Abstract
Background: People consume nitrates, nitrites, nitrosamines, and NOCs compounds primarily through processed food. Many studies have yielded inconclusive results regarding the association between cancer and dietary intakes of nitrates and nitrites. This study aimed to quantify these associations across the reported literature thus far. Methods: We performed a systematic review following PRISMA and MOOSE guidelines. A literature search was performed using Web of Science, Embase, PubMed, the Cochrane library, and google scholar up to January 2020. STATA version 12.0 was used to conduct meta-regression and a two-stage meta-analysis. Results: A total of 41 articles with 13 different cancer sites were used for analysis. Of these 13 cancer types/sites, meta-regression analysis showed that bladder and stomach cancer risk was greater, and that pancreatic cancer risk was lower with increasing nitrite intakes. Kidney and bladder cancer risk were both lower with increasing nitrate intakes. When comparing highest to lowest (reference) categories of intake, meta-analysis of studies showed that high nitrate intake was associated with an increased risk of thyroid cancer (OR = 1.40, 95% CI: 1.02, 1.77). When pooling all intake categories and comparing against the lowest (reference) category, higher nitrite intake was associated with an increased risk of glioma (OR = 1.12, 95% CI: 1.03, 1.22). No other associations between cancer risk and dietary intakes of nitrates or nitrites were observed. Conclusion: This study showed varied associations between site-specific cancer risks and dietary intakes of nitrate and nitrite. Glioma, bladder, and stomach cancer risks were higher and pancreatic cancer risk was lower with higher nitrite intakes, and thyroid cancer risk was higher and kidney cancer risk lower with higher nitrate intakes. These data suggest type- and site-specific effects of cancer risk, including protective effects, from dietary intakes of nitrate and nitrite.
Keywords: nitrate, nitrites, dietary intake, cancer, humans, systematic review
1. Introduction
Cancer is a leading cause of death worldwide, accounting for nearly 10.0 million (approximately one in six) deaths, in 2020 [1]. The global cancer burden is expected to be 28.4 million cases in 2040, a 47% rise from 2020 [1]. With this growing global burden, more evidence-based practice is needed in the identification and management of risk factors for cancer development. Although the causes of cancer are not completely understood, numerous factors are known to increase risk, including non-modifiable factors (e.g., gender, age, genetic factors) and modifiable factors (e.g., dietary, lifestyle) [2]. For instance, one-third of deaths from cancer are due to behavioral and dietary risks [3].
Over the past few years, new evidence has led a paradigm change in our understanding of the role of both dietary nitrate and nitrite on human health, particularly in relation to cancer risk [4,5,6,7]. Historically, a high intake of nitrate and nitrite were considered harmful food additives and listed as probable human carcinogens under conditions where endogenous nitrosation could take place and lead to formation of N-nitroso compounds (NOC) [2,4,8]. Nowadays, nitrate, nitrite and nitrosamine occur naturally in fruits and vegetables, which are regarded as an important part of a healthy diet due to the powerful evidence of beneficial health effects against cancer [9,10]. Numerous studies concluded that fruits and vegetables contribute over 80% of the daily dietary intake of nitrate [11,12,13] and nitrite [14,15,16,17], which represent the primary sources of exposure to nitrate and nitrite by humans.
Nitrates and nitrites are also widely used in food processing, such as in processed meats (e.g., sausages, hot dogs, luncheon meats, ham, and bacon) where they are used to reduce microbial spoilage and preserve meat products [18,19]. High consumption of both processed and fresh meat is linked to increased cancer risk (e.g., gastric [20], pancreatic [21], bladder [5], and colorectal [19]). It is the presence of nitrite, amides, and amines [8,22,23,24] in processed meats and heme iron in fresh meat [25,26,27,28,29] that is considered to be responsible for these risk effects. Moreover, there are other biological and dietary factors that may contribute to the effects that nitrates, nitrites, and their related compounds have on the body. For example, certain strains of oral bacteria have been identified that reduce nitrate found in food to nitrite [30]. This ”endogenous nitrosation” is known to occur mostly in the digestive system’s organs, especially the stomach, rectum, colon, and urinary bladder [31,32], but it can take place in any part of the body. Some of the NOC’s forms have been identified in human urine [33].
Despite the carcinogenic potential of NOCs, some epidemiologic studies found no association between dietary nitrate, nitrite, and NOC intake and cancer in humans [34,35,36]. Case control studies in Iowa [35] and Spain [36] found no association between long-term, average nitrate levels in public water supplies and bladder cancer. This might be due to the fact that nitrate in drinking water and their related compounds are in low concentrations [35,36,37]. Overall, epidemiologic studies that have examined associations between nitrate, nitrite, and NOC compounds and various types of cancer in humans have returned mixed and, in some cases, complex results. Some studies show positive associations [5,6,20,38,39], many show no association [35,36,37], and others show inverse associations, which may be due to confounding factors [31,37,40,41,42,43,44]. In this study, this systematic review aims to evaluate the association and clarify the relationship between dietary consumption of nitrate and nitrite and selected, site-specific cancer risks in humans.
2. Methods
Meta-analyses are typically used to estimate the overall/mean of an outcome of interest. However, inference about between-study variability, which is typically modeled using a between-study variance parameter, is usually an additional aim [45]. Meta-regression is a sensitivity analysis. In primary studies, we use regression, or multiple regression, to assess the relationship between one or more covariates (moderators) and a dependent variable. The same approach can be used with meta-analysis, except that the covariates are at the level of the study rather than the level of the subject, and the dependent variable is the effect size in the studies rather than subject scores. We use the term meta-regression to refer to these procedures when used in a meta-analysis [46].
2.1. Search Methods for Identifications of Studies
We performed a systematic review following PRISMA and MOOSE guidelines. This study evaluated the association and clarified the relationship between dietary nitrate, nitrite, and selected, site-specific cancers. Two investigators independently searched literature on the Web of Science, Embase, PubMed, the Cochrane library, and google scholar up to January 2020.
2.2. The Keywords and Search Terms Used
A literature search was performed on all the databases by using the following keywords: (Nitrate OR nitrates NO3-N OR NO3- OR nitrite OR nitrites OR N-nitroso compounds OR NO2-N OR NO2- OR sodium nitrate OR NaNO3 OR sodium nitrite OR NaNO2 OR ammonium nitrate OR NH4NO3 OR nitrosamine OR Nitrite amine OR NH2NO2 OR DMNA OR NDMA OR NDBA OR NMEA OR NDEA OR NPIP OR NPYR OR NMOR OR NDPA) AND (Neoplasm OR Tumor OR Tumour OR Cancer OR Carcinoma OR Carcinogenesis OR Malignant OR Adenocarcinoma OR Non-Hodgkin lymphoma OR Glioma) AND (Dietary OR Diet OR food). The full search strategy is provided in the online Supplementary Table S1. The bibliographies of original studies, reviews and relevant conferences were manually searched.
2.3. Inclusion and Exclusion Criteria
Inclusion criteria: Only articles reporting associations or outcomes between dietary nitrate, nitrite, and cancer in humans were used (both qualitatively and quantitatively). Exclusion criteria: All articles with animal experiments, articles not published in English, articles with a short commentary, short notes, no data or no records, or incomplete results and letters were excluded. Search results were screened for inclusion and exclusion criteria by four authors.
2.4. Data Extraction and Quality Assessment
Data collection and quality assessment processes were performed by four authors (Essien, Weihua, Kassim, and Abbas), and any disagreement was settled by group discussion. Qualitative analysis: the data were extracted using a self-developed data extraction form. For selected studies, data included the study characteristics: first author, year published and country, study design, exposure categories (nitrate and nitrite intake mg/day), reported Risk ratios (RR), odds ratios (OR), and hazard ratios (HR) with their 95% CIs, cancer sites, and adjustment. Quantitative analysis: the data included: Nitrate dosages and nitrite dosages from dietary intake; OR/RR/HR, with their 95% CI for each category of exposure.
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the included studies. Scores ranged from 0 to 9; studies with a score ≥7 were seen as high-quality studies [47].
2.5. Statistical Analysis
Software: STATA version 12.0 (StataCorp LP, College Station, TX, USA) was used to conduct meta-regression and meta-analysis (of both binary and continuous outcome variables, (Effect/CI)). (Statistical calculations and figures were produced with this software).
Analysis of data: All nitrate and nitrite dosage, and their related compounds, OR, RR, and HR, with 95% CIs were extracted (both crude and adjusted OR, RR, and HR,). The RRs and HRs were assumed to be the accurate estimates of ORs. The median intake of nitrate and nitrite dosage was calculated from the range given, (formula = lowest dosage (lower limit) + highest dosage (upper limit) divided by two in each given quartile). When the median intake was given, the data was used directly. When the interval of a quartile of any category of nitrate or nitrite dosage was not provided, the width of the class interval of quartile before this quartile was used to calculate and estimate the interval, (when the shortest or longest category was open-ended, it was assumed that the open-ended interval length had the same length as the adjacent interval). Logarithm of the ORs (LogOR) was calculated. Standard error was also calculated, (formula = (log 95% CI upper limit value—log 95% CI lower limit value) divided by 3.92) using Microsoft Office Excel.
Conversion of nitrate units and nitrite dosage: mg/day was the standard unit for nitrite and nitrate dosage from dietary intake used for analysis because most of the articles used these units in their analyses. The recommended daily calorie intake in the US is around 2500 kcal for men and 2000 kcal for women [48]; for this analysis, 2500 calories (also referred to as kcal) was used for the standard conversion of data. (a) mg/1000 kcal was converted to mg/day (formula = the dosage in mg/1000 kcal multiplied by 2.5 (2500 kcal = mg/day)). (b) Mcg (nanogram) was converted to mg/day; (formula = the dosage in mcg or micro/1000 kcals or microgram/1000 kcals or µg/1000 kcal/day is divided by 0.001 to be converted into mg/1000 kcals and then multiplied by 2.5. (c) Mcg/day was converted to mg/day; (formula = the dosage in mcg/day is divided by 0.001).
Meta-regression: The parameters for meta-regression calculation were the dependent variable (y) = LogOR; covariates = median (the median intake of nitrate and nitrite dosage); and within-study variability = standard error was used for analysis. Meta-regression analysis was conducted to determine the associations between nitrate and nitrite exposure and cancer risk. A meta-regression coefficient was considered statistically significant at p ≤ 0.05. A sensitivity analysis was performed whereby extreme dosages were removed, including their ORs. Any cancer site that had less than three studies was not included in the meta-regression (these included ovarian cancer and uterus corpus).
Meta-analysis: Meta-analysis was performed using two different approaches. The first approach compared the effect sizes (ORs (95% CI)) for site-specific cancer risk of the highest category of dietary nitrate and nitrite against the lowest (reference) category. were compared. The second approach pooled the effect sizes (ORs (95% CI)) for site-specific cancer risk in all intake categories and compared it against the lowest (reference) category. When I2 statistics do not present a notable heterogeneity (p > 0.05 or I2 ≤ 50%), we used a fixed-effects analysis described by Mantel-Haenszel [49]; otherwise, a random-effects analysis was conducted described by the DerSimonian and Laird method [50]. Any cancer site that had less than three studies was not included in the meta-analysis. Two types of cancers with similar sites or very close locations in the human body were merged for analysis (these included ovarian cancer and uterus corpus).
3. Results
3.1. Selection of the Studies
A total of 3348 records were identified through database searching. After screening titles and abstracts according to the inclusion and exclusion criteria, 98 records remained. The full texts of 74 articles were assessed for eligibility, upon which 31 articles were excluded. Therefore, 41 articles were eligible for meta-regression with 13 cancer sites. The process of selection of studies can be seen in Figure 1.
Figure 1.
Flow chart of study selection.
3.2. Results
3.2.1. Meta-Regression
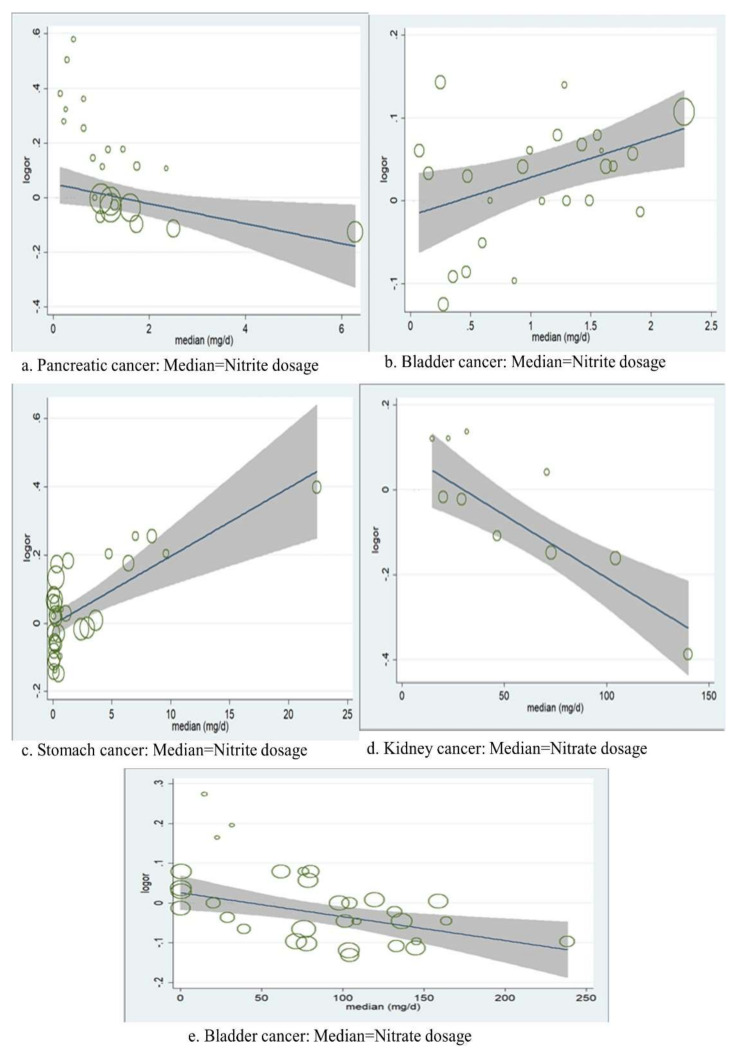
Meta-regression analyses showed that the risk of both bladder (t = 1.99, p = 0.056, adjusted R2 = 33.77%) and stomach cancers (t = 4.09, p = 0.000, adjusted R2= 74.06%) were positively associated with the dosage of dietary nitrite (Figure 2b,c, but that the risk of pancreatic cancer was inversely associated (t = −2.89, p = 0.007, adjusted R2 = 33.37%) (Figure 2a. In relation to the dosage of dietary nitrate, the risk of both kidney (t = −4.02, p = 0.002, adjusted R2 = 100%) and bladder cancers (t = −2.78, p = 0.008, and R2 =58.38%) were inversely associated (Figure 2d,e). No other significant associations were observed by meta-regression analyses.
