Abstract
Cardiovascular disorders (CVDs) are the leading risk factor for death worldwide, and research into the processes and treatment regimens has received a lot of attention. Tilianin is a flavonoid glycoside that can be found in a wide range of medicinal plants and is most commonly obtained from Dracocephalum moldavica. Due to its extensive range of biological actions, it has become a well-known molecule in recent years. In particular, numerous studies have shown that tilianin has cardioprotective properties against CVDs. Hence, this review summarises tilianin’s preclinical research in CVDs, as well as its mechanism of action and opportunities in future drug development. The physicochemical and drug-likeness properties, as well as the toxicity profile, were also highlighted. Tilianin can be a natural lead molecule in the therapy of CVDs such as coronary heart disease, angina pectoris, hypertension, and myocardial ischemia, according to scientific evidence. Free radical scavenging, inflammation control, mitochondrial function regulation, and related signalling pathways are all thought to play a role in tilianin’s cardioprotective actions. Finally, we discuss tilianin-derived compounds, as well as the limitations and opportunities of using tilianin as a lead molecule in drug development for CVDs. Overall, the scientific evidence presented in this review supports that tilianin and its derivatives could be used as a lead molecule in CVD drug development initiatives.
Keywords: tilianin, cardiovascular disorders, cardioprotection, molecular mechanism, drug-likeness, drug development
1. Introduction
The circulatory system, also known as the cardiovascular system, is the organ system that distributes blood to and from all regions of the body through vessels. It is the most important component of the human body consisting of the heart, blood vessels, veins, and arteries circulating throughout the human body. The circulatory system is operated by the heart which is responsible for distributing nutrients, oxygen, hormones, and waste products from cells throughout the body. A resting heart circulates approximately five litres of blood every minute throughout the body. The common cause of death related to the cardiovascular system worldwide is known as cardiovascular disease (CVD). CVD is a broad term that encompasses a variety of various conditions. Several of them may occur concurrently or may even result in the development of additional disorders or diseases. According to the World Health Organization (WHO), globally, CVD constitutes a significant cause of death, claiming 17.9 million lives in 2019, and accounting for 32% of all premature deaths documented. Heart attacks and strokes were responsible for 85% of these deaths [1]. By 2030, the WHO estimates that CVD-related deaths may increase to 23.6 million/year by 2030. CVD is known to affect both men and women equally [1].
There are numerous complications that can occur within the cardiovascular system, including endocarditis, rheumatic heart disease and abnormalities in the conduction system. CVD refers to four entities, which are: (1) coronary artery disease (CAD), commonly known as coronary heart disease (CHD), is a condition caused by reduced myocardial perfusion, which leads to angina, myocardial infarction (MI) and/or heart failure; (2) cerebrovascular disease is a broad term that encompasses stroke and transient ischemic attack (TIA); (3) peripheral arterial disease (PAD) is a condition that affects the peripheral arteries, particularly arterial illness affecting the limbs, which can cause claudication and (4) aortic atherosclerosis that includes thoracic and abdominal aneurysms [2]. Cardioprotection (CP) is a strategy for protecting the heart against various insults such as ischemia, ischemia/reperfusion injury (I/R) and chemical, metabolic and physical stresses, as well as lowering the risk of heart failure (HF) and death. Cardioprotection is defined as “all systems and techniques that contribute to the heart’s preservation by minimising or even preventing myocardial damage” [3]. Since acute ischemia-reperfusion injury causes myocardial damage, cardioprotection is an endogenous system that can decrease or prevent this damage, acting by reducing myocardial damage caused by coronary artery surgery and acute MI, both of which have high morbidity and cause death. In addition to current best-practice treatment, new cardioprotective treatments must be able to prevent or reduce myocardial damage. Cardioprotection in ischemic heart disease has come a long way in the last 40 years where re-oxygenating a blocked artery can minimise the severity of a MI.
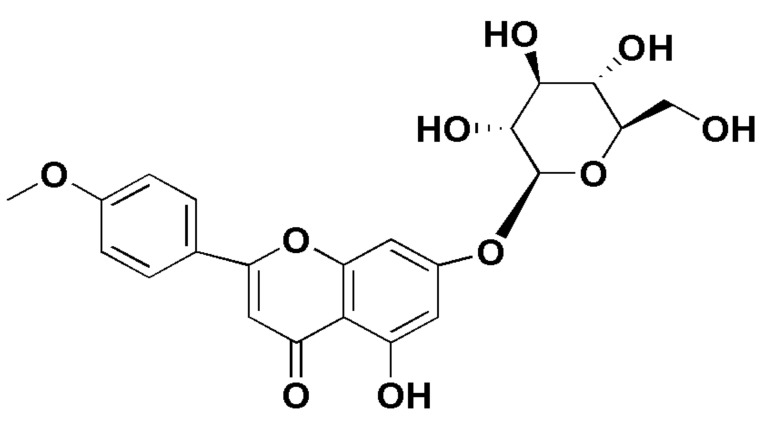
Medicines from natural products or herbs are becoming increasingly common in the medical field in the treatment of diseases defined as phytomedicine. Since ancient times, phytomedicines show a wide range of biological actions and is therefore utilised in the prevention and treatment of diseases globally [4]. The development of a novel drug molecule is a time-consuming and expensive process. One of the most important tasks in the drug discovery and development process is identification of a lead molecule, i.e., a molecule with a particular degree of potency that can be chemically changed to improve its biological activity, alter the metabolism, and pharmacokinetic profiles. Natural products from terrestrial sources (plants and fungus), microorganisms, and marine species, have a wide range of such molecules with structural diversity, making them a good source of new drug leads and therapeutic agents [5,6,7,8,9]. Researchers are intrigued by the prospect of finding novel biologically active compounds in natural products, particularly in herbs, for the treatment of diseases, especially CVD. Tilianin (acacetin-7-glucoside) is an active flavonoid glycoside (Figure 1) obtained from numerous medicinal plants and especially from Dracocephalum moldavica. It is also found in various common medicinal herbal plants including Agastache Mexicana, Agastache rugosa, Dracocephalum moldavia, Dracocephalum tanguticum, Dracocephalum moldavica, Lygodium japonicum and Discocleidion rufescens [10,11]. These plants are found in the East Asia region including China, Japan, Korea and Mexico. It was reported for wide range of biological activities including antidiabetic [12], anti-inflammatory [13], antioxidant [14], anti-depressant [15], cardioprotection [16,17] and neuroprotection [18].
Figure 1.
Chemical structure of tilianin.
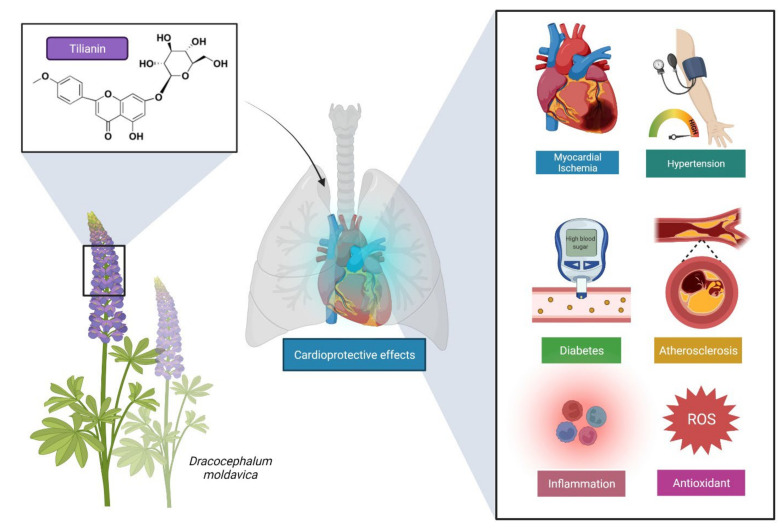
Scientific evidence clearly indicated that tilianin is a potential natural compound against CVDs such as atherosclerosis, coronary heart disease, angina pectoris, hypertension and myocardial ischemia (Figure 2). Hence, the present review aimed to provide the experimental evidence of tilianin against CVDs. The toxicity profile, as well as the physicochemical and drug-likeness properties, were also highlighted in this review. We also conducted molecular docking studies with selected proteins to validate its cardioprotection mechanism in order to strengthen this review. Along with that, we discuss tilianin-derived molecules, as well as the limitations and possible opportunities of using tilianin as a lead molecule in the drug development for CVDs.
Figure 2.
Tilianin, obtained mainly from Dracocephalum moldavica acts as a cardioprotectant and lowers the risk of CVDs.
