Abstract
Nanotechnology offers new opportunities for the development of novel materials and strategies that improve technology and industry. This applies especially to agriculture, and our previous field studies have indicated that zinc oxide nanoparticles provide promising nano-fertilizer dispersion in sustainable agriculture. However, little is known about the precise ZnO-NP effects on legumes. Herein, 1 mg·L−1 ZnO-NP spray was dispersed on lentil plants to establish the direct NP effects on lentil production, seed nutritional quality, and stress response under field conditions. Although ZnO-NP exposure positively affected yield, thousand-seed weight and the number of pods per plant, there was no statistically significant difference in nutrient and anti-nutrient content in treated and untreated plant seeds. In contrast, the lentil water stress level was affected, and the stress response resulted in statistically significant changes in stomatal conductance, crop water stress index, and plant temperature. Foliar application of low ZnO-NP concentrations therefore proved promising in increasing crop production under field conditions, and this confirms ZnO-NP use as a viable strategy for sustainable agriculture.
Keywords: lentil seeds, zinc oxide nanoparticles, nano-fertilizers, foliar application, physiological indexes, essential and beneficial nutrients
1. Introduction
Legumes are nutritionally valuable and most important in the human diet by providing proteins, complex carbohydrates and dietary fiber [1,2]. Moreover, lentil legumes are especially high in proteins and essential amino acids, fiber and minerals and low in fat and cholesterol [1,3]. Lentil is an annual crop with moderate resistance to high temperatures, drought [4] and salinity [5], and it is acknowledged to have a long photoperiod [6]. Lentils are classified in the two main Chilean and Persian categories, dependent on seed size and weight. The seeds also come in various colors, and herein we used the black Lens culinaris ‘Beluga’ seeds in our field experiments because these provide an important source of beneficial nutrients [7].
Sufficient amounts of micronutrients are required for optimal lentil growth and development [8]. These include zinc (Zn) because its deficiency negatively affects plant reproductive functions and pollen development [9], reduces leaf area and induces chloroses and abnormal growth of plant structures. This deficiency can also result in higher sensitivity to biotic and abiotic stress [10]. Most importantly, Zn agronomic management includes foliar application and soil fertilization [11,12,13].
Zinc is also an essential micro-nutrient in general metabolism [13,14]. It forms a fundamental part of more than 300 enzymes [15], and it is involved in photosynthesis, DNA replication and transcription [13], hydrocarbon metabolism [16], regulation of auxin-production [17] and cell membrane integrity [13].
Macro-sized and soluble-Zn-ion fertilizers are traditionally applied in agriculture [18], and both ZnS and ZnO nanoparticles are promising novel fertilizer nutrients for crops [11,19,20,21].
The ZnO zincite mineral mostly occurs in wurtzite-type crystal symmetry which provides superior physical properties [22]. These include high electron mobility and wide band-gap energy, which both enable strong zincite photocatalytic reactivity [23,24]. Therefore, its morphological, spatial and structural forms in glass, electronics, energy, pigments, rubber textiles, cosmetics, food additives and pharmaceutics and medicine are complemented by its use in other technology [22,24,25].
ZnO-NP agricultural application provides great benefits for plant physiology and consequent production [11,12,20]. The physiological value is reflected in improved crop water stress index (CWSI), and in stomatal conductance (Ig), which is a function of plant water stress and leaf water potential level [26,27]. ZnO-NPs also influence plant temperature and temperature variations which are useful indicators of stress response, plant transpiration and energy balance [28].
However, currently available studies on ZnO-NP stress properties lack essential data. This especially applies to translocation, the metal-residues in plant tissues and their impact on seed quality and nutritional balance. This balance in fully ripe lentil seeds is important for higher phytic acid content, because this absorbs further micronutrients and contains phosphor as an anti-nutrient. Intestinal digestion further negatively affects nutrient bioavailability [29].
Although many experiments have investigated ZnO-NP and plant interaction during germination and early growth under greenhouse conditions [24,30,31], field lentil production critically depends on local climate and temperature [6]. Therefore, applied ZnO-NP field experiments are important to understand its effects on lentil production and physiological parameters. These affect all the following plant yield and thousand-seed weight, number of pods per plant, stomatal conductance, crop water stress index and additional temperature-derived lentil parameters. This lack of research inspired us to apply foliar ZnO-NPs to lentils under field conditions to acquire new information on the effects of ZnO-NP on these plants.
2. Materials and Methods
2.1. Plant Material
We chose the Lens culinaris ´Beluga´ legume. This variety originates in southwest Asia, is grown mainly in Canada [32] and has the third highest fiber and protein content of all legumes, with low-fat and low glycemic index [3,32,33]. Lens culinaris prefers a moderate climate with low humidity and long photoperiodic lighting, and does not require excess heat to thrive [34]. It adapts perfectly to loamy, well-drained and loose soils and those treated with compost before planting. The optimal soil pH for growth ranges from 6 to 6.5 [35].
2.2. Colloidal Properties of ZnO Nanoparticles
The ZnO-NP mineralogical specifications of size, morphology, crystal symmetry and unit cell dimensions have previously been reported [12]. Herein, we analyzed the colloidal properties of a solution prepared for foliar application. The hydrodynamic diameter and zeta-potential were measured by nanoPartica SZ-100 analyzer (Horiba, Kyoto, Japan). This was arranged with a microprocessor unit which directly calculated the zeta-potential at neutral pH and 25 °C laboratory conditions under the generalized Smoluchowski equation.
2.3. Experimental Site Description
Field experiments were performed in the Hostie Village near Zlaté Moravce in Slovakia. This lies 310 m above sea level in the north-eastern part of Žitavská pahorkatina Upland. The Pohronský Inovec Mts. lie to the east and the Nitrianska pahorkatina Wold and Tribeč Mts. are on the western aspect. Geomorphic classification indicates that the experimental field is in an upland area with rendzic leptosols and prior annual rotation of maize and potatoes. Soil availability of Zn, Cu, Mn and Fe was measured by DTPA extraction [36]. The content of soil carbonates, H2O and KCl pH, hydrolytic acidity, sum of exchangeable basic cations, sorption capacity, soil organic carbon and electrical conductivity were all measured as in Hrivňáková, et al. [37], and shown here in Table 1.
Table 1.
Selected soil features from the Hostie experimental locality at Zlaté Moravce in Slovakia prior to experimental treatments.
| pHH2O | pHKCl | 1CC (%) | 2HA (mmol·kg−1) | 3SEBC (mmol·kg−1) | 4TSC (mmol·kg−1) | 5DSCBC (%) | 6EC (S) | 7C org (%) |
|---|---|---|---|---|---|---|---|---|
| 7.1 ± 0.1 | 6.9 ± 0.02 | 4.6 ± 1.0 | 5.0 ± 0.3 | 465 ± 13 | 470 ± 13 | 98.9 ± 0.1 | 310 ± 46 | 2.5 ± 0.5 |
1CC—carbonates’ content; 2HA—hydrolytic acidity; 3SEBC—sum of exchangeable basic cations; 4TSC—total sorption capacity; 5DSCBC—degree of saturation of complex with basic cations; 6EC—electrical conductivity, 7C org—soil organic carbon
The detailed geographic, climate and soil conditions were previously reported by Kolenčík et al. [12], and the local monthly air temperature and precipitation are recorded from Meteoblue Web Weather Server. These are highlighted in Figure 1.
