Abstract
We assessed the value of a new digoxigenin (DIG)-labeled generic probe mix in a PCR–enzyme-linked immunosorbent assay format to screen for the presence of human papillomavirus (HPV) DNA amplified from clinical specimens. After screening with this new generic assay is performed, HPV DNA-positive samples can be directly genotyped using a reverse blotting method with product from the same PCR amplification. DNA from 287 genital specimens was amplified via PCR using biotin-labeled consensus primers directed to the L1 gene. HPV amplicons were captured on a streptavidin-coated microwell plate (MWP) and detected with a DIG-labeled HPV generic probe mix consisting of nested L1 fragments from types 11, 16, 18, and 51. Coamplification and detection of human DNA with biotinylated β-globin primers served as a control for both sample adequacy and PCR amplification. All specimens were genotyped using a reverse line blot assay (13). Results for the generic assay using MWPs and a DIG-labeled HPV generic probe mix (DIG-MWP generic probe assay) were compared with results from a previous analysis using dot blots with a radiolabeled nested generic probe mix and type-specific probes for genotyping. The DIG-MWP generic probe assay resulted in high intralaboratory concordance in genotyping results (88% versus 73% agreement using traditional methods). There were 207 HPV-positive results using the DIG-MWP method and 196 positives using the radiolabeled generic probe technique, suggesting slightly improved sensitivity. Only one sample failed to test positive with the DIG-MWP generic probe assay in spite of a positive genotyping result. Concordance between the two laboratories was nearly 87%. Approximately 6% of samples that were positive or borderline when tested with the DIG-MWP generic probe assay were not detected with the HPV type-specific panel, perhaps representing very rare or novel HPV types. This new method is easier to perform than traditional generic probe techniques and uses more objective interpretation criteria, making it useful in studies of HPV natural history.
Some types of human papillomavirus (HPV) are widely accepted as causative agents for cervical cancer (3, 19). There are more than 40 HPV viral types that are commonly found in the genital tract, and approximately one-third of these are associated with cervical cancer and anal neoplasia. The anogenital HPV types are generally categorized as being either “high risk” or “low risk.” High-risk types are associated with high-grade precancerous lesions and invasive cancer, while low-risk types are found in asymptomatic or benign conditions such as genital warts. However, the distribution and prevalence of types vary somewhat by geographic region and other demographic factors. Because the significance of the variation in type distribution is still being elucidated, studies of HPV epidemiology need to employ a methodology that can detect the entire spectrum of viral types. One of the most common means to detect and characterize new HPVs has been by PCR using consensus primers, along with a broad-spectrum detection method such as gel electrophoresis or dot blotting techniques using a generic probe mix. In this way, any HPV DNA present in a specimen is amplified and detected and can subsequently be characterized. Generic probe detection on dot blots has been used in epidemiological studies and normally utilizes a mixture of radiolabeled or biotin-labeled HPV fragments as probes (1, 2, 5, 14, 16). This method can be highly sensitive and has the capability of testing large numbers of samples quickly. But traditional dot blots often suffer from inconsistent sensitivity or background noise because of the low stringency of the hybridization reaction between the generic probe and PCR-amplified products and require subjective criteria to determine specimen positivity. In fact, this approach normally calls for additional confirmation of HPV positivity, such as by gel electrophoretic analysis. Specific genotyping information necessitates either the sequencing of amplified genetic material, restriction fragment length polymorphism analysis, or hybridization to type-specific probes under stringent conditions (11). Studies which involve screening large numbers of samples using a generic probe detection method with subsequent characterization often require multiple PCR amplifications, followed by numerous detection procedures with various levels of stringency, specificity, and sensitivity. While effective, this approach can be cumbersome, time-consuming, and a source of laborious data interpretation or experimental error.
One advance in the rapid genotyping of large numbers of specimens was the development of a reverse line blot system that could detect up to 27 different HPV types from the MY09/MY11/HMB01 consensus PCR system with a single hybridization procedure (7, 13). However, screening samples for the presence of additional HPV types still requires gel electrophoretic analysis or generic probe blotting. We describe here a simple method for a broad-spectrum HPV screening assay; the method uses a generic probe mix composed of digoxigenin (DIG)-labeled fragments from four HPV types (11, 16, 18, and 51) on microwell plates (MWP) and a DIG-MWP detection kit from Roche Molecular Biochemicals. The assay utilizes the same biotinylated amplification products used in the MY09/MY11/HMB01 reverse line blot genotyping techniques, eliminating the need for additional PCR. We demonstrate here that the HPV generic probe assay with the DIG-MWP kit (DIG-MWP assay) has a sensitivity equivalent to those of other PCR methods, with the added benefits of a standardized protocol and algorithm for determining HPV positivity, ease of format for detection of PCR products, and avoidance of radioactivity. In addition, we demonstrate further support for the use of an improved primer system for consensus PCR, the PGMY primer set (12), which affords the greatest specificity and consistency of amplification of types across the genital HPV spectrum.
MATERIALS AND METHODS
Population studied.