Figure 2.
Meta-regression: the association between the risk of logarithm ORs and median dosage of dietary nitrite and nitrate for selected site-specific cancers; (a). Pancreatic cancer Median Nitrite dosage, (b). Bladder cancer: Median = Nitrite dosage, (c). Stomach cancer: Median = Nitrite dosage, (d). Kidney cancer: Median = Nitrate dosage, (e). Bladder cancer: Median = Nitrate dosage.
3.2.2. Meta-Analysis
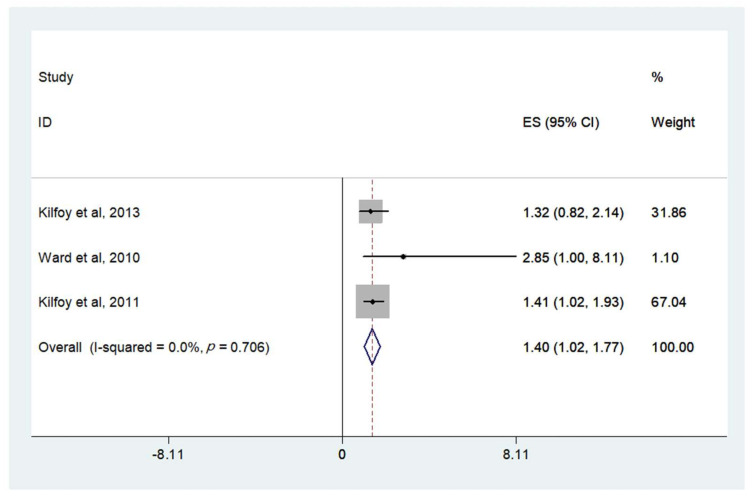
When comparing highest to lowest (reference) categories of intake, meta-analysis of studies showed that high nitrate intake was associated with an increased risk of thyroid cancer (OR = 1.40, 95% CI: 1.02, 1.77) (Figure 3 and Table 1). Little heterogeneity was observed (I2 = 0.0%, p = 0.706). There was no evidence of association between the risk of cancers of the reproductive organs (ovary and uterine corpus), breast, non-Hodgkin’s lymphoma, stomach, pancreatic, esophageal, bladder, kidney, colon, or rectal cancer and dietary nitrate and nitrite (Table 1).
Figure 3.
ORs (95% CI) for thyroid cancer of the highest versus lowest category of dosage of dietary nitrate consumption for the following selected studies .
Table 1.
Meta-analysis of pooled ORs (95% CI) of the highest versus lowest category and all combined higher versus the lowest category of dietary nitrate and nitrite consumption for selected, site-specific cancers.
| Type of Cancer | Highest versus the Lowest (Reference) Category | All Combined Highest versus the Lowest (Reference) Category | Publication Bias | |||
|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | I-Squared (I2) and p-Value | Pooled OR (95% CI) | I-Squared (I2) and p-Value | Egger’s Test p-Value |
Begg’s Test p-Value |
|
| (a) Ovarian and uterine corpus (nitrate) | 1.03, (0.84, 1.22) | 28.7%, p = 0.240 | 0.97, (0.75, 1.19) | 80.6%, p = 0.001 | 0.067 | 0.090 |
| (b) Breast (nitrate) | 0.91, (0.81, 1.00) | 0.0%, p = 0.526 | 0.92, (0.87, 0.96) | 42.7%, p = 0.175 | 0.310 | 0.144 |
| (c) Thyroid (nitrate) | 1.40, (1.02, 1.77) | 0.0%, p = 0.706 | 1.27, (0.85, 1.69) | 62.0%, p = 0.072 | 0.064 | 0.325 |
| (d) Glioma (nitrate) | 1.11, (0.91, 1.31) | 0.0%, p = 0.546 | 1.11, (0.94, 1.29) | 66.9%, p = 0.049 | 0.132 | 0.040 |
| (e) Glioma (nitrite) | 1.17, (0.98, 1.37) | 0.0%, p = 0.646 | 1.12, (1.03, 1.22) | 0.0%, p = 0.661 | 0.442 | 0.060 |
| (f) Non-Hodgkin’s Lymphoma (nitrate) | 0.82, (0.69, 0.94) | 27.1%, p = 0.195 | 0.83, (0.75, 0.91) | 35.6%, p = 0.124 | 0.163 | 0.728 |
| (g) Non-Hodgkin’s Lymphoma (nitrite) | 1.21, (0.78, 1.64) | 63%, p = 0.019 | 1.11, (0.85, 1.38) | 71.4%, p = 0.004 | 0.496 | 0.702 |
| (h) Pancreatic (nitrate) | 0.96, (0.84, 1.09) | 35.9%, p = 0.167 | 0.95, (0.89, 1.00) | 48.0%, p = 0.087 | 0.722 | 0.399 |
| (i) Pancreatic (nitrite) | 0.87, (0.76, 0.97) | 44.3%, p = 0.095 | 1.04, (0.85, 1.24) | 76.1%, p = 0.000 | 0.000 | 0.000 |
| (j) Bladder (nitrate) | 0.94, (0.84, 1.04) | 0.0%, p = 0.615 | 0.94, (0.84, 1.03) | 70.7%, p = 0.001 | 0.089 | 0.322 |
| (k) Bladder (nitrite) | 1.07, (0.94, 1.19) | 0.0%, p = 0.574 | 1.05, (0.92, 1.18) | 79.2%, p = 0.000 | 0.045 | 0.338 |
| (l) Kidney (nitrate) | 0.79, (0.17, 1.41) | 64.7%, p = 0.059 | 0.84, (0.52, 1.16) | 73.1%, p = 0.024 | 0.019 | 0.016 |
| (m) Kidney (nitrite) | 0.92, (0.62, 1.23) | 0.0%, p = 0.586 | 1.10, (0.78, 1.48) | 76.7%, p = 0.014 | 0.322 | 0.245 |
| (n) Colon (nitrate) | 0.99, (0.91, 1.08) | 40.1%, p = 0.100 | 1.00, (0.96, 1.04) | 0.0%, p = 0.593 | 0.332 | 0.284 |
| (o) Colon (nitrite) | 1.02, (0.92, 1.11) | 44.8%, p = 0.093 | 1.02, (0.93, 1.11) | 67.2%, p = 0.006 | 0.027 | 0.141 |
| (p) Rectal (nitrate) | 1.01, (0.88, 1.14) | 33.4%, p = 0.161 | 1.10, (0.96, 1.24) | 68.8%, p = 0.002 | 0.367 | 0.930 |
| (q) Rectal (nitrite) | 1.09, (0.79, 1.39) | 68.2%, p = 0.008 | 1.06, (0.87, 1.26) | 82.3%, p = 0.000 | 0.841 | 0.952 |
| (r) Esophageal (nitrate) | 0.75, (0.57, 0.94) | 46.1%, p = 0.073 | 0.83, (0.72, 0.94) | 33.7%, p = 0.159 | 0.983 | 0.446 |
| (s) Esophageal (nitrite) | 1.01, (0.78, 1.23) | 0.0%, p = 0.689 | 0.93, (0.81, 1.05) | 0.0%, p = 0.881 | 0.197 | 0.714 |
| (t) Stomach (nitrate) | 0.81, (0.70, 0.92) | 0.0%, p = 0.776 | 0.81, (0.75, 0.87) | 22.0%, p = 0.234 | 0.000 | 0.006 |
| (u) Stomach (nitrite) | 1.06, (0.92, 1.20) | 32.9%, p = 0.127 | 1.04, (0.91, 1.11) | 54.7%, p = 0.012 | 0.308 | 0.382 |
I-squared (I2), a statistic representing the amount of total variation attributed to heterogeneity; p-value of Cochran’s Q test for heterogeneity.
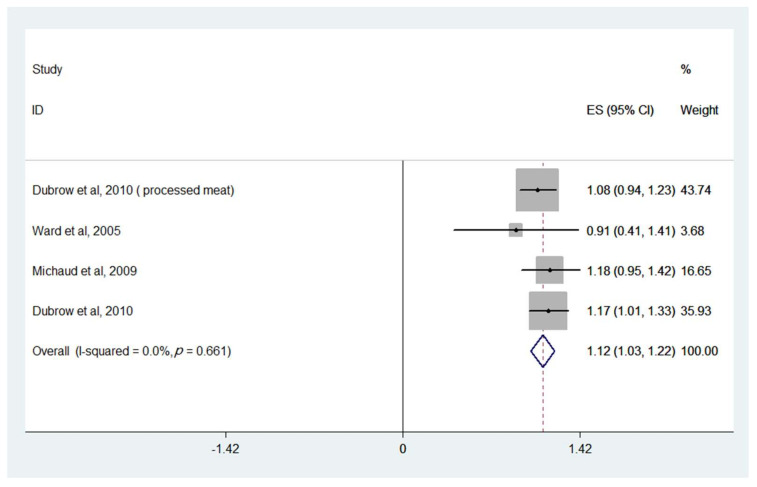
When pooling all intake categories and comparing against the lowest (reference) category, higher nitrite intake was associated with an increased risk of glioma (OR = 1.12, 95% CI: 1.03, 1.22) (Figure 4 and Table 1). Little heterogeneity was observed (I2 = 0.0%, p = 0.661). There was no evidence of association between the risk of cancers of the reproductive organs (ovary and uterine corpus), breast, non-Hodgkin’s lymphoma, stomach, pancreatic, esophageal, bladder, kidney, colon and rectal cancer, and dietary nitrate or nitrite consumption.
Figure 4.
ORs (95% CI) for glioma of all combined higher dosages versus the lowest category of dietary nitrite consumption for the following selected studies.
All results are shown in Table 1, and the remaining figures are shown in the Supplementary Figures S1 and S2.
3.2.3. Publication Bias
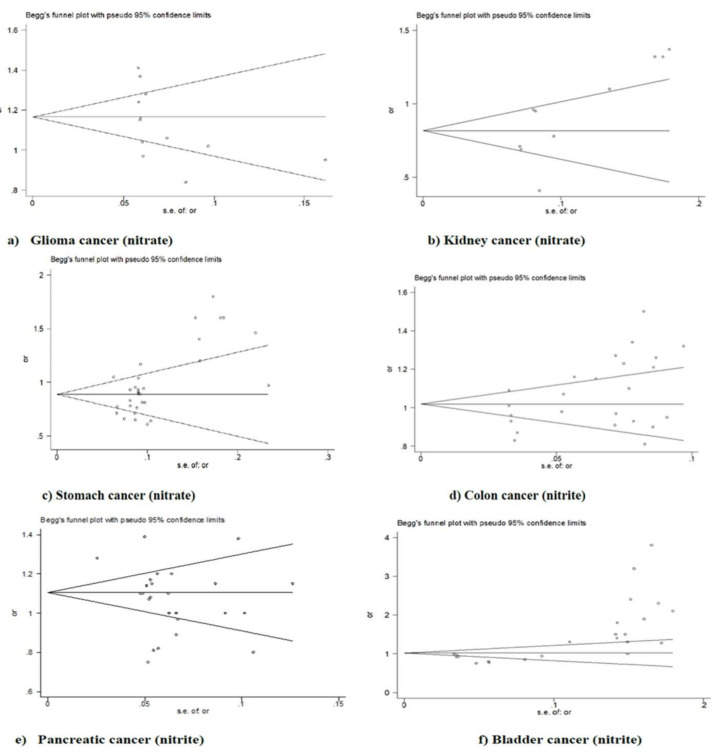
Both the Egger and Begg tests of bias indicated asymmetry (publication bias) for both cancer of the kidney (Egger, p = 0.019; Begg, p = 0.016; Figure 5b and Table 2), and stomach (Egger, p = 0.000; Begg, p = 0.006; Figure 5c and Table 2) with nitrates, and pancreatic cancer with nitrites (Egger, p = 0.000; Begg, p = 0.000; Figure 5e and Table 2). The Egger’s test showed statistical evidence of bias for colon cancer and nitrites (p = 0.027; Figure 5d and Table 2) but the Begg’s test did not (p = 0.141). The Begg’s test showed statistical evidence of bias for glioma and nitrates (p = 0.040; Figure 5a and Table 2) but the Egger’s test did not (p = 0.132). No other evidence of bias was indicated (Table 3). The remaining figures are shown in the Supplementary Figure S3.
Figure 5.
Funnel plot of nitrates and (a) glioma, (b) kidney, and (c) stomach cancer risk; nitrites and (d) colon, (e) pancreatic and (f) bladder cancer risk for publication bias.
Table 2.
