Abstract
Magnesium (Mg) may have several beneficial effects on human health outcomes. One hypothesized mechanism eliciting such effects is the action of Mg on serum inflammatory parameters. However, studies on this topic to date have several important limitations. Therefore, the present systematic review and meta-analysis aimed to summarize the current state of the art of all randomized control trials (RCTs) investigating the effects of Mg supplementation versus placebo on serum parameters of inflammation. We searched several databases until 23 November 2021 for RCTs. Eligible studies were RCTs investigating the effect of oral Mg supplementation vs. placebo and having serum inflammatory markers as an outcome. Among 2484 papers initially screened, 17 randomized controlled trials (889 participants; mean age: 46 years; females: 62.5%) were included. Generally, a low risk of bias was present. In meta-analysis, Mg supplementation significantly decreased serum C reactive protein (CRP) and increased nitric oxide (NO) levels. In descriptive findings, Mg supplementation significantly reduced plasma fibrinogen, tartrate-resistant acid phosphatase type 5, tumor necrosis factor-ligand superfamily member 13B, ST2 protein, and IL-1. In conclusion, Mg supplementation may significantly reduce different human inflammatory markers, in particular serum CRP and NO levels.
Keywords: magnesium, inflammation, C reactive protein, tumor necrosis factor, randomized controlled trial, meta-analysis
1. Introduction
The literature regarding the health benefits of magnesium (Mg) is exponentially increasing [1]. In an umbrella review with 16 meta-analyses and 50 independent outcomes findings suggested that Mg is associated with several positive health outcomes [1]. It is widely known that Mg is involved in more than 600 enzymatic reactions [2], consequently having a wide spectrum of actions in pregnancy [3,4,5], as well as in cardiovascular [6,7], gastrointestinal [8], infectious [9], and metabolic diseases [10], such as diabetes [11,12].
It should be acknowledged that several papers have reported that Mg has positive effects on medical events likely owing to improving inflammatory parameters [13,14,15]. For example, one observational study reported an inverse relationship between dietary magnesium intake and inflammatory parameter levels (in particular C-reactive protein, CRP) in people affected by obesity [13]. However, observational studies on this topic to date have been limited by research design or heterogeneity of the participants included (e.g., gender, ethnicity, age, etc.) and likely underpowered to achieve comprehensive and reliable conclusions [14,15]. Two meta-analyses have also shown that Mg supplementation can have differing effects on some indices of inflammatory and anti-inflammatory indexes, such as CRP [14,15]. Despite the importance of these two meta-analyses [14,15], many other studies are now available although the evidence is limited to CRP as an outcome.
Although the pathophysiological mechanisms through which Mg may improve inflammatory status is not clear yet, it has been demonstrated in, animal models and human studies that Mg deficiency acts as a trigger for the inflammatory process [15]. A possible explanation is that the reduction of Mg levels stimulates macrophages and influx of calcium ions into cells [16]. The increased cell calcium levels increase the Mg necessary to block the influx of calcium ions with an increased stimulation of N-methyl-D-aspartate receptors that present high permeability to calcium [16]. Thus, the stimulation of these receptors lead to the opening of non-selective channels to cations with a consequent rise of calcium ions in neuronal cells [16]. The result is the releasing of neurotransmitters and cytokines as IL-6 that, in turn, enhance CRP release starting the inflammatory response [17].
Given this background, the present systematic review and meta-analysis aimed to summarize the current state of the art of all randomized control trials (RCTs) investigating the effects of Mg supplementation versus placebo on serum parameters of chronic inflammation.
2. Materials and Methods
This systematic review adhered to the PRISMA statement [18] and followed a pre-planned, but unpublished protocol enclosed in the Supplementary Material.
2.1. Data Sources and Searches
Two investigators (NV and DP) independently conducted a literature search using several databases including PubMed/Medline, EMBASE, EBSCO, Web of Science from database inception until 23 November 2021, including RCTs investigating the effect of oral Mg vs. placebo on serum inflammatory parameters (outcome).
In PubMed, the following search strategy was used: (‘magnesium’) AND (‘inflammation’ OR ‘inflammatory’ OR ‘interferons’ OR ‘interferon’ OR ‘TNF’ OR ‘tumor necrosis factor’ OR ‘IL’ OR ‘interleukin’ OR ‘TGF’ OR ‘transforming growth factor’ OR ‘CRP’ OR ‘C-reactive protein’ OR ‘cytokines’ OR ‘cytokine’) AND (‘clinical trial’ OR ‘randomized controlled trial’ OR ‘placebo’), adapting the search according to the database. Any inconsistencies were resolved by consensus with a third author (LS).