2. Cardioprotective Effect of Tilianin
CVDs contribute to a significant cause of death in the world. Based on epidemiological research, many important risk factors for heart diseases are of environmental and biological origins [19]. The major cause of MI is oxidative damage as induced by cholesterol in the oxidation of low-density lipoprotein [20]. Jiang et al. [21] demonstrated that tilianin can ameliorate oxygen–glucose deprivation/re-oxygenation (OGD/R)-induced cardiomyocyte injury and maintain cardiac function in coronary artery ischemia/reperfusion (I/R)-injured hearts. Tilianin interacts with calcium/calmodulin-dependent protein kinase II (CaMKII) in cardiomyocytes injured by myocardial ischemia reperfusion injury (MIRI), regulating the expressions of p-CaMKII and ox-CaMKII with an efficient binding performance and strong binding score as well as inhibiting the expressions p- and ox-CaMKII. Importantly, CaMKII abolished tilianin-mediated recovery of OGD/R-induced cardiomyocyte injury and the maintenance of cardiac function in I/R-injured hearts at the expense of mitochondrial function protection. Furthermore, tilianin’s protective effects on mitochondrion-associated pro- and anti-apoptotic protein balancing as well as c-Jun N-terminal kinase (JNK)/nuclear factor (NF-) κB-related inflammation suppression were both abolished following the pharmacological inhibition of CaMKII [21].
In another study, administration of tilianin to rats reduced MI by: (1) improving the pathological morphology of the myocardium; (2) increasing the contents of ATP and NAD; (3) decreasing ADP and AMP levels as well as the ratio of AMP/ATP; (4) reducing the level of ROS and MDA and (5) up-regulating the expressions of AMPK, SIRT1, PGC-1α [22]. Furthermore, pre-administration of high dose tilianin reduced lactate dehydrogenase (LDH), malondialdehyde (MDA) and creatinine kinase-MB (CK-MB) release while increasing plasma superoxide dismuthase (SOD) levels and considerably reducing infarct size [17]. Moreover, western blot analysis revealed an increase in Bcl-2 and XIAP expressions in the myocardium, as well as a decreasing the expressions of Bax, Smac/Diablo, HtrA2/Omi as well as cleaved caspases-3, -7 and -9. Interestingly, the levels of phosphorylated Akt and PI3K were found to be increased in the presence of a high dose of tilianin.
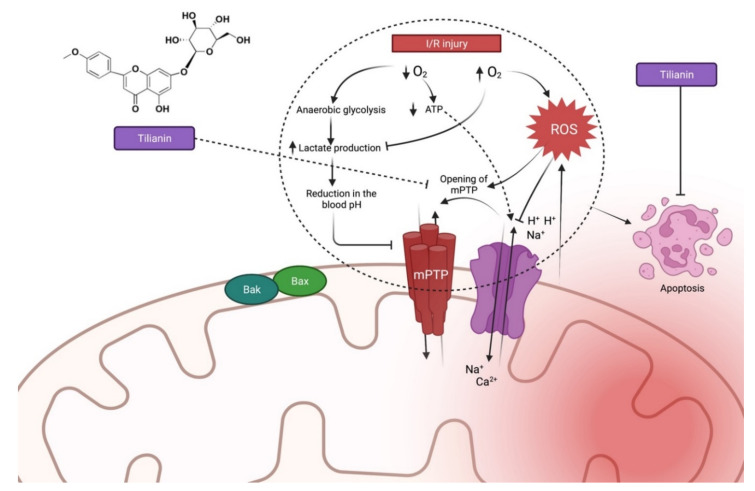
In rats, tilianin pretreatment has been found to inhibit apoptosis following I/R injury [23]. Tilianin also reduces mitochondrial damage, blocked 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (mPTP) opening and increased ATP generation. Additionally, the concentration of calcium (Ca2+) and ROS in the mitochondria was also reduced. Based on an apoptosis protein analysis by Wang et al. [23], treatment with tilianin resulted in the downregulation of apoptosis-inducing factor (AIF), as well as the suppression of cytochrome c leakage and caspase-3 activation. When tilianin was administered to MIRI rats, it enhanced ATPase activity and decreased serum nitric oxide (NO) and myocardial nitric oxide synthase (NOS) activities, both of which are important in the regulation of endothelial function. In addition, tilianin caused dose-dependent decreases in endothelin-1 and thromboxane B2 levels, as well as increases in calcitonin gene-related peptide and 6-keto prostaglandin F1a levels. Tilianin treatment reduced apoptosis, as confirmed by the increased Bcl 2 expression and decreased Bax and caspase 3 mRNA expression levels [24]. Similarly, pretreatment with tilianin in rats increased the myocardium’s ATP levels and protected the microstructures and functions of mitochondria in a dose-dependent manner. Additionally, tilianin’s cytoprotective action has been verified in vivo and in the H9c2 cardiomyoblast cell line as confirmed by the increased mitochondrial activity, regulation of Ca2+ and ROS and decreased caspase-3 and AIF productions in the cytoplasm. The study by Yuan et al. [25] further suggests tilianin’s clinical utility for cardiomyocyte and mitochondrial protection by suppressing myocardium energy metabolism and apoptosis during MIRI. Figure 3 summarises the possible mechanism of action of tilianin during I/R injury based on the abovementioned findings.
Figure 3.
Possible mechanism of action of tilianin during I/R injury [21,22,23,24,25]. In the ischemic phase, less oxygen is produced, resulting in an increase in lactate production, lowering the blood pH. Reduced oxygen also results in the opening of the mPTP, which increases ROS formation during reperfusion. The series of events affects intracellular H+, Na+ and Ca2+ distribution, eventually resulting in apoptosis. Abbreviations: ROS, Reactive oxygen species; mPTP, Mitochondrial permeability transition pore, Bax, Bcl-2 Associated X-protein; Bak, BCL2 Antagonist/killer.
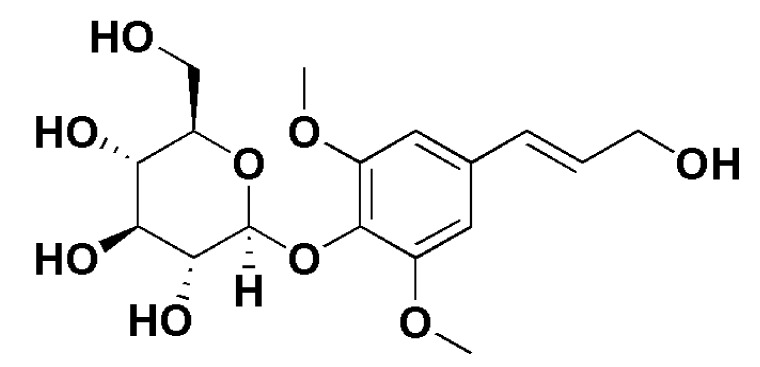
In diabetic rats and hyperglycemic-H9c2 cells, Yao et al. [26] investigated the combined effect of tilianin and syringin (50 and 60 mg/kg, i.p. respectively for eight weeks) on diabetic cardiomyopathy. They evaluated the cardiac function, inflammation, oxidative stress, apoptosis and mitochondrial function, as well as the contribution of TLR4/NF- κB/NLRP3 and PGC. The combination of tilianin and syringin (Figure 4) prevented the diabetic-induced cardiac functional, biochemical and histological alterations in diabetic cardiomyopathy in a synergistic manner. The conferred protection was further strengthened by crosstalk between the TLR4/NF-κB/NLRP3 and PGC1/SIRT3/mitochondrial pathways. All of the above findings suggested that tilianin could help in preventing ischemic heart disease, such as myocardial infarction, by inhibiting inflammatory reactions and oxidative stress [26].
Figure 4.
Chemical structure of syringin.
3. Atheroprotective Effect of Tilianin
Atherosclerosis is a multiphase process that significantly contributes to CHD and stroke and is the leading cause of mortality globally. Atherosclerosis has several pathophysiological processes and causes, the most significant of which are chronic inflammation and endothelial dysfunction [27]. Consequently, minimising endothelial damage may be a critical therapeutic strategy for atherosclerosis control and therapy. In vitro and in vivo studies conducted by Nam et al. [28] showed that tilianin has anti-atherogenic properties where the mice fed with a high-cholesterol diet supplemented with tilianin had considerably smaller lesions and lower cytokine levels when compared to control, while their serum cholesterol levels remained unchanged. In another study, the levels of TNF-α and IL-1β mRNA levels in primary cultured peritoneal macrophages from Ldlr−/− mice in response to LPS treatment were ameliorated by co-treatment with tilianin. Furthermore, electrophoretic mobility shift and NF-κB promoter experiments revealed that tilianin suppressed NF-κB activation. Tilianin prevented IκB kinase activation and the subsequent phosphorylation and degradation of IκBα protein upstream of NF-κB activation [28].