Characteristics of the included studies and reported associations between dietary nitrate (mg/day) and cancer risk.
| First Author, Year, Country | Study Design | Case | Control/Number of Person-Years | Exposure Categories Nitrate Intake (mg/day) | Reported OR/RR/HR 95 CI | Cancer Sites | Adjustment | NOS |
|---|---|---|---|---|---|---|---|---|
| Briseis Aschebrook-Kilfoya et al., 2012, USA [51] | Cohort study, 1995–1996 | 128 143 143 140 155 |
⁕ | 36.7 58.1 78.4 109.5 175.4 |
1 (Reference) 1.13 (0.89–1.44) 1.15 (0.9–1.44) 1.14 (0.89–1.46) 1.31 (1.01–1.68) |
Ovary | Age, race, total energy intake, family history of ovarian cancer, BMI, education, smoking status, menopausal status, parity, age at menarche, and total daily dietary vitamin C intake | 8 |
| Peter J. Weyer et al., 2001, USA [52] | Cohort study, 1986–1998 | 24 28 28 22 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 1.12 (0.65–1.94) 1.1 (0.63–1.92) 0.85 (0.47–1.55) |
Ovary | Age and total energy intake | 7 |
| 71 41 51 61 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 0.6 (0.41–0.88) 0.78 (0.54–1.12) 0.97 (0.68–1.39) |
Uterine corpus | ||||
| Maki Inoue-Choi et al., 2015, USA [12] | Population-based cohort, 1986–2010 | 59 73 54 74 55 |
⁕ | 3.87–65.43 65.44–92.04 92.05–121.96 121.97–165.48 165.48–209.2 |
1 (Reference) 1.18 (0.83–1.68) 0.86 (0.58–1.26) 1.21 (0.84–1.74) 0.85 (0.56–1.27) |
Ovary | Age, BMI, family history of ovarian cancer, number of live births, age at menarche, age at menopause, age at first live birth, oral contraceptive use, estrogen use, and history of unilateral oophorectomy, and total energy intake | 8 |
| Maki Inoue-Choi et al., 2012, USA [26] | Prospective cohort study, 1986–2008 | 604 541 575 601 554 |
⁕ | 3.9–65.2 65.2–91.8 91.8–121.8 121.8–165.6 165.6–209.9 |
1 (Reference) 0.86 (0.76–0.98) 0.9 (0.79–1.02) 0.96 (0.84–1.1) 0.86 (0.74–1.01) |
Breast | Age, total energy intake, BMI, WHR, education, smoking, physical activity level, alcohol intake, family history of breast cancer, education, smoking status, age at menopause, age at first live birth, estrogen use, total intake of folate, vitamin C and E intake and flavonoids, intake of cruciferae and red meat | 8 |
| Nadia Espejo-Herrera et al., 2016, Spain [53] | Multicase–Control Study, 2008–2013 | 387 349 348 |
⁕ | 0–90 90–138 138–186 |
1 (Reference) 0.9 (0.74–1.1) 0.9 (0.73–1.1) |
Breast | Study area, age, and education | 6 |
| Peter J. Weyer et al., 2001, USA [52] | Cohort study in Iowa, 1986–1998 | 253 252 265 254 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 0.98 (0.83–1.17) 1.04 (0.87–1.24) 0.99 (0.83–1.19) |
Breast | Age and total energy intake | 7 |
| Briseis Aschebrook-Kilfoy, et al., 2013, China [14] | Cohort study, 1996–2009 | 34 56 41 33 |
⁕ | 165.8 257.8 350.6 506.8 |
1 (Reference) 1.81 (1.18–2.76) 1.44 (0.92–2.28) 1.32 (0.82–2.14) |
Thyroid | Age, total energy intake, education, and history of thyroid disease | 8 |
| Mary H. Ward, et al., 2010, USA [54] | Cohort study, 1986–2004 | 6 10 10 14 |
77,806 86,270 89,707 83,454 |
0–17.4 17.5–27.7 27.8–41.1 41.1–54.4 |
1 (Reference) 1.65 (0.59–4.61) 1.69 (0.58–4.84) 2.85 (1–8.11) |
Thyroid | Age, total calories, vitamin C intake, and residence location | 8 |
| Briseis Aschebrook-Kilfoy et al., 2011, USA [55] | Prospective cohort study, 1995–2003 | 63 67 60 74 106 |
⁕ | 29.6 49.8 70.2 100.9 166.8 |
1 (Reference) 1.01 (0.72–1.43) 0.87 (0.61–1.24) 1.04 (0.74–1.45) 1.41 (1.02–1.93) |
Thyroid | Age | 8 |
| Dominique S. Michaud, et al. 2009, USA [32] | 3 prospective cohort studies, 1976–2005 | 67 74 60 59 75 |
Sub-cohort (PY) =815,155 833,168 811,541 822,304 818,945 |
⁑ 69.3 94.7 127.7 180 |
1 (Reference) 1.06 (0.76–1.48) 0.84 (0.57–1.22) 0.95 (0.46–1.98) 1.02 (0.66–1.58) |
Glioma | Age and caloric intake | 6 |
| *Robert Dubrow et al., 2010, USA [29] | Prospective cohort study, 1995–2003 | 98 114 135 126 112 |
⁕ | 48.38 74.8 102.38 143.5 237.13 |
1 (Reference) 1.16 (0.89–1.52) 1.41 (1.09–1.84) 1.37 (1.05–1.79) 1.28 (0.97–1.7) |
Glioma | Sex, age, race, energy intake, education, height, and history of cancer at baseline | 8 |
| 100 121 135 109 120 |
⁕ | 0.275 (nitrite plus nitrate) 0.725 1.225 1.925 3.575 |
1 (Reference) 1.15 (0.88–1.5) 1.24 (0.95–1.61) 0.97 (0.74–1.28) 1.04 (0.79–1.36) |
|||||
| Mary H. Ward et al., 2006, USA [56] | Case-control study, 1998–2000 | 156 116 111 80 |
98 98 98 97 |
0–76 76–113.9 114–169.9 170–225.9 |
1 (Reference) 0.75 (0.51–1.1) 0.71 (0.47–1.07) 0.54 (0.34–0.86) |
Non-Hodgkin’s lymphoma | Age, education, sex, study center, race, dietary vitamin C, and total energy | 7 |
| Mary H. Ward, et al., 1996, USA [57] | Case-control study, 1950–1987 | 35 38 20 11 |
82 106 86 64 |
0–13 13–19 19–26 26–33 |
1 (Reference) 1.1 (0.6–2.0) 0.8 (0.4–1.7) 0.7 (0.3–1.9) |
Non-Hodgkin’s lymphoma | Age, gender, family history of cancer, vitamin C, and carotenes | 7 |
| Peter J. Weyer, et al., 2001, USA [52] | Cohort study, 1986–1998 | 37 34 25 38 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 0.88 (0.55–1.4) 0.62 (0.37–1.04) 0.91 (0.56–1.46) |
Non-Hodgkin’s lymphoma | Age and total energy intake | 7 |
| Briseis Aschebrook-Kilfoya et al., 2012, USA [58] | Case-control, 1996–2008 | ⁕ | ⁕ | 0–62.8 62.8–95.9 95.9–141 141–186.1 |
1 (Reference) 1 (0.7–1.4) 1.1 (0.7–1.6) 1 (0.7–1.5) |
Non-Hodgkin’s lymphoma | Calories, age, family history, and vitamin C | 7 |
| Briseis Aschebrook-Kilfoya et al., 2013, USA [59] | Case-control, 1999–2002 | 100 83 80 72 |
115 115 114 115 |
46.5 80.5 110.9 178.5 |
1 (Reference) 0.9 (0.6–1.3) 0.9 (0.6–1.3) 0.8 (0.5–1.3) |
Non-Hodgkin’s lymphoma | Sex, age, body mass index, education, family history of cancer, vitamin C, and daily caloric intake | 6 |
| Brian C.-H. Chiu et al., 2008, USA [60] | Case-control study, 1983–1986 | 17 19 24 |
357 358 360 |
0–70 70–106 106–142 |
1 (Reference) 1 (0.5–1.9) 1.2 (0.6–2.4) |
Non-Hodgkin’s lymphoma | Age, sex, type of respondent, family history of cancer, and body mass index | 8 |
| 17 19 24 |
357 358 360 |
0–65 65–101 101–137 |
1 (Reference) 1 (0.5–1.9) 1.2 (0.6–2.4) |
|||||
| 36 28 23 |
357 358 360 |
0–70 70–106 106–142 |
1 (Reference) 0.8 (0.5–1.3) 0.7 (0.4–1.2) |
|||||
| 36 29 22 |
357 358 360 |
0–65 65–101 101–137 |
1 (Reference) 0.8 (0.5–1.3) 0.6 (0.3–1.1) |
|||||
| Briseis Aschebrook-Kilfoya et al., 2010, USA [25] | Case-control study, 1995–2001 | 274 317 |
352 355 |
Low High |
1 (Reference) 1.09 (0.86–1.39) |
Non-Hodgkin’s lymphoma | Age, family history of cancer, calories, vitamin C intake, vitamin E intake, and protein intake | 7 |
| Arbor J.L. Quist et al., 2018, USA [61] | Cohort study, 1986–2011 | 78 80 73 60 17 |
n = 8558 8552 8568 6849 1715 |
0–16.2 16.2–23.9 24–34.2 34.3–58.5 58.5–82.7 |
1 (Reference) 1.08 (0.78–1.48) 0.99 (0.7–1.39) 1.05 (0.72–1.52) 1.25 (0.71–2.21) |
Pancreas | Age, smoking category, calories, and mutually adjusted for either natural log-transported nitrate or nitrite | |
| Angela Coss, et al., 2004, USA [62] | Case-control study, 1960–1987 | 26 33 39 43 |
298 311 311 327 |
0–58 58–82 83–117 117–151 |
1 (Reference) 1.1 (0.63–1.9) 1.2 (0.7–2) 1 (0.6–1.8) |
Pancreas | Age, cigarette use, and caloric intake | |
| 39 33 24 26 |
164 157 158 160 |
0–63 63–90 91–126 126–161 |
1 (Reference) 0.99 (0.58–1.7) 0.64 (0.36–1.1) 0.53 (0.29–0.97) |
|||||
| Peter J. Weyer et al., 2001, USA [52] | Cohort study, 1986–1998 | 19 15 16 19 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 0.79 (0.4–1.56) 0.86 (0.44–1.69) 1.02 (0.52–1.99) |
Pancreas | Age and total energy intake | 7 |
| *Jiali Zheng et al., 2019, USA [63] | Case–control study, 2002–2009 | 283 236 192 271 |
235 234 235 234 |
9.18–73.5 69.4–101.1 92.8–133.6 119.1–715.9 |
1 (Reference) 0.93 (0.72–1.2) 0.76 (0.59–0.99) 1.08 (0.84–1.39) |
Pancreas | Age and energy intake | |
| Briseis Aschebrook-Kilfoy et al., 2011, USA [64] | Prospective cohort study, 1995–2006 | 370 330 360 340 322 |
⁕ | 34.8 56.9 75.0 95.3 150.3 |
1 (Reference) 0.91 (0.78–1.06) 1.02 (0.88–1.18) 0.99 (0.85–1.16) 1.01 (0.85–1.2) |
Pancreas | Age, race, total energy intake, smoking status, family history of cancer, family history of diabetes, body mass index, and intakes of saturated fat, folate, and vitamin C | |
| Rena R. Jones, et al., 2016, USA [8] | Cohort study, 1986–2010 | 67 68 64 59 |
n = 8467 8489 8, 506 8502 |
0–16.2 16.2–23.9 24–34.2 34.2–44.4 |
1 (Reference) 1 (0.72–1.41) 0.92 (0.66–1.3) 0.86 (0.6–1.22) |
Bladder | Age and total in-transformed dietary nitrite from all sources | |
| Maurice P. Zeegers et al., 2006, Netherlands [65] | Cohort study, 1986–1995 |
168 186 181 180 174 |
Subcohort (PY) =8512 8652 8706 8707 8564 |
2–69 69–88 88–107.5 107.5–135.3 135.3–451.1 |
1 (Reference) 1.14 (0.89–1.45) 1 (0.78–1.27) 1.02 (0.8–1.3) 1.01 (0.79–1.29) |
Bladder | Age and sex | |
| Chelsea E. Catsburg et al., 2014, USA [38] | Case-control study, 1987–1996 | 467 329 293 274 284 |
315 314 315 315 314 |
0–64.3 64.4–91.4 91.5–117.3 117.4–148.3 148.4–179.3 |
1 (Reference) 0.79 (0.63–1.01) 0.74 (0.57–0.97) 0.78 (0.58–1.06) 0.9 (0.6–1.35) |
Bladder | Smoking duration, smoking intensity, and smoking status | 8 |
| Mary H. Ward et al., 2003, USA [35] | Case-control study, 1986–1989. | ⁕ | ⁕ | 0–59 59–84 84–119 119–154 |
1 (Reference) 0.8 (0.7–1.1) 0.9 (0.7–1.2) 0.9 (0.7–1.1) |
Bladder | Age, education, and cigarette smoking, years chlorinated surface water, and study period | |
| ⁕ | ⁕ | 0–62 62–90 90–127 127–164 |
1 (Reference) 1.2 (0.8–1.9) 0.9 (0.5–1.4) 0.8 (0.5–1.3) |
|||||
| Peter J. Weyer, et al., 2001, USA [52] | Cohort study, 1986–1998 | 9 17 13 14 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 1.88 (0.84–4.24) 1.46 (0.62–3.47) 1.57 (0.66–3.75) |
Bladder | Age and total energy intake | 7 |
| Kathryn Hughes Barry et al., 2020, New England [66] | Case–control study, 1994–1996 and 2001–2004 | 227 230 225 183 172 |
247 245 243 246 244 |
0–21.9 21.19–28.28 28.28–36.10 36.10–47.21 >47.21 |
1 (Reference) 1.2 (0.88–1.5) 1.2 (0.92–1.6) 1.0 (0.75–1.4) 0.95 (0.69–1.3) |
Bladder | Adjusted for age, gender, smoking status, high-risk occupation, race, ethnicity, state, dietary vitamin C intake (per 1000 kcal—continuous), dietary vitamin B12 (per 1000 kcal—continuous), total energy intake (kcal—continuous), and total water intake (L/d—continuous); models for nitrate/nitrite from processed meat were additionally adjusted for total meat intake (per 1000 kcal—continuous) | |
| *Leah M. Ferrucci et al., 2010, USA [5] | Cohort study, 1995–2003 | 236 185 150 145 138 |
⁕ | 49.25 76 103.75 145 238.5 |
1 (Reference) 0.86 (0.71–1.06) 0.76 (0.6–0.95) 0.77 (0.6–0.99) 0.8 (0.58–1.1) |
Bladder | Age, gender, smoking, intakes of fruit, vegetables, beverages, and total energy | |
| 109 147 173 191 234 |
⁕ | 0.05 0.175 0.275 0.425 0.725 |
1 (Reference) 0.97 (0.76–1.24) 1.09 (0.87–1.38) 1.07 (0.85–1.36) 1.2 (0.95–1.51) |
|||||