Figure 1.
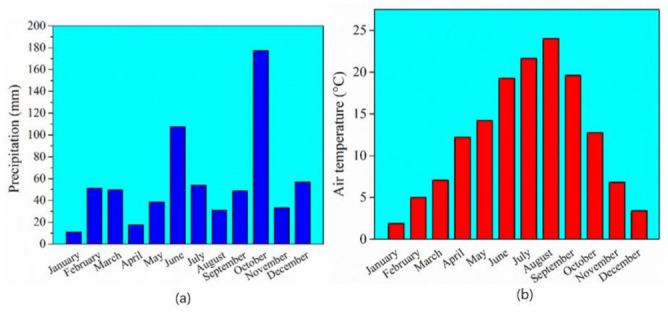
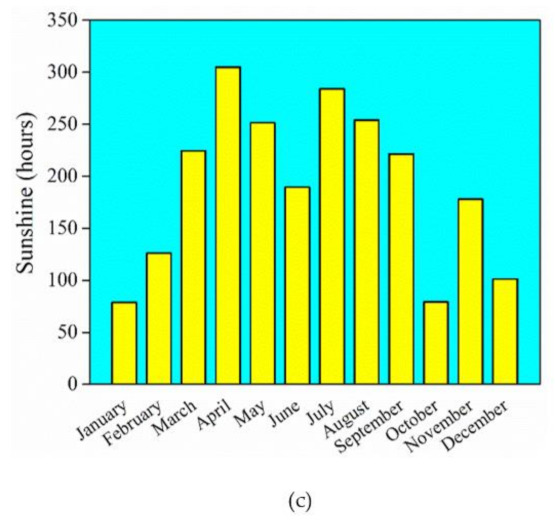
Monthly variations in (a) precipitation, (b) air temperature and (c) hours of sunshine during the 2020 vegetation season at the Hostie Village near Zlaté Moravce in Slovakia.
2.4. Field Experiment
The experiment was performed on 5 m2 parcels with silt loam soil [11]. Treatment and control plants were set in random orientation in the perpendicular blocks, and each had three parallel runs. Treatments lacking ZnO-NP foliar application formed the control.
The experimental field was treated with conventional moderate deep ploughing by a YMM 20 tractor after harvesting potatoes from the previous season. Rabbit and poultry manure fertilizer was then added, and the lentils were sown manually in lines with 25 mm sowing depth, 25 mm seeding distance and 20 mm inter-row spacing. The plots were finally pressed by roller [11].
The ZnO-NP solution was mixed with adjuvant SILWET STAR® (Chemtura Manufacturing UK Limited, Manchester, UK) to final 1 mg·L−1 NP concentration before direct foliar application. This eased nanoparticle penetration through the plant leaf wax sub-structure, and the adjuvant was applied without ZnO-NPs in the control experiment. The ZnO-NPs solution was applied twice during the growth season as recommended by Meier [38]. This occurred on the 45th and 60th day of the vegetation period when lentils achieved their ideal phenological growth stage (Figure 2). The solution was dispersed on the plant on a windless early morning by GAMMA5 pressure sprayer until the leaves were completely wet (GAMMA 5, Mythos Di Martino, Mussolente, Italy). The standard treatment included weed removal twice during the growing season by hand weeder. One liter of spray liquid was applied for each experiment replication.
Figure 2.
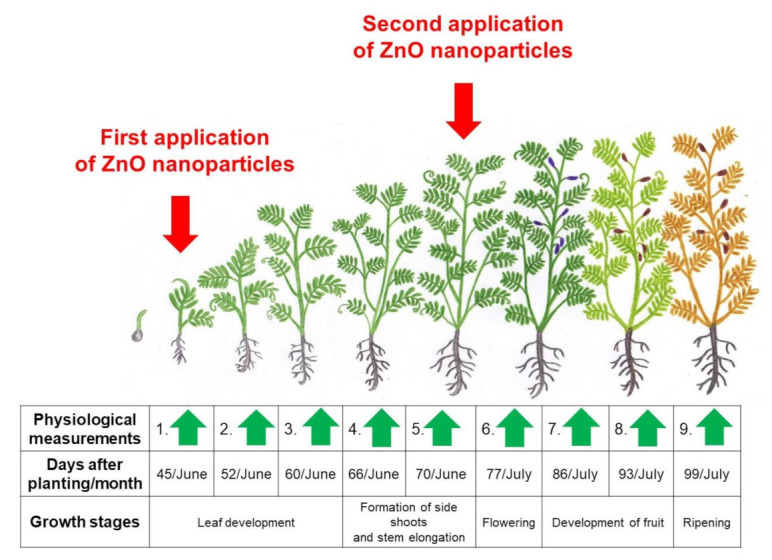
Schematic model of lentil growth stages. The red arrow shows the foliar application of 1 mg·L−1 ZnO-NPs, and the green arrows indicate the physiological measured time-intervals to assess lentil development.
2.5. Analysis of Quantitative Parameters; Water Stress, Stomatal Conductance, Plant Temperature and Temperature Difference
The lentil plants in each treatment were harvested manually when seeds attained physiological maturity. Approximately 10% of the plants began to change to yellow color at that time, and the lower-level pods turned to yellow-brown and brown [39]. The number of plants and number of pods per plants were calculated manually. The plant height was determined in millimeters by Texi 4007 laboratory equipment (Texi GmbH, Berlin, Germany). The seed yield was then determined in grams on the KERN PC3500-2 laboratory scale (Kern & Sohn, Balingen, Germany), and the thousand-seed weight was analyzed by NUMIREX equipment (MEZOS, Hradec Králové, Czech Republic).
Non-intrusive measurement of plant temperature (°C), temperature difference (°C), crop water stress index (CWSI) and stomatal conductance index (Ig) were performed from 11:00 a.m. to 1:00 p.m. periodically from the 46th to 100th day of the growth season. Ten measurements were made diagonally on each leaf of ten similar plants in each replication, as in Jones et al. [40].
