Abstract
The mesolimbic dopamine (DA) system reinforces behaviors that are critical for survival. However, drug dependence can occur when drugs of abuse, such as nicotine, highjack this reinforcement system. Pharmacologically targeting the DA system to selectively block drug reinforcement requires a detailed understanding of the neural circuits and molecular pathways that lead to the reward-based activation of mesolimbic circuits. Varenicline is an approved smoking cessation drug that has been shown to block nicotine-evoked DA increases in the nucleus accumbens (NAc) through action on nicotinic acetylcholine receptors. Because these receptors have been implicated in the reinforcement of other addictive substances, we explored the possibility that varenicline could broadly affect reward processing. We used in vivo fiber photometry to monitor midbrain DA neuron activity and striatal DA levels following either natural or drug rewards in mice treated with varenicline. We demonstrate that varenicline pretreatment enhances the suppression of nicotine-evoked DA release by attenuating DA neuron activity in the VTA. Varenicline’s ability to attenuate DA release is highly specific to nicotine, and varenicline slightly elevates DA release when co-administered with morphine or ethanol. Furthermore, varenicline has no effect on DA release in response to naturally rewarding behavior such as food intake or exercise. These results demonstrate the exquisite specificity with which varenicline blocks nicotine reward and highlight the complexity with which different rewards activate the mesolimbic DA system.
Keywords: Smoking cessation, nicotine, dopamine, calcium imaging, drug reward, natural reward
INTRODUCTION
Behaviors necessary for survival activate the brain’s reward system, increasing dopamine (DA) neuron activity and boosting striatal DA levels (Blackburn et al., 1992; Palmiter, 2008; Volkow et al., 2017). Drugs like nicotine, morphine and alcohol can co-opt these circuits to facilitate drug dependence (Gysling and Wang, 1983; Gessa et al., 1985; Grenhoff et al., 1986; Pidoplichko et al., 1997), a state characterized by dysregulated DA signaling (Volkow and Morales, 2015). Blocking the ability of drugs to act on the DA system is a logical intervention for substance use disorders (Volkow et al., 2004). However, this method is complicated by the fact that natural rewards such as food, sex, and exercise are also reinforced by DA release (Blackburn et al., 1992; Palmiter, 2008; Volkow et al., 2017). Determining the circuits and molecular mechanisms through which drugs and natural rewards differentially activate the DA system may enable the development of therapeutics that specifically reduce the reinforcing properties of drugs while potentially minimizing unwanted side effects.
Common interventions such as methadone or nicotine replacement therapy mimic drug effects on the mesolimbic DA pathway. While these methods are effective in alleviating withdrawal symptoms and reducing cravings, it is difficult to discontinue their use and relapse is common (Magura and Rosenblum, 2001; Volkow et al., 2019). A more desirable approach involves simultaneously reducing drug craving as well as blocking specific drug rewards. Varenicline is a smoking cessation aid approved by the Food and Drug Administration (Coe et al., 2005) that targets the DA system. Varenicline competes with nicotine for binding at nicotinic acetylcholine receptors (nAChRs) (Rollema et al., 2007). In the central nervous system, the dominant receptor subtypes are α4β2-containing nAChRs and homomeric α7 nAChRs (Benowitz, 2009) and activation of nAChRs increases firing of ventral tegmental area (VTA) DA neurons (Picciotto et al., 1998; Maskos et al., 2005). When administered independently, varenicline modestly enhances DA in the nucleus accumbens (NAc). By contrast, varenicline blunts the normal DA response when given in combination with nicotine (Coe et al., 2005). This effect is likely a result of the drug impeding the ability of nicotine to bind the α4β2 receptor and stimulate DA neuron activity (Jordan and Xi, 2018). By modestly boosting DA levels and attenuating nicotine-evoked DA release varenicline is thought to simultaneously ease drug craving and significantly decrease smoking satisfaction (Aubin et al., 2008; Ciano et al., 2016).
Given the ubiquitous activity of the DA system in response to rewards, could varenicline be used to curb other addictions? Rodent studies and a small-scale clinical study on heavy alcohol consumers suggest varenicline may also attenuate ethanol intake (Steensland et al., 2007; McKee et al., 2009; Lacroix et al., 2017). Additionally, varenicline administration decreased sucrose intake in rats (Shariff et al., 2016). However, because subpopulations of VTA DA neurons have been described (Lammel et al., 2014; Poulin et al., 2018), it is plausible that different rewards act on discrete neural circuits or receptors on VTA subpopulations.
Furthermore, the effects of varenicline on DA responses to ethanol and other drugs are controversial (Ericson et al., 2009; Feduccia et al., 2014) and it remains unknown if varenicline alters either the time course or the magnitude of DA signals in response to natural rewards.
We tested the ability of varenicline to alter VTA DA neuron activity and NAc DA release following acute administration of nicotine, other drug rewards, or natural rewards such as food and exercise. We demonstrate that varenicline strongly blunts nicotine-evoked DA release, but not DA released in response to other drugs. Further, we find that varenicline does not affect food- or exercise-evoked DA release. These results demonstrate that distinct pathways and receptor subtypes are involved in reinforcing natural and drug rewards, and have important implications for the clinical use of varenicline.
EXPERIMENTAL PROCEDURES
Experimental Model
Mice were group housed on a 12 hr light/dark cycle with ad libitum access to chow (LabDiet, 5001) and water unless otherwise noted. C57BL/6J and DAT-IRES-Cre (B6.SJL-Slc6a3tm1.1(cre)Bkmn/J) (Bäckman et al., 2006) heterozygous male and female mice greater than 8 weeks old were habituated to handling and experimental conditions prior to use. All mice received all experimental conditions for within-subject analyses and mice were randomly assigned to experimental condition for between-subject analyses. Individual mice underwent between one and three counter-balanced experiments, with at least 24 hr separating each assay. In total, 83 mice were used across all studies. For details on mice used in each experiment, see Table S1. All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Viral Injections and Fiber Optic Implantations
The following rAAV vectors were used: AAV1-Syn-Flex-GCaMP6f-WPRE-SV40 (Addgene #100833, titer: 4.216e13 GC/mL) and AAV9-hSyn-GRAB_DA1h (Addgene #113050 2.4e13 GC/mL). Syn, Synapsin promoter. hSyn, human Synapsin 1 promoter. SV40, sequence motif promoting polyadenylation and termination. Flex, Cre-dependent flip-excision switch. WPRE, woodchuck hepatitis virus response element. GCaMP, genetically encoded calcium indicator resulting from a fusion of GFP, M13 and Calmodulin. GRAB, G protein-coupled receptor activation-based dopamine sensor. DA1h, dopamine 1 high affinity.
Viral injections were performed as previously described (Su et al., 2017). Briefly, mice were pretreated with subcutaneous injections of Meloxicam (5mg/kg, Norbrook Laboratories, 55529–040-11) and bupivacaine (2mg/kg, Moore Medical, 52683). Mice were anesthetized with isoflurane (3%, Clipper, 0010250) and placed into a stereotaxic frame (Stoelting, 51725D) with continuous isoflurane (1–2%). Viral injections were performed as previously described (Alhadeff et al., 2019). Briefly, for ventral tegmental area (VTA) fiber photometry, 200 μL of AAV1-Syn-Flex-GCaMP6f-WPRE-SV40 was injected unilaterally at the following coordinates: bregma −3.2 mm, midline ± 1.2 mm, and skull −4.4 mm at a 10° angle from vertical in the medial/lateral axis. For nucleus accumbens (NAc) and dorsal striatum (DS) fiber photometry, 200 μL of AAV9-hSyn-GRAB_DA1h was injected unilaterally at the following coordinates: NAc: bregma +1.0 mm, midline ± 1.2 mm, and skull −4.1–4.0 mm; DS: bregma +1.0 mm, midline ± 1.3 mm, and skull −2.7 mm. A ferrule-capped optical fiber (400-mm core, NA 0.48, Doric, MF2.5, 400/430–0.48) was implanted 0.2 mm above the injection site and secured to the skull with low auto-fluorescent cements: Metabond cement (Parkell, S380) and dental cement (Lang Dental Manufacturing, Ortho-jet BCA Liquid, B1306 and Jet Tooth Shade Powder, 143069). Mice were given at least 2 weeks for recovery and viral expression before experiments were performed.
