Abstract
A quantitative, fluorescence-based PCR assay (TaqMan-based system) was developed for detection of human herpesvirus 8 (HHV-8) DNA in clinical specimens. Primers and probes chosen from each of five 10-kb segments from the unique region of the HHV-8 genome were evaluated for sensitivity with dilution series of DNA extracted from a cell line (BCBL-1) that harbors HHV-8 DNA. Although several of the primer-probe sets performed similarly with BCBL-1 DNA that had been diluted in water, their performance differed when target DNA was diluted in a constant background of uninfected cell DNA, an environment more relevant to their intended use. The two best primer-probe combinations were specific for HHV-8 relative to the other known human herpesviruses and herpesvirus saimiri, a closely related gammaherpesvirus of nonhuman primates. PCRs included an enzymatic digestion step to eliminate PCR carryover and an exogenous internal positive control that enabled discrimination of false-negative from true-negative reactions. The new assays were compared to conventional PCR assays for clinical specimens (saliva, rectal brushings, rectal swab specimens, peripheral blood lymphocytes, semen, and urine) from human immunodeficiency virus-positive patients with or without Kaposi's sarcoma. In all instances, the new assays agreed with each other and with the conventional PCR system. In addition, the quantitative results obtained with the new assays were in good agreement both for duplicate reactions in the same assay and between assays.
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS)-associated herpesvirus, is the etiologic agent of KS; it has been associated with a subset of body cavity lymphomas known as primary effusion lymphoma and multicentric Castleman's disease (reviewed in references 8, 13, and 15). Many aspects of HHV-8 natural history are unknown, especially with regard to viral load in various bodily fluids in relation to time after infection, immune function, and therapy directed either at the virus, the end-organ disease, or the immune system. Study of such relationships requires reproducible, accurate, and precise methods for determining DNA concentrations. Semiquantitative and robust quantitative systems have been described, but in general they are not easily adapted for high throughput and are susceptible to PCR contamination due to both the number of manipulations required and the need to manipulate amplified products to enable detection (2, 3, 6, 7).
Methods have been developed for the detection of PCR products that enable continuous monitoring of product accumulation during PCR, e.g., TaqMan, LightCycler, fluorescence resonance energy transfer probes (1, 5), and molecular beacons (17); these methods are collectively referred to as real-time PCR. These methods do not require specimen manipulation after reaction assembly, thereby substantially reducing the possibility of PCR carryover contamination and reducing net labor. In addition, these systems can be adapted to yield accurate and precise quantitative information over a dynamic range that spans 6 orders of magnitude. Several assays based on the TaqMan system have been described for HHV-8 (4, 11, 12). While these systems have clear utility, many aspects of their performance have not been defined, and only a few primer-probe combinations have been evaluated.
Here we describe the development and validation of a quantitative TaqMan-based system that is based on primer-probe combinations chosen from primer-probe sets derived from five different segments of the HHV-8 genome. Methods were incorporated for preventing amplimer carryover contamination and for evaluating amplification efficiency. The optimized assays were compared to each other and to a standard qualitative PCR system that used radioactivity-based Southern blot amplimer detection.
MATERIALS AND METHODS
Patient specimens.
Patient specimens were obtained from human immunodeficiency virus-positive patients, some with KS (P. E. Pellett et al., unpublished data). Specimens included peripheral blood lymphocytes, semen, urine, rectal swab specimens, rectal brushings, and saliva. Appropriate institutional approvals and informed consents were obtained.
Primers and probes.
Primers and probes were chosen using Primer Express software (PE Applied Biosystems, Foster City, Calif.). Primers and probes were synthesized by standard phosphoramide chemistry techniques at the Biotechnology Core Facility at the Centers for Disease Control and Prevention. Probes were labeled at the 5′ end with the reporter molecule 6-carboxyfluorescein (FAM) and at the 3′ end with the quencher 6-carboxytetramethylrhodamine. All TaqMan probes were synthesized with a 3′ phosphate group to block extension by Taq polymerase.
TaqMan PCR.
