Abstract
Background:
Obese children with asthma are more vulnerable to air pollution, especially fine particulate matter (PM2.5), but reasons are poorly understood. We hypothesized that differences in breathing patterns (tidal volume, respiratory rate, and minute ventilation) due to elevated body mass index (BMI) may contribute to this finding.
Objective:
To investigate the association of BMI with breathing patterns and deposition of inhaled PM2.5.
Methods:
Baseline data from a prospective study of children with asthma was analyzed (n=174). Tidal breathing was measured by a pitot-tube flowmeter, from which tidal volume, respiratory rate, and minute ventilation were obtained. The association of BMI z-score with breathing patterns was estimated in a multivariable model adjusted for age, height, race, sex, and asthma severity. A particle dosimetry model simulated PM2.5 lung deposition based on BMI-associated changes in breathing patterns.
Results:
Higher BMI was associated with higher tidal volume (adjusted mean difference [aMD] between obese and normal-range BMI of 25 mL, 95% confidence interval [CI] 5–45 mL) and minute ventilation (aMD 453 mL/min, 95%CI 123–784 mL/min). Higher tidal volumes caused higher fractional deposition of PM2.5 in the lung, driven by greater alveolar deposition. This translated into obese participants having greater per-breath retention of inhaled PM2.5 (aMD in alveolar deposition fraction of 3.4%; 95%CI 1.3—5.5%), leading to worse PM2.5 deposition rates.
Conclusions:
Obese children with asthma breathe at higher tidal volumes that may increase the efficiency of PM2.5 deposition in the lung. This finding may partially explain why obese children with asthma exhibit greater sensitivity to air pollution.
INTRODUCTION
Exposure to air pollution, particularly PM2.5 (fine particulate matter with an aerodynamic diameter smaller than 2.5μm), is harmful to pulmonary health1–3. In children, it is a known driver of chronic bronchitis, incident asthma, and asthma exacerbation4,5.
Obesity may increase susceptibility to air pollution. Epidemiologic associations between ambient air pollution and respiratory symptoms are stronger among obese versus normal weight children and adults6,7. Obese children with asthma report worse symptoms with equivalent levels of exposure to indoor PM2.5 and secondhand smoke8,9.
Increased susceptibility to equal indoor concentrations of PM2.5, via greater deposition of inhaled particles, is a potential pathway to at least partly explain disparate asthma morbidity in obese versus non-obese children. Bennett and Zeman reported among 36 healthy children that obesity was associated with greater fractional deposition of inhaled particles in the respiratory system during tidal breathing, and that this relationship was most strongly related to obese children having higher tidal volumes10. However, breathing patterns in children with asthma are unknown. Further, changes in anatomic location of particle deposition within the respiratory system due to obesity have not been examined.
The objective of this study was to examine the association of (1) BMI to respiratory patterns, (2) breathing patterns to PM2.5 particle deposition characteristics, and (3) BMI to PM2.5 particle deposition characteristics within a cohort of children with asthma. We hypothesize that obese children with asthma will breathe at higher tidal volumes, that higher tidal volumes will translate to more efficient deposition of PM2.5 in the respiratory system, and that obese children will therefore receive a greater “dose” of inhaled particles compared with normal weight children.
METHODS
Study cohort
AIRWEIGHS is a single-center, randomized, parallel-assignment, quadruple-masked clinical trial of an air purifier intervention for obesity-associated asthma (NCT02763917). A pre-specified secondary objective of the study was to examine breathing patterns in normal weight and obese children with asthma. Participants were 8–17 years of age, non-smokers, and had physician-diagnosed asthma by National Asthma Education and Prevention Program (NAEPP) criteria with at least one exacerbation in the prior year11. Those who were underweight or had other significant cardiopulmonary disease were excluded. The study was approved by the Institutional Review Board of Johns Hopkins University School of Medicine (IRB00074171). All participants gave assent, and all primary caretakers gave written informed consent.
Potential participants who lived within the Baltimore, Maryland region were identified from patients seen in Johns Hopkins outpatient clinics and the pediatric emergency department, community engagement activities, recruitment flyers posted in public locations, and a registry of previous study participants. Enrollment was not explicitly stratified by obesity as similar proportions of non-obese and obese children were anticipated based on historical recruitment data from our research center. Baseline characterization and measurement of breathing patterns occurred as part of the lead-in period prior to randomization, and thus this study includes participants who were lost to follow-up or disenrolled prior to randomization. A diagram of participant flow is included as eFigure 1.
