Abstract
The FoxQ1 is an oncogenic transcription factor that is overexpressed in basal-like and luminal-type human breast cancers when compared to the normal mammary tissue. The FoxQ1 is implicated in mammary tumor progression. However, the mechanism by which FoxQ1 promotes mammary tumorigenesis is not fully understood. In this study, we present experimental evidence for a novel function of FoxQ1 in regulation of complex I activity of the electron transport chain. The RNA-seq data from FoxQ1 overexpressing basal-like SUM159 cells revealed a statistically significant increase in the expression of complex I subunits NDUFS1 and NDUFS2 when compared to the empty vector (EV) transfected control cells. Consistent with these results, the basal and ATP-linked oxygen consumption rates were significantly increased by FoxQ1 overexpression in SUM159 and luminal-type MCF-7 cells. The FoxQ1 overexpression in both cell lines resulted in increased intracellular levels of pyruvate, lactate and ATP that was associated with overexpression of pyruvate dehydrogenase and pyruvate carboxylase proteins. Activity and assembly of complex I were significantly enhanced by FoxQ1 overexpression in SUM159 and MCF-7 cells that correlated with increased mRNA and/or protein levels of complex I subunits NDUFS1, NDUFS2, NDUFV1, and NDUFV2. The chromatin immunoprecipitation assay revealed recruitment of FoxQ1 at the promoters of both NDUFS1 and NDUFV1. The cell proliferation of SUM159 and MCF-7 cells was increased significantly by overexpression of NDUFS1 as well as NDUFV1 proteins. In conclusion, we propose that increased complex I-linked oxidative phosphorylation is partly responsible for oncogenic role of FoxQ1 at least in human breast cancer cells.
Keywords: FoxQ1, complex I, NDUFS1, NDUFV1, breast cancer
1. INTRODUCTION
Breast cancer remains a leading cause of cancer-related deaths worldwide. In the United States of America alone, the American Cancer Society estimates the number of new cases and deaths in the year 2021 to be 284,200 and 44,130, respectively.1 Breast cancer is a molecularly heterogeneous disease that is broadly classified into different major sub-types like luminal-type, human epidermal growth factor receptor-2 overexpressing (HER2+), basal like (mostly triple-negative), and normal-like.2 The luminal-type breast cancers are characterized by expression of estrogen and progesterone receptors and are responsive to selective estrogen receptor modulators (e.g., tamoxifen), selective estrogen receptor downregulators (e.g., fulvestrant), and aromatase inhibitors like exemestane.3,4 The HER2+ breast tumors are treated with an antibody against this receptor (trastuzumab).4 The triple-negative subtype is a complex, heterogeneous, and aggressive form of breast cancer that does not express estrogen receptor, progesterone receptor or HER2. Multiple therapeutic targets have been identified for triple-negative breast cancer including androgen receptor, epidermal growth factor receptor, poly-(ADP-ribose) polymerase, protein tyrosine kinases, phosphatases, proteases, and PI3K/Akt signaling pathway.4
The FoxQ1 belongs to the forkhead box family of transcription factors that are characterized by a conserved DNA-binding domain frequently referred to as forkhead or winged-helix domain.5,6 Normal physiological functions of FoxQ1 include regulation of hair differentiation and control of mucin expression/granule content in stomach surface mucous cells.5,6 Recent studies have implicated FoxQ1 in tumor progression for multiple solid tumors including breast cancer.7 Overexpression of FoxQ1 has been reported in basal-like and luminal-type human breast cancers in comparison with normal mammary tissues.8,9 An oncogenic function of FoxQ1 in breast cancer was first reported by Zhang et al.10 in regulation of epithelial-mesenchymal transition (EMT) through cross-species gene expression profiling strategy. Overexpression of FoxQ1 in HMLE cells resulted in increased cell migration and invasion in vitro, whereas knockdown of this protein in the 4T1 mouse mammary carcinoma cell line reversed theses effects.10 Pulmonary metastasis in vivo was also observed upon FoxQ1 overexpression.10 Moreover, the FoxQ1 overexpressing cells exhibited increased propensity for pulmonary metastasis in vivo.10 In high-grade basal-like human breast cancers, the FoxQ1 overexpression was associated with poor clinical outcomes.11 The FoxQ1 has also been shown to promote mammary stem cell-like phenotype like mammosphere multiplicity.11 Our own work revealed promotion of mammary cancer stem cell-like phenotype by overexpression of FoxQ1 in luminal-type MCF-7 cells and basal-like SUM159 cells.8 Several downstream targets of FoxQ1 have been identified including E-cadherin, dachshund homolog 1, platelet-derived growth factor receptors α/β, interleukin (IL)-1α, IL-8, and vascular endothelial growth factor.8–12
Recently, we performed RNA-seq analysis using FoxQ1 overexpressing SUM159 cells and the cells transfected with empty vector to identify additional mechanistic targets of this transcription factor.9 From the Gene Ontology (GO) pathway analysis, FoxQ1 overexpression was associated with upregulation of genes linked to locomotion, axon development, cellular component movement, cell substrate and adherens junction, focal adhesion, etc.9. On the other hand, genes linked to cell cycle regulation, mitotic cell cycle phase transition, nuclear division, nuclear chromosome segregation, microtubule, cell cycle G2/M phase transition, etc. were downregulated by FoxQ1 overexpression.9 In the present study, we explored a novel function of FoxQ1 in regulation of oxidative phosphorylation.