2.2. Study Selection
Inclusion criteria for this meta-analysis were: (i) RCT; (ii) double-blind design; (iii) use of oral Mg supplementation; (iv) assessment of serum inflammatory parameters at follow-up evaluation; (v) written in English. Studies were excluded if: (i) did not include humans; (ii) used a control group taking other substances than placebo; (iii) lack of sufficient information regarding serum inflammatory parameters.
2.3. Data Extraction
Two independent investigators (NV and DP) extracted key data from the included articles in a standardized Excel spread sheet and a third independent investigator (LS) checked the data. For each article, we extracted data on author names, year of publication, country, condition, study design (crossover or parallel), Mg daily dosage, and follow-up duration (in weeks). Moreover, we extracted data by Mg or placebo in relation to mean age, body mass index (BMI), and number of females at baseline.
2.4. Outcomes
The primary outcomes were the values of serum parameters of inflammatory markers after treatment with Mg compared to placebo.
2.5. Quality Assessment
Two authors (NV and DP) completed scoring using the risk of bias (RoB) tool suggested by the Cochrane group [19]. This tool assesses several domains of the quality of each RCT, including: adequacy of random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete data outcome (assessment of dropouts), selective outcome reporting, and the presence of other sources of bias. The potential answers were, as the Cochrane Handbook suggests, low risk of bias, high or unclear [20].
2.6. Data Synthesis and Analysis
All analyses were performed using STATA version 14.0 (StataCorp, College Station, TX, USA). Outcomes with at least three studies were meta-analyzed, whilst outcomes with less than three studies were reported descriptively.
The primary analysis compared serum parameters of inflammatory markers between participants treated with oral Mg supplementation vs. placebo at the follow-up evaluation. We calculated the difference between the means of the treatment and placebo groups using follow-up data through standardized mean differences (SMD) with their 95% confidence intervals (CIs), applying a random-effect model [21]. Heterogeneity across studies was assessed by the I2 metric and χ2 statistics. Given significant heterogeneity (I2 ≥ 50%, p < 0.05) and for outcomes having at least ten studies, we conducted a series of meta-regression analyses, according to follow-up (weeks), daily Mg dose, and differences at the baseline evaluation between treated with Mg and placebo in mean BMI, age, CRP serum levels, and percentage of females.
Publication bias was assessed by visually inspecting funnel plots and using the Begg–Mazumdar Kendall tau [22] and the Egger bias test [23].
For all analyses, a p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Search Results
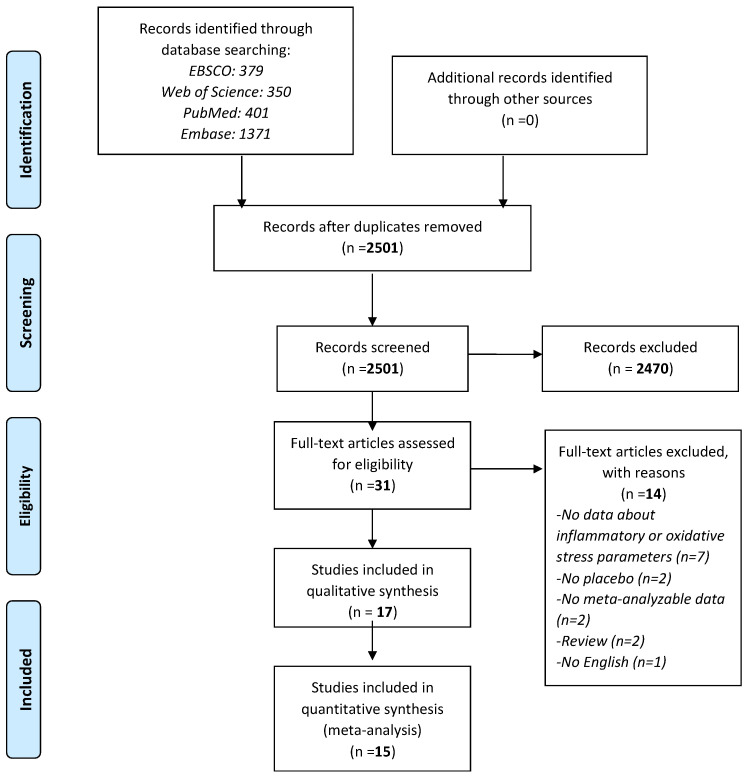
As shown in Figure 1, among 2501 records initially screened, 31 were retrieved as full-texts: of them, 17 papers [17,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] were included in the systematic review with 15 contributing to the meta-analysis.
