Abstract
Background:
For patients with acute, serious neurological conditions presenting to the emergency department (ED), prognostication is typically based on clinical experience, scoring systems and patient co-morbidities. Because estimating a poor prognosis influences caregiver decisions to withdraw life-sustaining therapy, we investigated the consistency of prognostication across a spectrum of neurology physicians.
Methods:
Five acute neurological presentations (2 with large hemispheric infarction; 1 with brainstem infarction, 1 with lobar hemorrhage, and 1 with hypoxic-ischemic encephalopathy) were selected for a department-wide prognostication simulation exercise. All had presented to our tertiary care hospital’s ED, where a poor outcome was predicted by the ED neurology team within 24 hours of onset. Relevant clinical, laboratory and imaging data available before ED prognostication were presented on a web-based platform to 120 providers blinded to the actual outcome. The provider was requested to rank-order, from most to least likely, the predicted 90-day modified Rankin Scale (mRS) score. To determine the accuracy of individual outcome predictions we compared the patient’s the actual 90-day mRS score to highest ranked predicted mRS score. Additionally, the group’s “weighted” outcomes, accounting for the entire spectrum of mRS scores ranked by all respondents, were compared to the actual outcome for each case. Consistency was compared between pre-specified provider roles: neurology trainees versus faculty; non-vascular versus vascular faculty.
Results:
Responses ranged from 106–110 per case. Individual predictions were highly variable, with predictions matching the actual mRS scores in as low as 2% of respondents in one case and 95% in another case. However, as a group, the weighted outcome matched the actual mRS score in 3 of 5 cases (60%). There was no significant difference between subgroups based on expertise (stroke/neurocritical care versus other) or experience (faculty versus trainee) in 4 of 5 cases.
Conclusion:
Acute neuro-prognostication is highly variable and often inaccurate among neurology providers. Significant differences are not attributable to experience or subspecialty expertise. The mean outcome prediction from group of providers (“the wisdom of the crowd”) may be superior to that of individual providers.
Keywords: neuroprognostication, neurological emergencies, outcome prediction, brain injury
Introduction
Neurologists are consulted in the emergency department (ED) and intensive care units to estimate prognosis, guide management, and participate in goals-of-care discussions. In acute settings such as in the ED, prognostication is particularly challenging because of factors such as limited knowledge of disease evolution, or patient preferences. Outcome prediction tools are often used to drive end of life decision-making, yet they are not designed for such use. Traditional prognostic scoring methods have focused on subacute or 1-year survival or short-term functional outcomes.(1–4) However, families or other surrogate decision-makers value long-term prognostic information in addition to mortality predictions.(5,6) Many prognostic scoring systems are limited by lack of incorporation of in-hospital parameters and further confounded by the “self-fulfilling prophecy,” in which life-sustaining measures are withdrawn prematurely.(7,8)
Acute neuro-prognostication has a profound impact on decision making and therefore mortality and is commonly based on a combination of prognostic scales, test performance and experience(9,10) in the context of known patient goals of care. Prediction of poor outcome often results in withdrawal of life-sustaining therapy (WLST) and withholding potentially efficacious life-saving treatments.(11) When acting as surrogate decision-makers, families face a multitude of concerns about a patient’s wishes and implications of medical decision making.(12,13) Surrogate decision makers often rely on neurologists to communicate their estimation of recovery and to recommend a direction of care, as patients often lack the capacity to communicate their preferences.(14)
Despite the frequency of this consultation question, the accuracy of early prognostication in the emergency neurology setting is not well studied. Prospective studies are limited because prediction of poor outcome and communication of that prognosis undoubtedly influences the plan of care, such that a poor prognosis may result in refusing treatments and a self-fulfilling prophecy of a poor outcome.(15–17) In this study, we sought to investigate prognostic accuracy among providers with different levels of experience and subspecialty training.
Methods
Study Design
All physicians (trainees and faculty) in the Neurology Department at Massachusetts General Hospital were invited to participate in an online simulation exercise. Five clinical cases of acute neurological injury were presented via a web-based platform to 120 providers. Physicians were instructed to provide estimates of 90-day outcome.
Clinical Cases and Vignettes
Cases were selected from patients presenting to our ED with acute, severe neurological conditions in which an estimate of long-term prognosis and management decisions were made within 24 hours of symptom onset by a neurology consultant and the patients had 90-day outcome information available. Cases were selected to represent typical presentations of acute neurological injury as well as atypical presentations that are often seen at academic medical centers. Information provided in clinical vignettes for this web-based simulation exercise reflected information available to neurology consultants at the time of evaluation in the ED, including presenting symptoms, past medical history, clinical examination findings, acute clinical course, laboratory test results, and key images from the cardiac, neuroimaging and electro-encephalogram studies obtained in the ED. To avoid confounding from WLST based on acute decision-making, we selected patients where care was not withdrawn, and actual 90-day outcomes were available to compare against the predicted outcomes.
