Abstract
It was first posited, more than 5 decades ago, that the etiology of schizophrenia involves overstimulation of dopamine receptors. Since then, advanced clinical research methods, including brain imaging, have refined our understanding of the relationship between striatal dopamine and clinical phenotypes as well as disease trajectory. These studies point to striatal dopamine D2 receptors, the main target for all current antipsychotic medications, as being involved in both positive and negative symptoms. Simultaneously, animal models have been central to investigating causal relationships between striatal dopamine D2 receptors and behavioral phenotypes relevant to schizophrenia. We begin this article by reviewing the circuit, cell-type and subcellular locations of dopamine D2 receptors and their downstream signaling pathways. We then summarize results from several mouse models in which D2 receptor levels were altered in various brain regions, cell-types and developmental periods. Behavioral, electrophysiological and anatomical consequences of these D2 receptor perturbations are reviewed with a selective focus on striatal circuit function and alterations in motivated behavior, a core negative symptom of schizophrenia. These studies show that D2 receptors serve distinct physiological roles in different cell types and at different developmental time points, regulating motivated behaviors in sometimes opposing ways. We conclude by considering the clinical implications of this complex regulation of striatal circuit function by D2 receptors.
Introduction:
The relationship between dopamine and symptoms of schizophrenia is complex. Increased dopamine release capacity in the associative regions of the striatum correlates with positive symptoms (hallucinations and delusions), and occupancy of dopamine D2 receptors (D2Rs) in the striatum predicts treatment response to antipsychotic medications that inhibit D2Rs1–4. In contrast, D2R occupancy in the ventral region of the striatum negatively correlates with the severity of negative symptoms, including motivational deficits5. Brain imaging studies have also linked altered density of striatal D2Rs to substance use disorder, attention deficit hyperactivity disorder, and obesity6, 7. However, how altered D2R expression and function affect striatal circuit function and behavior is not well understood. To model changes in D2R function in mice, D2R expression level has been manipulated in different subregions, at different times of development, and in different cell types. Here, we will summarize findings from the last 15 years using these models, focusing on striatal circuit function and motivated behavior. To date, mouse models for the study of schizophrenia have been limited to cognitive and negative symptoms, due to the inherently complex nature of positive symptoms. However, human studies suggest that hallucinations and delusions are caused by aberrations in perceptual processing8–10, a process that can be studied in rodents. Indeed, hallucination-like perceptions have been measured in mice and found to be mediated by striatal dopamine11. Future studies can determine how striatal D2Rs regulate perceptual abnormalities, addressing the link between enhanced D2R occupancy positive symptoms in patients.
Dopamine D2Rs are present in various projections, cell types and subcellular locations of striatal circuitry
The striatum is the input nucleus of the basal ganglia. Its principal neurons, the GABAergic spiny projection neurons (SPNs), are classified into the striato-pallidal and striato-nigral output pathways. While striato-nigral SPNs project directly to the basal ganglia output nuclei; the substantia nigra pars reticulata (SNr) and internal segment of the globus pallidus (GPi), striato-pallidal SPNs project to these regions indirectly via the external segment of the globus pallidum (GPe)12 (Figure 1). The two output pathways are often described as functionally opposing because the striato-pallidal pathway ultimately inhibits thalamo-cortical projections, while the striato-nigral pathway has a disinhibitory effect13. Historically, these pathways were described as anatomically segregated. However, striato-nigral neurons possess axon collaterals that innervate the striato-pallidal target area, the GPe14–16. Moreover, the GPe, which is often depicted as an intermediate station between the striatum and the GPi/SNr, projects back to the striatum as well as directly towards the thalamus and cortex17–19. These projections originate from distinct subpopulations within the GPe that have recently been defined using molecular tools17–19. While a similar organization exists in ventral striatum, including the nucleus accumbens (NAc)20, 21, the neuroanatomical and functional segregation of the two pathways is less pronounced than in dorsal regions22. Like the dorsal output pathways, the accumbo-pallidal SPNs project directly to the ventral pallidum (VP), which in turn projects to the SNr, the ventral tegmental area (VTA), and mediodorsal thalamus. As in the dorsal striatum, a substantial proportion of accumbo-nigral SPNs send projections to the VP, in addition to their direct projections to the basal ganglia output nuclei22–24.
Figure 1.
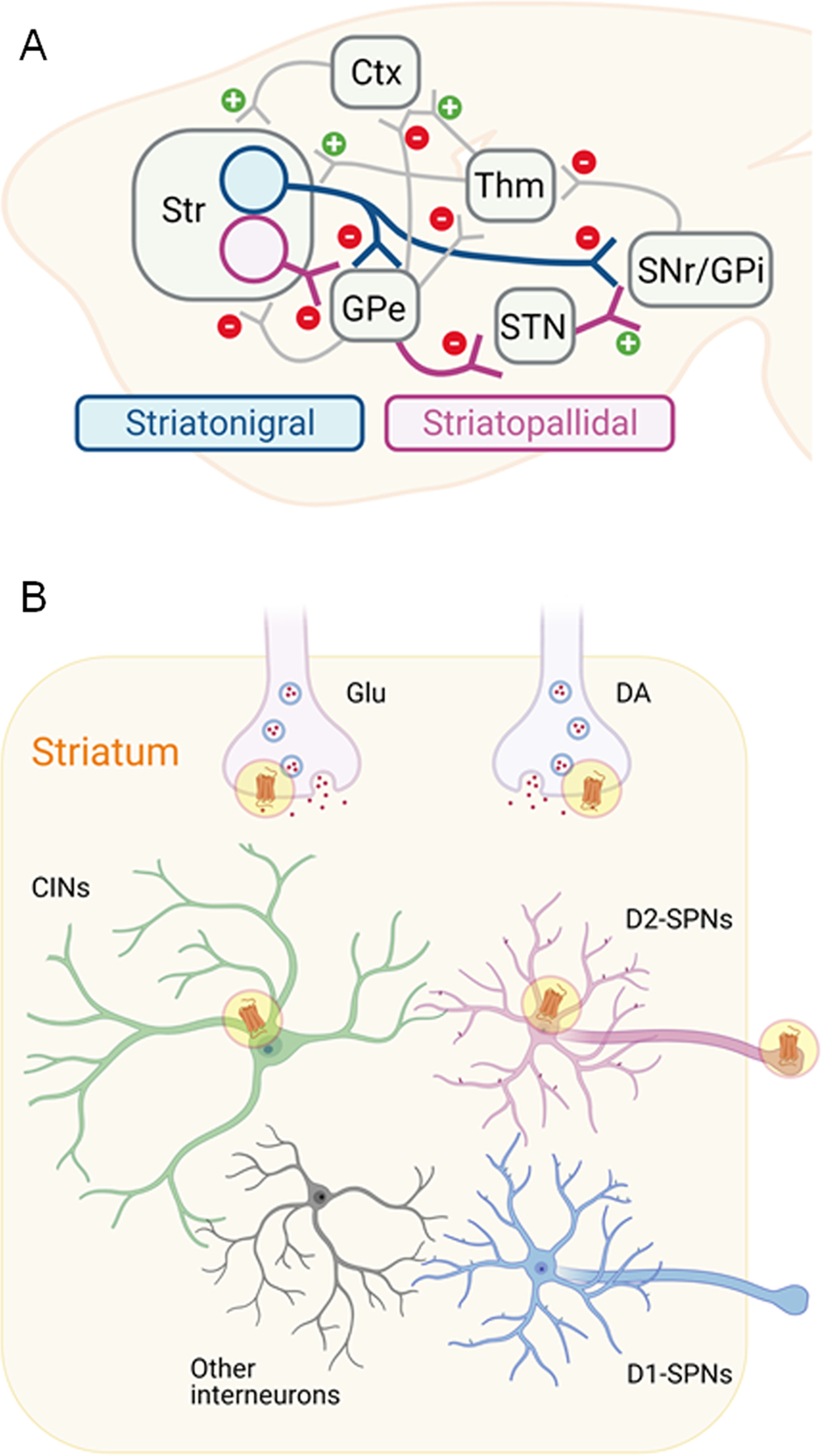
A. Schematic of the basal ganglia circuitry. The striatum receives excitatory input from the cortex (Ctx) and thalamus (Thm). Neurons of the striatonigral pathway innervate substantia nigra (GPi) and internal globus pallidum (GPi), resulting in disinhibition of thalamocortical function. Striatonigral pathway neurons also send inhibitory projections to the canonical target of the striatopallidal pathway, the external globus pallidum (GPe). GPe neurons project to classical striatopallidal downstream targets such as the subthalamic nucleus (STN) that lead to activation of the SNr/GPi and thalamocortical inhibition. In addition, GPe neurons project to striatum, thalamus, and cortex. Although there are quantitative differences in the strengths of projections the organization of dorsal and ventral striatal circuitry is similar. B. Within striatum, different neuronal populations express D2Rs, including cholinergic interneurons (CINs) and spiny projection neurons (SPNs) of the striatopallidal pathway, which exhibit somatodendritic as well as axonal presence of D2Rs. In addition, D2Rs may be found in axon terminals of glutamatergic and dopaminergic afferents.
