Abstract
Poly(butylene adipate-co-terephthalate) (PBAT) is a biodegradable polymer synthesized from petrochemical resources. PBAT has an exceptionally high elongation at break values which makes it one of the most promising substitutes for LDPE packaging films. However, the applicability of PBAT films is still limited by low strength and high production costs. In this work, we used polyethylene glycol 600 (PEG-600) as a coating agent to modify the surface of calcium carbonate and improve compatibility with the polymer matrix. A series of PBAT/CaCO3 composite films having different CaCO3 particle size and content of coating agent was prepared using extrusion blow molding. The effect of particle size of CaCO3 filler and the content of a coating agent on the mechanical and rheological properties of composite films have been studied. The biodegradation properties have been tested by burying the samples in soil or keeping them in artificial seawater for 90 days. It was shown that the addition of PEG-600 improves compatibility between the matrix and CaCO3 filler as polar –OH groups of PEG have a high affinity toward the polar surface of CaCO3. Moreover, the hydrophilicity of PEG-600 increased the diffusivity of water molecules and facilitated PBAT degradation. This work provides experimental data and theoretical guidance that support the development of high-performance PBAT/calcium carbonate films for the single use packaging industry.
Keywords: PBAT film, PBAT/CaCO3, coating agent, buried soil biodegradation, simulated seawater biodegradation
1. Introduction
Poly(butylene adipate-co-terephthalate) (PBAT) is a biodegradable plastic with excellent mechanical properties. Although synthesized from petrochemical resources, it has been considered as the most viable substitute for LDPE [1,2,3,4,5,6,7,8]. Although PBAT appeared on the market about a decade ago, the use of neat PBAT film products is limited by the high production costs and lower strength compared with LDPE as the most used packaging film material [9,10,11].
The mechanical properties of PBAT films are often reinforced by the incorporation of low-cost fillers such as starch, CaCO3, and hydrotalcite into the polymer matrix [12,13,14,15,16,17]. Calcium carbonate is one of the most applied inorganic fillers in thermoplastics [18,19,20,21,22]. The properties of polymer/CaCO3 composites depend on the amount of filler added to the polymer matrix, and the application type determines the maximum loading of CaCO3. For example, mulching films require good light transmittance and therefore tolerate only small amounts of CaCO3 [23]. On the other hand, packaging films could be filled with up to 30% CaCO3 to reduce the price of polymer composite while maintaining satisfactory mechanical properties [24]. The particle size of CaCO3, its dispersibility in the polymer matrix, and the affinity of a polymer toward filler determine the overall mechanical properties of a composite [25,26,27]. However, the main issue with this type of composite is the agglomeration that originates from the incompatibility between the filler and a polymer.
The addition of coating agents greatly enhances the stability of composite materials [28]. There are two types of coating agents classified according to the type of intermolecular interactions: (1) dispersants, which interact with particle surface through non-covalent Van der Waals forces, and (2) coupling agents, which are attached to the particle surface via covalent bonds. Fatty acids are the most widely used dispersants for CaCO3 and were applied in LDPE/CaCO3, HDPE/CaCO3, and PP/CaCO3 composites [21,29,30]. Among coupling agents, silanes and titanates are often applied in the synthesis of composites [31].
We previously showed that the addition of a silane coupling agent effectively modified calcium carbonate and improved its compatibility with PBAT. Compared with neat PBAT/calcium carbonate films, the modified material had better mechanical properties and was more resistant to hydrolysis and photodegradation [32,33]. As mulching films should be stable during the long period of crop growth, the robustness and resistance to cracking and degradation of the silane-modified composite were beneficial for this purpose. However, polymer films for packaging and garbage bags have much shorter life cycle and require fast degradation once discarded in the environment. Therefore, we hoped that the addition of dispersant instead of coupling agent would improve the mechanical properties of PBAT/calcium carbonate composite films and accelerate biodegradation.
In this study, CaCO3 particles having different size (12, 6.5, and 5 μm) were modified with polyethylene glycol 600 (PEG-600). The effect of particle size and the amount of coating agent on mechanical and rheological properties of PBAT/CaCO3 composite films was studied in detail. Moreover, the biodegradation rate of these films was determined in soil and simulated seawater. It was anticipated that the hydrophilic PEG can increased the diffusivity of water molecules and facilitated PBAT degradation [34,35].
2. Experimental
2.1. Materials
PBAT (Ecoworld) was purchased from Jinhui Zhaolong Hi Tech Co., Ltd. (Shanxi, China). Three calcium carbonate powders with the particle size of 3000 mesh, 2300 mesh, and 1250 mesh were kindly supplied by Zhejiang Qintang Calcium Industry Co., Ltd. (Hangzhou, China). PEG-600 was purchased from Beijing Institute of Chemical Reagents Co. Ltd. (Beijing, China).
2.2. Preparation of PBAT/CaCO3 Composites
The composition of the mixtures prepared in this study is listed in Table 1. In a typical preparation procedure, CaCO3 was mixed with the coating agent in a high-speed mixer for 15 min to obtain surface-modified CaCO3. In the next step, dry PBAT was added, mixed thoroughly, and the mixture was transferred to the twin-screw extruder for melt blending, cooling, and granulation. Finally, the thin films were produced from composite pellets using the blowing film machine. More details about the preparation procedure can be found in previous work [32].
Table 1.