Figure 1.
PRISMA flow-chart.
3.2. Study and Patient Characteristics
Full details regarding descriptive findings are reported in Table 1.
Table 1.
Descriptive findings of the randomized controlled trials included.
| Magnesium | Placebo | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Country | Condition | Inflammatory Parameters | Daily Mg Doses (mg) |
Type of Mg | Follow-Up (Weeks) | Sample Size | Age (SD) (Years) | Women (%) | BMI (SD) |
Sample Size | Age (SD) (Years) | Women (%) | BMI (SD) |
| Alonso, 2020 [34] | USA | Cardiovascular Diseases |
CRP, NO, TAC, GSH, MDA, Tartrate-resistant acid phosphatase type 5, ST2 protein, Interleukin-1 receptor type 1 | 400 | Oxide | 10 | 24 | 62 ± 5 | 88 | 28.3 ± 5.01 | 28 | 62 ± 6 | 61 | 27.8 ± 4.2 |
| Asemi, 2015 [17] | Iran | Pregnancy | CRP, NO, TAC, MDA | 250 | Oxide | 6 | 35 | 29.1 ± 4.6 | 100 | 29.6 ± 5.4 | 35 | 29.4 ± 3.1 | 100 | 29.1 ± 3.5 |
| Chacko, 2010 [25] | USA | Overweight | CRP, IL-6, TNF-alfa | 500 | Citrate | 4 | 13 | 47 ± 13.8 | 43 | 28.3 ± 1.6 | 13 | 41.9 ± 12.7 | 24 | 28.1 ± 2.2 |
| Cosaro, 2014 [28] | Italy | Family history of metabolic syndrome |
CRP | 368 | Pidolate | 8 | 8 | 6 | ||||||
| Hosseini, 2016 [37] | Iran | Asthma | IL-17 | 340 | Citrate | 8 | 50 | 36.38 ± 9.72 | 50 | 25.6 ± 3.8 | 50 | 34.56 ± 8.28 | 44 | 26.19 ± 3.69 |
| Joris, 2017 [31] | The Netherlands | Overweight/ obese |
CRP, IL-6, IL-8, TNF-alfa, amyloid | 350 | Citrate | 24 | 26 | 25 | ||||||
| Kazaks, 2010 [24] | USA | Asthma | CRP | 340 | Citrate | 26 | 27 | 37 ± 2 | 50 | 29 ± 1 | 25 | 37 ± 2 | 61.1 | 28 ± 1 |
| Lima de Souza E Silva, 2014 [29] |
Brasil | Metabolic Syndrome | CRP | 400 | Chelate | 12 | 35 | 44.6 ± 9.7 | 35.5 ± 8.2 | 37 | 46.6 ± 12.3 | 35.1 ± 6.3 | ||
| Mortazavi, 2013 [27] | USA | Hemodialysis patients | CRP | 440 | Oxide | 24 | 27 | 56.93 ± 12.19 | 48.3 | 25 | 56.36 ± 11.15 | 48 | ||
| Moslehi, 2012 [36] | Iran | Overweight | CRP, IL-6, fibrinogen | 250 | Oxide | 8 | 35 | 100 | 27.9 ± 3.2 | 34 | 100 | 27.9 ± 3 | ||
| Mousavi, 2021 [39] | Iran | Polycystic ovary syndrome | CRP, TAC, MDA, TNF-alfa | 250 | Oxide | 8 | 21 | 25.6 ± 4.9 | 100 | 28.0 ± 3.2 | 20 | 26.2 ± 5.7 | 100 | 26.9 ± 3.8 |
| Razzaghi, 2018 [32] | Iran | Diabetic foot ulcer | CRP, NO, TAC, GSH, MDA, ERS | 250 | Oxide | 12 | 35 | 60.1 ± 11.1 | 37.1 | 28.2 ± 5.2 | 35 | 59 ± 10.1 | 31.4 | 26.3 ± 4.2 |
| Rodriguez-Hernandez, 2010 [35] | Mexico | Obese | CRP | 450 | Chloride | 16 | 19 | 44.2 ± 10.8 | 63.6 | 30.5 ± 4.4 | 19 | 43.2 ± 7.8 | 63.6 | 35.1 ± 7.9 |
| Simental-Mendia, 2012 [26] | Mexico | Prediabetes | CRP, IL-6, IL-10, TNF-alfa | 382 | Chloride | 12 | 11 | 44.2 ± 10.8 | 63.6 | 30.5 ± 4.4 | 11 | 43.2 ± 7.8 | 63.6 | 35.1 ± 7.9 |
