Abstract
Alhagi camelorum (AC) is an old plant with a significant therapeutic value throughout Africa, Asia, and Latin America. The overuse of cisplatin (Cis > 50 mg/m2) is associated with observed nephrotoxicity, ototoxicity, gastrotoxicity, myelosuppression, and allergic reactions. Remedial measures are needed for the protection of nephrotoxicity against cisplatin. Thus, we investigated the nephroprotective effects of AC plant extract to prevent cisplatin-induced nephrotoxicity in albino Wistar rats. The presence of polyphenols, phenolic compounds, tannins, and saponins was revealed during phytochemical investigation, and a significantly intense antioxidant activity was recorded. There were no toxicological symptoms in the treated rats, and no anatomical, physiological, or histological abnormalities were found compared to the control rats. The results of correcting cisplatin-induced nephrotoxicity revealed that the extract has a significant ability to treat kidney damage, with most parameters returning to normal after only three weeks of therapy. It is concluded that co-administration of cisplatin with AC extract showed exceptional nephroprotective effects at a dose of 600 mg/kg for Cis-induced nephrotoxicity.
Keywords: cisplatin, nephrotoxicity, reactive oxygen species, Alhagi camelorum
1. Introduction
According to emerging evidence, nephrotoxicity is one of the most persistent kidney problems with an 8–15% lifetime risk in Europe, 2–5% in Asia, and 20% in the Middle East [1]. Nephrotoxicity leads to a reduction in the glomerular filtration rate and an increase in creatinine and blood urea nitrogen in the serum, ultimately increasing the blood pressure and fluid retention in the body (over-hydration) [1,2]. Kidneys are the primary target organ to bear toxic effects of medication. Kidneys account for 25% of the heat output and are naturally exposed to circulatory drugs and chemicals as central excretion bodies. These nephrotoxic drugs contribute to acute kidney failure and increased morbidity and death [3,4]. Because of their functions in glomerular concentrations, drug delivery, and metabolism, the epithelial cells of the renal proximal convoluted tubules (PCT) are a crucial target for nephrotoxicants [5]. Nephrotoxic agents usually damage the renal tubular epithelial cells either by reacting indirectly (through metabolites) or directly with membrane components and cellular macromolecules [6,7].
Cisplatin (Cis) is the most commonly used potential chemotherapeutic agent against different solid tumors, including those in the head, neck, lung, breast, bladder, and ovary. Besides its multiple advantages, Cis is responsible for inducing several side effects, including ototoxicity, gastrotoxicity, myelosuppression, and allergic reactions [8,9]. According to emerging evidence, the main toxic effect of Cis is the dose-limiting nephrotoxicity that is responsible for mortality and morbidity [10,11]. Several previous studies indicated the nephrotoxicity of Cis at a single dose (50–100 mg/m2) [9,12]. Nephrotoxicity caused by Cis occurs mainly in renal PCT [13].
Nephrotoxicity is the most common adverse effect of Cis accumulation in kidneys after chemotherapy [14]. The Cis disturbs the equilibrium between antioxidants and peroxides, while renal fibrosis is closely related to a rise in oxidative damage [15,16]. The Cis-complex moves through the cell membranes in a unionized form due to its high chloride concentration in the plasma. Cl-plasma is higher than the intracellular concentration, and chloride ligands are displaced by water, resulting in a nephrotoxic formation of the positive platinum complexes. The Cis molecule binds to the guanine DNA base and inhibits DNA, RNA, and protein synthesis. Cis binds to the DNA interface, and an intrastrand is established, leading to a faulty genetic code model and the arrest of the formation and duplication of DNA replication [17,18].
During the past few decades, natural compounds have been considered among the promising therapeutic agents against cancer, cardiovascular diseases, aging, diabetes, and especially neurodegenerative disorders due to their wide variety of modes of action, efficiency, accuracy, and fewer side effects [19,20]. Several studies have focused currently on traditional herbal medicines to evaluate novel therapeutic drugs for acute kidney injury (AKI) therapy. Various herbal medicines, including pomegranate (Lythraceae), Prosthechea michoacana (Orchidaceae), Zingiber officinale (Zingiberaceae), and red ginseng (family Araliaceae), have protective effects against cisplatin-induced acute kidney injury with vivo experiments [21,22,23].
Alhagi camelorum (AC) is a traditional herb that belongs to the family Leguminosae [24]. AC is used to treat metabolic, digestive, and hepatic problems, autoimmune diseases, headaches, and infections [25]. AC treats stomach problems of animals, heartworm, and pyrexia [21,22,23]. The herb is regarded as a laxative, diuretic, purgative, and antipyretic [26]. The major phytonutrients in AC include proteins, glycosides, coumarins, flavonoids, phenolics, resin, saponins, steroids, terpenes, ascorbic acid, essential oils, salicylic acid, ascorbic acid, and gallic acid [27]. This drove us to explore the nephroprotective effect of AC against chemotherapeutics such as cisplatin.
However, to the best of our knowledge, the in vivo toxicological effect and the nephroprotective effects of AC plant extract have not been identified. The current nephroprotective study serves as a necessary basis for further studies developing herbal medicine from this plant.
2. Materials and Methods
2.1. Chemicals
The analytical-grade chemicals included cisplatin (Mylan S.A.S, CHATILLON SUR CHALARONNE, AUVERGNE RHONE ALPES, France), 2, 2-Diphenyl, 1-picrylhydrazle, formalin, ketamine, and xylazine, which were purchased from Prix Lab Lahore, Pakistan. Ethanol (99.2% pure), picric acid (99.5% pure), NaOH, and trichloroacetic acid (TCA; 97% pure) were obtained from Sigma-Aldrich, St. Louis, MO, USA. The chemicals were mixed with other chemicals and with distilled water depended on the parameters and their protocols.
2.2. Preparation of the Plant Extract
AC was collected from the agricultural fields of Head Muhammad Wala, Multan, Pakistan. The plant was authenticated by expert taxonomists in the Department of Botany, Bahauddin Zakariya University, Multan, Pakistan, with the voucher number (R.R. Stewart F.W. Pak.711/12) for further reference. AC plants were washed, dried (under a shield), and powdered with the help of a herbal blender. AC powder (1200 g) was soaked in a hydroalcoholic solvent (70:30 v/v) in an air-tight amber-colored bottle for nine days. A rotary evaporator (Heidolph Laborota 4000 efficient, Hamburg, Germany) was used to evaporate the filtrate at reduced pressure [28,29]. The obtained semi-solid residue was refrigerated before further analysis.
2.3. Animals
Albino Wistar male rats weighing 260–290 g were collected from the animal house of the Department of Life Sciences, Muhammad Institute of Medical and Allied Sciences, Multan, and kept in polycarbonate cages that were covered by raw dust that was changed every three days under standard laboratory conditions (27 ± 2 °C) in the Pharmacology Research Laboratory. The rats were given water and standard diet pellets ad libitum. All experiments performed were approved by the Animal Ethical Committee of the Muhammad Institute of Medical and Allied science, Multan, Pakistan [30], in accordance with the guidelines of the national research council [31].
2.4. In Vivo Experiments
A single dose of Cis (5 mg/kg) was injected into the rats to induce nephrotoxicity [32]. Rats were typically divided into four different groups of six animals each. Group-I was given normal saline by oral gavage for 21 days and used as a control. Group-II received Cis 5 mg/kg (i.p) on the first day and received saline for 21 days by oral gavage. Group-III was given Cis + AC extract (400 mg/kg; cisplatin was given on the first day and then the extract was given for 21 days by oral gavage), while Group-IV received Cis + AC extract (600 mg/kg; cisplatin was given on the first day and then the extract was given for 21 days by oral gavage). The plant extract was freshly suspended in distilled water before administration with the aid of Tween 80. The rational choice of dose and treatment time was based on a previous study on different plants [30].
