Abstract
Introduction: Photobiomodulation therapy (PBM) appears to induce osteogenesis and stimulate fracture repair; because of its capacity, it is considered a promising treatment, but the characteristics of response to different radiation doses must be investigated through in vivo studies to establish their safety and effectiveness. Thus, this paper aims to analyze the effects of the PBM at different doses on the repair of critical bone defects through histological and histomorphometric analyses.
Methods: Sixty 90-day-old adult rats (Rattus norvegicus, albinus, Wistar) weighing approximately 300 g were used. Critical bone defects of 5 mm in diameter were performed in their calvaria. The animals were randomly separated into 5 groups: C-Blood clot, L15-PBM 15J/cm2, L30-PBM 30 J/ cm2, L45-PBM 45 J/cm2, L60-PBM 60 J/cm2. Each group was subdivided according to observation periods of 30 and 60 days with 6 rats in each subgroup. Low-level gallium aluminum arsenide (GaAlAs) lasers were used at a 660 nm wavelength, 30 mW and 0.04 cm2 in area. The PBM was applied over 5 points; 4 points of application were distributed on the edges while one point of application was located in the center of the bone defect. PBM occurred right after the procedure. In 30 and 60 days, the animals were euthanized by anesthesia overdose and the analyses were performed. The data were analyzed statistically by the ANOVA, together with the Tukey test, whose significance level was 5%.
Results: As regards the treatment factor, the highest percentage of bone neoformation was achieved by group L45-60. The group with the highest closure, despite not having a statistically significant difference with the other doses, was 45 J with only 0.49 mm between edges.
Conclusion: Thus, the present study allowed concluding that the highest percentage of bone neoformation area was achieved at 45 J/cm2 in 60 days; that is, it was significantly effective in comparison with other doses.
Keywords: Low-Level Light Therapy, Bone Regeneration, Lasers, Rats
Introduction
Bone regeneration is a substantial component of clinical practice aimed at filling post-trauma defects, congenital defects, and tumor excisions. Regardless of numerous current clinical strategies that can be applied to these defects, incomplete repair of the defect remains a clinical challenge. 1,2 Traditionally, a critical-size defect is defined as the smallest tissue defect size that cannot be fully and naturally repaired during an animal’s lifetime. 3
Photobiomodulation therapy (PBM) is a promising treatment that induces osteogenesis and stimulates fracture repair. 4 Its action is based on light absorption by tissues that will, in turn, generate changes in cell metabolism, regulate osteogenic, inflammatory mediators, angiogenic and growth factors that contributes to bone formation. 5 When PBM is applied to tissues, light is absorbed by photocells located in cells. In this way, light is able to modulate chemical reactions and stimulate mitochondrial respiration, molecular oxygen production, and ATP synthesis. 6 These effects can enhance DNA and RNA synthesis and regulate the cell cycle of proteins by stimulating proliferation. 7
In vitro studies on osteoblastic cells have shown that PBM is able to increase the formation of bone nodules, 6 osteocalcin and osteopontin gene expression, and alkaline phosphatase activity. 8,9 This appears to exert a bio-stimulatory effect on bone tissue by enhancing osteoblastic proliferation. 10 Moreover, it can promote the repair process of fractures in rabbits and rats, as well as increasing callus volume and bone mineral density. 11
Laser beams at low intensities, i.e., 660 nm, can play a positive role in repairing bone defects. 12,3 In hard tissues, the PBM significantly rises the number of viable osteocytes, thus displaying a positive outcome on bone matrix production rendering the bone tissue around the grafts or in the peripheral region of the defects highly reactive and vital. 14
Nevertheless, there is a lack of literature about the effect of different or appropriate laser therapy doses on repairing critical-size bone defects. Many variables can affect the biostimulatory effects of the PBM, such as wavelength, energy, exposure time, power, and cell biological status. Photobiomodulation of human fibroblasts by PBM has been reported in morphological and cell studies (proliferation and collagen production), 15 in which power ranged from 0.1 to 24.7 mW/cm2 and total energy varied from 0.02 to 12 J/cm2. Based on irradiation conditions, the effects of collagen production were both stimulating and inhibiting, and cell proliferation was both positive and negative. 15
Most bone repair studies have assessed the effect of infrared laser light beam irradiation because it can achieve deep tissue penetration. 16-18 The influence of PBM at a wavelength of 660 nm and irradiation of 24.7 J/cm2 on the repair of bone defects in immunosuppressed rats has been reported. 19 Another study demonstrated that the PBM at 637 nm (4 J/cm2 for 7 days) had an effect on the repair rate of femur defects in rats. 20 Histopathological results have indicated that bone regeneration is accelerated under laser irradiation, mainly in the early stages of repair. 19,20
Low-level gallium aluminum arsenide (GaAlAs) laser therapy at 660 nm (57.14 J/cm2) effectively stimulated bone formation in critical-size bone defects in the calvaria of rats subjected to ovariectomy. 21 However, the PBM is still considered a controversial treatment because no therapeutic margin for dosimetry or action mechanisms has been determined. 22,23 Even though the positive effects of PBM on cell proliferation have been demonstrated, its effects on the repair of critical bone defects, as well as an ideal dose for repairing such defects, are still not well known. 24
Thus, before it can be reliably used as a therapeutic modality for critical-size bone defect treatment, the outcomes and irradiation response characteristics of PBM must be investigated through in vivo studies to establish its safety and efficacy. In these circumstances, the purpose of this study was to evaluate the effects of PBM at different doses when applied to critical-size bone defects through histological and histomorphometric analyses.
