Abstract
We report the first documented case of endocarditis associated with Bartonella clarridgeiae in any species. B. clarridgeiae was identified as a possible etiological agent of human cat scratch disease. Infective vegetative valvular aortic endocarditis was diagnosed in a 2.5-year-old male neutered boxer. Historically, the dog had been diagnosed with a systolic murmur at 16 months of age and underwent balloon valvuloplasty for severe valvular aortic stenosis. Six months later, the dog was brought to a veterinary hospital with an acute third-degree atrioventricular block and was diagnosed with infective endocarditis. The dog died of cardiopulmonary arrest prior to pacemaker implantation. Necropsy confirmed severe aortic vegetative endocarditis. Blood culture grew a fastidious, gram-negative organism 8 days after being plated. Phenotypic and genotypic characterization of the isolate, including partial sequencing of the citrate synthase (gltA) and 16S rRNA genes indicated that this organism was B. clarridgeiae. DNA extraction from the deformed aortic valve and the healthy pulmonic valve revealed the presence of B. clarridgeiae DNA only from the diseased valve. No Borrelia burgdorferi or Ehrlichia sp. DNA could be identified. Using indirect immunofluorescence tests, the dog was seropositive for B. clarridgeiae and had antibodies against Ehrlichia phagocytophila but not against Ehrlichia canis, Ehrlichia ewingii, B. burgdorferi, or Coxiella burnetii.
Bartonella species are emerging pathogens in humans, causing severe diseases in immunocompromised individuals (8). Based on 16S rRNA gene sequence comparison, the Bartonella genus has been classified in the alpha subgroup of the Proteobacteria (8). At least eight Bartonella species or subspecies are known to be pathogenic for humans, including B. bacilliformis, B. quintana, B. henselae, B. elizabethae, B. grahamii, B. vinsonii subsp. arupensis (1, 26, 52), B. vinsonii subsp. berkhoffii (45), and B. washoensis (8). Furthermore, based on serological evidence, B. clarridgeiae has been associated with cat scratch disease in humans (29, 38, 46). Among these Bartonella species or subspecies, B. quintana, B. henselae, B. elizabethae, and B. vinsonii subsp. berkhoffii have been identified as causative agents of human endocarditis and B. washoensis has been identified as a cause of myocarditis (1, 5, 8, 19, 20, 45, 49).
At present, only two Bartonella species have been identified to cause clinical diseases in dogs. B. vinsonii subsp. berkhoffii has been shown to cause endocarditis, arrhythmia, and myocarditis (6, 9, 31), as well as granulomatous lymphadenitis and granulomatous rhinitis (40), and B. henselae was recently associated with a case of peliosis hepatis (27). During an active investigation of canine endocarditis cases, we cultured a fastidious, gram-negative organism that we identified to be B. clarridgeiae from the blood of a dog with an aortic endocarditis. We describe the clinicopathologic and histopathologic features as well as the microbiologic and genotypic identification of the organism isolated from the dog's blood and detected by molecular methods in the infected aortic valve.
MATERIALS AND METHODS
Strain sources.
The type strains of B. vinsonii subsp. berkhoffii (ATCC 51672) and B. clarridgeiae (ATCC 51734) were obtained from the American Type Culture Collection (Rockville, Md.). Isolate B. henselae strain U4 was obtained from our culture collection at the University of California, Davis (UC Davis). Isolate UCD-dog1 was cultured from the blood of the dog described in this report.
Clinical samples.
Blood (6 ml) was collected aseptically from the dog's external jugular vein and both lateral saphenous veins at the time of acute third-degree atrioventricular (AV) block, infectious endocarditis, and just prior to death. Blood samples were submitted for aerobic and aero-anaerobic cultures, as well as for specific Bartonella isolation on fresh blood agar (5% defibrinated rabbit blood) (16). Serum was submitted for determination of Bartonella antibody titers and for detection of several tick-borne pathogens (Ehrlichia spp., Borrelia burgdorferi) and of Coxiella burnetii, a known agent of endocarditis in humans and animals.
Similarly, on 22 June 2000, blood (0.5 to 2 ml) was collected aseptically from the external jugular vein of three cats from the household of origin for Bartonella culture and serology. A serum sample was also obtained from the dog's owner for possible detection of Bartonella antibodies.
Tissue samples.
Fragments of the aortic and pulmonic valves were frozen for PCR and electron microscopic examination and fixed in 10% buffered formalin for histology. After 24 h, formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin or silver (Warthin-Starry) stain. Slides were examined under light microscopy to characterize the lesions.
Isolation procedure.