Outcomes
Based on available information, physicians were requested to rank-order the patient’s predicted modified Rankin Scale (mRS) score at 90 days. The mRS is a commonly used scale to measure the degree of disability or functional independence in daily life, with 0 representing no disability and 6 representing death.(18) Participants were given five options for the 90-day mRS score: 0–2 (none or mild disability), 3 (moderate disability, able to ambulate), 4 (moderate disability, unable to ambulate), 5 (severe disability), or 6 (death). For each case, the participant ranked their predicted outcome for all 5 options, from most likely (rank 1) to least likely (rank 5). Interclass correlation (ICC) was used to compare rater agreement between predefined subgroups: faculty in the stroke/neurocritical care division, faculty in other neurology divisions; trainees (residents and fellows). We additionally requested rank-ordered recommendations for acute management to confirm concordance with their predicted functional outcomes: intensive comfort measures now; defer prognostication for 48–72 hours while offering medical care only; treat aggressively including surgical intervention.
Data analysis
Responses were tabulated and each case was analyzed separately. There was strong agreement between the predicted mRS score and the recommended acute management, so our subsequent analysis focused only on the predicted mRS scores.
Provider-level predicted mRS outcomes across the full range of mRS scores were captured on a ranked scale of 1–5. The ranks were “weighted” by using their reciprocals on a scale from 0.2 to 1 where 1 represents the most favored response and 0.2 represents the least favored. Thus, for case 1, if provider A’s predicted 90-day mRS scores (in rank order from 1 to 5) were 5, 6, 4, 3, and 0–2, then mRS 5 received score 1, mRS 6 received score 0.5, mRS 4 received score 0.33, and so on. For provider B, if the predicted 90-day mRS scores for case 1 (ranked from 1 to 5) were 4, 5, 6, 0–2, and 3, then mRS 4 received score 1, mRS 5 received score 0.5, mRS 6 received score 0.33, and so on. For provider C, if the predicted 90-day mRS scores for case 1 (ranked from 1 to 5) were also 4, 5, 6, 0–2, and 3, then mRS 4 received score 1, mRS 5 received score 0.5, mRS 6 received score 0.33, and so on. Across the 3 providers A, B and C, the 90-day mRS score 4 received an average rank score 0.78 (i.e., 0.33+1+1 divided by 3); mRS score 5 received an average score of 0.67 (i.e., 1+0.5+0.5 divided by 3); and mRS 6 received an average score of 0.39 (i.e., 0.5+0.33+0.33 divided by 3), and so on; mRS score 4 was therefore the group’s collective predicted outcome at 90 days. The highest average reciprocal rank (i.e., weighted) scores across all providers were used to determine the group’s predicted 90-day mRS scores, ranked 1–5, for each of the 5 cases.(19)
Respondent data were classified according to level of training (trainee versus faculty) and subspecialty expertise (stroke/neurocritical care, versus other neurological subspecialties). The χ2 test was utilized to compare predicted outcomes against the actual 90-day outcome. Pearson’s correlation co-efficient was used to assess inter-class correlation with statistical significance considered at a p value <0.05. Pre-planned secondary analysis of dichotomized mRS was performed using Pearson’s correlation coefficient with clinical outcome categorized as favorable (mRS 0–4) or unfavorable (mRS 5–6).(20,21) Analyses were conducted using Stata version 13.1 (StataCorp, College Station, Texas 77845 USA).
Standard protocol approvals, registrations, and patient consents
This study was undertaken as a quality-improvement project to understand variability in acute prognostication and end-of-life decision making. Only de-identified data were used to protect patient confidentiality. Hence this study was not formally supervised by our Institutional Review Board as per their policies.
Data availability statement
All data used for analysis are presented in the tables and figures in this article. Anonymized data will be shared if requested by other investigators for the purpose of replicating the results.
Classification of evidence
This study provides Class IIb evidence about the accuracy of acute neuroprognostication.
Results
A total of 113 trainees and faculty members from the Department of Neurology provided responses in the web-based simulation exercise with respondents per case ranging from 106 to 110. The cases with predicted and actual outcomes are summarized below.
Case 1
A 43-year-old woman developed sudden inappropriate laughter followed by rapidly progressive quadriplegia and somnolence. Admission brain MRI showed a large acute infarct in the bilateral pons and medulla, and head-neck CT angiography (CTA) showed left vertebral artery dissection and basilar artery occlusion (Figure 1A). She was outside the time windows for intravenous thrombolytic and intra-arterial clot retrieval. Neurological examination findings were consistent with the ‘locked in’ syndrome (preserved vertical eye movements, quadriplegia).