The composition of PBAT/CaCO3 materials prepared in this study.
| Samples | PBAT (wt.%) | CaCO3 (wt.%) | PEG Coating Agent (wt.%) * | ||
|---|---|---|---|---|---|
| 1250 Mesh (12 μm) | 2300 Mesh (6.5 μm) | 3000 Mesh (5 μm) | |||
| PBAT | 100 | - | - | - | - |
| P7C3-1 | 70 | 30 | - | - | - |
| P7C3-1/S0.5 | 70 | 30 | - | - | 0.5 |
| P7C3-1/S1 | 70 | 30 | - | - | 1 |
| P7C3-1/S2 | 70 | 30 | - | - | 2 |
| P7C3-1/S3 | 70 | 30 | - | - | 3 |
| P7C3-2 | 70 | - | 30 | - | - |
| P7C3-2/S0.5 | 70 | - | 30 | - | 0.5 |
| P7C3-2/S1 | 70 | - | 30 | - | 1 |
| P7C3-2/S2 | 70 | - | 30 | - | 2 |
| P7C3-2/S3 | 70 | - | 30 | - | 3 |
| P7C3-3 | 70 | - | - | 30 | - |
| P7C3-3/S0.5 | 70 | - | - | 30 | 0.5 |
| P7C3-3/S1 | 70 | - | - | 30 | 1 |
| P7C3-3/S2 | 70 | - | - | 30 | 2 |
| P7C3-3/S3 | 70 | - | - | 30 | 3 |
* The wt.% of coating agent relative to CaCO3.
2.3. PBAT/CaCO3 Films Degradation in Simulated Seawater
To make an artificial seawater sample, the 2-cm thick bottom layer was prepared using the sand and mud from Ningbo Sea, and covered with the upper layer made of a 1:1 (v:v) mixture of artificial and natural seawater. Natural seawater was taken from Bohai Bay, PRC. The artificial seawater was prepared according to the previous reported work [36]. The temperature was held constant at 20 ± 2 °C during the degradation period of 90 days. The fresh aliquots were taken for analysis every 30 days.
2.4. The Degradation of PBAT/CaCO3 Films in Soil
The soil degradation experiments were conducted by burying the samples at the lake coast in the western part of Beijing, PRC. More details about this procedure can be found in our previous publication [37]. The pictures of the degradation environment are shown in Figure 1. The soil degradation experiment was conducted from 1 July 2021 to 1 October 2021, 92 days in total. At the end of each month, the samples were taken out from the soil, cleaned, and dried at room temperature before characterization.
Figure 1.
Soil biodegradation test site.
2.5. Characterization
A detailed procedure for structure characterization and performance evaluation is described in Supporting Information.
3. Results and Discussion
3.1. Effect of CaCO3 Particle Size and Coating Agent Content on the Properties of PBAT/CaCO3 Films
3.1.1. Mechanical Properties
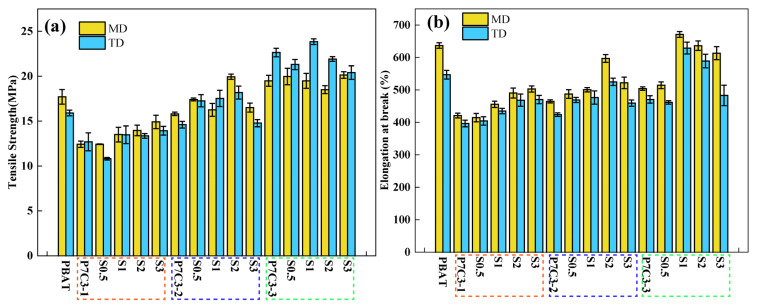
Tensile strength, elongation at break, and tearing strength of PBAT composites filled with 30 wt.% of PEG-coated CaCO3 are listed in Table 2. It was shown that these mechanical properties depend on the particle size of a filler and the amount of coating agent. To better understand these trends, the variations in tensile strength and elongation at break have been showed in Figure 2. All properties of extruded films have been measured in the machine direction (MD) and the transverse direction (TD). The results revealed that the mechanical properties measured in both directions improve with the decrease of particle size from 12 μm to 5 μm. The superior performance of a sample P7C3-3 with the smallest particles is in accordance with the results of our previous work [32].
Table 2.
The influence of particle size of CaCO3 filler and the amount of coating agent on the mechanical properties of PBAT/CaCO3 composite films.
| Sample Codes | Tensile Strength (MPa) | Elongation at Break (%) | Tearing Strength (KN/m) | |||
|---|---|---|---|---|---|---|
| MD | TD | MD | TD | MD | TD | |
| PBAT | 17.7 ± 0.81 | 15.9 ± 0.31 | 637 ± 8.5 | 547 ± 13.6 | 117.9 ± 5.21 | 110.8 ± 0.34 |
| P7C3-1 | 10.4 ± 0.17 | 9.4 ± 0.38 | 481.1 ± 12.76 | 383.5 ± 13.31 | 75.1 ± 0.63 | 74.8 ± 3.14 |
| P7C3-1/S0.5 | 12.42 ± 0.04 | 10.81 ± 0.14 | 414.57 ± 13.04 | 404.3 ± 13.12 | 87.8 ± 2.75 | 77.87 ± 1.61 |
| P7C3-1/S1 | 13.5 ± 0.82 | 13.47 ± 0.99 | 455.77 ± 9.82 | 434.85 ± 8.28 | 93.41 ± 5.56 | 88.35 ± 3.91 |
| P7C3-1/S2 | 13.96 ± 0.58 | 13.35 ± 0.26 | 490.61 ± 15.16 | 468.52 ± 18.90 | 93.36 ± 3.87 | 86.66 ± 3.24 |
| P7C3-1/S3 | 14.91 ± 0.74 | 13.93 ± 0.49 | 502.84 ± 9.53 | 470.34 ± 12.65 | 95.7 ± 1.06 | 96.22 ± 2.58 |
| P7C3-2 | 13.1 ± 1.71 | 13.4 ± 2.47 | 489.6 ± 11.32 | 466.0 ± 15.61 | 109.5 ± 1.64 | 115.9 ± 1.44 |
| P7C3-2/S0.5 | 17.41 ± 0.16 | 17.26 ± 0.68 | 487.49 ± 13.38 | 469.30 ± 7.76 | 116.5 ± 1.28 | 115.06 ± 0.51 |
| P7C3-2/S1 | 16.25 ± 0.72 | 17.52 ± 0.90 | 500.5 ± 6.70 | 476.58 ± 20.32 | 114.75 ± 5.37 | 118.36 ± 3.48 |
