokes than ischemic (RR 1.74, CI 1.42-2.11) and patients with preexisting qualitative atrophy (RR 1.66, CI 1.21-2.27).
Conclusion:
Several brain localizations and types of strokes were associated with delirium. Conclusions were in part limited by the heterogeneity of studies and broad or qualitative lesion descriptions. These results may assist in anticipating the risk of delirium in acute stroke and highlight brain networks and pathologies that may be involved in the pathophysiology of delirium.
Keywords: Stroke, Delirium, Localization, Meta-Analysis, Systematic Review
INTRODUCTION
Each year in the United States, 795,000 people have new or recurrent strokes.1 Approximately 25% of patients with acute stroke will also experience delirium, an acute and fluctuating disturbance in attention and awareness.2 Patients who suffer from delirium in the acute post-stroke setting have an increased risk of mortality,3 increased likelihood of discharge to post-acute care facilities, and increased disability compared to those without delirium.4 Therefore understanding who develops delirium after acute stroke impacts not only acute inpatient management, but also longer-term prognosis.
Although several predisposing and precipitating risk factors, such as age, cognitive reserve, and medications, have been associated with the development of delirium in patients with acute stroke,5 the role of the acute stroke lesion itself remains unclear. Prior literature has varied, with some papers reporting that right-sided brain lesions increase the risk of delirium,3,6 and others reporting that left-sided brain lesions increase the risk of delirium.7,8 Prior studies have also variably reported that delirium is associated with either anterior8 or posterior7 circulation lesions. It therefore remains unclear whether delirium arises solely from nonspecific, generalized brain dysfunction or instead can also be precipitated by lesions in specific regions. The results of single studies may be insufficient to address this question, given the variability of strokes and the heterogeneity of delirium, hindering our understanding of factors involved in the pathophysiology of delirium in acute stroke.
We therefore undertook a broad systematic review and meta-analysis to determine whether specific types of acute stroke are associated with an increased risk of delirium, with a particular emphasis on the role that lesion locations may play in the risk of delirium.
METHODS
Eligibility Criteria and Search Strategy
A recent systematic review and meta-analysis by Shaw et al studied the prevalence of delirium in acute stroke, reviewing the literature from January 2010 to June 2018.2 Given our goal to determine rates of delirium within subsets of patients with acute stroke, we both assessed the articles found by this systematic review and also chronologically expanded the search using the same criteria to include additional papers published more recently, between June 1, 2018-June 11, 2021. For our additional search, we used the same cross-disciplinary electronic databases as in the prior systematic review: MEDLINE, EMBASE, PsycINFO/PsycArticles, CINAHL, and Alois, all searched on June 11, 2021. Details are included in the supplement for the search strategy (Supplementary Appendix 1) and inclusion/exclusion criteria (Supplementary Appendix 2). We did not include studies where localization and imaging data were not reported, since post-hoc inclusion would include data that did not undergo peer review. The study was registered at https://www.crd.york.ac.uk/prospero/ (CRD42021238301); a protocol was not otherwise prepared. The following stroke classifications were added after protocol submission: cortical versus subcortical strokes and TOAST classification of ischemic stroke subtypes. Due to the nature of the work, institutional review board approval was not required.
Data Collection Process
Each article was screened independently by at least two of five investigators trained in systematic reviews (JR, MC, MM, SS, EK), using Covidence. Screening was first performed on titles and abstracts, and at a second stage on full text papers. Any discrepancies were discussed collectively, using prespecified inclusion and exclusion criteria, and agreement was always reached. The systematic review and meta-analyses were completed in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (Supplementary PRISMA Checklist).9
For each trial, we identified the number of patients with and without delirium, and then for each group extracted stroke characteristics including location (Supplementary Appendix 3). Most papers reported stroke locations using vascular territories (e.g., anterior circulation) rather than focal brain locations (e.g., frontal lobe), and most localizations were presented as single categories: e.g., right vs. left hemisphere, followed separately by other categories such as supratentorial vs. infratentorial location. When more specific data was given, we extracted categorical features to harmonize data across studies. As an example, a case of patient with an ischemic stroke in the right middle cerebral artery (MCA) territory was categorized according to delirium status into “ischemic” in the ischemic versus hemorrhagic category, “right” for the left versus right hemisphere category, and “anterior” in the vascular territory category. In the case of hemorrhage, the location or vascular territory feeding the hemorrhage as reported by the papers was used for categorization. Data extraction was performed in duplicate by two investigators (JR & EK) to ensure accuracy, and discrepancies were resolved by consensus discussion.
Meta-analysis Methods
The metabin function for R10 was utilized to calculate differences in the binary outcome of delirium across studies using random effects models, to analyze whether delirium risk was associated with particular locations or stroke types. The network meta-analysis nme function11 in R was used when more than two categories were present. Relative risk and 95% confidence intervals (CI) were calculated. Heterogeneity was assessed by I2, τ2, and Cochrane Q. Given the limited number of studies for many comparisons, no further analysis of heterogeneity was performed. Significance levels were set at p = 0.05. Corrections for multiple comparisons were not performed given the exploratory nature of this analysis. We displayed results graphically using forest plots for binary categories and using heat maps for multiple group analyses. Funnel plots were visualized to assess for possible reporting bias for binary comparisons.
Bias Assessment and Sensitivity Analyses
For sensitivity analyses, each paper was graded for risk of bias across eight different quality measures, including clearly defined inclusion criteria, clear definition of study subjects and setting, measurement of the exposure (stroke) in a valid and reliable way, use of objective and standard criteria for measurement of the affected stroke territories, identification of confounding factors and how those were dealt with, measurement of the outcome (delirium) in a valid and reliable way, and appropriate use of statistical analysis (Supplementary Appendix 4) using a modified bias appraisal tool12,13. Each paper was graded separately by two authors (JR and EK), and when there was disagreement, a discussion was held to achieve consensus. Papers at lower risk of bias were identified as those with a summed score of six or higher across all sensitivity measures. We then repeated metaanalyses using only these higher-quality studies.