Figure 1:
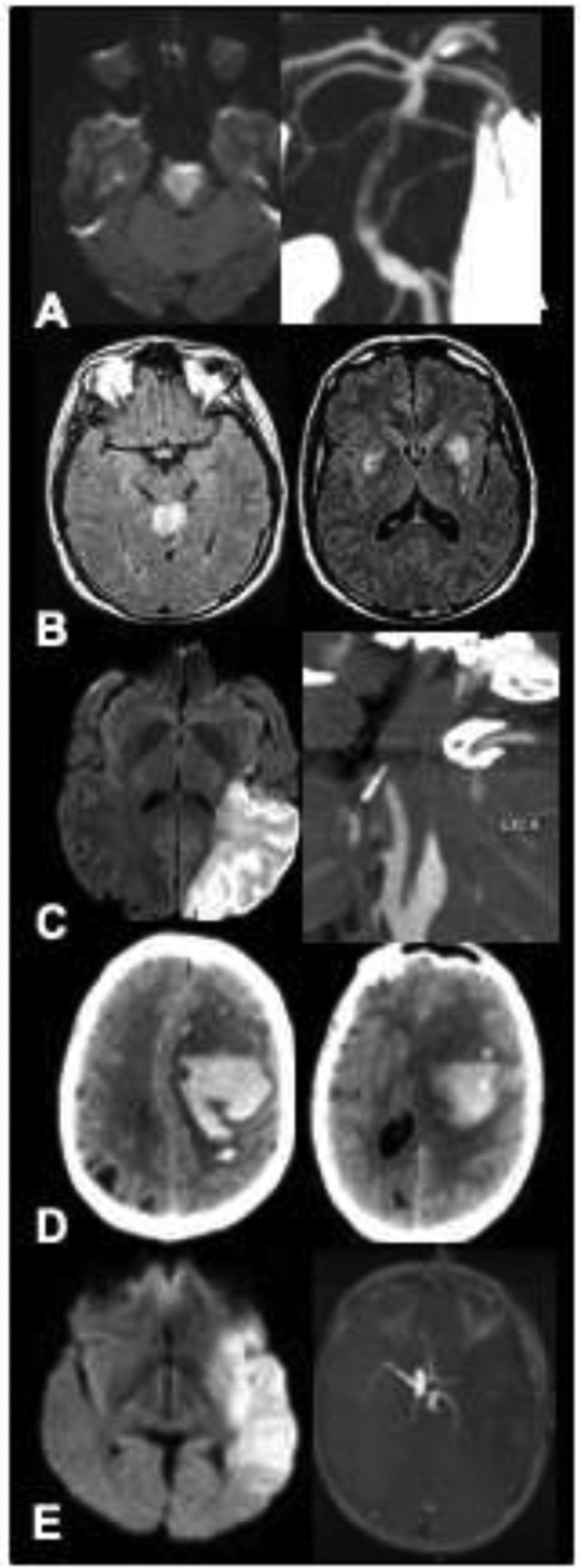
(A) Case 1: brain MRI with diffusion restriction in the pons and medulla (left); CTA of the head and neck with left vertebral dissection and basilar thrombus (right). (B) Case 2: brain MRI with FLAIR abnormalities in the cerebellar vermis (left); brain MRI with FLAIR abnormalities in the bilateral globus pallidus and caudate nuclei (right). (C) Case 3: brain MRI with large left MCA infarct on DWI (left); CTA with left internal carotid artery dissection (right). (D) Case 4: head CT with 70 cc lobar hemorrhage with extension into the subarachnoid space (left) and 11 mm midline shift (right). (E) Case 5: brain MRI with large infarct in left MCA territory (left); head CTA with proximal left MCA occlusion (right). Abbreviations: MRI, magnetic resonance imaging; CT, computerized tomography; CTA, computerized tomography angiogram; FLAIR, Fluid-attenuated inversion recovery; DWI, diffusion weighted imaging; MCA, middle cerebral artery.
Predicted outcome:
Most respondents (94%) listed mRS score 5 as their highest ranked 90-day mRS outcome. None (0%) ranked mRS 0–2; none (0%) ranked mRS 3, and only 2% ranked mRS 4 as the highest (most likely) predicted 90-day outcome. The highest group weighted outcome was 0.97 for 90-day mRS score 5 (Figure 2A).
Figure 2:
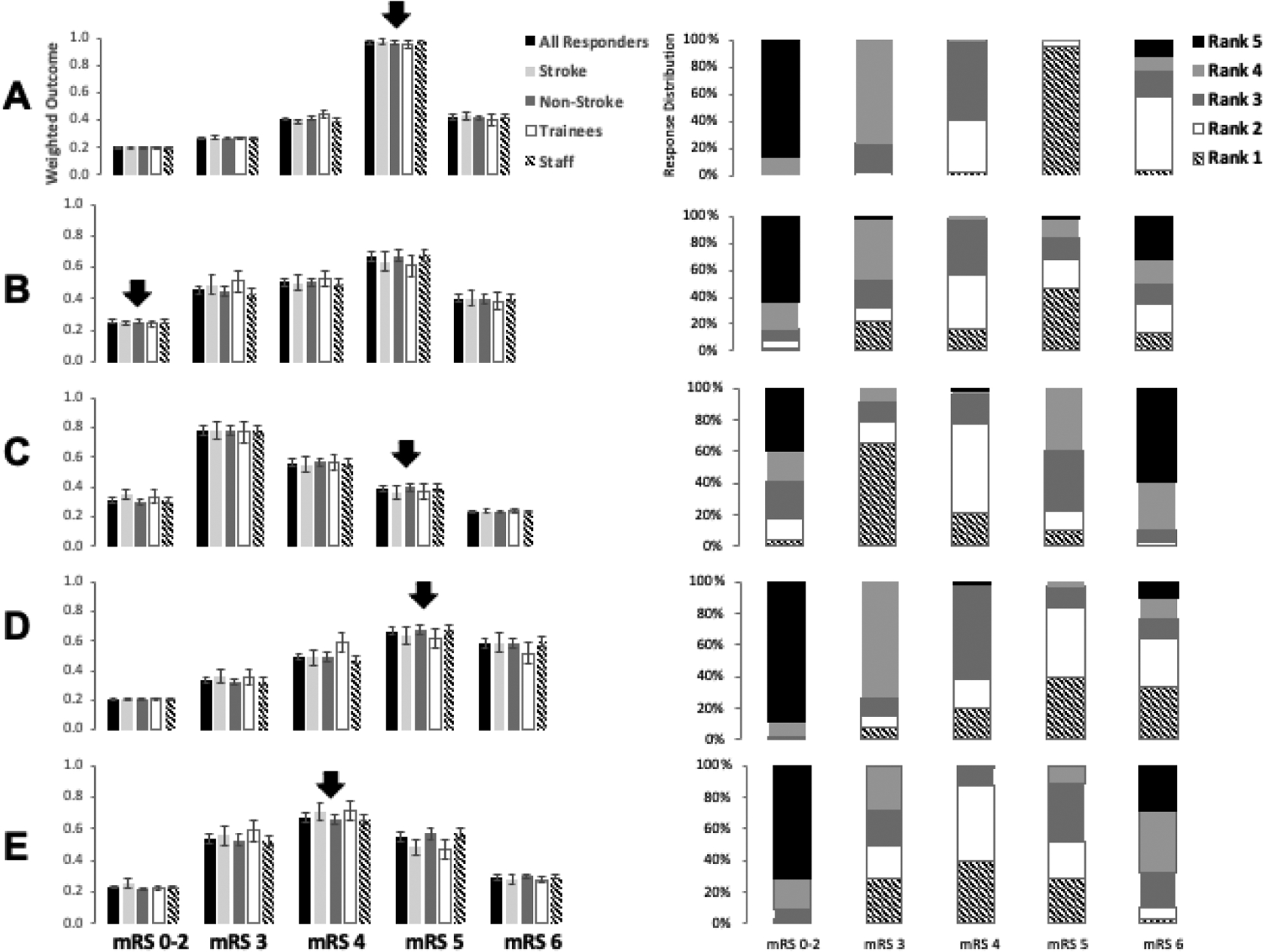
Left, Weighted outcome of modified Rankin scale (mRS) in Cases 1 through 5 (A thru E, respectively) with the arrow corresponding to the actual 90-day mRS score. Right, Distribution of responses by individual rank.
Actual outcome:
Decision-making about WLST was deferred for 3 weeks per family preference. The patient, with assistance from speech therapists and by using eye movements and letter boards, repeatedly and consistently communicated her strong desire to continue medical care. She was discharged to a nursing facility and remained with severe disability at 90 days, mRS 5 (Figure 2A). However, over the next decade this patient became partly independent with the assistance of a robotic arm device and wheelchair enabled by a brain-computer interface device. She became a brain injury advocate, and her story has been documented publicly. Notably, she reported that she was content with the choices she made.
Case 2
A 33-year-old fisherman was found unconscious in his boat 4 days after leaving for a fishing trip. Two accompanying fishermen were found dead in the cabin; their bodies were adjacent to a pile of decomposing squid. Shortly thereafter the patient had a witnessed generalized seizure. He was intubated, and eventually transferred to our ED. On arrival, he was comatose with intact brainstem reflexes, including eye movements and pupillary reaction to light. There were no spontaneous