| P7C3-2/S2 | 19.94 ± 0.29 | 18.18 ± 0.72 | 597.01 ± 11.90 | 524.93 ± 11.26 | 129.91 ± 1.83 | 125.4 ± 1.17 |
| P7C3-2/S3 | 16.50 ± 0.50 | 14.77 ± 0.40 | 521.81 ± 17.92 | 459.42 ± 9.56 | 122.85 ± 2.95 | 117.65 ± 2.27 |
| P7C3-3 | 15.6 ± 0.57 | 15.3 ± 0.30 | 547.6 ± 12.72 | 530.3 ± 6.70 | 116.9 ± 2.30 | 112.9 ± 1.42 |
| P7C3-3/S0.5 | 19.97 ± 0.92 | 21.3 ± 0.56 | 514.54 ± 10.50 | 461.75 ± 5.26 | 137.73 ± 3.54 | 130.42 ± 2.94 |
| P7C3-3/S1 | 19.48 ± 0.84 | 23.84 ± 0.32 | 670.97 ± 8.84 | 629.1 ± 18.49 | 141.14 ± 1.15 | 143.65 ± 1.67 |
| P7C3-3/S2 | 18.5 ± 0.44 | 21.91 ± 0.26 | 636.38 ± 14.50 | 589.06 ± 20.68 | 127.46 ± 2.83 | 134.97 ± 0.27 |
| P7C3-3/S3 | 20.13 ± 0.37 | 20.4 ± 0.76 | 613.34 ± 20.20 | 483.09 ± 31.67 | 117.54 ± 1.07 | 112.5 ± 2.69 |
| P7C3-3/CA1-2 [32] | 21.0 ± 0.51 | 20.3 ± 0.76 | 695.1 ± 10.28 | 625.8 ± 6.54 | 128.9 ± 2.37 | 127.3 ± 2.38 |
Figure 2.
(a) Tensile strength and (b) elongation at break of PBAT/CaCO3 films.
Comparing two samples having the same mass, the one with a smaller particle size has a larger surface area, and it could be expected that more coating agent is required to effectively modify its surface. However, it was interesting to observe that the amount of coating agent needed for the material to reach the optimum mechanical performance decreased with the particle size reduction. For example, the optimal content of the coating agent was 3% in P7C3-1, 2% in P7C3-2, and 1% in the P7C3-3 sample. This effect can be explained by strong electrostatic interactions between smaller CaCO3 particles that impede the dispersion and surface modification of individual particles. As a result of this process, an excess PEG coating agent remains trapped in the polymer matrix and represents another solid phase in the system. Being highly hydrophilic, it is not compatible with the hydrophobic PBAT matrix and the excess PEG decreases the mechanical properties of PBAT/CaCO3 composite films.
3.1.2. Rheological Properties
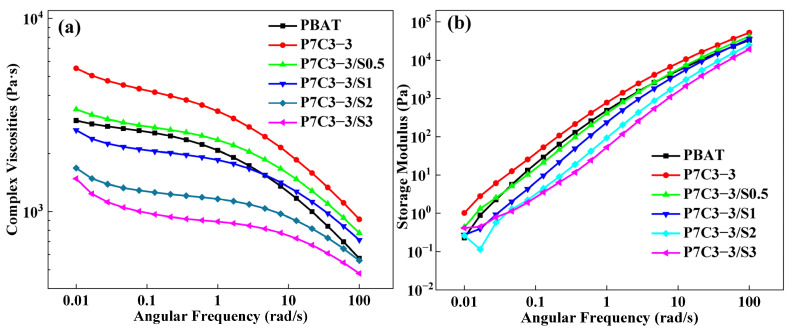
Rheological properties of molten composites strongly depend on the dispersibility of filler particles within the polymer matrix and the nature of intermolecular interactions. The effect of the coating agent on the structure and dispersion state of CaCO3 within the polymer matrix has been studied by measuring the complex viscosity and storage modulus of several composites modified by different amounts of PEG. These parameters have been compared with the values for neat PBAT (Figure 3). According to the results shown in Figure 3a, the viscosity of the modified P7C3-3 sample is higher than the value for neat PBAT. This happens due to formation of a “filler network” structure consisting of a significant amount of CaCO3 which agglomerates and aggregates [21]. This effect reduces the molecular mobility and free volume of polymer chains. Upon the addition of PEG, the complex viscosity drops in a concentration-dependent manner due to the lubricating effect of the coating agent.
Figure 3.
(a) Complex viscosity and (b) storage modulus of PBAT/CaCO3 films prepared using calcium carbonate with particle size 300 mesh and different amounts of PEG coating agent.
The decreased mobility of PBAT chains also results in longer relaxation times and the increased storage modulus of PBAT/CaCO3 composites compared with neat polymer (Figure 3b). The addition of PEG coating agent improves the mobility of a polymer and decreases the storage modulus of P7C3-3 composites.
3.1.3. Surface Morphology
The changes in the surface microstructure of PBAT films upon the addition of CaCO3 filler and PEG coating agent have been studied using SEM. The SEM images of modified and unmodified composite films captured at two magnifications are shown in Figure S1 (1000×) and Figure S2 (3000×). The surface of unmodified PBAT/CaCO3 films appeared relatively rough with abundant particle aggregates. Such a heterogeneous structure reduces the mechanical performances of material and might explain the results obtained in Section 3.1.1. On the other hand, composite films modified by the PEG coating agent exhibited relatively flat and smooth surface with good particle dispersion and without pronounced agglomeration. This confirms that the coating agent effectively increases the compatibility between PBAT and fillers.