The blood samples were collected in plastic 2-ml EDTA tubes (Becton Dickinson, Franklin Lakes, N.J.) and frozen at −70°C until plated. The blood samples were cultured on heart infusion agar containing 5% rabbit blood and were incubated in 5% CO2 at 35°C for up to 4 weeks. Identification of the isolates as B. clarridgeiae was initially based on morphological and growth characteristics, as previously described (25, 29). The isolates were then confirmed by PCR-restriction fragment length polymorphism (RFLP) analysis and sequence analysis of the citrate synthase (gltA) and 16S rRNA genes.
Microscopic and biochemical analysis. (i) DNA extraction.
The bacterial isolate obtained from the dog's blood was heated at 95°C for 10 min in sterile water. The supernatant was used as DNA template, as previously described (13). DNA was also extracted from 26 mg of aortic valve tissue and 20 mg of pulmonic valve tissue using the DNeasy Tissue Kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions.
(ii) PCR assay.
A 5-μl aliquot (diluted 1:10 in sterile water) of extracted DNA from the bacterial isolate was added to a 45-μl reaction mixture consisting of 15 mM Tris-HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, 1.25 U of AmpliTaq LD (low DNA) DNA polymerase (Perkin-Elmer, Foster City, Calif.), and 0.5 μM concentrations of each primer. The primers used for the gltA gene were 5′-GGGGACCACTCATGGTGG-3′ and 5′-AATGCAAAAAGAACAGTAAACA-3′ (39). Thermocycling was performed in an MJ PTC-100 apparatus (Watertown, Mass.) under the following conditions: 94°C for 5 min, 45 cycles of 94°C for 0.5 min, 55°C for 1 min, and 72°C for 1 min, followed by 72°C for 10 min. For the 16S rRNA gene, the primers used were 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-AAGGAGGTGATGCAGCCGCA-3′ (23), and the running cycles were as follows: 93°C for 5 min, 35 cycles of 93°C for 0.5 min, 60°C for 1 min, and 72°C for 1 min, followed by 72°C for 8 min.
For the DNA extracted from tissue, a 5-μl aliquot of extracted DNA (undiluted or a 1:5 or 1:10 dilution in sterile water) was added to a 45-μl reaction mixture as described above for the gltA and 16S rRNA genes. To determine the presence of Ehrlichia spp. and B. burgdorferi DNA, tissue-extracted DNA was evaluated by real-time PCR using protocols specific for Ehrlichia phagocytophila and B. burgdorferi as previously described (35, 42).
(iii) RFLP.
An approximately 400-bp fragment amplified from the gltA gene was verified by gel electrophoresis and then digested with TaqI (Promega, Madison, Wis.) and HhaI (New England Laboratories, Beverly, Mass.) restriction endonucleases (4, 44). The digestion conditions used were the ones recommended by the enzymes' manufacturers. Banding patterns of the digests were compared with a domestic dog isolate, B. vinsonii. subsp. berkhoffii (ATCC 51672), B. henselae (strain U-4; UC Davis), and B. clarridgeiae (ATCC 51734).
(iv) DNA sequencing.
The extracted DNA was sequenced for the gltA and 16S rRNA genes, as previously described (15). The PCR products used for DNA sequencing were purified with Microcon centrifugal filter devices (Millipore Corp., Bedford, Mass.) and sequenced with a fluorescence-based automated sequencing system (Davis Sequencing, Davis, Calif.). The FastA program of the GCG software (version 10, Wisconsin Sequence Analysis Package; Genetics Computer Group) was applied first to determine the closest bacterial species or subspecies. Then, the GAP and PileUP programs were used for sequence alignments and determination of the percentage of DNA similarity.
IFA tests. (i) Bartonella spp.
B. henselae, B. clarridgeiae, and B. vinsonii subsp. berkhoffii indirect immunofluorescent antibody (IFA) tests were performed as previously described (15, 16). The intensity of bacillus-specific fluorescence was scored subjectively from 1 to 4, and a fluorescence score of ≥2 at a dilution of 1:64 was reported as a positive result, as previously described (16). The same two readers performed a double-blinded reading of each slide. Negative and positive serum control samples were obtained from two laboratory dogs before and after infection with B. vinsonii subsp. berkhoffii and from cats before and after infection with B. henselae and B. clarridgeiae.
(ii) Ehrlichia spp., B. burgdorferi, and C. burnetii
Antibodies to Ehrlichia equi/Ehrlichia phagocytophila, Ehrlichia canis, and Ehrlichia ewingii were evaluated using commercially available IFA substrate slides (VMRD Inc., Pullman, Wash.) or purified E. equi-infected equine neutrophils, according to a previously described protocol (37). Known positive (a known-infected dog) and negative serum samples were run as controls on the same slide. B. burgdorferi antibodies were assessed by kinetic enzyme-linked immunosorbent assay and Western blotting at a reference laboratory (Diagnostic Laboratory, College of Veterinary Medicine, Cornell University, Ithaca, N.Y.).
Antibodies against phase I and phase II of C. burnetii were evaluated using a commercially available IFA substrate slide (Fuller Laboratories, Fullerton, Calif.) at a 1:64 dilution.