2. MATERIALS AND METHODS
2.1. Reagents and cell lines
Reagents for cell culture [fetal bovine serum, media, phosphate-buffered saline (PBS), and antibiotic mixture] were purchased from Invitrogen Life Technologies (Thermo Fisher Scientific, Waltham, MA). Antibodies against NDUFS1 and NDUFV1 were purchased from Proteintech (Rosemont, IL). Antibodies against pyruvate carboxylase (PC), malate dehydrogenase 2 (MDH2), pyruvate dehydrogenase (PDH), and pyruvate dehydrogenase kinase 1 (PDHK1) were from Cell Signaling (Danvers, MA). The anti-β-Actin antibody was purchased from Sigma-Aldrich (St.Louis, MO). The kits for measurement of lactate (K607–100), pyruvate (K609–100), and ATP (K354–100) were purchased from BioVision (Milpitas, CA). The MCF-7 cell line was purchased from the American Type Culture collection (Manassas, VA) whereas SUM159 cells were purchased from Asterand (Detroit, MI). Both cell lines were last authenticated by us in the year 2017. The MCF-7 and SUM159 cell lines stably transfected with the pCMV6 empty vector or the same vector encoding FoxQ1 have been described by us previously.8
2.2. Measurement of oxygen consumption rate (OCR)
The OCR was measured using a Seahorse XF24 Extracellular Flux Analyzer according to the manufacturer’s protocol. Briefly, cells were seeded in 96-well plates at a density of 50,000 cells/well. After 24 hours of cell attachment, cell culture media was replaced with unbuffered DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 2 mM GlutaMax™-1, 25 mM glucose, 1 mM sodium pyruvate, 32 mM sodium chloride and 15 mg phenol red. Basal oxygen consumption rate was measured prior to and after injection of oligomycin (2 μM), carbonyl cyanide-p- trifluoromethoxyphenyl-hydrazon (FCCP; 0.5 μM), 2-deoxy-D-glucose (2-DG; 10 mM), and a combination of rotenone and antimycin A (1 μM and 0.1 μM, respectively). All reagents were from Sigma-Aldrich. Data are expressed as pmole/min/5× 104 cells.
2.3. Quantitation of pyruvate, lactate, and ATP levels
Intracellular levels of lactate, pyruvate and ATP were determined using commercially available kits as recommended by the manufacturer.
2.4. Western blotting
Western blotting was done as described by us previously.8 Blots were stripped and re-probed with anti-β-Actin antibody for protein normalization. Densitometric quantitation was performed using UN-SCAN-IT software (Silk Scientific, Orem, UT).
2.5. Measurement of complex I activity
Complex I activity was determined as described by us previously with some minor modifications.13 Cell lysate protein (10–20 μg protein) was added to a reaction mixture containing 50 mM potassium phosphate (pH 7.5), 0.1 mM NADH, 3 mg/ml bovine serum albumin, and 0.5 mM potassium cyanide. The reaction was initiated by the addition of 60 μM ubiquinone to the mixture and the reaction product was monitored at 340 nm for 5 minutes. Subsequently, 10 μM rotenone was added to the same mixture, and the rate was measured for an additional 5 minutes at 340 nm. Complex I activity was calculated by subtracting the activity in the presence of rotenone from the total activity and normalized to the activity of citrate synthase.