3.2. Degradation Behavior of PBAT/CaCO3 Composites in Soil and Simulated Seawater Environment
The biodegradation of plastic films in soil or seawater is a complex process governed by the interplay of biological and physicochemical degradation mechanisms. According to the literature, three parameters govern the biodegradation rate of plastics: (1) the molecular structure and the thickness of polymer film; (2) the type and abundance of microbes in the environment, and (3) the physicochemical parameters of the environment including but not limited to temperature, acidity, humidity, and the presence of nutrients [38]. Previous studies have described that the biodegradation rate of samples buried in soil was mainly controlled by the soil microorganisms and moisture, where the microbial activity depends on temperature and moisture content in the soil [39]. The degradation processes that occur in seawater are somewhat different from those in the soil as seawater is rich in inorganic salts, deficient in organic nutrients, and has a weakly alkaline pH. In addition, the concentration of dissolved O2 is much lower in seawater compared with the soil environment which also influences the biodegradation rate of plastics [40]. In this study, we examined the biodegradation of PBAT/CaCO3 composites in soil and seawater.
3.2.1. Visual Inspection of Structural Changes upon Biodegradation of PBAT/CaCO3 Composites in Soil and Simulated Seawater Environment
The initial thickness of the composite film was 0.055 ± 0.015 mm. After 30 days in the soil environment, we observed partial fragmentation and erosion holes, which was accompanied by the decrease of film thickness to 0.046 ± 0.018 mm. After another 30 days of environmental exposure, the composite films were essentially broken into fragments and the average film thickness was reduced to 0.029 ± 0.013 mm. The P7C3-2/S0.5, P7C3-2/S1, and P7C3-3 samples were almost completely degraded which complicated the sampling for the analysis. After 90 days in soil, all samples except P7C3-1, P7C3-3/S2, and P7C3-3/S3 were almost completely decomposed and the film thickness was reduced to 0.012 ± 0.008 mm.
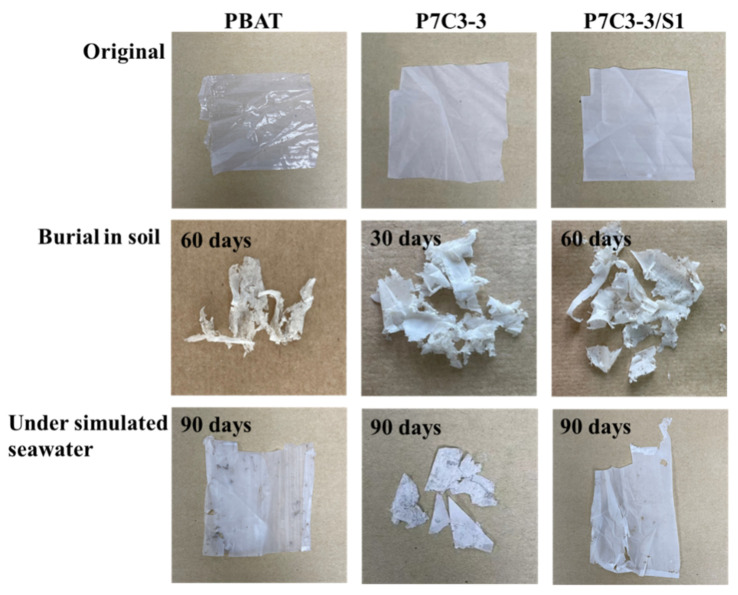
The biodegradation processes in simulated seawater environment were much slower compared to soil. It took 60 days to observe the first changes in film thickness, and it was reduced from the initial value to 0.050 ± 0.012 mm. After 90 days in seawater, PBAT/CaCO3 composites changed the color to brown and some signs of fragmentation occurred. Moreover, the film thickness dropped to 0.044 ± 0.016 mm. The images of neat PBAT, P7C3-3, and P7C3-3/S1 films taken before and after biodegradation in soil and seawater are given in Figure 4.
Figure 4.
The optical images of neat PBAT, P7C3-3, and P7C3-3/S1 films before and after biodegradation in soil and simulated seawater.
3.2.2. The Changes in Molecular Weight of Polymer upon Biodegradation Treatments
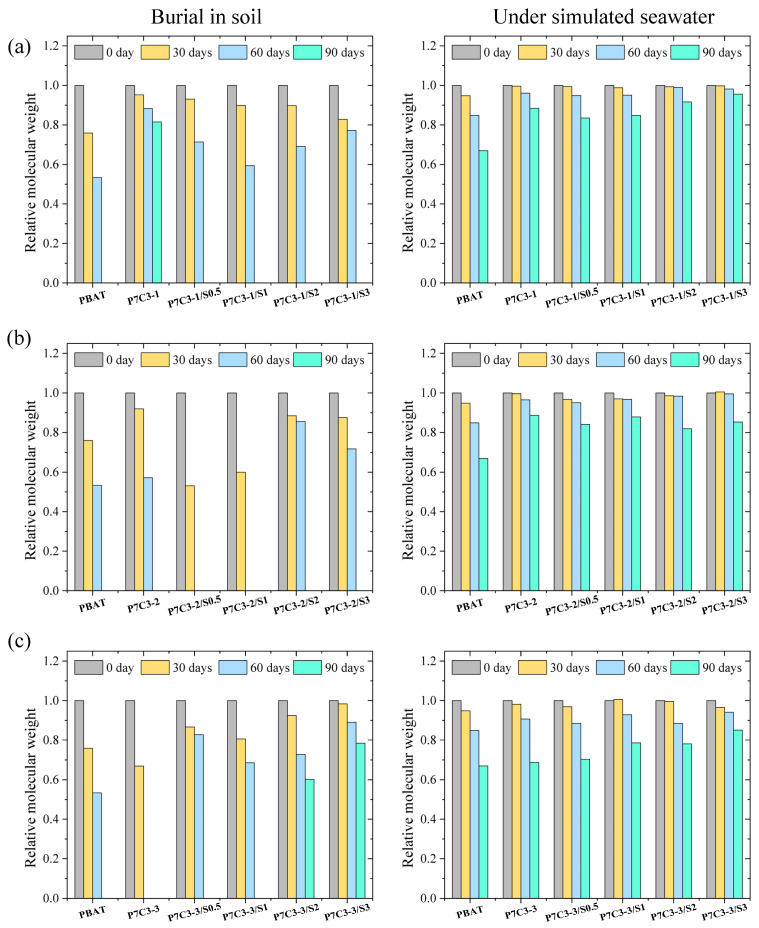
The molecular weight (MW) of the polymer was measured to further characterize the decomposition of neat PBAT films and PBAT/CaCO3 composites after two biodegradation treatments mentioned before. The number average (Mn) and weight-average MW (Mw) of all films exposed to soil and seawater biodegradation for different periods are listed in Tables S1 and S2. The data from Tables S1 and S2 have been visualized in Figure 5 to observe how Mn changes with degradation time.