Genital specimens from women enrolled in The Canadian Women's HIV Study (5, 15) were selected on the basis of initial results for HPV detection obtained with a standard PCR test (see below) using MY09, MY11, and HMB01 primers and isotopic probes. This selection ensured the inclusion of all HPV types detected in a standard consensus PCR test, as well as at least 150 HPV-positive samples. The remaining samples selected for this study were consecutively collected specimens from the Canadian Women's HIV Study. This cohort study investigates the relationships between genital HPV infection and cervical disease progression, in relation to human immunodeficiency virus-induced immune deficiency (5, 15). Two hundred eighty-seven genital specimens (109 vaginal tampons and 178 cervicovaginal lavages) from 248 women were included in this evaluation of the DIG-MWP HPV generic probe assay. For 39 women, a cervicovaginal lavage and a vaginal tampon were obtained at the same visit. All samples were tested without knowledge of the results of the corresponding PCR and clinical status. Written informed consent was obtained from each participant, and the Canadian Women's HIV Study has the approval of the ethics committees of the institutions involved.
Processing of clinical samples.
Before the physical examination, the participant was asked to insert and immediately withdraw a vaginal tampon (Meds regular; Johnson & Johnson). The vaginal tampon was placed in a sterile jar containing 50 ml of 10 mM Tris-HCl (pH 7.5), 50 mM EDTA, and 150 mM NaCl (9). During the pelvic examination, a cervicovaginal lavage was obtained with 10 ml of phosphate-buffered saline (pH 7.4) sprayed on the ectocervix with a syringe and aspirated from the posterior vaginal fornix (4, 18). Specimens were refrigerated within 1 h. The delay between sampling and processing never exceeded 7 days.
Vaginal tampons were squeezed to obtain a cellular suspension of genital cells. Cells from vaginal tampons and cervicovaginal lavages were pelleted after centrifugation at 2,500 rpm (IEC CENTRA-8R) for 10 min at 4°C, resuspended in 500 μl of 10 mM Tris-HCl (pH 8.2), and stored frozen at −70°C until processed. Cell suspensions were thawed, lysed by addition of Tween 20 at a final concentration of 0.8% (vol/vol), and digested with 250 μg of proteinase K/ml for 2 h at 45°C (6, 7). Cell lysates were boiled at 95°C for 10 min and stored at −70°C until tested. Five microliters of processed sample was tested in each PCR assay. Samples selected for this evaluation had all tested positive initially for β-globin with PC04 and GH20 primers (1, 6, 7).
Standard consensus PCR testing at the Montreal laboratory.
HPV DNA was amplified under standard conditions with the MY09, MY11, and HMB01 consensus HPV primers, as previously described (6, 8, 16). Amplification of HPV and β-globin DNA was performed in separate reactions. The amplification mixture contained 6.5 mM MgCl2; 50 mM KCl; 2.5 U of Taq DNA polymerase (Amplitaq; Roche Molecular Diagnostics, Mississauga, Ontario, Canada); 200 μM (each) dATP, dCTP, dGTP, and dTTP; and 50 pmol of each primer. Negative, weakly positive (10 HPV18 DNA copies), and strongly positive controls (HPV types 6 or 11, 16, 31, 33, 35, 39, and 45), were included in each run to monitor contamination and overall end point sensitivity. Measures to avoid false-positive reactions caused by contamination have been described elsewhere (6). Amplifications were performed in a TC9600 thermocycler (Perkin-Elmer Cetus, Montréal, Canada) for 40 cycles with the following cycling parameters: 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Amplified products were spotted onto nylon membranes and were hybridized under stringent conditions as described in previous publications with 32P-labeled oligonucleotide probes for types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, and 58 (1, 2, 16). PCR products spotted onto nylon membranes were also hybridized with an HPV generic probe mixture under low-stringency conditions as previously described (1, 2, 14, 16). The generic probe mixture was generated by amplification in separate reactions of HPV16, HPV18, and HPV31 plasmids with type-specific nested primers (14) and 32P-labeled deoxynucleotides. Amplified nested L1 fragments were mixed and used as a generic probe that efficiently detects common genital types (5, 14). Samples positive with the radioactive generic probe assay but negative with all of the type-specific probes were assumed to contain untyped HPV.
Consensus PCR with generic detection and reverse line blot genotyping at the California laboratory.
Amplification was performed using the improved PGMY and β-globin primers as previously described (12) with slight modifications noted below. HPV and β-globin DNA are coamplified in this protocol. The amplification mixture contained 1× PCR buffer II (Perkin-Elmer, Foster City, Calif.); 4.0 mM MgCl2; 7.5 U of AmpliTaq gold DNA polymerase (Perkin-Elmer); 200 μM (each) dATP, dCTP, and dGTP; and 600 μM dUTP. In addition, 100 pmol of each primer pool (5′-biotinylated PGMY09 and PGMY11) was added; the PGMY09 and PGMY11 pools consist of equimolar amounts of 10 and 5 primers, respectively, at a total concentration of 50 μM. Finally, 2.5 pmol each of the 5′-biotinylated β-globin primers GH20 and PC04 was included in the PCR. Amplifications were performed in a Perkin-Elmer TC9600 thermocycler using a 9-min AmpliTaq gold activation at 95°C followed by 40 cycles with the following cycling parameters: 95°C for 30 s, 55°C for 1 min, and 72°C for 1 min. This was followed by a final extension for 5 min at 72°C, and the reaction mixtures were subsequently stored at 4°C. To measure the effect of the newer PGMY primer system on the final results, amplification was also performed in our laboratory using biotinylated degenerate MY09, MY11, and HMB01 primers with the same parameters, except that the MgCl2 concentration was 6 mM and 50 pmol of each degenerate primer was used in the reaction mixture. Results from both experiments were compared internally and to the Montreal laboratory data set.