Shen et al. [29] investigated the anti-atherosclerotic mechanism of tilianin by developing in vitro models with macrophages, vascular smooth muscle cells (VSMC) and human umbilical vein endothelial cells, all of which contribute to atherosclerosis progression. Tilianin decreased TNF-α levels, generated VSMC proliferation and migration while suppressing LPS-induced inflammatory responses on macrophages. Furthermore, tilianin’s anti-inflammatory action on macrophages and VSMCs was demonstrated to be mostly contributed by the downregulation of the TNF-α/NF-κB pathway. Moreover, their findings showed that tilianin reduced the development of oxidized low-density lipoprotein (ox-LDL)-induced foam cells in macrophages by suppressing SR-A1 mRNA expression and inducing the expression of genes involved in cholesterol efflux, such as SRB-1 and ABCA1. Cao et al. [30] investigated the effects of tilianin on rat vascular smooth muscle cells (VSMCs) induced with angiotensin II on the proliferation, migration and the TGF-β/Smad signalling pathway (Ang II). In VSMCs induced by Ang II, tilianin suppresses the proliferation and expression of intracellular PCNA in a dose-dependent manner. Tilianin also inhibits the migration and expression of intracellular ICAM-1, VCAM-1, MMP-2 and MMP-9 in a dose-dependent manner. Additionally, tilianin suppresses TGF-β, Smad2, Smad3, Smad2/3 and PSmad2/3 in Ang II-induced VSMCs. Overall, tilianin’s inhibitory properties support its usage in the prevention and treatment of atherosclerosis. Based on the aforesaid data, Figure 5 summarises the possible mechanism of action of tilianin against atherosclerosis.
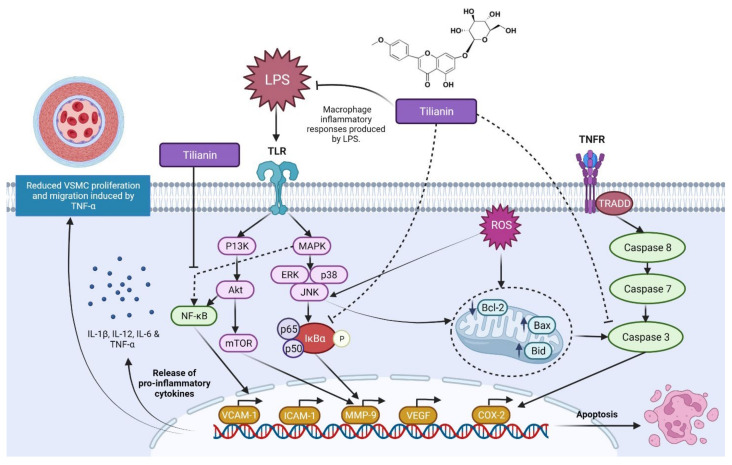
Figure 5.
Possible mechanism of action of tilianin against atherosclerosis [29,30]. Tilianin inhibits the activation of NF-κB, as well as the activation, phosphorylation and degradation of IkB kinase. This bioactive compound also inhibits TNF-induced VSMC proliferation and migration while lowering LPS-induced macrophage inflammation. Abbreviations: TLR, Toll-like receptor; TNFR, Tumor necrosis factor receptor; MAPK, Mitogen-activated protein kinase; P13K, Phosphatidylinositol-3-Kinase; ERK, Extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinases; IκBα, Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; Akt, Ak strain transforming; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; mTOR, Mammalian target of rapamycin; VCAM-1, Vascular cell adhesion protein 1; ICAM-1, Intercellular Adhesion Molecule 1; MMP-9, Matrix metallopeptidase 9; VEGF, Vascular endothelial growth factor; COX-2, Cyclooxygenase-2; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 Associated X-protein; Bid, BH3 interacting-domain death agonist; IL-1β, -12, -6, Interleukin-1 beta,-12,-6; TNF-α, Tumour necrosis factor alpha; VSMC, Vascular smooth muscle cell.
4. Antihypertensive and Vasorelaxant Effects of Tilianin
Despite recent advancements in the prevention, detection and treatment of high blood pressure, hypertension remains a major public health concern [31]. Hypertension affects approximately one billion individuals worldwide [32]. It is linked with an increased incidence of stroke, coronary heart disease, congestive heart failure and end-stage renal disease. Its occurrence can be decreased by reducing risk factors for its development, which include obesity, physical inactivity, smoking, poor potassium intake and excessive alcohol consumption. Uncontrolled hypertension raises the chance of serious health issues and is a major trigger for MI, blindness, renal disease and stroke [33]. Administration of tilianin (50 mg/kg, single dose, p.o.) resulted in significant reduction in systolic and diastolic blood pressures. Tilianin induces relaxation primarily through an endothelium-dependent pathway, likely due to NO release, as well as an endothelium-independent pathway by activating K+ channels, both of which confer the antihypertensive effect [34] (Figure 6).
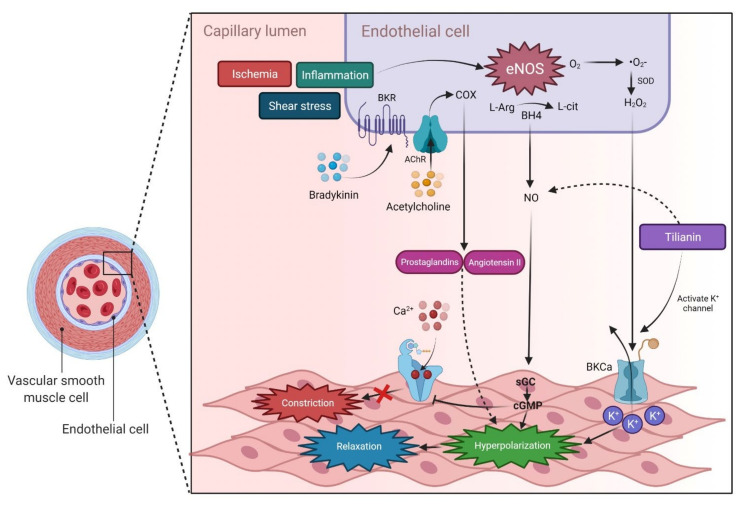
Figure 6.
The endothelial cell is involved in a variety of physiological processes, including the regulation of VSMC via a balance of vasodilators and vasoconstrictors. After being activated by endogenous stimuli such as acetylcholine, bradykinin, inflammation, shear stress and ischemia, NO diffuses into smooth muscle cells and sGC to become cGMP, which promotes smooth muscle relaxation. Tilianin stimulates K+ channels opening in vascular smooth muscle cells and NO release, resulting in hyperpolarization of the cell membrane and tissue relaxation [34]. Abbreviations: VSMC, Vascular smooth muscle cell; eNOS, Endothelial nitric oxide synthase; COX, Cyclooxygenase; SOD, Superoxide dismutases; O2, Oxygen; •O2−, superoxide; H2O2, Hydrogen peroxide; L-Arg, L-arginine; BH4, Tetrahydrobiopterin; L-cit, L-citrulline; AChR, Acetylcholine receptor; BKR, Bradykinin receptor; NO, Nitric oxide; BKCa, Ca2+-activated K+ channel; sGC, Soluble guanylate cyclase; cGMP, Cyclic guanosine monophosphate; K+, Potassium.
Furthermore, in aortic rings pre-contracted with noradrenaline (NA, 0.1 µM) and the presence of serotonin (5-HT, 100 µM), tilianin (0.002–933 µM) induced significant relaxation in concentration- and endothelium-dependent and -independent manners. Moreover, tilianin (130 µM) caused a significant shift to the left in the sodium nitroprusside (SNP; 0.32 nM–0.1 µM)-induced relaxation curve. Tilianin also caused significant in vitro NO overproduction in the rat aorta. Pre-treatment with tetraethylammonium (TEA, 5 µM) and 2-aminopyridine (2-AP, 0.1 µM) altered the relaxant curve induced by tilianin to the right [35]. Moreover, the antihypertensive impact of tilianin was dose-dependent with the calculated effective dose 50% (ED50) (53.51 mg/kg) being lower than the calculated lethal dose 50% (LD50) (6624 mg/kg), implying a wide range of pharmacology–toxicology patterns [10]. The findings indicate that tilianin should be explored further as an antihypertensive agent in randomized clinical trials. Carmona-Castro et al. [35] and Hernández-Abreu et al. [36] reported that the methanolic extract of aerial parts of Agastache mexicana has higher tilianin concentrations with potential vasorelaxant effects indicating that various parts of the plants have differing effects. The in vivo studies investigated the efficacy of tilianin against CVDs were summarized in Table 1.
Table 1.