Participant characterization
Sociodemographic information was obtained by participant or caregiver report. Asthma control was represented by the maximum symptom day (the maximum of the number of days in the prior two weeks a participant had slowed activity, wheezing, or coughing or chest tightness due to asthma) and by the Asthma Therapy Assessment Questionnaire (ATAQ) score, which ranges from 0–4, with scores ≥1 indicating uncontrolled asthma12,13. Body weight and height were measured in triplicate and each averaged, from which body mass index (BMI) was calculated and converted to a normalized z-score based on the 2000 Centers for Disease Control growth charts14,15. We chose z-score instead of percentile due to substantial negative skew in the BMI percentile distribution from a high proportion of obese participants. Gravimetric concentration of PM2.5 in the participant’s bedroom was measured by a Personal Environmental Monitor (model 200, MSP Corporation; Shoreview, MN) containing a 37-mm polytetrafluroethylene filter (Teflo R2PI025, Pall Corporation; New York, NY) connected to a personal aerosol sampling pump (model AFC 400S, BGI Incorporated; Waltham, MA) running at 4 L/min. Sampling was performed prior to randomization for one week.
Breathing patterns
Up to ten minutes of at-rest breathing were measured by a calibrated flow meter while participants passively watched videos on a multimedia device16. The apparatus was comprised of a pitot-tube flow sensor connected to a full-face neoprene mask. The design allowed integrated continuous measurements of oral and nasal airflow, minimized mechanical dead space, and allowed participants to breathe to their comfort.
Breath-by-breath tidal volume and respiratory rate were calculated from the airflow waveforms utilizing a semi-automated time-series analysis (WaveMetrics; Portland, OR), from which tidal volume and respiratory rate were measured. Minute ventilation was derived as the product of tidal volume and respiratory rate. More details are included in the supplement.
Particle deposition
We applied the multi-path particle dosimetry (MPPD) model version 3.04 (Applied Research Associates; Raleigh, NC) to estimate the effects of breathing patterns on PM2.5 deposition. This computational model estimates region-specific particle deposition characteristics in a five-lobe respiratory system by applying theoretical equations of particle impaction, sedimentation, and diffusion, which are described in more detail elsewhere17. The model incorporates age-specific differences in airway morphometry originally described by Mortensen and colleagues18,19. MPPD estimates have been shown to agree with empirical measurements of total deposition among individuals aged 3 months to 21 years old, and it is a commonly-used model in dosimetry research in children20–22.
We input each participant’s own tidal volume and respiratory rate into the model. Other parameters, chiefly body position and functional residual capacity (FRC), were left at default values. Estimates of fractional deposition (ratio of particles deposited to particles inhaled) and deposition rate (particle mass deposited per minute) for the upper respiratory tract (URT), defined as nose to trachea; tracheobronchial (TB), defined as trachea to the 16th airway generation; and alveolar regions were calculated. To allow between-participant comparisons of deposition rate attributable to BMI, calculations of deposition rate assumed a constant indoor air PM2.5 concentration equal to the mean observed in-home concentration.
We performed sensitivity analysis of two key model assumptions. First, the model estimates participant FRC using an age-specific equation based on the work of Hoffman23. Because obesity in children is associated with a reduction in FRC, we performed a parallel analysis by forcing a 20% reduction in model-estimated FRC among obese participants, which is the approximate magnitude based on a meta-analysis of studies24. Second, due to lack of empirical data specifically for nasal deposition efficiency in children, the model assumed different values for nasal deposition efficiency among children <12, 12–15, and ≥16 years of age. We initially stratified estimates by age group and investigated if this assumption caused systematic differences in the relationship of BMI to deposition characteristics between age groups. After determining that they were not, unstratified estimates were reported. Further details are included in the supplement.
Statistical analysis
Univariable comparisons between obese and non-obese participants were performed with unpaired t-tests for continuous variables and chi-square tests for categorical variables. The association of BMI z-score with tidal volume, respiratory rate, and minute ventilation was estimated by multivariable linear regression. We adjusted for covariates predictive of lung function – age, age2, height, height2, sex, and race – as well as asthma severity by NAEPP criteria25. Because there was a non-linear association between BMI z-score and respiratory rate, BMI was represented as a linear and squared term. Statistical association was assessed by a Wald test against the hypothesis that the coefficient of BMI z-score and BMI z-score2 were jointly equal to zero. Contrasts between an obese (BMI z-score of 2.2, corresponding to the median z-score among obese participants in this study) and normal weight participant (z-score of 0) were estimated. For deposition analysis, statistical relationships between BMI z-score, respiratory pattern, and deposition pattern were estimated by univariable regression.
All statistical analyses were performed in Stata 15 (StataCorp; College Station, TX). A two-sided p-value of <0.05 was considered statistically significant.
RESULTS
Cohort description
One-hundred and seventy-four participants had available anthropometry and respiratory pattern data for inclusion in this study (Table 1). Mean age was 11, and the majority were of male sex and Black race. Despite most participants receiving moderate-to-high doses of inhaled corticosteroids, 81% of the 155 participants with recorded ATAQ scores had uncontrolled asthma. The mean in-home PM2.5 concentration was 20.1 μg/m3. The BMI z-score range was −1.7 (5th percentile) to 2.9 (>99th percentile), and approximately half of the participants were obese by CDC criteria. When stratified by obesity, there were no differences in baseline characteristics in univariable comparisons except for higher minute ventilation among obese participants.
