Abstract
Background:
The Postoperative cognitive dysfunction (POCD) model was constructed by resection of the left hepatic lobe in aged mice to determine the behavioral effects of the POCD model in aged mice and the relationship between NF-κB and POCD in apoptosis and autophagy. Provide a theoretical basis for POCD prevention and treatment.
Methods:
This study was carried out in Ningbo No. 6 Hospital, Zhejiang, China, from Jun 2019 to Dec 2020. The POCD model was constructed after resection of the left extrahepatic lobe in aged mice and randomly divided into 6 groups: sham operation group, operation group (normal saline control group, solvent group, YC-1 group, PDTC group and 3-MA group). Related indicators of behavioral changes, neuronal inflammatory responses, apoptosis, and autophagy were examined.
Results:
The escape latency of the aged mice in the surgical group was significantly prolonged at three time points compared with the control group, and the number of insertions decreased significantly. Microglia are activated and the inflammatory response is increased, whereas PDTC has an inhibitory effect. It was demonstrated that apoptosis and necrosis of neurons can be induced by the NF-κb pathway, and autophagy can be promoted, whereas autophagy occurs before apoptosis.
Conclusion:
Activation of NF-κb pathway in neurons after POCD causes neuronal apoptosis and autophagy, and cognitive impairment occurs. PDTC, a NF-κb pathway inhibitor, can effectively reduce neuronal apoptosis induced by secondary brain injury after POCD. Necrosis, to protect the brain tissue.
Keywords: Postoperative cognitive dysfunction (POCD), Neuronal apoptosis, Autophagy
Introduction
Postoperative cognitive dysfunction (POCD) is a decline in cognitive function especially memory, attention after anesthesia and surgery. Concern has been growing regarding that POCD as an important complication of patients after surgery. Elderly patients in particular are vulnerable to memory disturbances and other types of cognitive impairment after surgical operations (1,2). The aging of the population and new developments in medicine both imply the number of elderly patients undergoing surgery and anesthesia will keep rising. This is an important issue in perioperative care. POCD has received more and more attention from scholars.
Although aging is regarded as the only one clear risk of POCD, the causes of higher POCD morbidity in aging is still unclear. Cytokines play a crucial role in the pathogenesis of POCD (3). These cytokines may enter the hippocampus back through the damaged blood-brain barrier, leading to neuronal damage in the hippocampus, affecting learning, memory and cognitive function. In the inflammatory response of the central nervous system, the NF-KB transcription factor is a central regulatory element, and the promoters of IL-2, IL-6, and NOS all have a DNA binding region of p50-p65 (4,5). This inflammatory response has been validated in the neurotoxicological response mechanism of heavy metals. NF-kB plays an extremely important role in the process of synaptic remodeling and protection as well as apoptosis caused by stress.
When HIF-la is activated, it regulates the transcription of many genes involved in energy metabolism, angiogenesis, apoptosis, and other genes, regulates oxygen delivery, or promotes related metabolism to adapt to hypoxia, which acts as a major regulator of steady-state hypoxic response in cells and systems. Therefore, HIF-1a plays an important role in embryonic angiogenesis, tumor angiogenesis, and pathophysiology of ischemic diseases. In addition, HIF-la plays a very important role in ischemic brain injury and hypoxic brain damage, which is reduced in ischemic brain damage and hypoxic brain damage, and HIF signaling pathway plays a role in neuroprotection (6,7). The role of HIF-1a expression up-regulation and maintenance can alleviate cognitive dysfunction. HIF-1a was inhibited, hippocampal cell apoptosis increased, cognitive function decreased, suggesting that HIF-1a has neuroprotective effects in subarachnoid hemorrhage, may be an effective therapeutic target (8). However, to date, the expression of HIF-1a in the development and progression of postoperative cognitive dysfunction remains controversial and needs further study.
Based on the current research basis, we evaluated the POCD model of elderly mice with left hepatectomy, and then determined the behavioral effects of POCD model in aged mice and the role and apoptosis of NF-κB and POCD. The relationship between autophagy and thus provides a theoretical basis for the prevention and treatment of POCD.
