Serum alpha-fetoprotein (AFP), a well-established biomarker for hepatocellular carcinoma (HCC), increases the sensitivity of ultrasound-based surveillance programs for early-stage HCC detection.1, 2 Multiple factor, including tumor burden, can affect AFP levels in patients with HCC.3 Non-tumoral factors, such as race/ethnicity and liver disease etiology, are also known to be associated with elevated AFP.3 With the increasing trend of earlier stage HCC detection and shift from viral to non-viral etiology, we hypothesized that AFP level at HCC diagnosis would decrease in the United States (US).
There are no contemporary, large-population level studies investigating AFP levels at HCC diagnosis, with most studies conducted within single-center tertiary care referral cohorts, which likely represents a biased population. We used the National Cancer Database (NCDB) to investigate recent trends and factors associated with elevated AFP in the US. The NCDB is a large clinical oncology database that represents more than 70 percent of newly diagnosed cancer cases nationally with more than 34 million historical records. We identified all HCC patients in the NCDB between January 2010 and December 2017. HCC diagnosis was based on the International Classification of Disease-Oncology-3rd Edition code C22.0 and histology codes 8170–8175. NCDB reports pre-treatment AFP in two different ways: 1) ranges with the lowest range <20 ng/mL, and 2) category (normal, borderline elevated, and elevated). In this study, we used the former range variable in most (98%) patients, with elevated AFP defined as ≥20 ng/mL, and used the category variable in those with missing AFP ranges.
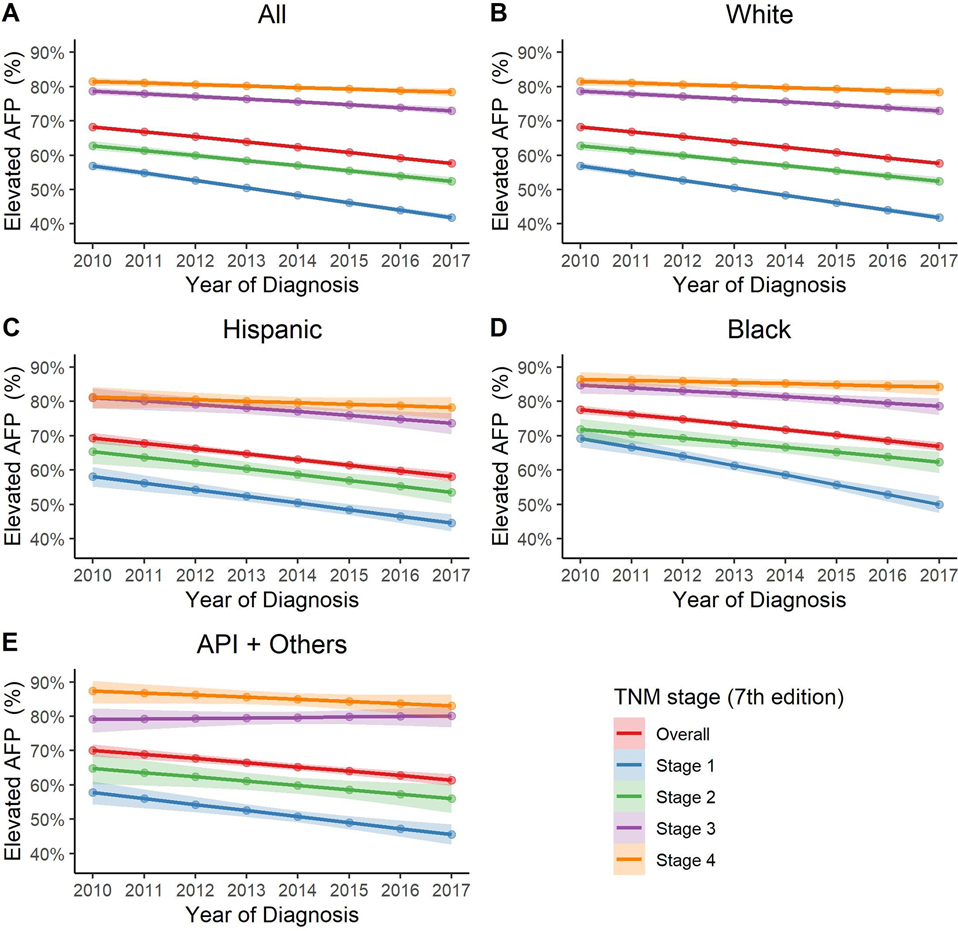
Of 133,542 patients with HCC, AFP level was reported in 108,353 patients (81%). Baseline characteristics were comparable between patients with and without AFP levels (Supplementary Table 1). The median range of AFP at HCC diagnosis has trended down from 70–79 ng/mL in 2010 to 30–39 ng/mL in 2017 (p < 0.001). Overall, 62.6% of patients with HCC had elevated AFP levels, although there has been a decline in the percentage of patients with AFP elevation from 2010 to 2017 (68.2% vs. 57.5%): - 6.6% annual change in likelihood (95% confidence interval [CI]: −7.1%, −6.0%). (Figure 1A). The decline was most notable among early-stage HCC from 55.7% in 2010 to 40.7% in 2017 with −8.7% (95% CI: −9.6%, −7.7%) annual percent changes (all p < 0.01 for pairwise comparisons between Stage 1 vs. 2, 3, 4, respectively). Similar trends were found across different race/ethnicity groups (Figure 1B–F).