D2Rs are expressed in different neuronal cell types within the striatum, including the striato-pallidal SPNs and cholinergic interneurons (CINs), but not the striatonigral SPNs, which primarily express D1 receptors13. D2Rs are also present in neurons that send afferents to the striatum, including dopamine neurons in the midbrain and glutamatergic neurons in the thalamus and cortex25–27. Quantitative ligand binding studies in cell type-selective D2R knock-out mice suggest that about 50% of D2Rs in the striatum are expressed in striato-pallidal SPNs, 20% on dopaminergic terminals and 10% on CINs, with the remaining D2Rs possibly coming from cortical and thalamic terminals27, 28. At the cellular level, D2Rs are found in somatodendritic and axonal compartments. The functional significance of this heterogeneous localization is particularly evident in midbrain dopamine neurons, which express autoreceptors that inhibit dopamine neuron firing patterns at the soma and dopamine release in axon terminals29, 30.
D2 receptor ligand binding has pleiotropic effects that vary across cell types and striatal regions.
D2Rs are G protein-coupled receptors (GPCRs) that belong to the D2-like dopamine receptor family along with D3 and D4 receptors. Canonically, these receptor complexes recruit heterotrimeric Gαi/o/z proteins such that dopamine binding leads to inhibition of adenylyl cyclase and decreased cAMP production, which regulates behavior via protein kinase A mediated phosphorylation of DARPP32 (dopamine and cAMP regulated phospho-protein Mw 32 KDa) and other effectors31, 32 (Figure 2). In addition, the G protein beta-gamma complex (Gβγ) mediates activation of G protein-coupled inwardly rectifying potassium channels (GIRK) channels, decreasing neuronal excitability33. By engaging phospholipase C-dependent mechanisms, Gβγ signaling further evokes Ca2+ release from intracellular stores, which in turn activate the phosphatase calcineurin, thereby regulating Ca2+ channels, such as L and N-type, within the striatum34, 35. In dopamine neurons, presynaptic D2Rs inhibit synaptic transmission via Gβγ-mediated regulation of voltage-gated calcium and/or potassium channels36–38. Similar mechanisms are proposed for striato-pallidal neurons where intra-striatal and striato-pallidal transmission is inhibited by presynaptic D2Rs39–43. In striatal CINs, D2R agonists not only reduce N-type Ca2+ currents, which promote neurotransmitter exocytosis, but they also decrease voltage-gated Na+ channel currents that support spontaneous pacemaking activity35. Optogenetic stimulation of DA neurons inhibit striatal CINs in a G protein-dependent manner, presumably through GIRK channels44. This inhibition of CINs by DA in brain slices is abolished in CIN-selective D2R knock-out mice45, 46.
Figure 2. Signaling pathways elicited by D2R activation.
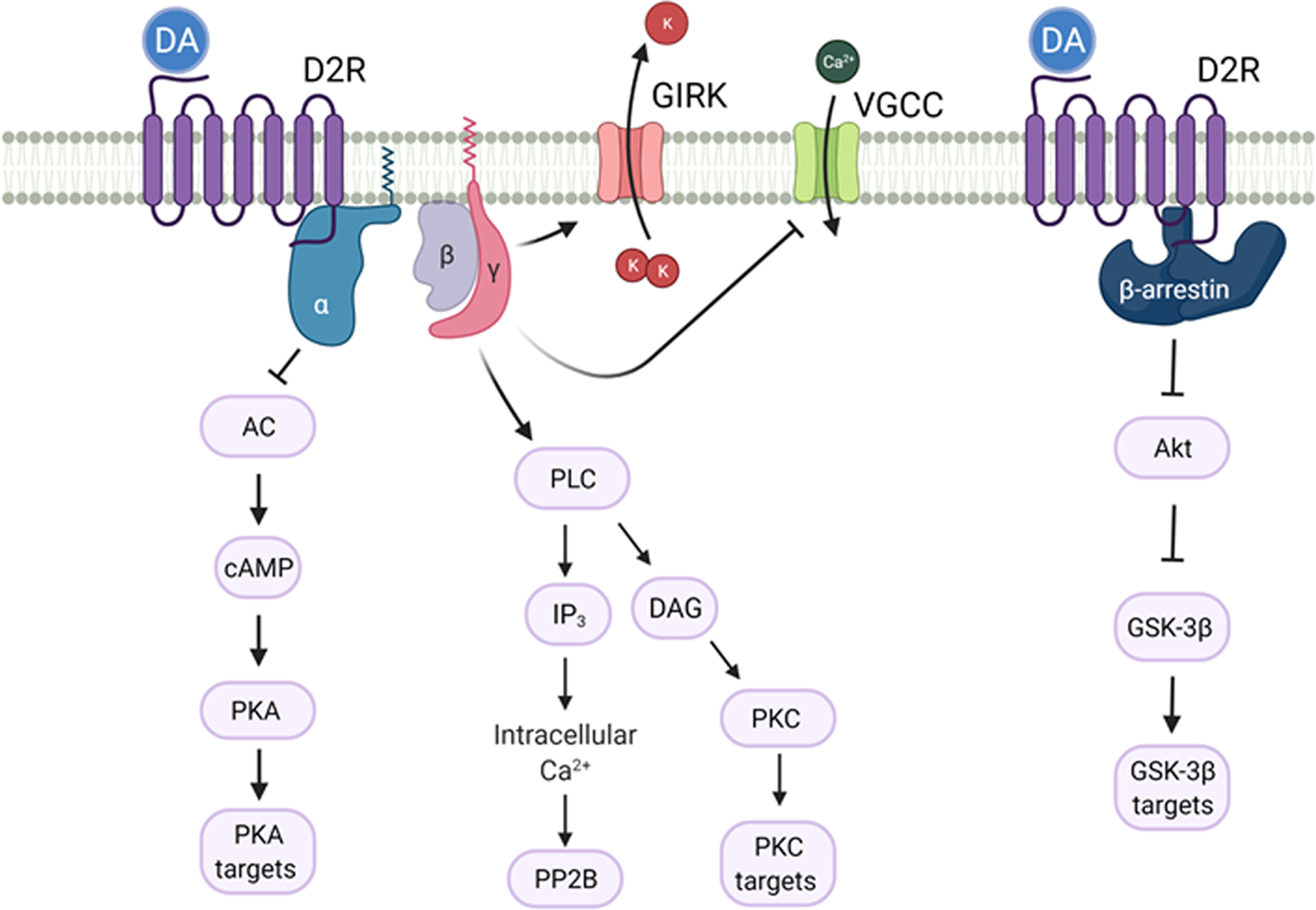
Canonical G protein-mediated signaling leads to inhibition of adenylyl cyclase (AC) and PKA activity. Activation of βγ subunits results in increased Ca2+ and PKC activation, as well as activation of G protein-coupled inwardly rectifying K+ channels (GIRK) and inhibition of voltage-gated Ca2+ channels (VGCC). G-protein independent signaling downstream of D2Rs can be mediated by β-arrestin, which inhibits Akt activity to increase GSK-3β function.