Materials and Methods
Grouping and administration of mice
This study was carried out in the Central laboratory of Ningbo No. 6 Hospital, Zhejiang, China, from Jun 2019 to Dec 2020.One week after adaptive feeding, the mice were randomly divided into 4 groups: control group, model group (model group, model+YC-1 group and model+PDTC group). There were 6 rats in each group. In the model+YC-1 group, mice were intraperitoneally injected with 2 mg/kg of YC-1 (YC-1 dissolved in DMSO) 30 min before and 24 h after liver partial resection; model+PDTC group was injected with 20 mg/kg (PDTC dissolved in DMSO) 30 min before and 24 h after liver partial resection; the control group was injected with the same dose of normal saline in the PDTC group.
Ethics approval
This study was approved by the ethics committee of Ningbo NO.6 Hospital (with approval number EC2020-007).
Animal model establishment
The mice were anesthetized with 10% chloral hydrate at a body mass of 0.04 mL/10g. After the righting reflex disappeared, the supine position was fixed and disinfected, and a 1.5–2.0 cm incision was made in the midline of the lower abdomen of the xiphoid process. The left lobe liver was isolated, and the left hepatic lobe was removed after ligation at the distal pedicle with a No.1 silk thread. After the hemostasis was completely stopped, the abdomen was closed layer by layer. Strict aseptic operation was maintained during the operation, and intraperitoneal injection of penicillin sodium 50 U/g, 1 time/d for 2 days to prevent infection.
Mouse learning and memory function test
Mice were tested for learning and memory ability on the first day after surgery using the Morris water maze system. Positioning navigation experiment: lasted for 5 days, divided into two periods, upper and afternoon, respectively. From two different marking points, the mice were placed in the water facing the pool wall, and the time required to find the platform within 1 min of the mouse was recorded. (ie escape the incubation period). If the platform fails to find the platform within 1 min after entering the water, place it on the platform for 15 s and record the escape latency as 1 min. Take the average of the time twice a day as the value of the day. Escape the incubation period to reflect the animal’s ability to gain experience that is, learning ability. Space exploration test: After the navigation test was completed, the platform was removed. The mouse was placed in the water at the entrance point. The mouse was recorded in the original platform quadrant for 1 min. The space exploration experiment was only performed once. Space exploration experiments reflect the ability of animals to maintain experience, that is, their ability to remember. Data collection and processing was done by Morris software.
Specimen collection
After the end of the water maze experiment, all the rats were anesthetized with sevoflurane until the righting reflex disappeared. After the rats were fixed, the heart was perfused, the head was broken, and the brain was quickly removed on the ice. The right hemisphere was separated and the right side was separated. The hippocampus tissue was placed in a cryotube and stored in a −80 °C freezer.
Western blot
Hippocampal tissue protein expression levels were determined by immunoblotting. Hippocampus tissue was added to cell lysate and protease inhibitor PMSF for 30 min on ice. After ultrasonic lysis and centrifugation at 4°C, the supernatant was taken and denatured in a 100 °C water bath for 10 min and frozen at −20°C. Protein was separated by 8% polyacrylamide gel electrophoresis and transferred to PVDF membrane. 5% skim milk powder was blocked at room temperature for 1 h, and β-Actin antibody (1:5000), iNOS, NF-κb, HIF-1α, Bcl- were added. Bax, Caspase-3, Beclin, BECN1 and p62 primary antibody (1:500) were placed on a shaker, incubated at 4 °C for 12 h, TBST was fully rinsed and then added with horseradish peroxidase-labeled secondary antibody at room temperature shaker 2 h, TBST was rinsed and developed with ECL.
Statistical analysis
Analysis was performed using SPSS (Chicago, IL, USA) 18.0 statistical software. The measurement data were expressed as mean ± standard deviation (x±s). The comparison within the group was analyzed by repeated measures data analysis. The comparison between groups was performed by group t test, and the count data was tested. P <0.05 was considered statistically significant.