| Simental-Mendia, 2014 [30] | Mexico | Prediabetes | CRP | 382 | Chloride | 12 | 29 | 39.8 ± 16 | 55.2 | 30.5 ± 5.7 | 28 | 41.1 ± 13.1 | 60.7 | 30 ± 5.7 |
| Talari, 2019 [33] | Iran | Diabetic hemodialysis |
CRP, NO, TAC, GSH, MDA | 250 | Oxide | 24 | 27 | 58.8 ± 10.1 | 51.9 | 27.2 ± 5.6 | 27 | 61.8 ± 10.2 | 55.6 | 26.2 ± 4.4 |
| Zanforlini, 2021 [38] | Italy | Chronic obstructive pulmonary disease |
CRP, TNF-alfa | 300 | Citrate | 24 | 21 | 73 ± 8.9 | 24 | 26.9 ± 4.3 | 20 | 72.2 ± 11 | 20.8 | 26.9 ± 3.8 |
| Total | Median = 12 | 447 | 47.1 ± 9.3 | 62.5 | 29.0 ± 4.4 | 442 | 46.8 ± 8.7 | 59.6 | 29.2 ± 4.4 | |||||
Among the 17 RCTs included, six were conducted in Asia, eight in North or South America, and three in Europe. The conditions ranged from metabolic disorders (including diabetes, pre-diabetes, overweight/obesity) present in 12 RCTs, pregnancy (n = 1), cardiovascular (n = 1), and respiratory conditions (n = 2). The median follow-up was 12 weeks, with a range between 4 and 26. The majority of the studies (n = 7) used a quantity of 250 mg/daily of Mg oxide (n = 5).
Altogether, 447 participants were randomized to Mg treatment: these participants had a mean age of 47.1 ± 9.3 years, were mainly female (=62.5%) with a mean BMI of 29.0 kg/m2. Conversely, 442 participants were randomized to the placebo group, having a similar mean age (46.8 ± 8.7 years), % of females (59.6%), and mean BMI (29.2 kg/m2) to the intervention group (Table 1).
3.3. Meta-Analysis of Mg Supplementation versus Placebo on Serum Inflammatory Parameters
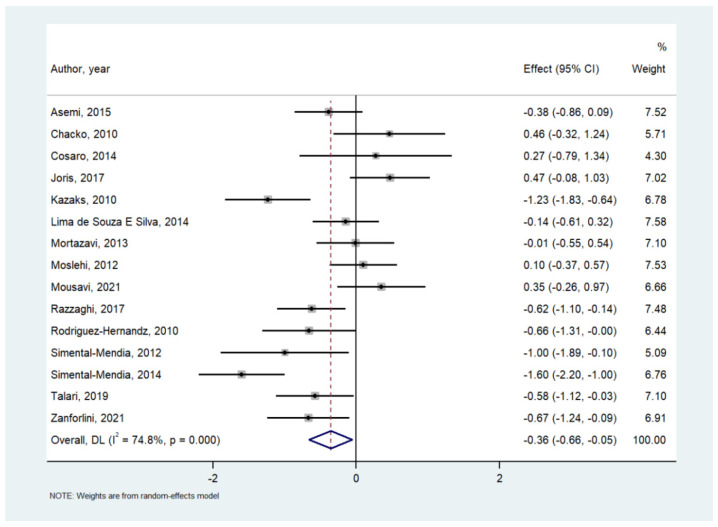
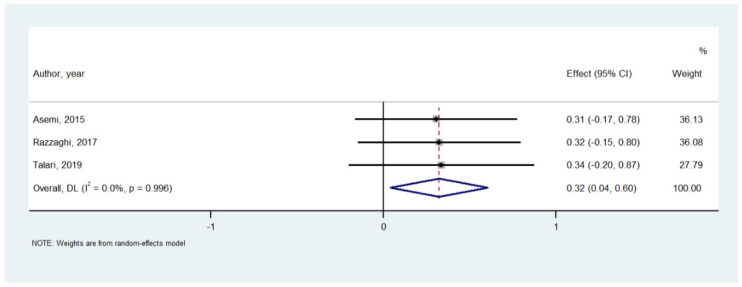
Table 2 shows the effect of Mg on serum inflammatory parameters. Among 737 participants in 15 RCTs, compared to placebo, Mg supplementation significantly decreased serum CRP (SMD = −0.356; 95% CI: −0.659 to −0.054; p = 0.02) (Figure 2), although a high heterogeneity (I2 = 74.8%) was observed. Similarly, Mg supplementation increased nitric oxide levels (n = 3 studies; 194 participants; SMD = 0.321; 95% CI: 0.037 to 0.604; p = 0.026; I2 = 0%) (Figure 3). On the contrary, in the meta-analyses performed with at least three studies, Mg supplementation did not affect the serum levels of IL-6, total antioxidant capacity, glutathione (GSH), tumor necrosis factor alpha, whilst the effect on malondialdehyde (MDA) was at the limits of statistical significance (SMD = −0.604; 95% CI: −1.224 to 0.017; p = 0.057; I2 = 77.8%) (Table 2). Visual inspection of funnel plots and the Begg–Mazumdar Kendall tau and the Egger bias tests did not suggest the presence of publication bias.