Genotyping was performed on all 287 samples using the reverse line blot detection system as previously described (13). The PCR product was denatured in 0.4 N NaOH and hybridized to an immobilized probe array containing probes for 27 HPV types plus the human β-globin gene. Positive hybridization was detected by a streptavidin-horseradish peroxidase-mediated color precipitation on the membrane at the probe line. An auxiliary genotyping strip with probes for 12 additional viral types was used to gather more information about what types are detectable with this generic probe assay but are not identified by the standard line blot assay. The novel HPV types represented on this additional strip are HPV61, -62, -64, -67, -69, -70, -71, -72, -81, CP6108, and IS39 (17).
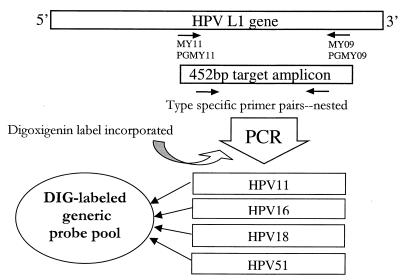
A schematic for the generation of the DIG-labeled generic probe is shown in Fig. 1. Each DIG-labeled probe fragment was synthesized via PCR using purified template DNA from HPV types 11, 16, 18, and 51 and using 5 pmol of each type-specific primer for each target (Table 1) per PCR. Other components and conditions for PCR were the same as those used for the target amplification (above), except that a nucleotide PCR-DIG labeling mix (Roche Molecular Biochemicals, Indianapolis, Ind.) was substituted for the nucleotides in order to incorporate DIG into the probe amplicon. Each DIG-labeled probe was tested against an extensive panel of HPV targets individually (data not shown). The four DIG-labeled probes were pooled together and stored at −20°C. The probe does not require any postamplification purification and is stable for at least 3 months under these conditions. The pooled probe was tested against a panel of HPV genotypes diluted down to single-copy target per PCR to evaluate the sensitivity of the detection (data not shown). A control probe for detection of the human β-globin amplification product was generated in a similar fashion. A human genomic-DNA template was used in a PCR with 5 pmol each of two gene-specific primers (AILA63+BTAG [5′-GGGTTGGCCAATCTACTCC −3′] and AILA213−BTAG [5′-TGAGGAGAAGTCTGCCGTTA-3′]). All other conditions and components for amplification were identical to those for the HPV generic probe PCR, except that the annealing temperature during thermocycling was increased to 60°C. The resulting amplicon was a DIG-labeled 170-bp human β-globin fragment.
FIG. 1.
Generation of DIG-labeled generic probe.
TABLE 1.
Primers used to make the generic probe
| HPV type | Primer name | DNA sequence (5′–3′) |
|---|---|---|
| 11 | 11.1+ | ATTTGCTGGGGAAACCACT |
| 11.3− | ATTCACTTGAAAACTTTTCT | |
| 16 | MY74 | CATTTGTTGGGGTAACCAAC |
| MY75 | TAGGTCTGCAGAAAACTTTTC | |
| 18 | MY76 | TGTTTGCTGGCATAATCAAT |
| MY77 | TAAGTCTAAAGAAAACTTTTC | |
| 51 | 51.1+ | CATTTGCTGGAACAATCAG |
| 51.2− | TAAATCTAAAGAAAATCGTTCC |
Generic probe detections were performed using the commercially available PCR–enzyme-linked immunosorbent assay DIG detection kit (Roche Molecular Biochemicals) according to the manufacturer's instructions with the following modifications. Both the DIG-labeled probe and target PCR product were denatured for 5 min using equal volumes of DNA solution and a 1.6% NaOH solution. Twenty microliters of denatured target DNA (biotinylated amplicon) was added to the streptavidin-coated microtiter wells, followed by the addition of 200 μl of hybridization buffer and finally 20 μl of the denatured generic probe pool. Hybridizations were performed at 37°C for 1 h in a dry-air incubator in accordance with the manufacturer's instructions. Following color development, absorbance was measured at 405 nm, and the background, defined as the average value for blank cells containing no PCR product, was subtracted from all values (for our laboratory plate reader, this was 0.15 on average). A specimen was considered positive if the corrected A405 was greater than 0.5, negative if the value was less than 0.2, and borderline in the range between 0.2 and 0.499. Borderline samples were reamplified and tested again with the DIG-MWP format generic probe assay. Human β-globin control detections were performed in microtiter wells separate from those for HPV generic probe detections.