TaqMan reagents and enzymes were obtained from PE Applied Biosystems. Each 50-μl PCR contained 1× TaqMan universal PCR master mix containing uracil-N-glycosylase (AmpErase), 500 nM each HHV-8 primer, 100 nM HHV-8 probe, 0.2 fg of an exogenous internal positive control DNA (TaqMan exogenous internal positive control), 1× TaqMan exogenous IPC primer and probe (VIC-labeled probe) mix, and 5 μl of template DNA. Following 2 min of incubation at 50°C for the activation of uracil-N-glycosylase, the polymerase (Amplitaq Gold) was activated at 95°C for 10 min. Forty cycles of PCR were done, each consisting of 95°C for 15 s and 60°C for 1 min. Amplification was carried out with an ABI Prism 7700 sequence detection system (PE Biosystems, Foster City, Calif.), which permitted continuous automated reading of fluorescence intensities during PCR. Each PCR run contained numerous controls along with the standard dilution curve, including six no-amplification controls and six no-template controls. The dilutions of control template used to generate the standard curve were run in duplicate reactions, as were the unknown specimens. Data were analyzed in both real-time and end-point plate read modes.
Control template for standard curve.
Purified BCBL-1 DNA (QIAamp blood kit; Qiagen, Valencia, Calif.) was used to prepare the standard curve. The HHV-8 genomic copy number was determined by Southern blot comparisons between dilutions of the purified BCBL-1 DNA and a plasmid of known concentration that contained an HHV-8 genomic fragment by use of a phosphorimager. As determined in a Southern blot reconstruction experiment, relative to a purified plasmid containing an HHV-8 genomic segment, there were approximately 25 copies of HHV-8 DNA per cell in the preparation used for these experiments (E. C. Mar, personal communication). The standard curve was generated based on dilutions of this DNA (5 × 106 copies to 0.5 copy per reaction) in a constant background of 500 ng of DNA purified from uninfected HLF cells per reaction and 0.2 fg of the exogenous internal positive control DNA.
Internal positive control.
A 0.2-fg quantity of TaqMan exogenous internal positive control was spiked into each unknown sample and control template. This control enables true negatives to be distinguished from false negatives due to PCR inhibition. In the PCR, the target and internal positive control DNA are amplified simultaneously. A negative result for the target and a positive result for the internal positive control DNA indicate that no target sequence is present. A negative result for each suggests PCR inhibition. The amounts of internal positive control DNA and primers included in each reaction were chosen by titration to be sufficient for consistent detection of the internal positive control DNA amplification without compromising the efficiency of the target DNA amplification. A post-PCR plate read (end point) was done to collect one fluorescence scan per well for detection of the internal positive control DNA amplification. The internal positive control DNA is detected with a VIC-labeled probe, allowing it to be discriminated from the HHV-8 DNA detected with the FAM-labeled probe. Data acquisition and analysis were done using sequence detection system software (version 1.6.3; PE Applied Biosystems).
RESULTS
Selection and preliminary evaluation of primers and probes.
Computer-based methods for choosing primers and probes do not take into account important parameters, such as the sequence composition of the complex mixture of DNA that is represented in a virus-infected eucaryotic cell or the influence of secondary structure on the ability of a segment of DNA to be amplified. Thus, experiments must be done to evaluate the relative utility of computer-chosen primer-probe combinations (10). Primer-probe combinations were chosen that were among the best predicted within each of five 10-kb segments that together span 50 kb of the HHV-8 genome. The different genomic segments were targeted to obtain at least two primer-probe sets derived from different genomic regions, to provide greater assurance that potentially infectious virus is being detected, and to ensure that the chosen sets represent theoretically and experimentally evaluated nearly optimal sequences derived from sampling of a substantial portion of the viral genome. Sequence analyses have indicated that this portion of the genome is generally highly conserved among HHV-8 genomes obtained on different continents and from different diseases (9). Criteria for the initial selection of primers included a G+C content of between 30 and 80%, avoidance of identical runs of a nucleotide, amplimer lengths of between 60 and 150 bp, a Tm of ∼58 to 60°C, and the absence of more than two G's or C's at the 3′ end. Probes were chosen to avoid identical runs of a nucleotide, to not have a G at the 5′ end, and to have a Tm ∼10°C higher than that of the corresponding primers (∼68 to 70°C). In addition, several primer sets were chosen so that their reaction temperatures would be the same for ease during optimization and eventual simultaneous use. The amplimers chosen for experimental evaluation ranged in length from 67 to 125 bp (Table 1).