Daily food and water consumption was regularly measured with the body weights of the rats. The body weight was measured before the experiment, while kidney weight was measured by sacrificing the rats. The urine was collected on days 0, 7th, 14th, and 21st from all experimental groups of rats by placing each rat on a plastic dish for sodium (Na), potassium (K), and creatinine level analysis [33]. The rats were kept for 24 h in metabolic cages with tap water, and we measured the total intake of water and the amount of urine. The collected urine samples were stored at −30 °C for the estimation of creatinine, Na, and K levels. Similarly, the blood samples of all experimental groups were collected on days 0, 7th, 14th, and 21st in EDTA tubes and centrifuged at 2300 rpm to collect the plasma to estimate the creatinine, Na, and K levels.
2.5. Phytochemical Screening
Phytochemical screening of the secondary metabolites and active compounds present in the AC extract was done using the standard protocols [34].
2.6. HPLC Analysis
The standard USP and ICH guidelines were carried out for the HPLC analysis to estimate phenolic acids and polyphenolic compounds [35]. The wavelength used for polyphenol identification was 280 nm, while the temperature of the furnace column was adjusted to 35 °C. Ultimate 3000 liquid chromatography incorporating a 5 cm flow cell DAD and Chromeleon system management for HPLC analytics were used. The reversed-phase Acclaim C18 column (5-micron particle size, 250 mm/4.6 mm) was used to differentiate components. In total, 30 mg dry methanol and water extract were dissolved separately in 25 mm of the mobile phase solvent. The 0.45 μm membrane filter filtered the sample solution before injection (methanol: 0.5% acetate acid in water: 1.9) in the HPLC system. High-performance liquid chromatography analysis was conducted using the methanol phase containing the mobile solvent (Solvent A) and acetic acid solutions (Solvent B), with 105 min for each sample. The HPLC spectrum library recorded and stored each standard. With respect to the identification criteria for Alhagi camelorum, chemicals were determined when the retention time and spectrum of unknown compounds were compared to the HPLC standard library. In the extracts, phenolic acids and flavonoids were measured by applying the calibration graph by drawing spikes against the relevant standard control sample. The data are reported as the standard ± error means for three independent assessments.
2.7. Acute Oral Toxicity Dose Test
The acute oral toxicity of AC was evaluated in 12 rats. Rats were divided into six groups; each group contained four rats. Rats were fasted for 24 h and dosed in the following manner: 500, 1000, 1500, 2000, 2500, 3000 mg/kg body weight. After the dosing, the rats were observed for 14 days for lethargy, jerkiness, and death [36].
2.8. DPPH Assay
To assess the antioxidant activity of the crude extract, photo-colorimetric methods were used to determine the free radical DPPH (2,2-Diphenyl-1-picrylhydrazyle) [37]. Samples of the ethanolic plant extract were diluted to 500, 1000, 1500, 2000, 2500, and 3000 ppm; 1 mL of each sample was added to the DPPH solution and prepared up to 5 mL with methanol, and then incubated for 40 min. Mixtures were kept at 25 °C, and the measurements were carried out using a spectrophotometer at 517 nm (spectrophotometer UV-340 G, Gehaka Santa Clara, CA, USA), and ascorbic acid was used as a standard. The tests were performed three times for all samples, and the mean value was calculated.
2.9. Biochemical Analyses
Blood samples were screened to determine various biochemical parameters.
2.10. Measurement of Plasma and Urine Sodium and Potassium Levels
For the screening of electrolytes or an acid–base imbalance, the electrolyte profile was used to check the effects of any treatment. Electrolytes include sodium, potassium, chloride, and bicarbonates for the diagnosis of any condition or disease [38]. Sodium and potassium concentrations in plasma and urine were measured by using a flame photometer (Sherwood Model 410, Nottingham, UK).
The samples were diluted (1:200) for the measurement of sodium in urine and plasma samples, and for the measurement of potassium in plasma samples, the same dilution was used, but for the potassium levels in urine, the dilution was 1:1000. All samples were measured in triplicate.
2.11. Urine Flow Rate
The urine flow rate is the amount of urine excreted per unit time and it was calculated using the following formula in µL/min/100 g of BW:
| Urine output (mL/24 h) × 1000 × 100 |
| Urine flow rate (µL/min/100 g of BW) = 1440 × Wt. of animal (grams) |
2.12. Measurement of Plasma and Urine Creatinine Levels
Creatinine is a catabolic product of creatine phosphate in muscles, and depending upon the muscle mass, it is usually produced at a fairly constant rate in the body [39].
Plasma and urinary creatinine concentrations were measured spectrophotometrically (Jaffe’s reaction) by a method described before [40].
Urinary samples were diluted up to 50 times with distilled water. Both plasma and urine samples were deproteinized using trichloroacetic acid (1.2 M/L) along with centrifugation, and the supernatant was used for the measurement. The principle of this assay is based on the reaction between creatinine in the sample and picric acid in an alkaline medium to form a colored complex. This complex can be detected by a spectrophotometer at 520 nm wavelength. The complex formation should be measured in a short period after preparation to avoid interference. The preparation of the assay component is shown below in the table. The total volume of the sample, blank, and standard was transferred to a 96-well microtiter plate and incubated for 20 min at room temperature. Following the incubation period, the absorbance of the mixture was measured using a microplate reader (Synergy HT BioTek ® Pittsburgh, PA, USA). All samples were analyzed in duplicate, and the concentration of creatinine in plasma and urine was calculated using the following formula:
| Abs. of sample—Abs. of blank × Conc. of standard |
| Plasma conc. (mg/dL) = Abs. of standard − Abs. of blank |
| Abs. of sample—Abs. of blank × Conc. of standard × 50 |
| Urine conc. (mg/dL) = Abs. of standard—Abs. of blank |
Abs = Absorbance.
2.13. Histopathological Analysis
The histopathological analysis of the kidney was performed using Olympus light microscopy (×400) with an Olympus digital camera. Kidneys preserved in formalin were dehydrated, cleared in xylene, and placed in paraffin, and the kidney was cut into 5 µm sections and stained with periodic acid Schiff and hematoxylin. First, specific sections (5 µm each) of the kidneys were rehydrated and desalinized with alcohol. Next, these sections were treated with a particular amount of endogenous peroxidases for 0.5 h at 37 °C and were rinsed three times in phosphate buffer saline (PBS) at pH 7.00. After rinsing, the kidney sections were heated with 0.01 M sodium citrate buffer at pH 6.00 for 25 min and incubated for 1 h with 1% BSA. After incubation, the sections were condensed with hematoxylin, dehydrated with alcohol, and cleaned with xylene [37,38].
2.14. Statistical Analysis
One-way analysis of variance (ANOVA) and Bonferroni’s post hoc analysis were performed for all data (mean ± SEM (n = 5)) to determine the methodological significance between different experimental groups. Statistically, significance was considered at p < 0.05 [36].