Like other GPCRs, D2Rs recruit β-arrestins. Arrestins were first implicated in terminating G protein signaling and facilitating clathrin-mediated receptor internalization47. Additional roles for arrestins include providing docking sites for signaling proteins and activating signal transduction pathways independently of G proteins48. D2Rs, acting via β-arrestin-2, can lead to the inactivation of the kinase Akt and the subsequent activation of glycogen synthase kinase 3 (GSK-3)49, 50. How this affects the physiology of striatal neurons is still unclear but may involve the membrane trafficking of AMPA receptors51.
Increased developmental striatal D2Rs can dampen motivation for food rewards in adulthood.
While decreased D2R occupancy in the ventral striatum correlates with negative symptoms such as passivity, apathy, and social withdrawal5, 52, human studies cannot determine if these factors are causally related. A large body of work using behavioral pharmacology in rodents demonstrates that depleting dopamine or acutely antagonizing dopamine receptors in the ventral striatum results in decreased motivation53. However, longer term changes in dopamine function, starting during development, also may be important because elevated striatal dopamine function in individuals with prodromal symptoms predates the onset of schizophrenia54. To determine if a chronic increase in D2R expression early in development can drive negative symptoms in adulthood, Kellendonk, Simpson et al. created a transgenic mouse (D2R-OE) that beginning in early development overexpresses D2Rs in striatal SPNs by approximately the same increment (15%) observed in patients55, 56. By using a bi-transgenic system, D2R overexpression can be switched off at any time point55. This aspect of the model makes it possible to determine which phenotypes are due to the current state of the brain versus those that are the result of irreversible compensatory changes that occur during development.
Like patients with schizophrenia, D2R-OE mice display deficits in select cognitive domains (working memory, cognitive flexibility and interval timing)55, 57–59 and a reduction in motivation57, 60–62. When the transgene is switched off after development, cognitive deficits persist in adulthood, whereas motivation is rescued. Here, we focus on the nature of the motivation deficit and related cellular and anatomical changes.
Testing motivation in animals is commonly done using a progressive ratio (PR) schedule of reinforcement where the number of lever presses required to earn a food reward is increased by some factor for each successive reward63. Figure 3 shows that when the cost of each reward doubles and the PR session ends when the mice stop pressing, D2R-OE mice give up trying to earn rewards much sooner than controls57 (Table 1). Switching off the transgene in adulthood using doxycycline rescued performance. Animals may quit early on a PR schedule for a number of reasons. A series of experiments determined that D2R-OE mice did not quit sooner because they were less hungry, had a diminished hedonic reaction to food reward, could not tolerate delays to reward, or differently respond to conditions of non-reward (extinction)60. Consistently, the use of novel conditioning paradigms that can dissociate effort-based and value-based decision making61 revealed that the D2R-OE mice evaluate the effort required to obtain a reward differently than controls, whereas their determination of the value of potential rewards is intact62. However, D2R-OE mice do not show reluctance to expend all types of effort. In a task requiring mice to hold a lever down for an increasing length of time, rather than pressing the lever an increasing number of times64, D2R-OE mice showed no deficit in earning rewards62. When given a choice, D2R-OE mice earn more rewards holding down a lever, even if it takes longer than a reward could be earned by making multiple presses62. This reluctance to repeatedly initiate action, explains why D2R-OE mice quit sooner than controls in PR tasks.
Figure 3. Schematic diagram of the operant progressive ratio (PR) task.
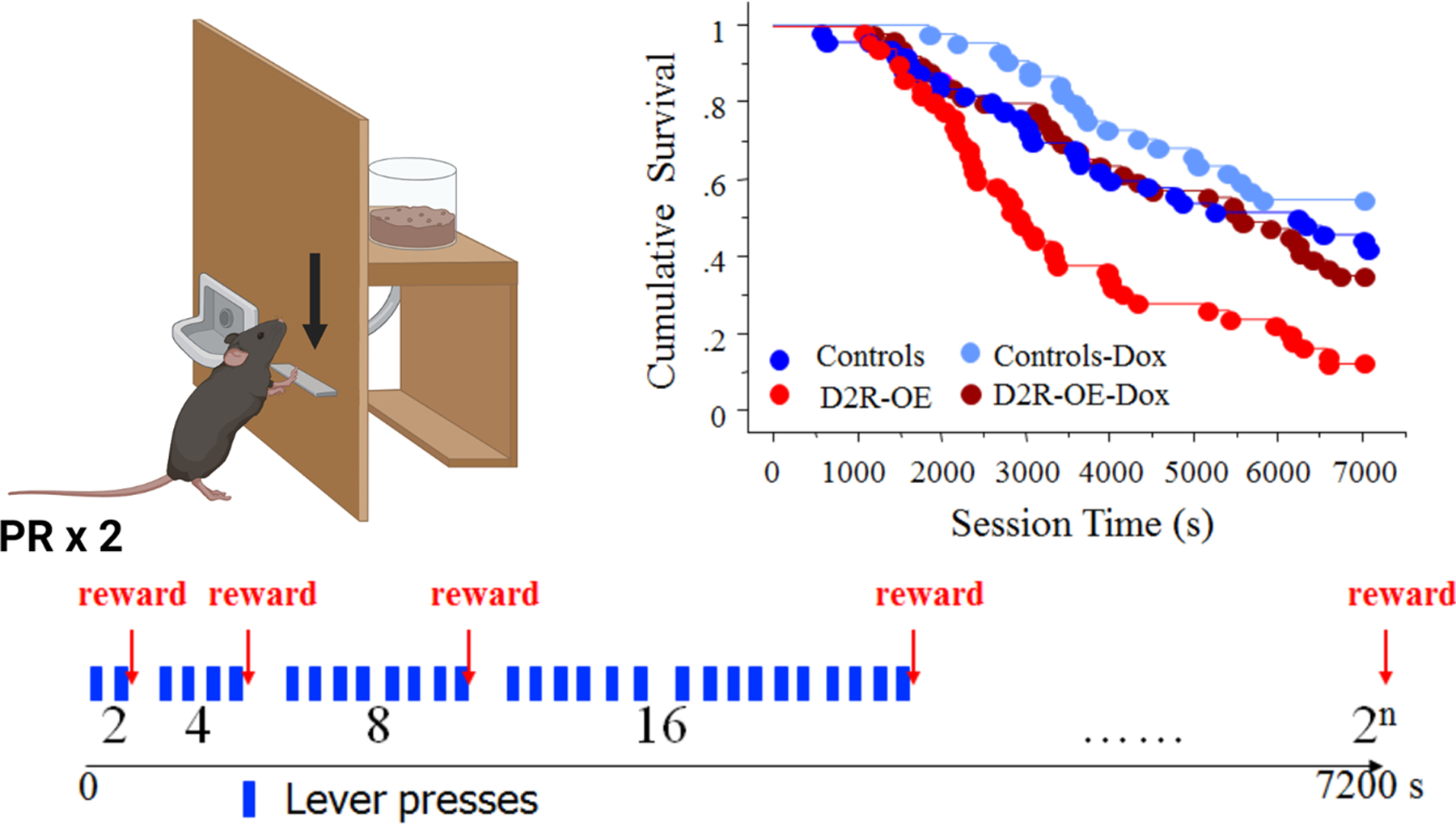
In this task mice press a lever an increasing number of times to earn food reinforcement (left). In this example, the lever press requirement is doubled after each reinforcer earned (bottom). The D2R-OE transgenic mice show reduced cumulative survival (proportion of mice that are still pressing the lever as a function of session time) compared to wildtype littermates or D2R-OE mice treated with doxycycline (Dox) to switch off the transgene (Right).