RESULTS
Case report.
On 4 April 2000, a 2.5-year-old, male neutered boxer was referred to the UC Davis Veterinary Medical Teaching Hospital with the diagnosis of acute third-degree AV block. The dog was acutely weak, lethargic, and nonambulatory.
The dog had been previously diagnosed by a board-certified veterinary cardiologist with a grade IV/VI left basilar systolic heart murmur due to valvular aortic stenosis at 16 months of age. Medical treatment consisted of a selective beta-blocker, atenolol (25 mg per os [p.o.] every 12 h [q12 h]). The dog was then referred to UC Davis Veterinary Medical Teaching Hospital for possible balloon valvuloplasty of the stenotic aortic valve on 28 September 1998. Echocardiography confirmed severe valvular aortic stenosis and mild aortic insufficiency, with a left ventricular-to-aortic pressure gradient of 120 mm Hg. The left ventricular papillary muscles were hyperechogenic, indicative of myocardial fibrosis. The electrocardiogram demonstrated an increased amplitude of the R waves consistent with left ventricular hypertrophy and an ST segment depression suggestive of regional myocardial hypoxia. An aortic balloon valvuloplasty was subsequently performed on 2 October 1998 without complication. The procedure was moderately successful, producing a decrease in the left ventricular-to-aortic pressure gradient to 80 mm Hg. Subsequent echocardiograms revealed static moderate valvular aortic stenosis with no changes in the left ventricular dimensions.
The dog remained free of clinical signs until March 2000, when he collapsed after exercise. An echocardiogram revealed thickened hyperechogenic aortic cusps that had restricted systolic movement and appeared calcified. The left ventricular-to-aortic pressure gradient had increased to 100 mm Hg, indicating severe progressive valvular aortic stenosis. The aortic insufficiency had increased from mild to moderate. The atenolol dose was increased from 25 mg p.o. q12 h to 50 mg p.o. q12 h.
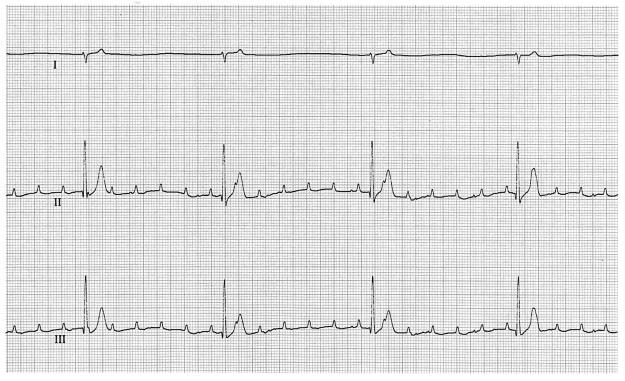
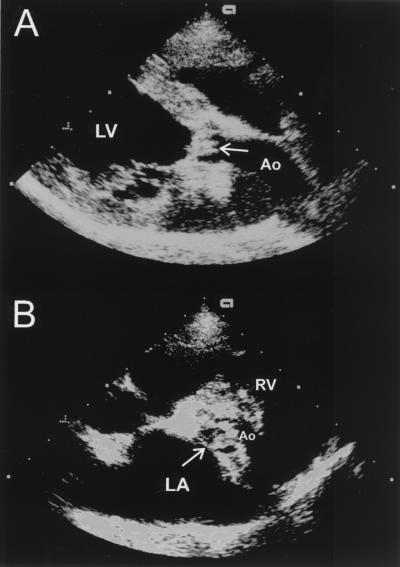
The dog was presented on 4 April 2000 as a cardiac emergency. Abnormalities on physical examination included severe weakness and depression, anisocoria, hyperthermia (39.9°C [103.5°F]), tachypnea (56 breaths/min), severely diminished femoral pulses, severe bradycardia (27 beats/min), pale mucous membranes, and a grade IV/VI left basilar systolic murmur. The electrocardiogram revealed a third-degree AV block with a ventricular rate of 28 beats/min and an atrial rate of 160 beats/min (Fig. 1). An echocardiogram again revealed severe valvular aortic stenosis (Fig. 2A and B). However, at this examination, the valvular thickening had progressed dramatically. In addition, the cusps had a shaggy, hyperechoic appearance consistent with severe infective endocarditis. The aortic insufficiency was only trivial.
FIG. 1.
Electrocardiogram showing third-degree AV block. Paper speed, 50 mm/s; gain, 10 mm/mV.
FIG. 2.
Two-dimensional echocardiograms recorded in right parasternal long axis (A) or short axis basilar (B), showing aortic valve proliferative lesions (arrows) characteristic of infective endocarditis. LA, left atrium; Ao, Aorta; RV, right ventricle. LV, left ventricule.