3. Results
3.1. Phytochemical Analysis and Antioxidant Activity
The phytochemical examination of the ethanol plant extract showed that flavonoids, phenolic compounds, tannins, and saponins were present, whereas triterpenoids, steroids, and anthocyanins were not detercted. The results of different antioxidant assays showed that the concentration-dependent DPPH radical scavenging activity of the ethanol extract of A. camelorum was highest at 3000 mg/mL (96.07%), whereas the lowest (26.07%) was measured at 500 μg/mL. The highest percentage decrease was measured at 3000 μg/mL with respect to the power reduction of the plant extract (108.9 percent). At a lower concentration of 500 μg/mL, the lowest percentage reduction power (60.1 percent) was measured. The nitric oxide activity of A. camelorum showed that increasing concentrations of the extract markedly increased the nitric oxide scavenging. At 3000 μg/mL of the plant extract, the highest activity was observed. In hydrogen peroxide scavenging, when the plant extract concentration increased, the activity of scavenging increased. The highest activity at 3000 μg/mL and the lowest activity at 500 μg/mL were recorded. The SOD test results showed a higher percentage of inhibition of the plant extract at 3000 μg/mL (79.8%) and a lower percentage at the dose of 500 μg/mL (21.0%). Table 1 shows the phytoconstituents of the ethanol extract, while Table 2 summarizes the antioxidant activity of various assays.
Table 1.
Phytoconstituents in the ethanol extract of the A. camelorum plant.
| Serial No | Test | Ethanolic Extract |
|---|---|---|
| 1 | Flavonoids | +++ |
| 2 | Phenols | +++ |
| 3 | Tannins | +++ |
| 4 | Saponins | +++ |
| 5 | Triterpenoids | - |
| 6 | Steroids | - |
| 7 | Anthocyanins | - |
+++ = Highly present, - = absent.
Table 2.
Outcomes of the antioxidant activity of the A. camelorum plant extract using the disparate test.
| Concentration (μg/mL) | Inhibition of the Extract | Inhibition of the Standard | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | Reducing Power | NO | H2O2 | SOD | DPPH | Reducing Power | NO | H2O2 | SOD | |
| 500 | 26.07 | 60.1 | 29 | 26 | 21.0 | 31.26 | 62.4 | 31.7 | 28 | 20.2 |
| 1000 | 46.60 | 71.6 | 39.4 | 36 | 31.68 | 51.47 | 71.7 | 43.3 | 40 | 33.7 |
| 1500 | 61.89 | 87.05 | 51.3 | 51 | 48.18 | 64.53 | 84.8 | 54.6 | 52.6 | 44.08 |
| 2000 | 79.64 | 91.3 | 62 | 68 | 59.3 | 83.49 | 91.6 | 66.8 | 70 | 64.10 |
| 2500 | 88.24 | 96.9 | 71.6 | 94 | 66.2 | 88.97 | 95.8 | 75.5 | 102 | 71.87 |
| 3000 | 96.07 | 108.9 | 75.9 | 128 | 79.86 | 96.93 | 105.8 | 79.8 | 131.6 | 86.03 |
DPPH (2,2-Diphenyl-1-picrylhydrazyl), NO (Nitric oxide), SOD (superoxide dismutase), H2O2 (Hydrogen peroxide).
3.2. HPLC Analysis
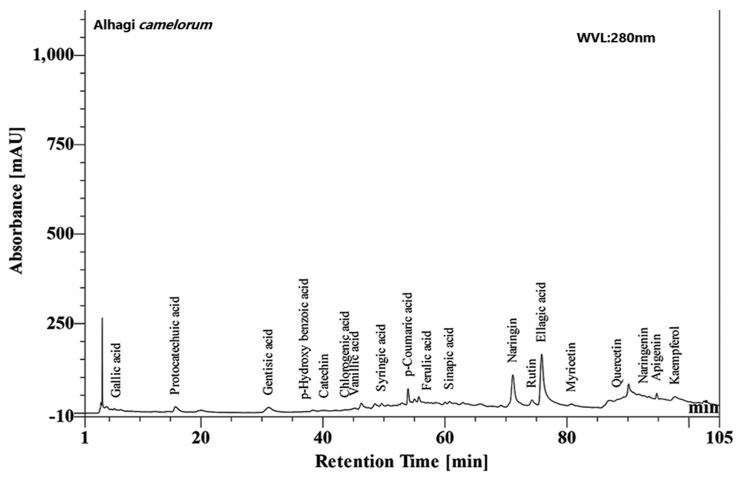
The HPLC analysis revealed many phytoconstituents in varying concentrations at a 280 nm wavelength, as shown in (Figure 1).
Figure 1.
HPLC chromatogram of the hydroalcoholic plant extract of Alhagi camelorum showing gallic acid, rutin, etc.
3.3. Acute Oral Toxicity Dose Test
The current study was performed in compliance with OECD. Guideline 423 found that the maximum dose was preserved at 3000 mg/kg. The acute oral toxicity of the extract was evaluated in 24 rats. They were divided into six groups, and each group contained four rats that were fasted for 24 h and then dosed in the following manner: 500, 1000, 1500, 2000, 2500, and 3000 mg/kg body weight. After the dosing, the rats were observed for 14 days for lethargy, jerkiness, and death
Relative Organ Weight
Table 3 shows the average relative organ weights for rats treated with the maximal dose of the plant extract (6000 mg/kg). The relative weights of the kidneys, liver, heart, lungs, stomach, and spleen were not considerably different between the control and the treatment group.
Table 3.
The impact of the A. camelorum plant extraction the weight of organs of the rats.
| Treatment | Control Group | Treatment Group |
|---|---|---|
| Stomach | 0.93 ± 0.10 | 0.92 ± 0.28 |
| Heart | 0.76 ± 0.13 | 0.72 ± 0.48 |
| Liver | 5.34 ± 0.76 | 6.26 ± 0.76 |
| Kidney | 3.4 ± 0.32 | 3.5 ± 0.35 |
| Spleen | 1.47 ± 0.35 | 1.65 ± 0.39 |
| Lung | 1.06 ± 0.49 | 1.93 ± 0.29 |
Organ/body weight (%). Mean ± SEM values (n = 5).
3.4. Effect of the A. camelorum Plant Extract on the Body Weight and Plasma K of Cis-Treated Rats
Nephrotoxicity is caused by a unilateral dose of cisplatin (5 mg/kg i.p) that was biochemically shown by increases (p ≤ 0.05) in body weight, urine output, urinary sodium, urinary potassium, plasma creatinine, and kidney weight. In contrast, the cisplatin caused a decrease (p ≤ 0.05) in plasma Na and K and urinary creatinine. Table 4 shows the body and plasma potassium levels between the control and cisplatin treatment and after the experiment with Ac extracts on days 0, 7, 14, and 21 after dose administration. Compared with the control, there was a decrease in body weight after cisplatin treatment on days 7, 14, and 21. Treatment with Ac protected against a loss in body weight. It was observed that the body weight increased up to the 14th day and then started to decrease, and maximum B.W. and plasma potassium were measured after 14th days of observation with both plant extracts. It was also observed that higher dose administration of the extract increased the B.W. and plasma potassium levels of rats compared to a lower dose. However, the decrease in B.W. and plasma potassium indicated the presence of nephrotoxicity.
Table 4.