limb movements, no myoclonus or overt seizure activity. Brain MRI showed symmetric ischemic lesions on diffusion-weighted imaging (DWI) and T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences in the cerebellar vermis, globus pallidus, and caudate nuclei (Figure 1B). An EEG showed a burst-suppression pattern with minimal alteration in background rhythm to stimulation. The bursts contained rare isolated generalized sharp waves and generalized diffuse spindle-like activities. He was diagnosed with anoxic encephalopathy due to inhalation of hydrogen sulfide (a mitochondrial toxin, similar to hydrogen cyanide and carbon monoxide, which is released by decomposing squid).
Predicted outcome:
Only 29% of respondents ranked mRS 5 as their highest (most likely) mRS score at 90 days. Only 2% ranked mRS 0–2; 22% ranked mRS 3, 9% ranked mRS 4 as the highest (most likely) predicted 90-day mRS score. The highest group weighted outcome was 0.66 for 90-day mRS score 5 (Figure 2B).
Actual outcome:
The patient’s family opted to pursue medical care, partly because the patient’s wife was in the final stages of pregnancy. He received ventilatory support and was treated for several weeks in the intensive care unit and inpatient floors. He regained consciousness after 3 weeks and was later able to interact with his wife, who had delivered healthy twins. At the time of discharge, he had a cerebellar tremor and an unsteady gait but was able to walk with 2-person assistance and had mild cognitive deficits. Upon 90-day follow up, his mRS score was 2 (Figure 2B).
Case 3
A 41-year-old man had a witnessed collapse on the sidewalk while walking at 09:00 AM. Paramedics transported him to a nearby hospital where he was found to be aphasic with right hemiplegia. Head and neck CTA showed left internal carotid artery (ICA) dissection and early ischemic changes in the left middle cerebral artery (MCA) territory (Figure 1C). Intravenous tissue plasminogen activator was withheld due to the large size of the ischemic lesion. He was transferred to our hospital with persistent deficits and a National Institutes of Health Stroke Scale score of 12. Repeat CT/CTA 4.5 hours after onset showed findings similar to the prior scan; brain MRI showed an established large left MCA infarct, and head/neck MR-angiography showed occlusion of the cavernous left ICA and the distal left MCA. He was deemed ineligible for intra-arterial clot retrieval because of large infarct size.
Predicted outcome:
65% of respondents listed mRS 3 as their highest ranked mRS score at 90 days; 4% ranked mRS 0–2; 21% ranked mRS 4, and 10% ranked mRS 5 as the highest (most likely) predicted 90-day mRS score. The highest group weighted outcome was 0.78 for 90-day mRS score 3 (Figure 2C).