Results
Mouse cognitive impairment associated with hippocampal microglia activated
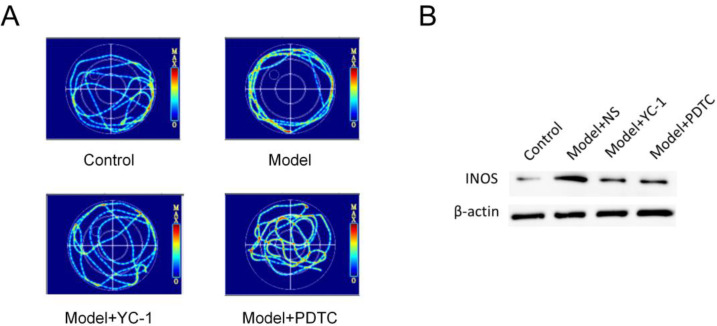
The results of spatial memory test showed that compared with the control group, the mice treated with the two treatments had better spatial memory ability than the model mice (Fig. 1A). Western blot results showed that the expression of iNOS after POCD increased significantly from 24 h after surgery (Fig. 1B). It was confirmed that after POCD, microglia were activated and the inflammatory response was increased, and PDTC inhibited this.
Fig. 1:
(A) Effects of YC-1 and PDTC interventions on cognitive function in aged mice. (B) Effects of YC-1 and PDTC interventions on activation of hippocampal microglia after POCD modeling
After POCD, NF-κb is activated and hippocampal neurons undergo apoptosis
After we cultured neurons, we added NF-κb inhibitor PDTC and HIF-1α inhibitor YC-1, respectively, to make NF-κb protein expression in neurons, and then observe the cell expression. To judge the expression of NF-κb protein.
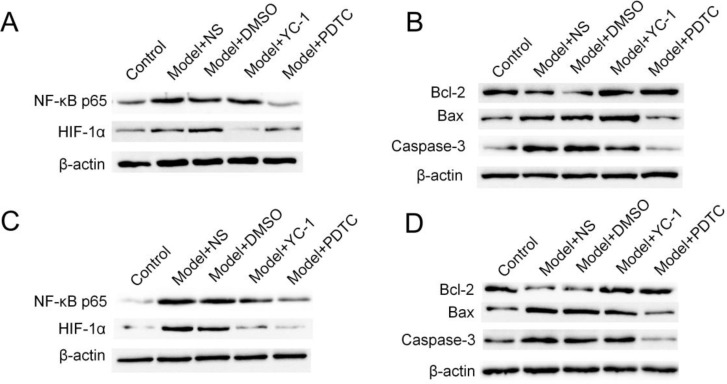
In this experiment, the expression of NF-κb protein in the PDTC group was lower than that in the surgery group (P<0.01). This indicated that POCD can activate neurons to express NF-κb protein, and the expression of NF-κb protein increases gradually from 24h (Fig. 2 A), and peaks at 72h (Fig. 2 C).
Fig. 2:
Effects of YC-1 and PDTC interventions on hippocampal neurons after POCD modeling. (A) The Western blotting assay of NF-κB and HIF-1α at 24h. (B) The Western blotting assay of Bcl-2, Bax and Caspase-3 at 24h. (C) The Western blotting assay of NF-κB and HIF-1α at 72h. (D) The Western blotting assay of Bcl-2, Bax and Caspase-3 at 72h
In the detection of Bcl-2, Bax and caspase-3 expression, in the PDTC group, the time point of detection was statistically significant compared with the surgery group (P<0.05), thus indicating that PDTC can inhibit the induction of NF-κb pathway. Apoptosis; apoptosis expression was lower than the surgery group but higher than the blank group, which was statistically significant (P < 0.01) (Fig. 2 B and D). This also indicated that PDTC does not completely inhibit the apoptosis induced by the NF-κb pathway. The inhibitory effect of YC-1 was weaker than that of PDTC.
In summary, we demonstrate that neuronal apoptosis and necrosis can be induced by NF-κb pathway, and we believe that PDTC can effectively reduce neuronal apoptosis and necrosis caused by secondary brain injury after POCD, and protect the brain. The role of the organization.