The temperature difference is calculated from atmospheric and plant temperatures (Equation (1)). The plant is under stress when the temperature difference has zero or positive value, and it has greater environmental stress resistance when the value is negative. The lower plant temperatures are due to intensive transpiration.
| Tdif = Tleaf − Tair | (1) |
The CWSI calculation was performed as in Jones et al. [40] using EasIR-4 thermo-camera (Bibus AG, Fehraltorf, Switzerland). The measurements included leaf temperature parameters (Tleaf), dry (Tdry) and wet (Twet) leaf surface and atmospheric moisture (Equation (2)).
| (2) |
The thermal lentil measurements were recorded from 2 m distance and 150 cm height, with the auto-focused field at 20.6 × 15.5 [12]. This provided the stomatal conductance index calculation in Equation (3).
| (3) |
2.6. Seed Mineral Composition Analysis
The final lentil seed mineral concentration was analyzed after digestion of 0.15–0.30 g seed samples. This was performed by the Anton Paar Multiwave 3000 microwave digestion system (Graz, Austria) in PTFE pressure vessels using the concentrated HNO3 and H2O2 mixture at 60 bar pressure. Iron, zinc, magnesium, calcium and phosphor content was determined by ICP-OES (Varian Vista MPX, Mulgrave, Victoria, Australia) with yttrium as the internal standard. Potassium, copper and manganese were then provided by F-AAS (Perkin-Elmer Model 1100, Waltham, MA, USA) [41]. Finally, the Kjeldahl method determined total nitrogen, and colorimetry established sulfur content.
2.7. Soil X-ray Diffraction Analysis and Verification of Zinc Oxide Nanoparticles
X-ray powder diffraction analyzed (XRD) the soil mineral composition. This was accomplished by Philips, Netherlands PW 1710 diffractometer with Cu-anode and graphite monochromator at 40 kV voltage and 20 mA electric current.
X-ray diffraction by a Bruker D8 DISCOVER diffractometer (Bruker, MA, USA) verified zinc oxide nanoparticles. The measurement conditions were 12 kW, 40 kV and 300 mA with a Cu-anode similar to that in Kolenčík et al. [11].
2.8. Scanning Electron Microscopy of ZnO-NPs
The NP-suspension was diluted and transferred to a copper mesh with a carbon/formvar membrane. The ZnO-NP grid was then placed in a holder and microscope chamber after drying. The ZnO-NPs morphology and size were observed in transmission mode under the JEOL JSM 7610F+ scanning electron microscope with Schottky cathode (SEM—JEOL, Tokyo, Japan). Dark- and bright-field observation at 30 keV accelerating voltage provided comparison.
2.9. Statistical Analysis
Statistical analysis was performed by STATISTICA software (StatSoft, Tulsa, OK, USA). The normality of experimental data was then assessed by Shapiro–Wilks test, and statistically significant differences between treatments were achieved using the Student’s two-sample t-test and ANOVA followed by Fisher’s least-significant difference.
3. Results and Discussion
3.1. Colloidal Properties of ZnO-NPs Foliar Application
The applied ZnO-NP physical–chemical properties have previously been reported [11]. Herein, we determined the wurtzite-type ZnO-NP crystal structure (Figure 3b). The mean diameter of individual particles was 17.3 ± 0.1 nm [11] with spherical, hexagonal, columnar, rod-like and cuboidal shape (Figure 3a).
Figure 3.
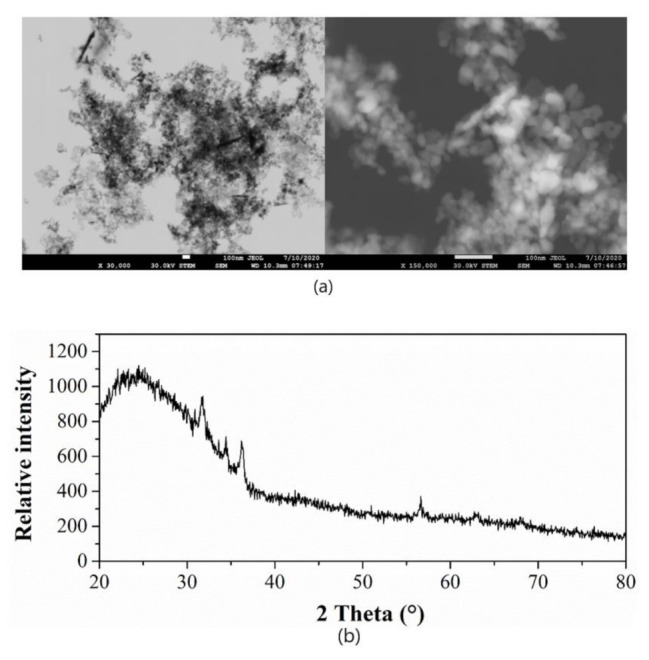
(a) Scanning transmission electron microscopy (STEM) visualized the zinc oxide nanoparticles (ZnO-NPs) used for foliar application to the lentils. The left STEM micrograph is bright-field, and dark-field is on the right, and (b) X-ray diffraction analysis shows the zinc oxide nanoparticles have wurtzite-structural symmetry.
The detected particle size has a profound effect on ZnO-NP availability. Although NPs usually enter plants through the stomata or cuticle, cuticular access is size-restricted to particles with diameter under 5 nm, and therefore ZnO-NPs have only stomatal transport available [42]. Transport velocity is also partly limited. However, ZnO-NP exposure to sunlight can initiate photo-corrosion, which is usually associated with transformation to more bioavailable Zn2+ species. These ions are complexed to organic acids and transported to plant tissues through cuticle access [43].
The individual NP size range is less than the 282.5 nm hydrodynamic diameter determined in the solution prepared for foliar application (Table 2).
Table 2.
ZnO-NP colloidal properties in foliar application.
| Hydrodynamic Diameter (nm) |
Zeta-Potential (mV) |
Electrophoretic Mobility (cm2·V−1·s−1) |
Conductivity (mS·cm−1) |
|---|---|---|---|
| 282.5 ± 6.9 | −33.3 ± 0.8 | −25.7·10−5 ± 0.8·10−5 | 0.070 ± 0.002 |
Hu et al. [44] recorded that smaller inorganic NPs with higher zeta-potential can penetrate the leaf’s lipid bilayers more efficiently for greater effect on the plant. Bhattacharjee [45] also reported that zeta-potentials below −30 mV indicate NP colloidal instability in solution. Therefore, our determined zeta-potential of −33.3 mV in Table 2 should prevent ZnO-NP aggregation and sedimentation.
3.2. ZnO-NP Effect on Lentil Quantitative Parameters
Zinc is required by plants for carbohydrate metabolism and gene expression in response to environmental stress [46], and ZnO-NPs are applied as nano-fertilizers and agrochemicals to enhance crop production [20,47,48]. Liu and Lal [49] recorded that the advantage of nano-domains is their gradual release of beneficial nutrients, and this encourages the most effective plant growth and development. Photocatalytic effects similar to those reported in TiO2-NP use induce photosynthesis during that slow release [50,51]. Singh et al. [52] noted that ZnO-NPs’ positive effect on plant growth is achieved in laboratory conditions at NP concentrations as high as 60 mg·L−1, and Prasad et al. [47] proposed even higher effective concentrations.