Table 1.
Participant characteristics (n=174)
| Characteristic | Non-Obese (n=88) | Obese (n=86) | p-value |
|---|---|---|---|
| Age, mean±SD | 11.1±2.5 | 10.9±2.5 | 0.49 |
| Female sex, n (%) | 30 (34) | 39 (45) | 0.13 |
| Black race, n (%) | 74 (84) | 75 (87) | 0.56 |
| ATAQ score+ | 2.2±1.9 | 2.8±2.0 | 0.08 |
| Severe persistent | 14 (16) | 17 (20) | |
| FEV1/FVC | 0.81±0.09 | 0.81±0.08 | 0.94 |
| Minute ventilation (L/min) | 3.4±1.0 | 3.9±0.9 | <0.01 |
20/174 missing;
19/174 missing;
ATAQ: Asthma Therapy Assessment Questionnaire; SD: standard deviation; FEV1: forced exhaled volume in the first second; FVC: forced vital capacity
Association of BMI with tidal volume, respiratory rate, and minute ventilation
As shown in Figure 1, we identified a linear relationship between increasing BMI and increasing tidal volume (adjusted mean difference [aMD] between obese and normal-range BMI of 25 mL, 95% confidence interval [CI] 5–45 mL). Respiratory rate had a U-shaped relationship with BMI, with those at the extremes of observed values having higher respiratory rates. However, the magnitude of change in tidal volumes outweighed the change in respiratory rate, such that the relationship of BMI to minute ventilation remained approximately linear, with increasing BMI associated with increasing minute ventilation (aMD 453 mL/min, 95% CI 123–784 mL/min). A child at the 99th percentile for BMI (z-score of 2.2) was predicted to have approximately 17% higher minute ventilation compared to a child at the 50th percentile (z-score of 0).
Figure 1.
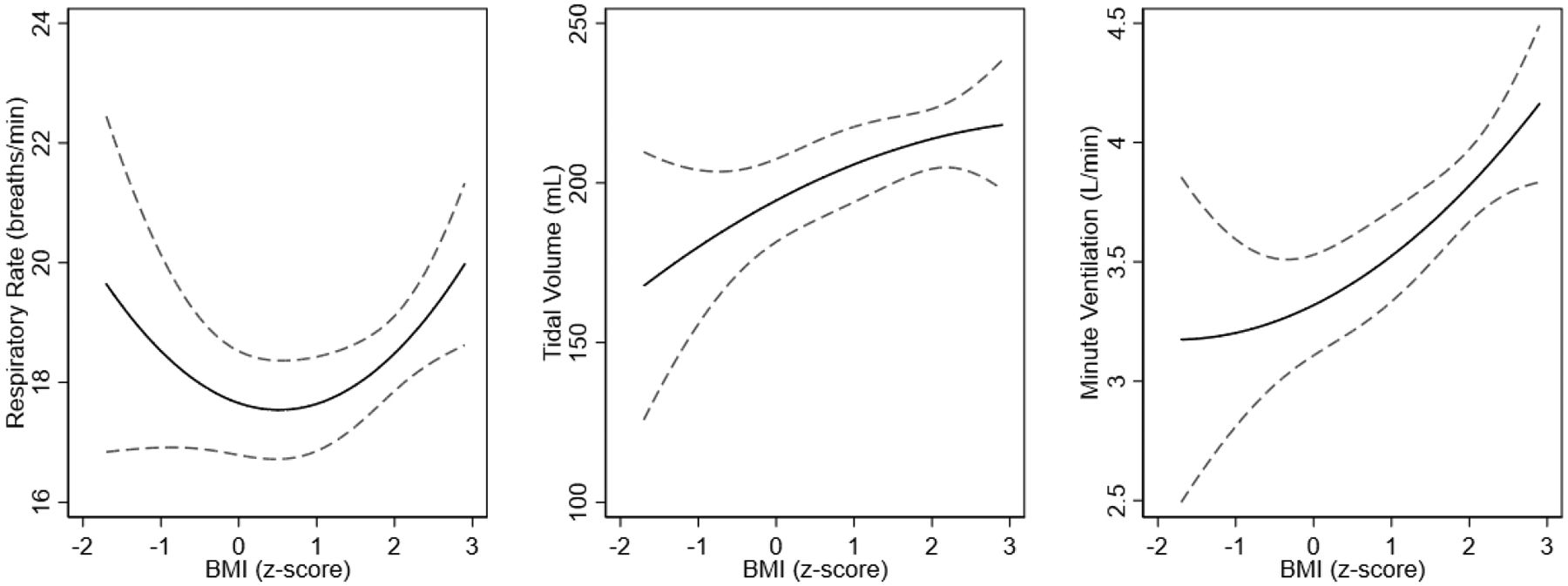
Point estimates (solid line) and 95% confidence intervals (dashed lines) of the association of BMI with respiratory rate, tidal volume, and minute ventilation across the range of observed BMI. Models are adjusted for age, age2, height, height2, race, sex, and asthma severity.