Figure 1: Trend of elevated serum AFP levels at the time of HCC diagnosis, both overall and in race/ethnicity subgroups.
A. Overall.
From 2010 to 2017, there has been a decline in the percentage of HCC tumors with AFP elevation with −6.6% annual change (95% CI: −7.1%, −6.0%). This decline was most significant in early stage tumor: - 8.7% (95% CI: −9.6%, −7.7%), −6.1% (95% CI: −7.3%, −4.8%), −4.5% (95% CI: −5.9, −3.1%), −2.7% (95% CI:−4.3%, −1.1%), annual percent changes in TNM stage 1, 2, 3, 4, respectively (all p < 0.01 for pairwise comparisons between Stage 1 vs. 2, 3, 4, respectively).
B. White
In White, there has been a decline in the percentage of HCC tumors with AFP elevation with −6.2% annual change (95% CI: −6.9%, −5.5%). This decline was most significant in early stage tumor: −8.6% (95% CI: −9.8%, −7.4%), −6.0% (95% CI: −7.6%, −4.5%), −4.1% (95% CI: −5.8%, −2.3%), −2.5% (95% CI: −4.5%, −0.5%), annual percent change in TNM stage 1, 2, 3, 4, respectively (p=0.05 for Stage 1 vs. 2 and all p < 0.01 for pairwise comparisons between Stage 1 vs. 3, 4, respectively).
C. Hispanic
In Hispanic, there has been a decline in the percentage of HCC tumors with AFP elevation with −7.0% annual change (95% CI: −8.6%, −5.5%). Annual percent changes were −7.8% (95% CI: −10.4%, −5.1%), −7.1% (95% CI: −10.5%, −3.7%), −6.1% (95% CI: −10.3%, −2.0%), −2.7% (95% CI: −7.4%, 2.0%), in TNM stage 1, 2, 3, 4, respectively. (p=0.99 for Stage 1 vs. 2, p=0.92 for Stage 1 vs. 3 and p = 0.25 for Stage 1 vs. 4).
D. Black
In Black, there has been a decline in the percentage of HCC tumors with AFP elevation with −7.7% annual change (95% CI: −9.2%, −6.2%). This decline was most significant in early stage tumor: −11.6% (95% CI: −14.2%, −9.0%), −6.2% (95% CI: −9.7%, −2.7%), −5.9% (95% CI: −9.6%, −2.1%), −2.6% (95% CI: −6.9%, 1.7%), annual percent change in TNM stage 1, 2, 3, 4, respectively (p=0.07 for Stage 1 vs. 2, p=0.06 for Stage 1 vs. 3 and p < 0.01 for Stage 1 vs. 4).
E. API and others
In API and others, there has been a decline in the percentage of HCC tumors with AFP elevation with −5.5% annual change (95% CI: −7.4%, −3.6%). Annual percent changes were −7.0% (95% CI: −10.1%, −4.0%), −5.3% (95% CI: −9.6%, −0.9%), 0.9% (95% CI: −3.9%, 5.8%), −4.9% (95% CI: −11.5%, 1.7%) in TNM stage 1, 2, 3, 4, respectively (p=0.92 for Stage 1 vs. 2, p=0.03 for Stage 1 vs. 3 and p = 0.94 for Stage 1 vs. 4).
Multiple demographic and clinical features were associated with increased AFP (Supplementary Table 2). Among demographic variables, race/ethnicity had the strongest association with increased AFP. Black (AOR: 1.59, 95% CI: 1.53, 1.66), Asian (AOR: 1.30, 95% CI: 1.24, 1.36) and Hispanic patients (AOR: 1.11, 95% CI: 1.06, 1.16) were more likely to have elevated AFP compared to White patients. Among non-tumoral clinical variables, lower Charlson/Deyo Comorbidity Score (AOR: 0.85, 95% CI: 0.82, 0.88), higher MELD scores (AOR: 1.09, 95% CI: 1.07, 1.10), clinical diagnosis of HCC (AOR: 1.45, 95% CI:1.41, 1.49) were associated with elevated AFP levels.