Data Availability
Data not published within this article will be made available by request from qualified investigators.
RESULTS
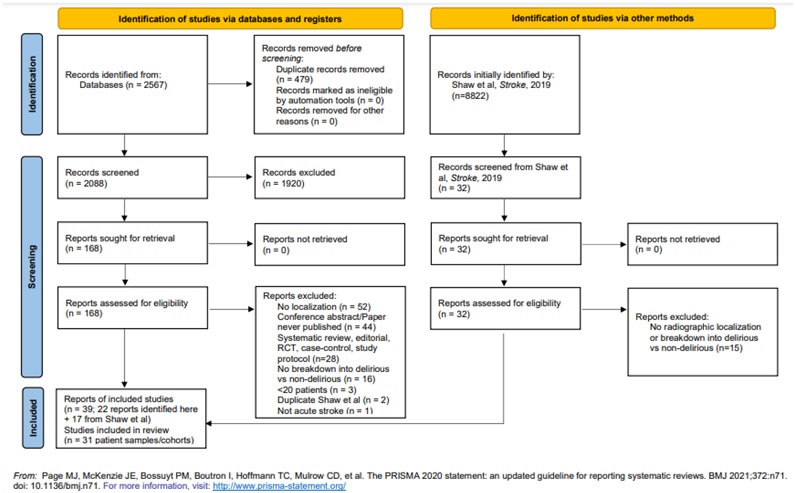
The literature search yielded a total of 39 papers for inclusion, of which 8 papers included data from previously published patient samples. We therefore combined the papers to yield 31 unique patient samples (which we will refer to as “cohorts”, moving forward) (Figure 1), representing a total of 8,329 patients. Common reasons for papers to be excluded at the full text stage were because they did not have radiographically determined localization information or did not break down patients into those with and without delirium.
Figure 1.
Flow diagram of the systematic review
The final list of included studies and their relevant characteristics are included in Table 1. Most papers measured delirium using the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria or Confusion Assessment Method (CAM) based assessment, though occasionally other measures were also used. Quality and risk of bias assessments for each paper are presented in Supplementary Appendix 5.
Table 1.
Characteristics of patient cohorts included in the meta-analysis
| Study | Country | n | Clinical Setting |
Type of Stroke |
Delirium Assessment |
Excluded Stroke Impairments |
Excluded Psychiatric Illness |
Mean age, y |
Women, n (%) |
Delirium cases , n |
Delirium cases , % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abawi et al 201617 | Netherlands | 103 | Inpatient | Ischemic | DSM | No | No | 80±8 | 53 (51%) | 15 | 15 |
| Aizen et al 201938 | Israel | 110 | Geriatric Rehabilitation Hospital | All stroke | CAM | No | No | 80.2±8 | 57 (51.8%) | 30 | 27.3 |
| Alvarez-Perez & Paiva 201739 | Portugal | 1072 | Stroke Service | All stroke | Case note review DSM | No | No | 68 (median), range: 77–83 | 507 (47.3%) | 118 | 10.2 |
| Caeiro et al 200540 | Portugal | 218 | Acute Stroke Unit | All stroke (including SAH) | DSM | Not reported | Not reported | 57±13 | 88 (40.4%) | 29 | 13 |
| Gustafson et al 199141 | Sweden | 145 | Stroke Unit | All stroke, TIA | DSM | Yes, decreased GCS, aphasia | Not reported | 73, range: 40–101 | 55 (37.9%) | 69 | 48 |
| Gustafson et al 199342 | Sweden | 83 | Stroke Unit | Supratentorial cerebral infarction | DSM | Yes, decreased GCS | Yes | 74.7±8.1 | 31 (37.3%) | 35 | 42 |
| Haight & Marsh 202019 | USA | 102 | Intensive Care Unit | Ischemic stroke or ICH | CAM-ICU | Coma without recovery, presented outside of 72 hours, withdrawal of care on admission | Not reported | 65, range: 26-97 | 49 (48%) | 51 | 50 |
| Henon et al 199921 | France | 202 | Stroke Unit | All stroke | DSM | No | Yes | 75 (median), range: 45–101 | 105 (52.0%) | 49 | 24.3 |
| Hosoya et al 201814 | Japan | 269 | Stroke Unit | Any | ICDSC | No | No | 75 ± 1.3 Delirium, 69.3 ± 1.0 Control | 123 (45.7%) | 97 | 36 |
| Kara et al 201343 | Turkey | 150 | Neurology department | Unspecified | DSM | Yes, aphasia | Not reported | 68.0±1.9 | 45 (30.0%) | 42 | 28 |
| Kostalova et al 201215 | Czech Republic | 100 | Stroke Unit | All stroke | Clinical | Not reported | Yes | 73.5±11.5 | 47 (47.0%) | 43 | 43 |
| Kotfis et al (combined)16,44 | Poland | 760 | Stroke Unit | Ischemic | CAM-ICU | No | No | 75.95 ± 13.49 Delirium, 0.82 ± 12.15 Control | Not available for subgroup | 121 | 15.9 |
| Kozak et al 201745 | Turkey | 60 | Stroke Unit | Acute ischemic stroke | DSM, DRS | Yes, aphasia | Yes | 66.2±12.5 | 31 (51.7%) | 11 | 18.3 |
| Kutlubaev et al 201346 | Russia | 96 | Stroke Unit | Unspecified | DSM | Not reported | Yes | 68.0±10.5 | 46 (47.9%) | 22 | 23 |
| Kutlubaev et al 201523 | Russia | 73 | Stroke Unit | Ischemic stroke or IPH, but not TIA, SAH, SDH | DSM | Not reported | Yes | 74 (69.5-78) | 52 (71.2%) | 33 | 45.2 |
| Lim et al 201747 | Korea | 576 | Stroke Unit | All stroke | CAM | Not reported | Not reported | 65.2 (median), range: 23.0–93.0 | 208 (36.1%) | 38 | 6.7 |
| Matsuzono et al 202048 | Japan | 461 | N/A | Ischemic | Unspecified | Not reported | Not reported | Medians: 80 delirium, 69 No delirium | 45.5 % delirimu 37.7 % no delirium | 119 | 25.8 |
| Miu and Yeung 202049 | Japan | 314 | Stroke Unit | All stroke | CAM | Not reported | Yes | 72.9±10.3 | 151 (48.1%) | 86 | 27.4 |