Tidal volume and respiratory rate have opposing effects on alveolar fractional deposition
MPPD analysis demonstrated that higher tidal volumes were associated with greater fractional deposition of inhaled PM2.5 within the respiratory system (eFigure 2, open circles). This was predominantly driven by more efficient deposition within the alveolar region, where for every 100 mL increase in tidal volume, fractional deposition in this region increased by approximately 4% (eFigure 2, closed circles). Associations with other regions are listed in eTable 1.
Higher respiratory rates were associated with the opposite effect. With increasing respiratory rate, total fractional deposition decreased (eFigure 3, open circles), which was again driven by decreased alveolar fractional deposition (eFigure 3, closed circles). Higher respiratory rate was also statistically associated with lower fractional deposition in TB region and higher fractional deposition in the URT region, but these were of negligible magnitude (eTable 1).
Overall, the effect of obesity was an increase in total and alveolar fractional deposition (Figure 2, open and closed circles). An obese child was predicted to have a 12% higher alveolar fractional deposition compared to a normal-weight child, corresponding to an adjusted mean difference of 3.4% (95% confidence interval 1.3—5.5%). We did not find a relationship between BMI and fractional deposition within the TB or URT regions.
Figure 2.
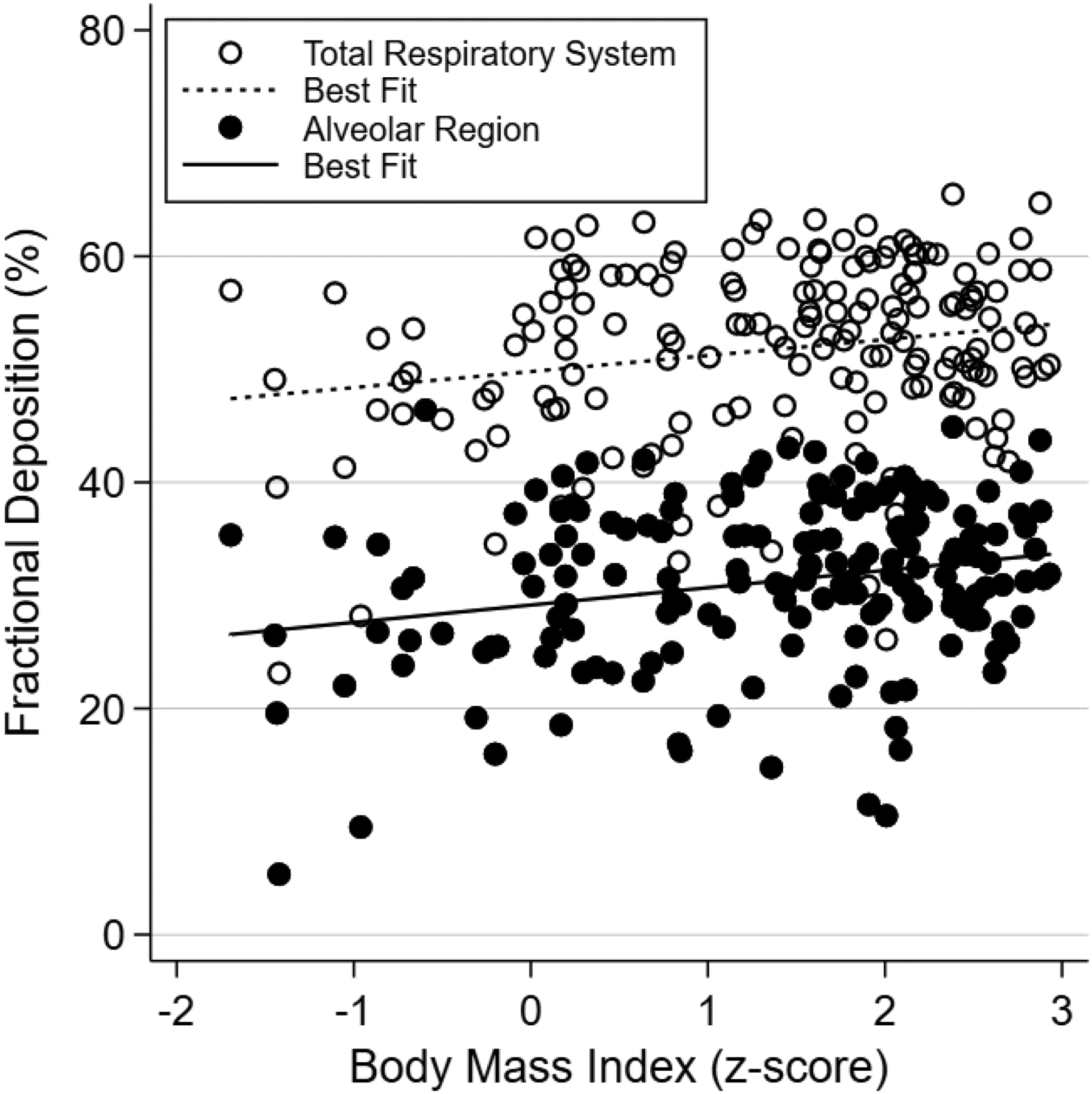
Scatterplot of PM2.5 fractional deposition by body mass index for the total respiratory system and alveolar regions, with respective lines of best fit.