Table 1.
A summary of the effect on performance in a food reinforced progressive ratio schedule of various manipulations to dopamine D2 receptors or dopamine neurons. NAc (nucleus accumbens), CPu (caudate putamen), SPNs (spiny projection neurons), βarr (βarrestin biased).
| PR | Type of manipulation | Region | Cell-type | D2R | Citation |
|---|---|---|---|---|---|
| DECREASE | DA depletion (6-OHDA) | NAc(Core) | DA neurons | - | 65 |
| D2 Antagonist infusion | NAc(Core) | - | - | 66 | |
| Developmental D2R-OE | Striatum | SPNs | WT | 57 | |
| NOT AFFECTED | DA depletion (6-OHDA) | NAc(Shell) | DA neurons | - | 67 |
| D2 Antagonist infusion | NAc(Shell) | - | - | 66 | |
| Viral D2R-OE in adult | CPU | Untargeted | WT | 68 | |
| Viral D2R-OE in adult | NAc | Indirect SPNs | βarr | 69 | |
| Viral D2R-OE in adult | NAc | ChINs | WT | 43 | |
| INCREASED | Viral D2R-OE in adult | NAc | Untargeted | WT | 68 |
| Viral D2R-OE in adult | NAc | Indirect SPNs | WT | 43 | |
| D2R knockout | VTA/NAc | DA neurons | WT | 70 | |
| D2R shRNA knockdown | VTA/NAc | DA neurons | WT | 71 |
The effort-based motivation deficit identified in D2R-OE mice is strikingly similar to observations made in patients72. The motivational deficit in patients with schizophrenia is not due to an inability to experience pleasure in the moment, as hedonic reaction appears intact73. Instead, the motivational deficit stems from impaired effort allocation74, which is associated with the severity of negative symptoms and poor functioning75, 76. These convergent findings identify developmental alterations in D2R level or function as a potential pathophysiological mechanism underlying effort-based motivation deficits.
There are a number of factors that may be responsible for the changes in motivated behavior observed in the D2R-OE mice. Concurrent transgene expression in the SPNs leads to increased membrane excitability and a decrease in the length and complexity of their dendritic arbors. Both phenotypes are driven by changes in the expression of the inward rectifier potassium channels (Kir2.1/2.3)77. Increased SPN excitability changes the anatomy of striatonigral neurons, increasing the density of collateral projections to the globus pallidus that “bridge” the direct and indirect pathways, thereby regulating the functional balance of the basal ganglia15. This enhancement in indirect pathway collaterals was reversed not only by switching off the transgene but also by chronic treatment with the antipsychotic D2R receptor blocker haloperidol15. However, in contrast to switching off the transgene, chronic haloperidol failed to reverse the deficit in motivated behavior, most likely because haloperidol blocks all D2Rs and not just upregulated D2Rs78. In addition to changes in SPN function, D2R upregulation also led to a decrease in tonic and burst activity of VTA DA neurons. Decreased tonic activity was reversed after normalizing D2R levels and may explain the decrease in extracellular DA levels measured in the D2R-OE mice during goal directed behavior, which likely also contributes to the motivational deficits in these mice62, 79. In contrast, the decrease in VTA burst activity was not reversible in D2R-OE mice and may explain their persistent cognitive deficits79. Indeed, disturbed firing patterns of VTA DA neurons are associated with cognitive deficits in D2R-OE mice59. During a working memory task, D2R-OE mice did not show typical synchrony of VTA DA neurons or phase-locking of 4-Hz oscillations between VTA-PFC, and these VTA abnormalities scaled with the deficits in working memory59.
Upregulation of dopamine D2Rs in spiny projection neurons in the adult mouse
That motivation impairments in D2R-OE mice are reversed by switching off the transgene in adulthood suggests that D2R overexpression during adulthood contributes to the phenotype57, 60, 78. This finding, however, is counter to expectation as acute treatment with D2R antagonists decrease motivation in the adult animal80. Therefore, to determine the effects of D2R upregulation in the absence of altered D2R levels during development, D2Rs were upregulated in the striatum of adult mice using a viral strategy. Targeted viral upregulation in theNAc core versus dorsal striatum further defined the striatal subregion(s) involved in the phenotype. Increased D2Rs selective to the NAc were tested in two models, one that was non-cell type specific and one in which expression was restricted to indirect pathway neurons. Both manipulations enhanced PR performance43, 68, 81 consistent with the pharmacological studies64, 80, 82. In addition to increased breakpoints in PR, when presented the choice between free chow and working for a preferred caloric reward (milk), adult D2R-OE mice chose the higher effort option more often than controls, again, an effect opposite to that in the developmental D2-OE model43, 68. Sucrose preference was unaltered in these mice81 suggesting that, like in the developmental model, increased D2Rs altered effort and not value computations. These data are consistent with pharmacological studies showing that local infusion of D2R antagonists into the NAc decreases the willingness to work for rewards80. Rather than affecting the hedonic value of the reward, NAc dopamine promotes behavioral activation by modulating how work-related response costs enter into cost-benefit decision making53, 83. Indeed, mice with genetic deletion of D2Rs selectively from iSPNs show normal acquisition in an appetitive Pavlovian conditioning task but poor performance in an instrumental task when the response requirement is high84. In contrast to upregulation in the NAc adult overexpression of D2Rs in dorsal striatum did not enhance motivation suggesting that this effect is specific to NAc68 (though see:85).