2.6. Blue native-polyacrylamide gel electrophoresis (BN-PAGE)
Intact mitochondria were isolated using a commercially available kit from Abcam (Waltham, MA). Isolated mitochondria (30 μg) were dissolved in cold native PAGE buffer containing 1 % digitonin. After centrifuge at 20,000g for 30 minutes, supernatants were used for electrophoresis using Pre-cast NativePAGE™ 3–12% bis-tris gels (Thermo Fisher Scientific). Other details of BN-PAGE have been described by us previously.14
2.7. Real time-polymerase chain reaction (RT-PCR)
Total RNA from cells was extracted using RNeasy kit (Qiagen) and cDNA was synthesized using Superscript Reverse Transcriptase (Invitrogen-Life Technologies) with oligo (dT)20 primer. The RT-PCR was performed using 2x SYBR Green qPCR kit (Thermo Fisher Scientific) with 95°C (15 seconds), 60°C (30 seconds), and 72°C (20 seconds) for 40 cycles. Primers for NDUFS1 and NDUFV1 were purchased from GeneCopoeia (Rockville, MD). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used an internal control with the following primer. forward: 5’-GGACCTGACCTGCCGTCTAGAA-3’, reverse: 5’-GGTGTCGCTGTTGAAGTCAGAG-3’. Relative gene expression was calculated using the method described by Livak and Schmittgen.15
2.8. Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using a commercially available kit (Magnetic ChIP kit, Thermo Fisher Scientific) and according to supplier’s protocol. In Brief, cells were treated with 1% formaldehyde for 15 minutes at room temperature followed by addition of 0.125 M glycine to quench the crosslinking reaction. The DNA-protein complex was sonicated to generate 200–500 bp DNA fragments and then immunoprecipitated with anti-FoxQ1 antibody or control IgG for overnight. After reversal of cross-linking, DNA fragments were purified using spin columns. The putative FoxQ1-binding sites at the NDUFS1 and NDUFV1 promoter were amplified (60°C, 1 minute, 40 cycles) with the following primers: NDUFS1 site 1, forward: 5’-CAGCATATCTGTTCCAACTTTATCA-3’ and reverse: 5’-TGCCTACAGTTTCAACCTTTCC-3’; site 2, forward: 5’-CCTTAGCCAAACAAAACTACAGATA-3’ and reverse: 5’-CTGGTCTGCAGCTGTTCTTAT-3’; NDUFV1 site 1, forward: 5’-GGAAGCAGGTCAGAGAGCAG-3’ and reverse: 5’-GCAACCCACCCCTACAAAT-3’; site 2, forward: 5’-CTTCCGCAGTTTTTGTGTCC-3’ and reverse: 5’-TGAAACATGTGCTGTGTCAACT-3’; site 3, forward: 5’-TCTCTTGCTTTTCCCCACAC-3’ and reverse: 5’-AGCCCTTTGGACGTCACCT-3’; site 4, forward: 5’-AGAAAGTGGAACACACCAGGA-3’ and reverse: 5’-TCTTTCGCTCCCTCCTTAGC-3’. Fold enrichment was normalized to the input.
2.9. Transient transfection
The SUM159 and MCF-7 cells were transiently transfected with empty pCMV6 vector or the same vector encoding NDUFS1 (OriGene, Rockville, MD) or NDUFV1 (OriGene) using FUGUNE 6. Twenty-four hours after transfections, the cells were collected and processed for western blotting and cell proliferation assay. For determination of proliferation, the cells were seeded (3,000 cells/well) in 96-well plates and maintained for 48 hours. Cell Proliferation was determined using a non-radioactive cell proliferation kit from Promega.
2.10. Statistical analysis
Statistical analyses were performed using GraphPad Prism (v 7.02). For two sample comparisons, an unpaired Student’s t-test was used.
3. RESULTS
3.1. FoxQ1 overexpression resulted in induction of NDUFS1 and NDUFS2 mRNA
Initially, we examined mRNA expression of some subunits of complex I from the RNA-seq data from FoxQ1 overexpressing SUM159 cells and the same cell line transfected with empty vector (hereafter abbreviated as EV).9 There was a trend of an increase in expression of NDUFV1 and NDUFV2 upon overexpression of FoxQ1 in SUM159 cells in comparison with EV cells, but the difference was not significant (Figure 1A). On the other hand, overexpression of FoxQ1 resulted in a statistically significant increase in expression of NDUFS1 and NDUFS2 (Figure 1A). These analyses of the previously published RNA-seq data9 prompted us to determine the effect of FoxQ1 overexpression on oxidative phosphorylation. Because overexpression of FoxQ1 is yet to be demonstrated in HER2+ human breast cancers, cell lines representative of only luminal-type (MCF-7) and basal-like (SUM159) were used for the functional experiments discussed below.
Figure 1.