A summary of in vivo studies investigated the efficacy of tilianin against CVDs.
| Animal, Sex | Model | Dose of Tilianin Dose (mg/kg/Day) | Route of Administration | Duration of Treatment | Mechanism of Action | Therapeutic Effects | References |
|---|---|---|---|---|---|---|---|
| SD rats, male | Isolated heart ischemia/reperfusion | -- | -- | -- | - By inhibiting CaMKII-mediated mitochondrial apoptosis - By inhibiting JNK/NF-κB inflammation |
Cardioprotection | Jiang et al. [21] |
| SD rats, male | Myocardial ischemia reperfusion injury |
5 | Intragastic | 7 Days | - By improving mitochondrial energy metabolism - By reducing oxidative stress via AMPK/SIRT1/PGC-1α signaling pathway |
Cardioprotection | Tian et al. [22] |
| SD rats, male | Myocardial ischemia reperfusion injury |
2.5, 5, 10 | p.o. | 14 Days | - By activating the PI3K/Akt signalling pathway - By inhibiting myocardial apoptosis |
Cardioprotection | Zeng et al. [17] |
| SD rats, male | Myocardial ischemia reperfusion injury |
1.25, 2.5, 5 | p.o. | 7 Days | - By alleviating apoptosis of cardiomyocytes - By protecting myocardium through protection of mitochondria and repression of mitochondrial apoptotic pathways |
Cardioprotection | Wang et al. [23] |
| SD rats, male | Myocardial ischemia reperfusion injury |
1.5, 2.5, 5 | p.o. | 7 Days | - By relieving calcium overload - By correcting energy metabolism - By improving endothelial function - By inhibiting cell apoptosis |
Cardioprotection | Guo et al. [24] |
| SD rats, male | Myocardial ischemia reperfusion injury |
1.25, 2.5, 5 | p.o. | 7 days | -By raising the levels of ATP of the myocardium - By protecting micro-structures and functions of mitochondria |
Cardioprotection | Yuan et al. [25] |
| Ldlr−/− mice, male | High-cholesteroldiet | high-cholesterol diet supplemented with 0.05% (w/w diet) of tilianin |
p.o. | 7 weeks | - By inhibiting NF-κB-dependent pro-inflammatory cytokines (TNF-α and IL-1β) - By inhibiting IκB kinase activity |
Atheroprotection | Nam et al. [28] |
| SHR rats, Male | Spontaneously hypertensive rats | 50 | p.o. | Single dose | - By endothelium-dependent manner, probably due to NO release - By endothelium-independent pathway by opening up K+ channels |
Anti-hypertensive | Hernández-Abreu et al. [34] |
The main body text covers all of the abbreviations.
5. Overview of the Mechanisms of Action for Tilianin against CVDs
The basic mechanism of tilianin in protecting the heart from CVDs in this review include: (1) vascular protection; (2) blood pressure modulation; (3) cholesterol level reduction; (4) platelet-blocking function; (5) lipid-metabolism regulation; (6) oxidative stress reduction and (7) ischemia or reperfusion reduction.
Pre-treatment with tilianin confers cardioprotective effect, with PI3K/Akt signalling playing a key part in the process as indicated by the improved cardiac damage recovery and a lower rate of myocardial apoptosis. It is critical to avoid apoptosis by limiting the cardiac damage produced by myocardial ischemia reperfusion injury (MIRI). MIRI is a type of tissue damage that develops during early coronary flow and returns to the heart promptly after ischemia, increasing myocardial injury. Based on previous research, tilianin has a cardioprotective effect and its fundamental mechanism is mitochondria-dependent Bax/Bcl-2, cytochrome c, caspase and PI3K/Akt signalling that regulates LDH, MDA, CK-MB and SOD factors. Furthermore, a study by Zeng et al. [17], stated that tilianin recovers MIRI by initiation of PI3K/Akt signalling which prevents myocardial apoptosis (caspase-3). Tilianin has an anti-atherosclerotic effect by repairing tissue as well as controlling inflammation. It is a common risk for an individual with a heart condition or MI, to have an underlying atherosclerotic condition where hypertension, diabetes and dyslipidemia are important risk factors. In addition, atheroprotection is another area where tilianin has potential since tilianin ameliorates all inflammatory responses, oxidative stress, lipid dysregulation and epigenetic disorders that contribute to the condition. Based on Nam et al. [28], the mechanism involved is regulation of inflammatory cytokines (TNF-α, IL-1β) and TGF-β/Smad/NF-κB signallings. In addition, tilianin also confers antihypertensive and vasorelaxant effects [34].
Hypertension is a common disorder that contributes to MI, stroke, kidney debilities and blindness [33]. A study performed on Agastache mexicana extract, an important source of crude tilianin, revealed to act as anti-hypertensive and a vasorelaxant [34]. Furthermore, tilianin cause a dose-dependent reduction in systolic and diastolic blood pressures through endothelium-independent mediated by potassium (K+) channel opening and NO/cGMP pathways. All the evidence points to tilianin’s potential protective effect against CVD disorders since tilianin can regulate oxidative stress, lipid metabolism, inflammation, exert positive influences on macrophage, arterial endothelial cells and prevent VSMC from excessive proliferation. Nevertheless, although tilianin shows remarkable pharmacological importance as a cardioprotection, more studies are required to confirm tilianin’s potential as an agent against CVDs, especially in randomized clinical trials.
6. In-Silico Molecular Docking Study of Tilianin
Moreover in this review, an in silico molecular docking study was conducted by us with CavAb voltage-gated calcium channel, angiotensin II type 2 receptor and human β1 adrenergic receptor to support the cardioprotection potential and mechanism of action of tilianin. Ca2+ antagonists are commonly utilised in the treatment of CVDs. 1,4-dihydropyridines are used to treat hypertension and angina pectoris, and they are thought to act as allosteric modulators of voltage-dependent Ca2+ channel activation, whereas phenylalkylamines and benzothiazepines are used to treat cardiac arrhythmias, and they are thought to physically block the pore [37]. The renin-angiotensin system (RAS) is blocked, which lowers the risk of cardiovascular events. The cardio-protective benefits of RAS inhibitors are not only dependent on hypertension control. The renin-angiotensin-aldosterone system (RAAS) effector peptide angiotensin II mediates a range of effects on blood pressure and body fluid balance and has been identified as a pathogenic factor at several points along the CVD continuum [38,39]. The sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS), particularly angiotensin II, suppress NRG-1 expression. RAAS inhibition may enhance cardiomyocyte survival pathways in patients undergoing HER2 inhibitor therapy by lowering angiotensin II levels. Carvedilol, nebivolol, and alprenolol are β-blockers that inhibit the β-1 adrenergic receptor and transactivate β-arrestin. Patients with proteasome inhibitors-related cardiac dysfunction are currently treated with β-blockers and RAAS inhibitors, but patients with light chain amyloidosis often do not tolerate these medications well [40,41]. Hence, all these three targeted protein structures (CavAb voltage-gated calcium channel, angiotensin II type 2 receptor and human β1 adrenergic receptor) were retrieved from the Protein Data Bank and processed for docking analysis using Molegro Virtual Docker 6.0 [42]. The docking results were inspected using the Pose Organizer and the ligand energy inspector tool, and the results were tabulated and the docked view was extracted (Table 2 and Figure 7). It was revealed that the MolDock score with the lowest values had the highest binding affinity to the target proteins. According to the findings, tilianin affinity for CVD protein targets was determined to be 7BU6 > 5KLB > 6JOD. Furthermore, the tilianin molecular docking scores were quite comparable to those of the standard drugs currently used to treat CVDs. This finding supports tilianin’s cardioprotective potential, perhaps advancing this physiologically active molecule to the next stage of drug discovery and development. In silico methodologies are playing an ever-increasing role in drug discovery that are critical in the cost-effective identification of promising drug candidates. However, the in silico result is not reliable to the researchers/scientists since it is a computer-generated simulation result. So, there must be a validation with in vitro and in vivo studies with experimental evidence to prove the data obtained from in silico are trustworthy and reliable. The overall literature evidence of in vitro and in vivo study results of tilianin reported against CVDs are well correlated with the present in silico docking results, which proves the predictability of the in silico model is reliable and can be used in the early stage of drug discovery and development of small molecules for many disorders including CVDs.
Table 2.
