Abstract
Background:
Despite a better understanding of the epidemiology, pathogenesis, and management of patients with anaphylaxis, there remain knowledge gaps. Enumerating and prioritizing these gaps would allow limited scientific resources to be directed more effectively.
Objective:
To systematically describe and appraise anaphylaxis knowledge gaps and future research priorities based on their potential impact and feasibility.
Methods:
We convened a 25-member multidisciplinary panel of anaphylaxis experts. Panelists formulated knowledge gaps/research priority statements in an anonymous electronic survey. Four anaphylaxis themed writing groups were formed to refine statements: 1) Population Science, 2) Basic & Translational Sciences, 3) Emergency Department Care/Acute Management, and 4) Long-Term Management Strategies & Prevention. Revised statements were incorporated into an anonymous electronic survey and panelists were asked to rate the impact and feasibility of addressing statements on a continuous 0-100 scale.
Results:
The panel generated 98 statements across the four anaphylaxis themes: Population Science (29), Basic & Translational Sciences (27), Emergency Department Care/Acute Management (24), and Long-Term Management Strategies & Prevention (18). Median scores for impact and feasibility ranged from 50.0-95.0 and from 40.0-90.0. Key statements based on median rating for impact/feasibility included the need to refine anaphylaxis diagnostic criteria, identify reliable diagnostic, predictive, and prognostic anaphylaxis bioassays, develop clinical prediction models to standardize post-anaphylaxis observation periods and hospitalization criteria, and determine immunotherapy best practices.
Conclusions:
We identified and systematically appraised anaphylaxis knowledge gaps and future research priorities. This study reinforces the need to harmonize scientific pursuits to optimize the outcomes of patients with and at risk of anaphylaxis.
Keywords: Allergy, anaphylaxis, basic science, emergency department, feasibility, impact, population science, research, translational science
Capsule Summary
We established and appraised anaphylaxis knowledge gaps and future research priorities; multinational, multidisciplinary collaborations are needed to resolve these gaps with the ultimate goal of optimizing patient outcomes and lessening the societal burden of anaphylaxis.
Introduction
Anaphylaxis is an acute, potentially life-threatening systemic allergic reaction.1 The most common triggers of anaphylaxis include foods, medications, insect stings, as well as allergen immunotherapy.2 Although a precise estimate of global burdens are unknown, the incidence of anaphylaxis is increasing in the US and abroad.3–6 Rising case counts are attributed to medications such as chemotherapy, monoclonal antibodies, and non-steroidal anti-inflammatory drugs as well as rising rates of food induced anaphylaxis in children and adolescents.4–8 During the past decade, ED visits for anaphylaxis in the US doubled among all patients and tripled among children.9 The estimated lifetime individual risk of anaphylaxis is between 1% and 3%, and although rare, fatal anaphylaxis is a pressing and pervasive concern for at-risk patients and their families.10–12
In 2006, the National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network (NIAID/FAAN) developed a (now) widely accepted definition of anaphylaxis and established clinical diagnostic criteria.1 These guidelines helped standardize anaphylaxis diagnosis and management.2 However, anaphylaxis management frequently relies on a one-size-fits all approach, despite evidence that anaphylaxis is a heterogeneous condition with differences in clinical presentation, host susceptibility and mechanistic responses that necessitate personalized short and long-term management strategies to optimize clinical care and patient outcomes.13–16 These gaps led to a proposed refinement of the NIAID/FAAN criteria by the World Allergy Organization (WAO) in 2020,17,18 yet it is unclear whether global consensus will be achieved for the recommended changes.
Although there have been promising advances to reduce the risk of anaphylaxis among high-risk patients (e.g. through allergen immunotherapy),19–21 acute anaphylaxis management has not changed significantly since the advent of epinephrine auto-injectors in the 1980s.22 Additionally, we lack a clear understanding of the global epidemiology of anaphylaxis, including factors associated with increased disease incidence across broad populations and geographies.3,23 Knowledge gaps also exist regarding anaphylaxis pathogenesis including genetic risk factors and humoral and cellular responses, which is particularly evident with respect to human IgE-independent disease pathways.16,24–27 Furthermore, in clinical care there are no validated clinical or biomarker-based models which reliably predict disease courses or outcomes. Such tools could be used by providers to inform decisions about acute clinical management and the potential benefits of long-term risk-reduction strategies, including immunotherapy.13
To optimize clinical care and patient outcomes, it is paramount that we elucidate and devise strategies to collectively address these as well as other anaphylaxis knowledge gaps. In pursuit of these goals, the objective of this study was to systematically establish and appraise anaphylaxis knowledge gaps and future research priorities based on their perceived potential impact and feasibility. Dissemination and assimilation of these findings by clinicians, researchers, patients/families, policymakers, and funders alike will support a comprehensive, deliberative approach to conduct practice-changing research to optimize patient outcomes and diminish the societal burden of anaphylaxis.
Methods
From September 2020 through May 2021, we convened a 25-member panel of experts in the field of anaphylaxis, including allergists/immunologists and general and pediatric emergency medicine specialists from the United States (22), Australia (1), Germany (1), and the United Kingdom (1).28,29 Panelists were selected based on their clinical expertise, prior published research, expert recommendations, and membership in research networks and anaphylaxis interest groups.28,29 Panelists were asked to submit anaphylaxis knowledge gaps, research strategies, and future research priority statements (hereafter referred to as statements) via an anonymous electronic survey to ensure all panel members felt comfortable contributing ideas no matter their seniority or prior contributions to the field.30 The primary investigator (TD) combined survey responses, removed duplicate statements, and organized statements into the following four predetermined themes: 1) Population Science, 2) Basic & Translational Sciences, 3) Emergency Department (ED) Care/Acute Management, and 4) Long-Term Management Strategies & Prevention.
Conference call
A conference call was conducted to ensure panel members agreed with the four proposed themes and whether there was need to reclassify statements under different themes or propose additional statements that were omitted from the initial survey. An audio recording of the call was made available to panel members who could not join the live call.
Writing groups
Following the conference call, the primary investigator solicited volunteers to serve on one of four writing groups: Population Science (JW, CC, KM, PC), Basic & Translational Sciences (JS, HS), ED /Acute Management (DV, DG, RC, MN, MP), and Long-Term Management Strategies & Prevention (MS, SR). Writing groups reviewed theme-specific statements for content, clarity, and to provide background/contextual information and were encouraged to generate additional statements if potentially important topics were omitted from the initial survey. After the draft statements were finalized, the complete list of statements was distributed to panel members for feedback and revisions to ensure statements were written clearly and to elicit additional statements. Writing groups revised the updated statements which were incorporated in the survey as described below.30 Following data analysis, writing groups reviewed theme-specific results and summarized key topics.
Survey
Statements finalized by writing groups were incorporated into an anonymous electronic REDCap survey.30 Panel members were asked to answer two questions specific to each statement: 1) the Impact (e.g. how important would it be to answer this question to advance knowledge, drive the field, and/or improve patient outcomes directly or indirectly) of addressing the statement on a continuous 0-100 scale (0 = no impact to 100 = highest impact), and 2) the Feasibility (e.g. accounting for logistics, infrastructure, sample size, cost, and/or ethical considerations) of addressing the statement on a continuous 0-100 scale (0 = not feasible to 100 = highest feasibility). Panel members were encouraged to provide free text comments for statements and could choose “not applicable” if they did not have the experience or knowledge about a particular statement. Survey results for impact and feasibility were presented as median with corresponding interquartile ranges (IQRs).
The institutional review board at Cincinnati Children’s Hospital Medical Center approved this study.