| Mary H. Ward et al., 2007, USA [27] | Case-control study, 1986–1989. | 109 83 84 57 |
471 472 471 472 |
0–59.32 59.32–86.62 86.63–122 122–157.77 |
1 (Reference) 0.71 (0.52–0.98) 0.69 (0.5–0.95) 0.41 (0.28–0.6) |
Kidney | Age, gender, sodium, and total calories | |
| Rena R. Jones et al., 2017, USA [67] | Cohort study, 1986–2010 |
67 65 66 43 15 |
n = 8467 8489 8, 506 6803 1699 |
0–16.2 16.2–23.9 23.91–34.27 34.28–58.64 58.6–82.96 |
1 (Reference) 0.96 (0.68–1.4) 0.95 (0.67–1.4) 0.78 (0.51–1.2) 1.1 (0.59–2) |
Kidney | Age, smoking status, pack-years of smoking, in-transformed total energy intake, body mass index, and total in-transformed total dietary nitrate or nitrite | |
| Peter J. Weyer et al., 2001, USA [52] | Cohort study from 1986–1998 | 12 15 14 14 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 1.32 (0.62–2.83) 1.32 (0.6–2.89) 1.37 (0.61–3.06) |
Kidney | Age and total energy intake | |
| Mary H. Ward et al., 2008, USA [68] | Case-control study from 1988–1994 |
14 17 28 39 |
99 99 99 100 |
0–3.8 3.8–5.7 5.7–8.3 8.3–10.9 |
1 (Reference) 0.7 (0.3–1.6) 1.7 (0.7–4.1) 2.2 (0.9–5.7) |
Esophagus | Year of birth, gender, body mass index, smoking, alcohol, total calories, vitamin A, folate, riboflavin, zinc, protein, and carbohydrate | |
| 29 27 18 24 |
99 99 99 100 |
0–16.9 16.9–26.2 26.2–38.8 38.8–51.4 |
1 (Reference) 0.9 (0.5–1.8) 0.6 (0.3–1.3) 0.8 (0.3–1.8) |
|||||
| Andra’s P. Keszei et al., 2013, The Netherlands [69] | Cohort study, 1986–2002 |
24 21 14 |
Sub-cohort (PY) 8383 9015 9050 |
68.1 100.8 146.2 |
1 (Reference) 0.82 (0.45–1.48) 0.54 (0.28–1.05) |
Esophagus | Age | |
| 39 36 39 |
8383 9015 9050 |
68.1 100.8 146.2 |
1 (Reference) 0.86 (0.54–1.37) 0.92 (0.58–1.46) |
|||||
| 15 18 15 |
9607 10,175 9996 |
66.4 98.5 142.7 |
1 (Reference) 1.17 (0.58–2.35) 1.02 (0.49–2.14) |
|||||
| 14 13 4 |
9607 10,175 9996 |
66.4 98.5 142.7 |
1 (Reference) 0.89 (0.42–1.92) 0.29 (0.09–0.89) |
|||||
| *Amanda J. Cross, et al., 2011, USA [24] | Cohort study, 1995–2006 | 22 25 15 25 41 |
⁕ | 0.605 0.1673 0.2818 0.4363 0.745 |
1 (Reference) 1.06 (0.59–1.91) 0.6 (0.3–1.18) 0.9 (0.49–1.67) 1.3 (0.72–2.35) |
Esophagus | Age, sex, BMI, education, ethnicity, tobacco smoking, alcohol drinking, usual physical activity at work, vigorous physical activity, daily intake of fruit, vegetables, saturated fat, and calories | |
| 47 61 68 89 112 |
⁕ | 0.605 0.1673 0.2818 0.4363 0.745 |
1 (Reference) 0.97 (0.66–1.43) 0.91 (0.62–1.35) 1.01 (0.7–1.47) 1.1 (0.75–1.6) |
|||||
| A. J. M. van Loon et al., 1998, The Netherlands [70] | Cohort study, 1986–1992 | 69 61 45 49 58 |
Sub-cohort (PY) 3784 3813 3814 3813 3796 |
55.8 79.4 98.7 120.7 172.2 |
1 (Reference) 0.93 (0.64–1.33) 0.65 (0.44–0.96) 0.71 (0.48–1.04) 0.83 (0.58–1.2) |
Stomach | Age and sex | |
| Raúl U. Hernández-Ramírez et al., 2009, Mexico [71] | Case-control study, 2004–2005 | ⁕ | ⁕ | 0–90.4 90.4–141.7 141.7–193 |
1 (Reference) 0.93 (0.62–1.39) 0.61 (0.39–0.96) |
Stomach | Energy, age, gender, H. pylori CagA status, schooling, and consumptions of salt, chili, and alcohol. | |
| Andra´s P. Keszei et al., 2013, The Netherlands [69] | Cohort study, 1986–2002 | 49 47 43 |
Sub-cohort (PY) 8383 9015 9050 |
68.1 100.8 146.2 |
1 (Reference) 0.89 (0.59–1.35) 0.81 (0.53–1.24) |
Stomach | Age | |
| 111 125 93 |
8383 9015 9050 |
68.1 100.8 146.2 |
1 (Reference) 1.05 (0.79–1.39) 0.77 (0.57–1.04) |
|||||
| 7 7 10 |
9607 10,175 9996 |
66.4 98.5 142.7 |
1 (Reference) 0.97 (0.34–2.81) 1.46 (0.54–3.93) |
|||||
| 59 46 55 |
9607 10,175 9996 |
66.4 98.5 142.7 |
1 (Reference) 0.76 (0.51–1.13) 0.95 (0.64–1.4) |
|||||
| Carlo La Vecchia, et al., 1994, Italy [72] | Case-control study, 1985–1992 | ⁕ | ⁕ | 62.95 80.7 96.33 116.88 |
1 (Reference) 0.71 (0.53–0.96) 0.66 (0.47–0.92) 0.78 (0.54–1.12) |
Stomach | Age, sex, education, family history of gastric cancer, body mass index, total energy intake, plus all above variables | 5 |
| * Amanda J. Cross et al., 2011, USA [24] | Cohort study, 1995–2006 | 50 48 50 56 73 |
⁕ | 0.605 0.1673 0.2818 0.4363 0.745 |
1 (Reference) 0.9 (0.6–1.35) 0.89 (0.59–1.33) 0.91 (0.61–1.37) 1.04 (0.69–1.55) |
Stomach | Age, sex, BMI, education, ethnicity, tobacco smoking, alcohol drinking, usual physical activity at work, vigorous physical activity, daily intake of fruit, vegetables, saturated fat, and calories. | |
| 39 57 36 61 62 |
⁕ | 0.605 0.1673 0.2818 0.4363 0.745 |
1 (Reference) 1.17 (0.77–1.77) 0.64 (0.4–1.02) 0.94 (0.61–1.45) 0.81 (0.52–1.25) |
|||||
| Mary H. Ward, et al., 2008, USA [68] | Case-control study from 1988–1994 |
19 31 25 29 |
99 99 99 100 |
0–3.8 (nitrite plus nitrate) 3.8–5.7 5.7–8.3 8.3–10.9 |
1 (Reference) 1.6 (0.8–3.2) 1.8 (0.8–3.8) 1.6 (0.7–3.7) |
Stomach |
Year of birth, gender, education, smoking, alcohol, total calories, vitamin C, fiber, and carbohydrate. | |
| 24 28 26 26 |
99 99 99 100 |
0–16.9 16.9–26.2 26.2–38.8 38.8–51.4 |
1 (Reference) 1.2 (0.6–2.5) 1.4 (0.7–2.9) 1.6 (0.7–3.6) |
|||||
| Curt T. Della Valle et al., 2014, China [44] | Prospective cohort study, 1996 to 2007 | 83 70 65 87 78 |
⁕ | 98.7 144.1 182.4 229 313.2 |
1 (Reference) 0.9 (0.65–1.25) 0.84 (0.59–1.2) 1.13 (0.77–1.66) 0.98 (0.59–1.63) |
Colon | Age, energy intake, education, physical activity, dietary vitamin C intake, carotene, and folate | |
| Nadia Espejo-Herrera et al., 2016, Spain [53] | Case-control study, 2008–2013 | 388 394 371 |
⁕ | 0–83 83–133 133–183 |
1 (Reference) 1.04 (0.87–1.24) 0.9 (0.74–1.1) |
Colon | Sex, age, education, physical activity, non-steroidal anti-inflammatory drugs use, family history of colorectal cancer, body mass index, and intake energy | |
| Rena R. Jones et al., 2019, USA [41] | Cohort study, 1986–2010 | 324 324 321 355 |
n = 8676 8674 8685 8673 |
0–9.8 9.81–13.8 13.81–19.29 19.29–24.77 |
1 (Reference) 0.98 (0.84–1.15) 0.97 (0.83–1.13) 1.11 (0.94–1.3) |
Colon | Age, heme iron, red meat, and total dietary nitrate or nitrite | |
| Peter J. Weyer et al., 2001, USA [52] | Cohort study, 1986–1998 | 98 78 90 97 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 0.79 (0.59–1.07) 0.93 (0.69–1.24) 1 (0.74–1.34) |
Colon | Age and total energy intake | 7 |
| * Amanda J. Cross et al., 2010, USA [29] | Prospective cohort study, 1994–2003 | 341 344 386 439 485 |
⁕ | 0.0598 0.1633 0.274 0.423 0.723 |
1 (Reference) 0.93 (0.8–1.08) 0.99 (0.86–1.16) 1.08 (0.93–1.25) 1.13 (0.97–1.32) |
Colon | Gender, education, BMI, smoking, and intake of total energy, fiber, and dietary calcium | |
| * L. M. Ferrucci et al., 2012, USA [73] | Multi-center, randomized controlled trial, 1993–2001. | 150 165 203 254 |
⁕ | 0.15 (nitrite plus nitrate) 0.425 0.9 2.1 |
1 (Reference) 0.98 (0.77–1.23) 1.07 (0.84–1.35) 1.16 (0.9–1.5) |
Colon | Age, study center, gender, ethnicity, education, family history of colorectal cancer, BMI, NSAIDs use, physical activity, smoking status, alcohol intake, dietary calcium, supplemental calcium, dietary fibre, and total energy intake | |
| Anneclaire J. De Roos et al., 2003, USA [28] | Case-control study, 1986–1990 | (n(%)) 89 (32) 68 (24) 68 (24) 55 (20) |
(n(%)) 261 (27) 241 (25) 246 (25) 234 (24) |
0–59.3 59.3–86.5 86.6–121.9 122–157 |
1 (Reference) 0.8 (0.6–1.2) 0.8 (0.5–1.1) 0.7 (0.4–1) |
Colon | Age, sex, and chlorinated surface water | |
| Yun Zhu et al. 2014 [74] | Case-control study, 1997–2006 | 127 153 122 137 122 |
517 480 489 479 516 |
56.94 91.45 124.81 169.59 264.14 |
1 (Reference) 1.25 (0.93–1.66) 0.9 (0.66–1.23) 1.06 (0.78–1.48) 0.75 (0.54–1.05) |
Colon | Age, sex, energy intake, BMI, cigarette smoking status, education attainment, reported colon screening procedures, NSAID use, multivitamin supplement use, folate supplement use, vegetable intake, and province of residence | |
| 109 113 128 122 114 |
517 480 489 479 516 |
56.94 91.45 124.81 169.59 264.14 |
1 (Reference) 1.07 (0.78–1.48) 1.24 (0.9–1.71) 1.31 (0.94–1.83) 1.01 (0.71–1.45) |
|||||
| Curt T. Della Valle et al., 2014, China [44] | Prospective cohort study, 1996–2007 | 46 39 41 51 59 |
⁕ | 98.7 144.1 182.4 229 313.2 |
1 (Reference) 0.9 (0.58–1.4) 0.95 (0.6–1.5) 1.17 (0.72–1.9) 1.26 (0.69–2.32) |
Rectum | Age, energy intake, education, physical activity, dietary vitamin C intake, carotene, and folate | |
| Nadia Espejo-Herrera et al., 2016, Spain [53] | Case-control study, 2008–2013. | 195 161 151 |
⁕ | 0–83 83–133 133–183 |
1 (Reference) 0.85 (0.66–1.08) 0.76 (0.58–1) |
Rectum | Sex, age, education, physical activity, non-steroidal anti-inflammatory drugs use, family history of colorectal cancer, body mass index, and intake energy | |
| Rena R. Jones et al., 2019, USA [41] | Cohort study, 1986–2010 | 79 81 71 94 |
n = 8676 8674 8685 8673 |
0–9.8 9.81–13.8 13.81–19.29 19.29–24.77 |
1 (Reference) 1.03 (0.76–1.41) 0.91 (0.66–1.26) 1.27 (0.93–1.74) |
Rectum | Age and total dietary nitrate or nitrite | |
| Peter J. Weyer et al., 2001, USA [52] | Cohort study, 1986–1998. | 28 39 27 28 |
⁕ | 0–11.6 11.6–18 18.1–27.2 27.2–36.3 |
1 (Reference) 1.42 (0.87–2.31) 1.01 (0.59–1.73) 1.06 (0.61–1.83) |
Rectum | Age and total energy intake | 7 |
| * Amanda J. Cross et al., 2010, USA [29] | Prospective cohort study, 1994–2003 | 110 126 144 170 174 |
⁕ | 0.0598 0.1633 0.274 0.423 0.723 |
1 (Reference) 1.08 (0.83–1.4) 1.18 (0.91–1.52) 1.31 (1.01–1.68) 1.26 (0.97–1.63) |
Rectum | Gender, education, BMI, smoking, and intake of total energy, fiber, and dietary calcium. | |
| * L. M. Ferrucci et al., 2012, USA [73] | Multi-center, randomized controlled trial, 1993–2001 | 44 64 75 80 |
⁕ | 0.15 (nitrite plus nitrate) 0.425 0.9 2.1 |
1 (Reference) 1.31 (0.88–1.95) 1.38 (0.92–2.07) 1.27 (0.8–1.99) |
Rectum | Age, study center, gender, ethnicity, education, family history of colorectal cancer, BMI, NSAIDs use, physical activity, smoking status, alcohol intake, dietary calcium, supplemental calcium, dietary fiber, and total energy intake | |
| Anneclaire J. De Roos et al., 2003, USA [28] | Case-Control study, 1986–1990 | (n(%)) 56 (22) 67 (27) 66 (27) 60 (24) |
(n(%)) 261 (27) 241 (25) 246 (25) 234 (24) |
0–59.3 59.3–86.5 86.6–121.9 122–157 |
1 (Reference) 1.3 (0.9–1.9) 1.2 (0.8–1.8) 1.1 (0.8–1.7) |
Rectum | Age, sex, and chlorinated surface water | |
| Yun Zhu, et al. 2014 [74] | Case-control study, 1997–2006 | 118 126 130 133 118 |
517 480 489 479 516 |
56.94 91.45 124.81 169.59 264.14 |
1 (Reference) 1.12 (0.83–1.53) 1.23 (0.9–1.69) 1.34 (0.94–1.85) 1.03 (0.73–1.46) |
Rectum | Age, sex, energy intake, BMI, cigarette smoking status, education attainment, reported colon screening procedures, NSAID use, multivitamin supplements use, folate supplement use, vegetable intakes and province of residence. |
* Original exposure categories of nitrates from studies converted to mg/day for meta-regression calculation (explained under statistics analysis); ⁕ missing cases/controls/person-years in sub-cohort from the studies; ⁑ missing nitrate dosage.
Table 3.