Actual outcome:
Maximal medical management was pursued. He remained aphasic with right hemiparesis, and the 90-day mRS score was 5 (Figure 2C).
Case 4
A 57-year-old woman with chronic hypertension and diabetes developed sudden headache, difficulty speaking, and right-sided weakness. Head CT showed a 70-cc left hemispheric lobar hemorrhage with extension into the subarachnoid space, and 11-mm midline shift (Figure 1D). She was treated with intravenous mannitol, levetiracetam, intubated, and transferred to our hospital via med flight. En route she developed fixed pupils, hypertension and bradycardia. On arrival to our ED, 23% saline was administered, with improvement in neurological status. She was now somnolent but arousable to pinch, had weak eye opening to pain, followed 1-step commands (e.g., show 2 fingers on the left, wiggle toes), localized pain with the left arm, but had no movement and no withdrawal to pain of the right arm and leg. Emergent CTA and transfemoral cerebral angiography showed no underlying vascular lesions. She was admitted to the ICU and continued on hyperosmolar therapy. A follow-up head CT showed interval increase in size of the brain hemorrhage with increased mass effect (midline shift now 18 mm), and evidence for left uncal herniation (Figure 1D).
Predicted outcome:
40% of respondents listed mRS 5 as their highest (most likely) predicted 90-day mRS score. 34% respondents ranked mRS 6; 0% ranked mRS 0–2; 8% ranked mRS 4 as the highest (most likely) predicted outcome at 90 days. The highest group weighted outcome was 0.67 for 90-day mRS score 5 (Figure 2D).
Actual outcome:
The patient eventually underwent hematoma evacuation and hemicraniectomy. A pathological specimen obtained at the time of surgery showed cerebral amyloid angiopathy. Her family declined tracheostomy and gastric tube placement for artificial nutrition, and she was later discharged to a hospice facility with an mRS 5 (Figure 2D).
Case 5
A 38-year-old right-handed woman was witnessed by her husband to develop global aphasia and dense right hemiplegia. She had reported a mild frontal headache for one week. She was transported to a local hospital, where head CT obtained 50 minutes after onset showed a dense left MCA sign but no parenchymal lesions or early ischemic signs. Head/neck CTA showed left ICA dissection with embolic occlusion of the proximal left MCA. Brain MRI obtained 1.5 hours post-onset showed a large infarction involving the left frontal, temporal, and parietal lobes. An emergent neurology telestroke video-consultation was obtained; she was deemed ineligible for thrombolysis and transferred to our tertiary care center. Upon arrival here she was drowsy, had left gaze deviation, global aphasia, and dense right hemiplegia. The admission NIHSS score was 20. Repeat CT/CTA showed left ICA dissection with a tapering occlusion; left MCA (M1) occlusion; and a large established infarct in almost the entire left MCA territory (Figure 1E). A follow up brain MRI showed a large left MCA infarct without mass effect. She was deemed ineligible for intra-arterial clot retrieval therapy due to the large infarct volume. She was admitted to the ICU on hyperosmolar therapy, with plans to monitor closely for signs of herniation and worsening neurological exam.
Predicted outcome:
39% of respondents ranked mRS 4 as the highest (most likely) mRS score at 90 days; 1% ranked mRS 0–2; 29% ranked mRS 3; 29% ranked mRS 5; 3% ranked mRS 6 as the highest (most likely) predicted outcome at 90 days. The weighted outcome was 0.67 for 90-day mRS score 4 (Figure 2E).
Actual outcome:
She received maximal medical management and improved such that she was able to repeat phrases and follow commands, but she continued to have a significant right hemiparesis. She was discharged to an inpatient rehabilitation hospital. Her mRS score at 90 days was 4 (Figure 2E). Of note, this case was encountered prior to the publication of trials showing efficacy of decompressive hemicraniectomy.(22)
Group-level Outcome Prediction
The cases were sent to 120 neurologists at different stages of training and different areas of specialization with 113 of those neurologists providing responses to cases. Twenty-eight participants had stroke or neurocritical care subspecialty training, while 82 were in other subspecialties or general neurology. Twenty-two participants were undergoing training (residency or fellowship), and 88 were attending physicians.
Across all cases, the group’s collective median 90-day mRS score prediction matched the actual score in 3 of 5 cases (60%) (Cases 1, 4 and 5, see Table 1). Interestingly, in Case 3, which involved a young person with a left hemispheric stroke, the group collectively predicted a better outcome by a difference of 2 on the mRS, but in Case 2, which involved another young person with bilateral acute brain injury, the group collectively predicted a worse outcome by a difference of 3 on the mRS. There was no significant difference between subgroups based on expertise (stroke/neurocritical care versus other) or experience (faculty versus trainee (Table 2). Interclass correlation (ICC), used to compare rater agreement between sub-groups, showed moderate correlation among groups between cases: all groups (0.42), neurocritical care and stroke specialists (0.45), other neurology specialties (0.42), attendings (0.43), and residents (0.56) (Table 3).