Docked study results of tilianin and standard drugs with the CVD target proteins.
| S. No. | Protein Data Bank ID | Name of the Protein | Ligand | MolDock Score | Rerank Score | HBond |
|---|---|---|---|---|---|---|
| 1. | 5KLB | CavAb voltage-gated calcium channel | Tilianin | −106.31 | −96.8228 | −14.7418 |
| Amlodipine | −129.418 | −40.768 | −1.49788 | |||
| Verapamil | −86.916 | −42.9639 | 0 | |||
| 2. | 6JOD | Angiotensin II type 2 receptor | Tilianin | −97.5636 | −87.5858 | −8.44394 |
| Azilsartan | −124.389 | −77.9133 | −9.92896 | |||
| Losartan | −116.448 | −51.6799 | −8.43829 | |||
| 3. | 7BU6 | Human β1 adrenergic receptor | Tilianin | −115.036 | −95.7438 | −5.83387 |
| Nebivolol | −100.61 | −80.3808 | −5.56831 | |||
| Atenolol | −84.7246 | −65.7884 | −6.11871 |
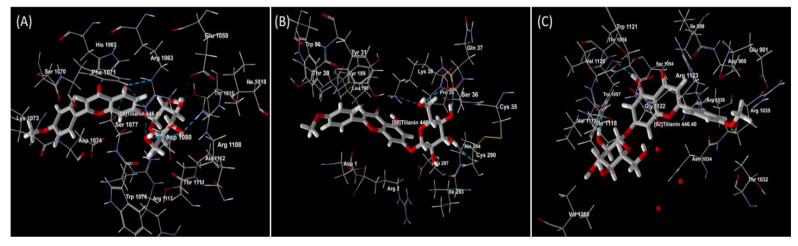
Figure 7.
Docked view of Tilianin on (A) PDB ID: 5KLB (CavAb voltage-gated calcium channel), (B) PDB ID: 6JOD (Angiotensin II type 2 receptor) and (C) PDB ID: 7BU6 (Human beta-1 adrenergic receptor).
7. Toxicity Profile of Tilianin
The experimental mice given tilianin at doses up to 1000 mg/kg showed no evidence of toxicity, according to Hernández-Abreu et al. [10]. Experimental animals given doses more than 1000 mg/kg, on the other hand, displayed lethargy for the first 5 h following administration, but then returned to normal. This data indicates that tilianin is not toxic to experimental animals and is completely safe. Furthermore, they found that tilianin has a wide range of pharmacology-toxicology reactions as an effective antihypertensive that is dose-dependent with a low ED50 (53.51 mg/kg) compared to the LD50 (6624 mg/kg). Using a toxicity estimation software tool with QSAR methodologies consensus method, hierarchical clustering method, and nearest neighbour method, the oral rat LD50 mg/kg (predicted value) of tilianin was found to be 1060.12 mg/kg, 755.40 mg/kg, and 2622.95 mg/kg, respectively [43]. All of these findings support that tilianin is toxic free upto the mentioned dose level and should be explored for the development of a drug molecule to treat CVDs. Jiang et al. [16] found that tilianin minimized the impact of OGD/R-induced damage in H9c2 cells in another investigation. Furthermore, there were no differences in cell viability between control and tilianin-treated cells, indicating that the conditions were toxicity-free.
8. Pharmacokinetics and Bioavailability of Tilianin
In a study by Yuan et al. [44], tilianin’s pharmacokinetics shows a two compartment model in rats following oral administration of microemulsion. The half-life (t1/2) of tilianin reduced dramatically with increasing dosages (25–50 mg/kg), although the area under the curve [AUC (0–t)], maximum concentration (Cmax) and rate constant (k) were higher than those reported at high doses. The absolute bioavailability of tilianin following oral administration of its microemulsion at 25 and 50 mg/kg, was 3.4% and 3.2%, respectively, while the relative bioavailabilities were 147.2% and 168.2%, respectively. The use of microemulsion can markedly increase the bioavailability of tilianin [44]. Acacetin-7-glucuronide and sulfate are the primary metabolites of tilianin detected in mouse plasma where the latter is found in higher concentrations in Bcrp1 type FVB mice (−/−) when compared to wild-type FVB mice. In the near future, more pharmacokinetics and bioavailability studies on tilianin are needed to obtain a greater understanding of its absorption, distribution, metabolism, and excretion (ADME) abilities. Furthermore, improved bioavailability of tilianin is likely to propel this promising natural molecule to the forefront of therapeutic medicines for the treatment of human disease, particularly CVDs.
9. Challenges and Opportunities of Tilianin to Be Developed as a Drug Molecule for the Treatment CVDs
Plant-based drug discovery initiatives continue to be key sources of new drug indicators, despite a number of hurdles such as obtaining and verifying plant materials, implementing high-operational evaluation bioassays and the yield of bioactive lead molecules. Beyond scientific discoveries and innovations, the barrier to developing new cardiovascular medicines is tremendous. Despite the fact that CVD is becoming more prevalent in developing countries, there is still time to prevent the pandemic from reaching its maximum potential. In this context, there are challenges and opportunities for tilianin to become a drug molecule for CVD treatment. Tilianin looks to be a promising drug-like molecule with the potential to be a good therapeutic agent for a variety of disorders, including CVDs, according to data acquired from the DruLiTo software [45] (Table 3). One of the challenges is the commercial availability of tilianin. As stated by Wang et al. [46] some tilianin flavonoids namely acacetin-7-glucuronide and sulfate are extremely limited. Furthermore, although tilianin has a protective mechanism on atherosclerosis [29], it remains unclear in some studies. Nevertheless, to date, there is no definitive study that stated the fact that it can be used as an anti-atherosclerotic agent and more studies are recommended [30] to point its role in this direction. In addition, there is yet insufficient direct confirmation for tilianin’s potential to reverse endothelial cell dysfunction, especially in view of the fact that the pathology and risk factors that lead to the onset of CVDs are broad. Based on the literature reviewed, tilianin is confirmed to have a significant cardioprotective mechanism [17,21,22,23,24,25] and anti-hypertension [34]. Nevertheless, although tilianin has various therapeutic potentials against CVDs, the majority of studies remain in the preclinical stage, where the availability of randomised controlled trials can help confirm the findings.
Table 3.
Physicochemical and drug-likeness properties of tilianin.
| Property/Rule | Result |
|---|---|
| Molecular formula | C22H22O10 |
| Molecular weight | 446.12 |
| Hydrogen bond donors | 5 |
| Hydrogen bond acceptors | 10 |
| Rotatable bonds | 5 |
| Log P (Partition coefficient, Predicted value) | 0.153 |
| Molar refractivity | 117.89 cm3 |
| Topological polar surface area | 155.14 Å2 |
| Lipinski’s Rule of Five | Passed |
| Unweighted QED | Passed |
| Weighted QED | Passed |
QED, Quantitative estimate of drug-likeness.