Data analysis.
Results from both laboratories were imported into a common database for comparison (Access; Microsoft). The crude percent agreement between the DIG-MWP format generic probe assay, the radioactive generic probe standard PCR assay, and the reverse line blot genotyping assay for the detection of HPV DNA was calculated as the percentage of pairwise samples with identical results. Specimens with borderline results by the new DIG-MWP method were treated in two different ways, depending on the analysis. When compared to the radioactive generic probe assay results, borderline results were considered to be negative results to obtain the most conservative estimate of the DIG-MWP generic probe assay sensitivity. However, for comparison with genotyping a borderline value was considered to be a positive result, since all specimens resulting in a corrected absorbance value greater than 0.2 would be subjected to follow-up genotyping. Cohen's unweighted κ statistic was calculated to measure the level of agreement between HPV detection methods (10). In general, a κ value from 0.61 to 0.80 represents a substantial agreement beyond chance, while a value 0.81 represents almost perfect agreement. The asymptotic-variance method was used to compute approximate 95% confidence intervals (CI). The level of significance for unequal distribution of discordant results was assessed using McNemar's chi-square test. Sensitivity and specificity of the generic probe assay were calculated considering either the results from the standard radioactive generic probe assay or the reverse line blot genotyping assay as a “gold standard” in separate comparisons.
RESULTS
HPV positivity by the DIG-MWP method versus the radioactive generic probe method.
There were 5 specimens that resulted in equivocal β-globin detection in the reverse line blot assay in our laboratory; therefore, those samples were omitted from the final analysis, leaving 282 samples for analysis. As indicated in Table 2, agreement between the two laboratories was 90.4% (κ = 0.77; CI, 0.68 to 0.85) when the radioactive generic probe method was compared to the detection of PGMY primer amplification followed by DIG-MWP generic probe detection. Of the 27 discordant results, 19 were positive with the DIG-MWP assay while eight were positive with the radioactive generic probe method (McNemar χ2 = 3.70; P = 0.054). In addition, the DIG-MWP generic probe technique appeared to be slightly more sensitive than the standard dot blot technique by detecting an additional 6.6% (19 of 282 samples) HPV-positive samples, although this difference is not statistically significant. Since the borderline values were treated as negative results in this comparison, the sensitivity of the assay may have been slightly underestimated. Borderline specimens accounted for 5.3% (15 of 282 samples) of results in this particular study. Seven of the 15 samples yielding borderline results contained HPV DNA detectable using the reverse line blot, including three specimens containing HPV type 52 infections, one with type 45, one with type 53, one with type 61, and one sample containing both type 58 and 73. Four of the borderline specimens gave a positive result using the radioactive generic probe assay previously, including two samples with an identifiable genotype (one type 52 and the other sample containing types 58 and 73). Gel agarose analysis conducted during the original standard PCR testing in Montreal gave a positive HPV band for only 2 of these 15 specimens, both of which contained a verified HPV infection as determined by the strip genotyping result. A repeat PGMY amplification reaction and detection with the DIG-MWP generic probe resolved several of these borderline specimens: six samples were unequivocally negative when retested, five samples were still borderline, and four samples were clearly positive. Using the standard PCR and radioactive generic probe detection as a comparative standard, the DIG-MWP generic probe method had a sensitivity of 95.9%. The calculated specificity was 73.6% for this comparison; however, this estimate did not account for any true positives not detected using the radioactive generic probe. Of the 19 samples falsely positive by the DIG-MWP assay in comparison to the radioactive generic probe method, 15 were in fact true positives, as verified by genotyping. Detection levels of the DIG-MWP generic probe assay using purified HPV DNA dilutions were at or below levels of sensitivity previously described (12) across a broad spectrum of genital genotypes (i.e., at or below 10 copies/PCR for many HPV genotypes).
TABLE 2.
Comparison between DIG-labeled generic probe MWP assay and standard radiolabeled-generic-probe methoda
| DIG-MWPb result | No. of samplesc with indicated result by standard methodd
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 188 | 19 | 207 |
| Negative | 8 (4) | 67 (11) | 75 (15) |
| Total | 196 | 86 | 282 |
K = 0.77 (CI, 0.68 to 0.85).
DIG-MWP assay with primers PGMY09 and PGMY11.
Numbers in parentheses are numbers of borderline results included.
Primers MY09, MY11, and HMB01 were used.
Comparisons with genotyping.