FoxQ1 overexpression increased OCR in breast cancer cells. A, mRNA expression of complex I subunits in SUM159 cells stably transfected with FoxQ1 or empty vector (EV). B, oxygen consumption rate (OCR) profiling using EV cells or FoxQ1 overexpressing SUM159 cells. The OCR analysis starts from basal respiration and after the addition of oligomycin (Oligo), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), 2-deoxy-D-glucose (2-DG) and a combination of rotenone (rot) and antimycin A (AA). The bar graphs show basal and ATP-linked OCR in SUM159 (C) and MCF-7 cells (D). Data shown are mean ± SD (n=3–4). All data are representative of two independent experiments. *Statistically significant (P < 0.05) compared to EV cells by Student’s t-test.
3.2. FoxQ1 overexpression increased OCR
A role of FoxQ1 in regulation of metabolic reprogramming has not been studied previously. In order to investigate this possibility, we measured OCR using FoxQ1 overexpressing SUM159 and MCF-7 cells and corresponding EV cells. Figure 1B shows OCR in EV or FoxQ1 overexpressing SUM159 cells. The basal OCR was 2.3- and 1.6-fold higher, respectively, in FoxQ1 overexpressing SUM159 and MCF-7 cells in comparison with corresponding EV cells (Figure 1C, D). Similarly, the ATP-linked respiration was significantly higher in FoxQ1 overexpressing SUM159 and MCF-7 cells in comparison with corresponding EV cells (Figure 1C, D). These results showed an increase in OCR in breast cancer cells overexpressing FoxQ1.
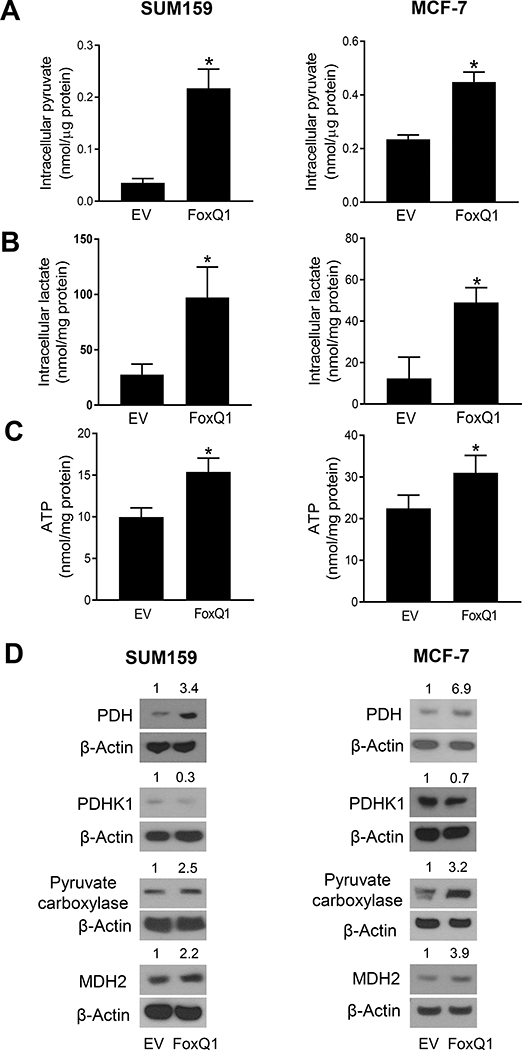
3.3. FoxQ1 overexpression increased intracellular levels of pyruvate, lactate, and ATP
We measured the intracellular levels of pyruvate and lactate which are the final products of glycolysis. As can be seen in Figure 2A, the intracellular level of pyruvate was significantly increased in FoxQ1 overexpressing cells compared with EV cells. Likewise, the intracellular level of lactate also was higher in FoxQ1 overexpressing cells with about 3.5-fold (SUM159) or 4-fold (MCF-7) increase in FoxQ1 overexpressing cells compared with EV cells (Figure 2B). The ATP level was significantly elevated by FoxQ1 overexpression in SUM159 and MCF-7 cell lines (Figure 2C). Increased pyruvate level in FoxQ1 overexpressing cells was associated with overexpression of PDH, PC, and MDH2 (Figure 2D). Together, these results indicated that FoxQ1 overexpressing cells preferentially relied on oxidative phosphorylation to generate ATP.
Figure 2.