Hematologic abnormalities included mild reticulocytosis (96,000/μl) and nucleated red blood cells (7/100 white blood cells), mild thrombocytopenia (117,000/μl), and mild leukocytosis (19,000/μl) with neutrophilia (15,960/μl) and a regenerative left shift (380 bands with slight toxicity). There was a mild azotemia (creatinine, 2.7 mg/dl [normal range, 0.5 to 1.6 mg/dl]; BUN, 55 mg/dl [normal range, 8 to 31 mg/dl]) and hyperphosphatemia (9.6 mg/dl [normal range, 3.0 to 6.2]). A urinalysis was not available to determine if the azotemia was renal or prerenal in origin. Blood gas analysis revealed severe metabolic acidosis and a moderately elevated venous blood lactate concentration (pH, 7.22; PCO2, 48.8 mm Hg; PO2, 22 mm Hg; HCO3, 18.4 mmol/liter; blood lactate, 3.4 mmol/liter). In addition to the routine blood work, six blood samples were collected aseptically from three different venous sites over 1 h and submitted for aerobic and anaerobic cultures of common microbiologic organisms and for Bartonella isolation.
Atropine (0.04 mg/kg of body weight, intravenous) was administered without a positive chronotropic effect. An intravenous infusion of dopamine was initiated at a dose of 5 μg/kg/min and was incrementally increased to 10 μg/kg/min over 20 min with no improvement in the heart rate. Despite the grave prognosis, an attempt was made to install a temporary pacemaker via the right jugular vein. The patient's neurologic abnormalities worsened, and during the procedure the dog died of cardiopulmonary arrest.
Macroscopic and microscopic lesions.
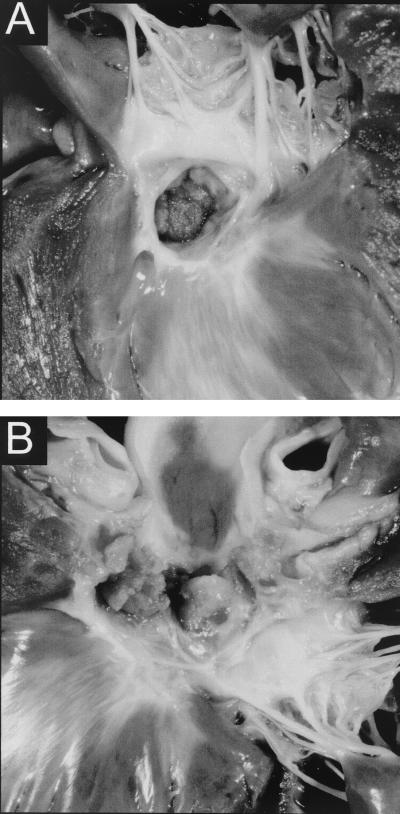
Gross examination at necropsy revealed severe infective endocarditis. Although all aortic cusps were involved, the most severely affected one was the right coronary cusp (Fig. 3A and B). The noncoronary cusp lesion infiltrated the atrial septum and protruded into the right atrium near the region of the AV node. No myocardial infarcts were noted. There was a mild fibrous subaortic ring present and moderate left ventricular concentric hypertrophy. Histopathology of the aortic valve revealed chronic, active, multifocal, moderate suppurative endocarditis. The aortic valve cusps contained fibroblasts, neutrophils, and hemosiderin-laden macrophages. However, Warthin-Starry staining failed to reveal bacteria. Similarly, no bacteria were visualized by electron microscopy on a fragment of the damaged aortic valve.
FIG. 3.
(A) Gross pathological specimen of aortic valve vegetative endocarditis. (B) Gross pathological specimen (close-up) of aortic valve endocarditis showing vegetative lesions.
The dog's environment.
The dog lived in central California in a household with several other pets, including three previously stray cats that were adopted 2.5 to 3 years prior to the onset of the dog's illness. The cats were 3 to 3.5 years old at the time of this study. The dog and the cats were mainly indoor pets, but they spent several hours a day outside in a fenced yard. All animals were infested with fleas. The animals lived in an area where ticks are endemic, but there was no history of tick infestation. The pet's owner is a human health care professional employed by an area hospital.