Materials and Methods
Sixty 90-day-old adult rats (Rattus norvegicus, albinus, Wistar) weighing around 300 g were supplied by Unesp Central Vivarium – Botucatu Campus (SP). They were kept in a suitable enclosure and provided a regular diet and water. The animals were randomly separated into five groups: C, L15, L30, L45, and L60 (Table 1). The groups were subdivided in accordance with the observation periods of 30 and 60 days, and at the end, each group contained six rats.
Table 1. Groups and Treatment Modalities of Bone Defects.
| Control Group (C) | Bone defect + Blood clot |
| Group irradiated with LLLT 15 J/cm2 (L15) | Bone defect + LLLT 15 J/cm2 |
| Group irradiated with LLLT 30 J/cm2 (L30) | Bone defect + LLLT 30 J/cm2 |
| Group irradiated with LLLT 45 J/cm2 (L45) | Bone defect + LLLT 45 J/cm2 |
| Group irradiated with LLLT 60 J/cm2 (L60) | Bone defect + LLLT 60 J/cm2 |
Abbreviation: LLLT, low level laser therapy.
Anesthetic Procedure
For surgery and euthanasia, the animals were anesthetized with a solution of 13 mg/kg of 2-(2,6-xylidine)-5-6-dihydro-4H-1,3 xylazine (Rompun-Bayer, Brazil) with 33 mg/kg ketamine (Dopalen, Agribands, Brazil) administered intramuscularly.
Surgical Procedure
The periosteum was incised, and soft tissues were removed with the aid of retractors of a size suitable to the surgical region. Critical bone defects 5 mm in diameter were performed using an electric engine that allowed a controlled speed of 800 rpm and a trephine drill under continuous irrigation with sterile saline solution. 25 During the surgical procedure, care was taken to avoid damage to the dura mater or the superior sagittal sinus. Subsequently, PBM was performed. The skin was sutured with 4.0 silk threads and an analgesic medication was applied. The day of the surgery was considered day 0.
Photobiomodulation Therapy
Low-level GaAlAs (Laser DUO, MMOptics Ltda., São Carlos, SP, Brazil) were employed with a continuous laser beam emission (CW) at a 660 nm wavelength, 30 mW, and 0.04 cm2 area. The PBM was performed using a laser pen nib in a single trans-surgical application directly at the edges of the bone defect. The irradiation was distributed punctually at five points so that the whole surgical wound received treatment evenly. Four points of the application were divided along the surgical wound edges in a clockwise direction at 3, 6, 9, and 12 o’clock. Moreover, an application point was situated in the middle of the bone defect. 26
In Group C, there was no PBM application. In group L15, each point was irradiated for 4 s (total exposure time of 20 seconds) with a total energy density of 15 J/cm2. In group L30, each point was irradiated for 8 seconds (total exposure time of 40 seconds) with a total energy density of 30 J/cm2. In group L45, each point was irradiated for 12 seconds (total exposure time of 1 minute) with a total energy density of 45 J/cm2. In group L60, each point was irradiated for 16 seconds (total exposure time of 1 minute and 20 seconds) with a total energy density of 60 J/cm2 (Table 1).
In 30 and 60 days, the animals were euthanized by general anesthesia overdose, and their calvariae were then removed for analysis.
Tissue Processing
For histological and histomorphometric analyses, the surgical area was immersed in 10% formaldehyde for up to 48 hours. Afterward, the decalcification process was initiated with 10% EDTA at a pH of 7.8 at room temperature.
After the partial decalcification, every specimen was divided into two blocks along the central line of the original bone defect in a macro view. Formic acid (20%) was used at room temperature to complete the demineralization of the specimens. Once decalcification was complete, each specimen originated two blocks that were immersed in paraffin and cut from their central regions.
Serial 5-μm-thick sections were made longitudinally, starting from the center of the original surgical wound. The slides were stained with hematoxylin-eosin and examined under a light microscope.
Histological and Histomorphometric Analyses
Three equidistant histological sections representing the surgical wound center were chosen for histological and histomorphometric analyses to enhance data reliability. 27
Images of sections were captured with an Axiophot 2 light microscope (Carl Zeiss Oberköchen, Germany) coupled to an AxioCam MRc 5 digital camera (Carl Zeiss Oberköchen, Germany). These were then exported to AxioVision release 4.7.2. software. A single researcher was responsible for capturing the images that received codes, which were subsequently analyzed by another examiner duly calibrated (Student’s t test: 0.46, P value = 0.658 > 0.05), thus allowing data to be blinded.
The histological analysis was initially performed at low magnification to ensure the overall visibility of the sections. AxioVision release 4.7.2 software was used to conduct a histomorphometric analysis of the images.
Histomorphometric Parameters
The criteria to standardize the analysis of digital images were the same as those adopted by previous studies, 26,27 and the total area was determined in mm2. Then, the following measurements were obtained 26 :
Total neoformation area (TNA): proportion of newly formed bone within the limits determined of the total area of the defect.
Edge neoformation area (ENA): area of bone neoformation in mm2 of the right and left edges of the defect.
Central neoformation area (CAN): area of bone neoformation in mm2 in the defect center.
Distance between the edges of the neoformed bone (DBE-NB): measurement of the remaining distance between the edges of the neoformed bone in mm.