Results
Panelists generated 98 statements across the four anaphylaxis themes: Population Science (29), Basic & Translational Sciences (27), Emergency Department Care/Acute Management (24), and Long-Term Management Strategies & Prevention (18). Survey results – including the number of responses for each statement, median scores for impact and feasibility with associated IQRs, as well as free text comments – are presented in Table E1 in the online repository. Median scores for impact and feasibility across the four themes ranged from 50.0-95.0 and from 40.0-90.0, respectively. Figures 1–4 depict the top 10 statements for summed median impact and feasibility scores for each anaphylaxis theme: Population Science (Figure 1), Basic & Translational Sciences (Figure 2), Emergency Department Care/Acute Management (Figure 3), and Long-Term Management Strategies & Prevention (Figure 4). A graphical representation of statements accounting for impact and feasibility scores is presented in Figure 5 (an online interactive version of the figure is available at http://dribin.pemcincinnati.com/).
Figure 1.
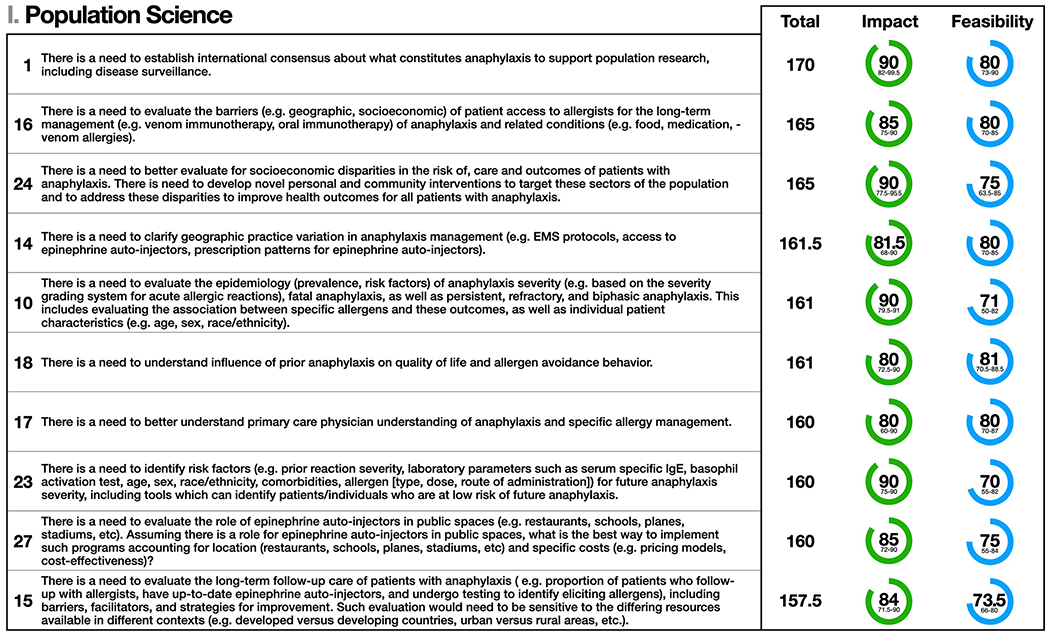
Population Science: the top 10 statements for summed median and impact and feasibility scores (maximum possible score = 200). Statement numbers corresponding to statements in the Online Repository are displayed to the left of each statement. Interquartile ranges are presented below median scores for impact and feasibility. Statement text may be abridged for clarity; a complete list of statements with unabridged text as well free text comments are detailed in Table E1 in the Online Repository.
Figure 4.
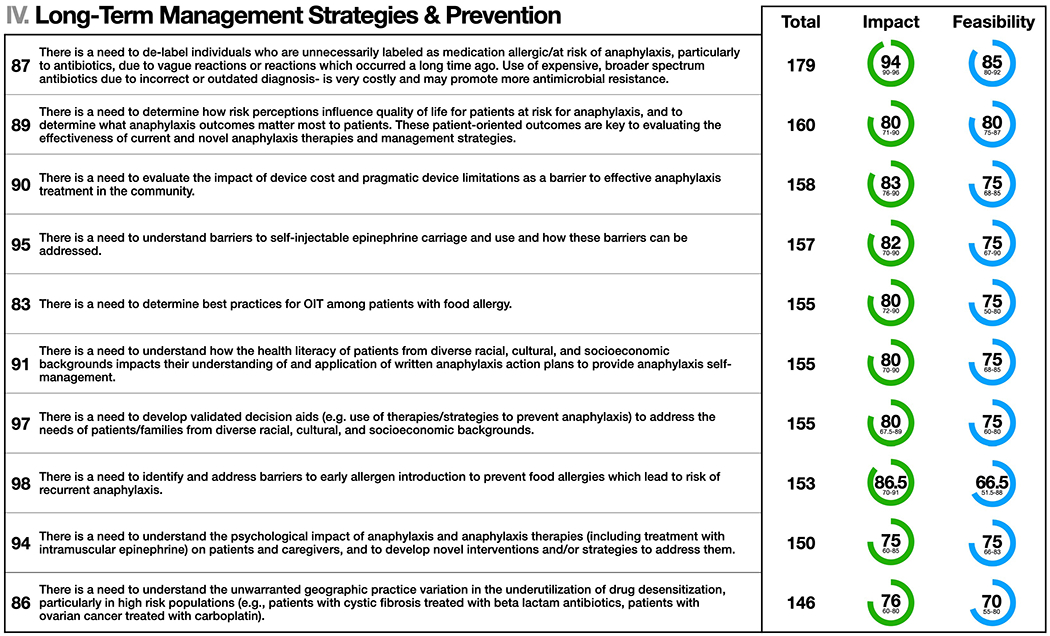
Long-Term Management Strategies & Prevention: the top 10 statements for summed median and impact and feasibility scores (maximum possible score = 200). Statement numbers corresponding to statements in the Online Repository are displayed to the left of each statement. Interquartile ranges are presented below median scores for impact and feasibility. Statement text may be abridged for clarity; a complete list of statements with unabridged text as well free text comments are detailed in Table E1 in the Online Repository.
Figure 2.
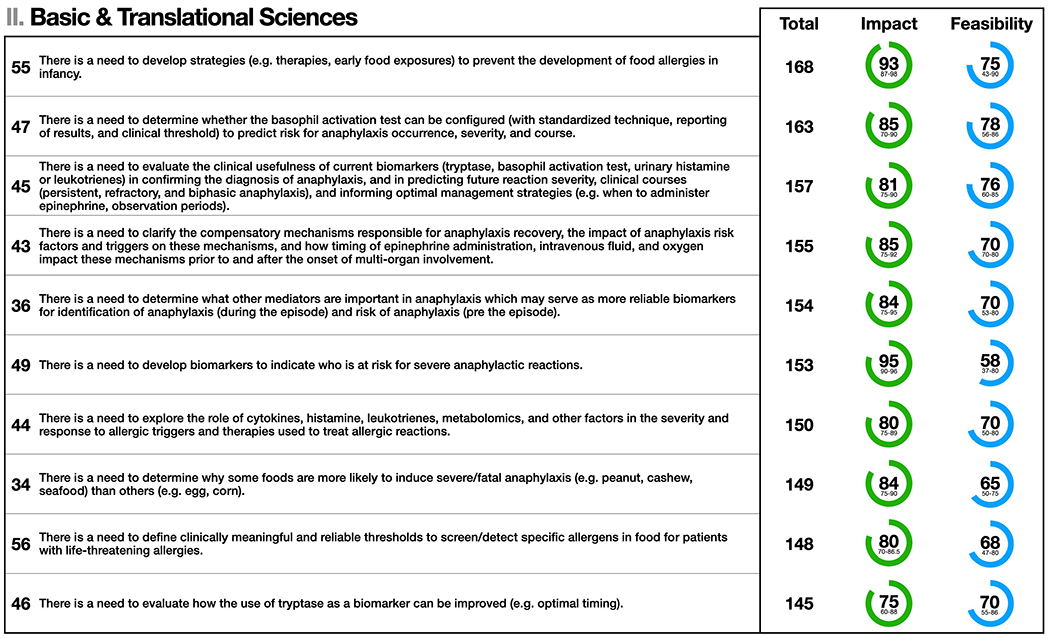
Basic & Translational Sciences: the top 10 statements for summed median and impact and feasibility scores (maximum possible score = 200). Statement numbers corresponding to statements in the Online Repository are displayed to the left of each statement. Interquartile ranges are presented below median scores for impact and feasibility. Statement text may be abridged for clarity; a complete list of statements with unabridged text as well free text comments are detailed in Table E1 in the Online Repository.