Characteristics of the included studies and reported associations between dietary nitrite (mg/day) and cancer risk.
| First Author, Year, Country | Study Design | Case | Control | Exposure Categories Nitrite intake mg/day) |
Reported OR/RR/HR 95 CI | Cancer sites | Adjustment | NOS |
|---|---|---|---|---|---|---|---|---|
| * Robert Dubrow et al., 2010, USA [29] | Prospective cohort study, 1995–2003 | 100 121 135 109 120 |
⁕ | 0.275 (nitrite plus nitrate) 0.725 1.225 1.925 3.575 |
1 (Reference) 1.15 (0.88–1.5) 1.24 (0.95–1.61) 0.97 (0.74–1.28) 1.04 (0.79–1.36) |
Glioma | Sex, age, race, energy intake, education, height, and history of cancer at baseline | 8 |
| 101 129 106 118 131 |
⁕ | 1.13 1.43 1.63 1.85 2.25 |
1 (Reference) 1.25 (0.96–1.63) 1.03 (0.79–1.36) 1.16 (0.89–1.52) 1.32 (1.01–1.71) |
|||||
| Mary H. Ward et al., 2005, USA [75] | Case-control study, 1983–1994 | 38 27 23 33 |
67 74 71 73 |
0–0.7 0.7–0.94 0.94–1.19 1.19–1.44 |
1 (Reference) 0.8 (0.4–1.7) 1.0 (0.4–2.3) 1.2 (0.5–3.2) |
Glioma | Age, gender, respondent type, education, ever live/work on a farm, education, beta-carotene, fiber, and calories | 6 |
| Dominique S Michaud et al., 2009, USA [32] | 3 prospective cohort studies, 1976–2005 | 55 65 71 69 75 |
Sub-cohort (PY) =812,763 812,974 844,064 810,417 820,895 |
⁑ 1.4 1.6 1.8 2.03 |
1 (Reference) 1.11 (0.72–1.71) 1.2 (0.84–1.71) 1.14 (0.73–1.78) 1.26 (0.89–1.79) |
Glioma | Sex, age, race, energy intake, education, height, and history of cancer at baseline | 8 |
| Mary H. Ward et al., 2006, USA [56] | Case-control study, 1998- 2000 | 82 108 110 166 |
98 98 98 97 |
0–0.71 0.71–0.909 0.91–1.209 1.21–1.509 |
1 (Reference) 1.5 (1–2.3) 1.7 (1.1–2.7) 3.1 (1.7–5.5) |
Non-Hodgkin’s lymphoma | Age, education, sex, study center, race, dietary vitamin C, and total energy | |
| Briseis Aschebrook-Kilfoya et al., 2012, USA [58] | Case-control study, 1996–2008 | ⁕ | ⁕ | 0–0.8 0.8–1.1 1.1–1.4 1.4–1.7 |
1 (Reference) 1.2(0.8–1.6) 0.8 (0.6–1.3) 1 (0.6–1.6) |
Non-Hodgkin’s lymphoma | Calories, age, family history, and vitamin C | |
| Briseis Aschebrook-Kilfoya et al., 2013, USA [14] | Case-control study, 1999–2002 | 82 90 68 95 |
114 115 116 114 |
0.9 1.2 1.5 1.7 |
1 (Reference) 1.2 (0.8–1.8) 0.8 (0.5–1.3) 1.3 (0.8–1.9) |
Non-Hodgkin’s lymphoma | Sex, age, body mass index, education, family history of cancer, vitamin C, and daily caloric intake | |
| Brian C.-H. Chiu et al., 2008, USA [60] | Case-control study, 1983–1986 | 14 15 31 |
357 358 360 |
0–1 1–1 1–2 |
1 (Reference) 1.1 (0.5–2.4) 2.8 (1.3–6.1) |
Non-Hodgkin’s lymphoma | Age, sex, type of respondent, family history of cancer, and body mass index | 8 |
| 39 25 23 |
357 358 360 |
0–1 1–1 1–2 |
1 (Reference) 0.7 (0.4–1.1) 0.6 (0.3–1.2) |
|||||
| Briseis Aschebrook-Kilfoya et al., 2010, USA [25] | Case-control study, 1995–2001 | 248 345 |
349 355 |
Low High |
1 (Reference) 1.37 (1.04–1.79) |
Non-Hodgkin’s lymphoma | Age, family history of cancer, calories, vitamin C intake, vitamin E intake, and protein intake | |
| Arbor J.L. Quist et al., 2018, USA [61] | Cohort study, 1986–2011 | 88 67 70 68 15 |
n = 8501 8505 8753 6761 1722 |
0–0.86 0.86–1.11 1.12–1.43 1.44–2.05 2.05–2.66 |
1 (Reference) 0.85 (0.59–1.22) 0.94 (0.62–1.42) 1.3 (0.79–2.14) 1.28 (0.59–2.79) |
Pancreas | Age, smoking category, calories, and mutually adjusted for either natural log-transported nitrate or nitrite | |
| Angela Coss et al., 2004, USA [62] | Case-control study, 1960–1987 | 15 22 40 64 |
233 307 333 374 |
0–0.75 0.75–0.98 0.99–1.3 1.3–1.61 |
1 (Reference) 1 (0.52–2) 1.5 (0.81–2.9) 1.5 (0.79–3) |
Pancreas | Age, cigarette use, and caloric intake | |
| 9 22 60 50 |
264 282 359 342 |
0–0.22 0.22–0.31 0.32–0.53 0.53–0.74 |
1 (Reference) 2.1 (0.95–4.8) 3.8 (1.8–8) 2.3 (1.1–5.1) |
|||||
| 18 32 32 40 |
144 146 168 181 |
0–0.56 0.56–0.71 0.72–0.93 0.93–1.13 |
1 (Reference) 1.8 (0.94–3.4) 1.4 (0.72–2.6) 1.3 (0.65–2.5) |
|||||
| 13 32 26 51 |
148 164 147 180 |
0–0.13 0.13–0.18 0.19–0.26 0.26–0.33 |
1 (Reference) 2.4 (1.2–4.7) 1.9 (0.94–4) 3.2 (1.6–6.4) |
|||||
| * Jiali Zheng et al., 2019, USA [63] | Case–control study, 2002–2009 | 291 226 225 215 |
235 234 235 234 |
0.025–1.475 1.375–2.1 2.075–2.925 2.9–9.65 |
1 (Reference) 0.8 (0.62–1.03) 0.77 (0.6–1) 0.75 (0.59–0.91) |
Pancreas | Age and energy intake | |
| Briseis Aschebrook-Kilfoy et al., 2011, USA [64] | Prospective cohort study, 1995- 2006 | 361 361 331 348 321 |
⁕ | 0.8 1.0 1.2 1.2 1.6 |
1(Reference) 0.99 (0.86–1.16) 0.92 (0.79–1.08) 0.97 (0.83–1.14) 0.92 (0.78–1.08) |
Pancreas | Age, race, total energy intake, smoking status, family history of cancer, family history of diabetes, body mass index, and intakes of saturated fat, folate, and vitamin C | |
| Rena R. Jones et al., 2016, USA [8] | Cohort study, 1986–2010 | 63 66 73 56 |
n = 8450 8514 8487 8513 |
0–0.86 0.86–1.12 1.13–1.43 1.44–1.74 |
1 (Reference) 1.15 (0.78–1.7) 1.38 (0.89–2.16) 1.15 (0.65–2.03) |
Bladder | Age, smoking status, pack-years of smoking, in-transformed total energy intake, and total in-transformed dietary nitrate from all sources | |
| Kathryn Hughes Barry et al., 2020, New England [76] | Case–control study, 1994–1996, 2001–2004 | 222 212 202 217 184 |
243 245 244 248 245 |
0–0.48 0.48–0.56 0.56–0.63 0.63–0.72 >0.72 |
1 (Reference) 1.0 (0.77–1.4) 1.0 (0.74–1.3) 1.1 (0.80–1.4) 0.97 (0.71–1.3) |
Bladder | Adjusted for age, gender, smoking status, high-risk occupation, race, ethnicity, state, dietary vitamin C intake (per 1000 kcal—continuous), dietary vitamin B12 (per 1000 kcal—continuous), total energy intake (kcal—continuous), and total water intake (L/d—continuous); models for nitrate/nitrite from processed meat were additionally adjusted for total meat intake (per 1000 kcal—continuous) | |
| Mary H. Ward et al., 2003, USA [35] | Case-control study, 1986–1989 | ⁕ | ⁕ | 0–0.81 0.81–1.06 1.06–1.39 1.39–1.72 |
1 (Reference) 1.1 (0.9–1.4) 1.2 (0.9–1.5) 1.2 (0.9–1.6) |
Bladder | Age, education, and cigarette smoking, years chlorinated surface water, and study period | |
| ⁕ | ⁕ | 0–0.58 0.58–0.75 0.75–0.98 0.98–1.21 |
1 (Reference) 1 (0.6–1.5) 0.8 (0.5–1.3) 1 (0.7–1.6) |
|||||
| * Leah M. Ferrucci et al., 2010, USA [5] | Cohort study, 1995– 2003 | 176 181 164 161 172 |
⁕ | 1.15 1.425 1.625 1.85 2.275 |
1 (Reference) 1.17 (0.9–1.45) 1.1 (0.89–1.37) 1.14 (0.91–1.44) 1.28 (1.02–1.61) |
Bladder | Age, gender, smoking, intakes of fruit, vegetables, beverages, and total energy | |
| 109 147 173 191 234 |
⁕ | 0.025 0.075 0.15 0.25 0.475 |
1 (Reference) 1.15 (0.9–1.46) 1.08 (0.85–1.37) 1.39 (1.11–1.74) 1.07 (0.85–1.36) |
|||||
| * Chelsea E. Catsburg et al., 2014, USA [38] | Case-control study, 1987– 1996 | 400 287 302 314 344 |
314 316 313 315 315 |
0–0.234 0.235–0.311 0.312–0.4 0.401–0.532 0.532–0.664 |
1 (Reference) 0.75 (0.59–0.94) 0.81 (0.63–1.03) 0.82 (0.64–1.07) 0.89 (0.66–1.2) |
Bladder | Smoking duration, smoking intensity, and smoking status | 8 |
| Mary H. Ward et al., 2007, USA [27] | Case-control study, 1986–1989 | 92 74 78 89 |
471 472 471 472 |
0–0.7 0.7–0.93 0.94–1.25 1.26–1.57 |
1 (Reference) 0.82 (0.58–1.17) 0.84 (0.57–1.22) 0.82 (0.5–1.33) |
Kidney | Age, gender, sodium, total fat, and total calories | |
| 64 90 88 91 |
471 472 471 472 |
0–0.18 0.18–0.28 0.29–0.47 0.48–0.66 |
1 (Reference) 1.37 (0.95–1.95) 1.24 (0.85–1.83) 1 (0.63–1.59) |
|||||
| Rena R. Jones et al., 2017, USA [67] | Cohort study, 1986–2010 | 57 68 69 49 13 |
n = 8450 8514 8487 1704 6809 |
0–0.86 0.86–1.12 1.13–1.43 1.44–2.06 2.06–2.68 |
1 (Reference) 1.3 (0.87–1.9) 1.4 (0.89–2.3) 1.4 (0.77–2.5) 1.6 (0.7–3.8) |
Kidney | Age, smoking status, pack-years of smoking, in-transformed total energy intake, body mass index, and total in-transformed total dietary nitrate or nitrite | |
| Mary H. Ward et al., 2008, USA [68] | Case-control study, 1988–1994 |
23 28 17 30 |
94 102 101 100 |
0–0.36 0.36–0.52 0.52–0.67 0.67–0.82 |
1 (Reference) 1.1 (0.5–2.3) 0.6 (0.2–1.3) 1 (0.4–2.4) |
Esophagus | Year of birth, gender, body mass index, smoking, alcohol, total calories, vitamin A, folate, riboflavin, zinc, protein, and carbohydrate | |
| 19 31 25 29 |
99 99 99 100 |
0–3.8 (nitrite plus nitrate) 3.8–5.7 5.7–8.3 8.3–10.9 |
1 (Reference) 0.7 (0.3–1.6) 1.7 (0.7–4.1) 2.2 (0.9–5.7) |
|||||
| Andra´s P Keszei et al., 2013, The Netherlands [69] | Cohort study, 1986–2002 | 17 19 23 |
Sub-cohort (PY) 8665 8895 8890 |
0.03 0.12 0.28 |
1 (Reference) 1.18 (0.61–2.3) 1.49 (0.78–2.87) |
Esophagus | Age | |
| 42 38 34 |
8665 8895 8890 |
0.03 0.12 0.28 |
1(Reference) 0.9 (0.57–1.43) 0.81 (0.5–1.31) |
|||||
| 16 18 14 |
10,009 10,016 9752 |
0.02 0.08 0.2 |
1 (Reference) 1.17 (0.59–2.32) 0.96 (0.46–2) |
|||||
| 12 12 7 |
10,009 10,016 9752 |
0.02 0.08 0.2 |
1 (Reference) 1.05 (0.47–2.36) 0.64 (0.25–1.64) |
|||||
| * Amanda J. Cross et al., 2011, USA [24] | Cohort study, 1995–2006 | 20 30 19 28 31 |
⁕ | 0.0303 0.0865 0.1535 0.2573 0.498 |
1 (Reference) 1.36 (0.76–2.43) 0.82 (0.43–1.57) 1.15 (0.63–2.11) 1.21 (0.67–2.2) |
Esophagus | Age, sex, BMI, education, ethnicity, tobacco smoking, alcohol drinking, usual physical activity at work, vigorous physical activity, daily intake of fruit, vegetables, saturated fat, and calories | |
| 50 60 66 81 120 |
⁕ | 0.0303 0.0865 0.1535 0.2573 0.498 |
1 (Reference) 0.89 (0.61–1.3) 0.82 (0.56–1.2) 0.88 (0.61–1.27) 1.19 (0.84–1.68) |
|||||
| Lawrence S. Engel et al., 2003, USA [77] | Case–control study, 1993–1995 | ⁕ | ⁕ | 1.8–5.55 5.65–7.2 7.3–9.5 9.6–35.2 |
1 (Reference) 1.5 (1–2.4) 1.8 (1.1–3) 2.5(1.4–4.3) |
Stomach | Geographic center, age, sex, race, income, respondent type, energy intake, and the other factors included in the table | |
| A. J. M. van Loon et al., 1998, The Netherlands [70] | Cohort study, 1986–1992 | 47 51 58 46 80 |
Sub-cohort (PY) 3873 3706 3829 3844 3760 |
0.01 0.04 0.09 0.16 0.35 |
1 (Reference) 1.15 (0.76–1.74) 1.21 (0.81–1.83) 0.87 (0.57–1.33) 1.49 (1.01–2.2) |
Stomach | Age and sex | |
| Raúl U. Hernández-Ramírez et al., 2009, Mexico [71] | Case–control study, 2004–2005 | ⁕ | ⁕ | 0–1 1–1.2 1.2–1.4 |
1 (Reference) 1.07 (0.69–1.65) 1.52 (0.99–2.34) |
Stomach | Energy, age, gender, H. pylori CagA status, schooling, and consumptions of salt, chili, and alcohol | |
| Andra´s P Keszei et al., 2013, The Netherlands [69] | Cohort study, 1986–2002 | 47 39 53 |
Sub-cohort (PY) 8665 8895 8890 |
0.03 0.12 0.28 |
1 (Reference) 0.83 (0.53–1.29) 1.14 (0.75–1.72) |
Stomach | Age | |
| 98 109 122 |
8665 8895 8890 |
0.03 0.12 0.28 |
1 (Reference) 1.17 (0.87–1.58) 1.36 (1.01–1.82) |
|||||
| 9 9 6 |
10,009 10,016 9752 |
0.02 0.08 0.2 |
1 (Reference) 1.05 (0.41–2.67) 0.73 (0.26–2.07) |
|||||
| 56 50 54 |
10,009 10,016 9752 |
0.02 0.08 0.2 |
1 (Reference) 0.94 (0.63–1.39) 1.06 (0.71–1.57) |
|||||
| Carlo La Vecchia et al., 1994, Italy [72] | Case-control study, 1985–1992 | ⁕ | ⁕ | 1.91 2.41 2.94 3.64 |
1 (Reference) 0.96 (0.69–1.32) 0.97 (0.7–1.35) 1.02 (0.73–1.43) |