As described above, D2Rs have been shown to inhibit synaptic transmission at indirect pathway terminals39–42. Consistent with this, D2R upregulation in striato-pallidal SPNs decreased synaptic transmission from the NAc to the ventral pallidum (VP), the main distal target of striato-pallidal SPNs43. Acute inhibition of synaptic transmission at VP terminals using the designer receptor hM4Di also enhanced PR performance, suggesting that a decrease in inhibition of VP neurons underlies the increase in incentive motivation seen in adult D2R-overexpressing mice43, 85.
Inhibiting the striato-pallidal pathway, therefore, will shift the functional balance of the two striatal output pathways in favor of the striato-nigral pathway, supporting the simple model that the direct pathway promotes action initiation whereas the indirect pathway constrains it. However, studies combining fiber photometry to monitor Ca2+ transients with optogenetic inhibition have shown that both pathways are concurrently activated during a motivation task, which is necessary for PR performance.86. While brief optogenetic activation of striato-pallidal neurons increases motivation and brief inhibition reduces motivation87, prolonged pharmacogenetic inhibition of striato-pallidal neurons resulted in increased PR performance43, 85. Together, these results argue that the timing and duration of SPN inhibition or excitation influences behavioral outcomes. It is possible that the calculation of the work-related response costs involves longer lasting SPN activity spanning entire trials or even sessions88.
Further evidence that VP disinhibition, as seen following D2R upregulation in striato-pallidial SPNs, alters effort computation rather than value computation comes from behavioral pharmacology experiments. Infusions of the GABAA agonist muscimol into the VP decrease effort-related choice, without altering the preference for palatable food53, 89. However, activation of a specific subpopulation of VP neurons that innervate the NAc shell (arkypallidal neurons), induces reward consumption and is thought to enhance the hedonic value of food reward90, pointing to a role of some VP neurons in value computations. Subtle differences in the NAc sub-areas targeted may explain this discrepancy. Indeed, viral D2R upregulation was mostly restricted to the NAc core, which primarily targets the dorsolateral VP, whereas the NAc shell mainly projects to ventromedial VP20.
Another factor contributing to the effects of D2R on motivation is the impact of striatal dopamine on energy metabolism, which may be independent from its effect on physical activity91–93. Local injection of the D2R agonist bromocriptine into the NAc has been shown to increase glucose intolerance92. Similarly, D2R upregulation in adult NAc striato-pallidal neurons increases glucose intolerance94, a phenotype that was also observed in the transgenic D2R-OE model95. Altered glucose metabolism could be mediated via NAc-projections to the lateral hypothalamus, which regulates food intake, metabolism and energy balance96. Independent of the underlying mechanisms it will be important to further study the role of striato-pallidal D2Rs in energy homeostasis and determine how this may impact motivated behavior.
Upregulation of dopamine D2Rs in cholinergic interneurons of the adult mouse
Within the striatum, D2Rs are not only expressed in striato-pallidal projection neurons but also in CINs where they inhibit neuronal activity via K+ and other ion channels44, 97, 98. In contrast to upregulation in striato-pallidal neurons, upregulation of D2Rs in CINs of the NAc core did not affect PR performance43. However, overexpression of D2Rs in CINs in the NAc was not without impact on operant behavior. This manipulation specifically delayed learning to suppress responding in a Go/NoGo paradigm, suggesting that D2Rs in CINs may play a role in balancing the opposing drives between action and inaction without an effect on PR performance99.
Inactivation of D2Rs in dopaminergic neurons
In midbrain dopaminergic neurons, D2Rs act as autoreceptors inhibiting neuronal activity, dopamine synthesis and dopamine DA release29, 33, 100. In line with this, genetic inactivation of D2 autoreceptors in midbrain dopamine neurons enhanced striatal DA release70, 101. D2R knock-out mice also showed enhanced performance in the PR task, which may be due to a greater activation of NAc D1Rs and/or D2Rs by enhanced DA release. This enhanced performance is likely due, at least in part, to ongoing D2R activation in the adult animal because shRNA-mediated inhibition of D2Rs in the VTA of adult mice recapitulates the higher incentive motivation71. In conclusion, D2Rs in different neuronal cell types can regulate motivated behaviors in opposite ways (striato-pallidal SPNs vs DA neurons) but do not affect PR performance in all cell types (e.g. in CINs). This has important implications for pharmacological interventions, including antipsychotic medications, which simultaneously target D2Rs in all neuron subtypes.
D2R downstream signaling and motivated behavior
As described above, D2Rs signal via G protein-dependent and β-arrestin-dependent pathways. To differentiate the effects of these pathways, there has been an effort to develop functionally biased ligands that preferentially engage G-protein-dependent versus β-arrestin-dependent signaling102, 103. However, since these ligands lack cellular specificity, D2R mutant constructs that predominantly recruit one or the other signaling pathway with different degrees of bias have been targeted to select cell types69, 104. Replacing endogenous striatal D2Rs with these biased receptors has revealed the relative role of each signaling pathway on various behaviors. G protein signaling was sufficient to support nestlet shredding, a measure of motor skill behavior, while normal locomotor activity depended on both G protein and β-arrestin signaling104. Moreover, D2R-linked β-arrestin signaling in striato-pallidal SPNs rescued the blunted effect of cocaine-induced hyperlocomotion in D2R knock-out mice69. However, studies using striato-pallidal D2R knockout mice have implicated inhibition of collateral transmission from striato-pallidal to striato-nigral neurons in the locomotor stimulating effects of cocaine42. It is therefore likely that both the canonical G protein-dependent and the non-canonical β-arrestin-dependent signaling pathways are involved in regulating locomotor activity.
Despite comparable locomotor effects in the open field, overexpression of wild-type but not β-arrestin-biased D2Rs in striato-pallidal SPNs of wild-type mice enhanced performance in the PR task69. This result suggests that the various components of motivated behavior, including drive and response type are differentially supported by G protein-dependent and β-arrestin-dependent D2R signaling. For example, β-arrestin signaling may be sufficient to enhance arousal, leading to higher activity in the open field, whereas G protein-signaling, likely by regulating neurotransmitter release in the VP, is required for enhancing goal-directed, reward-driven behavior. Future experiments in which G protein-biased D2Rs are expressed in a similar fashion will allow this hypothesis to be tested more directly.
Conclusions:
Several conclusions can be drawn from cell type-specific genetic approaches to target D2Rs.
D2Rs in different cell types play distinct roles: D2Rs play different cell type-specific roles in regulating motivated behavior. In DA neurons of the VTA, D2Rs inhibit neuronal activity and DA release, thereby constraining PR performance. In contrast, inhibition of striato-pallidal projections neurons by D2Rs enhances PR performance. These findings are consistent, as DA release inhibits striato-pallidal SPNs and inhibition of striato-pallidal transmission enhances motivation99. Dopamine release may also enhance motivation by attenuating collateral inhibitory transmission from D2R-expressing SPNs to D1R-expressing SPNs, which enhances striato-nigral pathway function. Although in vivo Ca2+ imaging did not provide direct evidence for disinhibited D1R SPN activity in adult NAc D2R-overexpressing mice, this may be due to the limited sensitivity of the Ca2+ imaging method99. Results in striato-pallidal D2R knockout mice further point to a crosstalk between striato-pallidal and striato-nigral neurons at the level of signaling, as the efficiency of D1R signaling is enhanced in D2R knockout mice105. How much such chronic, adaptive crosstalk contributes to regulating the willingness to work for food still remains to be addressed.