Table 1:
Comparison of predicted versus actual 90-day mRS score
| Case No. | Total N, Respondents | Actual 90-day Outcome | Accurate prediction | Most frequent prediction | Group weighted outcome |
|---|---|---|---|---|---|
| 1 | 109 | mRS 5 | n=104 (95%) | mRS 5 (n=104, 95%) | 0.97 for mRS 5 |
| 2 | 110 | mRS 2 | n=2 (2%) | mRS 5 (n=51, 46%) | 0.66 for mRS 5 |
| 3 | 104 | mRS 5 | n=11 (10%) | mRS 3 (n=68, 65%) | 0.78 for mRS 3 |
| 4 | 106 | mRS 5 | n=42 (40%) | mRS 5 (n=42, 40%) | 0.67 for mRS 5 |
| 5 | 106 | mRS 4 | n=42 (40%) | mRS 4 (n=42, 40%) | 0.67 for mRS 4 |
Abbreviation: mRS, modified Rankin Scale
Table 2:
Comparison of 90-day mRS outcome prediction by subspecialty and level of training.
| Case | N | Actual 90-d mRS | Neurocritical Care / Stroke | Other Sub-specialty | P-value | Attending | Trainee | P-value |
|---|---|---|---|---|---|---|---|---|
| 1 | 109 | 5 | 26/28 (93%) | 77/81 (95%) | 0.22 | 84/88 (95%) | 19/21 (90%) | 0.44 |
| 2 | 110 | 2 | 0/28 (0%) | 2/82 (2%) | 0.86 | 2/88 (2%) | 0/22 (0%) | 0.77 |
| 3 | 104 | 5 | 2/27 (7%) | 9/77 (12%) | 0.66 | 9/85 (11%) | 2/19 (11%) | 0.30 |
| 4 | 106 | 5 | 10/27 (37%) | 32/79 (41%) | 0.17 | 36/87 (41%) | 6/19 (32%) | 0.59 |
| 5 | 106 | 4 | 13/27 (48%) | 29/79 (37%) | 0.12 | 32/86 (37%) | 10/20 (50%) | 0.10 |
Note: N/N (%) indicates number (%) with accurate outcome predictions (numerator) from the total number of respondents in that category (denominator)
Table 3:
Interclass correlation among groups
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | ICC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | mean | SD | N | mean | SD | N | mean | SD | N | mean | SD | N | mean | SD | ||
| All | 109 | 5.02 | 0.23 | 110 | 4.49 | 1.00 | 104 | 3.38 | 0.73 | 106 | 4.99 | 0.92 | 106 | 4.03 | 0.85 | 0.42 |
| By sub-specialty | ||||||||||||||||
| Neurocritical / Stroke | 28 | 5.00 | 0.27 | 28 | 4.5 | 1.04 | 27 | 3.33 | 0.68 | 27 | 5.04 | 0.94 | 27 | 3.85 | 0.86 | 0.45 |
| Other subspecialties | 81 | 5.02 | 0.22 | 82 | 4.48 | 1.04 | 77 | 3.39 | 0.75 | 79 | 5.00 | 0.91 | 79 | 4.1 | 0.84 | 0.42 |
| By position | ||||||||||||||||
| Attending | 88 | 5.02 | 0.21 | 88 | 4.52 | 1.03 | 85 | 3.39 | 0.73 | 87 | 5.06 | 0.89 | 86 | 4.07 | 0.88 | 0.43 |
| Fellow | 10 | 5.10 | 0.32 | 12 | 4.5 | 1.24 | 11 | 3.64 | 0.81 | 11 | 4.73 | 1.01 | 12 | 4.08 | 0.79 | 0.23 |
| Resident | 11 | 4.91 | 0.30 | 11 | 4.18 | 0.87 | 9 | 3.00 | 0.5 | 9 | 4.67 | 1 | 9 | 3.56 | 0.53 | 0.56 |
Discussion
We selected 5 cases of emergent neurological injuries to evaluate the accuracy and consistency of outcome predictions by neurologists at various levels of training and subspecialty. Our results show that prognostication in acute neurological injury, especially within 24 hours, is often inaccurate. Neurologists, regardless of level of training or area of expertise, often predicted similar outcomes for patients, with a tendency to predict outcomes that were more pessimistic than the actual 90-day outcomes. Subgroups within the neurology community (trainees, faculty, stroke/ICU faculty) had no statistically significant difference in outcome predictions.
The results of this study highlight several interesting phenomena in clinical application of prognostication. First, physicians performed poorly in estimating functional outcomes and mortality. The results of our study show that the outcome predictions made by individuals across providers of all levels of subspecialty training and years of experience are highly variable and are very often accurate. Our results indicate that individual responders were inaccurate in predicting specific levels of disability and predicting severe disability or death across all cases.
Ranked responses of the group, taken collectively through weighted outcome, reflected accuracy of the weighted group response in 3 of 5 cases in our study. Our study’s use of highest weighted outcome, based on respondent’s ranked outcome scores, takes into account the full spectrum of outcome possibilities and can also demonstrate directionality of disability prediction. For example, if the highest weight outcome reflects an mRS of 4 and the second highest weighted outcome reflects an mRS of 5, then that would indicate a trend towards greater disability.