Stomach | Age, sex, education, family history of gastric cancer, body mass index, total energy intake, plus all the above variables | 5 |
| * Amanda J. Cross et al., 2011, USA [24] | Cohort study, 1995–2006 | 44 40 55 61 55 |
⁕ | 0.0303 0.0865 0.1535 0.2573 0.498 |
1 (Reference) 0.72 (0.47–1.11) 0.88 (0.58–1.32) 0.87 (0.58–1.31) 0.71 (0.47–1.08) |
Stomach | Age, sex, BMI, education, ethnicity, tobacco smoking, alcohol drinking, usual physical activity at work, vigorous physical activity, daily intake of fruits, vegetables, saturated fat, and calories | |
| 54 44 48 67 64 |
⁕ | 0.0303 0.0865 0.1535 0.2573 0.498 |
1 (Reference) 0.77 (0.51–1.15) 0.79 (0.53–1.18) 1.04 (0.71–1.52) 0.93 (0.63–1.37) |
|||||
| Mary H. Ward, et al., 2008, USA [68] | Case-control study, 1988–1994 |
23 22 29 30 |
94 102 101 100 |
0–0.36 0.36–0.52 0.52–0.67 0.67–0.83 |
1 (Reference) 1.1 (0.4–2.7) 0.8 (0.3–2.2) 1.1 (0.3–3.4) |
Stomach | Year of birth, gender, education, smoking, alcohol, total calories, vitamin C, fiber, and carbohydrate | |
| 19 31 25 29 |
99 99 99 100 |
0–3.8 3.8–5.7 5.7–8.3 8.3–10.9 |
1 (Reference) 1.6 (0.8–3.2) 1.8 (0.8–3.8) 1.6 (0.7–3.7) |
|||||
| Curt T. DellaValle et al., 2014, China [44] | Prospective cohort study, 1996–2007 | 72 81 75 80 75 |
⁕ | 0.56 0.74 0.87 1.01 1.23 |
1 (Reference) 1.27 (0.92–1.76) 1.23 (0.88–1.73) 1.34 (0.94–1.9) 1.26 (0.85–1.86) |
Colon | Age, energy intake, education, physical activity, dietary vitamin C intake, carotene, and folate | |
| Rena R. Jones et al., 2019, USA [41] | Cohort study, 1986– 2010 | 345 342 320 317 |
n = 8588 8655 8974 8491 |
0–0.57 0.58–0.65 0.66–0.74 0.74–0.82 |
1 (Reference) 0.93 (0.8–1.08) 0.83 (0.71–0.97) 0.87 (0.74–1.02) |
Colon | Age, heme iron, red meat, and total dietary nitrate or nitrite | |
| * Amanda J. Cross et al., 2010, USA [29] | Prospective cohort study, 1994–2003 | 344 359 397 441 454 |
⁕ | 0.0298 0.0843 0.1493 0.2498 0.4853 |
1 (Reference) 0.96 (0.83–1.12) 1.01 (0.88–1.18) 1.09 (0.94–1.26) 1.09 (0.94–1.26) |
Colon | Gender, education, BMI, smoking, and intake of total energy, fiber, and dietary calcium | |
| * L. M. Ferrucci et al., 2012, USA [73] | Multi-center, randomized controlled trial 1993–2001 | 150 165 203 254 |
⁕ | 0.15 (nitrite plus nitrate) 0.425 0.9 2.1 |
1 (Reference) 0.98 (0.77–1.23) 1.07 (0.84–1.35) 1.16 (0.9–1.5) |
Colon | Age, study center, gender, ethnicity, education, family history of colorectal cancer, BMI, NSAIDs use, physical activity, smoking status, alcohol intake, dietary calcium, supplemental calcium, dietary fiber, and total energy intake | |
| Anneclaire J. De Roos et al., 2003, USA [28] | Case-Control study, 1986–1990 | (n(%)) 90 (32) 73 (26) 48 (17) 69 (25) |
(n(%)) 311 (32) 251 (26) 220 (22) 200 (20) |
0–0.704 0.705–0.93 0.94–1.25 1.26–1.57 |
1 (Reference) 1.1 (0.8–1.6) 0.9 (0.6–1.3) 1.5 (1–2.1) |
Colon | Age, sex, and chlorinated surface water | |
| Yun Zhu et al. 2014 [74] | Case-control study, 1997–2006 | 131 145 126 120 139 |
536 496 520 474 455 |
0.65 0.89 1.12 1.4 1.92 |
1 (Reference) 1.15 (0.86–1.54) 0.91 (0.66–1.26) 0.81 (0.56–1.18) 0.95 (0.63–1.43) |
Colon | Age, sex, energy intake, BMI, cigarette smoking status, education attainment, reported colon screening procedures, NSAID use, multivitamin supplements use, folate supplement use, vegetable intakes, and province of residence | |
| 107 112 101 132 134 |
536 496 520 474 455 |
0.65 0.89 1.12 1.4 1.92 |
1 (Reference) 0.97 (0.7–1.34) 0.93 (0.65–1.32) 1.21 (0.82–1.78) 1.32 (0.85–2.04) |
|||||
| Curt T. Della Valle et al., 2014, China [44] | Prospective cohort study, 1996–2007 | 57 45 48 42 44 |
⁕ | 0.56 0.74 0.87 1.01 1.23 |
1 (Reference) 0.87 (0.58–1.29) 0.94 (0.63–1.42) 0.81 (0.52–1.25) 0.8 (0.49–1.29) |
Rectum | Age, energy intake, education, physical activity, dietary vitamin C intake, carotene, and folate | |
| Rena R. Jones et al., 2019, USA [41] | Cohort study, 1986– 2010 | 93 74 91 67 |
n = 8588 8655 8974 8491 |
0–0.57 0.58–0.65 0.66–0.74 0.74–0.82 |
1 (Reference) 0.75 (0.55–1.02) 0.88 (0.65–1.18) 0.68 (0.49–0.94) |
Rectum | Age and total dietary nitrate or nitrite | |
| * Amanda J. Cross et al., 2010, USA [29] | Prospective cohort study, 1994–2003 | 113 129 157 162 163 |
⁕ | 0.0298 0.0843 0.1493 0.2498 0.4853 |
1 (Reference) 1.07 (0.83–1.38) 1.23 (0.96–1.58) 1.21 (0.94–1.55) 1.16 (0.9–1.5) |
Rectum | Gender, education, BMI, smoking, and intake of total energy, fiber, and dietary calcium | |
| * L. M. Ferrucci et al., 2012, USA [73] | Multi-center, randomized controlled trial 1993–2001 | 44 64 75 80 |
⁕ | 0.15 (nitrite plus nitrate) 0.425 0.9 2.1 |
1 (Reference) 1.31 (0.88–1.95) 1.38 (0.92–2.07) 1.27 (0.8–1.99) |
Rectum | Age, study center, gender, ethnicity, education, family history of colorectal cancer, BMI, NSAIDs use, physical activity, smoking status, alcohol intake, dietary calcium, supplemental calcium, dietary fiber, and total energy intake | |
| Anneclaire J. De Roos et al., 2003, USA [28] | Case-control study, 1986–1990 | (n(%)) 74 (30) 62 (25) 43 (17) 70 (28) |
(n(%)) 311 (32) 251 (26) 220 (22) 200 (20) |
0–0.705 0.705–0.93 0.94–1.25 1.26–1.57 |
1 (Reference) 1.1 (0.7–1.6) 0.9 (0.6–1.4) 1.7 (1.1–2.5) |
Rectum | Age, sex, and chlorinated surface water | |
| Yun Zhu et al. 2014 [74] | Case-control study, 1997–2006 | 95 120 124 145 141 |
536 496 520 474 455 |
0.65 0.89 1.12 1.4 1.92 |
1 (Reference) 1.26 (0.91–1.73) 1.2 (0.84–1.71) 1.51 (1.02–2.22) 1.45 (0.94–2.24) |
Rectum | Age, sex, energy intake, BMI, cigarette smoking status, education attainment, reported colon screening procedures, NSAID use, multivitamin supplements use, folate supplement use, vegetable intakes, and province of residence |
* Original exposure categories of nitrite from studies converted to mg/day for meta-regression calculation (explained under statistics analysis); ⁕ missing cases/controls/person-years in sub-cohort from the studies; ⁑ missing nitrite dosage.
4. Discussion
This systematic literature review and meta-analysis aimed to quantify the associations between cancer risk and dose of dietary nitrate and nitrite reported in the literature to date. Using 41 eligible articles, we conducted meta-analyses using two different approaches to compare the risk of 13 different site-specific cancers across different categories of dietary intake. Moreover, we conducted a meta-regression analysis to examine associations between site-specific cancer risk and dosage of dietary nitrates and nitrites.
Firstly, when comparing highest to lowest (reference) categories of intake, meta-analysis showed that high nitrate intake was associated with an increased risk of thyroid cancer (OR = 1.40, 95% CI: 1.02, 1.77). When pooling all intake categories and comparing against the lowest (reference) category, higher nitrite intake was associated with an increased risk of glioma (OR = 1.12, 95% CI: 1.03, 1.22).
Meta-regression analysis showed that bladder and stomach cancer risk was greater, and that pancreatic cancer risk was lower, with increasing nitrite intakes. Kidney and bladder cancer risk were both lower with increasing nitrate intakes. No other associations between cancer risk and dietary intakes of nitrates or nitrites were observed. These data suggest type- and site-specific effects on cancer risk, including protective effects, from dietary intakes of nitrate and nitrite.
These findings from a meta-analysis of the literature are an important contribution, as individual studies on their own have reported seemingly inconsistent findings. Some studies have shown positive, and others, negative associations with cancer risk at different intakes of dietary nitrate and nitrite. For example, Kilfoya et al. [51] reported an association between ovarian cancer and a daily nitrate intake of 175.4 mg/day, HR: 1.31 (95% CI: 1.01, 1.68) in a 10-year prospective cohort study of women (aged 50–71 years), with a total of 709 incident epithelial ovarian cancer cases. This same study did not show any association between ovarian cancer and total nitrate intake, yet there was a relationship between a nitrate intake of 0.33 mg/1000 kcal from animal sources HR: 1.34 (95% CI: 1.05, 1.69). In contrast, Inoue-Choi et al., [5] did not show any association with the same range of nitrate daily intake 165.48–209.2 mg/day, HR: 0.85 (95% CI: 0.56, 1.27) in a similar cohort study of women aged 55–69 years. This is a good comparison because these two studies have almost the same daily nitrate intake and the same demographic characteristics, which is sometimes difficult to find. Three studies on breast cancer did not show any associations [12,52,53]. More research is needed to study the association between nitrate and nitrite intake and breast cancer from both food and water, especially since it is the second leading cause of cancer death in 92% of women. Non-Hodgkin’s lymphoma studies showed an association between daily nitrite intake and the disease [25,56,60]. Most studies had daily intake ranges of 0–2 mg/day; one study did not report the daily intake [25]. There was no association between daily nitrate intake and this cancer. Stomach cancer was the most studied cancer among the articles retrieved for this systematic review, yet only three of these studies showed any relationship with dietary nitrite intake. This study’s meta-regression showed an association between dietary nitrite exposure and stomach cancer. Daily nitrate intake was not associated with stomach cancer in the meta-regression analysis. As for other cancers (cancer of the colon, rectum, esophagus, pancreas, kidney, thyroid, and glioma), one or two studies showed positive associations with dietary nitrate and nitrite intake.
Many studies have shown that a long period of exposure/daily dietary intake that contains nitrate, nitrite, and NOC compounds can lead to specific health issues, but these results are still contradictory. Keszei, et al. (2013) [69] conducted a cohort study with 16.3 years of follow-up in the Netherlands, from 1986 to 2002 for men and women aged 55–69 years. In this study, esophageal squamous cell carcinoma (ESCC) risk was associated with nitrite intake (HR for 0.1-mg/day increase: 1.19; 95% CI: 1.05, 1.36; p-trend = 0.06). Positive associations were observed between N-nitrosodimethylamine intake and esophageal squamous cell carcinoma (ESCC) risk (HR for 0.1 micro gram/day increase in intake: 1.15; 95% CI: 1.05, 1.25; p-trend = 0.01 based on tertiles of intake) and gastric non-cardia adenocarcinoma (GNCA) risk (1.06; 95% CI: 1.01, 1.10; p-trend = 0.09) in men. Meanwhile Cross et al., 2011, [53] conducted a cohort study with 10 years of follow-up in the USA, from 1995 to 2006 for men and women aged 50–71 years, which showed nitrate and nitrite were not associated with esophageal or gastric cancer. Some case-control studies and ecological studies have yielded inconclusive results about different types of cancers.