Fiber Photometry
GCaMP6 was imaged using dual-wavelength fiber photometry (FP) as we have previously described (Su et al., 2017; Alhadeff et al., 2019). GRAB DA1m was imaged using identical parameters except the 405-nm wavelength was not used. Briefly, for dual wavelength FP, two excitation wavelengths (470 nm and 405 nm) were modulated at 211 and 566 Hz to avoid contamination from overhead lights and cross-talk between excitation lights. Excitation illumination was generated though fiber-coupled LEDs (Thorlabs, M470F3 and M405F1) and modulated by a real-time amplifier (Tucker-Davis Technologies, RZ5P or RZ10x). Excitation lights were filtered and combined by a fluorescence mini cube (Doric, FMC4_IE(400–410)_E(460–490)_F(500–550)_S). The combined excitation light was delivered through a 400 μm core, 0.48 NA, low-fluorescence optical fiber (Doric, MFP_400/430/1100–0.48_1.5_FCM-MF) to an implanted fiber (Doric, MFC_400/430–0.66_6.5mm_MF2.5_FLT) secured using a clamp (Thorlabs, ADAF2). LED power was set between 20 and 40 μW emitted from the fiber tip to minimize bleaching. GCaMP6f emission fluorescence was collected through the mini cube and focused onto a femtowatt photoreceiver (Newport, Model 2151, gain set to DC LOW). Fluorescence was sampled at 1017 Hz, and demodulated by the processor. LEDs were externally controlled by Synapse (Tucker-Davis Technology), and synchronized cameras (Ailipu Technology, ELP-USB100W05MT-DL36) were used to video-record mice during experiments.
Photometry during drug administration.
Mice were habituated to handling, injections, and tethering prior to all fiber photometry experiments. For ‘co-administration’ experiments, drugs were injected after a 5 min baseline period. IP injections were given approximately 30 s after the SC injection to allow the solution to diffuse prior to restraint. Recordings continued for 10–30 min after injections. Traces were aligned to the IP injection. For pretreatment experiments, SC saline or varenicline were administered after a 5 min baseline period. IP injections were given 30 min later, and the recording continued for 30 min after IP injections. Traces were aligned to the IP injection. Experimental conditions were counter-balanced, and at least 24 hr separated each recording.
Photometry during food intake.
Food was removed 24 hr prior to recordings. After recording a 5 min baseline, mice were given SC injections of saline or varenicline followed by placing a pellet of standard chow in the cage. For VTA recordings, the pellet was given 30 s after the injections. For NAc recordings, the pellet was given 10 min after injection. Recordings continued for 5 min following food presentation, and traces were aligned to the moment the food was placed in the cage.
Photometry during exercise.
A motorized treadmill (Exer-6; Columbus Instruments, Columbus, OH) was used for exercise experiments. All mice were habituated to the treadmill for 3 days prior to exercise. Habituation day 1: 3 hr rest on treadmill; habituation day 2: 2 hr rest on treadmill followed by 15 min at 6 m/min, 5 min at 8 m/min, and 5 min at 10 m/min; habituation day 3: 1 hr rest on treadmill followed by 15 min at 10 m/min, 5 min at 8 m/min, and 15 min at 10 m/min.
Mice were placed on the treadmill for a 1 hr acclimation period prior to recordings. Following acclimation, mice were treated with a SC injection of 1.5 mg/kg varenicline or saline and placed back on the treadmill. Recording began 25 min after injection while mice were still stationary, and running began 5 min later at 30 min post injection. Mice ran for 10 min at 10 m/min. The treadmill was stopped and recording continued for 15 min following the run. Recordings were aligned to the start of exercise to plot traces, calculate average ΔF/F, and calculate the maximum ΔF/F during exercise. Recordings were aligned to the end of exercise to calculate maximum ΔF/F after exercise. Experimental conditions were counter-balanced and separated by at least 24 hr.
Fiber Photometry Analysis
Data were exported to MATLAB (MathWorks) from Synapse using a script provided by Tucker-Davis Technology. Custom MATLAB scripts were used to independently normalize the demodulated 470-nm and 405-nm signals. ΔF/F was calculated (F-Fbaseline)/Fbaseline, with Fbaseline being the median of the 300 s before the stimulus. Data were down-sampled to 1 Hz. Subsequent plotting and analysis were performed in MATLAB and Prism 8 (GraphPad). Mean ΔF/F was calculated by integrating ΔF/F over a period of time after the stimulus and then dividing by the integration time. Maximum ΔF/F were calculated by taking ΔF/F value for each mouse at the maximum of the average trace.
Drugs
Varenicline tartate (1.5 mg/kg, Toronto Research Chemicals, V098490), nicotine hydrogen tartate (0.1, 0.5, and 1.5 mg/kg, Glentham Life Sciences, GL9693), and morphine (10 mg/kg, Sigma-Aldrich, M8777) were dissolved in saline. GTS 21 (4 mg/kg, Abcam, ab120560), a partial agonist of the α7 nAChR, was dissolved in DMSO. Stock solutions were aliquoted and frozen. Prior to experiments, stock solutions were diluted with saline to the appropriate concentrations for injection. 200 proof ethanol (2 g/kg) was diluted 1:4 in saline. Mice received injections of 100 μL solution per 10 g of body weight. A varenicline dose that was previously shown to increase NAc DA and attenuate nicotine-evoked NAc DA in vivo in rodents was chosen (Ericson et al., 2009). Varenicline also decreases ethanol consumption at the dose selected (Steensland et al., 2007). Drug doses that we have shown to significantly elevate GRAB-DA signal in the NAc were used (Alhadeff et al., 2019). Nicotine doses for feeding experiments were selected based on prior work showing nicotine’s long-term anorexic effects in mice (Mineur et al., 2011).
Food Intake Experiments
Cages were lined with a KimTech bench protector to allow for collection of crumbs to accurately measure food intake. Mice were habituated to the special caging conditions before experiments. Food was removed 24 hr prior to experiments. For varenicline experiments, mice were given SC injections of 1.5 mg/kg varenicline or saline and placed in a cage with a weighed pellet of chow (LabDiet, 5001) or high-fat, high-sugar diet (Research Diets, D12492). Food was weighed at 15 min, 30 min, and 1 hr, accounting for crumbs. For nicotine experiments, mice were given IP injections of saline, 0.1, 0.5, or 1.5 mg/kg nicotine. Chow was weighed at 1 and 24 hr, accounting for crumbs. Experiments were counter-balanced and separated by at least 72 hr to allow mice to return to their baseline body weights.
Histology and Imaging
Mice were deeply anesthetized with isoflurane (Clipper, 0010250) and transcardially perfused with 10 mL 0.1 M Dulbecco’s phosphate-buffered saline (PBS, HyClone, SH30013.04) followed by 10 mL 4% paraformaldehyde (MP Biomedicals, 150146). Brains were removed and post-fixed overnight then transferred to PBS. 200 μm coronal sections were cut with a vibrating blade microtome (Leica, VT1000S). Epifluorescence images were taken on a Leica SPE microscope to verify viral expression and fiber placements.