TABLE 1.
Primer-probe combinations used in TaqMan-based assays for the detection of HHV-8 DNA
| Gene | Primer or probe | Oligonucleotide sequence | 5′ coordinatea | Tm (°C) | ΔRnb, c
|
Ctb
|
Correlation coefficientb, d
|
Detection limitbe
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | − | + | |||||
| K5 | Forward | ACAAGGACCGTCAATTCGATGT | 25924 | 59 | ||||||||
| Reverse | CGCGTAAGTGGCACTGTGA | 25997 | 58 | |||||||||
| Probe | CCCGGGTGTTATTTGCCGCGTATAAT | 26027 | 68 | 1.0 | 1.0 | 14 | 14 | 0.97 | 0.97 | 5 | 5 | |
| orf25 | Forward | CCACCCTCGAATGCACAAC | 44138 | 59 | ||||||||
| Reverse | GTCGGGATCGGGAAAAGCT | 44186 | 60 | |||||||||
| Probe | CCACCCAGTCAGCCCAGGCACTAAAC | 44158 | 70 | 1.2 | 1.2 | 14 | 13 | 0.98 | 0.99 | 5 | 5 | |
| orf37 | Forward | TCGGTGGCGATGCTTTAGAC | 58537 | 60 | ||||||||
| Reverse | TGAAGCAGACGATGCTTTGC | 58614 | 59 | |||||||||
| Probe | TCGTAACCCCCGTCTACTTTCCCCG | 58585 | 69 | 1.2 | 1.5 | 14 | 13 | 0.98 | 0.99 | 5 | 5 | |
| orf47 | Forward | CAAACTTCGTTGTAAATACCACAACA | 69586 | 58 | ||||||||
| Reverse | TGAATGGGTTTAATCTGAGGTCATT | 69668 | 59 | |||||||||
| Probe | CCCAATCTTCGAACGACCGCGACTAA | 69641 | 70 | 1.3 | 1.5 | 14 | 14 | 0.97 | 0.97 | 50 | 50 | |
| orf56 | Forward | AGGACGCGTGCACTTTCC | 79821 | 58 | ||||||||
| Reverse | TTCCTGAGCGCCAAATGC | 79928 | 59 | |||||||||
| Probe | CCTGGCTCAGAACGAAATTTGTTACCGC | 79851 | 69 | 1.0 | 1.0 | 14 | 14 | 0.98 | 0.98 | 5 | 5 | |
Position relative to positions in the sequence under GenBank accession number U75698 (HHV-8 unique segment) (14).
Result obtained in the absence (−) or presence (+) of 10 ng per μl of uninfected cell DNA.
ΔRn values are from Ct values.
Linearity of response over a dilution series ranging from 0.5 to 5 × 106 HHV-8 genomic copies.
Number of HHV-8 genomes in the lowest dilution yielding a positive reaction in a dilution series ranging from 0.5 to 5 × 106 HHV-8 genomic copies.
In initial experiments, primer concentrations and ratios were titrated using a constant amount of purified BCBL-1 DNA (equivalent to 2,400 copies) to determine the minimum primer concentrations that yielded the maximum magnitude of the signal generated (ΔRn). Primer concentrations tested were 250, 500, and 750 nM. All probes were used at a concentration of 100 nM. The following primer concentrations (for the forward and reverse primers, respectively) worked best: K5, 250 and 500 nM; orf25, 500 and 500 nM; orf37, 500 and 500 nM; orf47, 500 and 750 nM; and orf56, 500 and 500 nM.