Studies have also shown that predictive accuracy does not correspond to the individual’s level of confidence Several comparative studies of experience based prognostication versus prediction scales of post-stroke outcomes, including of iScore, ASTRAL, DRAGON and SEDAN scores, found that physicians were outperformed by prognostic models.(23,24) Though a multitude of prognostic scales have been developed for predicting mortality and functional outcome for brain injury,(25, 26) the degree to which these scales are used clinically and to which they change management remains uncertain. Cognitive bias, including anchoring and overconfidence, may contribute to reliance of clinical intuition rather than objective prognostic scales.(28) A recent systematic review found that use of calculated prognostic estimation resulted in shorter length of stay and increased change to DNR status compared to experience-based estimates but no overall change in in-hospital mortality.(29)
Further, clinicians tend to underestimate favorable outcomes in patients with acute brain injury. One explanation for this trend is the “disability paradox,” in which a patient in a disabled state reports a good quality of life whereas observers would believe this was an unacceptable state, showing that functional outcome and disability do not always correlate with perceived quality of life, as was evident in Case 1.(30) This inherent bias of the observers (in our case, the providers) may color conversations with family regarding goals of care. In the most severe cases, this influence can lead to WLST.(31) In milder cases, this influence could result in less effort towards movement and mobility in therapy and therefore development of spasticity and contractures.
Another important consideration is the treating physicians’ experience with acute management and lack of experience with long-term outcomes after brain injury. Often, patients with brain injuries are seen by a neurologist for risk factor modification or follow up testing, but chronic management of their impairments are left to a primary care physician or physiatrist. Therefore, inpatient neurologists who are formulating prognoses and communicating these predictions to families may have limited experience with chronic states of brain injury, which could affect their attitude towards life-sustaining measures.(32)
Our study has several limitations. First, given that this study was performed at a single tertiary-care medical center with high volume of neurological injuries, all providers have significant experience with treating these conditions and thus their prediction may overestimate the diagnostic accuracy and inter-rater reliability of providers. Second, as noted above, the patients had an assortment of outcomes after significant neurological injury but none died; cases with 90-day survival were intentionally selected to avoid using cases where life-sustaining therapies were withdrawn and survival outcomes unknown. Third, some of the cases (e.g. Case 2, hydrogen sulfide toxicity) may be considered rare. We justified their inclusion since such cases are often encountered at tertiary care academic medical centers where prognostic decisions are routinely made despite the relative lack of knowledge concerning long-term outcomes. Fourth, our survey did not ask for additional variables that may have influenced the providers’ perceptions of prognosis. These variables could be important in elucidating how prognostic decisions are made. And finally, because our study was a quality improvement project, we used a convenience sample of Department of Neurology faculty and trainees rather than prepare an a-priori power calculation for sample size.
The results of this study support prior studies that have shown predictive accuracy is limited in the first 24 hours after presentation; these results inspired our department to change our process for prognostication and making goals of care determinations in the Emergency Department. Unless all care providers uniformly agree on a poor outcome, we have implemented a 48–72 hour period of observation and life-sustaining medical care prior to making decisions to limit intensive treatment strategies.
Acknowledgments:
We would like to acknowledge Lori Chibnik, Ph.D. for her biostatistical guidance and the patients whose stories of recovery continue to inspire us.
Study Funding and Author disclosures:
Dr. Sloane is supported by Thomas B. McCabe and Jeannette E. Laws McCabe Fund of the University of Pennsylvania and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001879. Dr. Edlow is supported by NIH Director’s Office (DP2HD101400), NIH National Institute of Neurological Disorders and Stroke (R21NS109627), James S. McDonnell Foundation, and Tiny Blue Dot Foundation. Dr. Rosenthal is supported by NINDS 1R01NS117904, NINDS 1R01NS113541, NINDS K23NS105950, and U.S. Army W81XWH-18-DMRDP-PTCRA. Dr. Singhal is supported by grants from the NIH-NINDS (U10 NS086729, U01NS095869) and CRICO-Risk Management Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke. 2008;39(8):2304–9. [DOI] [PubMed] [Google Scholar]
- 2.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH Score. Stroke. 2001;32(4):891–7. [DOI] [PubMed] [Google Scholar]
- 3.Muehlschlegel S, Ayturk D, Ahlawat A, Izzy S, Scalea TM, Stein DM, et al. Predicting survival after acute civilian penetrating brain injuries: The SPIN score. Neurology. 2016;87(21):2244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saposnik G, Kapral MK, Liu Y, Hall R, O’Donnell M, Raptis S, et al. IScore: A risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123(7):739–49. [DOI] [PubMed] [Google Scholar]
- 5.Quinn T, Moskowitz J, Khan MW, Shutter L, Goldberg R, Col N, et al. What Families Need and Physicians Deliver - Contrasting Communication Preferences between Surrogate Decision- Makers and Physicians during Outcome Prognostication in Critically-Ill TBI Patients. Neurocritical. 2017;27(2):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fins JJ. Rights Come to Mind: Brain Injury, Ethics, and the Struggle for Consciousness. 1st ed. New York, NY: Cambridge University Press; 2015. 394 p. [Google Scholar]