| Naidech et al 201318 | USA | 114 | Stroke Unit | ICH | CAM | Not reported | Not reported | 63.0±13.8 | 52 (45.6%) | 31 | 27 |
| Ng et al 20198 | Australia | 280 | Stroke Unit, General Medicine | Ischemic stroke | CAM | No | No | 63.6±13.7 | 94 (33.6%) | 36 | 12.9 |
| Oldenbeuving et al 20116 | The Netherlands | 527 | Stroke Unit | All stroke | CAM | Not reported | Not reported | 72.0 (median), range: 29.0–96.0 | 239 (45.4%) | 62 | 11.8 |
| PROPOLIS Combined4,22,50-53 | Poland | 750 | Stroke Unit | All stroke | CAM | Not reported | Not reported | 71.8±13.1 | 398 (53.1%) | 203 | 27.1 |
| Qu et al 20185 | China | 261 | Neurology Service | Ischemic | CAM | No | No | 61.3 (IQR 14.8) | 77 (29.2%) | 38 | 14.5 |
| Reimann et al 202154 | Austria | 276 | Neurology Intensive Care Unit | Non-traumatic SAH | ICDSC | Not reported | Not reported | 56, range; 47-67 | 171 (62.0%) | 65 | 23.6 |
| Reznik et al (combined)20,28,55 | USA | 311 | Neurology Intensive Care Unit | ICH | CAM-ICU | No | No | 70.1±15.8 | 139 (50%) | 157 | 55.3 |
| Rollo et al 202124 | Italy | 120 | Stroke unit | Ischemic or ICH | CAM-ICU | No | No | 71.8±12.6 | 48 (40%) | 36 | 39 |
| Sauvigny et al 201956 | Germany | 212 | Intensive Care Unit | SAH | RASS | No | No | 53.8 ± 13.4 (Control), 56.6 ± 13.4 (Delirium) | 134 (63.2%) | 78 | 34.6 |
| Sheng et al 200657 | Australia | 156 | Stroke Unit | All stroke | Clinical | Not reported | Yes | 79.2±6.7 | 73 (46.8%) | 39 | 25 |
| Shih et al 20077 | Taiwan | 29 | Neurology Service | Ischemic stroke in PCA territory | No official measurement | No official territory | Not reported | 66.3 ± 13.05 (Delirium), 64.8 ± 9.85 (Control) | 4 (13.8%) | 14 | 48.3 |
| Turco et al 201358 | Italy | 176 | Rehabilitation unit | Unspecified | CAM | No | No | 81.7±6.4 | 118 (67.0%) | 58 | 33 |
| Zaitoun et al 20193 | Egypt | 74 | ICU and Stroke Unit | All stroke | DSM-IV | Not reported | Not reported | 60.7±11.5 | 34 (45.9%) | 15 | 20.3 |
Given the number of different stroke features analyzed, a summary of the results below is presented in Table 2. Funnel plots for each binary analysis are presented in Supplementary Appendix 6, and corresponding sensitivity analyses are presented in Supplementary Appendix 7.
Table 2.
Summary of Meta-Analysis
| Patients
(n) with Delirium/Group (%) |
RR [95% CI] | p-value | |
|---|---|---|---|
| Supra- vs. Infra-tentorial Strokes (n=10) | |||
| Supratentorial | 605/2447 (24.7%) | 2.01 [1.49, 2.71] | 0.001 |
| Infratentorial | 53/530 (10.0%) | 1 | |
| Anterior vs. Posterior Territory Strokes (n=18) | |||
| Anterior | 722/3061 (23.6%) | 1.41 [1.13, 1.78] | 0.005 |
| Posterior | 152/1030 (14.8%) | 1 | |
| Cortical vs. Subcortical Strokes (n=8) | |||
| Cortical | 262/1080 (24.3%) | 1.54 [1.25, 1.89] | 0.019 |
| Subcortical | 185/1115 (16.6%) | 1 | |
| Right vs. Left Sided Strokes (n=18) | |||
| Right | 391/1574 (24.8%) | 0.99 [0.77, 1.28] | 0.740 |
| Left | 410/1870 (21.9%) | 1 | |
| OCSP Vascular Classification (n=11) | |||
| Total anterior circulation infarct (TACI) | 162/556 (29.1) | 3.95 [2.57, 6.15] | <0.05 |
| Partial anterior circulation infarct (PACI) | 99/629 (15.7) | 1.50 [0.95, 2.35] | ns |
| Posterior circulation infarct (POCI) | 49/448 (10.9) | 1.25 [0.76, 2.01] | ns |
| Lacunar Infarct (LACI) | 65/977 (6.7) | 1 | Ref |
| TOAST Classifications (n=5) | |||
| Cardioembolic | 86/246 (35.0%) | 2.69 [1.75, 4.51] | <0.05<0.05Ref |
| Large Vessel | 123/355 (34.6%) | 2.57 [1.58, 4.02] | |
| Small Vessel | 58/422 (13.7%) | 1 | |
| Hemorrhagic vs. Ischemic Strokes (n=9) | |||
| Hemorrhagic | 198/578 (34.2%) | 1.74 [1.42, 2.11] | <0.05 |
| Subarachnoid | 21/66 (31.8%) | 1.52 [0.83, 2.31] | ns |
| Ischemic | 641/3326 (19.3%) | 1 | Ref |
| Qualitative Atrophy (n=2) | |||
| Present | 37/199 (18.6%) | 1.66 [1.12, 2.27] | 0.031 |
| Absent | 7/71 (9.9%) | 1 | |
| Leukoaraiosis (n=4) | |||
| Present | 115/629 (18.3%) | 1.33 [0.58; 3.01] | 0.353 |
| Absent | 41/298 (13.8%) | 1 |
The relative risk (RR) of delirium according to different types or localizations of strokes were calculated using random-effects models (ns = not significant, i.e. p>0.05). Ref = reference, to clarify the reference group against which all other groups were compared for the purposes of this table in network meta-analyses across 3 or more groups. In all other cases, an RR value of 1 indicates the reference group.