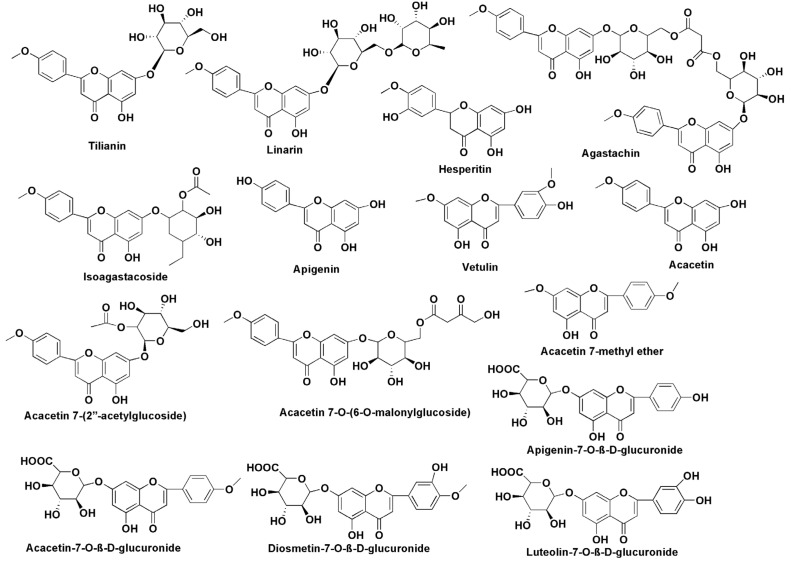
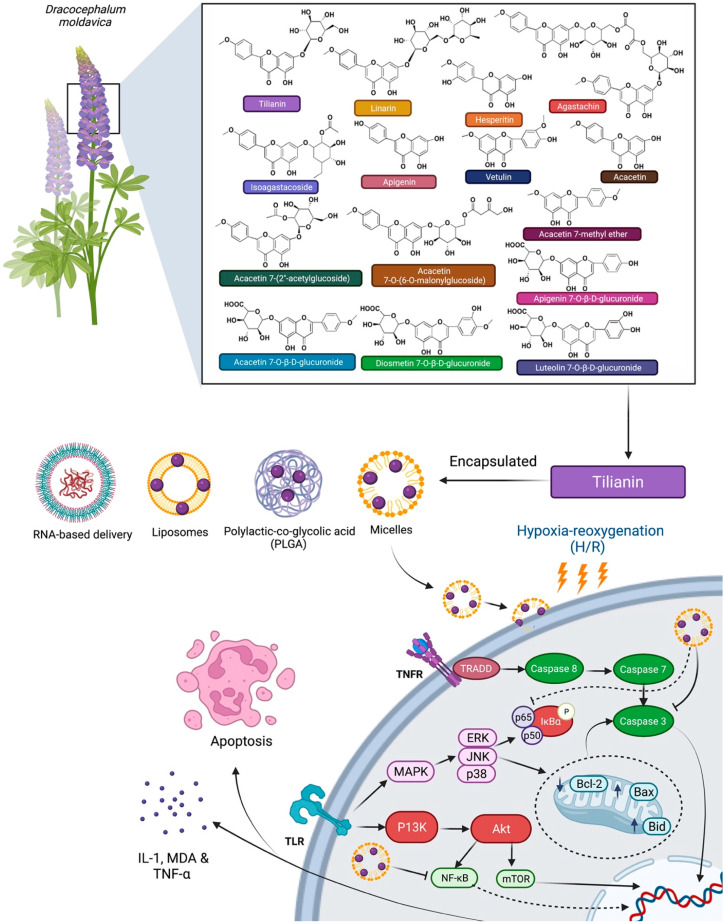
In addition, structural changes within tilianin can improve its physicochemical, bioavailability, and pharmacokinetic properties. Molecules related to tilianin have changes in the 7th position of the flavonoid ring where the glycoside ring is attached. Major non-volatile metabolites related to tilianin are hesperidin, apigenin, acacetin, linarin, isoagastachoside, agastachin, acacetin 7-(2″-acetylglucoside), acacetin 7-O-(6-O-malonylglucoside), acacetin 7-methyl ether, vetulin, luteolin-7-O-β-D-glucuronide, apigenin-7-O-β-D-glucuronide, diosmetin-7-O-β-D-glucuronide and acacetin-7-O-β-D-glucuronide (Figure 8) [47,48,49,50]. Hesperidin is one of the tilianin-related molecules that has been extensively researched against CVDs [51]. Apart from that, apigenin and acacetin have been associated with reduction in CVD risk [30,52]. In an attempt to increase its bioavailability, tilianin has been encapsulated in a hydrophobic shell to produce micro micelles [46]. Tilianin loaded micelles (70 nm) are effective hydrogen peroxide scavengers that also inhibit caspase-3 activity, thereby protecting cells from H/R-induced cytotoxicity. In a hypoxia-reoxygenation (H/R) model, tilianin loaded micelles lowered MDA, IL-1 and TNF-α, inhibited apoptosis, TLR4 and NF-κB p65 protein expression. By improving drug targeting, pharmacokinetics, efficacy and cellular uptake, nanomedicine has the ability to bridge the gap between pharmaceutical restrictions and natural phytochemical therapeutic potentials [53,54,55,56,57,58]. Nanoparticles in drug delivery such as polylactic-co-glycolic acid (PLGA), polyethylene glycol (PEG), liposomal delivery methods, and RNA-based delivery have various advantages, including: (1) enhanced adsorption; (2) selective targeting; (3) simple encapsulation (4) an increase in bioavailability; (5) decrease in adverse effects and (6) enhanced stability for CVDs [59,60,61]. These new delivery systems provide a number of approaches for achieving tissue selectivity and reducing system exposure, which are both necessary for deploying new pharmacological compounds derived from tilianin for improved CVD treatment (Figure 9).
Figure 8.
Chemical structure of tilianin derived/related compounds.
Figure 9.
Possible drug delivery methods of tilianin enclosed within nanocarriers, i.e., micelles, to increase its anti-apoptotic effect. Abbreviations: TLR, Toll-like receptor; TNFR, Tumor necrosis factor receptor; MAPK, Mitogen-activated protein kinase; P13K, Phosphatidylinositol-3-Kinase; ERK, Extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinases; IκBα, Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; Akt, Ak strain transforming; NF-kB, Nuclear factor kappa-light-chain-enhancer of activated B cells; mTOR, Mammalian target of rapamycin; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 Associated X-protein; Bid, BH3 interacting-domain death agonist; IL-1, Interleukin-1; TNF-α, Tumour necrosis factor alpha; MDA, Melanoma differentiation associated.
10. Conclusions and Future Perspectives
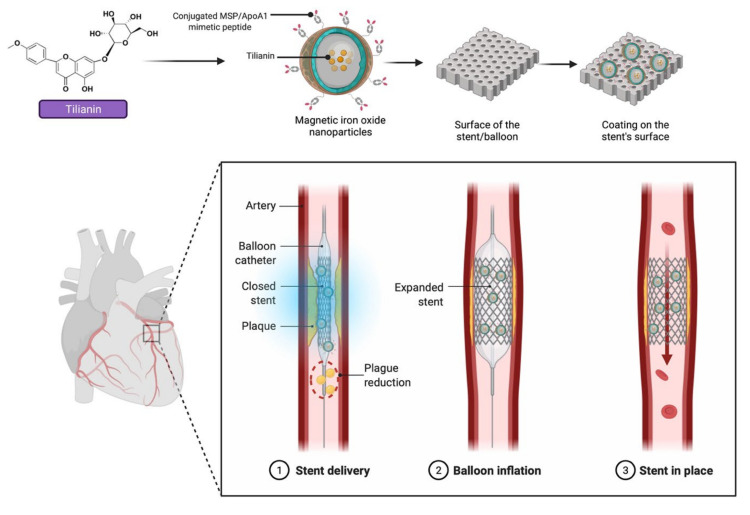
Nature has provided many drug molecules that are currently on the market. Furthermore, natural products from terrestrial sources (plants and fungus), microorganisms, and marine species, have been employed to provide a lead molecule for a new drug design, development, and therapy. Overall, the current review reported that tilianin is a potential natural lead molecule against CVDs including coronary heart disease, angina pectoris, hypertension and myocardial ischemia. Tilianin’s protective effects are attributed to several mechanisms notably free radical scavenging, inflammation control, mitochondrial function regulation and related signalling pathways. To understand the role of tilianin in the treatment of peripheral artery disease, cardiomyopathies, coronary artery disease, ischemic stroke, dyslipidemias, aortic aneurysm, diabetic cardiovascular complications, atherosclerosis, cardiac hypertrophy, and heart failure, future research should focus on the exploration and integration of innovative, organically generated drug prospects in the health and therapeutic sectors. Altogether, the findings of this review indicate that tilianin appears to be a promising molecule for new drug development in the prevention and treatment of CVDs. However, further preclinical research on tilianin and its derivatives is needed to validate its potential benefit and mechanism in the prevention of CVDs. Pharmacokinetic and bioavailability studies are also required before this molecule may be used in drug development. In terms of drug delivery, magnetic nanoparticles have recently sparked interest, particularly for those utilizing drug-eluting stent (DES) technologies, as they potentially can cause a paradigm shift with major therapeutic improvements. Earlier research has indicated that these strategies minimise the risk of reobstruction after stenting, a condition known as in-stent restenosis. Nevertheless, current DESs have some limitations such as the reduced bioavailability and the inability to alter the drug’s dosage and pharmacokinetics, especially imposed by the variability in the disease condition of the treated artery. A proposed strategy for overcoming the addressed limitations is to use magnetic iron oxide nanoparticles conjugated with MSP/ApoA1, a synthetic gene derived from a component of high-density lipoprotein (HDL), which can aid in preventing inflammation, oxidative stress and cholesterol efflux to reduce arterial fat formation that can lead to blockage. As discussed in the review, incorporation of tilianin may assist in minimising the growth of ox-LDL-induced foam cells and prevent further acceleration of the signalling cascade involved in atherosclerosis formation. The nanoparticles chosen can increase the utility of tilianin’s entrapment and distribution. Due to their physical features, they may be deposited onto the surface of a stent/balloon and dissociated for release for optimum localised drug administration to the afflicted area (Figure 10). We anticipate that the strategy can be a novel approach to drug delivery technology. Altogether, we believe that tilianin is a potential natural lead molecule for a new drug design, development, and therapy for CVDs, based on the scientific evidence presented in this review.
Figure 10.
Tilianin-loaded magnetic iron oxide nanoparticles augmented with surface decorated MSP/ApoA1, deposited onto the surface of the stent/balloon for in situ targeting approach for effective plague reduction activities in the future.
Acknowledgments
We would like to thank the Deanship of Scientific Research (RGP: 1/275/1442), King Khalid University, Abha, Saudi Arabia. All the authors of this manuscript are thankful to their respective Departments/Universities for the successful completion of this study. The figures in this manuscript have been created with the support of https://biorender.com (accessed on 3 November 2021) under a paid subscription (Ref: C08A1A0B-0002).