Statistically significant differences for quantitative parameters were observed when 1 mg·L−1 suspension of ZnO-NPs was applied. These included the number of pods per plant, seed yield and thousand-seed weight. This contrasted with our NP-free control, and there was also no statistical difference observed in the number of plants or their height in the NP-suspension experiment (Table 3). Our results are supported by the foliar application of 20 mg·L−1 ZnO-NPs to Abelmoschus esculentus which increased its pod number and improved plant growth and yield [53].
Table 3.
Comparison of the quantitative and physiological parameters of ZnO-NP-treated lentils and the NP-free control recorded in the 2020 vegetation season. The values state the means and standard deviation, and these were compared by Fisher’s least-significant difference (significance: * p-value < 0.05; ** p-value < 0.001.).
| ZnO-NPs Foliar Applied Variant | Control Variant (Without ZnO-NPs Application) | |
|---|---|---|
| Quantitative parameters | ||
| Number of plants (pcs) | 110 ± 3 | 109.3 ± 5 |
| Height of plants (mm) | 515 ± 42 | 535 ± 35 |
| Number of pods per plant (pcs) | 49 ± 5 * | 35 ± 6 * |
| Weight of thousand seeds | 17.4 ± 1.0 * | 14.2 ± 1.0 * |
| Seed Yield (g) | 35.5 ± 5.1 * | 21.5 ± 4.3 * |
| Physiological parameters | ||
| Temperature of plants (°C) | 22.57 ± 0.4 ** | 24.04 ± 0.2 ** |
| Temperature difference (°C) | −1.51 ± 0.05 ** | −0.04 ± 0.03 ** |
| Stomatal conductance index (Ig) | 1.9 ± 0.1 ** | 0.8 ± 0.1 ** |
| Crop water stress index (CWSI) | 0.4 ± 0.1 * | 0.7 ± 0.2 * |
3.3. Soil Nutrient Source, Bioavailability and Nutritional Quality of Harvested Lentil Seeds
The quality of lentil seeds can be determined by various parameters [54], including the essential and beneficial nutrient content [8,55]. Unfortunately, there is little knowledge of the nano-material effect on mineral nutrient uptake and overall crop nutritional quality [55].
The experimental area has not been exposed to widespread or long-term application of chemical fertilizers. Therefore, soil release of macro, micro and trace nutrients to lentil plants is partly governed by their spontaneous release from the naturally occurring soil minerals (Figure 4). The dominant soil minerals determined at the experimental field were quartz (SiO2), muscovite [KAl2(Si3Al)O10(OH,F)2], calcite (CaCO3), dolomite (MgCO3), and chlorite [(X,Y)4−6(Si,Al)4O10(OH,O)8, where the position of “X” and “Y” correspond to elements such as Fe2+, Fe3+, Ni2+, Zn2+, Al3+, Li+, or Ti+4], K-feldspar (KAlSi3O8), Na-feldspar (NaAlSi3O8) and Ca-feldspar (CaAl2Si2O8).
Figure 4.
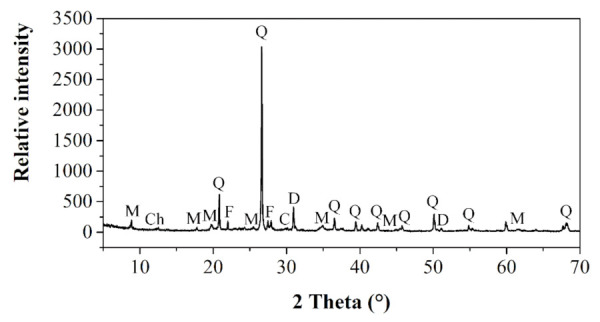
X-ray diffraction powder patterns of soil collected at the Hostie experimental locality at Zlaté Moravce in Slovakia; dominant quartz (Q), muscovite (M), chlorite (Ch), calcite (C), dolomite (D) and feldspar (F) minerals.
Table 4 lists the plant micronutrients available from the extractable fraction of the experiment soil. All determined bioavailable Cu, Fe and Mn values correspond to results reported by Bloem et al. [56]. Zinc, however, is one exception because its higher content most likely originated from residues deposited in the soil after ZnO-NP foliar application.
Table 4.
Content of soil-extractable micronutrients expressed as mg·kg−1 soil.
| Elements | Extractable Micronutrients (mg·kg−1 Soil) |
|---|---|
| Zinc | 15 ± 1.1 |
| Manganese | 27.5 ± 1.4 |
| Copper | 2.28 ± 0.1 |
| Iron | 24.7 ± 1.1 |
Several studies have evaluated NP effects on lentil production. These include biosynthesized Au-NPs [57], SiO2-NPs [58], TiO2-NPs [59], ZnO-NPs [60] and Ag-NPs [61]. The studies, however, do not consider the changes in content of nutrients such as minerals which affect final lentil seed quality. Our foliar application of low ZnO-NPs concentration did not significantly affect the lentil concentration of some essential and beneficial nutrients, including Zn (Table 5). The Zn concentration accumulated in the seeds is not statistically different in the ZnO-NP-treated plants and the control. In addition, the Zn concentration indicated in Table 5 is similar to that noted by Grusak [62]. Although it appears that foliar ZnO-NP application did not affect lentil seed Zn content, it may have influenced other physiological parameters such as photosynthesis [63], because zinc can be a fundamental component in photosynthetic processes [13]. Erdal et al. [64] noted that Zn fertilization led to decreased phosphor content, and it was further reported that the ZnO-NPs interrupted iron uptake and encouraged nitrogen assimilation [55]. In contrast, the nitrogen concentration in both variants corresponded to the typical nitrogen content in lentil seeds [8]. This was despite our results indicating that the nitrogen content in lentil seeds was lower when the plants were treated with ZnO-NPs. This ZnO-NP exposure resulted in decreased iron content in the lentil seeds, and although lentil is usually high in iron [65], both treated and control plants had lentil seed concentrations significantly below the typical average value [8]. This was most likely due to internal factors in our experiment plant variety [29]. Finally, there were no statistically significant differences in phosphor content as a seed anti-nutrient in ZnO-NP-treated plants and the control. The values corresponded with the reported average [62].
Table 5.