We next reduced FRC among obese participants and found that associations between BMI and worsened fractional deposition were maintained (eFigure 4). The difference in alveolar fractional deposition between an obese and normal-weight child was qualitatively similar at 5.2% (95% CI 2.7—7.7%). In more comprehensive sensitivity analyses (not shown), we found that inferences were insensitive to perturbations in FRC.
Obesity is associated with higher rates of particle deposition
We next modeled rates of PM2.5 deposition at a concentration of 24.1 μg/m3 for each participant, the mean indoor PM2.5 concentration from the 148 available measurements in this cohort (eFigure 1). As shown in Figure 3 (open circles), higher BMI was strongly associated with higher rates of particulate deposition within the respiratory system. This was driven predominantly by higher rates of deposition within the alveolar region (Figure 3, closed circles), where an obese child was predicted to have a 29% higher rate of PM2.5 deposition compared to a normal weight child, corresponding to an adjusted mean difference of 7.1 ng/min (95% CI 3.9—10.2 ng/min), with smaller increases in deposition rates within the URT and TB regions (eTable 2).
Figure 3.
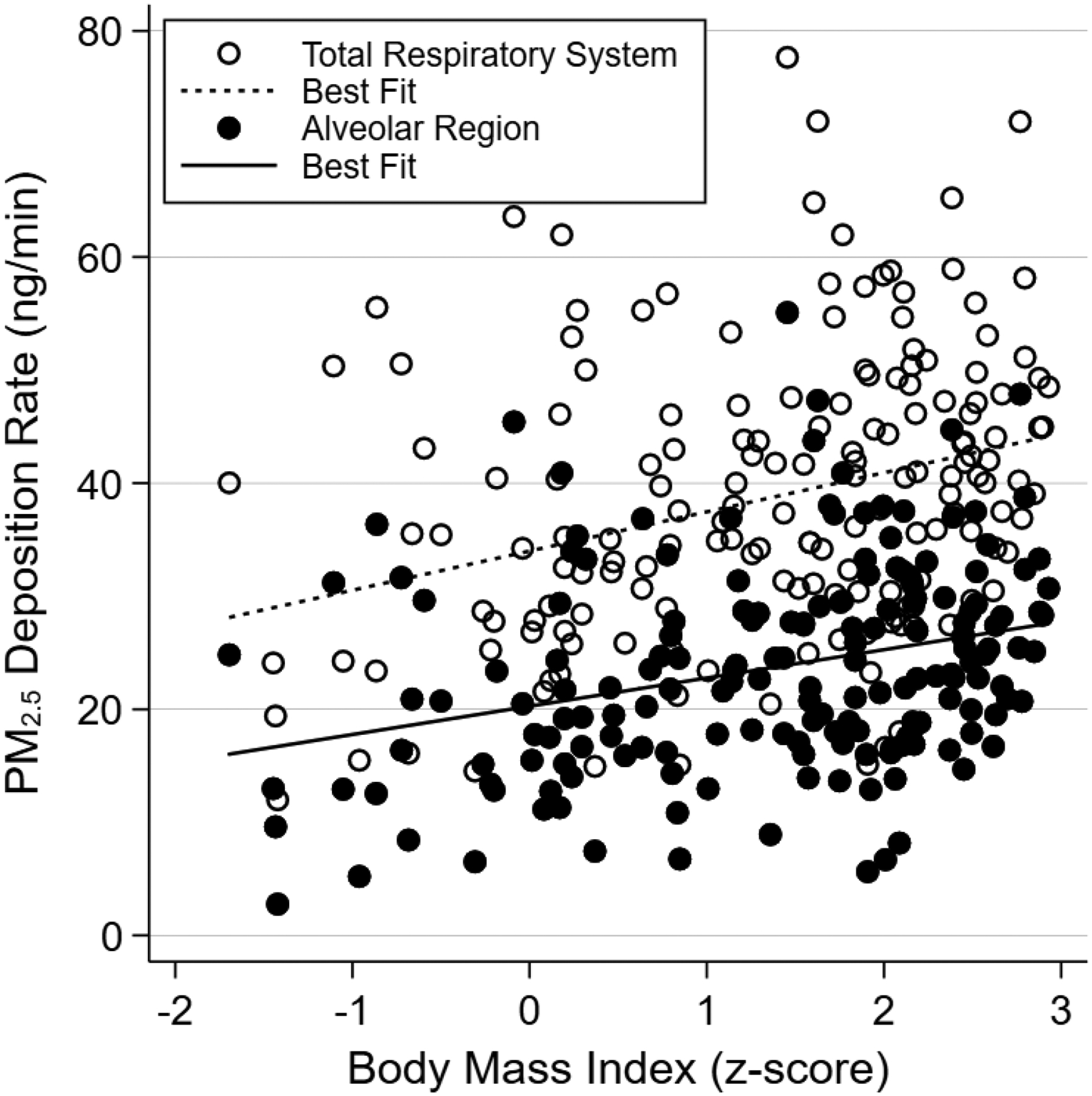
Scatterplot of PM2.5 deposition rate by body mass index for the total respiratory system and alveolar regions, with respective lines of best fit.