Table 2.
Meta-analysis of magnesium supplementation on serum inflammatory parameters.
| Inflammatory Parameter | Number of Comparisons | Number of Participants | SMD | 95% CI | p Value | I2 | Egger’s Test (p-Value) | |
|---|---|---|---|---|---|---|---|---|
| CRP | 15 | 737 | −0.356 | −0.659 | −0.054 | 0.02 | 74.8 | −0.28 (0.92) |
| IL-6 | 3 | 142 | −0.258 | −1.083 | 0.567 | 0.54 | 81.3 | 0.94 (0.38) |
| NO | 3 | 194 | 0.321 | 0.037 | 0.604 | 0.03 | 0 | 0.67 (0.40) |
| TAC | 4 | 235 | 0.189 | −0.491 | 0.869 | 0.59 | 84.8 | 8.86 (0.53) |
| GSH | 3 | 194 | −0.181 | −0.463 | 0.102 | 0.21 | 0 | 3.00 (0.61) |
| MDA | 3 | 194 | −0.604 | −1.224 | 0.02 | 0.06 | 77.8 | −13.9 (0.68) |
| TNF-a | 3 | 112 | 0.168 | −0.433 | 0.768 | 0.58 | 58.8 | 3.84 (0.68) |
Figure 2.
Forrest plot of the effect of magnesium versus placebo on serum C-reactive protein [17,24,25,26,27,28,29,30,31,32,33,35,36,38,39].
Figure 3.
Forrest plot of the effect of magnesium versus placebo on serum nitric oxide [17,32,33].
Among the inflammatory parameters having less than three RCTs, Mg supplementation significantly reduced plasma fibrinogen, tartrate-resistant acid phosphatase type 5, tumor necrosis factor ligand superfamily member 13B, Tumorigenicity 2 protein, and IL(interleukin)-1, while no significant variations were observed on IL-8, IL-10, IL-17, erythrocyte sedimentation rate, and serum amyloid.
3.4. Risk of Bias
The risk of bias assessment is fully reported in Supplementary Table S1. In general, the risk of bias was generally low. Only one RCT [35] had a suspicious high risk of bias in sequence generation, allocation concealment and blinding of participants, personnel and outcome assessors. However, in the sequence generation 7/17, in the allocation concealment and in blinding of participants, personnel and outcome assessors 3 RCTs over 17 were at unclear risk of bias.
3.5. Meta-Regression Analysis
Supplementary Table S2 reports the data of the meta-regression taking the difference between treated with Mg and treated with placebo in serum CRP at the follow-up evaluation, as outcome. The only factor that was able to explain the heterogeneity of this outcome (I2 = 75.6%) was the difference in the percentage of women between the two groups (beta = 0.06; 95% CI: 0.004 to 0.11; p = 0.03) meaning that each increase in one percentage point in the difference of women between groups corresponded to an increase in 0.06 units of CRP (R2 = 39%).
4. Discussion
The present meta-analysis including 17 RCTs with more than 800 participants found that, when compared to placebo, Mg supplementation significantly reduced serum CRP levels, thus supporting previous literature [14,15]. In addition, we found that Mg supplementation increased NO levels.
Considering the inflammatory markers, assessed at least from three RCTs, no other significant variations were reported comparing the treated vs. placebo groups. Interestingly, although not statistically significant, three such important markers IL-6, GSH and MDA showed a reduction in treated groups compared to the baseline indicating that further studies are required to confirm these findings and render possible the formulation of stronger conclusions. In particular, it is important to understand the effects of Mg intake on IL-6 levels [40], which is secreted by T cells and macrophages and acts as both a pro-inflammatory and an anti-inflammatory cytokine. Therefore, IL-6 may be both an indicator of acute inflammation, or undetected infection [40]. In addition, as this topic is attracting increasing attention, recent evidence reported that Mg supplementation significantly improved the reduction of plasma fibrinogen [36,41], tartrate-resistant acid phosphatase type 5, TNF ligand superfamily member 13B, ST2 protein, and IL-1 [34,42].