FoxQ1 overexpression increased intracellular levels of pyruvate, lactate, and ATP. Intracellular levels of pyruvate (A), lactate (B) and ATP (C) in EV cells or FoxQ1 overexpressing SUM159 and MCF-7 cells. Results are shown as mean ± SD (n = 3). *Statistically significant (P < 0.05) compared to EV cells by Student’s t-test. D, Immunoblotting for PDH, PDHK1, PC, and MDH2 proteins using lysates from EV cells or FoxQ1 overexpressing SUM159 and MCF-7 cells. Numbers on top of bands are fold change in protein level relative to corresponding EV cells. Results were consistent in replicate experiments. PDH, pyruvate dehydrogenase; PDHK1, pyruvate dehydrogenase kinase 1; PC, pyruvate carboxylase; MDH2, malate dehydrogenase 2.
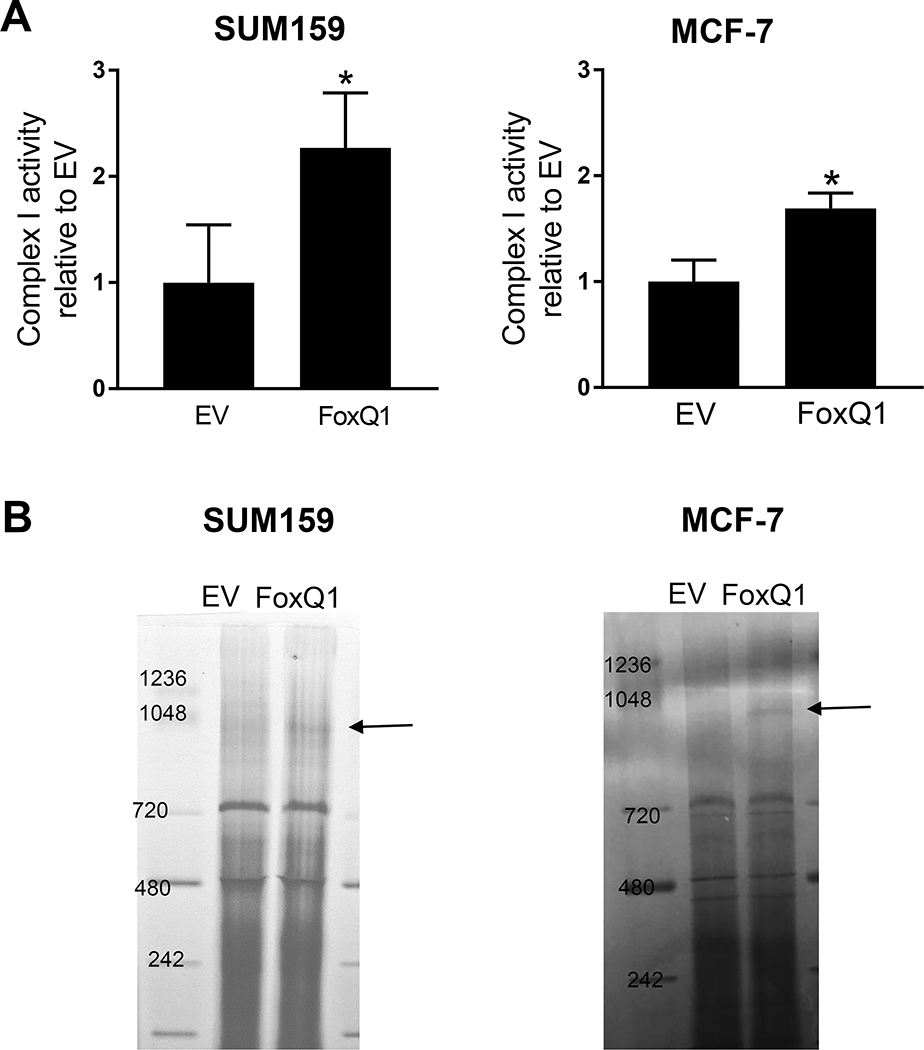
3.4. FoxQ1 overexpression increased complex Ⅰ activity
Next, we measured respiratory chain complex I enzyme activity using isolated mitochondrial from FoxQ1 overexpressing and EV cells. As shown in Figure 3A, FoxQ1 overexpressing cells showed a significant increase in complex Ⅰ activity, but the effect was more pronounced in the SUM159 cell line compared to MCF-7 cells. We next performed BN-PAGE, which is a technique to monitor assembly of mitochondrial complexes. The assembly of complex I was also increased upon overexpression of FoxQ1 in both SUM159 and MCF-7 cells (Figure 3B).
Figure 3.