Effect of crude extracts of Ac on B.W & plasma potassium of cisplatin-treated rats.
| Body Weight (g) | Observation (Day) | |||
|---|---|---|---|---|
| Groups | 0 | 7 | 14 | 21 |
| A. camelorum (Ac) | ||||
| Control | 264 ± 9.7 | 286 ± 10.4 | 348 ± 10.2 | 326 ± 10.7 |
| Cis | 268 ± 9.3 | 206 ± 10.1 (*) | 209 ± 11 (*) | 184 ± 10.6 (*) |
| Cis + Aca | 287 ± 10.6 | 230 ± 9.3 (&) | 267 ± 10.8 (&) | 250 ± 12 (&) |
| Cis + Acb | 289 ± 10.8 | 240 ± 7.7 (#) | 304 ± 10.6 (#) | 289 ± 10.3 (#) |
| Plasma potassium (mEq/L) | ||||
| Control | 8.3 ± 0.58 | 7.9 ± 0.69 | 8.5 ± 0.58 | 8.3 ± 0.48 |
| Cis | 8.0 ± 0.32 | 3.1 ± 0.44 (*) | 4.6 ± 0.45 (*) | 4.6± 0.52 (*) |
| Cis + Aca | 8.0 ± 0.66 | 5.5 ± 0.46 (&) | 5.8 ±0.48 (&) | 5.1 ± 0.35 (&) |
| Cis + Acb | 8.3 ± 0.47 | 6.7 ± 0.44 (#) | 6.3± 0.53 (#) | 7.2 ± 0.57 (#) |
Mean ± SEM (n = 6), where Cis is cisplatin (5 mg/kg i.p), Cis + Aca is cisplatin + Alhagi camelorum extract (400 mg/kg/21 days), Cis + Acb is cisplatin + Alhagi camelorum extract (600 mg/kg/21 days. The results are considered significant (*) if p < 0.005. * p < 0.05 vs. normal control, & p < 0.05 vs. cisplatin, and # p < 0.05 vs. cisplatin + Alhagi camelorum on corresponding days.
3.5. Effect of Ac Extracts on Plasma Creatinine and Plasma Sodium of Cis-Treated Rats
Table 5 presents the effect of Ac extracts on urinary creatinine and plasma sodium of cisplatin-treated rats. Results were observed after 0, 7, 15, and 21 days of dose administration. In addition, at 600 mg/kg, co-administration of Cis + AC affected the urinary creatinine and plasma sodium after the 21st day of observation. Overall, cisplatin treatment resulted in increased plasma creatinine and decreased plasma sodium; however, the co-administration of Cis + Aca and Cis + Acb significantly reduced the plasma creatinine and increased plasma sodium at rates of 400 and 600 mg/kg. However, it was concluded that Cis + Aca and Cis + Acb at 600 mg/kg showed better results than those observed at 400 mg/kg.
Table 5.
Effect of crude extracts of Ac. on urinary creatinine and plasma Na of cisplatin-treated rats.
| Plasma Sodium (mEq/L) | Observation (Day) | |||
|---|---|---|---|---|
| Groups | 0 | 7 | 14 | 21 |
| Ac | ||||
| Control | 163 ± 8.4 | 201 ± 8.3 | 202 ± 6.7 | 163 ± 8.7 |
| Cis | 172 ± 7.3 | 108± 10.4 (*) | 135 ± 8 (*) | 127± 9.2 (*) |
| Cis + Aca | 168 ± 7.2 | 147 ± 8.3 (&) | 154 ± 7.6 (&) | 148 ± 9.6 (&) |
| Cis + Acb | 173 ± 6.6 | 174 ± 7.9 (#) | 179 ± 7.0 (#) | 168 ± 8.8 (#) |
| Plasma creatinine (mg/dL) | ||||
| Control | 2.4 ± 0.47 | 2.4 ± 0.47 | 2.6 ± 0.41 | 2.6 ± 0.56 |
| Cis | 2.5 ± 0.40 | 6.7 ± 0.30 (*) | 7.7 ± 0.47 (*) | 6.0 ± 0.59 (*) |
| Cis + Aca | 2.6 ± 0.41 | 5.7 ± 0.28 (&) | 6.4 ± 0.58 (&) | 6.4 ± 0.52 (&) |
| Cis + Acb | 2.6 ± 0.56 | 4.9 + 0.28 (#) | 5.2 ± 0.30 (#) | 5.2 ± 0.45 (#) |
Mean ± SEM (n = 6), where Cis is cisplatin (5 mg/kg i.p), Cis + Aca is cisplatin + Alhagi camelorum extract (400 mg/kg/21 days), Cis + Acb is cisplatin + Alhagi camelorum extract (600 mg/kg/21 days. The results are considered significant (*) if p < 0.005. * p < 0.05 vs. normal control, & p < 0.05 vs. cisplatin, and # p < 0.05 vs. cisplatin + Alhagi camelorum on corresponding days.
3.6. Effect of Ac on Urine Output and Urinary Na in Cis-Treated Rats
Table 6 shows the effect of the Ac plant extract on urine output and urinary sodium (Na) in Cis-treated rats after 0, 7, 14, and 21 days of dose administration. Overall, cisplatin-treated rats showed a reduction in urine output and urinary Na; however, co-administration of Cis + Aca and Cis + Acb significantly increased urine output and urinary Na at a dose of 400 and 600 mg/kg. However, it was concluded that Cis + Aca and Cis + Acb at the 600 mg/kg rate showed better results than those observed in response to 400 mg/kg.
Table 6.
Effect of crude extract of Ac on urine output and urinary Na of cisplatin-treated rats.
| Urine Output (mL) | Observation (Day) | |||
|---|---|---|---|---|
| Groups | 0 | 7 | 14 | 21 |
| Ac | ||||
| Control | 9.6 ± 4.0 | 9.4 ± 4.0 | 9.9 ± 3.0 | 9.0 ± 2.6 |
| Cis | 9.4 ± 4.0 | 38± 4.6 (*) | 35 ± 6.4 (*) | 40 ± 2.9 (*) |
| Cis + Aca | 10.4 ± 3.1 | 30 ± 4.0 (&) | 29 ± 10.7 (&) | 35 ± 2.6 (&) |
| Cis +Acb | 9.6 ± 3.0 | 21 ± 3.8 (#) | 25 ± 3.9 (#) | 29 ± 1.3 (#) |
| Urinary sodium (mEq/L) | ||||
| Control | 197 ± 6.4 | 195 ± 10.6 | 201 ± 9.7 | 196 ± 10.8 |
| Cis | 194 ± 6.9 | 320 ± 26 (*) | 323 ± 26 (*) | 372 ± 21 (*) |
| Cis + Aca | 197 ± 10.4 | 286 ± 10.6 (&) | 292 ±10.0 (&) | 310 ± 26 (&) |
| Cis + Acb | 188 ± 8.6 | 243 ± 10.7 (#) | 260 ± 11.0 (#) | 270 ± 10 (#) |
Mean ± SEM (n = 6), where Cis is cisplatin (5 mg/kg i.p), Cis + Aca is cisplatin + Alhagi camelorum extract (400 mg/kg/21 days), Cis + Acb is cisplatin + Alhagi camelorum extract (600 mg/kg/21 days. The results are considered significant (*) if p < 0.005. * p < 0.05 vs. normal control, & p < 0.05 vs. cisplatin, and # p < 0.05 vs. cisplatin + Alhagi camelorum on corresponding days.
3.7. Effect of Ac on Urinary K and Urine Flow Rate in Cis-Treated Rats
To further analyze the effect of the Ac extract, the level of urinary potassium (K) and urine flow rate were determined in each group of Cis-treated rats. Table 7 shows the results of the effect of the Ac extract on urinary K. The Cis-treated group showed a significant increase in urinary K from the first day to 21 days of observation compared to the control. Results revealed that the 400 and 600 mg/kg co-administration of Cis + Ac significantly reduced urinary K after 7 and 21 days of observation compared to the Cis group.
Table 7.