Figure 5.
The relative changes in number molecular weight (Mn) of neat PBAT, unmodified and coating agent modified PBAT/CaCO3 composites as a function of degradation time, expressed relative to day 0: (a) P7C3-1, (b) P7C3-2 and (c) P7C3-3.
For all samples, Mn steadily decreased with soil degradation time. Comparing the degree of degradation for neat PBAT and PBAT composites with CaCO3 having various particle size, it decreased in the following order: P7C3-3 > P7C3-2 ≈ PBAT > P7C3-1. Two samples with the smallest particle size, namely P7C3-2 and P7C3-3 were almost completely decomposed after 90 and 60 days, respectively. This trend might be explained by the larger surface area of composite films filled with smaller CaCO3 particles, providing more opportunities for contact with water and oxygen molecules, thus facilitating the diffusion of water and hydrolysis/biodegradation of PBAT.
Next, we analyzed the effect of the coating agent on the extent of PBAT/CaCO3 degradation. It was found that the addition of an appropriate amount of PEG promotes decomposition, and this effect was most pronounced for P7C3-1 and P7C3-2 systems. The coating of CaCO3 with the hydrophilic PEG facilitates the diffusion of water molecules into the polymer matrix, which might explain the observed trends in degradation rate. This result is in accordance with the previous study that has demonstrated the increased water absorptivity of coating agent-modified PBAT/CaCO3 films [41]. Nevertheless, we observed that the degradation rate of PBAT decreases when the amount of coating agent exceeds 1%, regardless of CaCO3 particle size. For example, the addition of 2% or 3% of PEG coating agent added in P7C3-3 composite leads to incomplete degradation after 90 days in soil. This trend could be related to the previous discussion on the mechanical properties of PBAT/CaCO3 composites. When the amount of coating agent exceeds optimum, the dispersion of coated reinforcements being less successful, and the diffusion of water is less favored, this effect reduces the hydrolysis/biodegradation of the PBAT matrix. These results highlight the importance of the optimization of coating agent loading for mechanical and biodegradation film performances.
The molecular weight measurements also confirmed that the degradation rate in seawater is much slower than in soil. Although particle size and coating agent influence the diffusivity of water in the polymer matrix, it was found that the degradation rate of P7C3-3 and P7C3-3/S0.5 was similar to that of neat PBAT film. These results demonstrate the dominant role of biodegradation in the overall decomposition mechanism of PBAT/CaCO3 composites while hydrolysis has a minor influence. Some studies also revealed that the type and size of the microbial community are the key parameters for the biodegradation rate of plastics [42].
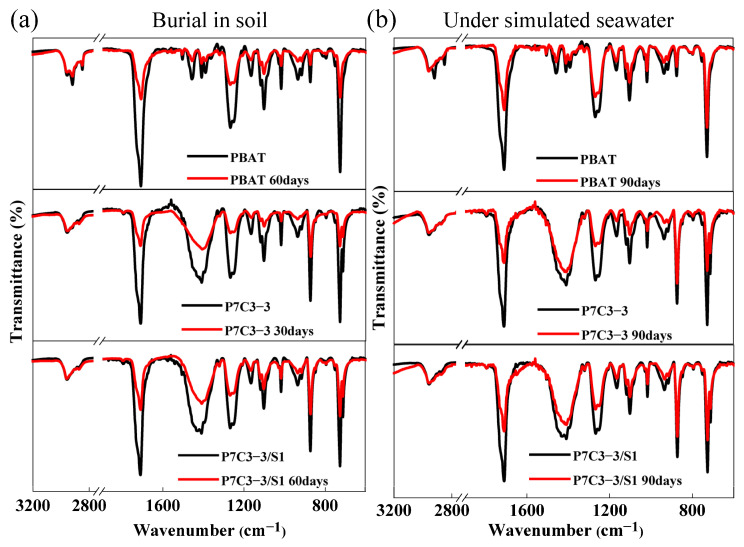
3.2.3. FTIR Analysis of Changes in the Chemical Structure of PBAT upon Biodegradation
The changes in the structure of PBAT polyester caused by soil or seawater degradation have been studied by FTIR. The FTIR spectra of pure PBAT and different PBAT/CaCO3 composite films are shown in Figure 6. Two intense peaks observed at 1710 cm−1 and 1250 cm−1 originate from the stretching vibrations of carbonyl (C=O) and aliphatic C-O-C bonds of ester groups [43]. Soil degradation causes a significant decrease in the intensity of two ester peaks after 30 days of treatment, and the effect intensifies after the additional 30 days. Seawater biodegradation also reduces the intensity of FTIR ester peaks of PBAT but to a much lower extent. Moreover, the changes in FTIR spectra are observed in the fingerprint region. It can be concluded that the biodegradation of PBAT polyester proceeds mainly through the hydrolysis of ester bonds.