NF-κb activation can induce autophagy in hippocampal neurons
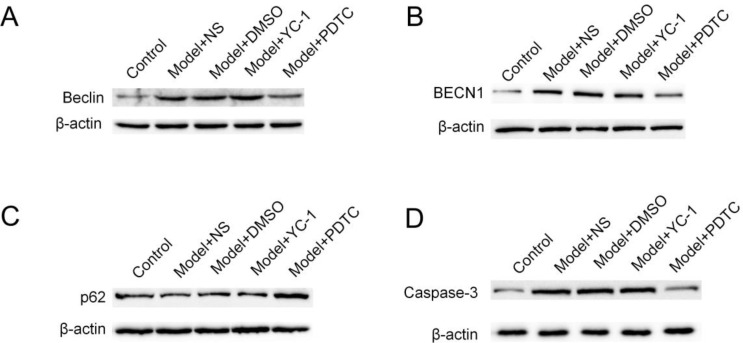
In this part of the experiment, we applied NF-κb inhibitor PDTC to neurons cultured in vitro at different time points, and compared the expression of Beclin1 protein with the surgery group to observe whether NF-κb signaling pathway is involved in autophagosomes. Formation process. From the experimental results, Beclinl, BECN1 and p62 were significantly different between the inhibition group and the operation group at 6h (P<0.05) (Fig. 3 A–C), while at this time, the caspase-3 was detected again (Fig. 3 D). Statistical differences have demonstrated that the NF-κb pathway promotes autophagy and that autophagy occurs before apoptosis.
Fig. 3:
Effects of MA-3 and PDTC interventions on hippocampal neurons after POCD modeling. (A) The Western blotting assay of Beclin1. (B) The Western blotting assay of BECN1. (C) The Western blotting assay of p62. (D) The Western blotting assay of Caspase-3
Discussion
According to United Nations statistics, the global elderly population has increased rapidly, reaching 1.4 billion by the end of the last century. In 1982, the elderly over 60 yr old accounted for 7.6% of the national population; in 1995, the number of people over 60 yr old reached 120 million, accounting for 9.76% of the total population. It is predicted that the elderly in China will grow at a rate of 3.37% per year. By 2025, the elderly population will reach 280 million, accounting for more than 16% of the national population (9). The increase in the elderly population has brought about an increase in the chance of surgery. Elderly patients often have coronary heart disease, diabetes, hypertension, chronic respiratory system, liver and kidney disease before surgery, which has a serious impact on the organ reserve function that has been reduced in elderly patients (10). Postoperative cognitive dysfunction (POCD) is one of the common neurological complications in the elderly. The patient is characterized by no mental abnormalities before surgery, postoperative cognitive decline, anxiety, personality changes, insanity, etc. (11, 12). POCD can lead to delayed recovery of surgery patients, increased medical costs, and affect the quality of life after surgery.
Although the harmfulness of POCD has been widely recognized, its specific pathogenesis and pathophysiology have not yet been elucidated. It may occur based on degeneration of the central nervous system in elderly patients. Surgical trauma leads to the release of a large number of inflammatory cytokines, which has serious clinical consequences. At the same time, elderly patients also have weakened cerebral vascular self-regulation function and low oxygen utilization rate of brain tissue (12). Under the influence of anesthesia and surgery, these factors are more likely to be aggravated and lead to POCD. Microglia, a congenital immune cell inherent in the Central Nervous System (CNS), has been thought to be involved in the pathological processes of neurodegenerative diseases and neuroinflammation for many years. More and more evidence indicates that trauma, peripheral inflammation and other factors stimulate microglia, which leads to the release of many neurotoxic substances after activation (13). These toxic substances promote each other, leading to neuronal apoptosis and damage. Our results showed that the expression of iNOS after POCD increased significantly from 24 h after surgery, microglia was activated, and the inflammatory response increased, while PDTC inhibited it.