Figure 3.
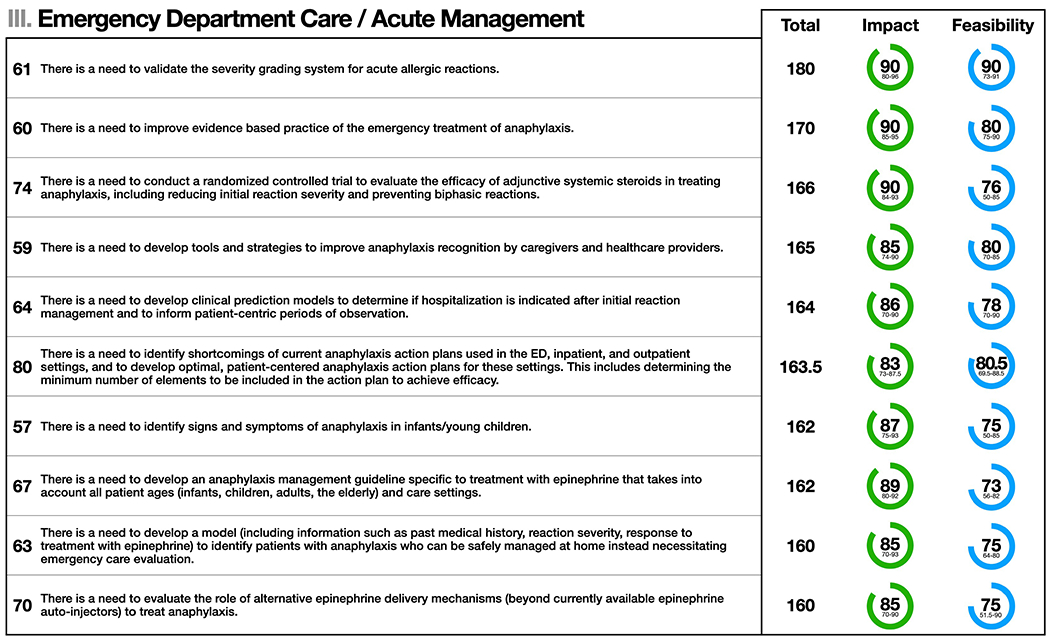
Emergency Department Care/Acute Management: the top 10 statements for summed median and impact and feasibility scores (maximum possible score = 200). Statement numbers corresponding to statements in the Online Repository are displayed to the left of each statement. Interquartile ranges are presented below median scores for impact and feasibility. Statement text may be abridged for clarity; a complete list of statements with unabridged text as well free text comments are detailed in Table E1 in the Online Repository.
Figure 5.
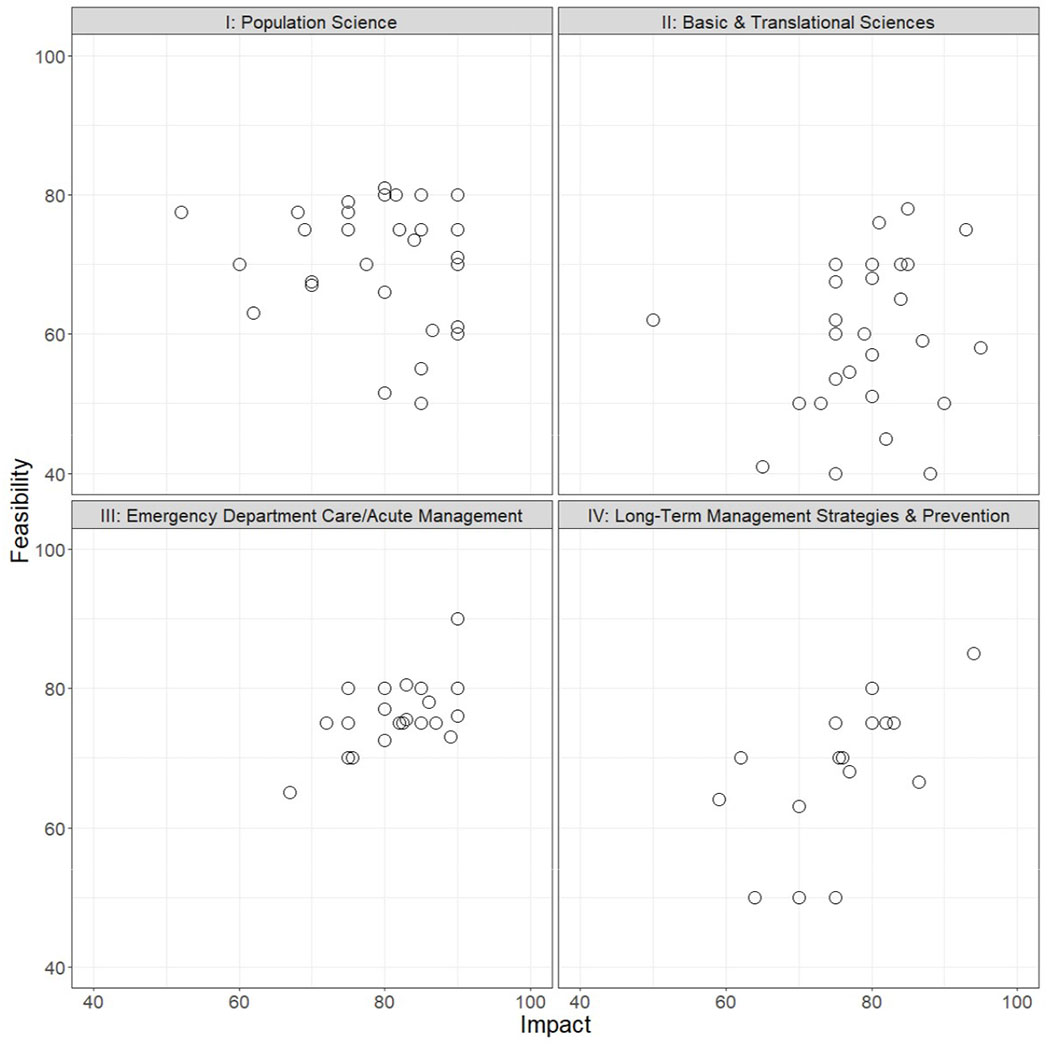
Distribution of anaphylaxis knowledge gaps and future research priorities accounting for the potential impact and feasibility of addressing statements across the four anaphylaxis themes: I) Population Science, II) Basic & Translational Sciences, III) Emergency Department Care/Acute Management, IV) Long-Term Management Strategies & Prevention.
Population Science
Current state
The incidence of anaphylaxis hospitalizations is increasing globally, especially for medication and food-induced anaphylaxis; but there is little data to indicate a parallel increase in anaphylaxis deaths.3 It is challenging to determine a precise estimate of disease burden globally (or to evaluate potential causes of increased disease incidence) because current estimates are often based on ED or hospital registries, which are susceptible to diagnosis code biases and do not account for patients who do not receive in-hospital care.3,12 This makes it difficult to establish accurate trends in anaphylaxis cases and outcomes28 across demographic groups and regions. In addition, variations in diagnostic criteria, miscoding and the lack of detail about specific triggers further confounds epidemiological analyses.3 Without accurate data, it is difficult to prioritize and maximize the impact of anaphylaxis investigations.