Figure 6.
FTIR spectra of neat PBAT, P7C3-3, and P7C3-3/S1 films after 30, 60, or 90 days of biodegradation in (a) soil and (b) simulated seawater environment.
3.2.4. The Changes in the Morphology of Polymers Caused by Biodegradation Revealed by SEM
The microstructure of neat PBAT, P7C3-3 composite, and composite with PEG-coated filler have been observed before and after each biodegradation test. According to SEM micrographs shown in Figure 7, the smooth surface of PBAT was preserved after 60 days of degradation in soil and only small pores and cracks occurred. Somewhat rougher surface of P7C3-3 films transformed into the structure with large pores and small cracks after 30 days in the soil environment. In case of surface-coated P7C3-3/S1 films, large cracks appeared at the surface after 60 days in soil.
Figure 7.
SEM images of (a) neat PBAT, (b) P7C3-3 and (c) P7C3-3/S1 films before and after biodegradation treatments. (a’–c’): burial in soil degradation; (a”–c”): under simulated seawater degradation.
The biodegradation in a simulated seawater environment resulted in significantly lesser changes in the microstructure of PBAT and composites. After 90 days of treatment, no visible signs of biodegradation have been observed in neat PBAT film. More pronounced structure defects appeared in P7C3-3 and P7C3-3/S1 films after 90 days in simulated seawater. The swelling of these polymers due to diffusion of water into the matrix resulted in the departure of CaCO3 particles and the occurrence of holes that could be observed from SEM images. In addition, no visible cracks were found for these samples. These results additionally support previous findings that CaCO3 as a filler and hydrophilic PEG as a coating agent promote the diffusion of water in a polymer matrix.
4. Conclusions
In this study, PEG was used as a dispersion agent for coating CaCO3 particles to improve mechanical properties and biodegradability of PBAT/CaCO3 composite films. Among three particle sizes of applied CaCO3 filler, the one with the smallest particles performed best in terms of mechanical and biodegradation properties. The addition of 1 wt.% of PEG coating agent further improved the performances of composite films. The results of biodegradation experiments in soil and simulated seawater revealed that the degradation is more pronounced in the soil environment, which highlights the importance of microbial activity in the overall mechanism of decomposition. The coating with PEG dispersant improved the compatibility between PBAT polymer and CaCO3 filler and facilitated the diffusivity of water molecules into the composite film resulting in faster degradation. After three months in soil, all PEG-modified samples were completely decomposed while the same period in seawater caused only minor structural changes. Using 3000 mesh CaCO3 particles and less than 1 wt.% of PEG coating agent gives a sample (P7C3-3/S1) with improved mechanical properties and similar degradation performance as neat PBAT. The developed composite is a promising substitute for LDPE as the low-cost, biodegradable single use packing material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14030484/s1, Characterizations; Figures S1 and S2: SEM images of composite films with different magnification; Table S1: Changes in molecular weight of PBAT/CaCO3 films under buried soil; Table S2: Changes in molecular weight of PBAT/CaCO3 films under simulated seawater degradation conditions.
Author Contributions
Conceptualization, C.Z.; methodology, X.D. and C.Z.; investigation, X.D.; data curation, X.D.; writing—original draft preparation, C.Z.; writing—review and editing, C.Z.; supervision, C.Z. and Y.W.; project administration, C.Z.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers: 52073004), Science and Technology Plan of Beijing Municipal Education Commission (general project: KM202110011008) and Beijing Science and Technology Plan Project (Z211100004321003, Z211100004321004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang T., Han W., Zhang C., Weng Y. Effect of chain extender and light stabilizer on the weathering resistance of PBAT/PLA blend films prepared by extrusion blowing. Polym. Degrad. Stabil. 2021;183:109455. doi: 10.1016/j.polymdegradstab.2020.109455. [DOI] [Google Scholar]
- 2.Khan H., Kaur S., Baldwin T.C., Radecka I., Jiang G., Bretz I., Duale K., Adamus G., Kowalczuk M. Effective Control against Broadleaf Weed Species Provided by Biodegradable PBAT/PLA Mulch Film Embedded with the Herbicide 2-Methyl-4-Chlorophenoxyacetic Acid (MCPA) ACS Sustain. Chem. Eng. 2020;8:5360–5370. doi: 10.1021/acssuschemeng.0c00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang G., Li H., Wang F. Structure of PBAT/PPC blends prepared by in-situ reactive compatibilization and properties of their blowing films. Mater. Today Commun. 2021;27:102215. doi: 10.1016/j.mtcomm.2021.102215. [DOI] [Google Scholar]
- 4.Chang C.C., Trinh B.M., Mekonnen T.H. Robust multiphase and multilayer starch/polymer (TPS/PBAT) film with simultaneous oxygen/moisture barrier properties. J. Colloid. Interface Sci. 2021;593:290–303. doi: 10.1016/j.jcis.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Jian J., Xiangbin Z., Xianbo H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020;3:19–26. doi: 10.1016/j.aiepr.2020.01.001. [DOI] [Google Scholar]