Effect of crude extracts of Ac on urinary K and urine flow rate of cisplatin-treated rats.
| Urinary Potassium (mEq/24 h) | Observation (Day) | |||
|---|---|---|---|---|
| Groups | 0 | 7 | 14 | 21 |
| Alhagi camelorum (Ac) | ||||
| Control | 6.3 ± 0.59 | 5.5 ± 0.49 | 5.8 ± 0.59 | 4.0 ± 0.43 |
| Cis | 6.8 ± 0.60 | 6.8 ± 0.43 (*) | 6.9 ± 0.53 (*) | 7.6 ± 0.58 (*) |
| Cis + Aca | 6.9 ± 0.40 | 6.6 ± 0.44 (&) | 5.9 ± 0.48 (&) | 4.0 ± 0.40 (&) |
| Cis +Acb | 6.8 ± 0.76 | 2.9 ± 0.44 (#) | 4.7 ± 0.35 (#) | 5.3 ± 0.57 (#) |
| Urine flow rate (µL/min/100 g of B.W.) | ||||
| Control | 4.5 ± 0.78 | 4.3 ± 0.67 | 3.9 ± 0.52 | 3.4 ± 0.65 |
| Cis | 4.4 ± 0.65 | 26± 3.6 (*) | 26 ± 3.4 (*) | 28 ± 3.4 (*) |
| Cis + Aca | 4.5 ± 0.67 | 9.8 ± 0.85 (&) | 9.0 ± 0.72 (&) | 8.7 ± 0.78 (&) |
| Cis +Acb | 3.3 ± 0.76 | 7.6 ± 0.97 (#) | 6.2 ± 0.66 (#) | 6.9 ± 0.59 (#) |
Mean ± SEM (n = 6), where Cis is cisplatin (5 mg/kg i.p), Cis + Aca is cisplatin + A. camelorum extract (400 mg/kg/21 days), Cis + Acb is cisplatin + Alhagi camelorum extract (600 mg/kg/21 days. The results are considered significant (*) if p < 0.005. * p < 0.05 vs. normal control, & p < 0.05 vs. cisplatin, and # p < 0.05 vs. cisplatin + Alhagi camelorum on corresponding days.
3.8. Effect of Ac on Urinary Creatinine and the Urinary Na/K Ratio in Cis-Treated Rats
Table 8 shows the nephroprotective effects of Ac on urinary creatinine and the urinary Na/K ratio in Cis-treated rats. The cis-treated rats showed a decline in urinary creatinine and an increase in the urinary Na/K ratio. It was observed that Cis + Ac co-administration at the rate of 600 mg/kg significantly increased urinary creatinine and decreased the urinary Na/K ratio level after 7, 14, and 21 days compared to cisplatin-treated rats; 600 mg/kg of Cis + Ac/significantly increased urinary creatinine and decreased the urinary Na/K ratio compared to the Cis group and the 400 mg/kg concentration.
Table 8.
Effect of crude extracts of Ac. on urinary creatinine and the urinary Na/K ratio of cisplatin-treated rats.
| Urinary Creatinine(mg/dL) | Observation (Day) | |||
|---|---|---|---|---|
| Groups | 0 | 7 | 14 | 21 |
| Alhagi camelorum (Ac) | ||||
| Control | 6.8 ± 0.57 | 7.4 ± 0.43 | 8.8 ± 0.72 | 8.2 ± 0.72 |
| Cis | 6.8 ± 0.57 | 4.2 ± 0.47 (*) | 4.3 ± 0.44 (*) | 3.6 ± 0.44 (*) |
| Cis + Aca | 7.0 ± 0.59 | 5.2 ± 0.45 (&) | 5.8 ±0.47 (&) | 5.5 ± 0.46 (&) |
| Cis + Acb | 6.7 ± 0.46 | 6.3 ± 0.46 (#) | 6.3 ± 0.56 (#) | 7.0 ± 0.39 (#) |
| Urinary Na/K ratio | ||||
| Control | 5.5 ± 0.70 | 4.8 ± 0.70 | 5.5 ± 0.69 | 6.1 ± 0.68 |
| Cis | 7.5 ± 0.60 | 38 ± 3.7 (*) | 43 ± 0.97 (*) | 44 ± 6 (*) |
| Cis + Aca | 8.4 ± 0.90 | 26 ± 3.8 (&) | 10.7 ± 0.95 (&) | 24 ± 3.6 (&) |
| Cis +Acb | 6.3 ± 0.66 | 8.0 ± 0.86 (#) | 6.0 ± 0.67 (#) | 10 ± 1.3 (#) |
Mean ± SEM (n = 6), where Cis is cisplatin (5 mg/kg i.p), Cis + Aca is cisplatin + Alhagi camelorum extract (400 mg/kg/21 days), Cis + Acb is cisplatin + Alhagi camelorum extract (600 mg/kg/21 days. The results are considered significant (*) if p < 0.005. * p < 0.05 vs. normal control, & p < 0.05 vs. cisplatin, and # p < 0.05 vs. cisplatin + Alhagi camelorum on corresponding days.
3.9. Impact of Ac Extracts on Kidney Weight in Cis-Treated Rats
Table 9 shows the Ac extract nephroprotective results on kidney weight in every group. Compared to normal regulation, the kidney weight of animals regarded as Cis was very high. Co-administration of the extract and Cis at varying concentrations led to a reduction in kidney weight.
Table 9.
Effect of crude extracts of Ac on kidney weight in Cis-treated rats.
| Groups | Kidney Weight (g) |
|---|---|
| Control | 0.65 ± 0.058 |
| Cis | 2.7± 0.035 (*) |
| Cis + Aca | 2.6 ± 0.0068 (&) |
| Cis +Acb | 0.98 ± 0.0066 (#) |
Statistical analysis was carried out using one-way analysis of variance (ANOVA) in all groups on respective days followed by Bonferroni’s posthoc test. The results are considered significant (*) if p < 0.005. * p < 0.05 vs. normal control, & p < 0.05 vs. cisplatin, and # p < 0.05 vs. cisplatin + Alhagi camelorum.
3.10. Histopathological Effects of Ac Extract in Cis-Treated Rats
The histopathological effects of AC were examined by H&E staining for histopathological abnormalities. Results showed that the normal kidney showed uniform tubules and normal glomeruli covered with an epithelial layer and showed no blockage, bleeding or interfacial injury. In the Cis group, distorted histology with atrophied glomerulus and collecting tubules with necrosis were observed. Several degenerative changes were also observed in the Cis group in the form of atrophic lining with tubular and eosinophilic casts in cytoplasmic vacuolization of cells. In addition, glomerular hypertrophy was also noted in Cis-treated rats. However, Cis + AC co-administration at the 400 mg/kg rate showed normal glomeruli with mild histopathological results of damage in tubules. On the other hand, Cis + AC co-administration at 600 mg/kg rate significantly reduced the histopathological abnormalities induced by Cis. Minor renal damage was found in the proximal and distal tubules compared to Cis + AC-treated groups as shown in Figure 2.
Figure 2.
Photomicrograph of kidney sections of rats in the (a) control, (b) Cis, (c) Cis + AC (400 mg/kg), and (d) Cis + AC (600 mg/kg) groups. BC (Bowman’s capsule), RT (renal tubules), DC (distal convoluted tubule), NC (necrosis).