Key statements summary
The top 10 statements for summed median impact and feasibility scores for Population Science are shown in Figure 1. The panel affirmed that understanding the causes and effects of anaphylaxis is dependent on a scientifically based, consensus definition of anaphylaxis, and approaches to recording and measuring it using population-based datasets and biomarkers. Developing such a definition is predicated on a clearer understanding of disease pathogenesis and epidemiology. The lack of consensus for a singular anaphylaxis definition (as well as variation in interpreting that definition) limits severity assessment/assignment and strategies to mitigate severe anaphylaxis outcomes.17,18 Addressing these issues will help elucidate disease and outcomes risk factors, and to determine the causes and impact of prevention, diagnostic, and treatment approaches. In particular, even with an incomplete understanding of anaphylaxis biology, clinical trials of different treatment strategies are warranted.
Next steps
A multispecialty group with broad international input and representation across key global organizations should refine anaphylaxis diagnostic criteria.1,17,18 Consideration should also be given to how such a definition relates to severity assessment,29,31,32 and how both anaphylaxis diagnostic criteria and severity assignment should incorporate recent evidence of disease pathogenesis and phenotypes.33,34 Additionally, the global epidemiology of anaphylaxis should be evaluated. Improved diagnostic code systems are needed to describe and detail reactions (including specific triggers) and to reflect our current understanding of allergic reaction severity and phenotypes. This would facilitate advanced disease surveillance and help evaluate barriers to improving outcomes. An improved understanding of population-based anaphylaxis trends (as well as trends in predisposing allergic conditions) will help researchers elucidate the complex, dynamic, and interdependent contributors of increased disease incidence. Studies evaluating public health measures and treatment approaches (including clinical trials) should be designed and undertaken. These advances will have the positive impact of informing not only novel public health paradigms but potentially agricultural, food production, and environmental policies to mitigate the societal burden of anaphylaxis.
Basic & Translational Sciences
Current state
There have been promising advances in our understanding of anaphylaxis pathogenesis over the past decade,35 including identifying and understanding the role of anaphylaxis mediators in disease severity.15,16 This includes characterizing genetic risk factors for food allergies27 and the role of the microbiome in allergic diseases including how the intestinal microbiome may be protective against food allergies and thus potentially against anaphylaxis.36,37 For example, a newly described genetic trait due to the duplication of alpha tryptase genes at the TPSAB1 gene locus on chromosome 16 and present in 4-6 % of the world’s population is the only known genetic risk factor for anaphylaxis.38,39 Still, we lack a clear understanding of conditions that increase the risk for anaphylaxis such as clonal and non-clonal mast cell activation disorders.40
Despite promising murine-based discoveries elucidating the complex pathogenesis of anaphylaxis (e.g. IgE dependent and independent pathways),24,25 it is difficult to extrapolate these findings to humans.41 Investigations in human subjects are challenging due to ethical concerns and the heterogonous nature of human anaphylaxis. This is further confounded by the fact that a key effector cell – mast cells – do not circulate in the blood and we currently lack simple, reliable, and minimally invasive techniques to obtain mast cells.42
Key statements summary
The top 10 statements for summed median impact and feasibility scores for Basic & Translational Sciences are shown in Figure 2. Despite recent advances, there is a need for further research before these and other discoveries can be integrated into routine clinical care. This includes evaluating the role of current biomarkers (histamine, tryptase, leukotrienes) and diagnostic modalities (e.g. basophil activation test) in improving anaphylaxis diagnosis (particularly where there is diagnostic ambiguity such as in fatal anaphylaxis), informing management, and in predicting clinical courses and future risk. There is also a need to identify other anaphylaxis mediators, which may serve as more reliable diagnostic, predictive and prognostic biomarkers. Complementary to this is the need to understand compensatory mechanisms responsible for anaphylaxis recovery,43,44 how these mechanisms relate to the risk of severe reactions, and how therapies (epinephrine, intravenous fluids, oxygen) – including their timing – impact outcomes. Such research is challenging given ethical constraints, and the need for large, prospective cohorts to obtain the requisite clinical and biological data from patients with severe, life-threatening reactions. There is also a need to develop novel preventive treatments including targeted therapies to block IgE and the release of anaphylaxis mediators from effector cells. Likewise, there is opportunity to improve strategies (e.g. therapies, early food exposures) to prevent the development of food allergies in infancy, which is the most common cause of anaphylaxis in children.2
Next steps
There is need for ongoing collaboration among basic scientists and clinical/translational researchers to develop, adapt, and integrate mechanistic animal-based research strategies and techniques to study human anaphylaxis. Given the difficulties of human studies, there is a need to prospectively enroll patients at risk of anaphylaxis to facilitate longitudinal clinical and biological data acquisition and the creation of robust biological repositories. This approach will support investigations to elucidate the pathogenesis of anaphylaxis and to translate these findings to bedside care in order to improve short and long-term management strategies and patient outcomes.
Emergency Department Care/Acute Management
Current state
Although the 2006 NIAID/FAAN anaphylaxis diagnostic criteria helped standardize anaphylaxis recognition and management1, they do not capture all anaphylaxis phenotypes and there is variation in definition interpretation (for example, how “persistent symptoms” is defined by clinicians in the context of an acute reaction). The WAO recently proposed refinements to the NIAID/FAAN criteria, yet the impact of these recommendations on acute management is uncertain.17,18 There is also variation in the use of intramuscular epinephrine to treat anaphylaxis, with significant underuse to treat anaphylaxis and overuse to treat non-anaphylaxis (such as isolated angioedema). Furthermore, there are no validated decision aids to determine which persistent and/or biphasic symptoms28 warrant treatment with epinephrine versus those that do not. There are also no prospectively derived and validated prediction models to inform the duration of ED observation periods or hospitalization criteria, nor have there been definitive randomized controlled trials (RCTs) to standardize the use of adjunctive anaphylaxis therapies.2,45 These knowledge gaps may contribute to underuse of epinephrine and overuse of ineffective medications as well as prolonged ED lengths of stay, unnecessary hospitalizations, and undue healthcare costs.2,45–48
Key statements summary
The top 10 statements for summed median impact and feasibility scores for Emergency Department Care/Acute Management are shown in Figure 3. The panel recognized the need to develop improved diagnostic and management strategies for infants and young children presenting to the ED with anaphylaxis, as signs and symptoms within this age group are often difficult to discern, and may overlap with normal behavior.49,50 The assignment of reaction severity and its utility in guiding acute treatment and/or need for in-hospital observation needs to be determined and validated.28,29,31,32 The panel identified the need to explore the mechanism of action, pharmacokinetics/pharmacodynamics, and clinical outcomes of epinephrine given by different routes/devices, including non-injectable delivery systems. This includes evaluating what constitutes delayed epinephrine administration and the degree to which this increases the risk for adverse outcomes (including refractory and/or biphasic reactions).28 There is also a need to clarify and disseminate data about shelf-life and temperature requirements of epinephrine. RCTs are needed to evaluate the efficacy of adjunctive anaphylaxis therapies including systemic steroids and H1 and H2 antagonists.2,51 Such studies will be resource intensive, as they require large patient enrollments and because of perceived lack of equipoise given the routine use of these medications in current practice.2 Lastly, the panel identified the need to evaluate how to best implement and standardize anaphylaxis action plans and epinephrine auto-injector (EAI) prescription programs.