Like the pathogenesis of Alzheimer’s disease, POCD may be a degenerative change induced or aggravated by external factors such as anesthesia and surgery on the basis of aging of the nervous system (14). Its pathogenesis is a combination of various factors. Studies have found that splenectomy causes glial cell activation leading to an inflammatory response in the hippocampus, accompanied by a decline in cognitive function (15–18). Neuroinflammation is characterized by excessive activation of microglia. Activated microglia release a large number of inflammatory mediators, such as cytokines, chemical factors, excitatory amino acids and reactive oxygen species (ROS), which damage peripheral neurons (19). Damaged neurons release intracellular proteins that bind to microglial membrane surface receptors, further activating microglia, which creates a vicious circle that leads to progressive neuronal loss and death. Therefore, inhibition of neuroinflammation may be one of the effective methods for treating POCD.
NF-κB is a protein molecule with multi-directional regulation, which can specifically bind to the NF-κB sequence of promoters and enhancers of various cellular genes, participate in the regulation of gene expression, regulate cellular immunity, regulate and regulate cancer (20). It plays an important role in the process of occurrence and apoptosis. Functional NF-κB complexes are present in various types of functional cells in the nervous system, such as neurons, astrocytes, microglia, and oligodendrocytes (21, 22). In this experiment, we used cell culture techniques to culture successful neurons, and then added NF-κB inhibitor PDTC and HIF-1α inhibitor YC-1, respectively, to make neurons express NF-κB protein for cellular immunoassay. The intensity of cell expression to determine the expression of NF-κB protein.
In this experiment, the expression of NF-κB protein in the PDTC group was lower than that of the NF-κB protein in the surgery group at 24 h, which was statistically significant (P<0.001). This indicated that POCD can activate neurons to express NF-κB protein, and the expression of NF-K B protein increases gradually from 24 h, and peaks at 72 h.
The NF-κB pathway is the main pathway involved in secondary brain injury after POCD (23). From our experimental results, it is concluded that NF-κB pathway can activate a large number of inflammatory factors, while inflammatory factors can stimulate NF-κB protein. The expression increases, thereby forming a loop that amplifies the NF-κB pathway effect. The expression of a large amount of NF-κB protein can induce various forms of death, including autophagy, apoptosis and necrosis.
NF-κB can induce neuronal damage by regulating the expression of various apoptosis-related genes. After brain injury, the expression of apoptosis-related genes induced by NF-κB is one of the pathways of neuronal apoptosis (24). In the detection of Bcl-2, Bax and caspase-3 expression, in the PDTC group, the time point of the test was statistically significant compared with the surgery group (P<0.05), thus indicating that PDTC can inhibit the induction of NF-κB pathway. Apoptosis; apoptosis expression was lower than the surgery group but higher than the blank group, which was statistically significant. PDTC does not completely inhibit the apoptosis induced by the NF-κB pathway. The inhibitory effect of YC-1 was weaker than that of PDTC. We have proved that apoptosis and necrosis of neurons can be induced by NF-kappa B pathway. At the same time, we believe that PDTC can effectively reduce apoptosis and necrosis of neurons caused by secondary brain injury after POCD and protect brain tissue.
There was also a close relationship between NF-κB and autophagic cell death. Just like apoptosis, many literature reported that down-regulation of NF-κB can inhibit autophagic cell death in cells (25, 26), but recently there have been some reports confirming activation of NF-κb can promote autophagy in cells (27, 28). Studies have shown that autophagy is activated after POCD, and autophagy has both protective effects on cell involvement and cell damage. This dual action depends on the location of autophagy after injury and the stage after injury. Autophagy is currently considered a double-edged sword (29–32). The double-sided nature of autophagy is of great significance in the study of traumatic brain injury. On the one hand, it can induce the interaction between autophagic cell death and apoptotic signals; on the other hand, autophagy may be in damaged nerve cells. Helps clear damaged organelles and prevent the initiation of apoptosis programs (33, 34). Therefore, in this part of the experiment, we designed NF-κB inhibitor PDTC to be added to the model group mice at different time points, and compared the expression of Beclinl, BECN1 and p62 protein with the operation group, respectively, to observe the NF-κB signal, testing whether the pathway is involved in the process of autophagosome formation, participate in the process of autophagosome formation. From the experimental results, Beclinl, BECN1 and p62 were significantly different between the inhibition group and the operation group at 6h (P<0.05), which proved that the NF-κB pathway can promote autophagy.