DBC-NB: measure of the remaining distance between the cortical already formed from the newly formed bone at the edges, in the upper (DBSC-NB) and lower region (DBIC-NB).
Statistical Analysis
Quantitative data were recorded as mean ± standard deviation (SD), and normality was tested using Shapiro-Wilk tests. The TNA, ENA, CAN, DBE-NB, DBSC-NB, and DBIC-NB values were tested using a two-way ANOVA to assess the differences within and between groups. This was accompanied by a Tukey test for multiple comparisons when the Shapiro-Wilk P value was ≥0.05. The data presenting Shapiro-Wilk P values < 0.05 were analyzed using a Friedman test and Mann-Whitney tests. A significance level of 0.05 was adopted.
Results
Qualitative Histological Analysis
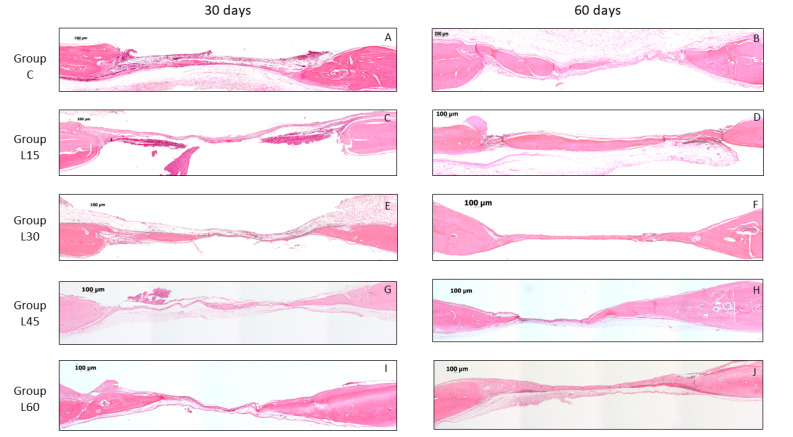
In 30 days, almost every specimen in all groups (C, L15, L30, L45, and L60) demonstrated bone neoformation areas that were observed in regions near the surgical defect edges. In 60 days, in many specimens from all groups (C, L15, L30, L45, and L60), it was possible to observe a narrow band of neoformed bone from the defect edges toward the center, almost extending throughout the extension of the surgical defect, though being narrower than the original calvarial bone. It was also possible to observe a dense connective tissue and chronic inflammatory infiltrate that extended throughout the surgical defect in all specimens (Figure 1).
Figure 1.
Photomicrography of Histomorphometric Analysis of Groups in Each Experimental Period. Group C (A and B), Group L15 (C and D), Group L30 (E and F), Group L45 (G and H) and Group L60 (I and J) in 30 and 60 days of the observation. Hematoxylin and eosin staining and 5× magnification.
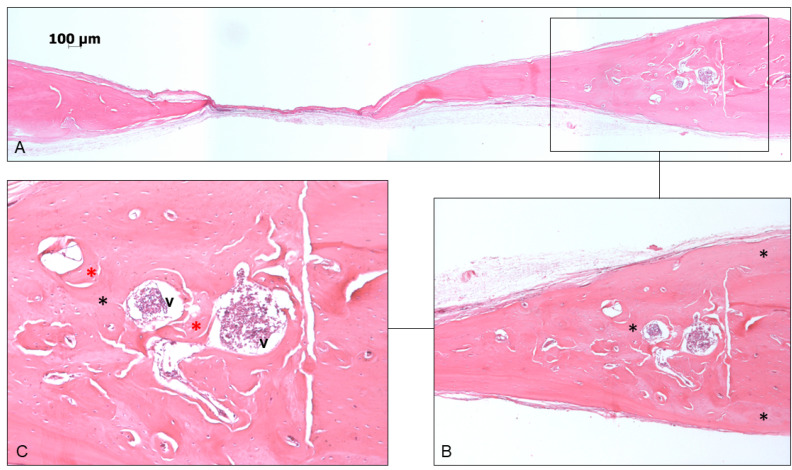
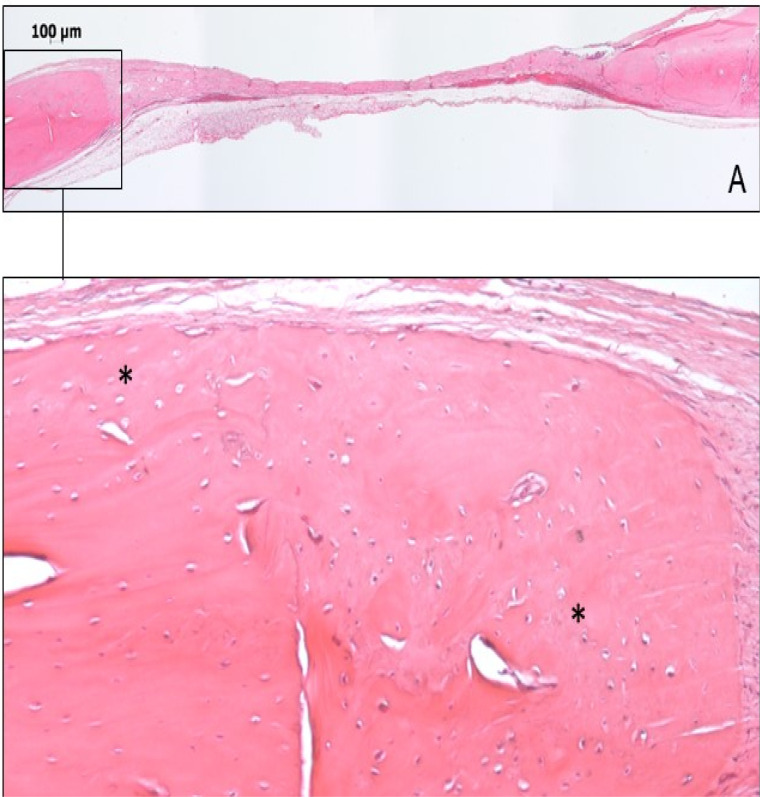
In 60 days, the newly formed bone could be observed above, below, and laterally at the edge of the surgical defect, with the organization of the osteoid matrix and blood vessels (Figures 2 and 3).