The time window of D2R action matters: It is striking that PR performance is decreased in the transgenic D2R-OE mice while it is increased in the adult D2R upregulation models. Notably, switching off the transgene in the adult D2R-OE mice reverses the behavioral phenotype. However, switching off excess D2R levels in a developmentally altered brain is different from regulating D2R levels in the unperturbed adult brain. Thus, early upregulation of D2Rs during development may alter striatal circuitry in a persistent way, affecting motivated behavior in the adult in a manner that can somehow be compensated by reducing D2R expression. Indeed, selective Gi activation of striato-pallidal neurons during the second postnatal week persistently altered the strength of cortico-striatal inputs for at least one week106. If these changes persist into adulthood, they could impact motivation in adult animals. As postnatal striatal development has been shown to be sensitive to dopamine levels107, future studies should address whether there are sensitive time windows during which D2Rs regulate striatal circuit maturation. In addition to varying the time window of expression, future studies should also more gradually titrate the levels of D2R expression. While in the transgenic model upregulation was by about 15% the upregulation using the viral vector was much more pronounced (3-fold in43). Larger degrees of upregulation may affect D2R signaling differently than lower degrees. In general, one must be careful when making comparisons between models that are generated using different methods. For example, transgenic mouse lines have the potential for ectopic offsite expression, depending on the faithfulness of the transgenic driver, whereas viral expression is more directly regionally targeted but usually leads to higher levels of overexpression. A careful analysis of transgene expression in the different models is therefore always required to aid the interpretation of outcomes.
D2R levels regulate effort-based and not value-based decisions: Here we focused mainly on the effect of various manipulations of D2Rs on a single behavior, PR, because this paradigm is the most common and best standardized test of motivated behavior that has been applied to all of the models discussed. However, additional behavioral analyses have revealed a more specific role of striatal D2Rs in motivated behavior. D2R-OE transgenic mice quit working for rewards due to an increased sensitivity to the effort requirement of the task and not a decrease in sensitivity to reward62. More specifically, it is the initiation of action, not persistence, perseverance or patience, which causes the mice to quit sooner and earn fewer rewards. In contrast, adult D2R-overexpressing mice choose to work harder for a preferred reward and inhibition of the indirect pathway enhanced the initiation of actions without affecting the sensitivity to reward43, 68, 85. Such dissection of behavioral phenotypes is extremely valuable because it permits a greater understanding of the impact on the circuits that regulate specific processes. This level of detail is also important for allowing translational researchers to better relate the phenotypes observed in animal models with clinical symptoms.
Clinical implications for schizophrenia:
Understanding differences in striatal dopamine function in patients at a detailed molecular level is still a work in progress. Early imaging and post-mortem studies found increased D2R availability and density in schizophrenia108. More recent results demonstrate that in medication-naïve patients, D2R availability is unchanged3, 109. Instead, imaging studies that aim to measure presynaptic DA have shown a well-replicated upregulation at the level of DA release capacity, as measured by [18F]-DOPA uptake and amphetamine-induced decreases in D2R tracer binding3, 109. This suggests a presynaptic origin of the enhanced DA, which leads to the increase in D2R occupancy that has been imaged using DA depletion studies2, 110. However, a recent meta-analysis found that when including data from several patient populations the mean value for D2R availability in patients does not differ from control subjects but is significantly more variable. This suggests that in a subgroup of patients changes in D2R availability may contribute to the disorder109. Regardless of the precise molecular origin of the dopamine changes in individual patients with schizophrenia, D2R upregulation models allow us to understand the relationship between enhanced D2R function resulting from enhanced D2R occupancy and/or availability, striatal circuit function and behavior.
The relationship between dopamine and symptoms of schizophrenia is not uniform across the entire striatum. PET imaging studies in humans are consistent with a tight link between dopamine in the associative striatum and positive or psychotic symptoms4. PET imaging studies further found an inverse relationship between ventral striatal dopamine levels and negative symptoms52, 111. These data are consistent with the adult D2R upregulation models, suggesting that enhanced D2R function in striato-pallidal neurons decreases passivity, as measured by open field activity, and enhances incentive motivation, as measured in the PR. The PET imaging studies cannot identify the neuronal cell types in which D2R occupancy with DA is enhanced in patients with schizophrenia. However, F-Dopa and psychostimulant-induced DA release studies suggest a presynaptic origin of DA upregulation, which a priori should affect D2Rs in striato-pallidal, CIN and DA neurons in the same way. The preclinical studies suggest that pharmacological treatments targeted to specific cell types will be required for effective treatment of motivational deficits.
Selectively targeting specific cell types is not the only potential avenue for improved treatments. Understanding how G protein-dependent vs. β-arrestin-dependent signaling pathways regulate behavior could also lead to more targeted treatments. While the effect of D2R G protein-mediated signaling is likely mediated by inhibiting synaptic transmission to VP, as discussed above, a role for β-arrestin-AKT-GSK-3β signaling in SPNs during motivated behavior remains to be determined. Notably, as discussed above, overexpression of a β-arrestin-biased D2R did not enhance PR performance although it did increase activity in the open field. D2R antagonists are efficacious in treating positive symptoms of schizophrenia, and despite the limitations of studying these symptoms in mice, it has been proposed that antipsychotic efficacy may be related to blocking β-arrestin signaling112. Therefore, based on the studies discussed here, therapeutic approaches that involve β-arrestin-biased antagonism while sparing G protein-dependent signaling might reduce positive symptoms while generating fewer adverse effects on incentive motivation, increasing therapeutic compliance compared with existing, less targeted medications.
Clinical studies show that dopamine dysregulation occurs early in the development of schizophrenia, prior to the diagnosis. Increased striatal D2R availability has been documented during patients’ first episode. Patients with high clinical risk psychosis also show increased striatal D2R occupancy52 and elevated striatal F-Dopa uptake, a radiological measure for DA release capacity. Moreover, the degree of prodromal increase in F-Dopa predicts later development of schizophrenia54. Interestingly, the topography of DA alterations in schizophrenia is not consistent across these studies. While increased D2R occupancy in first episode and chronic schizophrenia is most pronounced in the associative striatum, a recent PET study in patients at high clinical risk for developing schizophrenia, suggest that the earliest changes in D2R occupancy arise in the ventral striatum52. These early changes in ventral striatal DA may affect the maturation of striatal circuitry leading to persistent changes in striatal circuit function that impact motivation and cognitive behaviors later in life. Identifying critical time windows of striatal development may guide early intervention that could prevent the long-lasting consequences of early DA imbalance.
Although this review is focused on motivated behavior, successful targeting of the signaling pathways, cell types and circuits involved in motivation is likely to have a broader impact. While it is generally known that increasing motivation can positively impact cognition, there is specific evidence that striatal D2R modulation of motivation can enhance cognitive performance113. Improving motivation in D2-OE mice by switching of the transgene results in partial rescue of some of their cognitive deficits including behavioral flexibility and interval timing, cognitive domains that are disrupted in schizophrenia58, 113, 114. Given that the severity of both negative and cognition symptoms of schizophrenia are highly predictive of functional outcome, advancing treatments targeted to motivational deficits could have a significant, multilayered impact on quality of life for patients.