4. Discussion
Cisplatin is a commonly used platinum-containing antineoplastic drug used to treat solid tumors, including those in the breast, lung, head, and neck [41,42]. Despite its multiple advantages in cancer treatment, its uses are limited due to nephrotoxicity [43,44]. With increasing use of Cis, drug-based nephrotoxicity has been increasing day by day and causes almost 26% of acute kidney injuries (AKI) [45]. The emerging evidence suggested that a single dose of Cis up to 50 mg/m2 induces side effects in the kidney; however, an estimation across 40% of patients that received Cis higher than this limit suffered from acute or mild renal dysfunction [46,47]. It was observed that Cis-induced nephrotoxicity led to renal vasculature that alters renal hemodynamics [48].
Cis is modified due to intracellular hydration to form a reactive metabolite and alters the expression of many water channels and membrane transporters to inhibit the function of mitochondria, ultimately blocking ATP production and leading to nitrosative and oxidative stress [49]. These pharmacological effects lead to the reabsorption and uncoupling of water that precedes the excretion of electrolytes, including magnesium (Mg), sodium (Na), calcium (Ca), potassium (K), and calcium (Ca). Moreover, Cis attacks different organelles and interfaces in DNA replication, altering several biological mechanisms, including necrosis, apoptosis, inflammation, and tubular derangement [40,47]. Mechanisms through which Cis causes nephrotoxicity are complex and involve different biological pathways such as oxidative stress, apoptosis, and inflammation [48]. ROS production is increased by Cis in mitochondria; NADPH oxidase and the cellular xanthine oxidase system are involved in the pathogenesis of Cis-induced severe kidney failure [17]. The function of various renal antioxidant enzymes, including catalase (CAT), glutathione (GHx), and superoxide dismutase (SOD), is also reduced by Cis [49]. However, based on the side effects of Cis, there is a need to develop a drug to reduce the pathophysiology of Cis. Nowadays, a mixture of different chemicals and natural products are used as potential Cis-neuroreceptors to interfere with the nephrotoxicity of Cis [50].
In the present study, the synergistic effects of Cis with AC plant extracts were screened at 400 and 600 mg/kg. They showed a significant effect against Cis-induced nephrotoxicity, but the mechanism of action is not fully understood and may involve reducing inflammation, oxidative stress, or apoptosis. The results of the present study revealed that Cis significantly reduced the bodyweight of rats by increasing the kidney weight. On the other hand, the co-administration of Cis + AC at 400 and 600 mg/kg significantly (p < 0.001) increased the body weight and reduced the kidney weight (p < 0.005). The weight loss in the Cis group was strongly related to insufficient nutrition, an increase in metabolic processes, metabolic imbalances, or mental conflict in the Cis-treatment community [51]. In addition, Cis induced tubular necrosis through increased kidney weight in groups treated with Cis due to ischemia or proliferation [49]. In animals treated with Cis + AC (600 mg/kg), there was a substantial reduction in kidney weight similar to a previous study [52]. Similar results were observed by Singh et al. [53], who revealed that co-administration of Cis (30 mg/kg) + morin hydrate (40 mg/kg) significantly reduced the Cis-treated rat kidney weight as compared to the Cis group. Similarly, Sahu et al. [54,55] reported that supplementation of Cis + bai at the 50 mg/kg rate significantly decreased the relative kidney weight and increased the body weight compared to the Cis group. They also observed a significant reduction in the plasma creatinine level to almost equal to that in the control.
The present study showed that co-administration of Cis + AC extract at 400 and 600 mg/kg significantly (p < 0.001) improved renal function (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9); 400 and 600 mg/kg supplementation of Cis + Ac successfully (p < 0.005) increased the urinary sodium (Na), and potassium (K) level up to the control compared to the Cis-group that decreased the plasma Na and K levels after a single dose at 5 mg/kg Cis. In comparison, 400 and 600 mg/kg co-administration of Cis + AC improved (p < 0.005) the plasma creatinine, Na, and K levels that were reduced after intake of Cis. The weakening of membrane pumps such as Na-K is due to the nephrotoxicity caused by Cis. It leads to a decrease in salt reabsorption and hence increases the urine level [55,56,57]. In the present study, the Cis-group displayed hypernatriuria and hyperkaliuria. Co-administration of Cis + AC (400 and 600 mg/kg) allowed sodium and potassium levels to decrease to near normal values relative to the Cis groups (Table 6 and Table 7).
The results indicated that the AC extract has high nephroprotection. The best outcomes were noted after the 7th day of the experiment compared to the 14th and 21st days of observation. Our results are in agreement with the findings of Chtourou et al. [58]. They revealed that co-administration of Cis + Naringin100 significantly reduced the serum creatinine level up to 0.47 ± 0.02 mg/dL in rats compared to the Cis- group that showed a serum level of 0.97 ± 0.02 mg/dL after 5 mg/kg administration. Similarly, they observed that co-administration of Cis + Naringin (100 mg/kg) increased the urine creatinine level (6.27 ± 0.92 mg/dL) compared to the Cis group (4.05 ± 0.12 mg/dL). Fatima et al. [59] demonstrated that co-administration of Cis + A20 (EGCG + CoQ10) reduced the serum creatinine level (1.36 ± 0.30 mg/dL) compared to the Cis group (3.13 ± 0.25 mg/dL), with urine Na, K, Ca2+, and Mg2+ levels of 110 ± 2.56, 28 ± 3.01, 4.83 ± 0.05, and 27.4 ± 2.2 µmol/24 h, respectively.
Moreover, the histopathological analysis was performed to confirm the effect of both doses of AC on renal function. The results showed that after Cis (5 mg/kg) administration, the kidney exhibited glomerular hypertrophy, cytoplasmic vacuolization of cells, and atrophic lining with tubular and eosinophilic casts. However, after administration of Cis + AC at the rate of 400 and 600 mg/kg, a significant improvement was observed, indicating that Cis + AC effectively reduced the renal abnormalities associated with a single injection of Cis.
5. Conclusions
Based on our findings, it can be concluded that Alhagi camelorum seems to be safe and have high medicinal value. The ethanol extract showed the presence of flavonoids, phenols, tannins, and saponins and significantly high antioxidant activity. The treated rats did not show any anatomical, physiological or histopathological changes compared to the control. Kidney tissues appeared normal after the maximum dose of the extract with a possible alteration of distal tubules, proximal tubules, and glomerulus in the kidney tissue. The results of reversing Cis-induced nephrotoxicity suggest high potential for the extract for renal damage treatment, and most of the parameters retained to normal values after the administration of AC for only three weeks. The A. camelorum ethanol extract has great potential as an antioxidant and nephrotoxic therapeutic agent. Further studies are required to explore the exact molecular mechanism responsible for its nephroprotective effect, and LC-MS/MS spectrum analysis is recommended for further characterization.
Acknowledgments
We extend our gratitude to the laboratory staff and staff of the animal house of the Muhammad Institute of Medical and Allied Sciences, Multan, Pakistan, for their cooperation throughout the study. We are grateful to Shifa Clinical laboratory, Multan, for the research facilities.
Abbreviations
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| SOD | superoxide dismutase |
| NO | Nitric oxide |
| H2O2 | Hydrogen peroxide |
| Ac | Alhagi camelorum |
| CAT | catalase |
| SOD | Superoxide dismutase |
| GHx | Glutathione |
| EGCG | epigallocatechin gallate |
| CoQ10 | Coenzyme Q10 |
| Bai | Baicalein |
Author Contributions
Original draft, Supervision, Experimentation: M.O.I., M.M.A., I.A.K. Writing—original draft, Software, Editing. M.O.I., S.A. (Shafia Arshad), U.J., S.A. (Shumaila Andleeb), R.R., S.H.M., Z.M., M.M., A.M.; Formal analysis, Editing, & Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This is part of a Ph.D. thesis of the first author and received no funding from any funding agency.