We observed a good intralaboratory agreement of 87.9% between the DIG-MWP generic probe assay and genotyping using the reverse line blot methodology, which detects 27 HPV types (κ = 0.70, CI, 0.61 to 0.79). As shown in Table 3, when samples were screened for the additional 12 HPV types, the agreement between the DIG-MWP generic probe assay and genotyping for 39 HPV types was 93.3% (κ = 0.82; CI, 0.74 to 0.90). McNemar's test for unequal distribution of discordance in this comparison gave a statistically significant result (χ2 = 13.47; P < 0.001). Only a single sample containing HPV42 failed to test positive with the new generic probe assay, in spite of a positive genotyping result. The generic probe assay result for that sample was well below the cutoff and was not referred for reamplification. Approximately 6% (n = 18) of samples that were positive or borderline when tested with the DIG-MWP generic probe assay were not detected by the HPV type-specific probes in the panel. Of these 18 samples, there were 8 that also tested positive using the radioactive generic probe assay including 3 samples that had a positive genotyping result using the radiolabeled type-specific probes (one type 6 or -11, one was type 31, and one was type 56). The rest of the positives may represent either novel HPV types or low-level infections at the edge of the detection limit. Standard HPV PCR methods using dot blots with the radioactive generic probe and traditional radiolabeled type-specific probes for 14 HPV genotypes gave an intralaboratory agreement of 73.2% (κ = 0.48; CI, 0.39 to 0.57). Results from the radioactive generic probe assay were compared with the line blot genotyping results obtained in the California laboratory to test whether this difference in intralaboratory concordance is due to the higher number of genotypes included in the line blot method than in the dot blot genotyping strategy. There was an 86.5% agreement between the radioactive generic probe assay results and those obtained with the 27-probe genotyping strip (κ = 0.69; CI, 0.60 to 0.78). By including all 39 genotype results from the reverse line blots in the analysis, agreement increased to 88.7% (κ = 0.73; CI, 0.64 to 0.81). When a comparison between the two laboratory genotyping results was done, restricting the data to the 14 genotypes included in both data sets, the agreement was 89.0%.
TABLE 3.
DIG-MWP assay results compared to genotyping for 39 HPV typesa
| DIG-MWP resultb | No. of samplesc with indicated result by reverse line blot genotyping with PGMY primers
|
Total | |
|---|---|---|---|
| Positived | Negative | ||
| Positive | 204 (7) | 18 (8) | 222 (15) |
| Negative | 1 | 59 | 60 |
| Total | 205 | 77 | 282 |
K = 0.82 (CI, 0.74 to 0.90).
Primers PGMY09 and PGMY11 were used.
Numbers in parentheses are the numbers of borderline values included.
Samples were considered positive if one or more HPV genotypes were detected.
When the reverse line blot genotyping system with 27 HPV types represented was used as a reference method for evaluation of the generic probe assays, the sensitivity of the DIG-MWP assay was 99.5%, while specificity was 64.1%. Specificity was improved to 76.6% by including the additional 12 genotypes in the reverse line blot reference method, and sensitivity was not significantly changed (99.5%). As discussed above, 8 of the 18 “false positives” were also found to be positive for HPV according to radioactive generic probe results and 3 of those were verified with genotyping. The additional false positives may have been truly a result of low-copy-number infections or novel genotypes not represented in the genotyping strip probe array. By comparison, the radioactive generic probe assay had a sensitivity of 91.1% and a specificity of 77.2% using PGMY amplification plus detection with the 27-type strip as the reference method. In this case, there was an improvement to 86.8% specificity with the inclusion of the additional 12 genotypes in the reference method results. However, because the number of positive specimens by reverse line blot genotyping increased upon inclusion of additional novel HPV types, the calculated sensitivity of the radioactive generic probe assay dropped slightly, to 89.3%.
Effects of the PGMY primer system.
Results for amplification of HPV with each primer system are shown in Table 4, tabulated by genotype. The standard PCR test run in the Montreal laboratory included 14 genotypes, while the PCR tests with biotinylated primers PGMY09 and -11 and MY09 and -11 run in California used the 27-type reverse line probe system. For the PGMY-amplified samples, an additional array of probes for 12 HPV types were also used as described above. The total number of samples positive for any HPV was determined from the generic probe data, and the PGMY primer system detected the highest number of positive samples (n = 222, 77.4% of total). Amplification with nonbiotinylated MY09 and -11 detected 196 samples (68.3%), while the biotinylated MY09 and -11 primers detected 183 samples (63.8%).
TABLE 4.