Isolation and identification of Bartonella
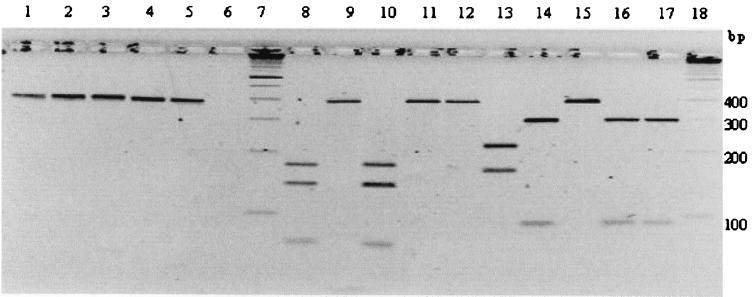
Three blood cultures for aerobic and anaerobic bacteria failed to grow bacteria. However, blood collected from the dog at the same time and cultured from the frozen-thawed EDTA tube grew a nonhemolytic gram-negative organism after 8 days, with only a few (seven), small (0.5 to 1 mm) white colonies. The colony growth characteristics and morphology were suggestive of Bartonella spp., based on time to appear, size, and color. Microscopic examination demonstrated elongated, slightly curved, gram-negative rods. PCR amplification with gltA primers produced a 400-bp fragment strongly suggestive of Bartonella. Digestion with TaqI and HhaI endonucleases produced an RFLP profile identical to that of B. clarridgeiae (ATCC 57134) and different from those of B. henselae or B. vinsonii subsp. berkhoffii (Fig. 4). Furthermore, partial sequencing of the gltA fragment (275 bp) indicated a sequence that was 100% identical to B. clarridgeiae (Table 1). The best score for the 16S rRNA gene fragment (741 bp) from the search by the FastA program yielded B. clarridgeiae as the closest match (Z score of 418.0, versus 407.7 for B. henselae). None of the three cat blood samples yielded any organisms on blood-enriched agar plates.
FIG. 4.
PCR (lanes 2 to 6) and PCR-RFLP (lanes 8 to 12, TaqI digestion; lanes 13 to 17, HhaI digestion) analysis of the gltA gene. Lanes 1, 8, and 13, B. henselae (strain U4, UC Davis); lanes 2, 9, and 14, B. clarridgeiae ATCC 57134; lanes 3, 10, and 15, B. vinsonii subsp. berkhoffii ATCC 51672; lanes 4, 11, and 16, dog isolate UCD-dog1; lanes 5, 12, and 17, DNA extracted from the aortic valve; lane 6, DNA extracted from the pulmonic valve; lanes 7 and 18, standard 100-bp molecular ladder.
TABLE 1.
DNA similarities, based on 275 bp of the gtlA gene
| DNA source | Similarity (%)
|
||||
|---|---|---|---|---|---|
| BI | AV | B. c. | B. v. b. | B. h. | |
| Blood isolate (BI) | 100 | 100 | 88 | 86 | |
| Aortic value DNA (AV) | 100 | 100 | 88 | 86 | |
| B. clarridgeiae (B. c.) | 100 | 100 | 88 | 86 | |
| B. vinsonii subsp. berkhoffii (B. v. b.) | 88 | 88 | 88 | 90 | |
| B. henselae (B. h.) | 86 | 86 | 86 | 90 | |
Serologic analysis.
Serum antibodies to B. clarridgeiae, B. vinsonii subsp. berkhoffii, and B. henselae were detected in the dog by IFA assay at reciprocal titers of 2,048 for B. clarridgeiae and 256 for the two other Bartonella species. The dog was also seropositive for E. equi/E. phagocytophila (reciprocal titer, 100) but was seronegative for E. canis, E. ewingii, B. burgdorferi, or C. burnetii. All three cats were seronegative for B. clarridgeiae, and two of the three cats had low titers for B. henselae (reciprocal titers of 128 and 64, respectively) and for B. vinsonii subsp. berkhoffii (1:64). The dog's owner was seronegative for all three Bartonella species tested.
PCR heart valve analysis.
Bartonella DNA was amplified from the aortic valve but not from the pulmonic valve. After digestion of the gltA fragment, the PCR-RFLP profile observed was characteristic of B. clarridgeiae (Fig. 4) and the partial sequence was identical to B. clarridgeiae (Table 1). Although a fragment was amplified using the primers for the Bartonella 16S rRNA gene, the product was not clean enough for sequence analysis. No Ehrlichia or Borrelia DNA was amplified from the damaged aortic valve and the pulmonic valve.