Conclusion
Apoptosis and autophagy increased with the increase of neuron expression of NF-kappa B protein, and autophagy occurred earlier than apoptosis. Therefore, we judged that apoptosis and autophagy coexist, and autophagy may transform to apoptosis after autophagy. We suggested that the mechanism may be that activation of neuronal NF-kappa B pathway induces the expression of a series of inflammatory factors, leading to autophagy and apoptosis in the early stage (1h–24h) and necrosis in the later stage (24h–48h).
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This work was supported by Zhejiang Province Traditional Chinese Medicine Project Funding (2020-20); Project of Yinzhou District (2018-33).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Tian Y, Guo S, Zhang Y, Xu Y, Zhao P, Zhao X. (2017). Effects of hydrogen-rich saline on hepatectomy-induced postoperative cognitive dysfunction in old mice. Mol Neurobiol, 54(4):2579–2584. [DOI] [PubMed] [Google Scholar]
- 2.Jiang P, Ling Q, Liu H, Tu W. (2015). Intracisternal administration of an interleukin-6 receptor antagonist attenuates surgery-induced cognitive impairment by inhibition of neuroinflammatory responses in aged rats. Exp Ther Med, 9(3):982–986. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Ding Y, Shi C, Chen L, et al. (2017). Effects of andrographolide on postoperative cognitive dysfunction and the association with nf?κb/mapk pathway. Oncol Lett, 14(6):7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng JW, Meng B, Li XY, et al. (2017). Nf-κb/p65 signaling pathway: a potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anestesia. Eur Rev Med Pharmacol Sci, 21(2):394–407. [PubMed] [Google Scholar]
- 5.Yu L, Sun L, Chen S. (2014). Protective effect of senegenin on splenectomy?induced postoperative cognitive dysfunction in elderly rats. Exp Ther Med, 7(4):821–826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Li Z, Cao Y, Li L, et al. (2014). Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem Biophys Res Commun, 449(1):74–80. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Li Z, Ma L, et al. (2018). Isoflurane?induced postoperative cognitive dysfunction is mediated by hypoxia?inducible factor?1α?dependent neuroinflammation in aged rats. Mol Med Rep, 17(6):7730–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang N, Liang Y, Yang P, Wang W, Zhang X, Wang J. (2016). Tnf-α receptor antagonist attenuates isoflurane-induced cognitive impairment in aged rats. Exp Ther Med, 12(1):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raju M. (2018). Population Ageing and the Elderly. Indian J Psychiatry, 60(Suppl 3):S295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Zhang Z, Chen T, Peng M, Xu X, Wang Y. (2015). Prolonged mechanical ventilation–induced neuroinflammation affects postoperative memory dysfunction in surgical mice. Crit Care, 19(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu JJ, Zhu LX, Zhang J, et al. (2017). Activation of nf-κb-autophagy axis by 2-hydroxyethyl methacrylate commits dental mesenchymal cells to apoptosis. Toxicol Sci, 157(1):100–111. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Huang L, Gong J, et al. (2017). Nf-κb pathway link with er stress-induced autophagy and apoptosis in cervical tumor cells. Cell Death Discov, 3:17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin B, Perkins N D. (2010). The skp2 promoter integrates signaling through the nf-κb, p53, and akt/gsk3β pathways to regulate autophagy and apoptosis. Mol Cell, 38(4):524–38. [DOI] [PubMed] [Google Scholar]
- 14.Razgonova MP, Veselov VV, Zakharenko AM, et al. (2019). Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review). Mol Med Rep, 19(4):2975–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su J, Liu F, Xia M, et al. (2015). P62 participates in the inhibition of nf-κb signaling and apoptosis induced by sulfasalazine in human glioma u251 cells. Oncol Rep, 34(1):235–43. [DOI] [PubMed] [Google Scholar]