DISCUSSION
In this study of children with asthma, we found positive associations between BMI and tidal volume, respiratory rate, and minute ventilation. We identified that obesity was associated with higher tidal volume and higher minute ventilation, which were in turn associated with greater breath-to-breath efficiency and higher rate of PM2.5 deposition in the lung. These results provide evidence for a unique mechanism which may partially explain why obese children with asthma demonstrate greater susceptibility to air pollution.
To our knowledge, this is the first study to examine obesity-related changes in breathing patterns among children with asthma. The combination of obesity precipitating a higher respiratory rate, with each breath being more efficient in depositing PM2.5 in the lung, outlines a “dual hit” scenario whereby obese children with asthma are cumulatively exposed to greater amounts of airborne particulate matter than their normal weight counterparts even when breathing the same ambient air.
Our results are consistent with a small experimental study in healthy children by Bennett and Zeman showing that increased BMI was associated with higher total fractional deposition of inhaled fine particles, which was in turn most associated with higher tidal volumes10. Our results extend this study by examining a larger number of children, focusing on those with prevalent asthma, and incorporating estimates of regional in addition to total fractional deposition. Additionally, we utilized a novel flow meter that allowed direct measurement of both oral and nasal flows. This device increased ventilatory measurement accuracy by minimizing mechanical dead space and avoided the need for a nose clip, which has been shown to alter breathing patterns26.
We utilized the MPPD model to predict consequences of BMI-associated changes in breathing patterns, which to our knowledge is the first application of a dosimetry model for this purpose. In this manner, we were able to relate obesity to more deleterious particle deposition characteristics. We emphasize that this inference was made indirectly through an analysis of tidal volumes in silico. An implicit assumption in this approach is that the effect of obesity on particle deposition occurs predominantly through changes in breathing patterns. Although we showed that the model was not particularly sensitive to perturbations in FRC, and we did not identify any other configurable parameter that may be conceivably altered by obesity, our study should not be interpreted as conclusive. Direct physiological measurement of regional particle deposition characteristics in non-obese and obese children with asthma is an important next step.
Due to increased metabolism and CO2 production, an increase in minute ventilation with higher BMI is perhaps unsurprising10,27. However, our study suggests that this was due in part to an increase in tidal volume, which is not immediately intuitive. Among adults, obesity has been associated with lower tidal volumes at rest and exercise, findings attributed to decreased respiratory system compliance and differences in autonomic regulation of breath timing27–30. These would seem consistent with well-described associations of obesity with a restrictive pattern on pulmonary function testing, characterized by reduced total lung capacity (TLC), FRC, and in cases of morbid obesity, a reduction in forced vital capacity (FVC). However, literature from adult bariatric studies have related weight-reduction surgery with a decrease in tidal volume despite an increase in FVC and reversal of other impairments seen in pulmonary function testing, reinforcing that lung function does not necessarily correlate with breathing patterns and suggesting that our finding may not be limited to children31,32. Further, in children, despite similar reductions in TLC and FRC, morbid obesity does not significantly affect FVC, suggesting that respiratory mechanics remain more preserved in youth24.
An obese child at the 99th percentile for BMI was predicted to have a 29% higher rate of alveolar PM2.5 deposition compared to a normal weight child the 50th percentile. Although the extrapolated difference of 10.2 μg per day is small, this estimate is conservative as it assumes calm, tidal breathing in an indoor environment at modest concentrations of PM2.5. In children with asthma, small differences in indoor PM2.5 concentration are associated with large differences in asthma symptoms, highlighting that asthma control is especially sensitive to environmental changes33. This may be explained by the particular harms of PM2.5, which by virtue of their small size are able to deposit deeper within the respiratory tree, exposing a large surface area of airway epithelium to inflammatory injury34.