- 6.Camani P.H., Souza A.G., Barbosa R.F.S., Zanini N.C., Mulinari D.R., Rosa D.S. Comprehensive insight into surfactant modified-PBAT physico-chemical and biodegradability properties. Chemosphere. 2021;269:128708. doi: 10.1016/j.chemosphere.2020.128708. [DOI] [PubMed] [Google Scholar]
- 7.Shar A.S., Zhang C., Song X., Weng Y., Du Q. Design of novel PLA/OMMT films with improved gas barrier and mechanical properties by intercalating OMMT interlayer with high gas barrier polymers. Polymers. 2021;13:3962. doi: 10.3390/polym13223962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Zhao L., Changyu H., Yancun Y. Biodegradable blends of poly (butylene adipate-co-terephthalate) and stereocomplex polylactide with enhanced rheological, mechanical properties and thermal resistance. Colloid Polym. Sci. 2020;298:463–475. doi: 10.1007/s00396-020-04636-1. [DOI] [Google Scholar]
- 9.Zhai X., Wang W., Zhang H., Dai Y., Dong H., Hou H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr. Polym. 2020;239:116231. doi: 10.1016/j.carbpol.2020.116231. [DOI] [PubMed] [Google Scholar]
- 10.Bai J., Pei H., Zhou X., Xie X. Reactive compatibilization and properties of low-cost and high-performance PBAT/thermoplastic starch blends. Eur. Polym. J. 2021;143:110198. doi: 10.1016/j.eurpolymj.2020.110198. [DOI] [Google Scholar]
- 11.Wei X., Ren L., Sun Y., Zhang X., Guan X., Zhang M., Zhang H. Sustainable composites from biodegradable poly (butylene succinate) modified with thermoplastic starch and poly (butylene adipate-co-terephthalate): Preparation and performance. New J. Chem. 2021;45:17384–17397. doi: 10.1039/D1NJ03208A. [DOI] [Google Scholar]
- 12.Xie J., Wang Z., Zhao Q., Yang Y., Xu J., Waterhouse G.I.N., Zhang K., Li S., Jin P., Jin G. Scale-Up Fabrication of Biodegradable Poly(butylene adipate-co-terephthalate)/Organophilic-Clay Nanocomposite Films for Potential Packaging Applications. ACS Omega. 2018;3:1187–1196. doi: 10.1021/acsomega.7b02062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Q., Buono P., Ruch D., Dubois P., Wu L., Wang W.-J. Biodegradable UV-Blocking Films through Core–Shell Lignin–Melanin Nanoparticles in Poly(butylene adipate-co-terephthalate) ACS Sustain. Chem. Eng. 2019;7:4147–4157. doi: 10.1021/acssuschemeng.8b05755. [DOI] [Google Scholar]
- 14.Rocha D.B., Souza de Carvalho J., de Oliveira S.A., dos Santos Rosa D. A new approach for flexible PBAT/PLA/CaCO3 films into agriculture. J. Appl. Polym. Sci. 2018;135:46660. doi: 10.1002/app.46660. [DOI] [Google Scholar]
- 15.Li J., Lai L., Wu L., Severtson S.J., Wang W.-J. Enhancement of Water Vapor Barrier Properties of Biodegradable Poly(butylene adipate-co-terephthalate) Films with Highly Oriented Organomontmorillonite. ACS Sustain. Chem. Eng. 2018;6:6654–6662. doi: 10.1021/acssuschemeng.8b00430. [DOI] [Google Scholar]
- 16.Girdthep S., Worajittiphon P., Leejarkpai T., Molloy R., Punyodom W. Effect of silver-loaded kaolinite on real ageing, hydrolytic degradation, and biodegradation of composite blown films based on poly(lactic acid) and poly(butylene adipate-co-terephthalate) Eur. Polym. J. 2016;82:244–259. doi: 10.1016/j.eurpolymj.2016.07.020. [DOI] [Google Scholar]
- 17.Mondal D., Bhowmick B., Mollick M.M.R., Maity D., Ranjan Saha N., Rangarajan V., Rana D., Sen R., Chattopadhyay D. Antimicrobial activity and biodegradation behavior of poly(butylene adipate-co-terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2014;131:400079. doi: 10.1002/app.40079. [DOI] [Google Scholar]
- 18.Zuiderduin W.C.J., Westzaan C., Huétink J., Gaymans R.J. Toughening of polypropylene with calcium carbonate particles. Polymer. 2003;44:261–275. doi: 10.1016/S0032-3861(02)00769-3. [DOI] [Google Scholar]
- 19.Xie X.-L., Liu Q.-X., Li R.K.-Y., Zhou X.-P., Zhang Q.-X., Yu Z.-Z., Mai Y.-W. Rheological and mechanical properties of PVC/CaCO3 nanocomposites prepared by in situ polymerization. Polymer. 2004;45:6665–6673. doi: 10.1016/j.polymer.2004.07.045. [DOI] [Google Scholar]
- 20.Zapata P.A., Palza H., Diaz B., Armijo A., Sepulveda F., Ortiz J.A., Ramirez M.P., Oyarzun C. Effect of CaCO(3) Nanoparticles on the Mechanical and Photo-Degradation Properties of LDPE. Molecules. 2018;24:10126. doi: 10.3390/molecules24010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantia F.P.L., Morreale M., Scaffaro R., Tulone S. Rheological and mechanical behavior of LDPE/calcium carbonate nanocomposites and microcomposites. J. Appl. Polym. Sci. 2013;127:2544–2552. doi: 10.1002/app.37875. [DOI] [Google Scholar]
- 22.Piekarska K., Piorkowska E., Bojda J. The influence of matrix crystallinity, filler grain size and modification on properties of PLA/calcium carbonate composites. Polym. Test. 2017;62:203–209. doi: 10.1016/j.polymertesting.2017.06.025. [DOI] [Google Scholar]
- 23.Titone V., La Mantia F.P., Mistretta M.C. The Effect of Calcium Carbonate on the Photo-Oxidative Behavior of Poly(butylene adipate-co-terephthalate) Macromol. Mater. Eng. 2020;305:2000358. doi: 10.1002/mame.202000358. [DOI] [Google Scholar]