Figure 2.
(A) Photomicrography of panoramic view of GL45 - 60. (B) Newly formed bone located around the edge of the surgical defect and highlighted with a black asterisk. (C) Newly formed bone located laterally to the surgical defect (black asterisks) with an organizing osteoid matrix (red asterisks), and blood vessels (v) (Hematoxylin and eosin staining, magnification, 5× in A, 10× in B and 20× in C).
Figure 3.
(A) Photomicrography of panoramic view of GL60 – 60. (B) Newly formed bone (black asterisks) located around the edge of the surgical defect (Hematoxylin and eosin staining, magnification, 5× in A, 10× in B).
Quantitative Histological Analysis
Regarding TNA, within the time range of 30 to 60 days, statistically significant differences were observed in all treatment modalities except in the 60 J group (30 days x 60 days; P = 0.39). In the intergroup comparison, within 60 days, there were differences between the doses of 45 J and C (P = 0.01), 45 J and 15 J (P < 0.001), and 45 J and 60 J (P = 0.001), where the dose of 45 J showed superior results (Table 2).
Table 2. Quantitative Results of Histomorphometric Analysis.
| Observation Time | C | L15J | L30J | L45J | L60J | |
| TNA (mm2) | 30 days | 0.38±0.19Aa | 0.22±0.24Aa | 0.42±0.22Aa | 0.36±0.29Aa | 0.51±0.36Aa |
| 60 days | 0.69±0.35Ba | 0.50±0.40Ba | 0.76±0.55Ba | 1.11±0.59Bb | 0.62±0.37Aa | |
| ENA (mm2) | 30 days | 0.27±0.33Aa | 0.20±0.24Aa | 0.30±0.20Aa | 0.2±0.14Aa | 0.23±0.16Aa |
| 60 days | 0.41±0.29Aab | 0.28±0.22Aab | 0.49±0.44Ba | 0.23±0.32Ab | 0.29±0.30Aab | |
| CAN (mm2) | 30 days | 0.07±0.13Aa | 0.01±0.04Aa | 0.08±0.14Aa | 0.07±0.10Aa | 0.06±0.07Aa |
| 60 days | 0.27±0.30Ba | 0.09±0.19Ba | 0.08±0.11Ba | 0.24±0.34Bb | 0.14±0.27Aa | |
| DBE-NB (mm) | 30 days | 1.70±1.13Aa | 2.38±1.40Aa | 2.13±1.09Aa | 1.96±1.23Aa | 1.63±1.20Aa |
| 60 days | 1.32±0.78Aab | 1.38±0.80Bab | 0.89±0.69Ba | 0.49±0.70Ba | 1.95±0.57Ab | |
| DBSC-NB (mm) | 30 days | 4.04±7.48Aa | 2.95±1.56Aa | 2.66±1.27Aa | 2.24±1.35Aa | 1.83±1.32Aa |
| 60 days | 3.17±1.16Aa | 1.81±0.73Aab | 1.19±0.71Aab | 0.60±0.72Bb | 2.11±0.67Aab | |
| DBIC-NB (mm) | 30 days | 2.40±1.47Aa | 2.95±1.60Aa | 2.81±1.19Aa | 2.31±1.43Aa | 1.91±1.39Aa |
| 60 days | 3.35±1Ba | 1.81±0.70Bb | 1.31±0.73Bbc | 0.65±0.74Bc | 2.07±0.74Ab |
Abbreviations: C, control group; L15, group irradiated with low level laser therapy (LLLT) 15 J/cm2; L30, Group irradiated with LLLT 30 J/cm2; L45, Group irradiated with LLLT 45 J/cm2; L60, Group irradiated with LLLT 60 J/cm2. TNA, total neoformation area; ENA, edge neoformation area; CAN, central neoformation area; DBE-NB, distance between the edges of neoformed bone; DBSC-NB, distance between the superior corticals formed in the neoformed bone; DBIC-NB, distance between the inferior corticals formed in the neoformed bone.
Note. Distinct capital letters - statistically significant difference (P = 0.05) in intragroup comparation –two-way ANOVA of repeated measures. Distinct lowercase letters - statistically significant difference (P = 0.05) in intergroup comparation – two-way ANOVA of repeated measures.
Comparing bone neoformation in ENA in 60 days, the comparison between the 30 J and 45 J dose groups showed a difference, where the 30 J group was superior (30 J x 45 J; P = 0.03).
Concerning CAN and observational times, a greater amount of bone tissue was observed in all groups in 60 days than in 30 days, except in the 60 J group. In 30 days after the application, there were no statistically significant differences between the doses received. However, in 60 days, the group that received 45 J showed results superior to all other doses of the study.