FoxQ1 overexpression increased complex Ⅰ activity. A, Complex Ⅰ activity was measured in mitochondria isolated from EV cells or FoxQ1 overexpressing SUM159 and MCF-7 cells. Complex Ⅰ activity was normalized to citrate synthase activity and the value are expressed as relative to EV cells. Results shown are mean ± S.D (n=3). *Significantly different (P < 0.05) compared to EV cells by Student’s t-test. B, Blue native-polyacrylamide gel electrophosresis using EV cells or FoxQ1 overexpressing SUM159 and MCF-7 cells. Complex Ⅰ is identified by an arrow. Each experiment was repeated at least three times with comparable results.
3.5. FoxQ1 regulated NDUFS1 and NDUFV1 expression in breast cancer cells
Complex Ⅰ is the first and the largest complex of the electron transport chain and consists of 44 subunits, including 14 “core” subunits and 30 “accessory” subunits.16 To investigate which molecules are critically involved in alterations in complex I activity by FoxQ1 overexpression, we determined the mRNA expression of four complex I subunits (NDUFS1, NDUFS2, NDUFV1, and NDUFV2), which are parts of the 7 nDNA-encoded subunits among 14 core subunits.16 As can be seen in Figure 4A, the mRNA levels NDUFS1, NDUFS2, NDUFV1, and NDUFV2 were significantly increased by FoxQ1 overexpression in SUM159 cells compared with EV cells. On the other hand, MCF-7-FoxQ1 cells showed increased expression of NDUFS1, NDUFS2, and NDUFV1 mRNA (Figure 4A). Western blotting confirmed overexpression of NDUFS1 and NDUFV1 protein expression in FoxQ1 overexpressing SUM159 and MCF-7-FoxQ1 cells when compared to corresponding EV cells (Figure 4B).
Figure 4.
FoxQ1 regulated expression of NDUFS1 and NDUFV1 in breast cancer cells. A, Quantitation of NDUFS1, NDUFS2, NDUFV1 and NDUFV2 mRNA expression by real-time qRT-PCR in EV or FoxQ1 overexpressing SUM159 and MCF-7 cells. Results shown are mean ± SD (n = 3). *Significantly different (P < 0.05) compared to EV cells by Student’s t-test. B, Western blotting for NDUFS1 and NDUFV1 proteins using lysates from EV or FoxQ1 overexpressing SUM159 and MCF-7 cells. Similar results were observed in replicate experiments
3.6. FoxQ1 was recruited to the promoters of NDUFS1 and NDUFV1
Next, we performed ChIP assays to determine whether NDUFS1 or NDUFV1 were direct regulatory targets of FoxQ1. The promoter of NDUFS1 contains two putative FoxQ1 binding consensus sequences of (A/T)TGTTTA(G/T) (Figure 5A).17 The ChIP assay revealed recruitment of FoxQ1 at both sites in SUM159 as well as MCF-7 cells (Figure 5B). Possible FoxQ1 binding consensus sequences were also identified at the promoter of NDUFV1 (Figure 5C). The ChIP assay confirmed recruitment of FoxQ1 at all four sites of NDUFV1 promoter (Figure 5D). Collectively, these observations indicated that FoxQ1 was a direct regulator of NDUFS1 and NDUFV1 expression in breast cancer cells.
Figure 5.
FoxQ1 was recruited to the promoters of NDUFS1 and NDUFV1. A, Putative FoxQ1 binding sequences at the NDUFS1 promoter. B,) The bar graphs from the ChIP assays show the recruitment of FoxQ1 at the NDUFS1 promoter in SUM159 and MCF-7 cells. C, Putative FoxQ1 binding sequences at the NDUFV1 promoter. D, ChIP assay using SUM159 and MCF-7 showing the binding of FoxQ1 to the NDUFV1 promoter. The results shown are mean ± SD (n = 3). *Significantly different (P < 0.05) compared to control by Student’s t-test.
3.7. NDUFS1 and NDUFV1 overexpression increased breast cancer cell proliferation
Next, to clarify whether NDUFS1 or NDUFV1 affected breast cancer cell proliferation, we performed ectopic expression of NDUFS1 or NDUFV1 by transient transfection. Increased Myc-flag-tagged level of NDUFS1 protein (Figure 6A) or NDUFV1 protein (Figure 6B) was confirmed by western blotting. In SUM159 cells, NDUFV1 ectopic expression resulted in a 70 % increase in cell proliferation compared with EV cells (Figure 6C). The NDUFS1 overexpressing cells also exhibited a significant increase in cell proliferation but not as much as NDUFV1 overexpressing cells (Figure 6C). In MCF-7 cells, both NDUFS1 and NDUFV1 overexpression caused a significant increase in cell proliferation (Figure 6C).