Individually, most of the research articles included in this systematic review did not find any association between nitrate and nitrite and any type of cancer in humans. However, when analyzed together, greater exposure to dietary nitrate and nitrite increased the risk of getting some cancers (glioma, bladder, stomach, and thyroid) and decreased the risk of getting others (pancreatic and kidney). Most of these cancers can be considered cancers of the digestive system. The risk of these types of cancers have been shown to be modified by other dietary and lifestyle factors. For example, some studies have shown an inverse association of vegetable and fruit intake with these cancers’ risk [78]. Other studies have shown that people who take in high vitamin C, high vitamin E, low red meat (or any type of meat), and folate while being exposed to nitrate or nitrite at the same time had a lower risk of having cancer, than those who did not [64,79]. However, not all studies showed these protective effects. Zeegers et al., 2006, [65] showed that vitamin C (p = 0.63) and vitamin E (p = 0.62), did not appear to be significant effect modifiers in the association between nitrate exposure from food and bladder cancer risk. Catsburg et al., (2014) [38] showed that among individuals with high nitrate intake, a positive association between high (i.e., above the median) heme intake and risk of bladder cancer was observed (highest category vs. lowest category OR = 1.76; 95% CI = 1.21–2.55; p trend = 0.007). Some studies showed no association at all [33,67,80].
Notwithstanding the null findings of some studies, it is widely accepted that it is important that people consume diets high in fresh fruits and vegetables that contain a lot of vitamins and essential minerals and reduce meat, fatty food, and processed food intake to improve their health. This may be important in modifying any harmful effects of dietary nitrates and nitrites on particularly susceptible tissues in the digestive system and elsewhere in the body. Cases of cancer are considered to be linked to nutritional factors. Scientific evidence suggests that food/diet is most convincingly linked to cancer of the lung, stomach, rectum, colon, pharynx, nasopharynx, esophagus, and mouth [81,82,83]. Filtration and purification of drinking water from both private and public sources before consumption is extremely important because several studies have shown that the consumption of nitrate and nitrite from drinking water, even in a very small amount, over a long period can lead to cancer (a chronic disease) and other health issues [31,84,85].
Most of the studies included in this systematic review were conducted in Europe and the U.S., and very few or no studies from South America, Africa, Australia, and Asia were retrieved. Many studies and awareness of early screening for different types of cancers are needed in South America, Africa, Australia, and Asia to better understand this issue on a larger scale with different demographics. A proper and comprehensive assessment of nitrate and nitrite from dietary intake, including inhibitors of endogenous nitrosation and intakes of antioxidants, are needed in future studies. Many studies lacked information about study participants’ water consumption and dietary intake that contain nitrate and nitrite simultaneously, which may be essential to better analyze the links between dietary intakes and cancers. Future studies should also pay close attention to the different duration or lengths (years) of food intake with nitrite and nitrate, especially to understand the effects of exposure. There is still no precise standard maximum contaminant level for nitrate and nitrite in food to protect people from non-communicable diseases like cancer. This might be because noncommunicable diseases, such as cancer, can take a long time to occur and the casualty has not yet been fully established.
Limitations
Using two different statistical analyses for each cancer site is the strength of this research. It can help to better understand the robustness of the associations. However, the study’s limitations are as follows; first, very few articles were available for each type of cancer, some with three or fewer studies, and so the results for this analysis should be treated with caution. More detailed, well-designed studies with accurate and precise information about study participants’ food intake and other lifestyle factors may be essential to more accurately estimate associated risk. Secondly, this research was unable to adjust for potential confounders or examine effect modification (e.g., of dietary vitamin C intake) due to lack of available data in the included studies. Some studies included vitamin C, D, and E, and folate and red/processed meat intake; however, most did not. We recommend that such research should include dietary vitamin C, D, and E, intake, as well as folate intake, polyphenols, red/processed meat, heme iron intake, and other nutrients/minerals and compounds (especially the dosage) from food and drinking water that could affect nitrosation in the body, to enable more precise estimation of risks and more sophisticated analyses.
Third, there was a wide range of nitrate and nitrite intake values from different studies, which resulted in different ranges across the analyses performed in this meta-analysis. For example, dietary nitrite ranged from 0 to 2.4 mg/day in the analysis against bladder cancer risk and from 0 to 22 mg/day in the analysis against stomach cancer risk. This variation in range in the independent variable across different analyses could be partly responsible for the variation in association with the dependent variables (the different site-specific cancer risks) observed in this study. A lack of standardized units for reporting dietary intakes of nitrates and nitrites in the literature made it necessary to convert the data to a common unit for meta-regression. Whilst this is not a major limitation, it has the potential to introduce error. Moreover, a lack of standardized units might pose a problem to efforts to implement a limit or precise standard maximum contaminant level to protect people from health risks of having a type of cancer. Lastly, many cohort and case-control studies should be done in different parts of the world to understand this topic better, especially noting other confounding factors and nutrient intake.
5. Conclusions
This study showed varied associations between site-specific cancer risks and dietary intakes of nitrate and nitrite. Glioma, bladder, and stomach cancer risks were higher, but pancreatic cancer risk was lower with higher nitrite intakes. Thyroid cancer risk was higher, but kidney and bladder cancer risks were lower with higher nitrate intakes. These data suggest type- and site-specific effects of cancer risk, including protective effects, from dietary intakes of nitrate and nitrite.
Acknowledgments
The authors would like to thank Mattheu Haren for his collaboration in reviewing and commenting on our manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14030666/s1, Table S1: Search Strategy of this study; Figure S1: Pooled ORs (95 % CI) of the highest dosage versus lowest category of dosage of nitrate and nitrite consumption from dietary intake for the following cancer type: (1) Reproductive organs, (2) Breast, (3) Thyroid, (4) Glioma, (5) Non-hodgkin, (6) Pancreatic, (7) Bladder, (8) Kidney, (9) Esophageal, (10) Stomach, (11) Colon, and (12) Rectum; Figure S2: Pooled ORs (95 % CI) of all combined higher dosages versus the lowest category of nitrate and nitrite consumption from dietary intake for each type of cancer; Figure S3: Funnel plot of nitrates and nitrites and for each type of cancer risk for publication bias.
Author Contributions
Conceptualization: K.S.A., E.E.E. and M.A.; methodology: E.E.E., K.S.A. and M.A.; formal analysis: E.E.E., K.S.A., M.A. and X.Y.; data curation: E.E.E., L.A., J.S. and W.X.; writing—original draft preparation: K.S.A., E.E.E. and M.A.; writing—review and editing; supervision and project administration: M.A., X.Y. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Centre de recherche en gestion des Services de Santé, University Laval and Riphah International University (Riphah-ORIC-21-22/FPS-51).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data set used/analyzed are available from the corresponding author on request.
Conflicts of Interest
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bryan N.S., van Grinsven H. The role of nitrate in human health. Adv. Agron. 2013;119:153–182. [Google Scholar]
- 3.GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2010;94:448. [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrucci L.M., Sinha R., Ward M.H., Graubard B.I., Hollenbeck A.R., Kilfoy B.A., Schatzkin A., Michaud D.S., Cross A.J. Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer. 2010;116:4345–4353. doi: 10.1002/cncr.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaud D.S., Holick C.N., Giovannucci E., Stampfer M.J. Meat intake and bladder cancer risk in 2 prospective cohort studies. Am. J. Clin. Nutr. 2006;84:1177–1183. doi: 10.1093/ajcn/84.5.1177. [DOI] [PubMed] [Google Scholar]
- 7.Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N.N., Norat T., Greenwood D.C., Riboli E., Vatten L.J., Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017;46:1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones R.R., Weyer P.J., Dellavalle C.T., Inoue-Choi M., Anderson K.E., Cantor K.P., Krasner S., Robien K., Freeman L.E.B., Silverman D.T., et al. Nitrate from Drinking Water and Diet and Bladder Cancer Among Postmenopausal Women in Iowa. Environ. Health Perspect. 2016;124:1751–1758. doi: 10.1289/EHP191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradbury K.E., Appleby P.N., Key T.J. Fruit, vegetable, and fiber intake in relation to cancer risk: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Am. J. Clin. Nutr. 2014;100:394S–398S. doi: 10.3945/ajcn.113.071357. [DOI] [PubMed] [Google Scholar]
- 10.González C.A., Lujan-Barroso L., Bueno-De-Mesquita H.B., Jenab M., Duell E.J., Agudo A., Tjønneland A., Boutron-Ruault M.C., Clavel-Chapelon F., Touillaud M., et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: A reanalysis of the European prospective investigation into cancer and nutrition (EPIC-EURGAST) study after a longer follow-up. Int. J. Cancer. 2012;131:2910–2919. doi: 10.1002/ijc.27565. [DOI] [PubMed] [Google Scholar]
- 11.Speijers G.J.A., Van Went G.F., Van Apeldoorn M.E., Montizaan G.F., Janus J.A., Canton J.H., Van Gestel C.A.M., Van Der Heijden C.A., Heijna-Merkus E., Knaap A.G.A.C., et al. Integrated Criteria Document Nitrate; Effects. Appendix to RIVM Report No. 758473012. National Institute for Public Health and the Environment; Bilthoven, The Netherlands: 1989. RIVM Report No. A758473012. [Google Scholar]
- 12.Inoue-Choi M., Jones R.R., Anderson K.E., Cantor K.P., Cerhan J.R., Krasner S., Robien K., Weyer P.J., Ward M.H. Nitrate and nitrite ingestion and risk of ovarian cancer among postmenopausal women in Iowa. Int. J. Cancer. 2015;137:173–182. doi: 10.1002/ijc.29365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hord N.G., Ghannam J.S., Garg H.K., Berens P.D., Bryan N.S. Nitrate and Nitrite Content of Human, Formula, Bovine, and Soy Milks: Implications for Dietary Nitrite and Nitrate Recommendations. Breastfeed. Med. 2011;6:393–399. doi: 10.1089/bfm.2010.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschebrook-Kilfoy B., Shu X.-O., Gao Y.-T., Ji B.-T., Yang G., Li H.L., Rothman N., Chow W.-H., Zheng W., Ward M.H. Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai women’s health study. Int. J. Cancer. 2013;132:897–904. doi: 10.1002/ijc.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joint FAO/WHO Expert Committee on Food Additives . World Health Organization. Evaluation of Certain Veterinary Drug Residues in Food: Fifty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization; Geneva, Switzerland: 2002. (WHO Technical Report Series No. 911). [Google Scholar]
- 16.World Health Organization . Boron in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 17.Gangolli S.D., van den Brandt P.A., Feron V.J., Janzowsky C., Koeman J.H., Speijers G.J., Spiegelhalder B., Walker R., Wishnok J.S. Nitrate, nitrite and N-nitroso compounds. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 18.Ward M.H., Cross A.J., Divan H., Kulldorff M., Nowell-Kadlubar S., Kadlubar F.F., Sinha R. Processed meat intake, CYP2A6 activity and risk of colorectal adenoma. Carcinogenesis. 2007;28:1210–1216. doi: 10.1093/carcin/bgm009. [DOI] [PubMed] [Google Scholar]
- 19.Santarelli R.L., Pierre F., Corpet D. Processed Meat and Colorectal Cancer: A Review of Epidemiologic and Experimental Evidence. Nutr. Cancer. 2008;60:131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keszei A.P., Schouten L., Goldbohm R.A., van den Brandt P.A. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann. Oncol. 2012;23:2319–2326. doi: 10.1093/annonc/mdr615. [DOI] [PubMed] [Google Scholar]
- 21.Heinen M.M., Verhage B.A., Goldbohm R.A., van den Brandt P.A. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int. J. Cancer. 2009;125:1118–1126. doi: 10.1002/ijc.24387. [DOI] [PubMed] [Google Scholar]
- 22.Cassens R.G. Use of sodium nitrite in cured meats today. Food Technol. 1995;49:72–80. [Google Scholar]
- 23.Larsson S.C., Orsini N., Wolk A. Processed Meat Consumption and Stomach Cancer Risk: A Meta-Analysis. J. Natl. Cancer Inst. 2006;98:1078–1087. doi: 10.1093/jnci/djj301. [DOI] [PubMed] [Google Scholar]
- 24.Cross A.J., Freedman N.D., Ren J., Ward M.H., Hollenbeck A.R., Schatzkin A., Sinha R., Abnet C. Meat Consumption and Risk of Esophageal and Gastric Cancer in a Large Prospective Study. Am. J. Gastroenterol. 2011;106:432–442. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilfoy B.A., Ward M.H., Zheng T., Holford T.R., Boyle P., Zhao P., Dai M., Leaderer B., Zhang Y. Risk of non-Hodgkin lymphoma and nitrate and nitrite from the diet in Connecticut women. Cancer Causes Control. 2010;21:889–896. doi: 10.1007/s10552-010-9517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue-Choi M., Ward M.H., Cerhan J., Weyer P.J., Anderson K.E., Robien K. Interaction of Nitrate and Folate on the Risk of Breast Cancer Among Postmenopausal Women. Nutr. Cancer. 2012;64:685–694. doi: 10.1080/01635581.2012.687427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward M.H., Rusiecki J.A., Lynch C.F., Cantor K.P. Nitrate in public water supplies and the risk of renal cell carcinoma. Cancer Causes Control. 2007;18:1141–1151. doi: 10.1007/s10552-007-9053-1. [DOI] [PubMed] [Google Scholar]