Quantification and Statistical Analysis
Data were expressed as means ± SEMs in FP traces and line graphs. For box and whisker plots, the box extends from the 25th to 75th percentiles, the line in the box represents the median, and the whiskers extend from the maximum to minimum values. Paired or unpaired two-tailed t-tests were performed as appropriate. One-way, two-way, and fixed-effect repeated measures ANOVA were used to make comparisons across more than two groups using Prism 8 (GraphPad). For unpaired t-tests, equal variance was not assumed, and Welch’s correction was used. For repeated measures two-way ANOVAs, sphericity was not assumed, and the Geisser-Greenhouse method was used to correct for violations of the sphericity assumption. The Holm-Šídák method was used to correct for multiple comparisons. Test, statistics, significance levels, and sample sizes for each experiment are listed in Table S1. ns p>0.05, t-tests and post-hoc comparisons: *p<0.05, **p<0.01, ***p<0.001; interaction: ∞p<0.05, ∞∞p<0.01, ∞∞∞p<0.001; main effect (group, condition or drug): ☼<0.05, ☼☼p<0.01, ☼☼☼p<0.001.
RESULTS
Varenicline attenuates nicotine-evoked NAc DA release by altering VTA DA neuron activity
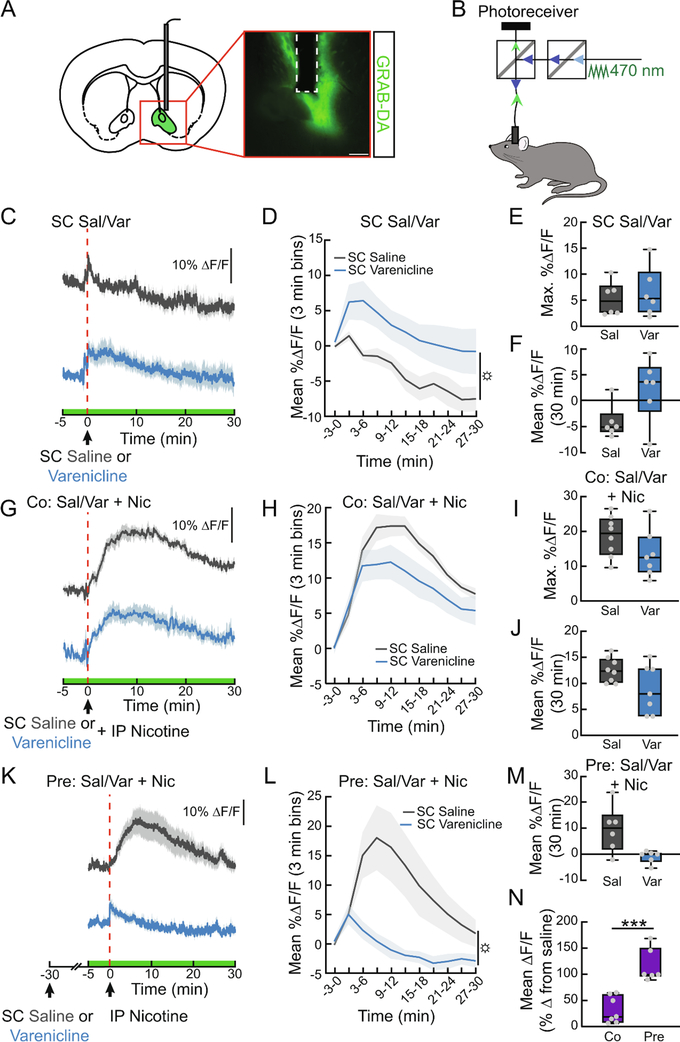
Modulating DA signaling has been an effective strategy for smoking cessation. To examine the time course with which varenicline influences DA signaling in real time, we used a genetically-encoded fluorescent sensor, GRAB-DA, for in vivo monitoring of dopamine levels (Sun et al., 2018). We expressed GRAB-DA in the NAc (Fig. 1A) and used fiber photometry to continuously monitor fluorescence as a proxy for DA levels (Fig. 1B). As previously observed, subcutaneous (SC) injections of varenicline (1.5 mg/kg) rapidly enhanced dopamine signaling in the NAc, peaking 5 min after injection and returning to baseline levels after 20–30 min (Fig. 1C–F, F1,10=5.197, p<0.05). We next determined the effects of varenicline on nicotine-evoked DA release. The NAc DA response to co-administration of varenicline and nicotine (1.5 mg/kg) was significantly different than nicotine alone (ANOVA interaction: F10,130=2.161, p<0.05), with a trend towards attenuated DA levels (Fig. 1G–J, F1,13=3.735, p=0.08). Because the utility of varenicline depends on its ability to block nicotine reward long term, we tested whether a pretreatment of varenicline would also reduce nicotine-evoked DA signaling. We found that pretreatment with varenicline 30 min before nicotine completely suppressed nicotine-evoked DA release in the NAc (Fig. 1K–M, F1,10=7.637, p<0.05), compared to the 32% suppression observed during co-administration (Fig. 1N, t9.6=5.158, p<0.001). These data provide a time course of varenicline action on the DA system and demonstrate a consistent, inhibitory effect on the DA response to nicotine.
Figure 1. Varenicline attenuates nicotine-evoked DA signaling in the NAc.
(A) Schematic and representative image showing GRAB-DA and fiber placement in the NAc. Scale bar, 200 μm. (B) Schematic showing the fiber photometry system used to monitor DA-induced fluorescent changes in awake, behaving mice. (C) Average ΔF/F of NAc GRAB-DA signals before, during, and following a subcutaneous (SC) injection of varenicline (1.5 mg/kg) or saline (0.9%). Saline was injected intraperitoneally (IP) immediately after SC injections to control for the extra handling in subsequent co-injection experiments. Signals are aligned to the IP injection. Blue, varenicline; grey, saline. Green on timeline represents duration of fiber photometry recording. (D) Mean ΔF/F (3-min bins) of GRAB-DA signals shown in (C) (n=6/group, two-way repeated measures ANOVA, main effect of group: p<0.05). (E) Maximum ΔF/F of the GRAB-DA signal shown in (C) (n=6, paired t-test, ns). (F) 30 min mean ΔF/F of the GRAB-DA signal shown in (C) (n=6, paired t-test, p=0.05). (G) Average ΔF/F of GRAB-DA signals of mice treated with either SC saline or varenicline and co-injected with IP nicotine (1.5 mg/kg). (H) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (G) (n=7–8/group, two-way repeated measures ANOVA). (I) Maximum ΔF/F of the GRAB-DA signal shown in (G) (n=7–8, unpaired t-test, ns). (J) 30 min mean ΔF/F of the GRAB-DA signal shown in (G) (n=7–8, unpaired t-test, p=0.07). (K) Average ΔF/F of GRAB-DA signals of mice injected with IP nicotine 30 min after a pretreatment with SC varenicline or saline. (L) Mean ΔF/F (3-min bins) of GRAB-DA signals shown in (K) (n=6/group, main effect of group: p<0.05). (M) 30 min mean ΔF/F of the GRAB-DA signal shown in (K) (n=6, paired t-test). (N) Percent suppression of GRAB-DA signal following nicotine injection with co- or pretreatment of varenicline (n=6–7/group, unpaired t-test, p<0.001). For photometry traces and line graphs, dark lines represent means and lighter, shaded areas represent SEM. For box and whisker plots, the box extends from the 25th to 75th percentiles, the line in the box represents the median, and the whiskers extend from the maximum to minimum values. Grey circles represent individual mice. t-tests: ***p<0.001. ANOVA main effect of group: ☼p<0.05. See Table S1 for details on statistical analyses.