ZnO-NP foliar application effects on g·kg−1 content of selected essential and beneficial nutrients in the harvested lentil seeds.
| ZnO-NPs-Treated Plants | Plants That Were Not Exposed to ZnO-NPs | |
|---|---|---|
| Zinc | 0.05 ± 0.003 | 0.05 ± 0.03 |
| Phosphor | 5.8 ± 0.3 | 6.1 ± 0.3 |
| Nitrogen | 42.7 ± 2.1 | 47.1 ± 2.4 |
| Potassium | 11.2 ± 0.6 | 10.8 ± 0.5 |
| Sulfur | 3.13 ± 0.32 | 3.38 ± 0.29 |
| Copper | 0.013 ± 0.005 | 0.013 ± 0.005 |
| Iron | 0.05 ± 0.002 | 0.06 ± 0.001 |
| Manganese | 0.009 ± 0.001 | 0.012 ± 0.002 |
| Calcium | 0.52 ± 0.03 | 0.58 ± 0.03 |
| Magnesium | 1.01 ± 0.05 | 1.04 ± 0.05 |
A similar trend was observed in potassium, calcium, sulfur, manganese, copper and magnesium. The seed contents did not deviate from expected values [8,62], and was unaffected by ZnO-NP treatment. There were no statistically significant differences in the analyzed seed nutrient contents of treated and untreated lentil plants, but all average content values except potassium were slightly higher in the control. This may have been due to improved stress response or dilution effect because the ZnO-NP biomass yield was higher. This effect has been noted by other authors [11,66,67].
3.4. Stress Response to ZnO-NPs Foliar Application of Lentil
We recorded statistically significant differences in stomatal conductance and transpiration efficiency in ZnO-NP-treated plants and the control (Table 3). Stomatal conductance was enhanced despite the applied low ZnO-NP concentration. Although Faizan et al. [63] applied a 50-fold higher ZnO-NP concentration, they recorded a similar response. Statistically significant difference in crop water stress index (CWSI) was also noted between the ZnO-NP-treated plants and the control (Table 3).
The higher control CWSI indicated that ZnO-NP application eased the general effect of environmental stress on the lentil plant, including its response to water-deficiency and higher air-temperatures [68,69]. Statistically significant ZnO-NP difference was also confirmed for plant temperature and temperature difference compared to the untreated controls (Table 3). Moreover, all physiological indices show that ZnO-NP foliar application affected photosynthesis. This is supported by the increased leaf chlorophyll content in wheat [70] and okra [71].
There were no statistically significant differences between ZnO-NP-treated plants and the controls during leaf development or prior to the first ZnO-NP application. However, statistically significant differences were detected in the selected physiological indicators following NP exposure and in the following lentil growth stages (Figure 5a–d).
Figure 5.
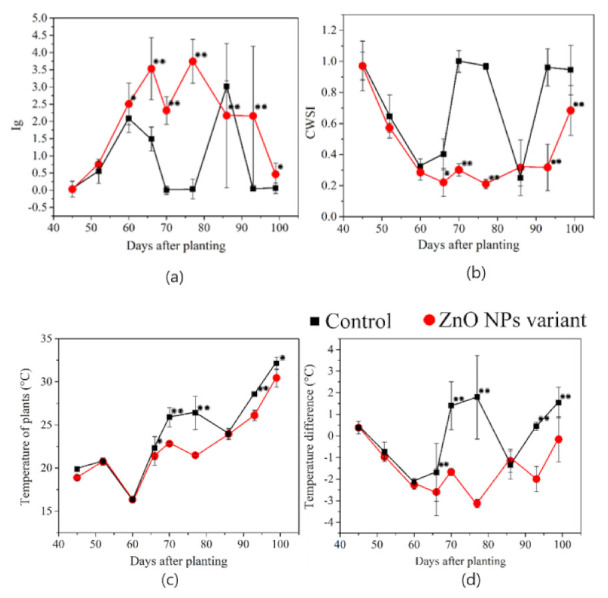
Seasonal effects of ZnO-NP foliar application on (a) stomatal conductance index (Ig), (b) crop water stress index (CWSI), (c) plant temperature (Tp) and (d) temperature difference (Td) compared to untreated controls (significance: * p-value < 0.05, ** p-value < 0.001).
The positive lentil response to NPs was apparent for all physiological indices within three weeks of ZnO-NP treatment. A similar response was confirmed in our previous study, where the common sunflower was exposed to low ZnO-NP foliar concentration [12]. The improved stress response was maintained after the second foliar application, and this highlighted increased lentil yield and thousand-seed weight (Table 3).
The stress incurred was due to altered environmental conditions, and NP treatment resulted in increased plant resistance. In addition, Salama et al. [72]. noted increased production and quality in this dry bean following exposure to 10–40 mg·L−1 ZnO-NPs.
Figure 5 shows the seasonal dynamics, and our results of the average daily air temperature and calculated CWSI, Ig, Tp and Td indices concur. Figure 5 statistically significant difference of p = 0.001 was recorded for all physiological indices when the average air temperature was above 19 °C (Figure 6a) and plants were exposed to 10 h minimum sunshine (Figure 6b). Lentil is sensitive to photo-thermal conditions and its response to temperature and photoperiod is expressly linear [6]. It is, therefore, highly likely that the improved stress response to NPs is enhanced when the photo-thermal conditions overcome the critical 10 h photoperiodic lighting and corresponding temperature, and the lentil seed yield is then higher [6].
Figure 6.
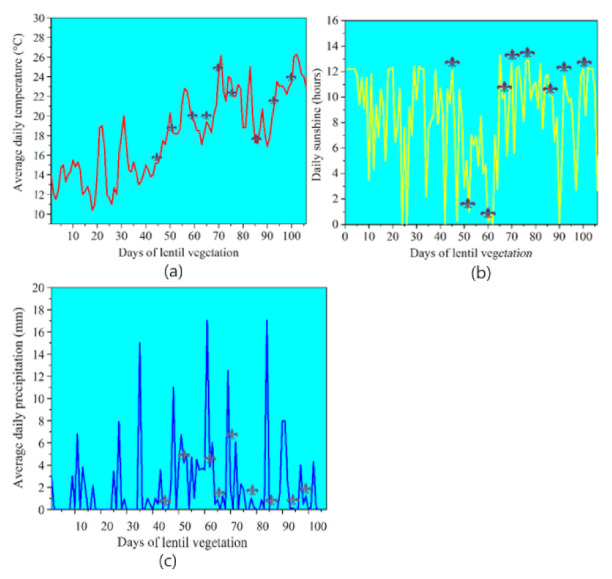
Average (a) daily air temperature, (b) daily sunshine, and (c) daily precipitation during the entire 106-day lentil vegetation season at the Hostie experimental locality at Zlaté Moravce in Slovakia. This period covered sowing on 20 April 2020 to harvesting on 3 August 2020. The inserted grey markers indicate the measurement day.
Rainfall precipitation also appears partly responsible for the observed effects. The statistically significant differences for almost all measured physiological indices were primarily noted when the average weekly precipitation was less than 18 mm (Figure 6c). This is important because weekly precipitation above 19 mm decreases average atmospheric temperature. Higher precipitation with subsequent slightly higher soil water content was confirmed on the 87th day when increased lentil transpiration was an observed trend. One stomatal conductance index was the only statistically significant difference at that time.