Moreover, it has been reported that Mg deficiency, in animal models, may increase the recruitment of phagocytic cells to perform their effector functions, which ultimately leads to the generation of reactive oxygen species leading to an increased production of several cytokines involved in the inflammatory cascade, such as TNF-α [43]. At the same time, the release of these cytokines is induced by an increased level of intracellular Ca2+, which is considered a signal to start the inflammatory process and this condition could occur in case of Mg deficiency [44]. Moreover, other in animal and in vitro studies have indicated that pro-inflammatory cytokine production induced in case of Mg deficiency involves the pathway of NFκβ, with a consequent higher production of TNF-α and IL-1β [45]. Therefore, it is likely that all the systems involved in Mg deficiency may affect the inflammatory response in several ways and, in particular, through a modulation of intracellular calcium that regulates several pathways involved in inflammation [42].
Furthermore, this is the first meta-analysis showing that Mg can improve NO levels. This find could be crucial not only in terms of inflammatory mechanisms, but also from a cardiovascular point of view with a potential clinical impact. Interestingly, there is evidence showing that low Mg levels are associated with increased atrial fibrillation and coronary heart disease risk, while Mg supplementation is implied in the secondary prevention of cardiac arrhythmias [46,47]. Indeed, in vitro and in vivo animal studies have discovered a number of new electrophysiological properties of NO [48].
Finally, another interesting result of our systematic review and meta-analysis is that in meta-regression analyses the difference in percentage of females at baseline between treated and placebo groups was associated with a higher effect of Mg supplementation on serum CRP levels. Whilst it is known that males and females have significantly different levels of Mg not only in serum, but also in other biological fluids [49], the exact reason of the effect of Mg being more efficacious in females than in males in lowering serum CRP levels is not known and, therefore, future research is warranted.
The results of this meta-analysis should be considered taking in account its limitations. First, the RCTs included were small in sample size, had a limited follow-up time, and used different doses and types of Mg; therefore, the clinical applicability of our findings should be confirmed in higher quality RCTs. Second, several studies did not include the assessment of Mg introduced in the diet. However, it may be hypothesized that no significant difference in this parameter was present between treated and placebo groups due to randomization. The role of dietary Mg on the effect of Mg supplementation should be better determined. Third, for several outcomes, we were not able to run a meta-analysis since these outcomes were included in less than 3 studies. Finally, when considering the outcome with the largest number of studies, i.e., serum CRP, it was characterized by a high heterogeneity that we were able to explain only partly with the meta-regression analyses.
5. Conclusions
This systematic review and meta-analysis showed the beneficial effects of Mg supplementation in significantly reducing different inflammatory markers, in particular CRP and increasing NO levels. These data open new scenarios in clinical practice suggesting the importance of considering Mg supplementation in patient categories, with particular focus on cardiovascular diseases. Considering the presence of inconsistent but potentially useful data regarding Mg supplementation, further studies are desirable to corroborate and better clarify these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14030679/s1, supplementary Table S1: Risk of bias in the randomized controlled trials included; supplementary Table S2: Meta-regression analyses.