Next steps
The panel emphasized the need for high-quality multisite prospective observational and interventional studies to improve patient outcomes. This line of research is less feasible owing to the challenge of timely ED based enrollment, randomization, and collecting accurate longitudinal data to power predictive models. Despite existing obstacles, developing and refining novel enrollment, data collection, and follow-up procedures will help the ED become not only a clinical laboratory to optimize management strategies but also a biological laboratory to support innovative translational research through refined biospecimen collection processes.
Long-Term Management Strategies & Prevention
Current state
As outlined in international anaphylaxis guidelines (including from the American College of Allergy, Asthma, and Immunology/American Academy of Allergy, Asthma & Immunology, the European Academy of Allergy and Clinical Immunology, and the WAO), existing strategies to prevent anaphylaxis are predicated on allergen avoidance, allergen immunotherapy, drug desensitization protocols, and precautionary observation for high risk medications and procedures.18–20,52,53 Although food allergen immunotherapy shows promise in reducing the risk of anaphylaxis from accidental ingestion, the evidence clearly indicates an increased risk of anaphylaxis during treatment.54 However, anaphylaxis in the context of a treatment dose given in a controlled setting (where there is anticipation of a reaction) may be distinct from anaphylaxis secondary to unintended allergen exposure in the community. Choosing preventative therapies requires patient-centered discussions regarding potential risks and benefits. The benefits of any allergen immunotherapy (which may include improved quality of life in addition to a reduced risk of anaphylaxis) must be weighed against potential risks, including treatment-related anaphylaxis.54–56 While current practice often requires universal in-clinic observation for patients to receive certain therapies (e.g. allergen immunotherapy), the health and economic impacts of such practices are not equally distributed across the population.57–59
Key statements summary
The top 10 statements for summed median impact and feasibility scores for Long-Term Management Strategies and Prevention are shown in Figure 4. The panel identified the need to improve our understanding of immunotherapy best practices, including the effectiveness of allergen immunotherapy (AIT) at more prolonged dosing intervals and the ideal duration of AIT in high-risk patients. Other priorities include determining best practices for AIT and identifying and addressing barriers to implementing research evaluating earlier allergen introduction as a food allergy prevention strategy. There is a need to explore the degree to which immunotherapy protocols could be adapted in specific patient-preference sensitive contexts – for example performing AIT maintenance dosing in the non-medically observed setting for eligible patients. Regarding the management of drug allergy, there is a need to understand how variations in practice contribute to a failure to de-label those with an incorrect diagnosis and under-utilization of drug desensitization. Knowledge gaps also exist related to patient/family perspectives about longer-term anaphylaxis management, specifically the need to determine how patient risk perceptions may drive clinically meaningful outcomes and the need to address barriers to EAI use (including cost). Finally, understanding the impact of the determinants of health literacy would promote the development of improved shared decision-making models and patient decision aids.
Next steps
Addressing these gaps requires a concerted and sustained effort (as well as the resources) to design and execute transformative studies. Clarifying patient-centered approaches to long-term anaphylaxis management requires multimodal and potentially multidisciplinary research approaches. Research programs should seek to incorporate mixed-methods study designs to ensure study outcomes and interventions are truly patient-centric and result in optimal, equitable health outcomes for all patients.60 Mixed-methods study designs (combining quantitative and qualitative research components) are ideal because they strengthen the conclusions, impact, and validity of research findings.61 Additionally, since anaphylaxis impacts a wide range of patients, clinicians, and community members, diverse stakeholder representation should be included when designing and conducting studies.
Discussion
We convened a multidisciplinary group of anaphylaxis experts to establish anaphylaxis knowledge gaps and future research priorities, appraise their potential impact and feasibility, and propose strategies to address them. Dissemination and uptake of the knowledge gaps and research priorities outlined in this manuscript by clinicians, researchers, funders, and policymakers will help harmonize, accelerate and direct research efforts to improve the care and outcomes of patients with and at risk of anaphylaxis.
Our study methodology improves upon smaller studies leveraging expert opinion, because we convened a multispecialty, multi-national panel representing diverse clinical and scientific experience, and because we applied a rigorous consensus methodology. Consequently, study results are less likely to be constrained to a limited patient population, clinical setting, causative agent, research concentration or study design. Additionally, we included statements that did not directly reference anaphylaxis but instead were specific to advancing our understanding of the pathogenesis, management, and/or prevention of conditions that predispose patients to anaphylaxis (e.g. food, medication, venom allergies). This was intentional, given that scientific questions related to common predisposing conditions are connected with advancing anaphylaxis research, and only by addressing these complementary topics will we optimize short and long-term patient outcomes. By considering impact and feasibility and encouraging panelists to provide contextual details specific to study execution (e.g. study design and limitations, patient population, cost, and ethical considerations), we hope our findings will serve as an actionable framework and tool for researchers and funders to identify, prioritize and strategically address the most pressing scientific questions in the field of anaphylaxis.
Towards a precision medicine model of anaphylaxis care
Clinical care and research is hampered by the lack of global, consensus anaphylaxis diagnostic criteria and the assignment of reaction severity and outcomes. There is a need to transform care away from the one-size-fits all care model to a precision medicine care model that accounts for host susceptibility (genetics, environmental exposures, comorbidities, socioeconomics), causative agents and host responses (phenotypes, endotypes) to inform targeted short and long-term management and therapeutic strategies and improved patient outcomes.13,62 Central to this is a need to better describe the global epidemiology of anaphylaxis, which will help align investigations and systematically evaluate their longitudinal impact. The International Classification of Diseases-11 is also an important advancement to improve the codification of anaphylaxis and may provide more accurate global epidemiological data.63 Although the 2006 NIAID/FAAN anaphylaxis diagnostic criteria are validated and widely used in clinical care and research,1,64,65 there is inconsistent interpretation and application of the criteria in clinical care.17,18,31 Specifically, there is a need to account for milder patient reported symptoms, symptoms specific to infants, the severity and duration of gastrointestinal symptoms (as well as the causative agent), the potential for patients to have delayed onset of symptoms after allergen exposure, and patients who develop acute, isolated respiratory compromise after known/likely allergen exposure.17,18 These definitional limitations contribute to variation in determining which persistent and/or recurrent symptoms necessitate treatment with epinephrine.
To advance clinical care we must move beyond relying solely on patient and reaction characteristics (e.g. triggers, phenotypes) and response to therapies to inform clinical decision making. Instead, there is need to understand the role of currently available but not widely used biomarkers and diagnostic modalities13,16,26,66 as well as novel mediators, and whether and how these biomarkers can be translated into routine bedside care to optimize management strategies. We imagine that clinicians will one day be able to obtain point-of-care biomarkers to confirm the diagnosis of anaphylaxis when there is diagnostic uncertainty and to standardize management decisions including the need for prolonged observation periods/hospitalization to monitor for biphasic reactions. These advances may also promote the discovery of biochemical targets for drug development including efficacious abortive (in cases of refractory anaphylaxis), preventive (e.g. novel biologies, Bruton’s tyrosine kinase inhibitors)67, and curative therapies.
Finally, crucial to the delivery of precision medical care is the need to evaluate and account for the perspectives and preferences of patients and families from diverse racial/ethnic, cultural, religious, and socioeconomic backgrounds. This will ensure that evidence-based practice parameters and care pathways will be tailored to the needs of patients/families in the context of the communities in which they live, learn, and grow. Such an approach will ensure that management and treatment strategies result in optimal, equitable health outcomes for all patients.68
To improve patient outcomes and decrease the societal burden of anaphylaxis, we encourage the further development of innovative, collaborative research networks with experts in basic science, clinical, translational, population, health services, and public health research, pharmaceuticals, bioengineering, and healthcare policy. Only through strategic, integrated research networks will we be able to advance our understanding of the epidemiology and pathogenesis of anaphylaxis and translate these discoveries into novel and efficacious diagnostic, management, and therapeutic strategies.