DISCUSSION
We report here the first isolation of B. clarridgeiae outside of its natural feline reservoir, as well as the first case of endocarditis associated with B. clarridgeiae. It is also the first clinical case associated with the isolation of the infectious agent, as human cases of cat scratch disease caused by B. clarridgeiae have only been established serologically (29, 38, 46). In this dog, several factors support causation, including (i) isolation of the organism from blood, (ii) failure to isolate other bacteria by conventional blood culture, and (iii) PCR amplification of B. clarridgeiae DNA from the abnormal aortic valve but not the normal pulmonic valve. Unfortunately, we were not able to confirm microscopically the presence of bacilli in the damaged valve. More likely, this negative result could be associated with the paucity of bacilli in the lesion as well as the testing of a very limited amount of the damaged tissue, most of it being used for PCR testing. The valvular lesion was located mainly on the aortic valve, as previously reported for Bartonella endocarditis cases in dogs (6, 9). As reported by those authors, endocarditis associated with Bartonella and other α-Proteobacteria mainly involves the aortic valve (70%), in contrast to a review of five studies of bacterial endocarditis in which the mitral valve was involved more often than the aortic valve (67% versus 23%). Increased predilection for aortic valve involvement could be explained by preexisting valvular disease, such as subaortic stenosis, for which boxers are congenitally predisposed. Similarly, in humans, in a series of 33 patients with Bartonella endocarditis, 29 (88%) had involvement of the aortic valve, including 2 with concurrent aortic and mitral valvular involvement, compared to only 4 patients with involvement only of the mitral valve (43). In a cohort of 15 patients with Bartonella endocarditis, more than half of the patients (53%) had preexisting valvular disease (34). Furthermore, the human case of endocarditis caused by B. vinsonii subsp. berkhoffii had a bicuspid aortic valve with thickened cusps and significant regurgitation (45), and the patient from whom B. elizabethae was isolated had large vegetations on the aortic valve (19). In humans, blood culture-negative endocarditis comprises between 5 and 30% of all cases of infective endocarditis (5, 50, 51). Bartonella endocarditis accounts for 3 to 10% of human endocarditis cases (10, 33). In one series, Bartonella infection accounted for 10 (3.3%) of 299 cases of endocarditis (10, 43), and in another series of 369 patients with endocarditis, 38 (10.3%) were diagnosed with Bartonella sp. endocarditis (33). Although the clinical incidence of infective endocarditis in dogs is unknown, reported prevalences at necropsy vary from 0.06 to 6.6% (48). However, as in human beings (43), negative blood cultures are obtained from a high number (24 to 75%) of canine endocarditis cases (11, 12, 47). Unfortunately, no data are available yet to determine the prevalence of Bartonella endocarditis among all infective canine endocarditis cases.
B. clarridgeiae has been reported as a potential zoonotic agent causing cat scratch disease, based on serologic evidence (29, 38). Recently, Sander et al. (46) indicated that 3.9% of 724 patients suffering from lymphadenopathy had antibodies specific for the FlaA protein of B. clarridgeiae. Human cases of endocarditis caused by B. clarridgeiae will likely be diagnosed in the near future, just as B. vinsonii subsp. berkhoffii was initially diagnosed in dogs and later in a human endocarditis case. It is noteworthy that in the present case the dog's owner was Bartonella seronegative and therefore unlikely to have been infected by Bartonella spp.
The source of infection and mode of transmission of B. clarridgeiae for this dog will most likely remain unexplained. The presence of several formerly stray cats in the household could be the source of infection, as domestic cats have been shown to be the main reservoir for B. clarridgeiae (17, 24, 28, 30). Although two of the three cats were B. henselae seropositive, none had B. clarridgeiae-specific antibodies and all three cats were Bartonella negative by blood culture, making it unlikely that they were the origin of the dog's infection. Bartonella infections have been shown to be vector borne, and cat fleas (Ctenocephalides felis) are the main vectors for cat-to-cat transmission of B. henselae (18, 22). Cat fleas are also considered to be the vector for B. clarridgeiae infection among cats (8). Fleas and flea dirt were observed on the household cats and therefore could have been potential vectors for the dog's Bartonella infection, as cat fleas are also the main flea species that infests dogs (3, 21). The dog was found to be not only Bartonella seropositive but also seropositive for E. phagocytophila/E. equi, an infectious agent known to be transmitted by ticks. The observation of past tick exposure in this dog raises the possibility of acquisition of the infection through a tick bite. Ticks have been suspected as the possible vector of B. vinsonii subsp. berkhoffii in dogs, based on epidemiological (41) and serological (2, 7, 32) evidence, as most B. vinsonii subsp. berkhoffii-seropositive dogs were also seropositive for E. canis or Babesia canis. Similarly, Davoust et al. reported that B. vinsonii subsp. berkhoffii-positive dogs were more likely to be infested by Rhipicephalus sanguineus ticks (P < 0.001) (B. Davoust, M. Drancourt, M. Boni, J. Signot, V. Roux, and D. Raoult, Eur. Working Group on Rickettsia-Am. Soc. Rickettsiol. Joint Meeting, Marseille, France, abstr. 232B, 1999). Tick transmission was also highly suspected in two human cases of B. henselae infection (36). Finally, we have been able to identify Bartonella DNA in Ixodes pacificus questing adult ticks from coastal northern California (14). However, more investigations are needed to better understand how B. clarridgeiae is transmitted among cats and dogs, the respective roles of fleas and ticks as vectors for this bacterium, and the potential risk for humans to acquire this infection.
ACKNOWLEDGMENTS
This project was supported in part by a grant from the Center for Companion Animal Health, School of Veterinary Medicine, UC Davis.
We thank Robert Munn, Amy Poland, Courtney Rand, and Christian Leutenegger for technical assistance. We also thank M. A. Greeley and R. J Higgins for performing the histopathology analysis.