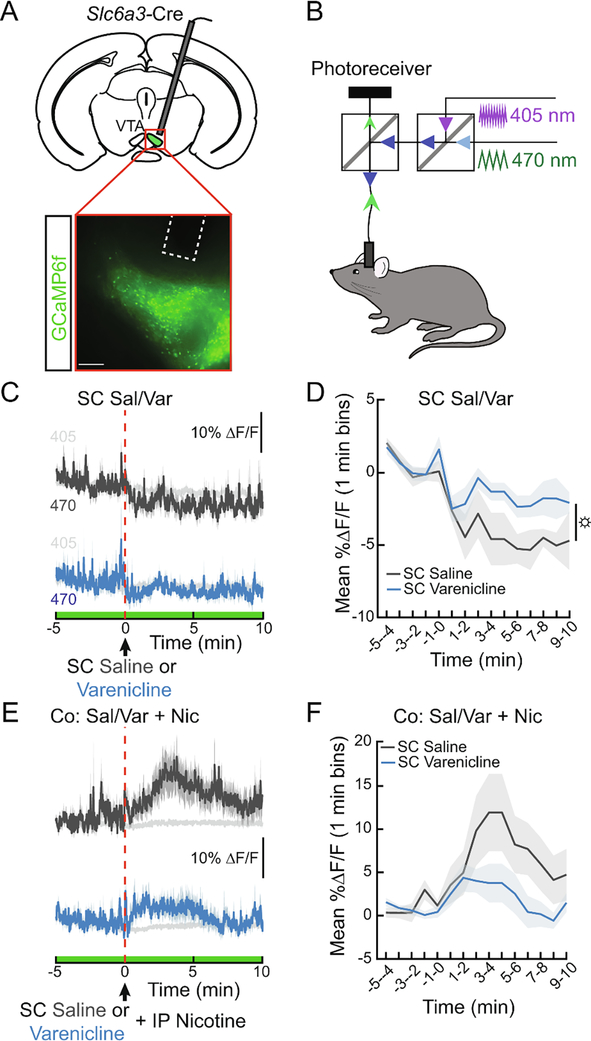
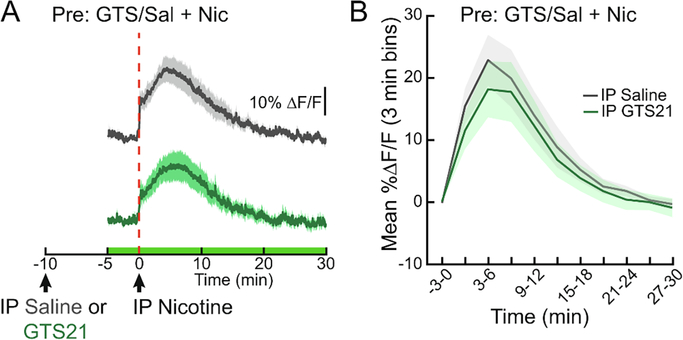
To determine if varenicline changes striatal DA levels by modulating the activity of VTA DA neurons, we engineered DAT::Cre mice to express the genetically encoded calcium indicator GCaMP6f in DAT-expressing neurons (Fig. 2A) and used dual-wavelength fiber photometry to continuously monitor in vivo calcium dynamics (Fig. 2B). Compared to controls, we observed increased DA neuron calcium dynamics for 10 min after SC varenicline administration (Fig. 2C, D, F1,10=5.68, p<0.05). When co-administered with nicotine, varenicline significantly altered the time course of VTA DA neuron activity (Fig. 2E, F, ANOVA interaction F14,140=2.072, p<0.05). GTS21, a partial agonist of the α7 nAChR, did not alter NAc DA following nicotine administration (Fig. 3). These experiments support prior work demonstrating that varenicline, by outcompeting nicotine at α4β2 receptors, reduces VTA DA neuron activity to block NAc DA release (Rollema et al., 2007; Perez et al., 2015).
Figure 2. Varenicline blunts nicotine-evoked VTA DA neuron activity.
(A) Schematic and representative image showing GCaMP6f expression and fiber placement in the VTA of DAT-Cre mice. Scale bar, 200 μm. (B) Schematic showing the dual-wavelength fiber photometry system used to monitor GCaMP fluorescence in awake, behaving mice. The 470 nm light excites calcium-dependent fluorescence while the 405 nm light serves as an isosbestic control (C) Average ΔF/F of GCaMP6f signals of mice during a subcutaneous (SC) injection of varenicline (1.5 mg/kg) or saline (0.9%). Saline was injected IP immediately after SC injections to control for the extra handling in subsequent co-injection experiments. Signals are aligned to the IP injection. Blue, 470 nm varenicline; dark grey, 470 nm saline; light grey, 405 nm. Green on timeline represents duration of fiber photometry recording. (D) Mean ΔF/F (3-min bins) of GCaMP6f signals shown in (C) (n=6/group, two-way repeated measures ANOVA, main effect of group: p<0.05). (E) Average ΔF/F of GCaMP6f signals of mice treated with SC varenicline or saline and co-injected with IP nicotine (1.5 mg/kg) (F) Mean ΔF/F (3-min bins) of GCaMP6f signals shown in (E) (n=6/group, two-way repeated measures ANOVA). Dark lines represent means and lighter, shaded areas represent SEM. ANOVA main effect of group: ☼p<0.05. See Table S1 for details on statistical analyses.
Figure 3. Partial agonism of α7 nAchRs does not affect nicotine-induced NAc DA.
(A) Average ΔF/F of GRAB-DA signals of mice during an intraperitoneal injection of nicotine (1.5 mg/kg) 10 min after a pretreatment of IP GTS 21 (4 mg/kg) or saline (0.9%). Signals are aligned to the second IP injection. Green, GTS 21; grey, saline. Green area represents duration of fiber photometry recording. (B) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (A) (n=6/group, two-way repeated measures ANOVA). Dark lines represent means and lighter, shaded areas represent SEM. See Table S1 for details on statistical analyses.
Varenicline pretreatment blocks nicotine-induced dorsal striatum DA release
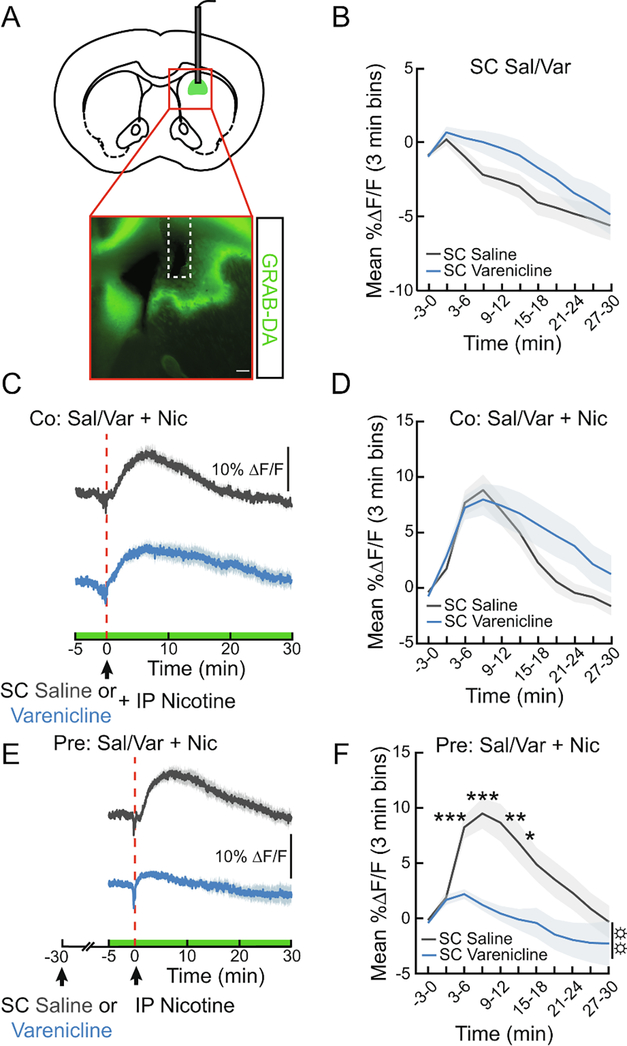
The dorsal striatum receives dopaminergic input from nicotine-responsive neurons and is believed to play a role in habitual drug-seeking (Zhang et al., 2009; Everitt and Robbins, 2013). To probe the effects of varenicline on dorsal striatal DA, we expressed GRAB-DA in the dorsal striatum (Fig. 4A). Unlike in the NAc, varenicline alone did not significantly modulate dorsal striatum DA signaling (Fig. 4B). Varenicline co-injected with nicotine significantly extended the time course of enhanced dorsal striatum DA levels (Fig. 4C, D ANOVA interaction F10,260=3.621, p<0.001). However, this effect was not observed when varenicline treatment occurred 30 min prior to nicotine, as pretreatment leads to a robust attenuation of dorsal striatum DA in response to nicotine (Fig. 4E, F, F1,22=9.53, p<0.01). These results reveal a slower acting but strong suppression of nicotine-induced DA release in the dorsal striatum by varenicline.