Major Stroke Locations
Supratentorial versus Infratentorial Lesions
Given the major neuroanatomical differences between supratentorial and infratentorial locations, we first analyzed whether supratentorial or infratentorial strokes were associated with the risk of delirium (n=10 cohorts). Patients with supratentorial strokes had a higher risk of delirium than patients with infratentorial strokes (RR 2.01, CI 1.49-2.72, p<0.001) (Figure 2A). Sensitivity analysis with only high-quality studies yielded similar results (n=7, RR 2.24, CI 1.63-3.08, p<0.001, Supplementary Appendix 7).
Figure 2. Relative Risks of Delirium by Stroke Locations.
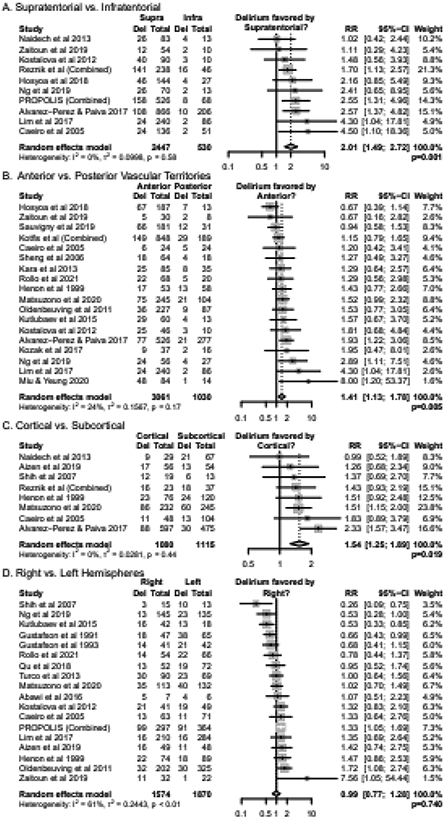
Results of random-effects meta-analysis are displayed as Relative Risks (RR) alongside 95% confidence intervals (CI).
Anterior versus Posterior Vascular Territory
We next analyzed whether the risk of delirium was associated with strokes in anterior (i.e., internal carotid artery, ACA, or MCA) versus posterior (i.e., vertebrobasilar or PCA) circulation territories (n=18 cohorts). Patients with anterior vascular territory strokes had a higher risk of delirium than patients with posterior vascular territory strokes (RR 1.41, CI 1.13-1.78, p=0.005) (Figure 2B). Sensitivity analysis with only high-quality studies yielded similar results (n=11, RR 1.72, CI 1.29-2.30, p=0.002, Supplementary Appendix 7).
Only four studies evaluated the risk of delirium within more specific intracranial arterial territories.8,14-16 Rates of delirium were not significantly different between anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA), and vertebrobasilar (VB) territory strokes (Supplementary Appendix 8).
Cortical versus Subcortical Stroke
We next analyzed the association of cortical or subcortical strokes with delirium (n=8 cohorts). Patients with cortical strokes had a higher risk of delirium than patients with subcortical strokes (RR 1.54, CI 1.25-1.89, p=0.002) (Figure 2C). Sensitivity analysis with only high-quality studies yielded similar results (n=4, RR 1.76, CI 1.19-2.60, p=0.019, Supplementary Appendix 7).
Right versus Left Hemisphere Strokes
We also evaluated whether right or left hemispheric strokes were associated with an increased risk of delirium (n=18 cohorts). There was no statistically significant difference in rates of delirium for right vs. left hemispheric strokes (RR 0.99, CI 0.77-1.28, p=0.930), although there was a high degree of heterogeneity among studies (Figure 2D). Sensitivity analysis with only high-quality studies yielded similar results (n=11, RR 1.04, CI 0.80-1.36, p=0.740, Supplementary Appendix 7).
Clinical Characteristics of Strokes
Lesion Volume
Data for lesion volumes by delirium status was reported in seven cohorts, though using different summary measurements in different patient populations. Three studies reported lesion volumes using medians and interquartile ranges in different populations (one post-procedural,17 one with hemorrhagic strokes,18 and one with ischemic strokes5). Lesions were significantly larger in patients with delirium in only one study5 (1/3). Two studies reported lesion volumes using means: lesions were significantly larger in patients with delirium in both (2/2).19,20 Two studies reported the proportions of patients who had lesions greater than or less than a cutoff volume. In one study using 40 ml as the cutoff, rates of delirium were not clearly significantly different between patients with larger and smaller lesions (p=0.069).15 In the second study using 2.5 ml as the cutoff, delirium was more likely in the patients with larger lesions (p<0.05).16 In summary, delirium was significantly associated with larger lesions in 4/7 studies, but study heterogeneity precluded more formal meta-analysis.