- 24.Luo Z., Wang Y., Jiang L., Xu X. Effect of nano-CaCO3-LDPE packaging on quality and browning of fresh-cut yam. LWT Food Sci. Technol. 2015;60:1155–1161. doi: 10.1016/j.lwt.2014.09.021. [DOI] [Google Scholar]
- 25.Sun S., Li C., Zhang L., Du H.L., Burnell-Gray J.S. Interfacial structures and mechanical properties of PVC composites reinforced by CaCO3 with different particle sizes and surface treatments. Polym. Int. 2006;55:158–164. doi: 10.1002/pi.1932. [DOI] [Google Scholar]
- 26.Oladele I.O., Ibrahim I.O., Akinwekomi A.D., Talabi S.I. Effect of mercerization on the mechanical and thermal response of hybrid bagasse fiber/CaCO3 reinforced polypropylene composites. Polym. Test. 2019;76:192–198. doi: 10.1016/j.polymertesting.2019.03.021. [DOI] [Google Scholar]
- 27.Zhao H., Yu Y., Han C., Liu Q., Liu H., Zhou G., Xu M. Improving the stereocomplexation and toughness of poly (L-lactic acid)/poly (D-lactic acid) blends via melt blending with ethylene/methyl acrylate/glycidyl methacrylate terpolymer. J. Macromol. Sci. Part A. 2021;58:419–430. doi: 10.1080/10601325.2021.1873071. [DOI] [Google Scholar]
- 28.Rong M., Zhang M., Ruan W. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol-Lond. 2006;22:787–796. doi: 10.1179/174328406X101247. [DOI] [Google Scholar]
- 29.Cao Z., Daly M., Clémence L., Geever L.M., Major I., Higginbotham C.L., Devine D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016;378:320–329. doi: 10.1016/j.apsusc.2016.03.205. [DOI] [Google Scholar]
- 30.Sahebian S., Zebarjad S.M., Sajjadi S.A., Sherafat Z., Lazzeri A. Effect of both uncoated and coated calcium carbonate on fracture toughness of HDPE/CaCO3 nanocomposites. J. Appl. Polym. Sci. 2007;104:3688–3694. doi: 10.1002/app.25644. [DOI] [Google Scholar]
- 31.Doufnoune R., Chebira F., Haddaoui N. Effect of titanate coupling agent on the mechanical properties of calcium carbonate filled polypropylene. Int. J. Polym. Mater. Polym. Biomater. 2003;52:967–984. doi: 10.1080/714975875. [DOI] [Google Scholar]
- 32.Zhang T., Zhang C., Yang Y., Yang F., Zhao M., Weng Y. Improved properties of poly(butylene adipate-co-terephthalate)/calcium carbonate films through silane modification. J. Appl. Polym. Sci. 2021;138:50970. doi: 10.1002/app.50970. [DOI] [Google Scholar]
- 33.Yang Y., Zhang C., Weng Y. Effects of CaCO3 surface modification and water spraying on the weathering properties of PBAT/CaCO3 films. Polym. Test. 2021;102:107334. doi: 10.1016/j.polymertesting.2021.107334. [DOI] [Google Scholar]
- 34.Chandel A.K.S., Kumar C.U., Jewrajka S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation–Release Performance of Biodegradable/Cytocompatible Agarose–Polyethylene Glycol–Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces. 2016;8:3182–3192. doi: 10.1021/acsami.5b10675. [DOI] [PubMed] [Google Scholar]
- 35.Nutan B., Chandel A.K.S., Jewrajka S.K. Liquid prepolymer-based in situ formation of degradable poly (ethylene glycol)-linked-poly (caprolactone)-linked-poly (2-dimethylaminoethyl) methacrylate amphiphilic conetwork gels showing polarity driven gelation and bioadhesion. ACS Appl. Bio. Mater. 2018;1:1606–1619. doi: 10.1021/acsabm.8b00461. [DOI] [PubMed] [Google Scholar]
- 36.Ravichandran R., Rajendran N. Electrochemical behaviour of brass in artificial seawater: Effect of organic inhibitors. Appl. Surf. Sci. 2005;241:449–458. doi: 10.1016/j.apsusc.2004.07.046. [DOI] [Google Scholar]
- 37.Weng Y.X., Wang L., Zhang M., Wang X.L., Wang Y.Z. Biodegradation behavior of P(3HB,4HB)/PLA blends in real soil environments. Polym. Test. 2013;32:60–70. doi: 10.1016/j.polymertesting.2012.09.014. [DOI] [Google Scholar]
- 38.Haider T.P., Volker C., Kramm J., Landfester K., Wurm F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. Engl. 2019;58:50–62. doi: 10.1002/anie.201805766. [DOI] [PubMed] [Google Scholar]
- 39.Martucci J.F., Ruseckaite R.A. Tensile properties, barrier properties, and biodegradation in soil of compression—Molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009;112:2166–2178. doi: 10.1002/app.29695. [DOI] [Google Scholar]
- 40.Laycock B., Nikolić M., Colwell J.M., Gauthier E., Halley P., Bottle S., George G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017;71:144–189. doi: 10.1016/j.progpolymsci.2017.02.004. [DOI] [Google Scholar]
- 41.Wang X.W., Wang G.X., Huang D., Lu B., Zhen Z.C., Ding Y., Ren Z.L., Wang P.L., Zhang W., Ji J.H. Degradability comparison of poly(butylene adipate terephthalate) and its composites filled with starch and calcium carbonate in different aquatic environments. J. Appl. Polym. Sci. 2019;136:46916. doi: 10.1002/app.46916. [DOI] [Google Scholar]
- 42.Wang G.X., Huang D., Ji J.H., Volker C., Wurm F.R. Seawater-Degradable Polymers-Fighting the Marine Plastic Pollution. Adv. Sci. (Weinh) 2020;8:2001121. doi: 10.1002/advs.202001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi R., Jones D.L., Liu Q., Liu Q., Li Z., Yan C. Field test on the biodegradation of poly (butylene adipate-co-terephthalate) based mulch films in soil. Polym. Test. 2021;93:107009. doi: 10.1016/j.polymertesting.2020.107009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.