- 28.de Roos A.J., Ward M.H., Lynch C.F., Cantor K.P. Nitrate in Public Water Supplies and the Risk of Colon and Rectum Cancers. Epidemiology. 2003;14:640–649. doi: 10.1097/01.ede.0000091605.01334.d3. [DOI] [PubMed] [Google Scholar]
- 29.Dubrow R., Darefsky A.S., Park Y., Mayne S.T., Moore S., Kilfoy B., Cross A.J., Sinha R., Hollenbeck A.R., Schatzkin A., et al. Dietary Components Related to N-Nitroso Compound Formation: A Prospective Study of Adult Glioma. Cancer Epidemiol. Biomark. Prev. 2010;19:1709–1722. doi: 10.1158/1055-9965.EPI-10-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiso M., Schechter A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE. 2015;10:e0119712. doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirvish S.S. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- 32.Michaud D.S., Holick C.N., Batchelor T.T., Giovannucci E., Hunter D.J. Prospective study of meat intake and dietary nitrates, nitrites, and nitrosamines and risk of adult glioma. Am. J. Clin. Nutr. 2009;90:570–577. doi: 10.3945/ajcn.2008.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tricker A., Pfundstein B., Theobald E., Preussmann R., Spiegelhalder B. Mean daily intake of volatile N-nitrosamines from foods and beverages in West Germany in 1989–1990. Food Chem. Toxicol. 1991;29:729–732. doi: 10.1016/0278-6915(91)90180-F. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 35.Ward M.H., Cantor K.P., Riley D., Merkle S., Lynch C.F. Nitrate in Public Water Supplies and Risk of Bladder Cancer. Epidemiology. 2003;14:183–190. doi: 10.1097/01.EDE.0000050664.28048.DF. [DOI] [PubMed] [Google Scholar]
- 36.Espejo-Herrera N., Cantor K.P., Malats N., Silverman D.T., Tardon A., García-Closas R., Serra C., Kogevinas M., Villanueva C.M. Nitrate in drinking water and bladder cancer risk in Spain. Environ. Res. 2015;137:299–307. doi: 10.1016/j.envres.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Bryan N.S., Alexander D.D., Coughlin J.R., Milkowski A.L., Boffetta P. Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food and Chemical Toxicology. 2012;50:3646–3665. doi: 10.1016/j.fct.2012.07.062. [DOI] [PubMed] [Google Scholar]
- 38.Catsburg C.E., Gago-Dominguez M., Yuan J.-M., Castelao J.E., Cortessis V.K., Pike M.C., Stern M.C. Dietary sources of N-nitroso compounds and bladder cancer risk: Findings from the Los Angeles bladder cancer study. Int. J. Cancer. 2014;134:125–135. doi: 10.1002/ijc.28331. [DOI] [PubMed] [Google Scholar]
- 39.Wu J.W., Cross A.J., Baris D., Ward M.H., Karagas M.R., Johnson A., Schwenn M., Cherala S., Colt J.S., Cantor K.P., et al. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br. J. Cancer. 2012;106:1891–1898. doi: 10.1038/bjc.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eichholzer M., Gutzwiller F. Dietary Nitrates, Nitrites, and N-Nitroso Compounds and Cancer Risk: A Review of the Epidemiologic Evidence. Nutr. Rev. 1998;56:95–105. doi: 10.1111/j.1753-4887.1998.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 41.Jones R.R., DellaValle C.T., Weyer P.J., Robien K., Cantor K.P., Krasner S., Freeman L.E.B., Ward M.H. Ingested nitrate, disinfection by-products, and risk of colon and rectal cancers in the Iowa Women’s Health Study cohort. Environ. Int. 2019;126:242–251. doi: 10.1016/j.envint.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bingham S., Pignatelli B., Pollock J., Ellul A., Malaveille C., Gross G., Runswick S., Cummings J., O’Neill I. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17:515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 43.Jakszyn P., Gonzalez C.A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J. Gastroenterol. 2006;12:4296–4303. doi: 10.3748/wjg.v12.i27.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DellaValle C.T., Xiao Q., Yang G., Shu X.-O., Aschebrook-Kilfoy B., Zheng W., Li H.L., Ji B.-T., Rothman N., Chow W.-H., et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int. J. Cancer. 2014;134:2917–2926. doi: 10.1002/ijc.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veroniki A.A., Jackson D., Viechtbauer W., Bender R., Bowden J., Knapp G., Kuss O., Higgins J.P.T., Langan D., Salanti G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons; Oxford, UK: 2009. [Google Scholar]
- 47.Callisto M., Molozzi J., Barbosa J.L.E. Eutrophication: Causes, Consequences and Control. Springer; Dordrecht, The Netherlands: 2014. Eutrophication of lakes; pp. 55–71. [Google Scholar]
- 48.U.S. Department of Health and Human Services. U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans. USDA; Washington, DC, USA: HHS; Washington, DC, USA: 2015. [Google Scholar]
- 49.Rogers H.J., Swaminathan H. A Comparison of Logistic Regression and Mantel-Haenszel Procedures for Detecting Differential Item Functioning. Appl. Psychol. Meas. 1993;17:105–116. doi: 10.1177/014662169301700201. [DOI] [Google Scholar]
- 50.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aschebrook-Kilfoy B., Ward M.H., Gierach G.L., Schatzkin A., Hollenbeck A.R., Sinha R., Cross A.J. Epithelial ovarian cancer and exposure to dietary nitrate and nitrite in the NIH-AARP Diet and Health Study. Eur. J. Cancer Prev. 2012;21:65–72. doi: 10.1097/CEJ.0b013e328347622f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weyer P.J., Cerhan J., Kross B.C., Hallberg G.R., Kantamneni J., Breuer G., Jones M.P., Zheng W., Lynch C.F. Municipal Drinking Water Nitrate Level and Cancer Risk in Older Women: The Iowa Women’s Health Study. Epidemiology. 2001;12:327–338. doi: 10.1097/00001648-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Espejo-Herrera N., Gracia-Lavedan E., Pollan M., Aragones N., Boldo E., Perez-Gomez B., Altzibar J.M., Amiano P., Zabala A.J., Ardanaz E., et al. Ingested Nitrate and Breast Cancer in the Spanish Multicase-Control Study on Cancer (MCC-Spain) Environ. Health Perspect. 2016;124:1042–1049. doi: 10.1289/ehp.1510334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward M.H., Kilfoy B.A., Weyer P.J., Anderson K.E., Folsom A.R., Cerhan J. Nitrate Intake and the Risk of Thyroid Cancer and Thyroid Disease. Epidemiology. 2010;21:389–395. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilfoy B.A., Zhang Y., Park Y., Holford T.R., Schatzkin A., Hollenbeck A., Ward M.H. Dietary nitrate and nitrite and the risk of thyroid cancer in the NIH-AARP Diet and Health Study. Int. J. Cancer. 2010;129:160–172. doi: 10.1002/ijc.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward M.H., Cerhan J., Colt J.S., Hartge P. Risk of Non-Hodgkin Lymphoma and Nitrate and Nitrite from Drinking Water and Diet. Epidemiology. 2006;17:375–382. doi: 10.1097/01.ede.0000219675.79395.9f. [DOI] [PubMed] [Google Scholar]
- 57.Ward M.H., Mark S.D., Cantor K.P., Weisenburger D.D., Correa-Villasenor A., Zahm S.H. Drinking water nitrate and the risk of non-Hodgkin’s lymphoma. Epidemiology. 1996;7:465–471. doi: 10.1097/00001648-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Aschebrook-Kilfoy B., Ward M.H., Zheng T., Holford T.R., Boyle P., Leaderer B., Zhang Y. Dietary Nitrate and Nitrite Intake and Non-Hodgkin Lymphoma Survival. Nutr. Cancer. 2012;64:488–492. doi: 10.1080/01635581.2012.658136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aschebrook-Kilfoy B., Ward M.H., Dave B.J., Smith S.M., Weisenburger D.D., Chiu B.C.-H. Dietary nitrate and nitrite intake and risk of non-Hodgkin lymphoma. Leuk. Lymphoma. 2013;54:945–950. doi: 10.3109/10428194.2012.734613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiu B.C.-H., Dave B.J., Ward M.H., Fought A.J., Hou L., Jain S., Gapstur S., Evens A.M., Zahm S.H., Blair A., et al. Dietary factors and risk of t(14;18)-defined subgroups of non-Hodgkin lymphoma. Cancer Causes Control. 2008;19:859–867. doi: 10.1007/s10552-008-9148-3. [DOI] [PubMed] [Google Scholar]
- 61.Quist A.J., Inoue-Choi M., Weyer P.J., Anderson K.E., Cantor K.P., Krasner S., Freeman L.E.B., Ward M.H., Jones R.R. Ingested nitrate and nitrite, disinfection by-products, and pancreatic cancer risk in postmenopausal women. Int. J. Cancer. 2018;142:251–261. doi: 10.1002/ijc.31055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coss A., Cantor K.P., Reif J.S., Lynch C.F., Ward M.H. Pancreatic Cancer and Drinking Water and Dietary Sources of Nitrate and Nitrite. Am. J. Epidemiol. 2004;159:693–701. doi: 10.1093/aje/kwh081. [DOI] [PubMed] [Google Scholar]
- 63.Zheng J., Stuff J., Tang H., Hassan M.M., Daniel C.R., Li N. Dietary N-nitroso compounds and risk of pancreatic cancer: Results from a large case–control study. Carcinogenesis. 2019;40:254–262. doi: 10.1093/carcin/bgy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aschebrook-Kilfoy B., Cross A.J., Stolzenberg-Solomon R.Z., Schatzkin A., Hollenbeck A.R., Sinha R., Ward M.H. Pancreatic Cancer and Exposure to Dietary Nitrate and Nitrite in the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2011;174:305–315. doi: 10.1093/aje/kwr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeegers M.P., Selen R.F., Kleinjans J.C., Goldbohm R.A., van den Brandt P.A. Nitrate Intake Does Not Influence Bladder Cancer Risk: The Netherlands Cohort Study. Environ. Health Perspect. 2006;114:1527–1531. doi: 10.1289/ehp.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karwowska M., Kononiuk A. Nitrates/Nitrites in Food—Risk for Nitrosative Stress and Benefits. Antioxidants. 2020;9:241. doi: 10.3390/antiox9030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones R.R., Weyer P.J., Dellavalle C.T., Robien K., Cantor K.P., Krasner S., Freeman L.E.B., Ward M.H. Ingested Nitrate, Disinfection By-products, and Kidney Cancer Risk in Older Women. Epidemiology. 2017;28:703–711. doi: 10.1097/EDE.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward M.H., Heineman E.F., Markin R.S., Weisenburger D.D. Adenocarcinoma of the stomach and esophagus and drinking water and dietary sources of nitrate and nitrite. Int. J. Occup. Environ. Health. 2008;14:193–197. doi: 10.1179/oeh.2008.14.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keszei A.P., Goldbohm R.A., Schouten L.J., Jakszyn P., van den Brandt P.A. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2013;97:135–146. doi: 10.3945/ajcn.112.043885. [DOI] [PubMed] [Google Scholar]
- 70.van Loon A.J., Botterweck A.A., Goldbohm R.A., Brants H.A., van Klaveren J.D., van den Brandt P.A. Intake of nitrate and nitrite and the risk of gastric cancer: A prospective cohort study. Br. J. Cancer. 1998;78:129–135. doi: 10.1038/bjc.1998.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernández-Ramírez R.U., Galván-Portillo M.V., Ward M.H., Agudo A., González C.A., Oñate-Ocaña L.F., Herrera-Goepfert R., Palma-Coca O., López-Carrillo L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int. J. Cancer. 2009;125:1424–1430. doi: 10.1002/ijc.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.La Vecchia C., Ferraroni M., D’Avanzo B., Decarli A., Franceschi S. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol. Biomark. Prev. 1994;3:393–398. [PubMed] [Google Scholar]
- 73.Ferrucci L.M., Sinha R., Huang W.-Y., Berndt S.I., Katki H.A., Schoen R.E., Hayes R.B., Cross A.J. Meat consumption and the risk of incident distal colon and rectal adenoma. Br. J. Cancer. 2011;106:608–616. doi: 10.1038/bjc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y., Wang P.P., Zhao J., Green R., Sun Z., Roebothan B., Squires J., Buehler S., Dicks E., Zhao J., et al. Dietary N-nitroso compounds and risk of colorectal cancer: A case–control study in Newfoundland and Labrador and Ontario, Canada. Br. J. Nutr. 2014;111:1109–1117. doi: 10.1017/S0007114513003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward M.H., Heineman E.F., McComb R.D., Weisenburger D.D. Drinking Water and Dietary Sources of Nitrate and Nitrite and Risk of Glioma. J. Occup. Environ. Med. 2005;47:1260–1267. doi: 10.1097/01.jom.0000184879.67736.ae. [DOI] [PubMed] [Google Scholar]
- 76.Barry K.H., Jones R.R., Cantor K.P., Freeman L.E.B., Wheeler D.C., Baris D., Johnson A.T., Hosain G.M., Schwenn M., Zhang H., et al. Ingested Nitrate and Nitrite and Bladder Cancer in Northern New England. Epidemiology. 2020;31:136–144. doi: 10.1097/EDE.0000000000001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engel L.S., Chow W., Vaughan T.L., Gammon M.D., Risch H.A., Stanford J.L., Schoenberg J.B., Mayne S.T., Dubrow R., Rotterdam H., et al. Population Attributable Risks of Esophageal and Gastric Cancers. J. Natl. Cancer Inst. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 78.Pobel D., Riboli E., Cornée J., Hémon B., Guyader M. Nitrosamine, nitrate and nitrite in relation to gastric cancer: A case-control study in Marseille, France. Eur. J. Epidemiol. 1995;11:67–73. doi: 10.1007/BF01719947. [DOI] [PubMed] [Google Scholar]
- 79.Mayne S.T., Risch H.A., Dubrow R., Chow W.H., Gammon M.D., Vaughan T.L., Farrow D.C., Schoenberg J.B., Stanford J.L., Ahsan H., et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol. Biomark. Prev. 2001;10:1055–1062. [PubMed] [Google Scholar]
- 80.Li H., Duncan C., Townend J., Killham K., Smith L.M., Johnston P., Dykhuizen R., Kelly D., Golden M., Benjamin N., et al. Nitrate-reducing bacteria on rat tongues. Appl. Environ. Microbiol. 1997;63:924–930. doi: 10.1128/aem.63.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muñoz J. Salud 21 de la Región de Europa: Meta 11. Organización Mundial de la Salud; Copenhagen, Denmark: 2000. [Google Scholar]
- 82.Robertson A., Tirado C., Lobstein T., Jermini M., Knai C., Jensen J.H., Ferro-Luzzi A., James W.P. Food and Health in Europe: A New Basis for Action. WHO Regional Office for Europe; Copenhagen, Denmark: 2004. [PubMed] [Google Scholar]
- 83.Roberts J.A., Cumberland P., Sockett P.N., Wheeler J., Rodrigues L.C., Sethi D., Roderick P.J., Infectious Intestinal Disease Study Executive . A Report of the Study of Infectious Intestinal Disease in England. The Stationery Office; London, UK: 2000. [Google Scholar]
- 84.Morris R.D., Audet A.M., Angelillo I.F., Chalmers T.C., Mosteller F. Chlorination, chlorination by-products, and cancer: A meta-analysis. Am. J. Public Health. 1992;82:955–963. doi: 10.2105/AJPH.82.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Essien E.E., Abasse K.S., Côté A., Mohamed K.S., Baig M.M.F.A., Habib M., Naveed M., Yu X., Xie W., Jinfang S., et al. Drinking-water nitrate and cancer risk: A systematic review and meta-analysis. Arch. Environ. Occup. Health. 2020;77:51–67. doi: 10.1080/19338244.2020.1842313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data set used/analyzed are available from the corresponding author on request.