4. Conclusions
Our field experiment applied low 1 mg·L−1 ZnO-NP foliar spray dispersion to the Lens culinaris ´Beluga´ lentil. It provided positive ZnO-NP production responses, including increased lentil seed yield, thousand-seed weight and the number of pods per plant. This strongly contrasted with the untreated control. However, the Zn foliar supplementation did not affect its translocation to seeds regardless of Zn content, and therefore did not change the lentil seed nutrient quality.
Although there were no statistically significant differences in treated and untreated lentil seed nutrients, slightly higher average values were noted in the control, except for potassium. The ZnO-NP-treated plants had improved stress response within three weeks of NP exposure, and this included differences in stomatal conductance, crop water stress index, plant temperature and temperature difference.
In conclusion, our results justify the increasing trend of nano-fertilizer application in sustainable agriculture. The application of low NP concentrations has a significant impact on plant stress responses. This enhanced lentil seed quantitative properties. Finally, these procedures highlight the pro-environment character of the agricultural practices in our study, and the low ZnO-NP concentrations herein should not over-burden the increasing environmental nanoparticle input.
Author Contributions
M.K. (Marek Kolenčík) and D.E. investigation, writing—original draft preparation, supervision, proposed the topic; M.K. (Matej Komár) and D.E. performed the experiments; R.I., J.C., I,Č., Ľ.Ď., G.K. obtained formal analysis, figures, tables, and software—statistical implementations, and validation; H.F., Y.Q. writing—review and editing, and supervision; M.U. and M.Š. writing—review and editing, checked grammar, review and editing whole concept of manuscript; M.B. realized ICP-MS analysis with data evaluation; M.J. carried out soil analysis with data evaluation; K.Č.B. realized DSL analysis with data evaluation; G.K. performed SEM analysis; L.D. carried out F-AAS analysis; E.A. funding acquisition, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences (Vedecká grantová agentúra MŠVVaŠ SR a SAV) under the contracts No. VEGA 1/0747/20, and by the project from the Grant Agency of the Slovak University of Agriculture in Nitra No. 04-GASPU-2021.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Viadel B., Barberá R., Farré R. Uptake and retention of calcium, iron, and zinc from raw legumes and the effect of cooking on lentils in Caco-2 cells. Nutr. Res. 2006;26:591–596. doi: 10.1016/j.nutres.2006.09.016. [DOI] [Google Scholar]
- 2.Romano A., Gallo V., Ferranti P., Masi P. Lentil flour: Nutritional and technological properties, in vitro digestibility and perspectives for use in the food industry. Curr. Opin. Food Sci. 2021;40:157–167. doi: 10.1016/j.cofs.2021.04.003. [DOI] [Google Scholar]
- 3.Ramírez-Ojeda A.M., Moreno-Rojas R., Cámara-Martos F. Mineral and trace element content in legumes (lentils, chickpeas and beans): Bioaccesibility and probabilistic assessment of the dietary intake. J. Food Compos. Anal. 2018;73:17–28. doi: 10.1016/j.jfca.2018.07.007. [DOI] [Google Scholar]
- 4.Biju S., Fuentes S., Gupta D. Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol. Biochem. 2017;119:250–264. doi: 10.1016/j.plaphy.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Naser S., Mohsen J. Effect of nano-silicon particles application on salinity tolerance in early growth of some lentil genotypes. Ann. Univ. Mariae Curie-Skłodowska, C. Biol. 2015:39–55. [Google Scholar]
- 6.Summerfield R.J., Roberts E.H., Erskine W., Ellis R.H. Effects of Temperature and Photoperiod on Flowering in Lentils (Lens culinaris Medic.) Ann. Bot. 1985;56:659–671. doi: 10.1093/oxfordjournals.aob.a087055. [DOI] [Google Scholar]
- 7.Erbaş Köse Ö.D., Bozoğlu H., Mut Z. Quality Traits of Green Lentil (Lens culinaris Medik.) Cultivars and Lines. Yuz. Yil Univ. J. Agric. Sci. 2018;28:55–61. [Google Scholar]
- 8.Yadav S., McNeil D.L., Andrews M., Chen C., Brand J., Singh G., Shivakumar B., Gangaiah B. Soil nutrient management. In: Erskine W., Muehlbauer F.J., Sarker A., Sharma B., editors. The Lentil: Botany, Production and Uses. CAB International; Cambridge, MA, USA: 2009. pp. 194–212. [Google Scholar]
- 9.Pandey N., Pathak G.C., Sharma C.P. Zinc is critically required for pollen function and fertilisation in lentil. J. Trace Elem. Med. Biol. 2006;20:89–96. doi: 10.1016/j.jtemb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Obata H., Kawamura S., Senoo K., Tanaka A. Changes in the level of protein and activity of Cu/Zn-superoxide dismutase in zinc deficient rice plant, Oryza sativa L. Soil Sci. Plant Nutr. 1999;45:891–896. doi: 10.1080/00380768.1999.10414338. [DOI] [Google Scholar]
- 11.Kolenčík M., Ernst D., Komár M., Urík M., Šebesta M., Dobročka E., Černý I., Illa R., Kanike R., Qian Y., et al. Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica L.) under field conditions. Nanomaterials. 2019;9:1559. doi: 10.3390/nano9111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolenčík M., Ernst D., Urík M., Ďurišová Ľ., Bujdoš M., Šebesta M., Dobročka E., Kšiňan S., Illa R., Qian Y. Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials. 2020;10:1619. doi: 10.3390/nano10081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturikova H., Krystofova O., Huska D., Adam V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018;349:101–110. doi: 10.1016/j.jhazmat.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Salgueiro M.J., Zubillaga M., Lysionek A., Sarabia M.I., Caro R., De Paoli T., Hager A., Weill R., Boccio J. Zinc as an essential micronutrient: A review. Nutr. Res. 2000;20:737–755. doi: 10.1016/S0271-5317(00)00163-9. [DOI] [Google Scholar]
- 15.Alloway B.J. Zinc in Soils and Crop Nutrition. International Zinc Association and International Fertilizer Industry Association; Brussels, Belgium: 2008. [Google Scholar]
- 16.Kabata-Pendias A. Trace Elements in Soils and Plants. CRC press; Boca Raton, FL, USA: 2000. [Google Scholar]
- 17.Wang J., Moeen-ud-din M., Yang S. Dose-dependent responses of Arabidopsis thaliana to zinc are mediated by auxin homeostasis and transport. Environ. Exp. Bot. 2021;189:104554. doi: 10.1016/j.envexpbot.2021.104554. [DOI] [Google Scholar]