Within the DBE-NB, the groups presented statistically significant differences in 60 days of the application. There was a difference between the 30 J and 60 J groups (30 J x 60 J, P = 0.013) and between 45 J and 60 J (45 J x 60 J, P < 0.001). The 60 J group presented higher measurements than the other groups. The group with the highest closure, despite not having a statistically significant difference with the other doses, was 45 J with only 0.49 mm between edges.
In the DBSC-NB parameter, the 45 J dose group showed superior results to the control group 60 days after application (45 J x C; P = 0.02). In the DBIC-NB, the control group was inferior to all other groups in 60 days. The 45 J dose group was superior to all other groups except the 30 J dose group. The difference between them was not statistically significant (30 J x 45 J, P = 0.41) (Table 2).
Discussion
Currently, there are multiple PBM applications in tissue repair processes, including those concerning bone neoformation. However, few studies have investigated how different doses can influence the treatment. Thus, this study aimed to assess its effects at different doses on the repair of critical-sized bone defects in rats.
In the present paper, the highest proportion of bone neoformation was found in group L45-60, which was statistically significant in groups L15-60, L30-60, and L60-60. The present findings are in agreement with a study conducted by Altan et al who demonstrated that the PBM was significantly effective at increasing bone neoformation at irradiations of 5 J/cm2 and 6300 J/cm2 through suture expansion in rats. However, it did not reveal a significant effect on bone neoformation at 20 J/cm2. 28 Furthermore, Scalize et al demonstrated that there was an increase in bone neoformation in groups that underwent PBM at 20 J/cm2 and 30 J/cm2 in an experimental model with rats suffering from osteoporosis, although the group at 30 J/cm2 showed better results over time. 29 However, Bossini et al. used PBM at 830 nm and two different doses, 60 J/cm2 and 120 J/cm2. They found that they were effective at stimulating bone repair in osteoporotic rats with no difference in doses. 24
In the analysis of the remaining distances between the newly formed bone edges, it was noticed that the control group presented inferior results when compared to the groups with different laser dosages. This shows that PBM even at different doses has positive activity in the bone tissue. However, Nunes et al observed a statistically significant difference between the newly formed bone edges in only 21 days of observation, after which the remaining distances became similar between the groups. 30
The area of major bone formation was concentrated in the regions of the edge of the defect due to vascularization and cellular contribution from the native bone. In 60 days, the 45 J group showed the highest bone neoformation in the central region. This may be explained by the presence of osteocalcin, osteopontin, and vascular endothelial growth factor in the central region of the defect 7 days after laser application. 31
A few studies have shown that processes of cell proliferation and growth can stay unchanged at different doses of exposure. This can be observed in different cell types, such as osteoblasts and fibroblasts. 6 However, specific wavelengths and doses can influence different responses for each cell type. 32 The stimulation effects depend on doses, period, frequency, and radiation intensity. 33,34 Hence, there is some inconsistency between the findings of the studies on these parameters. 28 Jenkins and Carroll reported that irradiation and beam parameters are essential for the success of low-level laser therapy in clinical and laboratory studies, thus emphasizing the importance of reciprocity, concerning not only the parameters of wavelength, energy (J), or energy density (J/cm2), but also the information on the type of apparatus, irradiation parameters, and treatment parameters. This helps to ensure reproducibility by other researchers and clinicians. 35 Therefore, reproducibility is supported by the reciprocity of the parameters that guide the PBM principle.
De Freitas and Hamblin highlighted, in a review, how PBM produces better results when applied at low doses than when applied at high levels. 36 In addition, in some studies, those above 20 J/cm2 were considered to have an inhibitory effect. 37,38 However, Saito and Shimizu obtained positive results for bone neoformation despite the application of low level laser therapy (LLLT) at high doses (6.354 J/cm2 and 21 180 J/cm2). 33 Da Silva et al. also achieved positive results at 160 J/cm2. 39 Altan et al evaluated the effect of PBM doses compared to three levels: low (5 J/cm2), medium (20 J/cm2), and high (6300 J/cm2). 28 The PBM at doses 5 and 6300 J/cm2 was significantly effective, whereas it showed no significant effect on bone neoformation increase at 20 J/cm2. Thus, this shows that increased bone neoformation was found in 87% of the low dosage group (5 J/cm2), 40% of the group receiving the mean dosage (20 J/cm2), and 51% of the high-level group (6300 J/cm2). 28 These findings revealed that the therapy effectiveness is truly affected by dosage, thus corroborating the findings of the present study. Nevertheless, Brassolatti et al, in their review, observed that protocols that use lower energy values promote the same stimuli to the regeneration and/or repair process when compared to protocols that employ higher values because biological tissue responds similarly when the established parameters are concentrated within a therapeutic window. 40
The present study revealed that the highest bone neoformation percentage was obtained in the L45-60 group. This result can be supported by the biphasic dose-response curve, often called the Arndt-Schulz law. This curve explains that when energy is deficient, there is no response because a minimum threshold has not been reached. If more energy is applied, then the threshold is exceeded and bio-stimulation is achieved. But when energy is excessive, stimulation disappears and bio-inhibition occurs. 37
However, because PBM can be applied by using different protocols for each photobiomodulation, one can think of a maximum energy utilization threshold. Therefore, different tissue responses can occur in each case. Thus, in this paper, low energy for doses of 15 J/cm2 and 30 J/cm2 can be estimated and energy should be considered excessive at 60 J/cm2, given that 45 J/cm2 is considered optimal. In contrast, Schwartz-Filho et al. concluded that the PBM at 685 nm and 25, 77, and 130 J/cm2 did not influence cell growth and proliferation, although there was an alteration in the mineralization process pattern in osteogenic cell culture. It has led to a discussion on whether stimulus effects are dose-dependent or not. 32
The maximum threshold considered effective depends on the parameters used, as well as on the therapy target tissue. 28 Jenkins and Carroll reported that the PBM dose and beam parameters should be established for clinical and laboratory studies. They stressed that in addition to dose parameters being used, time, energy, and energy density and apparatus data should be observed. These include the beam release system, irradiation parameters, treatment parameters, exposure duration, number of irradiated points, application technique, and number and frequency of treatment sessions, among others. 35 Tissue penetration and spreading should also be factored in during the preparation of studies.