Figure 4. Varenicline pretreatment blunts nicotine-evoked DA signaling in the dorsal striatum.
(A) Schematic and representative image showing GRAB-DA and fiber placement in the dorsal striatum. Scale bar, 200 μm. (B) Mean ΔF/F (3 min bins) of GRAB-DA signals of mice given a subcutaneous (SC) injection of varenicline (1.5 mg/kg) or saline (0.9%). Saline was injected IP immediately after SC injections to control for the extra handling in subsequent co-injection experiments (n=12/group, two-way repeated measures ANOVA). (C) Average ΔF/F of GRAB-DA signals of mice treated with SC varenicline or saline and co-injected with IP nicotine (1.5 mg/kg). Signals are aligned to the IP injection. Blue, varenicline; grey, saline. Green on timeline represents duration of fiber photometry recording. (D) Mean ΔF/F (3-min bins) of GRAB-DA signals shown in (C) (n=14/group, two-way repeated measures ANOVA). (E) Average ΔF/F of GRAB-DA signals of mice injected with IP nicotine 30 min after a pretreatment with SC varenicline or saline. (F) Mean ΔF/F (3-min bins) of GRAB-DA signals shown in (E) (n=12/group, two-way repeated measures ANOVA, main effect of group: p<0.01). Dark lines represent means and lighter, shaded areas represent SEM. post-hoc comparisons: *p<0.05, **p<0.01, ***p<0.001. ANOVA main effect of group: ☼☼p<0.01. See Table S1 for details on statistical analyses.
Varenicline does not suppress the NAc DA responses to acute morphine or ethanol
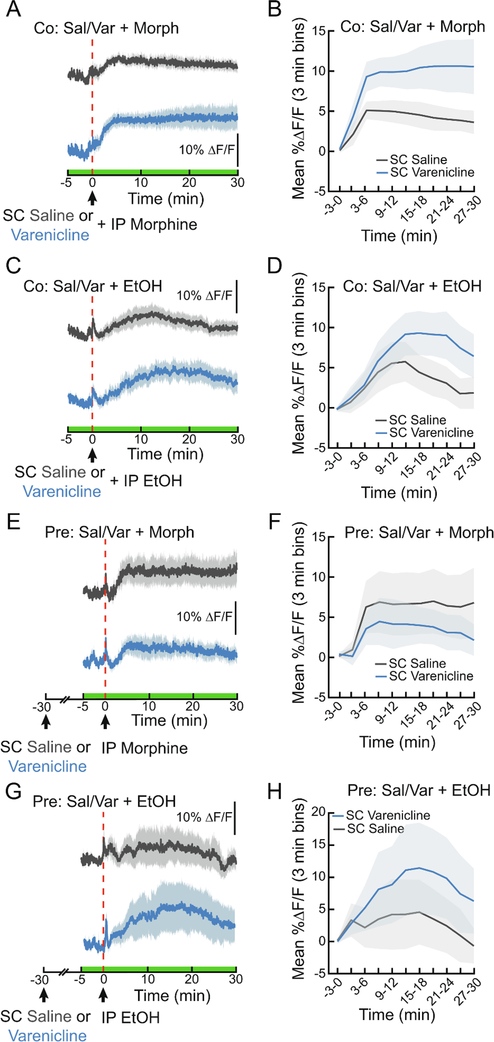
It has been suggested that varenicline may reduce the use of other addictive substances (Steensland et al., 2007; Hendrickson et al., 2010; Feduccia et al., 2014). In fact, reports show that varenicline decreases ethanol consumption in both rodents and humans (Steensland et al., 2007; McKee et al., 2009). How does varenicline affect the DA response to other drugs? As previously shown (Chiara and Imperato, 1986; Alhadeff et al., 2019), morphine and EtOH both significantly increased NAc DA levels (Fig. 5A–D, F2,44=11.25, p<0.001 (morphine), F2,57=10.51, p<0.001 (EtOH)). Varenicline affected the time course of DA signaling when co-administered with either morphine (10 mg/kg, Fig. 5A, B, ANOVA interaction F10,240=2.209, p<0.05) or ethanol (2 g/kg, Fig. 5C, D, ANOVA interaction F10,240=2.209, p<0.05). Varenicline treatment did not reduce DA signaling in response to either drug, rather there was a trend towards a larger DA signal (F1,24=4.026, p=0.06 (morphine), F1,24=1.245, n.s.(EtOH)). Varenicline did not affect drug-evoked DA release when given 30 min prior to morphine (Fig. 5E, F) or EtOH (Fig. 5G, H). These findings demonstrate that varenicline’s ability to attenuate drug-evoked dopamine release is specific to nicotine.
Figure 5. Varenicline enhances morphine- and ethanol-evoked DA signaling in the NAc.
(A) Average ΔF/F of GRAB-DA signals of mice during an IP injection of morphine (10 mg/kg) immediately following a subcutaneous (SC) injection of varenicline (1.5 mg/kg) or saline (0.9%). Signals are aligned to the IP injection. Blue, 470 nm varenicline; dark grey, 470 nm saline. Green on timeline represents duration of fiber photometry recording. (B) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (A) (n=13/group, two-way repeated measures ANOVA). (C) Average ΔF/F of GRAB-DA signals of mice during an IP injection of EtOH (2 g/kg) following an injection of SC saline or varenicline. (D) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (D) (n=13/group, two-way repeated measures ANOVA). (E) Average ΔF/F of GRAB-DA signals of mice injected with IP morphine 30 min after a pretreatment with SC varenicline or saline. (F) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (E) (n=6/group, two-way repeated measures ANOVA). (G) Average ΔF/F of GRAB-DA signals of mice injected with IP EtOH 30 min after a pretreatment with SC varenicline or saline. (H) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (G) (n=6/group, two-way repeated measures ANOVA). Dark lines represent means and lighter, shaded areas represent SEM. See Table S1 for details on statistical analyses.