Limitations
This study is subject to limitations, especially the potential that key statements/themes or research strategies (e.g. technologies such as e-health) were omitted.69 To address this issue, we sought input from a large panel of researchers with broad expertise and encouraged panel members to suggest additional statements during all study phases to ensure important topics were not omitted. Our study is also limited by the absence of perspectives from patients, clinicians from other specialties, allied health providers, and community members. Because the preponderance of panelists were from the US (with no representatives from Asia, Africa, or Latin America), we recognize that the statements outlined in this study do not encompass all anaphylaxis knowledge gaps and research priorities in different cultural or social backgrounds – and encourage researchers globally to build upon our findings to pursue other relevant and innovative research questions.
Panel members may have rated the impact and/or feasibility of statements differently based on the potential to employ different study designs to address the same statement. For example, a retrospective or prospective study could be used to address the statement, “There is need to validate the severity grading system for acute allergic reactions.”29 Although a prospective study would be more impactful than a retrospective study, it would be less feasible. The panel intentionally did not limit statements to only “gold standard” study designs given grant applications to conduct clinical practice changing research are often based on pilot data from lower impact/high feasibility studies. We attempted to account for potential differences in statement interpretation by including a large panel of experts (25), providing IQRs, and including free text comments. Additionally, some statements included specific study designs (e.g. an RCT to evaluate the efficacy of systemic steroids) if only one methodological approach could sufficiently address a research question, and thus results for these statements should be less subject to differences in interpretation.
Conclusions
We established and systematically appraised the potential impact and feasibility of addressing anaphylaxis knowledge gaps and future research priorities. Our intention is that this study will serve as a foundation and catalyst for purposeful, collaborative research to accelerate scientific discoveries and to translate these discoveries into novel management and therapeutic strategies to optimize patient care and clinical outcomes.
Supplementary Material
Clinical Implication.
The anaphylaxis knowledge gaps and research priorities outlined in this study provide a framework for clinicians and researchers to align and accelerate scientific discoveries to optimize patient outcomes.
Funding:
Division of Emergency Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2UL1TR001425 - 05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2KL2TR001426-05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure of potential conflict of interest:
T. E. Dribin receives funding from the NIH. D. Schnadower receives funding from the NIH. C. A. Camargo has consulted for Bryn Pharma and Kaleo. J. Wang receives research support from NIAID, Aimmune, DBV Technologies, and Regeneron, and consultancy fees from Aimmune, ALK Abello, DBV Technologies, and Genentech. K. A. Michelson receives funding from AHRQ. M. Shaker has been involved in research supported by DBV technologies, is a member of the Joint Task Force on Practice Parameters, and serves as an editorial board member for the Journal of Allergy and Clinical Immunology In Practice, Annals of Allergy, Asthma, and Immunology, and the Journal of Food Allergy. D. B. K. Golden has received financial support from Aquestive, Sandoz, ALK-Abelló, Genentech, Stallergenes Greer, Novartis, Allergy Therapeutics, and UpToDate. J. M. Spergel has grant support from DBV Technology, AImmune, and NIH; he has consulted for Kaleo. R. L. Campbell has consulted for Bryn Pharma, been a peer reviewer for EB Medicine, and an author for UpToDate. M. I. Neuman is an author for UpToDate and serves as an editorial board member of Pediatrics. M. Pistiner has served on advisory boards of DBV technologies, Kaleo and Novartis. He has received research funding from Kaleo, National Peanut Board and Egg Nutrition Center; Program funding from DBV Technologies, and is cofounder of AllergyHome and Allergy Certified Training. M. Castells is the BWH PI for the PIONEER BluPrint Clinical trial for Indolent Systemic Mastocytosis. D. C. Brousseau receives funding from the NIH and MCHB. L. C. Schneider has received research support from Regeneron Pharmaceuticals, DBV Technologies, Pfizer, and Genentech; has consulted for Aimmune Therapeutics and DBV Technologies; and is on the Medical Advisory Board of FARE (Food Allergy Research and Education), Scientific Advisory Boards for BioThea Pharmaceuticals, Inc. and Ukko, and DSMB for Alladapt Immunotherapeutics. A. H. Assa’ad has research grants from NIH, Aimmune, DBV technologies, Astellas, ABBVIE, and Sanofi. R. Mistry receives funding from the NIAID. D. E. Campbell reports grants from National Health and Medical Research Council Of Australia (paid to institution), funding for sponsor lead studies from Nestle Health Sciences (paid to institution), part time salary from DBV-Technologies, part time salary from Sydney Children’s Hospitals Network and consulting fees from AllerGenis and Westmead Fertility Centre. M. Worm has received fees for serving on advisory boards from Regeneron Pharmaceuticals, Aimmune, LEO Pharma, ALK, Eli Lilly, Abbvie, Mylan, Novartis Sanofi and Pfizer and consulting fees from Aimmune, ALK, DBV, LEO Pharma, Mylan, Sanofi and Novartis. P. J. Turner reports grants from UK Medical Research Council, NIHR/Imperial BRC, UK Food Standards Agency and JM Charitable Foundation; personal fees from UK Food Standards Agency, Aimmune Therapeutics, Allergenis and ILSI Europe, outside the submitted work. H. A. Sampson receives funding to his institution for grants from the NIH/NIAID and has received consulting fees from DBV Technologies, S.A., N-Fold Therapeutic, LLC, and Siolta, Inc. and stock options from DBV Technologies and N-Fold Therapeutics. S. A. Rudders, D. Vyles, P. S. Capucilli, J. Lee, K. A. Risma, J. K. Witry, Y. Zhang, and B. Sobolewski report no conflicts of interest, no other relationships, or activities that could appear to have influenced the submitted work.