In the present study, euthanasia was performed between 30 and 60 days. This is the period when all regulatory mechanisms by which the laser acts in the tissues have been already consolidated. The mechanisms determine mesenchymal cell differentiation and the initial phase of osteoblast and fibroblast proliferation. Laser light also promotes an increase in fibroblast growth factor levels that acts on cell differentiation, increases proliferation rate, stimulates maturation and bone matrix production. 41 Between 30 and 60 days, there was a statistically significant difference in the L45 group.
In the present investigation, energy with a wavelength of 660 nm was applied immediately after the surgical procedure. Ebrahimi et al. reported that in vitro studies on PBM at wavelengths of 670 nm and 830 nm resulted in increased expression of osteocalcin, osteopontin, and type I. Moreover, in animal studies, PBM accelerates the regeneration process at extraction sites, fracture defects, bone defects by experimental induction, and osteogenic distraction. 42 PBM application periods also vary widely in the literature. Scalize et al obtained bone neoformation after three sessions of PBM. 29 Marques et al applied PBM to calvarial defects every 48 hours for 15 postoperative days. 31 In contrast, Garcia et al applied the PBM only once to critical-size defects, whose model has been used herein. 19,21
Analyzing adverse systemic conditions, Nunes et al. in an animal model, demonstrated the potential of photobiomodulation in regulating bone metabolism as a negative factor of smoking. The animals that were treated with PBM presented a lower number of TRAP-positive cells than the untreated ones. This resulted in a better tissue repair process in the treated group. 30 In the treatment of chronic periodontitis in diabetic humans, photobiomodulation in clinical parameters did not show superior effects when compared to mechanical therapy only at the end of the follow-up. However, at 6 months the photobiomodulation group showed better results. Thus, reinforcing that laser therapy has immediate wound healing properties. 43
However, bone neoformation percentage after photobiomodulation depends on the target tissue and parameters, such as those described by de Freitas and Hamblin who described the regulation mechanisms of PBM. 36 Concerning the molecular regulation mechanisms of bone neoformation in photobiomodulation, the authors described that the receptor activator of nuclear factor-kB (RANK) and osteoprotegerin (OPG) ratio determines whether the bone is reabsorbed or formed during the remodeling process. Thus, the bone remodeling cycle consists of the elevated RANKL expression by osteoblasts and subsequent binding to the RANK receptor, which is highly expressed in osteoclast membranes. This leads to an increase in osteoclast progenitor cells, mononuclear progenitor cell differentiation, increased survival of these cells, their fusion into multinucleated osteoclasts, and eventually activation. Osteoblasts are capable of modulating this process through the OPG expression of a RANKL receptor inhibitor. Thus, the photobiomodulation process can occur through chromophore activation, signaling molecules, transcription activation factors, molecular effects, and cellular and tissue regulation mechanisms.
Taking this context into consideration, more studies on different doses and parameters are needed to establish new protocols in animals and for the subsequent evaluation of PBM clinical application and its efficient establishment.
Conclusion
Through this study, it was possible to conclude that the application of PBM at 45 J/cm2 promoted the highest percentage of bone neoformation area in critical-size defects in 60 days, along with greater closure of the lesion, which was significantly more effective than the other doses.
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2015/10376-3 – Scientific initiation scholarship).
Conflict of Interests
The authors declare that they have no conflicts of interest.
Ethics Considerations
This study was carried out in accordance with the Ethical Principles of Animal Experimentation adopted by the National Council for Control of Animal Experimentation (CONCEA). It was submitted and approved by the Research Ethics Committee of the Institute of Science and Technology of Sao Jose dos Campos/UNESP.
Please cite this article as follows: De Marco AC, Torquato LC, Gonçalves PR, Ribeiro TC, Nunes CM, Bernardo DV, Gomes MF, Jardini MAN, Santamaria MP. The effect of photobiomodulation therapy in different doses on bone repair of critical size defects in rats: a histomorphometric study. J Lasers Med Sci. 2021;12:e53. doi:10.34172/jlms.2021.53.