Varenicline does not suppress the DA response to natural rewards
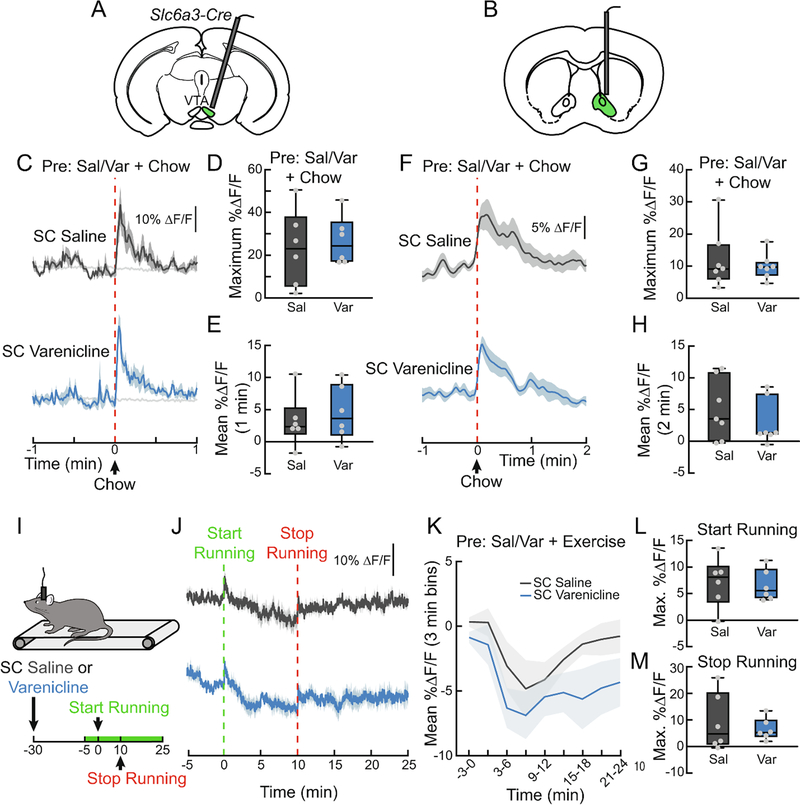
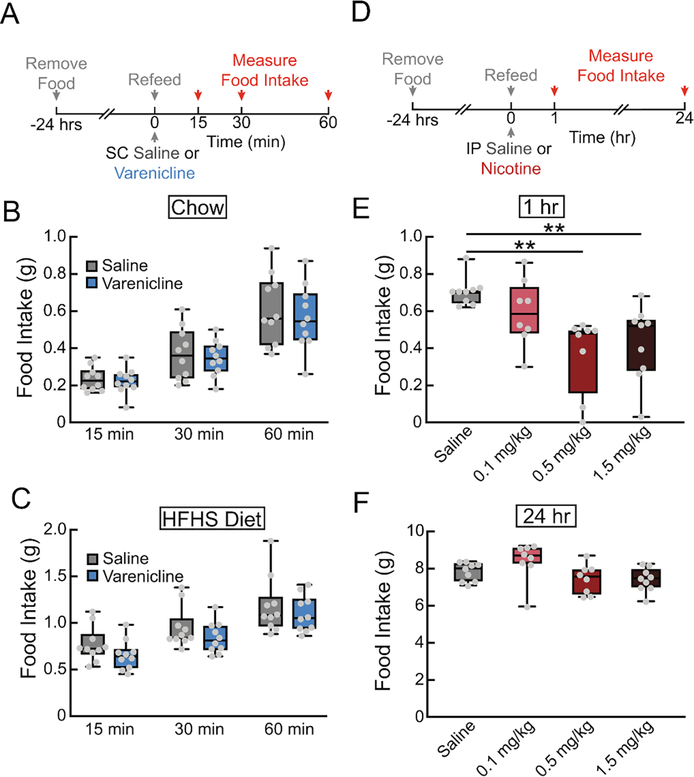
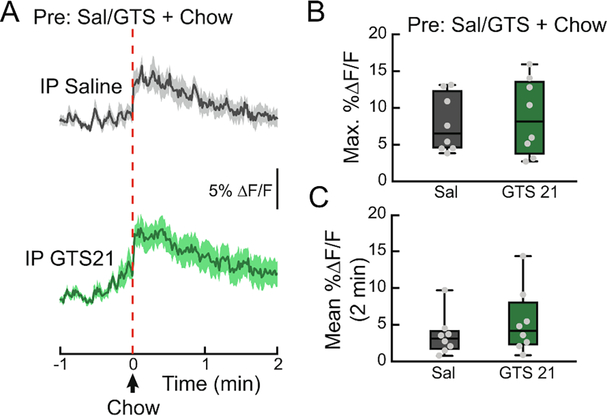
To determine if varenicline blocks the DA response to natural rewards, we monitored both VTA DA neuron activity (Fig. 6A) and NAc DA levels (Fig. 6B) while presenting hungry mice with a pellet of food after varenicline injection. Varenicline treatment did not alter food-evoked VTA dopamine neuron activity (Fig. 6C–E) or NAc dopamine release (Fig. 6F–H). Correspondingly, varenicline did not affect chow intake (Fig. 7A, B), or highly palatable high-fat, high-sugar food intake (Fig. 7A, C) in contrast to nicotine (Fig. 7D–F, F3,30=6.822, p<0.01). Additionally, the α7 partial agonist GTS21 did not alter the NAc DA spike in response to food (Fig. 8A–C), suggesting that neither partial agonism of the α4β2 nor α7 subunits of nAChRs affect the circuit mechanisms by which food stimulates dopamine release.
Figure 6. Varenicline does not affect DA responses to natural rewards.
(A) Schematic depicting GCaMP fiber photometry of VTA DAT neurons used in (C-E). (B) Schematic depicting GRAB-DA fiber photometry in the NAc of mice used in (F-H). (C) Average ΔF/F of VTA GCaMP6f signals of mice pretreated with SC injections of varenicline (1.5 mg/kg) or saline (0.9%) and then given a pellet of chow. Signals are aligned to chow presentation. Blue, 470 nm varenicline; dark grey, 470 nm saline; light grey, 450 nm. (D) Maximum ΔF/F of the 470 nm GCaMP6f signal shown in (C) (n=6/group, paired t-test). Blue, varenicline; grey, saline. (E) Mean ΔF/F of the 470 nm GCaMP6f signal shown in (C) (n=6/group, paired t-test). (F) Average ΔF/F of NAc GRAB-DA signals of mice pretreated with SC injections of varenicline or saline and then given a pellet of chow (n=7/group). (G) Maximum ΔF/F of the GRAB-DA signal shown in (F) (n=7/group, paired t-test). (H) Mean ΔF/F of the GRAB-DA signal shown in (F) (n=7/group, paired t-test). (I) Mice ran on a treadmill while their NAc DA signaling was monitored using fiber photometry. Mice were pretreated with SC varenicline or saline, ran for 10 min at 10 m/min, and then rested on the treadmill for another 15 min after running. Green on timeline represents duration of fiber photometry recording. (J) Average ΔF/F of GRAB-DA signals of mice from the experiment depicted in (I) (n=6/group). Signals are aligned to the start of running. (K) Mean ΔF/F (3 min bins) of GRAB-DA signals shown in (J) (n=6/group, two-way repeated measures ANOVA). (L) Maximum ΔF/F of the GRAB-DA signal shown in (J) during running (n=6/group, paired t-test). (M) Maximum ΔF/F of the GRAB-DA signal shown in (F) after running (n=6/group, paired t-test). Signals were aligned to the end of running. For photometry traces and line graphs, dark lines represent means and lighter, shaded areas represent SEM. For box and whisker plots, the box extends from the 25th to 75th percentiles, the line in the box represents the median, and the whiskers extend from the maximum to minimum values. Grey circles represent individual mice. See Table S1 for details on statistical analyses.
Figure 7. Varenicline, unlike nicotine, does not affect acute food intake in hungry mice.
(A) 24 hr food deprived mice were given SC injections of varenicline (1.5 mg/kg) or saline (0.9%), followed by a pellet of food. Food intake was measured at 15 min, 30 min, and 1 hr. (B) Chow intake following SC injections of varenicline or saline (n=10/group, two-way repeated measures ANOVA, ns). (C) High-fat, high-sugar diet intake following SC injections of varenicline or saline (n=10/group, two-way repeated measures ANOVA, ns). (D) 24 hr food deprived mice were given IP injections of saline or nicotine (0.1, 0.5, 1.5 mg/kg), followed be a pellet of food. Food intake was measured at 1 hr and 24 hr. (E) 1 hr chow intake following IP injections of saline or nicotine (n=8–9/group, one-way ANOVA, p<0.01). (F) 24 hr chow intake following IP injections of saline or nicotine (n=8–9/group, one-way ANOVA, p<0.05). For box and whisker plots, the box extends from the 25th to 75th percentiles, the line in the box represents the median, and the whiskers extend from the maximum to minimum values. Grey circles represent individual mice. post-hoc comparisons: **p<0.01. See Table S1 for details on statistical analyses.
Figure 8. Partial agonism of α7 nAchRs does not affect food-evoked NAc DA.
(A) Average ΔF/F of GRAB-DA signals of 24 h food-deprived mice given a pellet of chow 10 min after a pretreatment with intraperitoneal (IP) GTS 21 (4 mg/kg) or saline (0.9%). Signals are aligned to food presentation. Green, GTS 21; grey, saline. Dark lines represent means and lighter, shaded areas represent SEM. (B) Maximum ΔF/F of the GRAB-DA signal shown in (A) (n=8/group, paired t-test). (C) Mean ΔF/F of the GRAB-DA signal shown in (A) (n=8/group, paired t-test). For box and whisker plots, the box extends from the 25th to 75th percentiles, the line in the box represents the median, and the whiskers extend from the maximum to minimum values. Grey circles represent individual mice. See Table S1 for details on statistical analyses.