HPV types amplified with different primer sets and detected using the intralaboratory genotyping method
| HPV type | Results for:
|
|||||
|---|---|---|---|---|---|---|
| Montreal laboratory (standard PCR [MY09 and -11] and dot blot genotyping)
|
California laboratory
|
|||||
| PGMY primers (biotinylated) and reverse line blot genotyping
|
MY09 and -11 primers (biotinylated) and reverse line blot genotyping
|
|||||
| No. positive | % of total | No. positive | % of total | No. positive | % of total | |
| Any HPVa | 196 | 68.3 | 222 | 77.4 | 183 | 63.8 |
| 6 or 11 | 20 | 7.0 | 14 | 4.9 | 15 | 5.2 |
| 16 | 5 | 1.7 | 10 | 3.5 | 6 | 2.1 |
| 18 | 4 | 1.4 | 8 | 2.8 | 7 | 2.4 |
| 26 | NAb | 4 | 1.4 | 0 | 0.0 | |
| 31 | 17 | 5.9 | 14 | 4.9 | 12 | 4.2 |
| 33 | 10 | 3.5 | 7 | 2.4 | 8 | 2.8 |
| 35 | 13 | 4.5 | 13 | 4.5 | 3 | 1.0 |
| 39 | 18 | 6.3 | 23 | 8.0 | 14 | 4.9 |
| 40 | NA | 2 | 0.7 | 0 | 0.0 | |
| 42 | NA | 22 | 7.7 | 0 | 0.0 | |
| 45 | 11 | 3.8 | 14 | 4.9 | 7 | 2.4 |
| 51 | 9 | 3.1 | 5 | 1.7 | 7 | 2.4 |
| 52 | 22 | 7.7 | 26 | 9.1 | 6 | 2.1 |
| 53 | 19 | 6.6 | 18 | 6.3 | 19 | 6.6 |
| 54 | NA | 27 | 9.4 | 21 | 7.3 | |
| 55 | NA | 13 | 4.5 | 3 | 1.0 | |
| 56 | 27 | 9.4 | 21 | 7.3 | 11 | 3.8 |
| 58 | 19 | 6.6 | 14 | 4.9 | 12 | 4.2 |
| 59 | NA | 11 | 3.8 | 3 | 1.0 | |
| 61 | NA | 23 | 8.0 | NA | ||
| 62 | NA | 23 | 8.0 | NA | ||
| 64 | NA | 1 | 0.3 | NA | ||
| 66 | NA | 19 | 6.6 | 16 | 5.6 | |
| 67 | NA | 4 | 1.4 | NA | ||
| 68 | NA | 15 | 5.2 | 7 | 2.4 | |
| 69 | NA | 1 | 0.3 | NA | ||
| 70 | NA | 27 | 9.4 | NA | ||
| 71 | NA | 9 | 3.1 | NA | ||
| 72 | NA | 8 | 2.8 | NA | ||
| 73 | NA | 11 | 3.8 | 3 | 1.0 | |
| 81 | NA | 21 | 7.3 | NA | ||
| 82 | NA | 0 | 0.0 | 0 | 0.0 | |
| 83 | NA | 25 | 8.7 | 23 | 8.0 | |
| 84 | NA | 26 | 9.1 | 17 | 5.9 | |
| CP6108 | NA | 17 | 5.9 | NA | ||
| IS39 | NA | 1 | 0.3 | NA | ||
Detected using intralaboratory generic probe method.
NA, not available. Probes for all indicated types were not used for all detection methods.
Evaluation of the DIG-MWP β-globin control probe.
The DIG-MWP β-globin assay was used to test a series of positive and negative controls to assess performance. A randomly selected set of 72 clinical specimens from this study were tested in the β-globin DIG-MWP format to confirm that the algorithm for assessing positivity and the background levels for this probe would be identical to those for the HPV generic probe assay. Among the clinical specimens tested, there were six β-globin results that required follow-up testing: five values were borderline (optical density [OD] = 0.2 to 0.5), and a single value fell below the cutoff (OD = 0.104). All six of these samples resulted in positive signal for the β-globin control on the reverse line blot. In addition, all of these specimens were HPV positive and resulted in HPV generic DIG-MWP absorbance values greater than 1.9, suggesting high viral copy numbers that can result in competition in the PCR between the β-globin target and the HPV target. Further analysis of this phenomenon was not feasible in this particular study, since there were no β-globin-negative samples included in the initial sample selection.
DISCUSSION
Overall concordance between the two generic probe methods (i.e., standard PCR with radioactive generic probe dot blot versus PGMY PCR with DIG-MWP detection) was very good. As demonstrated by this comparative study, the results obtained from standard PCR for the detection of HPV DNA can be of high quality when performed with care. Nonetheless, standard PCR methods for HPV detection are also cumbersome and labor-intensive and require highly skilled technical expertise to correctly interpret the results. In the standard PCR testing of this sample set, HPV positivity by generic probe was determined by comparing the sample dots against a series of negative-control dots. There was no quantifiable cutoff for positivity, which makes the interpretation subjective. The default call in a questionable specimen would be HPV positive for such traditional dot blot methods, while additional analyses such as gel electrophoresis are routinely used for verification. The DIG-MWP assay also requires further investigation to resolve samples resulting in a borderline absorbance reading. However, unequivocal negative and positive cutoffs can be set to reduce the subjectivity of analysis, to triage samples that will be submitted for genotyping, and to eliminate some of the associated potential experimental error. The average number of borderline samples from this and other unpublished clinical data was on the order of 5% or less. This translates into fewer specimens requiring additional workup to resolve HPV status.