References
- 1.Giannoudis PV, Atkins R. Management of long-bone non-unions. Injury. 2007;38(Suppl 2):S1–2. doi: 10.1016/s0020-1383(07)80002-7. [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82(5):655–8. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;(205):299–308. [PubMed] [Google Scholar]
- 4.Gauthier O, Müller R, von Stechow D, Lamy B, Weiss P, Bouler JM. et al. In vivo bone regeneration with injectable calcium phosphate biomaterial: a three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials. 2005;26(27):5444–53. doi: 10.1016/j.biomaterials.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 5.Hosseinpour S, Fekrazad R, Arany PR, Ye Q. Molecular impacts of photobiomodulation on bone regeneration: A systematic review. Prog Biophys Mol Biol. 2019;149:147–159. doi: 10.1016/j.pbiomolbio.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Stein A, Benayahu D, Maltz L, Oron U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg. 2005;23(2):161–6. doi: 10.1089/pho.2005.23.161. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira P, Ribeiro DA, Pipi EF, Driusso P, Parizotto NA, Renno AC. Low level laser therapy does not modulate the outcomes of a highly bioactive glass-ceramic (Biosilicate) on bone consolidation in rats. J Mater Sci Mater Med. 2010;21(4):1379–84. doi: 10.1007/s10856-009-3945-4. [DOI] [PubMed] [Google Scholar]
- 8.Renno AC, McDonnell PA, Crovace MC, Zanotto ED, Laakso L. Effect of 830 nm laser phototherapy on osteoblasts grown in vitro on Biosilicate scaffolds. Photomed Laser Surg. 2010;28(1):131–3. doi: 10.1089/pho.2009.2487. [DOI] [PubMed] [Google Scholar]
- 9.Yaoita H, Orimo H, Shirai Y, Shimada T. Expression of bone morphogenetic proteins and rat distal-less homolog genes following rat femoral fracture. J Bone Miner Metab. 2000;18(2):63–70. doi: 10.1007/s007740050013. [DOI] [PubMed] [Google Scholar]
- 10.Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A. Effect of Low Level Laser Therapy on Proliferation and Differentiation of the Cells Contributing in Bone Regeneration. J Lasers Med Sci. 2014;5(4):163–70. [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto MA, Ferino RV, Monteleone GF, Ribeiro DA. Low-level laser therapy modulates cyclo-oxygenase-2 expression during bone repair in rats. Lasers Med Sci. 2009;24(2):195–201. doi: 10.1007/s10103-008-0544-4. [DOI] [PubMed] [Google Scholar]
- 12.Trelles MA, Mayayo E. Bone fracture consolidates faster with low-power laser. Lasers Surg Med. 1987;7(1):36–45. doi: 10.1002/lsm.1900070107. [DOI] [PubMed] [Google Scholar]
- 13.Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, da Cruz-Höfling MA. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B. 2003;70(2):81–9. doi: 10.1016/s1011-1344(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 14.Dörtbudak O, Haas R, Mailath-Pokorny G. Effect of low-power laser irradiation on bony implant sites. Clin Oral Implants Res. 2002;13(3):288–92. doi: 10.1034/j.1600-0501.2002.130308.x. [DOI] [PubMed] [Google Scholar]
- 15.van Breugel HH, Bär PR. Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med. 1992;12(5):528–37. doi: 10.1002/lsm.1900120512. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro AL, Limeira Júnior Fde A, Gerbi ME, Ramalho LM, Marzola C, Ponzi EA. et al. Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseus membrane. J Clin Laser Med Surg. 2003;21(5):301–6. doi: 10.1089/104454703322564523. [DOI] [PubMed] [Google Scholar]
- 17.Lirani-Galvão AP, Jorgetti V, da Silva OL. Comparative study of how low-level laser therapy and low-intensity pulsed ultrasound affect bone repair in rats. Photomed Laser Surg. 2006;24(6):735–40. doi: 10.1089/pho.2006.24.735. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro AL, Martinez Gerbi ME, de Assis Limeira F Jr , Carneiro Ponzi EA, Marques AM, Carvalho CM. et al. Bone repair following bone grafting hydroxyapatite guided bone regeneration and infra-red laser photobiomodulation: a histological study in a rodent model. Lasers Med Sci. 2009;24(2):234–40. doi: 10.1007/s10103-008-0556-0. [DOI] [PubMed] [Google Scholar]
- 19.Garcia VG, Sahyon AS, Longo M, Fernandes LA, Gualberto Junior EC, Novaes VC. et al. Effect of LLLT on autogenous bone grafts in the repair of critical size defects in the calvaria of immunosuppressed rats. J Craniomaxillofac Surg. 2014;42(7):1196–202. doi: 10.1016/j.jcms.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Markovic A, Kokovic V, Todorovic L. The influence of low-power laser on healing of bone defects: an experimental study. J Oral Laser Appl. 2005;5(3):169–72. [Google Scholar]
- 21.Garcia VG, da Conceição JM, Fernandes LA, de Almeida JM, Nagata MJ, Bosco AF. et al. Effects of LLLT in combination with bisphosphonate on bone healing in critical size defects: a histological and histometric study in rat calvaria. Lasers Med Sci. 2013;28(2):407–14. doi: 10.1007/s10103-012-1068-5. [DOI] [PubMed] [Google Scholar]
- 22.Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, e al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001;4(1):3–14. doi: 10.1034/j.1600-0544.2001.040102.x. [DOI] [PubMed] [Google Scholar]