Author Contributions
Conceptualization: N.V. and M.B.; data curation: D.P., L.S. and N.V.; formal analysis: N.V.; methodology: N.V.; supervision: M.B.; roles/writing—original draft: L.J.D. and N.V.; writing—review & editing: L.S. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the inclusion of already published works.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and the databases are available upon reasonable request to the Corresponding Author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Veronese N., Demurtas J., Pesolillo G., Celotto S., Barnini T., Calusi G., Caruso M.G., Notarnicola M., Reddavide R., Stubbs B. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2020;59:263–272. doi: 10.1007/s00394-019-01905-w. [DOI] [PubMed] [Google Scholar]
- 2.Caspi R., Altman T., Billington R., Dreher K., Foerster H., Fulcher C.A., Holland T.A., Keseler I.M., Kothari A., Kubo A. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2014;42:D459–D471. doi: 10.1093/nar/gkt1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs L., Molnar B., Huhn E., Bodis L. Magnesium substitution in pregnancy. A prospective, randomized double-blind study. Geburtshilfe Frauenheilkd. 1988;48:595–600. [PubMed] [Google Scholar]
- 4.Bartal M.F., Sibai B.M. Eclampsia in the 21st century. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.09.037. ahead of print . [DOI] [PubMed] [Google Scholar]
- 5.Fanni D., Gerosa C., Nurchi V., Manchia M., Saba L., Coghe F., Crisponi G., Gibo Y., Van Eyken P., Fanos V. The role of magnesium in pregnancy and in fetal programming of adult diseases. Biol. Trace Elem. Res. 2021;199:3647–3657. doi: 10.1007/s12011-020-02513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X., Wang K., Han D., He X., Wei J., Zhao L., Imam M.U., Ping Z., Li Y., Xu Y. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016;14:1–13. doi: 10.1186/s12916-016-0742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez L., Veronese N., Barbagallo M. Magnesium and Hypertension in Old Age. Nutrients. 2021;13:139. doi: 10.3390/nu13010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gröber U., Schmidt J., Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez L.J., Veronese N., Guerrero-Romero F., Barbagallo M. Magnesium in Infectious Diseases in Older People. Nutrients. 2021;13:180. doi: 10.3390/nu13010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbagallo M., Veronese N., Dominguez L.J. Magnesium in aging, health and diseases. Nutrients. 2021;13:463. doi: 10.3390/nu13020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veronese N., Watutantrige-Fernando S., Luchini C., Solmi M., Sartore G., Sergi G., Manzato E., Barbagallo M., Maggi S., Stubbs B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016;70:1354–1359. doi: 10.1038/ejcn.2016.154. [DOI] [PubMed] [Google Scholar]
- 12.Veronese N., Dominguez L.J., Pizzol D., Demurtas J., Smith L., Barbagallo M. Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients. 2021;13:4074. doi: 10.3390/nu13114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King D.E., Mainous A.G., III, Geesey M.E., Woolson R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005;24:166–171. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 14.Mazidi M., Rezaie P., Banach M. Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. AMS. 2018;14:707–716. doi: 10.5114/aoms.2018.75719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simental-Mendia L.E., Sahebkar A., Rodriguez-Moran M., Zambrano-Galvan G., Guerrero-Romero F. Effect of Magnesium Supplementation on Plasma C-reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2017;23:4678–4686. doi: 10.2174/1381612823666170525153605. [DOI] [PubMed] [Google Scholar]
- 16.Maier J.A., Castiglioni S., Locatelli L., Zocchi M., Mazur A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021;115:37–44. doi: 10.1016/j.semcdb.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Asemi Z., Karamali M., Jamilian M., Foroozanfard F., Bahmani F., Heidarzadeh Z., Benisi-Kohansal S., Surkan P.J., Esmaillzadeh A. Magnesium supplementation affects metabolic status and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2015;102:222–229. doi: 10.3945/ajcn.114.098616. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 23.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazaks A.G., Uriu-Adams J.Y., Albertson T.E., Shenoy S.F., Stern J.S. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: A randomized placebo controlled trial. J. Asthma. 2010;47:83–92. doi: 10.3109/02770900903331127. [DOI] [PubMed] [Google Scholar]
- 25.Chacko S.A., Sul J., Song Y., Li X., LeBlanc J., You Y., Butch A., Liu S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: A randomized, double-blind, controlled, crossover trial in overweight individuals. Am. J. Clin. Nutr. 2011;93:463–473. doi: 10.3945/ajcn.110.002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simental-Mendia L.E., Rodriguez-Moran M., Reyes-Romero M.A., Guerrero-Romero F. No positive effect of oral magnesium supplementation in the decreases of inflammation in subjects with prediabetes: A pilot study. Magnes. Res. 2012;25:140–146. doi: 10.1684/mrh.2012.0322. [DOI] [PubMed] [Google Scholar]
- 27.Mortazavi M., Moeinzadeh F., Saadatnia M., Shahidi S., McGee J.C., Minagar A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: A double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013;69:309–316. doi: 10.1159/000346427. [DOI] [PubMed] [Google Scholar]