The DIG-MWP method described here for detection of HPV from clinical samples also includes a primer pair for generating a DIG-labeled β-globin control probe. Samples can be evaluated for cellular adequacy or for PCR inhibition by amplification of this cellular control. The interpretation of the β-globin control result is identical to that of the HPV generic probe assay result. If the β-globin control absorbance value is negative or borderline (i.e., OD of 0.2 to 0.5), then the amplification must be repeated. However, β-globin-negative specimens have to be tested for HPV, since several of these samples contained HPV DNA sequences. In fact, the six specimens in this study that resulted in weak β-globin signals (including one negative result) all resulted in very strong HPV DIG-MWP absorbance readings and positive genotyping. A very high HPV copy number present in the coamplified specimen could compete with the β-globin amplification and result in a falsely negative PCR result for β-globin. One way of demonstrating this would be to test the samples for β-globin alone without HPV primers. We chose the reverse line blot β-globin control rather than the DIG-MWP β-globin value to evaluate specimen adequacy for this study because it is already well established. In the present analysis, there were five specimens with equivocal β-globin results. Since all samples had been β-globin positive when first tested in the Montreal laboratory prior to testing in California, this difference could be attributed to either sample degradation or PCR amplification differences. The β-globin and HPV amplifications were done separately in the standard PCR test and not by coamplification. A high HPV viral load did not interfere in the latter assay with β-globin amplification. Variation in amplification efficiencies can be due to a number of factors such as differences in thermocycler temperature controls and primer lots, user-introduced variation, and use in a coamplification reaction. Equivocal or negative samples need to be retested and omitted if still negative for β-globin and HPV.
Another advantage of the use of the DIG-MWP generic HPV assay with biotinylated primers is that genotyping can be performed on a reverse line blot array using the same PCR products without the need for further amplification. In large studies, particularly those with a low prevalence of HPV, a reliable generic probe assay can be invaluable in screening for the presence of HPV DNA, thereby reducing the cost of testing. Only those samples with a positive or borderline result would be further tested for genotype determination. The usefulness of a generic probe assay is also related to the prevalence of HPV infection. Studies on populations with a high prevalence of HPV infection will not benefit very much from the inclusion of a generic probe test if the majority of samples contain HPV DNA.
In the present study, only one sample was misclassified as HPV negative by the DIG-MWP generic probe assay. The most common explanation for such an erroneous event is that detection of a low-copy-number HPV sample lacks reproducibility because of sampling error. In this case, the same amplification product was used for both detections; however, in a low-copy-number situation the signal may be very near detection limits for either method. Given that caveat, a “false-negative” rate of <0.4% is acceptable. The assay was reliable and sufficiently sensitive to detect a broad spectrum of HPV types at low copy numbers. By comparison, the standard PCR plus radioactive generic probe detection failed to detect 17 samples that were positively genotyped using the reverse line blot genotyping array with probes for 27 HPV types. This “false-negative” rate for radioactive generic probe detection of 5.9% increased further to greater than 7% when 12 genotypes were added.
An evaluation of the effect of using a different priming strategy in PCR (i.e., PGMY versus degenerate MY09 and MY11) reinforces the idea that a nondegenerate pool of primers is more reliable than primers that were synthesized in a degenerate fashion (discussed in reference 12). The results generated for this study suggest that the PGMY primer system is more sensitive than the MY09-MY11 primer system, since it detected more HPV positives than either of the two MY09-MY11 amplifications. The difference is likely due to variation in the efficiencies with which the degenerate MY09-MY11 primer pair amplifies different HPV types since the systems for detection of PCR products were identical in the California laboratory and AmpliTaq gold was used with both primer pairs. From a comparison of the data in Table 4, it was found that there is a notable difference between types amplified efficiently by the two different lots of MY09-MY11 primers in the separate laboratories. In general, results from the Montreal laboratory are more consistent with the PGMY amplification results than were the results obtained with the biotinylated MY09-MY11 primer pair used in the California laboratory. However, there are still marked deficiencies in the detection of certain genotypes, such as HPV16 and -18. The performance of nonbiotinylated degenerate primers in the standard PCR (Montreal laboratory) appears to be significantly better than that of the biotinylated MY09-MY11 primer pair from the California laboratory. It is possible that different degenerate oligonucleotide syntheses resulted in primer pairs that were more effective at amplifying certain HPV types or that biotinylation of primers affects amplification efficiency. The difference could also be due to coamplification with β-globin in the California laboratory versus the two separate amplifications performed in the standard PCR. For example, this sample set contained a large number of HPV52-positive samples, most of which were not detected by the MY09-MY11 amplification in the California laboratory. Conversely, the HPV16 and HPV18 samples not detected in the Montreal amplification were detected by the biotinylated MY09-MY11 primer pair used in the California PCR.
In conclusion, we believe that the use of the PGMY primer system for consensus amplification of HPV from genital specimens provides the most comprehensive coverage of representative types. The use of generic probe detection by the DIG-MWP assay described here allows for rapid and reliable screening of large numbers of specimens to identify specimens for genotyping using a reverse probe array without additional amplification of the sample. It is our hope that this approach will facilitate further natural history and epidemiological studies tracking the biology of anogenital HPVs and their resultant infection and disease states.
ACKNOWLEDGMENTS
We are grateful to Patti Gravitt for valuable comments on this paper. We thank Diane Gaudreault and Diane Bronsard for processing genital samples and Pierre Forest for HPV testing with the standard PCR test.
The Medical Research Council of Canada and Health and Welfare Canada support The Canadian Women's HIV Study. F.C. is a clinical research scholar supported by the FRSQ. E.F. holds a Distinguished Scientist Award from the Medical Research Council of Canada.