Abbreviations
- AIT
allergen immunotherapy
- EAIs
epinephrine auto-injectors
- ED
emergency department
- IQR
interquartile range
- NIAID/FAAN
National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network
- OIT
oral immunotherapy
- RCT
randomized controlled trial
- VIT
venom immunotherapy
- WAO
World Allergy Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr., Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: Summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006. Feb 1;117(2):391–7. Available from: 10.1016/j.jaci.2005.12.1303 [DOI] [PubMed] [Google Scholar]
- 2.Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis--a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020. Apr 1;145(4):1082–123. Available from: 10.1016/j.jaci.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 3.Turner PJ, Campbell DE, Motosue MS, Campbell RL. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J Allergy Clin Immunol Pract. 2020;8(4):1169–76. Available from: http://www.sciencedirect.com/science/article/pii/S2213219819309675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J Allergy Clin Immunol Pract. 2016;4(3):497–504. Available from: https://www.sciencedirect.com/science/article/pii/S2213219816000118 [DOI] [PubMed] [Google Scholar]
- 5.Isabwe GAC, Garcia Neuer M, de las Vecillas Sanchez L, Lynch D-M, Marquis K, Castells M. Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J Allergy Clin Immunol. 2018;142(1):159–170.e2. Available from: https://www.sciencedirect.com/science/article/pii/S0091674918303063 [DOI] [PubMed] [Google Scholar]
- 6.Aun MV, Blanca M, Garro LS, Ribeiro MR, Kalil J, Motta AA, et al. Nonsteroidal Anti-Inflammatory Drugs are Major Causes of Drug-Induced Anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(4):414–20. Available from: https://www.sciencedirect.com/science/article/pii/S2213219814001354 [DOI] [PubMed] [Google Scholar]
- 7.Cianferoni A, Muraro A. Food-Induced Anaphylaxis. Immunol Allergy Clin North Am. 2012;32(1):165–95. Available from: https://www.sciencedirect.com/science/article/pii/S0889856111001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilaver LA, Chadha AS, Doshi P, O’Dweyer L, Gupta RS. Economic burden of food allergy- A systematic review. Ann Allergy, Asthma Immunol. 2019;122(4):373–80. Available from: 10.1016/j.anai.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 9.Michelson KA, Dribin TE, Vyles D, Neuman MI. Trends in emergency care for anaphylaxis. J Allergy Clin Immunol Pract. 2020;8(2):767–768.e2. Available from: 10.1016/j.jaip.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 10.Wood RA, Camargo CA, Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–7. Available from: http://www.sciencedirect.com/science/article/pii/S009167491301302X [DOI] [PubMed] [Google Scholar]
- 11.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J Allergy Clin Immunol Pract. 2017;5(5):1169–78. Available from: http://www.sciencedirect.com/science/article/pii/S2213219817305159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pouessel G, Turner PJ, Worm M, Cardona V, Deschildre A, Beaudouin E, et al. Food-induced fatal anaphylaxis: From epidemiological data to general prevention strategies. Clin Exp Allergy. 2018. Dec 1;48(12):1584–93. Available from: 10.1111/cea.13287 [DOI] [PubMed] [Google Scholar]
- 13.Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140:321–33. Available from: 10.1016/j.jaci.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 14.Pascal M, Perez-Gordo M, Caballero T, Escribese MM, Lopez Longo MN, Luengo O, et al. Microbiome and Allergic Diseases. Vol. 9, Frontiers in Immunology. 2018. p. 1584. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2018.01584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-Activating Factor, PAF Acetylhydrolase, and Severe Anaphylaxis. N Engl J Med. 2008. Jan 3;358(1):28–35. Available from: 10.1056/NEJMoa070030 [DOI] [PubMed] [Google Scholar]
- 16.Brown SGA, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, et al. Anaphylaxis: Clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132(5):1141–1149.e5. Available from: http://www.sciencedirect.com/science/article/pii/S0091674913009834 [DOI] [PubMed] [Google Scholar]
- 17.Turner PJ, Worm M, Ansotegui IJ, El-Gamal Y, Rivas MF, Fineman S, et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019. Oct 31;12(10):100066. Available from: https://pubmed.ncbi.nlm.nih.gov/31719946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ J. 2020;13(10):100472. Available from: https://www.sciencedirect.com/science/article/pii/S1939455120303756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral Immunotherapy for Treatment of Egg Allergy in Children. N Engl J Med. 2012. Jul 18;367(3):233–43. Available from: 10.1056/NEJMoa1200435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden DBK, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of Allergy to Insect Stings in Children, with and without Venom Immunotherapy. N Engl J Med. 2004. Aug 12;351(7):668–74. Available from: 10.1056/NEJMoa022952 [DOI] [PubMed] [Google Scholar]
- 21.Grzeskowiak LE, Tao B, Knight E, Cohen-Woods S, Chataway T. Adverse events associated with peanut oral immunotherapy in children – a systematic review and meta-analysis. Sci Rep. 2020;10(1):659. Available from: 10.1038/s41598-019-56961-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur G. Epinephrine: a short history. Lancet Respir Med. 2015. May 1;3(5):350–1. Available from: 10.1016/S2213-2600(15)00087-9 [DOI] [PubMed] [Google Scholar]
- 23.Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: An analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. 2015;135(4):956–963.e1. Available from: http://www.sciencedirect.com/science/article/pii/S0091674914015164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cianferoni A. Non--IgE-mediated anaphylaxis. J Allergy Clin Immunol. 2021. Apr 1;147(4):1123–31. Available from: 10.1016/j.jaci.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137(6):1674–80. Available from: https://www.sciencedirect.com/science/article/pii/S0091674916003821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SGA. Elevated serum cytokines during human anaphylaxis: Identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol. 2009;124(4):786–792.e4. Available from: http://www.sciencedirect.com/science/article/pii/S009167490901166X [DOI] [PubMed] [Google Scholar]
- 27.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127(3):661–7. Available from: http://www.sciencedirect.com/science/article/pii/S0091674911001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dribin TE, Sampson HA, Camargo CA Jr., Brousseau DC, Spergel JM, Neuman MI, et al. Persistent, refractory, and biphasic anaphylaxis: a multidisciplinary Delphi study. J Allergy Clin Immunol. 2020. Aug 24; Available from: 10.1016/j.jaci.2020.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dribin TE, Schnadower D, Spergel JM, Campbell RL, Shaker M, Neuman MI, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol. 2021. Jan 20;148. Available from: 10.1016/j.jaci.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stafford A, Patel N, Turner PJ. Anaphylaxis – moving beyond severity…. J Allergy Clin Immunol. 2021; Available from: https://www.sciencedirect.com/science/article/pii/S0091674921006588 [DOI] [PubMed] [Google Scholar]
- 32.Hogan SP. Commentary: Severity grading system for acute allergic reactions. Time for validation and assessment of best practices. J Allergy Clin Immunol. 2021; Available from: https://www.sciencedirect.com/science/article/pii/S0091674921007284 [DOI] [PubMed] [Google Scholar]
- 33.Francuzik W, Ruëff F, Bauer A, Briò MB, Cardona V, Christoff G, et al. Phenotype and risk factors of venom-induced anaphylaxis: A case-control study of the European Anaphylaxis Registry. J Allergy Clin Immunol. 2021;147(2):653–662.e9. Available from: https://www.sciencedirect.com/science/article/pii/S0091674920308381 [DOI] [PubMed] [Google Scholar]
- 34.Chong KW, Ruiz-Garcia M, Patel N, Boyle RJ, Turner PJ. Reaction phenotypes in IgE-mediated food allergy and anaphylaxis. Ann Allergy, Asthma Immunol. 2020;124(5):473–8. Available from: https://www.sciencedirect.com/science/article/pii/S1081120619315303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science (80-). 2019. Aug 30;365(6456):eaaw6433. Available from: http://science.sciencemag.org/content/365/6456/eaaw6433.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25(3):448–53. Available from: 10.1038/s41591-018-0324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DYM, Muraro A, et al. The microbiome in allergic disease: Current understanding and future opportunities--2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol. 2017. Apr 1;139(4):1099–110. Available from: 10.1016/j.jaci.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyons JJ, Chovanec J, O’Connell MP, Liu Y, Šelb J, Zanotti R, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020; Available from: http://www.sciencedirect.com/science/article/pii/S0091674920310290 [DOI] [PubMed] [Google Scholar]