Figure 6.
Effect of ectopic expression of NDUFS1 or NDUFV1 protein on cell proliferation. Immunoblotting for NDUFS1 (A) or NDUFV1 (B) protein using lysates from SUM159 and MCF-7 cells transiently transfected with the empty pCMV6 vector or the same vector encoding for NDUFS1 or NDUFV1. C, Overexpression of NDUFS1 or NDUFV1 promoted SUM159 and MCF-7 cells proliferation. The data presented are mean ± S.D (n=3 or 4). *Significantly different (P < 0.05) compared with EV cells by Student’s t-test. Comparable results were observed in repeated experiments.
4. DISCUSSION
The FoxQ1 is best known for its role in regulation of EMT and maintenance of stem-like cells.10,11 The FoxQ1-mediated promotion of EMT was accompanied by transcriptional repression of epithelial regulator E-cadherin.11 Consistent with these results, FoxQ1 overexpressing cells not only acquired a more aggressive phenotype such as increased pulmonary metastasis in vivo but also conferred resistance to chemotherapy-induced apoptosis.10,11 Depletion of FoxQ1 in basal-like MDA-MB-231 human breast cancer cell line led to increased apoptosis following treatment with chemotherapeutic agents 5-fluorouracil, paclitaxel, and camptothecin.11 The present study documents yet another novel function of FoxQ1 in regulation of complex I activity. FoxQ1 overexpression resulted in an increase in basal- and ATP-linked OCR and ATP levels that was not a cell line-specific phenomenon. Thus, increased ATP levels may also contribute to oncogenic functions of FoxQ1. Because FoxQ1 promotes maintenance of breast cancer stem-like cells,8,11 it would be worthwhile in future to determine its role in regulation of complex I activity in this population.
The pyruvate derived from glucose is either converted to lactate through catalytic mediation of lactate dehydrogenase or metabolized to acetyl-CoA, a reaction catalyzed by pyruvate dehydrogenase, that enters the tricarboxylic (TCA) cycle. We found an increase in the intracellular levels of both pyruvate and lactate in FoxQ1 overexpressing cells. The expression of PDH was increased upon overexpression of FoxQ1 in both MCF-7 and SUM159 cells. The PDH activity is negative regulated by PDHK1 whose expression was repressed in FoxQ1 overexpressing cells. The PDH is considered to have oncogenic role. One study concluded that breast cancer cells rely on the pyruvate to drive collagen-based remodeling of the extracellular matrix in the pulmonary metastatic niche.18 AMP-activated kinase-mediated phosphorylation of the alpha catalytic subunit of PDH was shown to drive TCA cycle and promote metastasis.19 We also found an increase in protein level of PC in FoxQ1 overexpressing cells. The PC was shown to be required for the growth of breast cancer that metastasized to the lungs.20 A small molecule inhibitor of PC inhibited breast cancer progression.21 Survival benefit was observed in breast cancer patients with lower expression of PC.21 The role of MDH2, which catalyzes the reversible conversion of malate and oxaloacetate, in breast cancer is not clear but this protein is differentially expressed in triple negative breast cancer when compared to luminal-type and HER2+ subtypes.22 Nevertheless, further studies are needed to determine whether PDH, PC, and MDH2 are direct transcriptional targets of FoxQ1 and if this protein also regulates TCA cycle.
We found that the expression of at least two subunits of complex I (NDUFS1 and NDUFV1) is regulated by FoxQ1 in both MCF-7 and SUM159 cells. The FoxQ1 is recruited at the promoters of both NDUFS1 and NDUFV1 and protein and mRNA levels of these subunits are increased upon stable overexpression of FoxQ1. Proliferation of both SUM159 and MCF-7 cells is increased significantly by ectopic expression of NDUFS1 and NDUFV1 proteins. The role of NDUFS1 or NDUFV1 in breast cancer is yet to be studied, but low NDUFS1 mRNA expression was shown to be a biomarker in clear renal cell carcinoma.23 Further studies are needed to determine if a similar association exists in breast cancer. Depletion of NDUFV1 in B16ρ0 mouse melanoma cells significantly diminished its ability to form tumors.24
In conclusion, the present study shows that FoxQ1 overexpression in basal-like and luminal-type human breast cancer cells increases complex I activity through direct transcriptional regulation of NDUFS1 and NDUFV1. The effects may contribute to the oncogenic function of FoxQ1.