Oxfordshire Community Stroke Project (OCSP) Clinical Subgroups
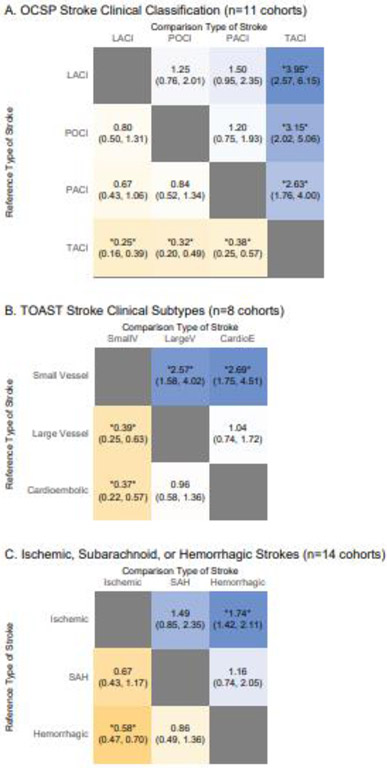
We analyzed whether the risk of delirium varied according to clinically identified stroke subgroups (n=11 cohorts). Patients with total anterior circulation (TACI) infarcts were more likely to have delirium compared to those with partial anterior circulation infarcts (PACI) (RR 2.63, CI 1.76-4.00), posterior circulation infarcts (POCI) (RR 3.15, CI 2.02-5.06), or lacunar infarcts (LACI) (RR 3.95, CI 2.57-6.15). No other comparisons were statistically significant (Figure 3A). Sensitivity analysis with only high-quality studies yielded similar results (Supplementary Appendix 7).
Figure 3. Relative Risks of Delirium by Types of Strokes.
Results of random-effects meta-analysis are displayed as Relative Risks alongside 95% confidence intervals in each cell, demonstrating a comparison between two groups as indicated. *= p<0.05.
Ischemic Stroke Clinical Subtypes
Data were organized by Trial of Org 10172 in Acute Stroke Treatment (TOAST) clinical subtypes, including cardioembolic, large vessel, or small vessel strokes (n=8 cohorts). Compared to small vessel strokes, there was a statistically significantly increased risk for delirium for both large vessel strokes (RR 2.57, CI 1.58-4.02, Figure 3B) and cardioembolic strokes (RR 2.69, CI 1.75-4.51, Figure 3B). Sensitivity analysis with only high-quality studies yielded similar results (Supplementary Appendix 7).
Ischemic versus Hemorrhagic Strokes
We analyzed whether rates of delirium were associated with ischemic or hemorrhagic strokes, including subarachnoid hemorrhage (n=14 cohorts). There was a higher risk of delirium in patients with hemorrhagic strokes than patients with ischemic strokes (RR 1.74, CI 1.42-2.11, Figure 3C). No other comparisons were statistically significant (Figure 3C). Sensitivity analysis with only high-quality studies yielded similar results (Supplementary Appendix 7).
Chronic Pathology
Several studies evaluated whether pre-stroke, chronic pathology was associated with the risk of delirium, specifically atrophy and white matter changes or leukoaraiosis.
Atrophy
When comparing patients with or without evidence of atrophy (n=2 cohorts), there was a higher risk of delirium for patients with atrophy versus those without (RR 1.66, CI 1.21-2.27, p=0.03) (Figure 4A). Neither study met the standards for high-quality for further sensitivity analysis. Five additional studies examined atrophy in patients with and without delirium using ordinal scales, and all found significantly higher atrophy scores in patients with delirium (5/5).5,6,21-23
Figure 4. Relative Risks of Delirium by Chronic Pathology.
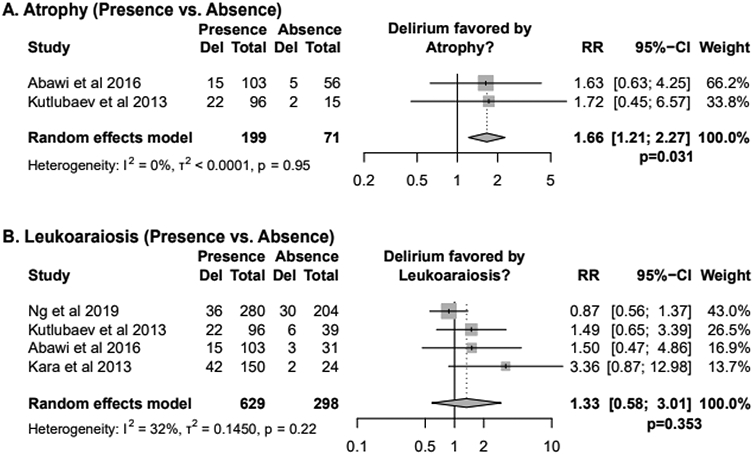
Results of random-effects meta-analysis are displayed as Relative Risks (RR) alongside 95% confidence intervals (CI).
Leukoaraiosis
When comparing patients with and without leukoaraiosis (n=4 cohorts), there was no clear effect of leukoaraiosis on the risk of developing delirium (RR of 1.33, CI 0.58-3.01, p = 0.353) (Figure 4B). Sensitivity analysis with only high-quality studies yielded similar results (n=2, RR 1.45, CI 0.00-5677.15, p=0.672, Supplementary Appendix 7).
Four additional studies examined white matter changes in patients with and without delirium using ordinal scales. One study found significantly greater white matter changes in patients with delirium in deep white matter, but not periventricular white matter.24 Another study found significantly greater white matter changes in patients with delirium in anterior white matter, but not posterior.23 The final two studies did not find significant differences in white matter changes between patients with and without delirium.5,22
DISCUSSION
Our study affirmed that strokes in supratentorial, anterior, and cortical locations were more likely to be associated with delirium, as was more extensive clinical involvement of the anterior circulation. Conversely, there was no association between delirium and right- or left-sided lesions. While delirium is often considered to be a disorder of generalized brain dysfunction, a recent meta-analysis has identified discrete localizations of other cognitive impairments in stroke,25 supporting the hypothesis that for delirium, an acute change in cognition, more specific localization may also be possible. The supratentorial compartment and anterior circulation comprise a broad network of cortical and subcortical structures, but papers did not consistently report more specific localizations within this territory. Further analysis, however, demonstrated that cortical strokes have a higher risk of delirium than subcortical strokes. Cortical networks in the anterior circulation, such as frontoparietal networks, are involved in higher-level cognitive skills including, but not limited to, attention, executive function, and language; deficits in these cognitive areas are core features in delirium. It remains unclear, however, whether anterior circulation strokes directly cause delirium, or rather increase the risk of delirium either by reflecting underlying comorbidities or by predisposing patients to further precipitating factors, such as infection or medication sensitivity. We were unable to evaluate these hypotheses due to the limited and heterogeneous reporting of covariates between studies in the context of localization.