Primer and probe sets were tested against dilutions of purified BCBL-1 DNA ranging from 5 × 106 copies/μl to 0.5 copy/μl. As summarized in Table 1, all of the primer-probe combinations performed well when the dilution was done in water. However, when the same template was diluted in a background of 10 ng per μl (500 ng per reaction) of DNA extracted from uninfected cells to mimic the actual conditions of low virus concentrations in human tissues and fluids, the primer-probe sets based on orf25 and orf37 had better values for both ΔRn and the threshold cycle of detection (Ct) (Table 1). Standard curves generated with these primers and a control template diluted in the presence of uninfected cell DNA had a correlation coefficient of approximately 0.99 over the range of 5 to 5 × 106 genomic copies (Table 1).
Exogenous internal coamplified positive control.
The use of an exogenous internal coamplified positive control enables same-tube evaluation of amplification efficiency, which reflects the presence of PCR inhibitors. This is particularly useful with specimens such as bodily fluids or tissues in which the cellular DNA load can vary widely and in which the detection of a cellular gene by standard PCR can give a false sense of the suitability of a template preparation for detecting a viral DNA target that may be present at fewer than 1 copy per 100,000 cells. We used a commercially available internal positive control DNA that can be detected with a VIC-labeled probe that does not interfere with the FAM-labeled probe used to detect HHV-8 DNA. Titrations of the internal positive template sequence and its primers and probes were done to identify concentrations that enabled reliable detection of the probe without interfering with the amplification of viral DNA (data not shown). The internal positive control DNA could be diluted 40-fold relative to the manufacturer's recommendations. The recommended concentration of the probe worked the best (data not shown). Under these conditions, the internal positive control could be detected with no discernible effect on HHV-8 detection limits.
Specificity.
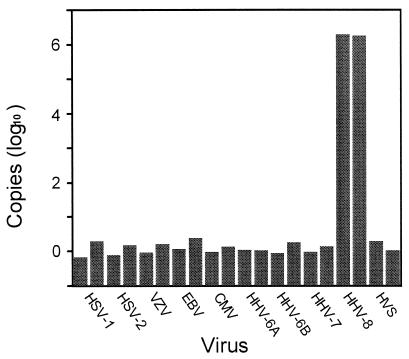
All of the primer-probe sequences were compared with sequences in GenBank by using BlastN and BlastX; no obviously significant matches to anything other than their intended HHV-8 targets were identified. The orf25 and orf37 primer-probe combinations were further tested for specificity in reactions that included 105 genomic copies of each of the other known human herpesviruses plus herpesvirus saimiri, a nonhuman primate gammaherpesvirus closely related to HHV-8. As shown for orf37 in Fig. 1, only HHV-8 gave a positive signal.
FIG. 1.
Sensitivity and specificity of the orf37 TaqMan-based assay. Specificity reactions (shown in duplicate) included 105 genomic copies of each of the following: herpes simplex virus type 1 (HSV-1), HSV-2, varicella-zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), HHV-6A, HHV-6B, HHV-7, HHV-8, and herpesvirus saimiri (HVS).
Comparison with conventional PCR.
Having optimized for sensitivity and specificity the primer-probe combinations, we compared the orf25 and orf37 TaqMan-based PCR assays to our conventional PCR assay. Patient specimens were obtained from human immunodeficiency virus-positive patients, some of whom had KS. Specimens included peripheral blood lymphocytes, semen, urine, rectal swab specimens, rectal brushings, and saliva and were chosen as a mixture of specimen types that had shown positive or negative reactivity in our conventional PCR assay (16). There was complete correlation between the TaqMan-based assays and the standard assay (Table 2). Viral loads for HHV-8-positive samples ranged from 13 copies/reaction to 1,530 copies/reaction. The results obtained from duplicate reactions were very consistent (less than 2% variation from the mean), and the quantitative values from the orf25 and orf37 assays were in good agreement (Table 2). These results are further indicators of the reproducibility of the assays.
TABLE 2.