- 18.Torabian S., Zahedi M., Khoshgoftar A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 2016;39:172–180. doi: 10.1080/01904167.2015.1009107. [DOI] [Google Scholar]
- 19.Pérez Velasco E.A., Betancourt Galindo R., Valdez Aguilar L.A., González Fuentes J.A., Puente Urbina B.A., Lozano Morales S.A., Sánchez Valdés S. Effects of the morphology, surface modification and application methods of ZnO-NPs on the growth and biomass of tomato plants. Molecules. 2020;25:1282. doi: 10.3390/molecules25061282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabir S., Arshad M., Chaudhari S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014;2014:925494. doi: 10.1155/2014/925494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh M.D., Kumar B.A. Bio efficacy of nano zinc sulphide (ZnS) on growth and yield of sunflower (Helianthus annuus L.) and nutrient status in the soil. Int. J. Agric. Sci. 2017;9:3795–3798. [Google Scholar]
- 22.Kołodziejczak-Radzimska A., Jesionowski T. Zinc oxide-from synthesis to application: A review. Materials. 2014;7:2833–2881. doi: 10.3390/ma7042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehman S., Ullah R., Butt A.M., Gohar N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009;170:560–569. doi: 10.1016/j.jhazmat.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 24.Park S.J., Das G.S., Schütt F., Adelung R., Mishra Y.K., Tripathi K.M., Kim T. Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater. 2019;11:8. doi: 10.1038/s41427-019-0107-0. [DOI] [Google Scholar]
- 25.Mishra Y.K., Adelung R. ZnO tetrapod materials for functional applications. Mater. Today. 2018;21:631–651. doi: 10.1016/j.mattod.2017.11.003. [DOI] [Google Scholar]
- 26.Kim H.-H., Goins G.D., Wheeler R.M., Sager J.C. Stomatal conductance of lettuce grown under or exposed to different light qualities. Ann. Bot. 2004;94:691–697. doi: 10.1093/aob/mch192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarco-Tejada P.J., González-Dugo V., Williams L.E., Suárez L., Berni J.A.J., Goldhamer D., Fereres E. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band airborne imagery and the CWSI thermal index. Remote Sens. Environ. 2013;138:38–50. doi: 10.1016/j.rse.2013.07.024. [DOI] [Google Scholar]
- 28.Jackson R.D., Idso S.B., Reginato R.J., Pinter Jr P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981;17:1133–1138. doi: 10.1029/WR017i004p01133. [DOI] [Google Scholar]
- 29.Thavarajah D., Thavarajah P., See C.-T., Vandenberg A. Phytic acid and Fe and Zn concentration in lentil (Lens culinaris L.) seeds is influenced by temperature during seed filling period. Food Chem. 2010;122:254–259. doi: 10.1016/j.foodchem.2010.02.073. [DOI] [Google Scholar]
- 30.Rizwan M., Ali S., Ali B., Adrees M., Arshad M., Hussain A., Zia ur Rehman M., Waris A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 31.Xiang L., Zhao H.-M., Li Y.-W., Huang X.-P., Wu X.-L., Zhai T., Yuan Y., Cai Q.-Y., Mo C.-H. Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Environ. Sci. Pollut. Res. 2015;22:10452–10462. doi: 10.1007/s11356-015-4172-9. [DOI] [PubMed] [Google Scholar]
- 32.Paranavitana L., Oh W.Y., Yeo J., Shahidi F. Determination of soluble and insoluble-bound phenolic compounds in dehulled, whole, and hulls of green and black lentils using electrospray ionization (ESI)-MS/MS and their inhibition in DNA strand scission. Food Chem. 2021;361:130083. doi: 10.1016/j.foodchem.2021.130083. [DOI] [PubMed] [Google Scholar]
- 33.Chelladurai V., Erkinbaev C. Lentils. In: Manickavasagan A., Thirunathan P., editors. Pulses: Processing and Product Development. Springer International Publishing; Cham, Switzerland: 2020. pp. 129–143. [Google Scholar]
- 34.Lake L., Sadras V.O. Lentil yield and crop growth rate are coupled under stress but uncoupled under favourable conditions. Eur. J. Agron. 2021;126:126266. doi: 10.1016/j.eja.2021.126266. [DOI] [Google Scholar]
- 35.Nleya T., Vandenberg A., Walley F., Deneke D. Lentil: Agronomy. In: Colin W., editor. Encyclopedia of Grain Science. Elsevier; Oxford, England: 2016. pp. 150–157. [Google Scholar]
- 36.Lindsay W.L., Norvell W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978;42:421–428. doi: 10.2136/sssaj1978.03615995004200030009x. [DOI] [Google Scholar]
- 37.Hrivňáková K., Makovníková J., Barančíková G., Bezák P., Bezáková Z., Dodok R., Grečo V., Chlpík J., Kobza J., Lištjak M., et al. The Uniform Methods of Soil Analysis. VÚPOP Bratislava; Ružinov, Slovakia: 2011. p. 136. [Google Scholar]
- 38.Meier U. Growth Stages of Mono-And Dicotyledonous Plants. Blackwell Wissenschafts-Verlag; Kurfürstendamm, Germany: 1997. [Google Scholar]
- 39.Abraham R. Lentil (Lens culinaris Medikus) Current status and future prospect of production in Ethiopia. Adv. Plants Agric. Res. 2015;2:00040. [Google Scholar]
- 40.Jones H.G., Serraj R., Loveys B.R., Xiong L., Wheaton A., Price A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009;36:978–989. doi: 10.1071/FP09123. [DOI] [PubMed] [Google Scholar]
- 41.Losak T., Hlusek J., Martinec J., Jandak J., Szostkova M., Filipcik R., Manasek J., Prokes K., Peterka J., Varga L., et al. Nitrogen fertilization does not affect micronutrient uptake in grain maize (Zea mays L.) Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011;61:543–550. [Google Scholar]
- 42.Kolenčík M., Nemček L., Šebesta M., Urík M., Ernst D., Kratošová G., Konvičková Z. Effect of TiO2 as plant-growth stimulating nanomaterial on crop production. In: Singh V.P., Singh S., Prasad S.M., Chauhan D.K., Tripathi D.K., editors. Plant Responses to Nanomaterials, Recent Interventions, and Physiological and Biochemical Responses. 1st ed. Springer International Publishing; Cham, Switzerland: 2021. pp. 129–144. [Google Scholar]