Our meta-analysis demonstrated that there is no clear indication that right hemispheric strokes are more associated with delirium than left hemispheric strokes, despite prior reports.3,6 It is possible that specific locations within the right hemisphere are associated with specific delirium symptoms, for example right MCA infarcts causing inattention in the context of neglect. However, due to the limited availability of combined hemisphere and vascular territory data, we were unable to compare the risk of delirium by more specific territories. Additionally, it remains unclear whether the precipitation of individual delirium symptoms such as inattention necessarily leads to development of the complete delirium syndrome26, reflecting a pathobiological encephalopathy27 superimposed on a vulnerable substrate. While delirium screening in stroke may have additional challenges that may be less common in more general medical contexts, delirium screens as used in the cited stroke studies have been validated according to reference standards28-30 and at times have been adjusted to take into account focal deficits such as aphasia.28
Different symptom domains of delirium may ultimately have different localizations themselves and mapping of specific symptoms to specific focal lesions may yield an incomplete picture of delirium. For example, even aphasia can localize to multiple territories and neural networks, and dysfunction in regions connected to the lesion site rather than the lesion itself may also cause the neurologic deficit. Lesion-associated networks have been critical in understanding lesion-induced symptoms that may not map to a single brain location.31 Such an approach may be even more valuable for delirium, in which broader network consequences of lesions associated with delirium may trigger the more complete syndrome.
In addition to delirium’s associations with the described locations, delirium was also more likely to occur in patients with hemorrhagic strokes than ischemic strokes. The pathophysiology for this unclear, but may relate to additional hypoxic or ischemic dysfunction in tissue surrounding the hemorrhagic injury;32 secondary phenomena, including inflammation, peripheral immune dysregulation, or a propensity for systemic infection;33 or higher rates of intensive care.
More broadly, the pathophysiology of delirium is thought to be a multifactorial interplay between the severity of acute precipitating factors and the susceptibility caused by chronic predisposition and brain vulnerability. Even in the context of acute stroke, it appears that both acute factors, such as more widespread anterior circulation involvement, and chronic factors, such as brain atrophy, are both associated with higher risks of delirium. We were unable to study the quantitative influence of both acute and chronic factors jointly, however, given heterogeneity in reporting stroke volumes and chronic pathology and limitations in how various features were reported independently. Prospective studies are needed to address the interactions between acute and chronic factors, including how any prior history of cognitive impairments may relate to the associations between stroke locations and delirium.
Our systematic review used a rigorous search strategy with strict inclusion and exclusion criteria, offering a representative summary of the published literature evaluating delirium and stroke localization. Our sensitivity analyses strengthened the results by confirming their significance, despite study heterogeneity in how stroke and/or delirium diagnoses were made. As with any meta-analysis and systematic review, however, our results are limited by the studies currently available. Variability in reporting limited some analyses, in particular analysis of the joint and/or independent effects of the identified delirium associations. Furthermore, though there was no statistically significant difference between left and right sided lesions, insufficient data made it difficult to evaluate whether more specific foci within either hemisphere are involved in delirium. Lastly, interactions between specific brain regions and important covariates known to affect delirium in the non-stroke context, such as age, previous cognitive status, and comorbidities, remains a topic for future study. These limitations highlight important considerations for future work and reporting. More rigorous localization using a more sophisticated approach, such as voxel-by-voxel association analyses may allow for greater inferences to be drawn about neural networks and patterns of dysfunction and could be the next step in better understanding the localization of delirium.34
SUMMARY/CONCLUSIONS
This meta-analysis lays the groundwork for anticipating the risk of delirium during acute stroke, based on the stroke location and type, as well as chronic atrophy. While all patients with stroke merit some delirium prevention, better and earlier anticipation of patients with stroke at higher risk of delirium could help target more intensive multicomponent delirium prevention interventions as they continue to be developed35-37. This work also suggests that stroke may be an informative context in which to study the brain networks involved in both the acute precipitating and chronic predisposing factors of the pathophysiology of delirium. Future research using more quantitative imaging and more detailed covariates may clarify whether delirium risk can be localized more precisely within specific anterior cortical locations or networks. A better understanding of specific localization of networks34 involved in delirium may ultimately lead to more further insights into the pathophysiology of clinical delirium in broader clinical contexts.
Supplementary Material
Acknowledgements
The authors would like to thank Lisa Liang Philpotts, Co-Director of the Treadwell Library at Massachusetts General Hospital for assistance in the initial search strategy of the meta-analysis; and Hang Lee, PhD, Associate Professor of Medicine, MGH Biostatics Center, Massachusetts General Hospital, for consultation on statistics related to the meta-analysis.
Funding
Dr. Kimchi has received funding from NIH (K08-MH116135). Dr. Fox has received funding from the NIH (R01MH113929, R21MH126271, and R56AG069086). Dr. Rost has received funding from the NIH (U19NS115388, R01NS086905, and R01NS082285)
The funders were not involved in the work or preparation of this manuscript.