The direct measurement of breathing patterns and application of the MPPD model to predict consequences of their changes due to obesity are key novelties of this investigation. However, some limitations are noted. Although we have shown that estimates from the MPPD model were robust to changes in assumed inputs, the model does not explicitly simulate airway obstruction. This limitation, however, is balanced by two factors. First, exposure studies of ultrafine particles in children and fine particles in adults have both shown that asthma is associated with higher total deposition, suggesting that our findings establish a conservative lower bound for estimates of fractional deposition35,36. Second, almost all participants with asthma in these other studies had obstruction on spirometry. This was not evident in our population, making significant departures from MPPD estimates less likely overall. Additionally, because AIRWEIGHS did not recruit participants who are underweight, this study cannot be extrapolated to children below the 5th percentile for BMI. Finally, our study population was predominantly Black and recruited from an urban center, with attendant limitations in generalizability to the overall population of pediatric asthma.
CONCLUSION
Among children with asthma, obesity was associated with higher tidal volumes which, in turn, was associated in a particle deposition model with more efficient deposition of PM2.5 within the lung generally and alveoli in particular. These findings characterize a mechanism for why obese children with asthma are more susceptible to air pollution, and thus rationale for why obesity is a risk factor for more severe and uncontrolled asthma. Follow-on investigation of particle deposition characteristics in children with asthma is warranted.
Supplementary Material
Funding Statement
Technology described in this manuscript was supported by an SBIR award from the National Heart, Lung, and Blood Institute (R44HL091687). EB, KK, NNH, and MCM are supported by the National Institute of Environmental Health Sciences (KK, NNH, MCM: P50ES018176, EB: K23ES029105) and by the United States Environmental Protection Agency (83615201 and 83615001). TDW is supported by the National Heart, Lung, and Blood Institute (K23HL151669) and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Center for Innovation in Quality, Effectiveness and Safety (CIN 13-413). This work is the sole responsibility of the authors and does not necessarily represent the views of the National Institutes of Health, the Department of Veterans Affairs, the Environmental Protection Agency, or the United States government.
ABBREVIATIONS
- aMD
Adjusted mean difference
- ATAQ
Asthma Therapy Assessment Questionnaire
- BMI
Body mass index
- FEV1
Forced exhaled volume in the first second
- FVC
Forced vital capacity
- MPPD
Multi-path particle deposition
- PM2.5
Fine particulate matter
- TB
Tracheobronchial
- TLC
Total lung capacity
- URT
Upper respiratory tract
BIBLIOGRAPHY
- 1.Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–913. doi: 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer-Oglesby L, Grize L, Gassner M, et al. Decline of ambient air pollution levels and improved respiratory health in Swiss children. Environ Health Perspect. 2005;113(11):1632–1637. doi: 10.1289/ehp.8159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilliland F, Avol E, McConnell R, et al. The Effects of Policy-Driven Air Quality Improvements on Children’s Respiratory Health. Res Rep Health Eff Inst. 2017;(190):1–75. [PMC free article] [PubMed] [Google Scholar]
- 4.Berhane K, Chang C-C, McConnell R, et al. Association of Changes in Air Quality With Bronchitic Symptoms in Children in California, 1993–2012. JAMA. 2016;315(14):1491–1501. doi: 10.1001/jama.2016.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack MC, Breysse PN, Matsui EC, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294–298. doi: 10.1289/ehp.11770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong GH, Qian Z, Liu M-M, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes (Lond). 2013;37(1):94–100. doi: 10.1038/ijo.2012.125 [DOI] [PubMed] [Google Scholar]
- 7.Epstein TG, Ryan PH, LeMasters GK, et al. Poor asthma control and exposure to traffic pollutants and obesity in older adults. Ann Allergy Asthma Immunol. 2012;108(6):423–428.e2. doi: 10.1016/j.anai.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu KD, Breysse PN, Diette GB, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131(4):1017–1023, 1023.e1–3. doi: 10.1016/j.jaci.2012.12.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu TD, Brigham EP, Peng R, et al. Overweight/obesity enhances associations between secondhand smoke exposure and asthma morbidity in children. J Allergy Clin Immunol Pract. 2018;6(6):2157–2159.e5. doi: 10.1016/j.jaip.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett WD, Zeman KL. Effect of body size on breathing pattern and fine-particle deposition in children. J Appl Physiol (1985). 2004;97(3):821–826. doi: 10.1152/japplphysiol.01403.2003 [DOI] [PubMed] [Google Scholar]