Institutional Review Board Statement
The Ethical Board of Muhammad Institute of Medical and Allied Health Sciences approved the study.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yadav N., Sharma S., Sharma S., Sharma K. Critical Analysis of protective role of plants against gentamicin induced nephrotoxicity. Indian J. Environ. Sci. 2017;21:1–34. [Google Scholar]
- 2.Saylor C., Tamayo-Ortiz M., Pantic I., Amarasiriwardena C., McRae N., Estrada-Gutierrez G., Parra-Hernandez S., Tolentino M.C., Baccarelli A.A., Fadrowski J.J. Prenatal blood lead levels and reduced preadolescent glomerular filtration rate: Modification by body mass index. Environ. Int. 2021;154:106414. doi: 10.1016/j.envint.2021.106414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller T., Dewitz C., Schmitz J., Schröder A.S., Bräsen J.H., Stockwell B.R., Murphy J.M., Kunzendorf U., Krautwald S. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell. Mol. Life Sci. 2017;74:3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah S., Leonard A.C., Harrison K., Meganathan K., Christianson A.L., Thakar C.V. Mortality and recovery associated with kidney failure due to acute kidney injury. Clin. J. Am. Soc. Nephrol. 2020;15:995–1006. doi: 10.2215/CJN.11200919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi M., McMillan K.L., Wu J., Gillings N., Flores B., Moe O.W., Hu M.C. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab. Investig. 2018;98:1105–1121. doi: 10.1038/s41374-018-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal M.O., Sial A.S., Akhtar I., Naeem M., Hazafa A., Ansari R.A., Rizvi S.A. The nephroprotective effects of Daucus carota and Eclipta prostrata against cisplatin-induced nephrotoxicity in rats. Bioengineered. 2021;12:12702–12721. doi: 10.1080/21655979.2021.2009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo H., Deng N., Dou L., Ding H., Criswell T., Atala A., Furdui C.M., Zhang Y. 3-D Human Renal Tubular Organoids Generated from Urine-Derived Stem Cells for Nephrotoxicity Screening. ACS Biomater. Sci. Eng. 2020;6:6701–6709. doi: 10.1021/acsbiomaterials.0c01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manohar S., Leung N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 9.Casanova A.G., Hernández-Sánchez M.T., López-Hernández F.J., Martínez-Salgado C., Prieto M., Vicente-Vicente L., Morales A.I. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur. J. Clin. Pharmacol. 2020;76:23–33. doi: 10.1007/s00228-019-02771-5. [DOI] [PubMed] [Google Scholar]
- 10.Miller R.P., Tadagavadi R.K., Ramesh G., Reeves W.B. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H., Tang J., Guo D., Zhao Q., Wen J., Zhang Y., Obianom O.N., Zhou S., Zhang W., Shu Y. Cadmium exposure enhances organic cation transporter 2 trafficking to the kidney membrane and exacerbates cisplatin nephrotoxicity. Kidney Int. 2020;97:765–777. doi: 10.1016/j.kint.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phung H.M., Lee S., Hwang J.H., Kang K.S. Preventive Effect of Muscone against Cisplatin Nephrotoxicity in LLC-PK1 Cells. Biomolecules. 2020;10:1444. doi: 10.3390/biom10101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Tang C., Cai J., Chen G., Zhang D., Zhang Z., Dong Z. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018;9:1–14. doi: 10.1038/s41419-018-1152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farooqui Z., Ahmed F., Rizwan S., Shahid F., Khan A.A., Khan F. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed. Pharmacother. 2017;85:7–15. doi: 10.1016/j.biopha.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 15.Qi Z., Li W., Tan J., Wang C., Lin H., Zhou B., Liu J., Li P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine. 2019;61:152862. doi: 10.1016/j.phymed.2019.152862. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Daim M.M., Aleya L., El-Bialy B.E., Abushouk A.I., Alkahtani S., Alarifi S., Alkahtane A.A., AlBasher G., Ali D., Almeer R.S. The ameliorative effects of ceftriaxone and vitamin E against cisplatin-induced nephrotoxicity. Environ. Sci. Pollut. Res. 2019;26:15248–15254. doi: 10.1007/s11356-019-04801-2. [DOI] [PubMed] [Google Scholar]
- 17.Fang C.-Y., Lou D.-Y., Zhou L.-Q., Wang J.-C., Yang B., He Q.-J., Wang J.-J., Weng Q.-J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021;42:1951–1969. doi: 10.1038/s41401-021-00620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez R.M., Gomez Y.G., Ramirez E.B. Nephroprotective activity of Prosthechea michuacana against cisplatin-induced acute renal failure in rats. J. Med. Food. 2010;13:911–916. doi: 10.1089/jmf.2009.0175. [DOI] [PubMed] [Google Scholar]
- 19.Cayir K., Karadeniz A., Simsek N., Yildirim S., Karakus E., Kara A., Akkoyun H.T., Sengul E. Pomegranate seed extract attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in rats. J. Med. Food. 2011;14:1254–1262. doi: 10.1089/jmf.2010.0286. [DOI] [PubMed] [Google Scholar]
- 20.Sultana S., Verma K., Khan R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J. Pharm. Pharmacol. 2012;64:872–881. doi: 10.1111/j.2042-7158.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 21.Xiang X., Guo C., Tang C., Cai J., Dong Z. Epigenetic regulation in kidney toxicity: Insights from cisplatin nephrotoxicity. Semin. Nephrol. 2019;39:152–158. doi: 10.1016/j.semnephrol.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty S., Majumdar S. Natural products for the treatment of pain: Chemistry and pharmacology of Salvinorin A, Mitragynine, and Collybolide. Biochemistry. 2020;60:1381–1400. doi: 10.1021/acs.biochem.0c00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazafa A., Rehman K.-U., Jahan N., Jabeen Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer. 2020;72:386–397. doi: 10.1080/01635581.2019.1637006. [DOI] [PubMed] [Google Scholar]
- 24.Khan F.M. Ethno-veterinary medicinal usage of flora of Greater Cholistan desert (Pakistan) Pak. Vet. J. 2009;29:75–78. [Google Scholar]
- 25.Marwat S.K. Ethnophytomedicines for treatment of various diseases in DI Khan district. Sarhad J. Agric. 2008;24:305–315. [Google Scholar]
- 26.Ballabh B., Chaurasia O.P. Traditional medicinal plants of cold desert Ladakh—Used in treatment of cold, cough and fever. J. Ethnopharmacol. 2007;112:341–349. doi: 10.1016/j.jep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Donkor S., Larbie C., Komlaga G., Emikpe B.O. Evaluation of the Acute Hepatoprotective Potential of Hydroethanolic Extract of Duranta erecta L. Parts. J. Toxicol. 2020;2020:8815719. doi: 10.1155/2020/8815719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irshad M., Subhani M.A., Ali S., Hussain A. Essential Oils-Oils of Nature. IntechOpen; London, UK: 2020. Biological importance of essential oils. [Google Scholar]
- 29.Singh B., Koley T., Maurya A., Singh P., Singh B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. sativus Schubl. & Martens) Physiol. Mol. Biol. Plants. 2018;24:899–907. doi: 10.1007/s12298-018-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute of Health . Guide for the Care and Use of Laboratory Animals. NIH Publication; Bethesda, MD, USA: 1985. No. 85-23. [Google Scholar]