REFERENCES
- 1.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baneth G, Breitschwerdt E B, Hegarty B C, Pappalardo B, Ryan J. A survey of tick-borne bacteria and protozoa in naturally exposed dogs from Israel. Vet Parasitol. 1998;74:133–142. doi: 10.1016/s0304-4017(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 3.Beresford-Jones W P. Prevalence of fleas on dogs and cats in an area of central London. J Small Anim Pract. 1981;22:27–29. doi: 10.1111/j.1748-5827.1981.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Birtles R J, Raoult D. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol. 1996;46:891–897. doi: 10.1099/00207713-46-4-891. [DOI] [PubMed] [Google Scholar]
- 5.Breathnach A S, Hoare J M, Eykyn S J. Culture-negative endocarditis: contribution of Bartonella infections. Heart. 1997;77:474–476. doi: 10.1136/hrt.77.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitschwerdt E B, Atkins C E, Brown T T, Kordick D L, Snyder P S. Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis or myocarditis. J Clin Microbiol. 1999;37:3618–3626. doi: 10.1128/jcm.37.11.3618-3626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitschwerdt E B, Hegarty B C, Hancock S I. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitschwerdt E B, Kordick D L. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–438. doi: 10.1128/cmr.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14:177–207. doi: 10.1128/CMR.14.1.177-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvert C A. Valvular bacterial endocarditis in the dog. J Am Vet Med Assoc. 1982;180:1080–1084. [PubMed] [Google Scholar]
- 12.Calvert C A, Greene C E, Hardie E M. Cardiovascular infections in dogs: epizootiology, clinical manifestations, and prognosis. J Am Vet Med Assoc. 1985;187:612–616. [PubMed] [Google Scholar]
- 13.Chang C C, Chomel B B, Kasten R W, Heller R, Kocan K M, Ueno H, Yamamoto K, Elmi C, Bleich V C, Pierce B M, Gonzales B J, Swift P K, Boyce W M, Jang S S, Boulouis H, Piemont Y. Isolation of Bartonella spp. from wild cervids, bovids, and domestic cattle in North America. Emerg Infect Dis. 2000;6:306–311. doi: 10.3201/eid0603.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C C, Chomel B B, Kasten R W, Romano V, Tietze N. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol. 2001;39:1221–1226. doi: 10.1128/JCM.39.4.1221-1226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang C C, Kasten R W, Chomel B B, Simpson D C, Hew C M, Kordick D L, Heller R, Piemont Y, Breitschwerdt E B. Coyotes (Canis latrans) as the reservoir for a human pathogenic Bartonella sp.: Molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J Clin Microbiol. 2000;38:4193–4200. doi: 10.1128/jcm.38.11.4193-4200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomel B B, Abbott R C, Kasten R W, Floyd-Hawkins K A, Kass P H, Glaser C A, Pedersen N C, Koehler J E. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995;33:2445–2450. doi: 10.1128/jcm.33.9.2445-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomel B B, Carlos E T, Kasten R W, Yamamoto K, Chang C C, Carlos R S, Abenes M V, Pajares C M. Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from The Philippines. Am J Trop Med Hyg. 1999;60:593–597. doi: 10.4269/ajtmh.1999.60.593. [DOI] [PubMed] [Google Scholar]
- 18.Chomel B B, Kasten R W, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield A N, Abbott R C, Pedersen N C, Koehler J E. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 21.Dryden M W, Rust M K. The cat flea: biology, ecology and control. Vet Parasitol. 1994;52:1–19. doi: 10.1016/0304-4017(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 22.Foil L, Andress E, Freeland R L, Roy A F, Rutledge R, Triche P C, O'Reilly K L. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol. 1998;35:625–628. doi: 10.1093/jmedent/35.5.625. [DOI] [PubMed] [Google Scholar]
- 23.Gurfield A N, Boulouis H J, Chomel B B, Heller R, Kasten R W, Yamamoto K, Piemont Y. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J Clin Microbiol. 1997;35:2120–2123. doi: 10.1128/jcm.35.8.2120-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller R, Kubina M, Mariet P, Riegel P, Delacour G, Dehio C, Lamarque F, Kasten R, Boulouis H J, Monteil H, Chomel B, Piemont Y. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int J Syst Bacteriol. 1999;49:283–288. doi: 10.1099/00207713-49-1-283. [DOI] [PubMed] [Google Scholar]
- 26.Kerkhoff F T, Bergmans A M C, Van Der Zee A, Rothova A. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol. 1999;37:4034–4038. doi: 10.1128/jcm.37.12.4034-4038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchell B E, Fan T M, Kordick D L, Breitschwerdt E B, Wollenberg G, Lichtensteiger C A. Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med. 2000;216:519–523. doi: 10.2460/javma.2000.216.519. [DOI] [PubMed] [Google Scholar]