Acknowledgements:
This manuscript has been based on research funded by NIH MH107648 for E.F.G., NIH MH068073 for P.B, NIH MH054137 and Hope for Depression Research Foundation for J.A.J. and NIH MH093672 for C.K. Figures were created with BioRender.com.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
References:
- 1.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46(1): 56–72. [DOI] [PubMed] [Google Scholar]
- 2.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia.[comment]. Proceedings of the National Academy of Sciences of the United States of America 2000; 97(14): 8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A et al. The Nature of Dopamine Dysfunction in Schizophrenia and What This Means for Treatment: Meta-analysis of Imaging Studies. Arch Gen Psychiatry 2012; 66(1): 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol Psychiatry 2017; 81(1): 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 2010; 67(3): 231–239. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 2009; 56 Suppl 1: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis 2004; 23(3): 39–53. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ. Hallucinations and perceptual inference. Behavioral and brain sciences 2005; 28(6): 764–766. [Google Scholar]
- 9.Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 2017; 357(6351): 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassidy CM, Balsam PD, Weinstein JJ, Rosengard RJ, Slifstein M, Daw ND et al. A Perceptual Inference Mechanism for Hallucinations Linked to Striatal Dopamine. Curr Biol 2018; 28(4): 503–514 e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmack K, Bosc M, Ott T, Sturgill JF, Kepecs A. Striatal dopamine mediates hallucination-like perception in mice. Science 2021; 372(6537). [DOI] [PubMed] [Google Scholar]
- 12.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989; 12(10): 366–375. [DOI] [PubMed] [Google Scholar]
- 13.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 2011; 34: 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci 2000; 23(10 Suppl): S20–27. [DOI] [PubMed] [Google Scholar]
- 15.Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 2014; 81(1): 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 2015; 521(7550): 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP et al. Dichotomous organization of the external globus pallidus. Neuron 2012; 74(6): 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez VM, Hegeman DJ, Cui Q, Kelver DA, Fiske MP, Glajch KE et al. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J Neurosci 2015; 35(34): 11830–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abecassis ZA, Berceau BL, Win PH, Garcia D, Xenias HS, Cui Q et al. Npas1(+)-Nkx2.1(+) Neurons Are an Integral Part of the Cortico-pallido-cortical Loop. J Neurosci 2020; 40(4): 743–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 1991; 41(1): 89–125. [DOI] [PubMed] [Google Scholar]
- 21.Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat--II. Compartmentation of ventral pallidal efferents. Neuroscience 1989; 30(1): 33–50. [DOI] [PubMed] [Google Scholar]
- 22.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci 2015; 18(9): 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience 1998; 82(3): 767–780. [DOI] [PubMed] [Google Scholar]
- 24.Baimel C, McGarry LM, Carter AG. The Projection Targets of Medium Spiny Neurons Govern Cocaine-Evoked Synaptic Plasticity in the Nucleus Accumbens. Cell Rep 2019; 28(9): 2256–2263 e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol 2002; 442(4): 392–404. [DOI] [PubMed] [Google Scholar]
- 26.Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 2004; 42(4): 653–663. [DOI] [PubMed] [Google Scholar]
- 27.Clark AM, Leroy F, Martyniuk KM, Feng W, McManus E, Bailey MR et al. Dopamine D2 Receptors in the Paraventricular Thalamus Attenuate Cocaine Locomotor Sensitization. eNeuro 2017; 4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bocarsly ME, da Silva ESD, Kolb V, Luderman KD, Shashikiran S, Rubinstein M et al. A Mechanism Linking Two Known Vulnerability Factors for Alcohol Abuse: Heightened Alcohol Stimulation and Low Striatal Dopamine D2 Receptors. Cell Rep 2019; 29(5): 1147–1163 e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghajanian GK, Bunney BS. Dopamine”autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol 1977; 297(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 30.Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014; 282: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Camilli P, Macconi D, Spada A. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature 1979; 278(5701): 252–254. [DOI] [PubMed] [Google Scholar]
- 32.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 1999; 23(3): 435–447. [DOI] [PubMed] [Google Scholar]
- 33.Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 1987; 392: 397–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H et al. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci 2000; 20(24): 8987–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol 1997; 77(2): 1003–1015. [DOI] [PubMed] [Google Scholar]
- 36.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 1996; 380(6571): 258–262. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 1996; 380(6571): 255–258. [DOI] [PubMed] [Google Scholar]
- 38.Martel P, Leo D, Fulton S, Berard M, Trudeau LE. Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS One 2011; 6(5): e20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper AJ, Stanford IM. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology 2001; 41(1): 62–71. [DOI] [PubMed] [Google Scholar]
- 40.Floran B, Floran L, Sierra A, Aceves J. D2 receptor-mediated inhibition of GABA release by endogenous dopamine in the rat globus pallidus. Neurosci Lett 1997; 237(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 41.Tecuapetla F, Koos T, Tepper JM, Kabbani N, Yeckel MF. Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci 2009; 29(28): 8977–8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA. Dopamine Regulation of Lateral Inhibition between Striatal Neurons Gates the Stimulant Actions of Cocaine. Neuron 2016; 90(5): 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun 2018; 9(1): 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron 2014; 81(4): 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D et al. Parkinsonism Driven by Antipsychotics Originates from Dopaminergic Control of Striatal Cholinergic Interneurons. Neuron 2016; 91(1): 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Augustin SM, Chancey JH, Lovinger DM. Dual Dopaminergic Regulation of Corticostriatal Plasticity by Cholinergic Interneurons and Indirect Pathway Medium Spiny Neurons. Cell Rep 2018; 24(11): 2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefkowitz RJ, Inglese J, Koch WJ, Pitcher J, Attramadal H, Caron MG. G-protein-coupled receptors: regulatory role of receptor kinases and arrestin proteins. Cold Spring Harb Symp Quant Biol 1992; 57: 127–133. [DOI] [PubMed] [Google Scholar]
- 48.Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 2011; 32(9): 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005; 122(2): 261–273. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci 2007; 27(4): 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J, Liu W, Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J Biol Chem 2010; 285(34): 26369–26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girgis RR, Slifstein M, Brucato G, Kegeles LS, Colibazzi T, Lieberman JA et al. Imaging synaptic dopamine availability in individuals at clinical high-risk for psychosis: a [(11)C]-(+)-PHNO PET with methylphenidate challenge study. Mol Psychiatry 2020. [DOI] [PubMed] [Google Scholar]
- 53.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007; 191(3): 461–482. [DOI] [PubMed] [Google Scholar]
- 54.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 2009; 66(1): 13–20. [DOI] [PubMed] [Google Scholar]
- 55.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning.[see comment]. Neuron 2006; 49(4): 603–615. [DOI] [PubMed] [Google Scholar]
- 56.Laruelle M Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Quarterly Journal of Nuclear Medicine 1998; 42(3): 211–221. [PubMed] [Google Scholar]
- 57.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci 2007; 27(29): 7731–7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci U S A 2008; 105(41): 16027–16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duvarci S, Simpson EH, Schneider G, Kandel ER, Roeper J, Sigurdsson T. Impaired recruitment of dopamine neurons during working memory in mice with striatal D2 receptor overexpression. Nat Commun 2018; 9(1): 2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology 2012; 37(7): 1699–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey MR, Chun E, Schipani E, Balsam PD, Simpson EH. Dissociating the effects of dopamine D2 receptors on effort-based versus value-based decision making using a novel behavioral approach. Behav Neurosci 2020; 134(2): 101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filla I, Bailey MR, Schipani E, Winiger V, Mezias C, Balsam PD et al. Striatal dopamine D2 receptors regulate effort but not value-based decision making and alter the dopaminergic encoding of cost. Neuropsychopharmacology 2018; 43(11): 2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hodos W Progressive ratio as a measure of reward strength. Science 1961; 134(3483): 943–944. [DOI] [PubMed] [Google Scholar]