- 23.Diniz JS, Nicolau RA, de Melo Ocarino N, do Carmo Magalhães F, de Oliveira Pereira RD, Serakides R. Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci. 2009;24(3):347–52. doi: 10.1007/s10103-008-0568-9. [DOI] [PubMed] [Google Scholar]
- 24.Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz Mde A. et al. Low level laser therapy (830nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol. 2012;47(2):136–42. doi: 10.1016/j.exger.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Calixto JC, Lima CE, Frederico L, Lima RP, Anbinder AL. The influence of local administration of simvastatin in calvarial bone healing in rats. J Craniomaxillofac Surg. 2011;39(3):215–20. doi: 10.1016/j.jcms.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 26. Torquato LC, Suárez EAC, Bernardo DV, Pinto ILR, Mantovani LO, Lemes Silva TI, et al. Bone repair assessment of critical size defects in rats treated with mineralized bovine bone (Bio-Oss®) and photobiomodulation therapy: a histomorphometric and immunohistochemical study. [published online ahead of print, 2021 Jan 5]. Lasers Med Sci 2021;10.1007/s10103-020-03234-5. 10.1007/s10103-020-03234-5 [DOI] [PubMed]
- 27.Messora MR, Nagata MJ, Dornelles RC, Bomfim SR, Furlaneto FA, de Melo LG. et al. Bone healing in critical-size defects treated with platelet-rich plasma activated by two different methods A histologic and histometric study in rat calvaria. J Periodontal Res. 2008;43(6):723–9. doi: 10.1111/j.1600-0765.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 28.Altan AB, Bicakci AA, Avunduk MC, Esen H. The effect of dosage on the efficiency of LLLT in new bone formation at the expanded suture in rats. Lasers Med Sci. 2015;30(1):255–62. doi: 10.1007/s10103-014-1645-x. [DOI] [PubMed] [Google Scholar]
- 29.Scalize PH, de Sousa LG, Regalo SC, Semprini M, Pitol DL, da Silva GA. et al. Low-level laser therapy improves bone formation: stereology findings for osteoporosis in rat model. Lasers Med Sci. 2015;30(5):1599–607. doi: 10.1007/s10103-015-1773-y. [DOI] [PubMed] [Google Scholar]
- 30.Nunes CMM, Ferreira CL, Bernardo DV, Oblack GB, Longo M, Santamaria MP. et al. The influence of LLLT applied on applied on calvarial defect in rats under effect of cigarette smoke. J Appl Oral Sci. 2019;27:e20180621. doi: 10.1590/1678-7757-2018-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marques L, Holgado LA, Francischone LA, Ximenez JP, Okamoto R, Kinoshita A. New LLLT protocol to speed up the bone healing process—histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med Sci. 2015;30(4):1225–30. doi: 10.1007/s10103-014-1580-x. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz-Filho HO, Reimer AC, Marcantonio C, Marcantonio E Jr, Marcantonio RA. Effects of low-level laser therapy (685 nm) at different doses in osteogenic cell cultures. Lasers Med Sci. 2011;26(4):539–43. doi: 10.1007/s10103-011-0902-5. [DOI] [PubMed] [Google Scholar]
- 33.Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111(5):525–32. doi: 10.1016/s0889-5406(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 34.Takeda Y. Irradiation effect of low-energy laser on alveolar bone after tooth extraction Experimental study in rats. Int J Oral Maxillofac Surg. 1988;17(6):388–91. doi: 10.1016/s0901-5027(88)80070-5. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins PA, Carroll JD. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg. 2011;29(12):785–7. doi: 10.1089/pho.2011.9895. [DOI] [PubMed] [Google Scholar]
- 36.de Freitas LF, Hamblin MR. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifi M, Shafeei HA, Daneshdoost S, Mir M. Effects of two types of low-level laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci. 2007;22(4):261–4. doi: 10.1007/s10103-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 38.Goulart CS, Nouer PR, Mouramartins L, Garbin IU, de Fátima Zanirato Lizarelli R . Photoradiation and orthodontic movement: experimental study with canines. Photomed Laser Surg. 2006;24(2):192–6. doi: 10.1089/pho.2006.24.192. [DOI] [PubMed] [Google Scholar]
- 39.da Silva AP, Petri AD, Crippa GE, Stuani AS, Stuani AS, Rosa AL. et al. Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med Sci. 2012;27(4):777–83. doi: 10.1007/s10103-011-0968-0. [DOI] [PubMed] [Google Scholar]
- 40.Brassolatti P, de Andrade ALM, Bossini PS, Orth DL, Duarte FO, Dos Anjos Souza AB. et al. Photobiomodulation on critical bone defects of rat calvaria: a systematic review. Lasers Med Sci. 2018;33(9):1841–1848. doi: 10.1007/s10103-018-2653-z. [DOI] [PubMed] [Google Scholar]
- 41.Pinheiro AL, Gerbi ME. Photoengineering of bone repair processes. Photomed Laser Surg. 2006;24(2):169–78. doi: 10.1089/pho.2006.24.169. [DOI] [PubMed] [Google Scholar]
- 42.Ebrahimi T, Moslemi N, Rokn A, Heidari M, Nokhbatolfoghahaie H, Fekrazad R. The influence of low-intensity laser therapy on bone healing. J Dent (Tehran) 2012;9(4):238–48. [PMC free article] [PubMed] [Google Scholar]
- 43.Castro Dos Santos N, Andere NMRB, Miguel MMV, Dos Santos LM, Santamaria M Jr, Mathias IF. et al. Photobiomodulation for the treatment of periodontal pockets in patients with type 2 diabetes: 1-year results of a randomized clinical trial. Lasers Med Sci. 2019;34(9):1897–1904. doi: 10.1007/s10103-019-02799-0. [DOI] [PubMed] [Google Scholar]