- 11.Busse WW, Boushey HA, Camargo CA, Evans D, Foggs MB, Janson SL. Expert panel report 3: Guidelines for the diagnosis and management of asthma. Washington, DC: US Department of Health and Human Services, National Heart Lung and Blood Institute. Published online 2007:1–417. [Google Scholar]
- 12.Wu TD, Perzanowski M, Peng RD, et al. Validation of the maximum symptom day among children with asthma. J Allergy Clin Immunol. 2019;143(2):803–805.e10. doi: 10.1016/j.jaci.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner EA, Diette GB, Algatt-Bergstrom PJ, et al. The Asthma Therapy Assessment Questionnaire (ATAQ) for children and adolescents. Dis Manag. 2004;7(4):305–313. doi: 10.1089/dis.2004.7.305 [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–1319. doi: 10.1002/sim.4780111005 [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 16.Kirkness JP, Verma M, McGinley BM, et al. Pitot-tube flowmeter for quantification of airflow during sleep. Physiol Meas. 2011;32(2):223–237. doi: 10.1088/0967-3334/32/2/006 [DOI] [PubMed] [Google Scholar]
- 17.Asgharian B, Hofmann W, Bergmann R. Particle deposition in a multiple-path model of the human lung. Aerosol Science & Technology. 2001;34(4):332–339. [Google Scholar]
- 18.Mortensen JD, Schaap R, Bagley B, et al. Final report: A study of age specific human respiratory morphometry. University of Utah Research Institute, UBTL Division, University of Utah, Salt Lake City, UT. Published online 1983:01525–01010. [Google Scholar]
- 19.Mortensen JD, Stout L, Bagley B, et al. Age related morphometric analysis of human lung casts. Extrapolation of dosimetric relationships for inhaled particles and gases. Published online 1989:59–68. [Google Scholar]
- 20.Patterson RF, Zhang Q, Zheng M, Zhu Y. Particle deposition in respiratory tracts of school-aged children. Aerosol and Air Quality Research. 2014;14(1):64–73. [Google Scholar]
- 21.Othman M, Latif MT, Matsumi Y. The exposure of children to PM2. 5 and dust in indoor and outdoor school classrooms in Kuala Lumpur City Centre. Ecotoxicology and environmental safety. 2019;170:739–749. [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg GL, Asgharian B, Kimbell JS, Ultman JS, Jarabek AM. Modeling approaches for estimating the dosimetry of inhaled toxicants in children. J Toxicol Environ Health A. 2008;71(3):166–195. doi: 10.1080/15287390701597889 [DOI] [PubMed] [Google Scholar]
- 23.Forno E, Han Y-Y, Mullen J, Celedón JC. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J Allergy Clin Immunol Pract. 2018;6(2):570–581.e10. doi: 10.1016/j.jaip.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 25.Gilbert R, Auchincloss JH, Brodsky J, Boden W. Changes in tidal volume, frequency, and ventilation induced by their measurement. J Appl Physiol. 1972;33(2):252–254. doi: 10.1152/jappl.1972.33.2.252 [DOI] [PubMed] [Google Scholar]
- 26.McCormack MC, Breysse PN, Matsui EC, et al. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol. 2011;106(4):308–315. doi: 10.1016/j.anai.2011.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8(1):E69–74. doi: 10.3978/j.issn.2072-1439.2016.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(4):1269–1276. doi: 10.1152/jappl.1983.55.4.1269 [DOI] [PubMed] [Google Scholar]
- 29.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–210. doi: 10.1155/2006/834786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17(1):43–49. doi: 10.1111/j.1440-1843.2011.02096.x [DOI] [PubMed] [Google Scholar]
- 31.Chlif M, Keochkerian D, Choquet D, Vaidie A, Ahmaidi S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir Physiol Neurobiol. 2009;168(3):198–202. doi: 10.1016/j.resp.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 32.Matos CMP, Moraes KS, França DC, et al. Changes in breathing pattern and thoracoabdominal motion after bariatric surgery: a longitudinal study. Respir Physiol Neurobiol. 2012;181(2):143–147. doi: 10.1016/j.resp.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Dávila-Cervantes A, Domínguez-Cherit G, Borunda D, et al. Impact of surgically-induced weight loss on respiratory function: a prospective analysis. Obes Surg. 2004;14(10):1389–1392. doi: 10.1381/0960892042583996 [DOI] [PubMed] [Google Scholar]
- 34.Xing Y-F, Xu Y-H, Shi M-H, et al. The impact of PM2.5 on the human respiratory system. JThorac Dis 2016; 8(1):E69. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svartengren M, Anderson M, Bylin G, Philipson K, Camner P. Regional deposition of 3.6-micron particles and lung function in asthmatic subjects. J Appl Physiol (1985). 1991;71(6):2238–2243. doi: 10.1152/jappl.1991.71.6.2238 [DOI] [PubMed] [Google Scholar]
- 36.Olvera HA, Perez D, Clague JW, et al. The effect of ventilation, age, and asthmatic condition on ultrafine particle deposition in children. Pulm Med. 2012;2012:736290. doi: 10.1155/2012/736290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