In this contemorary nationwide study of patients with HCC, we demonstrated a downtrend in percentages of HCC tumors with elevated AFP at the time of diagnosis, with the strongest trend seen in early-stage HCC. We also observed an association between elevated AFP and demographic, socioeconomic, and clinical features of patients.
Currently, semi-annual liver ultrasound and AFP are recommended for HCC surveillance in high-risk populations. However, it is well known that the sensitivity of ultrasound-based surveillance is limited, especially in patients with metabolic etiology.4 As the incidence of HCC attributed to non-viral etiology rises, which is also associated with lower accuracy of AFP3, a newer approach is needed to enhance the accuracy of liver cancer surveillance programs.
Currently, AASLD guideline recommends diagnostic multiphasic CT/MRI for further evaluation when AFP level is 20 ng/ml or higher in the surveillance test.5 However, our results suggest that the sensitivity of AFP as a biomarker for HCC at this threshold is not optimal for early-stage HCC. These changes in AFP values at HCC diagnosis are in part related to increased early tumor detection via wider surveillance implementation but also changes in liver disease etiology, with increased suppression of chronic viral hepatitis and an increasing proportion of cirrhosis patients having non-viral liver disease. Our data reinforce prior data that the threshold to consider diagnostic imaging may need to be lowered. A large phase 2 biomarker case-control study from the Early Detection Research Network (EDRN) reported that the optimal AFP cut-off value may be lower at 10.9 ng/mL with a higher value in viral etiology (14.1 ng/ml) vs. non-viral etiology (5.5 ng/ml), and a phase III biomarker study from Korea found the best threshold was 5 ng/mL for detection of early-stage HCC.6 7 Similarly, Gopal et al reported optimal AFP cut-offs likely vary by etiology with lower AFP cut-off for non-HCV etiology.8
Due to the limitation of AFP as a single biomarker for HCC, studies are evaluating novel biomarkers and biomarker panels that may have consistent performance across liver disease etiologies. For example, the biomarker panel, GALAD, has been shown to be a highly promising approach for early detection of HCC.9 Finally, cfDNA mutation/methylation and plasma extracellular vesicles are promising novel techniques for early detection of early-stage HCC, although phase 3 external validation studies are in progress.10
There are several limitations of our study. Some pertinent data were unavailable in the database analyzed (e.g., etiology of liver disease), which can impact AFP levels. NCDB does not have data on the Barcelona Clinic Liver Cancer (BCLC) classification system; however, stage-specific analysis was performed with the TNM system, which reflects tumor burden. Finally, we were unable to evaluate AFP cut-offs below 20 ng/mL, as this is the lowest threshold reported in the NCDB.
In conclusion, our large nationwide study demonstrates a downtrend of AFP levels at HCC diagnosis, with the most remarkable decline seen among those with early-stage HCC. These results are likely due to changes in the underlying liver disease etiology and increased early tumor detection. An increasing percent of HCC with normal AFP, particularly in early-stage cancer, highlights the urgent need for novel surveillance strategies.
Supplementary Material
Acknowledgments
Conflict of Interest Statement:
Dr. Roberts has consulted for AstraZeneca, Bayer, Exact Sciences, Gilead Sciences, GRAIL, QED Therapeutics, and TAVEC, and has received grant funding from Bayer, BTG International, Exact Sciences, Gilead Sciences, GlycoTest, Redhill, TARGET PharmaSolutions and FUJIFILM Medical Systems.
Dr. Singal has been on advisory boards and served as a consultant for Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, and TARGET RWE.
Dr. Yang provides a consulting service for Exact Sciences and Gilead.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aarshi Vipani, Cedars-Sinai Medical Center.
Marie Lauzon, Cedars-Sinai Medical Center.
Michael Luu, Cedars-Sinai Medical Center.
Lewis R. Roberts, Mayo Clinic.
Amit G. Singal, University of Texas Southwestern Medical Center.
Ju Dong Yang, Cedars-Sinai Medical Center.
References:
- 1.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706–1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colli A, Nadarevic T, Miletic D, et al. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev 2021;4:Cd013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JD, Dai J, Singal AG, et al. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol Biomarkers Prev 2017;26:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Kim GA, Han S, et al. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology 2019;69:1983–1994. [DOI] [PubMed] [Google Scholar]
- 8.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of α-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol 2016;14:875–886.e6. [DOI] [PubMed] [Google Scholar]
- 10.SunN Lee YT, Zhang RY, et al. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun 2020;11:4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.