- 39.Luskin KT, White AA, Lyons JJ. The Genetic Basis and Clinical Impact of Hereditary Alpha-Tryptasemia. J Allergy Clin Immunol Pract. 2021; Available from: https://www.sciencedirect.com/science/article/pii/S2213219821003068 [DOI] [PubMed] [Google Scholar]
- 40.Valent P, Akin C, Bonadonna P, Hartmann K, Brockow K, Niedoszytko M, et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J Allergy Clin Immunol Pract. 2019;7(4):1125–1133.e1. Available from: https://www.sciencedirect.com/science/article/pii/S221321981930056X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner PJ, Campbell DE. Epidemiology of severe anaphylaxis: can we use population-based data to understand anaphylaxis? Curr Opin Allergy Clin Immunol. 2016;16(5). Available from: https://journals.lww.com/co-allergy/Fulltext/2016/10000/Epidemiology_of_severe_anaphylaxis__can_we_use.6.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Vol. 6, Frontiers in Immunology. 2016. p. 620. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner PJ, Ruiz-Garcia M, Durham SR, Boyle RJ. Limited effect of intramuscular epinephrine on cardiovascular parameters during peanut-induced anaphylaxis: An observational cohort study. J Allergy Clin Immunol Pract. 2021;9(1):527–530.e1. Available from: https://www.sciencedirect.com/science/article/pii/S2213219820308734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Garcia M, Bartra J, Alvarez O, Lakhani A, Patel S, Tang A, et al. Cardiovascular changes during peanut-induced allergic reactions in human subjects. J Allergy Clin Immunol. 2021;147(2):633–42. Available from: https://www.sciencedirect.com/science/article/pii/S0091674920310241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michelson KA, Monuteaux MC, Neuman MI. Variation and Trends in Anaphylaxis Care in United States Children’s Hospitals. Acad Emerg Med. 2016;23(5):623–7. [DOI] [PubMed] [Google Scholar]
- 46.Dribin TE, Michelson KA, Monuteaux MC, Schnadower D, Neuman MI. Timing and predictors of repeat epinephrine administration among children hospitalized for anaphylaxis. J Allergy Clin Immunol Pract. 2020;8:1400–1402.e2. Available from: 10.1016/j.jaip.2019.09.028 [DOI] [PubMed] [Google Scholar]
- 47.Shaker M, Wallace D, Golden DBK, Oppenheimer J, Greenhawt M. Simulation of Health and Economic Benefits of Extended Observation of Resolved Anaphylaxis. JAMA Netw Open. 2019. Oct 23;2(10):e1913951–e1913951. Available from: 10.1001/jamanetworkopen.2019.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson LB, Arroyo AC, Faridi MK, Rudders SA, Camargo CA. Trends in US hospitalizations for anaphylaxis among infants and toddlers: 2006 to 2015. Ann Allergy, Asthma Immunol. 2021;126(2):168–174.e3. Available from: https://www.sciencedirect.com/science/article/pii/S1081120620310024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pistiner M, Mendez-Reyes JE, Eftekhari S, Carver M, Lieberman J, Wang J, et al. Caregiver Reported Presentation of Severe Food-induced Allergic Reactions in Infants and Toddlers. J Allergy Clin Immunol Pract. 2020. Nov 18; Available from: 10.1016/j.jaip.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 50.Greenhawt M, Gupta RS, Meadows JA, Pistiner M, Spergel JM, Camargo CA, et al. Guiding Principles for the Recognition , Diagnosis , and Management of Infants with Anaphylaxis : An Expert Panel Consensus. J Allergy Clin Immunol Pract. 2019;7(4):1148–1156.e5. Available from: 10.1016/j.jaip.2018.10.052 [DOI] [PubMed] [Google Scholar]
- 51.Dodd A, Hughes A, Sargant N, Whyte AF, Soar J, Turner PJ. Evidence update for the treatment of anaphylaxis. Resuscitation. 2021;163:86–96. Available from: https://www.sciencedirect.com/science/article/pii/S0300957221001507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castells M, Khan DA, Phillips EJ. Penicillin Allergy. N Engl J Med. 2019. Dec 11;381(24):2338–51. Available from: 10.1056/NEJMra1807761 [DOI] [PubMed] [Google Scholar]
- 53.Muraro A, Roberts G, Worm M, Briò MB, Brockow K, Fernández Rivas M, et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy Eur J Allergy Clin Immunol. 2014;69(8):1026–45. [DOI] [PubMed] [Google Scholar]
- 54.Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393(10187):2222–32. Available from: https://www.sciencedirect.com/science/article/pii/S0140673619304209 [DOI] [PubMed] [Google Scholar]
- 55.Wasserman RL, Factor J, Windom HH, Abrams EM, Begin P, Chan ES, et al. An Approach to the Office-Based Practice of Food Oral Immunotherapy. J Allergy Clin Immunol Pract. 2021; Available from: https://www.sciencedirect.com/science/article/pii/S2213219821002518 [DOI] [PubMed] [Google Scholar]
- 56.Goldberg MR, Nachshon L, Levy MB, Elizur A, Katz Y. Risk Factors and Treatment Outcomes for Oral Immunotherapy–Induced Gastrointestinal Symptoms and Eosinophilic Responses (OITIGER). J Allergy Clin Immunol Pract. 2020;8(1):125–31. Available from: https://www.sciencedirect.com/science/article/pii/S221321981930652X [DOI] [PubMed] [Google Scholar]
- 57.Greenhawt M, Shaker M. Keeping risk in context while rethinking the setting of asthma biologics in patient-centered care. Ann Allergy, Asthma Immunol. 2020;125(2):124–5. Available from: https://www.sciencedirect.com/science/article/pii/S1081120620303926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaker M, Briggs A, Dbouk A, Dutille E, Oppenheimer J, Greenhawt M. Estimation of Health and Economic Benefits of Clinic Versus Home Administration of Omalizumab and Mepolizumab. J Allergy Clin Immunol Pract. 2020;8(2):565–72. Available from: https://www.sciencedirect.com/science/article/pii/S2213219819308669 [DOI] [PubMed] [Google Scholar]
- 59.Shaker MS, Mosnaim G, Oppenheimer J, Stukus D, Abrams EM, Greenhawt M. Health and Economic Outcomes of Home Maintenance Allergen Immunotherapy in Select Patients with High Health Literacy during the COVID-19 Pandemic: A Cost-Effectiveness Analysis During Exceptional Times. J Allergy Clin Immunol Pract. 2020;8(7):2310–2321.e4. Available from: https://www.sciencedirect.com/science/article/pii/S2213219820304773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeJonckheere M, Lindquist-Grantz R, Toraman S, Haddad K, Vaughn LM. Intersection of Mixed Methods and Community-Based Participatory Research: A Methodological Review. J Mix Methods Res. 2018. Jun 8;13(4):481–502. Available from: 10.1177/1558689818778469 [DOI] [Google Scholar]
- 61.Schoonenboom J, Johnson RB. How to Construct a Mixed Methods Research Design. Kolner Z Soz Sozpsychol. 2017/07/05. 2017;69(Suppl 2):107–31. Available from: https://pubmed.ncbi.nlm.nih.gov/28989188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muraro A, Lemanske RF, Castells M, Torres MJ, Khan D, Simon H, et al. Precision medicine in allergic disease — food allergy , drug allergy , and anaphylaxis — PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy. 2017;72:1006–21. [DOI] [PubMed] [Google Scholar]
- 63.Tanno LK, Chalmers R, Jacob R, Kostanjsek N, Bierrenbach AL, Martin B, et al. Global implementation of the world health organization’s International Classification of Diseases (ICD)-11: The allergic and hypersensitivity conditions model. Allergy. 2020. Sep 1;75(9):2206–18. Available from: 10.1111/all.14468 [DOI] [PubMed] [Google Scholar]
- 64.Campbell RL, Hagan JB, Manivannan V, Decker WW, Kanthala AR, Bellolio MF, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129:748–52. Available from: 10.1016/j.jaci.2011.09.030 [DOI] [PubMed] [Google Scholar]
- 65.Brauer CEL, Motosue MS, Li JT, Hagan JB, Bellolio MF, Lee S, et al. Prospective Validation of the NIAID / FAAN Criteria for Emergency Department Diagnosis of Anaphylaxis. J Allergy Clin Immunol Pract. 2016;4:1220–6. Available from: 10.1016/j.jaip.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 66.Hemmings O, Kwok M, McKendry R, Santos AF. Basophil Activation Test: Old and New Applications in Allergy. Curr Allergy Asthma Rep. 2018;18(12):77. Available from: 10.1007/s11882-018-0831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J Clin Invest. 2020. Sep 1;130(9):4759–70. Available from: 10.1172/JCI138448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griffith DM. Precision Medicine Approaches to Health Disparities Research. Ethn Dis. 2020. Apr 2;30(Suppl 1):129–34. Available from: https://pubmed.ncbi.nlm.nih.gov/32269453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eysenbach G What is e-health? J Med Internet Res. 2001;3(2):e20. Available from: http://www.jmir.org/2001/2/e20/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