Another natural reward that increases VTA DA neuron activity is exercise (Herrera et al., 2016). We next sought to determine if varenicline alters exercise-evoked DA levels in the NAc. We observed a brief increase in NAc DA signaling at the onset of exercise and a slower, longer lasting elevation following 10 min of forced running (10 m/min, Fig. 6I, J). To determine whether varenicline would affect the DA dynamics observed during exercise, mice were pretreated with varenicline 30 min before running onset. Varenicline did not affect the initial DA surge during running or the elevation after cessation of running (Fig. 6I–M).
DISCUSSION
As demonstrated by the success of varenicline, modulating DA signaling in response to drug rewards is a viable therapeutic approach to reduce the use of addictive substances (Jordan and Xi, 2018). Our data support the notion that varenicline suppresses nicotine-evoked dopamine release by occluding VTA dopamine neuron activation in the presence of nicotine. We also revealed the second by second time course of this response, showing that varenicline is over three times more effective in blocking nicotine-evoked DA when given 30 min prior to the drug. The effects were mirrored in VTA DA neurons, suggesting that varenicline acts on α4β2 receptors to change VTA neuron activity and consequently NAc DA levels. Strikingly, varenicline did not block DA release in response to other drugs or natural rewards. Together, these results (1) highlight the precision with which varenicline attenuates DA signaling in response to nicotine and (2) have translational implications for the use of varenicline to treat substance use disorders.
Time course of varenicline action and implications for smoking cessation
Ventral striatal DA signaling in response to varenicline has previously been described (Coe et al., 2005). By performing real-time DA measurements, we provide new insights into how varenicline influences VTA DA neurons as well as striatal DA signaling. In the NAc, varenicline alone rapidly boosts DA signaling while diminishing the response to nicotine. In the dorsal striatum, the immediate effects are different. Varenicline alone fails to boost DA levels yet prolongs nicotine’s effects. After 30 min, however, varenicline strongly suppresses nicotine-induced DA surges in both regions. This is an important consideration for coursing varenicline treatment for maximal efficacy. For example, when varenicline is first introduced to smokers, our data suggest that abstaining from nicotine immediately after drug treatment may maximize efficacy. Additionally, it is thought that the transient increase in DA levels following varenicline administration provides a ‘nicotine reward’ that helps avoid nicotine use immediately following varenicline therapy. In fact, a therapeutic agent that evokes a larger DA response itself may be more effective in reducing drug craving during abstinence.
The differences observed between ventral and dorsal striatum responses to varenicline may be due to differential modulation of DA neurons in the VTA and substantia nigra (SN) that project to the NAc and dorsal striatum, respectively. nAChRs are differentially expressed in VTA and SN DA neurons as well as inputs to these regions (Azam et al., 2002; Keath et al., 2007), and VTA DA neurons are more sensitive to activation by nAChRs (Keath et al., 2007; Zhang et al., 2009). This could explain why varenicline alone enhances NAc DA but does not significantly affect dorsal striatum DA. Determining the different inputs and nAChRs responsible for the immediate and lasting effects of varenicline on VTA and SN DA neurons is an intriguing topic for future investigation.
Although it has been reported that varenicline is more effective in females than in males for short-term smoking cessation, it has been shown that females have lower quitting rates using nicotine replacement therapy (McKee et al., 2016). In rodents, sex differences have been reported in nicotine reward and nicotine-evoked changes in protein synthesis (Barrett et al., 2017; Lee et al., 2021). Though both male and female mice were used here (Table S1), our studies were not sufficiently powered to detect sex differences in the reported effects. The effect of sex on varenicline’s role in modulating DA responses to natural and drug rewards remains a compelling topic for future studies.
Varenicline as a treatment for substance use disorders
Varenicline pretreatment did not reduce the NAc DA response to drugs of abuse other than nicotine. Several reports have indicated that varenicline decreases alcohol intake in rodents and humans, but the neural mechanism remains unclear (Steensland et al., 2007; McKee et al., 2009). Some evidence points to the NAc as a site of action mediating this effect, while other evidence implicates the VTA (Ericson et al., 2009; Hendrickson et al., 2010; Feduccia et al., 2014). Interestingly, we found that varenicline pretreatment did not suppress the EtOH-induced DA surge in the NAc. Thus, the mechanisms by which varenicline decreases nicotine and EtOH consumption are likely distinct.
Additionally, we find that co-administration of varenicline with ethanol or morphine alters the time course of DA signaling in the NAc. These results suggest that varenicline has an additive effect on the DA system, likely because of non-overlapping mechanisms. While the precise mechanisms by which ethanol and morphine increase NAc DA are not fully understood, our results strongly suggest that the α4β2 nAChR is not critical. Future work, however, should investigate varenicline’s effects on DA signaling in mice with chronic drug exposure, as repeated exposure to ethanol or opioids dramatically alters neurotransmission in brain reward centers (Nestler and Lüscher, 2019).
nAchR involvement in food intake and food reward
Cigarette smokers are leaner than non-smokers (Albanes et al., 2011) – an effect that has been attributed to the ability of nicotine to suppress food intake in rodents (Grunberg et al., 1987). Nicotine may also decrease feeding through its action in the VTA, as altering DA signaling significantly alters food intake (Sotak et al., 2005). Here we show that partial agonism of two of the most commonly expressed nAChRs in the brain with varenicline (α4β2) and GTS21 (α7), did not affect food-evoked DA. Furthermore, varenicline, unlike nicotine, did not alter refeeding following food deprivation. Thus, nicotine likely alters feeding behavior by acting on other nAChRs in other brain regions. nAChRs are also expressed in hypothalamic areas critical for food intake control such as the arcuate nucleus. We recently showed nicotine has an inhibitory effect on orexigenic agouti-related protein (AgRP)-expressing neurons (Alhadeff et al., 2019), and others have shown an excitatory effect on anorexigenic pro-opiomelanocortin (POMC)-expressing neurons via the α3β4 nAChR (Mineur et al., 2011). These studies suggest nicotine action in the hypothalamus may be responsible for changes in feeding behavior independent of the DA system.
It is also possible that VTA nAChRs are involved in longer-term food intake and body weight control, neither of which was assessed here. Additionally, while a single varenicline injection did not affect food intake or VTA/NAc responses to food, the effects of long-term varenicline treatment on food intake need to be evaluated. This is particularly interesting given the fact that prolonged nicotine exposure results in weight loss, while smoking cessation causes hyperphagia, weight gain, and increased food reward (Spring et al., 2003; Audrain-McGovern and Benowitz, 2017). This may be explained by known changes in nAChR expression (Renda and Nashmi, 2014) following prolonged activation by nicotine, which could change neural responses to food and food cues.
Pharmacological agents as a tool to investigate natural and drug reward circuits
Beyond the therapeutic potential as a treatment for substance use disorders, drugs like varenicline can be important tools to dissect critical receptors, sites of action, and neural circuits for both natural and drug rewards. A 30 min pretreatment of varenicline virtually eliminated nicotine’s ability to increase striatal DA. Conversely, DA responses to natural rewards such as food and exercise were not affected by varenicline, indicating that α4β2 activation is not involved in signaling these rewards. In this way, a drug like varenicline that was developed to specifically target receptors necessary for nicotine reward can shed light on how endogenous cholinergic signaling in the DA system influences responses to naturally rewarding stimuli. Furthermore, these findings begin to suggest the possibility that different VTA neuron populations are activated to reinforce different rewards. Such differential activation may be therapeutically advantageous, as it makes it possible to selectively alter the effect of specific rewards on the DA system. Ultimately, a thorough understanding of the neural circuits involved in food reward, for example, could be used to treat obesity in a manner similar to varenicline’s effects on nicotine intake.