Comparison between conventional and TaqMan-based PCR assays for detection of HHV-8 in clinical specimens
| Specimen
|
Result in the following PCR:
|
|||
|---|---|---|---|---|
| No. | Typea | Conventionalb | TaqMan basedc
|
|
| orf37 | orf25 | |||
| 10 | PBL | − | ||
| 49 | Saliva | + | 361 | 501 |
| 48 | Semen | − | ||
| 27 | Rectal brushing | + | 12 | 28 |
| 89 | PBL | − | ||
| 110 | Saliva | + | 1,117 | 2,038 |
| 27 | Rectal swab specimen | − | ||
| 105 | Urine | − | ||
| 41 | PBL | + | 211 | 284 |
PBL, peripheral blood lymphocytes.
The conventional PCR results are from two independent assays whose results were in complete agreement. These assays were based on radiolabeled Southern blot detection (16).
Number of copies per 5 μl of DNA; mean of duplicates (variation from the mean was less than 2%).
The highest concentration of control BCBL-1 DNA template used to generate the standard curve typically results in an initial Ct value of 14 cycles, indicating a significant increase in the ΔRn. At the detection limit, the control specimen containing approximately five copies of HHV-8 DNA usually has a Ct value of approximately 33 cycles. All of the clinical specimens that were identified as positive in the conventional PCR assay had Ct values of between 26 and 32 cycles in the two TaqMan-based assays. All of the clinical specimens that were identified as negative in the conventional PCR assay had Ct values of greater than 35 cycles in the two TaqMan-based assays.
DISCUSSION
Quantitative, fluorescence-based assays were developed for PCR detection of HHV-8 DNA in various human body fluids. The new assays have numerous advantages over conventional PCR assays that require additional steps for amplimer detection, such as Southern blotting following gel electrophoresis or enzyme-linked immunosorbent assay. These advantages include (i) reduced opportunity for carryover contamination from prior PCR experiments because reactions are performed with closed 96-well plates that are never opened after reactions are set up, (ii) inclusion of an exogenous internal positive control that allows discrimination of true negatives from false negatives due to PCR inhibition, and (iii) ability to complete experiments in 1 day with a rigorous quantitative result. In addition, the chance of PCR carryover contamination was further reduced by inclusion of an enzymatic step to digest stray amplimers from previous reactions. The linear range of the assays was between 5 and 5 × 106 target copies; variability between duplicate specimens tested with the same assay was less than 2%, with independent quantitative assays being in agreement within a factor of approximately 2. The simplicity of the system makes it useful for application to large numbers of samples for the quantitation of HHV-8 in clinical specimens.
Lallemand et al. (4) recently described a TaqMan-based HHV-8 quantitative PCR system that was based on a single primer set derived from one HHV-8 gene (orf73). They quantitated cellular DNA in a separate reaction, a process which enabled them to report results in terms of viral genome copies per cell; for some purposes, this would be a straightforward useful addition to the method described here. However, the use of a cellular gene as a positive control target can provide false reassurance that the template is suitable for the amplification of low-copy-number targets, as exemplified by the possibility of only 1 in 100,000 cells being infected with HHV-8. To address this situation, we incorporated a titrated amount of an exogenous internal positive control that enabled us to discriminate false-negative from true-negative reactions, without competing for reaction resources or giving a false sense of security regarding template suitability.
ACKNOWLEDGMENTS
We thank Ruth Ann Tucker and David Swan for in-depth discussions and guidance during the initial stages of developing the TaqMan-based system, Eng-Chun Mar for determining the HHV-8 copy number in BCBL-1 DNA, Sheila Dollard for sharing results obtained by conventional PCR, and the Atlanta HHV-8 Working Group for collecting the clinical specimens.