- 28.Kordick D L, Breitschwerdt E B. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis. 1998;4:325–328. doi: 10.3201/eid0402.980225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kordick D L, Papich M G, Breitschwerdt E B. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob Agents Chemother. 1997;41:2448–2455. doi: 10.1128/aac.41.11.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kordick D L, Swaminathan B, Greene C E, Wilson K H, Whitney A M, O'Connor S, Hollis D G, Matar G M, Steigerwalt A G, Malcolm G B, Hayes P S, Hadfield T L, Breitschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 32.Kordick S K, Breitschwerdt E B, Hegarty B C, Southwick K L, Colitz C M, Hancock S I, Bradley J M, Rumbough R, McPherson J T, MacCormack J N. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998) J Clin Microbiol. 1999;37:1899–1905. doi: 10.1128/jcm.37.6.1899-1905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepidi H, Fournier P E, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114:880–889. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 35.Leutenegger C M, Pusterla N, Mislin C N, Weber R, Lutz H. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J Clin Microbiol. 1999;37:3390–3391. doi: 10.1128/jcm.37.10.3390-3391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucey D, Dolan M J, Moss C W, Garcia M, Hollis D G, Wegner S. Relapsing illness due to Rochalimaea henselae in immunocompetent host: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992;14:683–688. doi: 10.1093/clinids/14.3.683. [DOI] [PubMed] [Google Scholar]
- 37.Madigan J E, Hietala S, Chambers S, DeRock E. Seroepidemiologic survey of antibodies to Ehrlichia equi in horses in northern California. J Am Vet Med Assoc. 1990;196:1962–1964. [PubMed] [Google Scholar]
- 38.Margileth A M, Baehren D F. Chest-wall abscess due to cat-scratch disease (CSD) in an adult with antibodies to Bartonella clarridgeiae: case report and review of the thoracopulmonary manifestations of CSD. Clin Infect Dis. 1998;27:353–357. doi: 10.1086/514671. [DOI] [PubMed] [Google Scholar]
- 39.Norman A F, Regnery R, Jameson P, Greene C, Krause D C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pappalardo B L, Brown T, Gookin J L, Morrill C L, Breitschwerdt E B. Granulomatous disease associated with Bartonella infection in 2 dogs. J Vet Intern Med. 2000;14:37–42. doi: 10.1892/0891-6640(2000)014<0037:gdawii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Pappalardo B L, Correa M T, York C C, Peat C Y, Breitschwerdt E B. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58:467–471. [PubMed] [Google Scholar]
- 42.Pusterla N, Huder J B, Leutenegger C M, Braun U, Madigan J E, Lutz H. Quantitative real-time PCR for detection of members of the Ehrlichia phagocytophila genogroup in host animals and Ixodes ricinus ticks. J Clin Microbiol. 1999;37:1329–1331. doi: 10.1128/jcm.37.5.1329-1331.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton A M. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. . (Erratum, 127:249, 1997). [DOI] [PubMed] [Google Scholar]
- 44.Regnery R L, Anderson B E, Clarridge III J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roux V, Eykyn S J, Wyllie S, Raoult D. Report of Bartonella vinsonii subspecies berkhoffii as an agent of afebrile blood culture negative endocarditis in man. J Clin Microbiol. 2000;38:1698–1700. doi: 10.1128/jcm.38.4.1698-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sander A, Zagrosek A, Bredt W, Schiltz E, Piemont Y, Lanz C, Dehio C. Characterization of Bartonella clarridgeiae flagellin (FlaA) and detection of antiflagellin antibodies in patients with lymphadenopathy. J Clin Microbiol. 2000;38:2943–2948. doi: 10.1128/jcm.38.8.2943-2948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sisson D, Thomas W P. Endocarditis of the aortic valve in the dog. J Am Vet Med Assoc. 1984;184:570–577. [PubMed] [Google Scholar]
- 48.Sisson D, Thomas W P. Current veterinary therapy IX. Philadelphia, Pa: W.B. Saunders Company; 1986. pp. 402–406. [Google Scholar]
- 49.Spach D H, Callis K P, Paauw D S, Houze Y B, Schoenknecht F D, Welch D F, Rosen H, Brenner D J. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tunkel A R, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326:1215–1217. doi: 10.1056/NEJM199204303261809. [DOI] [PubMed] [Google Scholar]
- 51.Watanakunakorn C, Burkert T. Infective endocarditis at a large community teaching hospital, 1980–1990: a review of 210 episodes. Medicine. 1993;72:90–102. doi: 10.1097/00005792-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Welch D F, Carroll K C, Hofmeister E K, Persing D H, Robison D A, Steigerwalt A G, Brenner D J. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol. 1999;37:2598–2601. doi: 10.1128/jcm.37.8.2598-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]