- 7.Wartenberg KE, Hwang DY, Haeusler KG, Muehlschlegel S, Sakowitz OW, Madžar D, et al. Gap Analysis Regarding Prognostication in Neurocritical Care: A Joint Statement from the German Neurocritical Care Society and the Neurocritical Care Society. Neurocrit Care [Internet]. 2019;31(2):231–44. Available from: 10.1007/s12028-019-00769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahuranec DB, Fagerlin A, Sánchez BN, Roney ME, Thompson BB, Fuhrel-Forbis A, et al. Variability in physician prognosis and recommendations after intracerebral hemorrhage. Neurology. 2016;86(20):1864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg A, Callaway CW, Arnold RM, Cronberg T, Naito H, Dadon K, et al. Prognostication after cardiac arrest: Results of an international, multi-professional survey. Resuscitation [Internet]. 2019;138:190–7. Available from: 10.1016/j.resuscitation.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale CM, Sinuff T, Morrison LJ, Golan E, Scales DC. Understanding early decisions to withdraw life-sustaining therapy in cardiac arrest survivors: A qualitative investigation. Ann Am Thorac Soc. 2016;13(7):1115–22. [DOI] [PubMed] [Google Scholar]
- 11.Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56(6):766–72. [DOI] [PubMed] [Google Scholar]
- 12.Knies AK, Zhang Q, Juthani P, Tu S, Pach J, Martinez A, et al. Psychological Attachment Orientations of Surrogate Decision-Makers and Goals-of-Care Decisions for Brain Injury Patients in ICUs. Crit Care Expl. 2020;2(7):e0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang DY, Knies AK, Mampre D, Kolenikov S, Schalk M, Hammer H, et al. Concerns of surrogate decision makers for patients with acute brain injury: A US population survey. Neurology. 2020;94(19):e2054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geurts M, Macleod MR, van Thiel GJMW, van Gijn J, Kappelle LJ, van der Worp HB. End-of-life decisions in patients with severe acute brain injury. Lancet Neurol [Internet]. 2014;13(5):515–24. Available from: 10.1016/S1474-4422(14)70030-4 [DOI] [PubMed] [Google Scholar]
- 15.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: Do they exist in traumatic brain injury, too? Neurocrit Care. 2013;19(3):347–63. [DOI] [PubMed] [Google Scholar]
- 16.Turgeon AF, Lauzier F, Burns KEA, Meade MO, Scales DC, Zarychanski R, et al. Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: A survey of canadian intensivists, neurosurgeons, and neurologists. Crit Care Med. 2013;41(4):1086–93. [DOI] [PubMed] [Google Scholar]
- 17.Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation [Internet]. 2016;102:127–35. Available from: 10.1016/j.resuscitation.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swieten JC van Koudstaal PJ, Visser MC Schouten HJA, Gijn J van. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. [DOI] [PubMed] [Google Scholar]
- 19.Odu GO. Weighting methods for multi-criteria decision making technique. J Appl Sci Environ Manag. 2019;23(8):1449. [Google Scholar]
- 20.Neugebauer H, Creutzfeldt CJ, Hemphill JC, Heuschmann PU, Jüttler E. DESTINY-S: Attitudes of physicians toward disability and treatment in malignant MCA infarction. Neurocrit Care. 2014;21(1):27–34. [DOI] [PubMed] [Google Scholar]
- 21.Neugebauer H, Schnabl M, Lulé D, Heuschmann PU, Jüttler E. Attitudes of Patients and Relatives Toward Disability and Treatment in Malignant MCA Infarction. Neurocrit Care. 2017;26(2):311–8. [DOI] [PubMed] [Google Scholar]
- 22.Wijdicks EFM, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–38. [DOI] [PubMed] [Google Scholar]
- 23.Saposnik G, Cote R, Mamdani M, Raptis S, Thorpe KE, Fang J, et al. JURaSSiC: Accuracy of clinician vs risk score prediction of ischemic stroke outcomes. Neurology. 2013;(81):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ntaios G, Gioulekas F, Papavasileiou V, Strbian D, Michel P. ASTRAL, DRAGON and SEDAN scores predict stroke outcome more accurately than physicians. Eur J Neurol. 2016;23(11):1651–7. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K, Nohara Y, Soejima H, Yonehara T, Nakashima N, Kamouchi M. Stroke Prognostic Scores and Data-Driven Prediction of Clinical Outcomes after Acute Ischemic Stroke. Stroke. 2020;51(5):1477–83. [DOI] [PubMed] [Google Scholar]
- 26.Caplan LR. Scores of Scores. JAMA Neurol. 2013;70(2):252. [DOI] [PubMed] [Google Scholar]
- 27.Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10. [DOI] [PubMed] [Google Scholar]
- 28.Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak [Internet]. 2016;16(1):1–14. Available from: 10.1186/s12911-016-0377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basile M, Press A, Adia AC, Wang JJ, Herman SW, Lester J, et al. Does Calculated Prognostic Estimation Lead to Different Outcomes Compared With Experience-Based Prognostication in the ICU? A Systematic Review. Crit Care Explor. 2019;1(2):e0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larach DR, Larach DB, Larach MG. A life worth living: Seven years after craniectomy. Neurocrit Care. 2009;11(1):106–11. [DOI] [PubMed] [Google Scholar]
- 31.Fins JJ. The ethics of measuring and modulating consciousness: the imperative of minding time [Internet]. Vol. 177, Progress in Brain Research. Elsevier; 2009. 371–382 p. Available from: 10.1016/S0079-6123(09)17726-9 [DOI] [PubMed] [Google Scholar]
- 32.Rogge A, Witt VD, Valdueza JM, Borzikowsky C, Buyx A. Experience in Rehabilitation Medicine Affects Prognosis and End-of-Life Decision-Making of Neurologists: A Case-Based Survey. Neurocrit Care [Internet]. 2019;31(1):125–34. Available from: 10.1007/s12028-018-0661-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for analysis are presented in the tables and figures in this article. Anonymized data will be shared if requested by other investigators for the purpose of replicating the results.