REFERENCES
- 1.Bauer H M, Greer C E, Manos M M. Determination of genital human papillomavirus infection by consensus polymerase chain reaction amplification. In: Herrington C S, McGee J O D, editors. Diagnostic molecular pathology, a practical approach. Oxford, United Kingdom: IRL Press; 1992. pp. 131–152. [Google Scholar]
- 2.Bauer H M, Ting Y, Greer C E, Chambers J C, Tashiro C J, Chimera J, Reingold A, Manos M M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–477. [PubMed] [Google Scholar]
- 3.Bosch F X, Manos M M, Munoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 4.Burk R D, Kadish A S, Calderin S, Romney S L. Human papillomavirus infection of the cervix detected by cervicovaginal lavage and molecular hybridization: correlation with biopsy results and Papanicolaou smear. Am J Obstet Gynecol. 1986;154:982–989. doi: 10.1016/0002-9378(86)90733-7. [DOI] [PubMed] [Google Scholar]
- 5.Coutlée F, Hankins C, Lapointe N, Gill J, Romanowski B, Shafran S, Grimshaw R, Haase D, Schlech W, Sellors J, Smaill F, Boucher M, Chateauvert M, Falutz J, Lalonde R, Macleod J, Noel G, Routy J P, Toma E, Garber G, Victor G, Trottier S, Berge P. Comparison between vaginal tampon and cervicovaginal lavage specimen collection for detection of human papillomavirus DNA by the polymerase chain reaction. Canadian Women's HIV Study Group. J Med Virol. 1997;51:42–47. doi: 10.1002/(sici)1096-9071(199701)51:1<42::aid-jmv7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Coutlée F, Provencher D, Voyer H. Detection of human papillomavirus DNA in cervical lavage specimens by a nonisotopic consensus PCR assay. J Clin Microbiol. 1995;33:1973–1978. doi: 10.1128/jcm.33.8.1973-1978.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutlée F, Gravitt P, Richardson H, Hankins C, Franco E, Lapointe N, Voyer H The Canadian Women's HIV Study Group. Nonisotopic detection and typing of human papillomavirus DNA in genital samples by the line blot assay. J Clin Microbiol. 1999;37:1852–1857. doi: 10.1128/jcm.37.6.1852-1857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutlée F, Trottier A M, Ghattas G, Leduc R, Toma E, Sanche G, Rodrigues I, Turmel B, Allaire G, Ghadirian P. Risk factors for oral human papillomavirus in adults infected and not infected with human immunodeficiency virus. Sex Transm Dis. 1997;24:23–31. doi: 10.1097/00007435-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Fairley F K, Chen S, Tabrazi S N, Quinn M A, McNeil J J, Garland S. Tampons: a novel patient-administered method for the assessment of genital human papillomavirus infection. J Infect Dis. 1992;165:1103–1106. doi: 10.1093/infdis/165.6.1103. [DOI] [PubMed] [Google Scholar]
- 10.Fleiss J L. Statistical methods for rates and proportions. New York, N.Y: John Wiley and Sons Inc.; 1981. [Google Scholar]
- 11.Franco E L, Villa L L, Sobrinho J P, Prado J M, Rousseau M C, Desy M, Rohan T E. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–1423. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 12.Gravitt P E, Peyton C L, Alessi T Q, Wheeler C M, Coutlee F, Hildesheim A, Schiffman M H, Scott D R, Apple R J. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravitt P E, Peyton C L, Apple R J, Wheeler C M. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–3027. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero E, Daniel R W, Bosch F X, Castellsagué X, Munoz N, Gili M, Viladu P, Navarro C, Zubiri M L, Ascunce N, Gonzales L C, Tafur L, Izaraugaza I, Shah K V. Comparison of Virapap, Southern blot hybridization, and polymerase chain reaction methods for human papillomavirus identification in an epidemiological investigation of cervical cancer. J Clin Microbiol. 1992;30:2951–2959. doi: 10.1128/jcm.30.11.2951-2959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankins C, Coutlee F, Lapointe N, Simard P, Tran T, Samson J, Hum L The Canadian Women's HIV Study Group. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. Can Med Assoc J. 1999;160:185–191. [PMC free article] [PubMed] [Google Scholar]
- 16.Hildesheim A, Schiffman M H, Gravitt P E, Glass A G, Greer C E, Zhang T, Scott D R, Rush B B, Lawler P, Sherman M E. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 17.Peyton, C. L., P. E. Gravitt, W. C. Hunt, R. S. Hundley, M. Zhao, R. J. Apple, and C. M. Wheeler. Determinants of genital human papillomavirus detection in a US population. J. Infect. Dis. 183:1554–1564. [DOI] [PubMed]
- 18.Vermund S H, Schiffman M H, Goldberg G L, Ritter D B, Weltman A, Burk R D. Molecular diagnosis of genital human papillomavirus infection: comparison of two methods used to collect exfoliated cervical cells. Am J Obstet Gynecol. 1989;160:304–308. doi: 10.1016/0002-9378(89)90430-4. [DOI] [PubMed] [Google Scholar]
- 19.Walboomers J M, Jacobs M V, Manos M M, Bosch F X, Kummer J A, Shah K V, Snijders P J, Peto J, Meijer C J, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]