Modulating the dopaminergic system to influence behavior
The brain’s DA system is critical for reinforcing adaptive behaviors by increasing salience and promoting reward. Drugs co-opt this system, providing instant reward that can ultimately cause dependence and addiction. We show here that varenicline, a smoking cessation aid that was synthesized to boost DA levels while blocking nicotine’s effects on the DA system, is highly specific to nicotine reward. Varenicline did not attenuate the DA response to ethanol, morphine, or natural rewards like food and exercise. Our results highlight the complexity with which various rewards activate dopaminergic signaling and suggest that DA responses to individual drugs of abuse may be blocked by specific pharmaceutical interventions.
Supplementary Material
HIGHLIGHTS.
Dopamine sensing reveals time-dependent effects of varenicline
Varenicline blocks nicotine-evoked dopamine signaling in dorsal and ventral striatum
Varenicline transiently elevates dopamine levels as a nicotine substitute
Varenicline slightly prolongs dopamine release evoked by other drugs
Varenicline does not influence the dopamine response to natural rewards
ACKNOWLEDGMENTS
We thank Amber L. Alhadeff, Mariella De Biasi, and Peter Lee for comments on the manuscript and O. Park, A. McKnight, and C. Jaycox for experimental assistance.
FUNDING AND DISCOLSURE
This research was funded by the NIH (P01DK119130-01 to J.N.B. and T32NS105607 to N.G.), the NSF GFRP (DGE-1845298 to N.G.), the Klingenstein Fund and Simons Foundation (to J.N.B.), and the American Diabetes Association (118IBS116 to J.N.B.) and the University of Pennsylvania School of Arts and Sciences. The authors have nothing to disclose.
ABBREVIATIONS
- DA
dopamine
- nAChR
nicotinic acetylcholine receptor
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- FP
fiber photometry
- GCaMP
genetically encoded calcium indicator resulting from a fusion of GFP, M13 and Calmodulin
- GRAB
G protein-coupled receptor activation-based dopamine sensor
- DS
dorsal striatum
- SN
substantia nigra
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albanes D, Jones DY, Micozzi MS, Mattson ME (2011) Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health 77:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Goldstein N, Park O, Klima ML, Vargas A, Betley JN (2019) Natural and Drug Rewards Engage Distinct Pathways that Converge on Coordinated Hypothalamic and Reward Circuits. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, Williams KE, Reeves KR (2008) Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 63:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Benowitz N (2017) Cigarette Smoking, Nicotine, and Body Weight. Clin Pharmacol Ther 90:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444:260–274. [DOI] [PubMed] [Google Scholar]
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC (2006) Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44:383–390. [DOI] [PubMed] [Google Scholar]
- Barrett ST, Geary TN, Steiner AN, Bevins RA (2017) Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology 234:187–198. [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2009) Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Pharmacol Toxicol 49:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG (1992) Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol 39:247–279. [DOI] [PubMed] [Google Scholar]
- Chiara G, Imperato A (1986) Preferential Stimulation of Dopamine Release in the Nucleus Accumbens by Opiates, Alcohol, and Barbiturates: Studies with Transcerebral Dialysis in Freely Moving Rats. Ann Ny Acad Sci 473:367–381. [DOI] [PubMed] [Google Scholar]
- Ciano PD, Guranda M, Lagzdins D, Tyndale RF, Gamaleddin I, Selby P, Boileau I, Foll BL (2016) Varenicline-Induced Elevation of Dopamine in Smokers: A Preliminary [11C]-(+)-PHNO PET Study. Neuropsychopharmacol 41:1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW et al. (2005) Varenicline: An α4β2 Nicotinic Receptor Partial Agonist for Smoking Cessation. J Med Chem 48:3474–3477. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Söderpalm B (2009) The Smoking Cessation Medication Varenicline Attenuates Alcohol and Nicotine Interactions in the Rat Mesolimbic Dopamine System. J Pharmacol Exp Ther 329:225–230. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2013) From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37:1946–1954. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE (2014) Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Brit J Pharmacol 171:3420–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 348:201–203. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH (1986) Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand 128:351–358. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Popp KA (1987) Sex differences in nicotine’s effects on consummatory behavior and body weight in rats. Psychopharmacology 91:221–225. [DOI] [PubMed] [Google Scholar]
- Gysling K, Wang RY (1983) Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 277:119–127. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR (2010) Activation of α4* nAChRs is Necessary and Sufficient for Varenicline-Induced Reduction of Alcohol Consumption. J Neurosci 30:10169–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, Loetz E, Campeau S, Fleshner M, Greenwood BN (2016) Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur J Neurosci 43:1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Xi Z-X (2018) Discovery and development of varenicline for smoking cessation. Expert Opin Drug Dis 13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS (2007) Differential Modulation by Nicotine of Substantia Nigra Versus Ventral Tegmental Area Dopamine Neurons. J Neurophysiol 98:3388–3396. [DOI] [PubMed] [Google Scholar]
- Lacroix F, Pettorelli A, Maddux J-MN, Heidari-Jam A, Chaudhri N (2017) Varenicline Reduces Context-Induced Relapse to Alcohol-Seeking through Actions in the Nucleus Accumbens. Neuropsychopharmacol 42:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC (2014) Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Mansuri MS, Wilson RS, Lam TT, Nairn AC, Picciotto MR (2021) Sex Differences in the Ventral Tegmental Area and Nucleus Accumbens Proteome at Baseline and Following Nicotine Exposure. Front Mol Neurosci 14:657064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Rosenblum A (2001) Leaving methadone treatment: lessons learned, lessons forgotten, lessons ignored. Mt Sinai J Medicine New York 68:62–74. [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux J-P, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani I, Bemelmans A-P, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux J-P (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline Reduces Alcohol Self-Administration in Heavy-Drinking Smokers. Biol Psychiat 66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH (2016) Sex Differences in Varenicline Efficacy for Smoking Cessation: A Meta-Analysis. Nicotine Tob Res 18:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D, Diano S, Biasi MD, Horvath TL, Gao X-B, Picciotto MR (2011) Nicotine Decreases Food Intake Through Activation of POMC Neurons. Science 332:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Lüscher C (2019) The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 102:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD (2008) Dopamine Signaling in the Dorsal Striatum Is Essential for Motivated Behaviors. Ann Ny Acad Sci 1129:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Khroyan TV, McIntosh JM, Quik M (2015) Varenicline enhances dopamine release facilitation more than nicotine after long-term nicotine treatment and withdrawal. Pharmacol Res Perspectives 3:e00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux J-P (1998) Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390:401–404. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan CS, Dombeck DA, Deisseroth K, Awatramani R (2018) Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci 21:1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renda A, Nashmi R (2014) Chronic nicotine pretreatment is sufficient to upregulate α4* nicotinic receptors and increase oral nicotine self-administration in mice. Bmc Neurosci 15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, Williams KE (2007) Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994. [DOI] [PubMed] [Google Scholar]
- Shariff M, Quik M, Holgate J, Morgan M, Patkar OL, Tam V, Belmer A, Bartlett SE (2016) Neuronal Nicotinic Acetylcholine Receptor Modulators Reduce Sugar Intake. Plos One 11:e0150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD (2005) Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res 1061:88–96. [DOI] [PubMed] [Google Scholar]
- Spring B, Pagoto S, McChargue D, Hedeker D, Werth J (2003) Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Be 76:351–360. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc National Acad Sci 104:12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Alhadeff AL, Betley JN (2017) Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Reports 21:2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F et al. (2018) A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174:481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatr 9:557–569. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Jones EB, Einstein EB, Wargo EM (2019) Prevention and Treatment of Opioid Misuse and Addiction. Jama Psychiat 76:208–216. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M (2015) The Brain on Drugs: From Reward to Addiction. Cell 162:712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18:741–752. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou F-M, Dani JA (2009) Dopamine Signaling Differences in the Nucleus Accumbens and Dorsal Striatum Exploited by Nicotine. J Neurosci 29:4035–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.