- 28.Cosaro E., Bonafini S., Montagnana M., Danese E., Trettene M.S., Minuz P., Delva P., Fava C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014;24:1213–1220. doi: 10.1016/j.numecd.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Lima de Souza E.S.M.L., Cruz T., Rodrigues L.E., Ladeia A.M., Bomfim O., Olivieri L., Melo J., Correia R., Porto M., Cedro A. Magnesium replacement does not improve insulin resistance in patients with metabolic syndrome: A 12-week randomized double-blind study. J. Clin. Med. Res. 2014;6:456–462. doi: 10.14740/jocmr1580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simental-Mendia L.E., Rodriguez-Moran M., Guerrero-Romero F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: A clinical randomized double-blind placebo-controlled trial. Arch. Med. Res. 2014;45:325–330. doi: 10.1016/j.arcmed.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Joris P.J., Plat J., Bakker S.J., Mensink R.P. Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: A randomized controlled trial in overweight/obese adults. Sci. Rep. 2017;7:106. doi: 10.1038/s41598-017-00205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razzaghi R., Pidar F., Momen-Heravi M., Bahmani F., Akbari H., Asemi Z. Magnesium Supplementation and the Effects on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2018;181:207–215. doi: 10.1007/s12011-017-1056-5. [DOI] [PubMed] [Google Scholar]
- 33.Talari H.R., Zakizade M., Soleimani A., Bahmani F., Ghaderi A., Mirhosseini N., Eslahi M., Babadi M., Mansournia M.A., Asemi Z. Effects of magnesium supplementation on carotid intima-media thickness and metabolic profiles in diabetic haemodialysis patients: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2019;121:809–817. doi: 10.1017/S0007114519000163. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A., Chen L.Y., Rudser K.D., Norby F.L., Rooney M.R., Lutsey P.L. Effect of Magnesium Supplementation on Circulating Biomarkers of Cardiovascular Disease. Nutrients. 2020;12:1697. doi: 10.3390/nu12061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Hernandez H., Cervantes-Huerta M., Rodriguez-Moran M., Guerrero-Romero F. Oral magnesium supplementation decreases alanine aminotransferase levels in obese women. Magnes. Res. 2010;23:90–96. doi: 10.1684/mrh.2010.0204. [DOI] [PubMed] [Google Scholar]
- 36.Moslehi N., Vafa M., Rahimi-Foroushani A., Golestan B. Effects of oral magnesium supplementation on inflammatory markers in middle-aged overweight women. J. Res. Med. Sci. 2012;17:607. [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseini S.A., Fathi N., Tavakkol H., Yadollahpour A. Investigating the effects of oral magnesium citrate supplement on lung function, magnesium level and interlukine-17 in patients with asthma. Int. J. Pharm. Res. Allied Sci. 2016;5:86–92. [Google Scholar]
- 38.Zanforlini B.M., Ceolin C., Trevisan C., Alessi A., Seccia D.M., Noale M., Maggi S., Guarnieri G., Vianello A., Sergi G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin. Exp. Res. 2021;34:167–174. doi: 10.1007/s40520-021-01921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mousavi R., Alizadeh M., Jafarabadi M.A., Heidari L., Nikbakht R., Rezaei H.B., Karandish M. Effects of Melatonin and/or Magnesium Supplementation on Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2021;200:1010–1019. doi: 10.1007/s12011-021-02725-y. [DOI] [PubMed] [Google Scholar]
- 40.Rose-John S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018;10:a028415. doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padron-Monedero A., Rodríguez-Artalejo F., Lopez-Garcia E. Dietary micronutrients intake and plasma fibrinogen levels in the general adult population. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-83217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018;11:25. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libako P., Nowacki W., Rock E., Rayssiguier Y., Mazur A. Phagocyte priming by low magnesium status: Input to the enhanced inflammatory and oxidative stress responses. Magnes. Res. 2010;23:1–4. doi: 10.1684/mrh.2009.0201. [DOI] [PubMed] [Google Scholar]
- 44.Lin C., Tsai P., Hung Y., Huang C. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br. J. Anaesth. 2010;104:44–51. doi: 10.1093/bja/aep336. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto J., Romani A.M., Valentin-Torres A.M., Luciano A.A., Kitchen C.M.R., Funderburg N., Mesiano S., Bernstein H.B. Magnesium decreases inflammatory cytokine production: A novel innate immunomodulatory mechanism. J. Immunol. 2012;188:6338–6346. doi: 10.4049/jimmunol.1101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Gobbo L.C., Imamura F., Wu J.H., de Oliveira Otto M.C., Chiuve S.E., Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013;98:160–173. doi: 10.3945/ajcn.112.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arsenault K.A., Yusuf A.M., Crystal E., Healey J.S., Morillo C.A., Nair G.M., Whitlock R.P. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst. Rev. 2013;2013:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L. Role of nitric oxide in regulating cardiac electrophysiology. Exp. Clin. Cardiol. 2001;6:167. [PMC free article] [PubMed] [Google Scholar]
- 49.Fordyce A., Gouliouk V., Henkin R. Age and gender changes in calcium and magnesium metabolism. FASEB J. 2011;25:766–768. doi: 10.1096/fasebj.25.1_supplement.768.6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and the databases are available upon reasonable request to the Corresponding Author.