- 16.Tang MM, Lin WJ, Pan YQ, Li YC. (2018). Fibroblast Growth Factor 2 Modulates Hippocampal Microglia Activation in a Neuroinflammation Induced Model of Depression. Front Cell Neurosci, 12:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friess L, Cheray M, Keane L, Grabert K, Joseph B. (2021). Atg7 deficiency in microglia drives an altered transcriptomic profile associated with an impaired neuroinflammatory response. Mol Brain, 14(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Z, Zhou T, Sun X, et al. (2018). Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ, 25(1):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liwei W, Qinghui Z, Weiqi D, et al. (2017). Quercetin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy by inhibiting erk/nf-\r, κ\r, b pathway. Gastroenterol Res Pract, 2017:9724217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu BS, Xing CG, Lin F, et al. (2011). Blocking nf-κb nuclear translocation leads to p53-related autophagy activation and cell apoptosis. World J Gastroenterol, 17(4):478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen X, Ma L, Dong W, et al. (2016). Autophagy regulates intracerebral hemorrhage induced neural damage via apoptosis and nf-κb pathway. Neurochem Int, 96:100–12. [DOI] [PubMed] [Google Scholar]
- 22.Salminen A, Hyttinen JM, Kauppinen A, Kaarniranta K. (2012). Context-dependent regulation of autophagy by ikk-nf-κb signaling: impact on the aging process. Int J Cell Biol, 2012:849541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang DL, Cui HF, Yu S, Lin T, Liu ZH, Zhang HM. (2015). Effects of tanyu tongzhi formula on nf-κb pathway, autophagy and antiapoptosis of rats with myocardial ischemia-reperfusion injury. Chinese Journal of Information on Traditional Chinese Medicine. [Google Scholar]
- 24.Wei M, Li Z, Yang Z. (2014). Crosstalk between protective autophagy and nf-κb signal in high glucose-induced podocytes. Mol Cell Biochem, 394(1–2):261–73. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Li F, Zhao K, Yao J, et al. (2014). LFG-500 inhibits the invasion of cancer cells via down-regulation of PI3K/AKT/NF-κB signaling pathway. PLoS One, 9(3):e91332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhunchha B, Fatma N, Kubo E, et al. (2013). Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am J Physiol Cell Physiol, 304(7):C636–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Zhao YF, Liu RS, et al. (2019). Olanzapine induced autophagy through suppression of NF-κB activation in human glioma cells. CNS Neurosci Ther, 25(9):911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Ma Y, Yan J, Liu J, Li L. (2017). Geniposide promotes autophagy to inhibit insulin resistance in HepG2 cells via P62/NF κB/GLUT 4. Mol Med Rep, 16(5):7237–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Zhang L, Li J, Gao F, Zhou G. (2017). Hydrogen peroxide-induced change in meat quality of breast muscle of broilers is mediated by ros generation, apoptosis and autophagy in nf-κb signal pathway. J Agric Food Chem, 65(19):3986–3994. [DOI] [PubMed] [Google Scholar]
- 30.Ogata M, Hino S, Saito A, et al. (2006). Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol, 26(24):9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang YB, Li SX, Chen XP, et al. (2008). Autophagy is activated and might protect neurons from degeneration after traumatic brain injury. Neurosci Bull, 24(3):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong J, Kong Q, Dai L, Ma H, Cao X, Liu L, Ding Z. (2017). Autophagy activated by tuberin/mTOR/p70S6K suppression is a protective mechanism against local anaesthetics neurotoxicity. J Cell Mol Med, 21(3):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim CB, Fu PY, Ky N, et al. (2012). Nf-κb p65 repression by the sesquiterpene lactone, helenalin, contributes to the induction of autophagy cell death. BMC Complement Altern Med, 12:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djavaheri-Mergny M, Amelotti M, Mathieu J, et al. (2006). Nf-κb activation represses tumor necrosis factor-α-induced autophagy. J Biol Chem, 281(41):30373–82. [DOI] [PubMed] [Google Scholar]