REFERENCES
- 1.Bernard P S, Lay M J, Wittwer C T. Integrated amplification and detection of the c677T point mutation in the methylenetetrahydrofolate reductase gene by fluorescence resonance energy transfer and probe melting curves. Anal Biochem. 1998;255:101–107. doi: 10.1006/abio.1997.2427. [DOI] [PubMed] [Google Scholar]
- 2.Diamond C, Brodie S J, Krieger J N, Huang M L, Koelle D M, Diem K, Muthui D, Corey L. Human herpesvirus 8 in the prostate glands of men with Kaposi's sarcoma. J Virol. 1998;72:6223–6227. doi: 10.1128/jvi.72.7.6223-6227.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond C, Huang M L, Kedes D H, Speck C, Rankin G W J, Ganem D, Coombs R W, Rose T M, Krieger J N, Corey L. Absence of detectable human herpesvirus 8 in the semen of human immunodeficiency virus-infected men without Kaposi's sarcoma. J Infect Dis. 1997;176:775–777. doi: 10.1086/517299. [DOI] [PubMed] [Google Scholar]
- 4.Lallemand F, Desire N, Rozenbaum W, Nicholas J C, Marechal V. Quantitative analysis of human herpesvirus 8 viral load using a real-time PCR assay. J Clin Microbiol. 2000;38:1404–1408. doi: 10.1128/jcm.38.4.1404-1408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lay M J, Wittwer C T. Real time fluorescence genotyping of factor V Leiden during rapid-cycle PCR. Clin Chem. 1997;43:2262–2267. [PubMed] [Google Scholar]
- 6.Lock M J, Griffiths P D, Emery V C. Development of a quantitative competitive polymerase chain reaction for human herpesvirus 8. J Virol Methods. 1997;64:19–26. doi: 10.1016/s0166-0934(96)02139-8. [DOI] [PubMed] [Google Scholar]
- 7.Mendez J C, Procop G W, Espy M J, Paya C V, Smith T F. Detection and semiquantitative analysis of human herpesvirus 8 DNA in specimens from patients with Kaposi's sarcoma. J Clin Microbiol. 1998;36:2220–2222. doi: 10.1128/jcm.36.8.2220-2222.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Y-X, Pellett P E. Herpesviruses 6, 7, and 8. In: Ahmed R, Chen I, editors. Persistent viral infections. Sussex, England: John Wiley & Sons, Ltd.; 1999. pp. 269–296. [Google Scholar]
- 9.Neipel F, Albrecht J C, Ensser A, Huang Y Q, Li J J, Friedman-Kien A E, Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997;71:839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitsche A, Steuer N, Schmidt C A, Landt O, Ellerbrok H, Pauli G, Siegert W. Detection of human cytomegalovirus DNA by real-time quantitative PCR. J Clin Microbiol. 2000;38:2734–2737. doi: 10.1128/jcm.38.7.2734-2737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Leary J, Kennedy M, Howells D, Silva I, Uhlmann V, Luttich K, Biddolph S, Lucas S, Russell J, Bermingham N, O'Donovan M, Ring M, Kenny C, Sweeney M, Sheils O, Martin C, Picton S, Gatter K. Cellular localisation of HHV-8 in Castleman's disease: is there a link with lymph node vascularity? Mol Pathol. 2000;53:69–76. doi: 10.1136/mp.53.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Leary J J, Kennedy M, Luttich K, Uhlmann V, Silva I, Russell J, Sheils O, Ring M, Sweeney M, Kenny C, Bermingham N, Martin C, O'Donovan M, Howells D, Picton S, Lucas S B. Localisation of HHV-8 in AIDS related lymphadenopathy. Mol Pathol. 2000;53:43–47. doi: 10.1136/mp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellett P E, Dollard S C. Human herpesviruses 6, 7, and 8. In: Specter S, Hodinka R L, Young S A, editors. Clinical virology manual. Washington, D.C.: ASM Press; 2000. pp. 450–471. [Google Scholar]
- 14.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz T F. Epidemiology of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. Adv Cancer Res. 1999;76:121–160. doi: 10.1016/s0065-230x(08)60775-7. [DOI] [PubMed] [Google Scholar]
- 16.Spira T J, Lam L, Dollard S C, Meng Y X, Pau C P, Black J B, Burns D, Cooper B, Hamid M, Huong J, Kite-Powell K, Pellett P E. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J Clin Microbiol. 2000;38:2174–2180. doi: 10.1128/jcm.38.6.2174-2180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyagi S, Kramer F. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]