- 31.Chung I.-M., Rajakumar G., Lee J.-H., Kim S.-H., Thiruvengadam M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata. Appl. Microbiol. Biotechnol. 2017;101:5247–5257. doi: 10.1007/s00253-017-8363-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang R., Hassan W., Jabeen Q., Ahmed H., Iqbal O. Citrus aurantium ameliorates cisplatin-induced nephrotoxicity. BioMed Res. Int. 2019;2019:3960908. doi: 10.1155/2019/3960908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal M.O., Yahya E.B., Andleeb S., Ahmed M.M., Javaid M.U., Shakeel W., Iqbal I. In vivo assessment of reversing Cisplatin-Induced nephrotoxicity using Jatropha mollissima crude extract and its potential cytotoxicity. Saudi J. Biol. Sci. 2021;28:7373–7378. doi: 10.1016/j.sjbs.2021.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans W.C. Trease and Evans Pharmacognosy. Saunders; Edinburgh, UK: New York, NY, USA: 2009. [Google Scholar]
- 35.Seal T., Pillai B., Chaudhuri K. Effect of solvent extraction system on the antioxidant activity of some selected wild leafy vegetables of Meghalaya state in India. Int. J. Pharm. Sci. Res. 2013;4:1046–1051. [Google Scholar]
- 36.Kpemissi M., Metowogo K., Melila M., Veerapur V.P., Negru M., Taulescu M., Potârniche A.-V., Suhas D.S., Puneeth T.A., Vijayakumar S. Acute and subchronic oral toxicity assessments of Combretum micranthum (Combretaceae) in Wistar rats. Toxicol. Rep. 2020;7:162–168. doi: 10.1016/j.toxrep.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mensor L.L., Menezes F.S., Leitão G.G., Reis A.S., Santos T.C.D., Coube C.S., Leitão S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 38.Gowda S., Desai P.B., Kulkarni S.S., Hull V.V., Math A.A., Vernekar S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010;2:170. [PMC free article] [PubMed] [Google Scholar]
- 39.Jen J.F., Hsiao S.L., Liu K.H. Simultaneous determination of uric acid and creatinine in urine by an eco-friendly solvent-free high performance liquid chromatographic method. Talanta. 2002;58:711–717. doi: 10.1016/S0039-9140(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 40.Seeling H.P., Wust H. Colorimetric method for determination of creatinine. Arztl. Lab. 1969;15:34. [Google Scholar]
- 41.Hu S., Wang J., Wang J., Li S., Jiang W., Liu Y. Renoprotective effect of fucoidan from Acaudina molpadioides in streptozotocin/high fat diet-induced type 2 diabetic mice. J. Funct. Foods. 2017;31:123–130. doi: 10.1016/j.jff.2017.01.031. [DOI] [Google Scholar]
- 42.Izuwa Y., Kusaba J.-I., Horiuchi M., Aiba T., Kawasaki H., Kurosaki Y. Comparative study of increased plasma quinidine concentration in rats with glycerol-and cisplatin-induced acute renal failure. Drug Metab. Pharmacokinet. 2009;24:451–457. doi: 10.2133/dmpk.24.451. [DOI] [PubMed] [Google Scholar]
- 43.Kpemissi M., Eklu-Gadegbeku K., Veerapur V.P., Negru M., Taulescu M., Chandramohan V., Hiriyan J., Banakar S.M., Thimmaiah N., Suhas D.S. Nephroprotective activity of Combretum micranthum G. Don in cisplatin induced nephrotoxicity in rats: In-vitro, in-vivo and in-silico experiments. Biomed. Pharmacother. 2019;116:108961. doi: 10.1016/j.biopha.2019.108961. [DOI] [PubMed] [Google Scholar]
- 44.Ma Z.-N., Li Y.-Z., Li W., Yan X.-T., Yang G., Zhang J., Zhao L.-C., Yang L.-M. Nephroprotective effects of saponins from leaves of Panax quinquefolius against cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 2017;18:1407. doi: 10.3390/ijms18071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M., Jia Z., Sun Y., Zhang A., Yang T. A H2S Donor GYY4137 Exacerbates Cisplatin-Induced Nephrotoxicity in Mice. Mediat. Inflamm. 2016;2016:8145785. doi: 10.1155/2016/8145785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L., Jiang L., Yuan W., Liu Z., Liu D., Wei P., Zhang X., Yi T. Dual-Modality Detection of Early-Stage Drug-Induced Acute Kidney Injury by an Activatable Probe. ACS Sens. 2020;5:2457–2466. doi: 10.1021/acssensors.0c00640. [DOI] [PubMed] [Google Scholar]
- 47.Tsang R.Y., Al-Fayea T., Au H.-J. Cisplatin overdose. Drug Saf. 2009;32:1109–1122. doi: 10.2165/11316640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Mehta R.L., Awdishu L., Davenport A., Murray P.T., Macedo E., Cerda J., Chakaravarthi R., Holden A.L., Goldstein S.L. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88:226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashrafi F., Ebrahimi Z., Nematbakhsh M. Effect of short hydration on cisplatin-induced nephrotoxicity in cancer patients: A retrospective study. Int. J. Hematol. Oncol. Stem Cell Res. 2017;11:262. [PMC free article] [PubMed] [Google Scholar]
- 50.Sánchez-González P.D., López-Hernández F.J., López-Novoa J.M., Morales A.I. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. 2011;41:803–821. doi: 10.3109/10408444.2011.602662. [DOI] [PubMed] [Google Scholar]
- 51.Sancho-Martínez S.M., Prieto-García L., Prieto M., Lopez-Novoa J.M., López-Hernández F.J. Subcellular targets of cisplatin cytotoxicity: An integrated view. Pharmacol. Ther. 2012;136:35–55. doi: 10.1016/j.pharmthera.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Sancho-Martinez S.M., Prieto-García L., Prieto M., Fuentes-Calvo I., Lopez-Novoa J.M., Morales A.I., Martínez-Salgado C., Lopez-Hernandez F.J. N-acetylcysteine transforms necrosis into apoptosis and affords tailored protection from cisplatin cytotoxicity. Toxicol. Appl. Pharmacol. 2018;349:83–93. doi: 10.1016/j.taap.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Singh M.P., Chauhan A.K., Kang S.C. Morin hydrate ameliorates cisplatin-induced E.R. stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int. Immunopharmacol. 2018;56:156–167. doi: 10.1016/j.intimp.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 54.Sahu B.D., Kumar J.M., Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS ONE. 2015;10:e0134139. doi: 10.1371/journal.pone.0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao X., Panichpisal K., Kurtzman N., Nugent K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 56.Pabla N., Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 57.Chtourou Y., Aouey B., Aroui S., Kebieche M., Fetoui H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem.-Biol. Interact. 2016;243:1–9. doi: 10.1016/j.cbi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Landau S.I., Guo X., Velazquez H., Torres R., Olson E., Garcia-Milian R., Moeckel G.W., Desir G.V., Safirstein R. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 2019;95:797–814. doi: 10.1016/j.kint.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fatima S., Al-Mohaimeed N., Al-Shaikh Y., Tyagi P., Banu N., Hasan S., Arjumand S. Combined treatment of epigallocatechin gallate and Coenzyme Q10 attenuates cisplatin-induced nephrotoxicity via suppression of oxidative/nitrosative stress, inflammation and cellular damage. Food Chem. Toxicol. 2016;94:213–220. doi: 10.1016/j.fct.2016.05.023. [DOI] [PubMed] [Google Scholar]