Author Contributions
Writing—original draft: F.S.K., M.S., S.F. and N.K.F.; Conceptualization: F.S.K. and M.S.; Supervision: M.S., S.F. and N.K.F.; Resources: F.S.K., M.S., S.F., S.H.G., N.N.I.M.R., S.R., K.C., M.Y.B., A.K.A., S.J., A.D., L.T., P.T.L., V.S., Y.S.W., K.V.S. and N.K.F.; Data curation: F.S.K., M.S., S.F., S.H.G., N.N.I.M.R., S.R., K.C., M.Y.B., A.K.A., S.J., A.D., L.T., P.T.L., V.S., Y.S.W., K.V.S. and N.K.F.; Writing—review and editing: F.S.K., M.S., S.F., S.H.G., N.N.I.M.R., S.R., K.C., M.Y.B., A.K.A., S.J., A.D., L.T., P.T.L., V.S., Y.S.W., K.V.S. and N.K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Deanship of Scientific Research (RGP: 1/275/1442), King Khalid University, Abha 62529, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Cardiovascular Diseases. [(accessed on 24 September 2021)]. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1.
- 2.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy D.J., Yellon D.M. Cardioprotection (Oxford Cardiology Library) Oxford University Press; Oxford, UK: 2009. [Google Scholar]
- 4.Sajid M., Cameotra S.S., Khan M.S.A., Ahmad I. New Look to Phytomedicine. Elsevier; Amsterdam, The Netherlands: 2019. Nanoparticle-based delivery of phytomedicines: Challenges and opportunities; pp. 597–623. [Google Scholar]
- 5.Casertano M., Menna M., Imperatore C. The ascidian-derived metabolites with antimicrobial properties. Antibiotics. 2020;9:510. doi: 10.3390/antibiotics9080510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosén J., Gottfries J., Muresan S., Backlund A., Oprea T.I. Novel chemical space exploration via natural products. J. Med. Chem. 2009;52:1953–1962. doi: 10.1021/jm801514w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooi B.K., Chan K.-G., Goh B.H., Yap W.H. The role of natural products in targeting cardiovascular diseases via Nrf2 pathway: Novel molecular mechanisms and therapeutic approaches. Front. Pharmacol. 2018;9:1308. doi: 10.3389/fphar.2018.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ntie-Kang F., Svozil D. An enumeration of natural products from microbial, marine and terrestrial sources. Phys. Sci. Rev. 2020;5:20180121. doi: 10.1515/psr-2018-0121. [DOI] [Google Scholar]
- 10.Hernández-Abreu O., Torres-Piedra M., García-Jiménez S., Ibarra-Barajas M., Villalobos-Molina R., Montes S., Rembao D., Estrada-Soto S. Dose-dependent antihypertensive determination and toxicological studies of tilianin isolated from Agastache mexicana. J. Ethnopharmacol. 2013;146:187–191. doi: 10.1016/j.jep.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Wei J., Cao P., Wang J., Kang W. Analysis of tilianin and acacetin in Agastache rugosa by high-performance liquid chromatography with ionic liquids-ultrasound based extraction. Chem. Cent. J. 2016;10:76. doi: 10.1186/s13065-016-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Díaz J.A., Navarrete-Vázquez G., García-Jiménez S., Hidalgo-Figueroa S., Almanza-Pérez J.C., Alarcón-Aguilar F.J., Gómez-Zamudio J., Cruz M., Ibarra-Barajas M., Estrada-Soto S. Antidiabetic, antihyperlipidemic and anti-inflammatory effects of tilianin in streptozotocin-nicotinamide diabetic rats. Biomed. Pharmacother. 2016;83:667–675. doi: 10.1016/j.biopha.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Nam K.-H., Choi J.-H., Seo Y.-J., Lee Y.-M., Won Y.-S., Lee M.-R., Lee M.-N., Park J.-G., Kim Y.-M., Kim H.-C. Inhibitory effects of tilianin on the expression of inducible nitric oxide synthase in low density lipoprotein receptor deficiency mice. Exp. Mol. Med. 2006;38:445–452. doi: 10.1038/emm.2006.52. [DOI] [PubMed] [Google Scholar]
- 14.Oh H.M., Kang Y.J., Lee Y.S., Park M.K., Kim S.H., Kim H.J., Seo H.G., Lee J.H., Chang K.C. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264. 7 cells from hydrogen peroxide-induced injury. J. Ethnopharmacol. 2006;103:229–235. doi: 10.1016/j.jep.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Trujano M.E., Ponce-Muñoz H., Hidalgo-Figueroa S., Navarrete-Vázquez G., Estrada-Soto S. Depressant effects of Agastache mexicana methanol extract and one of major metabolites tilianin. Asian Pac. J. Trop. Med. 2015;8:185–190. doi: 10.1016/S1995-7645(14)60312-6. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H., Fang J., Xing J., Wang L., Wang Q., Wang Y., Li Z., Liu R. Tilianin mediates neuroprotection against ischemic injury by attenuating CaMKII-dependent mitochondrion-mediated apoptosis and MAPK/NF-κB signaling. Life Sci. 2019;216:233–245. doi: 10.1016/j.lfs.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Zeng C., Jiang W., Zheng R., He C., Li J., Xing J. Cardioprotection of tilianin ameliorates myocardial ischemia-reperfusion injury: Role of the apoptotic signaling pathway. PLoS ONE. 2018;13:e0193845. doi: 10.1371/journal.pone.0193845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gálvez J., Estrada-Reyes R., Benítez-King G., Araujo G., Orozco S., Fernández-Mas R., Almazán S., Calixto E. Involvement of the GABAergic system in the neuroprotective and sedative effects of acacetin 7-O-glucoside in rodents. Restor. Neurol. Neurosci. 2015;33:683–700. doi: 10.3233/RNN-140486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greiser K.H., Kluttig A., Schumann B., Kors J.A., Swenne C.A., Kuss O., Werdan K., Haerting J. Cardiovascular disease, risk factors and heart rate variability in the elderly general population: Design and objectives of the CARdiovascular disease, Living and Ageing in Halle (CARLA) Study. BMC Cardiovasc. Disord. 2005;5:33. doi: 10.1186/1471-2261-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pashkow F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflamm. 2011;2011:514623. doi: 10.4061/2011/514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H., Zeng L., Dong X., Guo S., Xing J., Li Z., Liu R. Tilianin extracted from Dracocephalum moldavica L. induces intrinsic apoptosis and drives inflammatory microenvironment response on pharyngeal squamous carcinoma cells via regulating TLR4 signaling pathways. Front. Pharmacol. 2020;11:205. doi: 10.3389/fphar.2020.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian L., Cao W., Yue R., Yuan Y., Guo X., Qin D., Xing J., Wang X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019;139:352–360. doi: 10.1016/j.jphs.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Yuan Y., Wang X., Wang Y., Cheng J., Tian L., Guo X., Qin D., Cao W. Tilianin post-conditioning attenuates myocardial ischemia/reperfusion injury via mitochondrial protection and inhibition of apoptosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:4490. doi: 10.12659/MSM.903259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X., Cao W., Yao J., Yuan Y., Hong Y., Wang X., Xing J. Cardioprotective effects of tilianin in rat myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2015;11:2227–2233. doi: 10.3892/mmr.2014.2954. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Y., Cao W., Hong Y., Guo X., Wang Y., Wang Y., Wang X., Hu P. Tilianin pretreatment prevents myocardial ischemia-reperfusion injury via preservation of mitochondrial function in rat heart. Phytomedicine. 2017;34:106–114. doi: 10.1016/j.phymed.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Yao J., Li Y., Jin Y., Chen Y., Tian L., He W. Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-κB/NLRP3 and PGC1a/SIRT3 pathways. Int. Immunopharmacol. 2021;96:107728. doi: 10.1016/j.intimp.2021.107728. [DOI] [PubMed] [Google Scholar]
- 27.Castellon X., Bogdanova V. Chronic inflammatory diseases and endothelial dysfunction. Aging Dis. 2016;7:81. doi: 10.14336/AD.2015.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam K.-w., Kim J., Hong J.-J., Choi J.-H., Mar W., Cho M.-H., Kim Y.-M., Oh S.-R., Lee H.-k., Nam K.-H. Inhibition of cytokine-induced IκB kinase activation as a mechanism contributing to the anti-atherogenic activity of tilianin in hyperlipidemic mice. Atherosclerosis. 2005;180:27–35. doi: 10.1016/j.atherosclerosis.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Shen W., Anwaier G., Cao Y., Lian G., Chen C., Liu S., Tuerdi N., Qi R. Atheroprotective mechanisms of tilianin by inhibiting inflammation through down-regulating NF-κB pathway and foam cells formation. Front. Physiol. 2019;10:825. doi: 10.3389/fphys.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao W., Hu N., Yuan Y., Cheng J., Guo X., Wang Y., Wang X., Hu P. Effects of tilianin on proliferation, migration and TGF-β/smad signaling in rat vascular smooth muscle cells induced with angiotensin II. Phytother. Res. 2017;31:1240–1248. doi: 10.1002/ptr.5846. [DOI] [PubMed] [Google Scholar]