Footnotes
Declaration of Competing Interest
none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RC, Walker G, Elliott E, Quinn TJ. Occurrence Rate of Delirium in Acute Stroke Settings: Systematic Review and Meta-Analysis. Stroke 2019;50:3028–3036. [DOI] [PubMed] [Google Scholar]
- 3.Zaitoun AM, Elsayed DAF, Ramadan BM, Gaffar HAA. Assessment of the risk factors and functional outcome of delirium in acute stroke. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2019;55. [Google Scholar]
- 4.Pasinska P, Wilk A, Kowalska K, Szyper-Maciejowska A, Klimkowicz-Mrowiec A. The long-term prognosis of patients with delirium in the acute phase of stroke: PRospective Observational POLIsh Study (PROPOLIS). J Neurol 2019;266:2710–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu J, Chen Y, Luo G, Zhong H, Xiao W, Yin H. Delirium in the Acute Phase of Ischemic Stroke: Incidence, Risk Factors, and Effects on Functional Outcome. J STROKE CEREBROVASC DIS 2018;27:2641–2647. [DOI] [PubMed] [Google Scholar]
- 6.Oldenbeuving AW, Kort PL, Jansen BP, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology 2011;76:993–999. [DOI] [PubMed] [Google Scholar]
- 7.Shih HT, Huang WS, Liu CH. Confusion or delirium in patients with posterior cerebral arterial infarction. Acta Neurol Taiwan 2007;16:136–142. [PubMed] [Google Scholar]
- 8.Ng BH, Law ZK, Remli R, Tan HJ, Ibrahim NM, Raymond AA, et al. Incidence and risk factors of delirium in patients with acute ischaemic stroke. Neurology Asia 2019;24:295–302. [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. Springer International Publishing, 2015. [Google Scholar]
- 11.Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Medical Research Methodology 2019;19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132–140. [DOI] [PubMed] [Google Scholar]
- 13.Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI, 2020. [Google Scholar]
- 14.Hosoya R, Sato Y, Ishida E, Shibamoto H, Hino S, Yokote H, et al. Association between Delirium and Prehospitalization Medication in Poststroke Patients. J STROKE CEREBROVASC DIS 2018;27:1914–1920. [DOI] [PubMed] [Google Scholar]
- 15.Kostalova M, Bednarik J, Mitasova A, Dusek L, Michalcakova R, Kerkovsky M, et al. Towards a predictive model for post-stroke delirium. Brain Inj 2012;26:962–71. [DOI] [PubMed] [Google Scholar]
- 16.Kotfis K, Bott-Olejnik M, Szylińska A, Rotter I. Could Neutrophil-to-Lymphocyte Ratio (NLR) Serve as a Potential Marker for Delirium Prediction in Patients with Acute Ischemic Stroke? A Prospective Observational Study. J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abawi M, Nijhoff F, Agostoni P, de Vries R, Slooter AJC, Emmelot-Vonk MH, et al. Effect of New Cerebral Ischemic Lesions on the Delirium Occurrence After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;68:1489–1490. [DOI] [PubMed] [Google Scholar]
- 18.Naidech AM, Beaumont JL, Rosenberg NF, Maas MB, Kosteva AR, Ault ML, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med 2013;188:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haight TN, Marsh EB. Identifying Delirium Early after Stroke: A New Prediction Tool for the Intensive Care Unit. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2020;29:105219. [DOI] [PubMed] [Google Scholar]
- 20.Reznik ME, Moody S, Murray K, Costa S, Grory BM, Madsen TE, et al. The impact of delirium on withdrawal of life-sustaining treatment after intracerebral hemorrhage. Neurology 2020;95:e2727–e2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henon H, Lebert F, Durieu I, Godefroy O, Lucas C, Pasquier F, et al. Confusional state in stroke: relation to preexisting dementia, patient characteristics, and outcome. Stroke 1999;30:773–9. [DOI] [PubMed] [Google Scholar]
- 22.Czyzycki M, Glen A, Slowik A, Chrzan R, Dziedzic T. Clinical utility of brain computed tomography in prediction of post-stroke delirium. J Neural Transm (Vienna) 2021;128:207–213. [DOI] [PubMed] [Google Scholar]
- 23.Kutlubaev MA, Bikbulatova LF, Akhmadsmall ie CLR. [Early diagnosing of delirium in the elderly with acute stroke]. Adv Gerontol 2015;28:493–499. [PubMed] [Google Scholar]
- 24.Rollo E, Callea A, Brunetti V, Vollono C, Marotta J, Imperatori C, et al. Delirium in acute stroke: A prospective, cross-sectional, cohort study. European journal of neurology 2021;28:1590–1600. [DOI] [PubMed] [Google Scholar]
- 25.Weaver NA, Kuijf HJ, Aben HP, Abrigo J, Bae HJ, Barbay M, et al. Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 10.1016/S1474-4422(21)00060-0. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. 5 edition. Washington, D.C: American Psychiatric Publishing, 2013. [Google Scholar]
- 27.Slooter AJC, Otte WM, Devlin JW, Arora RC, Bleck TP, Claassen J, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med 2020;46:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reznik ME, Drake J, Margolis SA, Moody S, Murray K, Costa S, et al. Deconstructing Poststroke Delirium in a Prospective Cohort of Patients With Intracerebral Hemorrhage. Crit Care Med 2020;48:111–118. [DOI] [PubMed] [Google Scholar]
- 29.Gusmao-Flores D, Salluh JIF, Chalhub RÁ, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 2012;16:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MB, Bednarik J, Lee P, Shehabi Y, Salluh JI, Slooter AJ, et al. Delirium Monitoring in Neurocritically Ill Patients: A Systematic Review. Crit Care Med 2018;46:1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MD. Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med 2018;379:2237–2245. [DOI] [PubMed] [Google Scholar]