FUNDING
This study was supported by the National Cancer Institute at the National Institutes of Health grant R01 CA219180 (to SVS). This study used the UPMC Hillman Cancer Center resources supported by the National Cancer Institute at the National Institutes of Health grant P30 CA047904.
ABBREVIATIONS:
- AA
antimycin A
- BN-PAGE
blue native-polyacrylamide gel electrophoresis
- ChIP
chromatin immunoprecipitation
- 2-DG
2-deoxy-D-glucose
- EV
empty vector transfected cells
- FCCP
carbonyl cyanide-p- trifluoromethoxyphenyl-hydrazon
- FoxQ1
forkhead box Q1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GO
gene ontology
- IL
interleukin
- MDH2
malate dehydrogenase 2
- Oligo
oligomycin
- OCR
oxygen consumption rate
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- PDHK1
pyruvate dehydrogenase kinase 1
- rot
rotenone
- RT-PCR
real-time quantitative polymerase chain reaction
- TCA cycle
tricarboxylic acid cycle
Footnotes
CONFLICT OF INTEREST
The authors do not declare any conflict of interest.
DATA AVAILABILITY STATEMENT
The data and the cell lines used for this publication are available upon formal request to the senior author. A material transfer agreement will be executed between the requester and the senior author before the material transfer.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021; 7:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 3.Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10:2293–2301. [DOI] [PubMed] [Google Scholar]
- 4.Nagini S Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem.2017;17:152–163. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson P, Mahlapuu M. Forkhead transcription factors: Key players in development and metabolism. Dev Biol. 2002;250:1–23. [DOI] [PubMed] [Google Scholar]
- 6.Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: Key players in health and disease. Trends Genet.2011;27:224–232. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Zhang Y, Yao Z, Li S, Yin Z, Xu M. Forkhead box Q1: A key player in the pathogenesis of tumors (Review). Int J Oncol.2016;49:51–58. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Kaschula CH, Priedigkeit N, Lee AV, Singh SV. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J Biol Chem. 2016;291:13495–13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Hahm ER, Singh KB, Singh SV. Novel mechanistic targets of forkhead box Q1 transcription factor in human breast cancer cells. Mol Carcinog. 2020;59:1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Meng F, Liu G, et al. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 2011;71:1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao Y, Jiang X, Lee ST, Karuturi RK, Hooi SC, Yu Q. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res. 2011;71:3076–3086. [DOI] [PubMed] [Google Scholar]
- 12.Meng F, Speyer CL, Zhang B, et al. PDGFRα and β play Critical roles in mediating Foxq1-driven breast cancer stemness and chemoresistance. Cancer Res. 2015;75:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehrawat A, Samanta SK, Hahm ER, St Croix C, Watkins S, Singh SV. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion. 2019;47:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 16.Rodenburg RJ. Mitochondrial complex I-linked disease. Biochim Biophys Acta. 2016;1857:938–945. [DOI] [PubMed] [Google Scholar]
- 17.Bieller A, Pasche B, Frank S, et al. Isolation and characterization of the human forkhead gene FoxQ1. DNA Cell Biol. 2001;20:555–561. [DOI] [PubMed] [Google Scholar]
- 18.Elia I, Rossi M, Stegen S, et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature. 2019;568:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Z, Li CF, Han F, et al. Phosphorylation of PDHA by AMPK drives TCA cycle to promote cancer metastasis. Mol Cell. 2020;80:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinde A, Wilmanski T, Chen H, Teegarden D, Wendt MK. Pyruvate carboxylase supports the pulmonary tropism of metastatic breast cancer. Breast Cancer Res. 2018;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, He Y, Wang X, et al. Targeting pyruvate carboxylase by a small molecule suppresses breast cancer progression. Adv Sci (Weinh). 2020;7:1903483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz DM, Böllner C, Thomas G, et al. Identification of differentially expressed proteins in triple-negative breast carcinomas sing DIGE and mass spectrometry. J Proteome Res. 2009;8:3430–3438. [DOI] [PubMed] [Google Scholar]
- 23.Ellinger J, Poss M, Brüggemann M, et al. Systematic expression analysis of mitochondrial complex I identifies NDUFS1 as a biomarker in clear-cell renal-cell carcinoma. Clin Genitourin Cancer. 2017;15:e551–e562. [DOI] [PubMed] [Google Scholar]
- 24.Dong LF, Kovarova J, Bajzikova M, et al. Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. Elife. 2017;6:e22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and the cell lines used for this publication are available upon formal request to the senior author. A material transfer agreement will be executed between the requester and the senior author before the material transfer.