- 31.Israili Z.H., Hernández-Hernández R., Valasco M. The future of antihypertensive treatment. Am. J. Ther. 2007;14:121–134. doi: 10.1097/01.pap.0000249915.12185.58. [DOI] [PubMed] [Google Scholar]
- 32.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr., Jones D.W., Materson B.J., Oparil S., Wright J.T., Jr. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 33.Finlay B.B., Falkow S. Free-form 3-D surface description in multiple scales. Microbiol. Mol. Biol. Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández-Abreu O., Castillo-España P., León-Rivera I., Ibarra-Barajas M., Villalobos-Molina R., González-Christen J., Vergara-Galicia J., Estrada-Soto S. Antihypertensive and vasorelaxant effects of tilianin isolated from Agastache mexicana are mediated by NO/cGMP pathway and potassium channel opening. Biochem. Pharmacol. 2009;78:54–61. doi: 10.1016/j.bcp.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Carmona-Castro G., Estrada-Soto S., Arellano-García J., Arias-Duran L., Valencia-Díaz S., Perea-Arango I. High accumulation of tilianin in in-vitro cultures of Agastache mexicana and its potential vasorelaxant action. Mol. Biol. Rep. 2019;46:1107–1115. doi: 10.1007/s11033-018-4570-4. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Abreu O., Durán-Gómez L., Best-Brown R., Villalobos-Molina R., Rivera-Leyva J., Estrada-Soto S. Validated liquid chromatographic method and analysis of content of tilianin on several extracts obtained from Agastache mexicana and its correlation with vasorelaxant effect. J. Ethnopharmacol. 2011;138:487–491. doi: 10.1016/j.jep.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 37.Tang L., El-Din T.M.G., Swanson T.M., Pryde D.C., Scheuer T., Zheng N., Catterall W.A. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature. 2016;537:117–121. doi: 10.1038/nature19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deedwania P. The Role of Angiotensin Receptor Blockers in Cardiovascular Protection Beyond Blood Pressure Control. US Cardiol. 2006;3:37–39. doi: 10.15420/usc.2006.3.2.37. [DOI] [Google Scholar]
- 39.Asada H., Inoue A., Kadji F.M.N., Hirata K., Shiimura Y., Im D., Shimamura T., Nomura N., Iwanari H., Hamakubo T. The crystal structure of angiotensin II type 2 receptor with endogenous peptide hormone. Structure. 2020;28:418–425.e4. doi: 10.1016/j.str.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Hahn V.S., Zhang K.W., Sun L., Narayan V., Lenihan D.J., Ky B. Heart failure with targeted cancer therapies: Mechanisms and cardioprotection. Circ. Res. 2021;128:1576–1593. doi: 10.1161/CIRCRESAHA.121.318223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiseman D.N., Samra N., Román Lara M.M., Penrice S.C., Goddard A.D. The Novel Application of Geometric Morphometrics with Principal Component Analysis to Existing G Protein-Coupled Receptor (GPCR) Structures. Pharmaceuticals. 2021;14:953. doi: 10.3390/ph14100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitencourt-Ferreira G., de Azevedo W.F. Docking Screens for Drug Discovery. Springer; Berlin/Heidelberg, Germany: 2019. Molegro virtual docker for docking; pp. 149–167. [DOI] [PubMed] [Google Scholar]
- 43.EPA . User’s Guide for T.E.S.T. (version 5.1) (Toxicity Estimation Software Tool): A Program to Estimate Toxicity from Molecular Structure. EPA; Cincinnati, OH, USA: 2020. [Google Scholar]
- 44.Yuan Y., Wang X., Chen W., Hong Y., Chen Q. Pharmacokinetics and bioavailability of tilianin microemulsion in rats. Chin. J. Hosp. Pharm. 2015;35:1815–1819. [Google Scholar]
- 45.NIPER Drug Likeness Tool (DruLiTo) [(accessed on 24 September 2021)]; Available online: http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_Reference.html.
- 46.Wang Y., Wang Y., Wang X., Hu P. Tilianin-loaded reactive oxygen species-scavenging nano-micelles protect H9c2 cardiomyocyte against hypoxia/reoxygenation-induced injury. J. Cardiovasc. Pharmacol. 2018;72:32–39. doi: 10.1097/FJC.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 47.Zielińska S., Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae) Phytochem. Rev. 2014;13:391–416. doi: 10.1007/s11101-014-9349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H.W., Ryu H.W., Baek S.C., Kang M.-G., Park D., Han H.-Y., An J.H., Oh S.-R., Kim H. Potent inhibitions of monoamine oxidase A and B by acacetin and its 7-O-(6-O-malonylglucoside) derivative from Agastache rugosa. Int. J. Biol. Macromol. 2017;104:547–553. doi: 10.1016/j.ijbiomac.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 49.Chaurasiya N.D., Zhao J., Pandey P., Doerksen R.J., Muhammad I., Tekwani B.L. Selective inhibition of human monoamine oxidase B by acacetin 7-methyl ether isolated from turnera diffusa (Damiana) Molecules. 2019;24:810. doi: 10.3390/molecules24040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu N., He C., Awuti G., Zeng C., Xing J., Huang W. Simultaneous determination of six active compounds in Yixin Badiranjibuya granules, a traditional Chinese medicine, by RP-HPLC-UV method. J. Anal. Methods Chem. 2015;2015:974039. doi: 10.1155/2015/974039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mas-Capdevila A., Teichenne J., Domenech-Coca C., Caimari A., Del Bas J.M., Escoté X., Crescenti A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients. 2020;12:1488. doi: 10.3390/nu12051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang W., Wu Q.-Q., Xiao Y., Jiang X.-H., Yuan Y., Zeng X.-F., Tang Q.-Z. Acacetin protects against cardiac remodeling after myocardial infarction by mediating MAPK and PI3K/Akt signal pathway. J. Pharmacol. Sci. 2017;135:156–163. doi: 10.1016/j.jphs.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Bonferoni M.C., Rossi S., Sandri G., Ferrari F. Nanoparticle formulations to enhance tumor targeting of poorly soluble polyphenols with potential anticancer properties. Semin. Cancer Biol. 2017;46:205–214. doi: 10.1016/j.semcancer.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Lagoa R., Silva J., Rodrigues J.R., Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020;38:107382. doi: 10.1016/j.biotechadv.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Rahimi H.R., Nedaeinia R., Shamloo A.S., Nikdoust S., Oskuee R.K. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomedicine. 2016;6:383. [PMC free article] [PubMed] [Google Scholar]
- 56.Siddiqui I.A., Sanna V. Impact of nanotechnology on the delivery of natural products for cancer prevention and therapy. Mol. Nutr. Food Res. 2016;60:1330–1341. doi: 10.1002/mnfr.201600035. [DOI] [PubMed] [Google Scholar]
- 57.Wang S., Su R., Nie S., Sun M., Zhang J., Wu D., Moustaid-Moussa N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014;25:363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pistollato F., Bremer-Hoffmann S., Basso G., Cano S.S., Elio I., Vergara M.M., Giampieri F., Battino M. Targeting glioblastoma with the use of phytocompounds and nanoparticles. Target. Oncol. 2016;11:1–16. doi: 10.1007/s11523-015-0378-5. [DOI] [PubMed] [Google Scholar]
- 59.Zuraini N.Z.A., Sekar M., Wu Y.S., Gan S.H., Bonam S.R., Rani N.N.I.M., Begum M.Y., Lum P.T., Subramaniyan V., Fuloria N.K. Promising nutritional fruits against cardiovascular diseases: An overview of experimental evidence and understanding their mechanisms of action. Vasc. Health Risk Manag. 2021;17:739–769. doi: 10.2147/VHRM.S328096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yap K.M., Sekar M., Fuloria S., Wu Y.S., Gan S.H., Rani N.N.I.M., Subramaniyan V., Kokare C., Lum P.T., Begum M.Y. Drug delivery of natural products through nanocarriers for effective breast cancer therapy: A comprehensive review of literature. Int. J. Nanomed. 2021;16:7891–7941. doi: 10.2147/IJN.S328135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davatgaran-Taghipour Y., Masoomzadeh S., Farzaei M.H., Bahramsoltani R., Karimi-Soureh Z., Rahimi R., Abdollahi M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017;12:2689. doi: 10.2147/IJN.S131973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.