- 64.Bailey MR, Jensen G, Taylor K, Mezias C, Williamson C, Silver R et al. Dissecting Goal-Directed Action and Arousal Components of Motivated Behavior. Behav Neurosci 2015; 129(3): 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav 1999; 64(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 66.Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 2005; 135(3): 959–968. [DOI] [PubMed] [Google Scholar]
- 67.Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav 1998; 59(3): 557–566. [DOI] [PubMed] [Google Scholar]
- 68.Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry 2013; 18(9): 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donthamsetti P, Gallo EF, Buck DC, Stahl EL, Zhu Y, Lane JR et al. Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol Psychiatry 2020; 25(9): 2086–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci 2011; 14(8): 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Jong JW, Roelofs TJ, Mol FM, Hillen AE, Meijboom KE, Luijendijk MC et al. Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation. Neuropsychopharmacology 2015; 40(9): 2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull 2012; 38(6): 1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 2007; 93(1–3): 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry 2013; 74(2): 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res 2015; 161(2–3): 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol 2014; 123(2): 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci 2012; 32(7): 2398–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S et al. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry 2011; 69(10): 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krabbe S, Duda J, Schiemann J, Poetschke C, Schneider G, Kandel ER et al. Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proc Natl Acad Sci U S A 2015; 112(12): E1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav 1998; 61(4): 341–348. [DOI] [PubMed] [Google Scholar]
- 81.Gallo EF, Salling MC, Feng B, Moron JA, Harrison NL, Javitch JA et al. Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology 2015; 40(7): 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayorga AJ, Popke EJ, Fogle CM, Paule MG. Similar effects of amphetamine and methylphenidate on the performance of complex operant tasks in rats. Behav Brain Res 2000; 109(1): 59–68. [DOI] [PubMed] [Google Scholar]
- 83.Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl) 2007; 191(3): 483–495. [DOI] [PubMed] [Google Scholar]
- 84.Augustin SM, Loewinger GC, O’Neal TJ, Kravitz AV, Lovinger DM. Dopamine D2 receptor signaling on iMSNs is required for initiation and vigor of learned actions. Neuropsychopharmacology 2020; 45(12): 2087–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carvalho Poyraz F, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA et al. Decreasing Striatopallidal Pathway Function Enhances Motivation by Energizing the Initiation of Goal-Directed Action. J Neurosci 2016; 36(22): 5988–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M et al. Ventrolateral Striatal Medium Spiny Neurons Positively Regulate Food-Incentive, Goal-Directed Behavior Independently of D1 and D2 Selectivity. J Neurosci 2017; 37(10): 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soares-Cunha C, Coimbra B, Sousa N, Rodrigues AJ. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci Biobehav Rev 2016; 68: 370–386. [DOI] [PubMed] [Google Scholar]
- 88.Olivetti PR, Balsam PD, Simpson EH, Kellendonk C. Emerging roles of striatal dopamine D2 receptors in motivated behaviour: Implications for psychiatric disorders. Basic Clin Pharmacol Toxicol 2020; 126 Suppl 6: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ et al. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience 2008; 152(2): 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vachez YM, Tooley JR, Abiraman K, Matikainen-Ankney B, Casey E, Earnest T et al. Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption. Nat Neurosci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S et al. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci 2016; 36(45): 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michaelides M, Miller ML, DiNieri JA, Gomez JL, Schwartz E, Egervari G et al. Dopamine D2 Receptor Signaling in the Nucleus Accumbens Comprises a Metabolic-Cognitive Brain Interface Regulating Metabolic Components of Glucose Reinforcement. Neuropsychopharmacology 2017; 42(12): 2365–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab 2017; 25(2): 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welch AC, Zhang J, Lyu J, McMurray MS, Javitch JA, Kellendonk C et al. Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Labouesse MA, Sartori AM, Weinmann O, Simpson EH, Kellendonk C, Weber-Stadlbauer U. Striatal dopamine 2 receptor upregulation during development predisposes to diet-induced obesity by reducing energy output in mice. Proc Natl Acad Sci U S A 2018; 115(41): 10493–10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav 2011; 104(1): 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Straub C, Tritsch NX, Hagan NA, Gu C, Sabatini BL. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci 2014; 34(25): 8557–8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wieland S, Du D, Oswald MJ, Parlato R, Kohr G, Kelsch W. Phasic dopaminergic activity exerts fast control of cholinergic interneuron firing via sequential NMDA, D2, and D1 receptor activation. J Neurosci 2014; 34(35): 11549–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gallo EF, Greenwald J, Teboul E, Martyniuk K, Li Y, Javitch JA et al. Dopamine D2 receptors modulate the cholinergic pause and inhibitory learning BioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 2016; 6(3): 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holroyd KB, Adrover MF, Fuino RL, Bock R, Kaplan AR, Gremel CM et al. Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues. Neuropsychopharmacology 2015; 40(6): 1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 2011; 108(45): 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urs NM, Gee SM, Pack TF, McCorvy JD, Evron T, Snyder JC et al. Distinct cortical and striatal actions of a beta-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci U S A 2016; 113(50): E8178–E8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rose SJ, Pack TF, Peterson SM, Payne K, Borrelli E, Caron MG. Engineered D2R Variants Reveal the Balanced and Biased Contributions of G-Protein and beta-Arrestin to Dopamine-Dependent Functions. Neuropsychopharmacology 2018; 43(5): 1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dobbs LK, Kaplan AR, Bock R, Phamluong K, Shin JH, Bocarsly ME et al. D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology 2019; 44(4): 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature 2012; 485(7400): 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lieberman OJ, McGuirt AF, Mosharov EV, Pigulevskiy I, Hobson BD, Choi S et al. Dopamine Triggers the Maturation of Striatal Spiny Projection Neuron Excitability during a Critical Period. Neuron 2018; 99(3): 540–554 e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong DF, Wagner HN Jr., Tune LE, Dannals RF, Pearlson GD, Links JM et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics.[erratum appears in Science 1987 Feb 6;235(4789):623]. Science 1986; 234(4783): 1558–1563. [DOI] [PubMed] [Google Scholar]
- 109.Brugger SP, Angelescu I, Abi-Dargham A, Mizrahi R, Shahrezaei V, Howes OD. Heterogeneity of Striatal Dopamine Function in Schizophrenia: Meta-analysis of Variance. Biol Psychiatry 2020; 87(3): 215–224. [DOI] [PubMed] [Google Scholar]
- 110.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry 2009; 65(12): 1091–1093. [DOI] [PubMed] [Google Scholar]
- 111.Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T et al. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry 2010; 68(7): 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR et al. Antagonism of dopamine D2 receptor/{beta}-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Avlar B, Kahn JB, Jensen G, Kandel ER, Simpson EH, Balsam PD. Improving temporal cognition by enhancing motivation. Behav Neurosci 2015; 129(5): 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci 2009; 123(4): 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]