- 32.Ostrowski RP, Stepien K, Pucko E, Matyja E. The efficacy of hyperbaric oxygen in hemorrhagic stroke: experimental and clinical implications. Arch Med Sci 2017;13:1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saand AR, Yu F, Chen J, Chou SH. Systemic inflammation in hemorrhagic strokes - A novel neurological sign and therapeutic target? J Cereb Blood Flow Metab 2019;39:959–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naidech AM, Polnaszek KL, Berman MD, Voss JL. Hematoma Locations Predicting Delirium Symptoms After Intracerebral Hemorrhage. Neurocrit Care 2016;24:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. Am J Geriatr Psychiatry 2018;26:1015–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice KL, Bennett MJ, Berger L, Jennings B, Eckhardt L, Fabré-LaCoste N, et al. A Pilot Randomized Controlled Trial of the Feasibility of a Multicomponent Delirium Prevention Intervention Versus Usual Care in Acute Stroke. J Cardiovasc Nurs 2017;32:E1–E10. [DOI] [PubMed] [Google Scholar]
- 37.Brown EG, Josephson SA, Anderson N, Reid M, Lee M, Douglas VC. Evaluation of a multicomponent pathway to address inpatient delirium on a neurosciences ward. BMC Health Serv Res 2018;18:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aizen E, Yalonnitsky I, Zalyesov E, Shugaev I. Cognitive and functional outcomes in elderly patients with post-stroke delirium. Aging Medicine and Healthcare 2019;10:122–127. [Google Scholar]
- 39.Alvarez-Perez FJ, Paiva F. Prevalence and Risk Factors for Delirium in Acute Stroke Patients. A Retrospective 5-Years Clinical Series. J Stroke Cerebrovasc Dis 2017;26:567–573. [DOI] [PubMed] [Google Scholar]
- 40.Caeiro L, Menger C, Ferro JM, Albuquerque R, Figueira ML. Delirium in acute subarachnoid haemorrhage. Cerebrovasc Dis 2005;19:31–8. [DOI] [PubMed] [Google Scholar]
- 41.Gustafson Y, Olsson T, Eriksson S, Asplund K, Bucht G. Acute Confusional States (Delirium) in Stroke Patients. Cerebrovasc Dis 1991;1:257–264. [Google Scholar]
- 42.Gustafson Y, Olsson T, Asplund K, Hagg E. Acute Confusional State (Delirium) Soon after Stroke is Associated with Hypercortisolism. Cerebrovasc Dis 1993;3:33–38. [Google Scholar]
- 43.Kara H, Bicakci S, Over MF, Calis N, Bicakci YK, Ozeren A, et al. Acute Confusional State at Early Stage of Stroke. Journal of Neurological Sciences [Turkish] 2013;30:21–29. [Google Scholar]
- 44.Kotfis K, Bott-Olejnik M, Szylinska A, Listewnik M, Rotter I. Characteristics, Risk Factors And Outcome Of Early-Onset Delirium In Elderly Patients With First Ever Acute Ischemic Stroke - A Prospective Observational Cohort Study. Clin Interv Aging 2019;14:1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozak HH, Uguz F, Kilinc I, Uca AU, Serhat Tokgoz O, Akpinar Z, et al. Delirium in patients with acute ischemic stroke admitted to the non-intensive stroke unit: Incidence and association between clinical features and inflammatory markers. Neurol Neurochir Pol 2017;51:38–44. [DOI] [PubMed] [Google Scholar]
- 46.Kutlubaev MA, Akhmadeeva LR, Bikbulatova LF. [Delirium in the acute phase of stroke: frequency and predisposing factors]. Zh Nevrol Psikhiatr Im S S Korsakova 2013;113:37–41. [PubMed] [Google Scholar]
- 47.Lim TS, Lee JS, Yoon JH, Moon SY, Joo IS, Huh K, et al. Cigarette smoking is an independent risk factor for post-stroke delirium. BMC Neurol 2017;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuzono K, Mashiko T, Ozawa T, Miura K, Suzuki M, Furuya K, et al. Management Effort for Delirium and Insomnia in Patients with Acute Ischemic Stroke (MEDIAS) Study. Psychiatry and clinical neurosciences 2020;74:279–280. [DOI] [PubMed] [Google Scholar]
- 49.Miu DK, Yeung JC. Incidence of post-stroke delirium and 1-year outcome. Geriatr Gerontol Int 2013;13:123–9. [DOI] [PubMed] [Google Scholar]
- 50.Pasinska P, Kowalska K, Klimiec E, Szyper-Maciejowska A, Wilk A, Klimkowicz-Mrowiec A. Frequency and predictors of post-stroke delirium in PRospective Observational POLIsh Study (PROPOLIS). J Neurol 2018;265:863–870. [DOI] [PubMed] [Google Scholar]
- 51.Pasinska P, Kowalska K, Klimiec E, Wilk A, Szyper-Maciejowska A, Dziedzic T, et al. Poststroke Delirium Clinical Motor Subtypes: The PRospective Observational POLIsh Study (PROPOLIS). J Neuropsychiatry Clin Neurosci 2019;31:104–111. [DOI] [PubMed] [Google Scholar]
- 52.Klimiec-Moskal E, Lis A, Pera J, Slowik A, Dziedzic T. Subsyndromal delirium is associated with poor functional outcome after ischaemic stroke. Eur J Neurol 2019;26:927–934. [DOI] [PubMed] [Google Scholar]
- 53.Klimiec E, Kowalska K, Pasinska P, Klimkowicz-Mrowiec A, Szyper A, Pera J, et al. Pre-stroke apathy symptoms are associated with an increased risk of delirium in stroke patients. Sci rep 2017;7:7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reimann F, Rinner T, Lindner A, Kofler M, Ianosi BA, Schiefecker AJ, et al. Hyperactive delirium in patients after non-traumatic subarachnoid hemorrhage. Journal of critical care 2021;64:45–52. [DOI] [PubMed] [Google Scholar]
- 55.Reznik ME, Kalagara R, Moody S, Drake J, Margolis SA, Cizginer S, et al. Common biomarkers of physiologic stress and associations with delirium in patients with intracerebral hemorrhage. Journal of critical care 2021;64:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauvigny T, Mohme M, Grensemann J, Duhrsen L, Regelsberger J, Kluge S, et al. Rate and risk factors for a hyperactivity delirium in patients with aneurysmal subarachnoid haemorrhage. Neurosurg Rev 2019;42:481–488. [DOI] [PubMed] [Google Scholar]
- 57.Sheng AZ, Shen Q, Cordato D, Zhang YY, Yin Chan DK. Delirium within three days of stroke in a cohort of elderly patients. J Am Geriatr Soc 2006;54:1192–8. [DOI] [PubMed] [Google Scholar]
- 58.Turco R, Bellelli G, Morandi A, Gentile S, Trabucchi M. The Effect of Poststroke Delirium on Short-Term Outcomes of Elderly Patients Undergoing Rehabilitation. Journal of Geriatric Psychiatry and Neurology 2013;26:63–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not published within this article will be made available by request from qualified investigators.