- 43.Li C., Wang P., van der Ent A., Cheng M., Jiang H., Read T.L., Lombi E., Tang C., de Jonge M.D., Menzies N.W., et al. Absorption of foliar-applied Zn in sunflower (Helianthus annuus): Importance of the cuticle, stomata and trichomes. Ann. Bot. 2019;123:57–68. doi: 10.1093/aob/mcy135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu P., An J., Faulkner M.M., Wu H., Li Z., Tian X., Giraldo J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano. 2020;14:7970–7986. doi: 10.1021/acsnano.9b09178. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharjee S. DLS and zeta potential–What they are and what they are not? J. Control. Release. 2016;235:337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Baker S., Volova T., Prudnikova S.V., Satish S., Prasad M.N. Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environ. Toxicol. Pharmacol. 2017;53:10–17. doi: 10.1016/j.etap.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Prasad T.N.V.K.V., Sudhakar P., Sreenivasulu Y., Latha P., Munaswamy V., Reddy K.R., Sreeprasad T.S., Sajanlal P.R., Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012;35:905–927. doi: 10.1080/01904167.2012.663443. [DOI] [Google Scholar]
- 48.Prasad R., Bhattacharyya A., Nguyen Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R., Lal R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015;514:131–139. doi: 10.1016/j.scitotenv.2015.01.104. [DOI] [PubMed] [Google Scholar]
- 50.Gao F., Hong F., Liu C., Zheng L., Su M., Wu X., Yang F., Wu C., Yang P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol. Trace Elem. Res. 2006;111:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- 51.Kamal R., Mogazy A.M. Effect of doping on TiO2 nanoparticles characteristics: Studying of fertilizing effect on cowpea plant growth and yield. J. Soil Sci. Plant Nutr. 2021:1–13. [Google Scholar]
- 52.Singh J., Kumar S., Alok A., Upadhyay S.K., Rawat M., Tsang D.C.W., Bolan N., Kim K.-H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019;214:1061–1070. doi: 10.1016/j.jclepro.2019.01.018. [DOI] [Google Scholar]
- 53.Keerthana P., Vijayakumar S., Vidhya E., Punitha V.N., Nilavukkarasi M., Praseetha P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti -infection and growth promotion in plants. Ind. Crops Prod. 2021;170:113762. [Google Scholar]
- 54.Bishaw Z., Makkawi M., Niane A.A. Seed quality and alternative seed delivery systems. In: Erskine W., Muehlbauer F.J., Sarker A., Sharma B., editors. The Lentil: Botany, Production and Uses. CAB International; Cambridge, MA, USA: 2009. pp. 350–367. [Google Scholar]
- 55.Peralta-Videa J.R., Hernandez-Viezcas J.A., Zhao L., Diaz B.C., Ge Y., Priester J.H., Holden P.A., Gardea-Torresdey J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014;80:128–135. doi: 10.1016/j.plaphy.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 56.Bloem E., Haneklaus S., Haensch R., Schnug E. EDTA application on agricultural soils affects microelement uptake of plants. Sci. Total Environ. 2017;577:166–173. doi: 10.1016/j.scitotenv.2016.10.153. [DOI] [PubMed] [Google Scholar]
- 57.Abd El-Aziz A.R., Al-Othman M.R. Gold nanoparticles biosynthesis using zingiber officinale and their impact on the growth and chemical composition of lentil (Lens culinaris Medic.) Pak. J. Bot. 2019;51:443–450. doi: 10.30848/PJB2019-2(21). [DOI] [Google Scholar]
- 58.Janmohammadi M., Sabaghnia N., Ahadnezhad A. Impact of silicon dioxide nanoparticles on seedling early growth of lentil (Lens culinaris Medik.) genotypes with various origins. Poljopr. I Sumar. 2015;61:19. doi: 10.17707/AgricultForest.61.3.02. [DOI] [Google Scholar]
- 59.Feizi H., Agheli N., Sahabi H. Titanium dioxide nanoparticles alleviate cadmium toxicity in lentil (Lens culinaris Medic) seeds. Acta Agric. Slov. 2020;116:59–68. doi: 10.14720/aas.2020.116.1.1116. [DOI] [Google Scholar]
- 60.Siddiqui Z., Khan A., Khan M., Abd-Allah E. Effects of zinc oxide nanoparticles (ZnO NPs) and some plant pathogens on the growth and nodulation of lentil (Lens culinaris Medik.) Acta Phytopathol. Entomol. Hung. 2018;53:195–211. doi: 10.1556/038.53.2018.012. [DOI] [Google Scholar]
- 61.Hojjat S.S., Hojjat H. Effects of silver nanoparticle exposure on germination of Lentil (Lens culinaris Medik.) Int. J. Farm. Allied Sci. 2016;5:248–252. [Google Scholar]
- 62.Grusak M.A. Nutritional and health-beneficial quality. In: Erskine W., Muehlbauer F.J., Sarker A., Sharma B., editors. The Lentil: Botany, Production and Uses. CAB International; Cambridge, MA, USA: 2009. pp. 368–390. [Google Scholar]
- 63.Faizan M., Bhat J.A., Chen C., Alyemeni M.N., Wijaya L., Ahmad P., Yu F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021;161:122–130. doi: 10.1016/j.plaphy.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Erdal I., Kaya M., Küçükyumuk Z. Effects of Zinc and Nitrogen fertilizations on grain yield and some parameters effecting Zinc bioavailability in lentil seeds. Legume Res. 2014;37:55–61. doi: 10.5958/j.0976-0571.37.1.008. [DOI] [Google Scholar]
- 65.Sebastiá V., Barberá R., Farré R., Lagarda M.J. Effects of legume processing on calcium, iron and zinc contents and dialysabilities. J. Sci. Food Agric. 2001;81:1180–1185. doi: 10.1002/jsfa.927. [DOI] [Google Scholar]
- 66.Mitchell M., Pritchard J., Okada S., Larroque O., Yulia D., Pettolino F., Szydlowski N., Singh S., Liu Q., Ral J.P. Oil Accumulation in Transgenic Potato Tubers Alters Starch Quality and Nutritional Profile. Front. Plant Sci. 2017;8:554. doi: 10.3389/fpls.2017.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raigond P., Raigond B., Kaundal B., Singh B., Joshi A., Dutt S. Effect of zinc nanoparticles on antioxidative system of potato plants. J. Environ. Biol. 2017;38:435–439. doi: 10.22438/jeb/38/3/MS-209. [DOI] [Google Scholar]
- 68.Franks P., Brodribb T.J. Vascular Transport in Plants. Elsevier; Amsterdam, The Netherlands: 2005. Stomatal control and water transport in the xylem; pp. 69–89. [Google Scholar]
- 69.Kirkham M. Stress-degree-day concept and crop water stress index. In: Kirkham M., editor. Principles of Soil and Plant Water Relations. Academic Press; Cambridge, MA, USA: 2014. pp. 437–453. [Google Scholar]
- 70.Adrees M., Khan Z.S., Hafeez M., Rizwan M., Hussain K., Asrar M., Alyemeni M.N., Wijaya L., Ali S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021;208:111627. doi: 10.1016/j.ecoenv.2020.111627. [DOI] [PubMed] [Google Scholar]
- 71.Alabdallah N.M., Alzahrani H.S. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020;27:3132–3137. doi: 10.1016/j.sjbs.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salama D.M., Osman S.A., Abd El-Aziz M.E., Abd Elwahed M.S.A., Shaaban E.A. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris) Biocatal. Agric. Biotechnol. 2019;18:101083. doi: 10.1016/j.bcab.2019.101083. [DOI] [Google Scholar]


