Abstract
Background
Patients hospitalized with COVID-19 often exhibit markers of a hypercoagulable state and have an increased incidence of VTE. In response, CHEST issued rapid clinical guidance regarding prevention of VTE. Over the past 18 months the quality of the evidence has improved. We thus sought to incorporate this evidence and update our recommendations as necessary.
Study Design and Methods
This update focuses on the optimal approach to thromboprophylaxis in hospitalized patients. The original questions were used to guide the search, using MEDLINE via PubMed. Eight randomized controlled trials and one observational study were included. Meta-analysis, using a random effects model, was performed. The panel created summaries using the GRADE Evidence-to-Decision framework. Updated guidance statements were drafted, and a modified Delphi approach was used to obtain consensus.
Results
We provide separate guidance statements for VTE prevention for hospitalized patients with acute (moderate) illness and critically ill patients in the ICU. However, we divided each original question and resulting recommendation into two questions: standard prophylaxis vs therapeutic (or escalated dose) prophylaxis and standard prophylaxis vs intermediate dose prophylaxis. This led to a change in one recommendation, and an upgrading of three additional recommendations based upon higher quality evidence.
Conclusions
Advances in care for patients with COVID-19 have improved overall outcomes. Despite this, rates of VTE in these patients remain elevated. Critically ill patients should receive standard thromboprophylaxis for VTE, and moderately ill patients with a low bleeding risk might benefit from therapeutic heparin. We see no role for intermediate dose thromboprophylaxis in either setting.
Key Words: COVID-19, DIC, DVT, hypercoagulability, pulmonary embolism, VTE
Abbreviations: LMWH, low-molecular-weight heparin; OSFD, organ support-free day; PE, pulmonary embolism; RR, risk ratio; UFH, unfractionated heparin
Summary of Recommendations
1. In hospitalized patients with acute illness with COVID-19 who have low risk of bleeding, with consideration for the remarks below, we suggest therapeutic dose heparin (UFH or LMWH) over current standard dose anticoagulant thromboprophylaxis (Conditional Recommendation, Ungraded Consensus-Based Statement).
Remarks: Providers should carefully weigh the risks of thrombosis and bleeding in making this decision. Patients with a significantly elevated D-dimer level (studies have previously defined this as 2-4× the upper limit of normal), those with prior VTE, or those with other comorbidities known to be associated with VTE may be at increased risk of thrombosis. Patients with high risk of bleeding include, but are not limited to, those with known bleeding within the last 30 days requiring ED presentation or hospitalization, known history of an inherited or acquired bleeding disorder, active dual antiplatelet therapy, recent ischemic stroke, intracranial malignancy, history of bleeding diatheses (eg, hemophilia), history of GI bleeding within previous 3 months, thrombolysis within the previous 7 days, presence of an epidural or spinal catheter, recent major surgery < 14 days, or uncontrolled hypertension (systolic BP > 200 mm Hg, diastolic BP > 120 mm Hg).
2. In hospitalized patients with acute illness with COVID-19 who are not receiving therapeutic dose heparin (UFH or LMWH), we recommend current standard dose anticoagulant thromboprophylaxis over intermediate dose anticoagulation (defined as LMWH bid or increased weight-based dosing that is less than recommended therapeutic doses) (Strong Recommendation, Ungraded Consensus-Based Statement).
3. In critically ill patients with COVID-19, we suggest current standard dose anticoagulant thromboprophylaxis (with UFH or LMWH) over therapeutic dose anticoagulation (Conditional Recommendation, Ungraded Consensus-Based Statement).
4. In critically ill patients with COVID-19, we suggest current standard dose anticoagulant thromboprophylaxis over intermediate dose anticoagulation (defined as LMWH bid or increased weight-based dosing that is less than recommended therapeutic doses) (Conditional Recommendation, Ungraded Consensus-Based Statement).
Background
Within the first few months of the COVID-19 global pandemic, it was recognized that patients hospitalized with SARS-CoV-2 often exhibited markers of a hypercoagulable state and had an increased incidence of VTE. Reports documented significantly elevated D-dimer levels that were associated with increased morbidity and mortality.1 , 2 This led many professional societies, including CHEST,3 to develop rapid guidance documents regarding the optimal strategy for prophylaxis of VTE in these patients.4, 5, 6, 7, 8, 9, 10 Initially, evidence was extremely limited and consisted of fewer than 30 retrospective cohort studies of varying size that reported on patients from varied geographic regions and clinical settings. There was no universal approach to screening or diagnosis. Perhaps most importantly, the thromboprophylaxis regimens varied across studies and were sometimes not reported at all. Based upon the limited, low-quality evidence suggesting patients with COVID-19 pneumonia had a higher risk of thrombosis than similarly ill patients without COVID-19, societal guidelines were uniform in their recommendation that all hospitalized patients with COVID-19 pneumonia receive pharmacologic thromboprophylaxis in the absence of a contraindication. Recommendations regarding the optimal dosing, however, varied. Some, including CHEST, recommended standard dose prophylaxis for all patients, while a few suggested that intermediate or therapeutic dosing could be considered, especially in patients admitted to the ICU. Table 1 provides a summary of these early recommendations.
Table 1.
Early Societal Guidelines Regarding Thromboprophylaxis in COVID-19
| Guideline | Outpatient | In-Hospital Noncritically Ill | In-Hospital Critically Ill | Postdischarge |
|---|---|---|---|---|
| Global COVID-19 Thrombosis Collaborative Group6 (April 17, 2020) | In the absence of high-quality data, pharmacologic prophylaxis should be reserved for those patients at highest risk, including those with limited mobility and history of prior VTE or active malignancy | Prophylactic daily LMWH or subcutaneous UFH bid | Prophylactic daily LMWH or subcutaneous UFH bid | It is reasonable to employ individualized risk stratification for thrombotic and hemorrhagic risk, followed by consideration of extended prophylaxis (for up to 45 d) for patients with elevated risk of VTE who have low risk of bleeding |
| International Society of Thrombosis and Haemostasis10 (May 27, 2020) | NA | A universal strategy of routine thromboprophylaxis with standard dose UFH or LMWH should be used after careful assessment of bleed risk, with LMWH as the preferred agent. Intermediate dose LMWH may also be considered | Routine thromboprophylaxis with prophylactic dose UFH or LMWH should be used after careful assessment of bleed risk. Intermediate dose LMWH can also be considered in high-risk patients | Extended postdischarge thromboprophylaxis should be considered for all hospitalized patients with COVID-19 who meet high VTE risk criteria |
| Chest Guideline and Expert Panel Report3 (June 2, 2020) | NA | In hospitalized patients with acute illness with COVID-19, we recommend current standard dose anticoagulant thromboprophylaxis over intermediate or full treatment dosing, per existing guidelines | In critically ill patients with COVID-19, we suggest current standard dose anticoagulant thromboprophylaxis over intermediate or full treatment dosing, per existing guidelines | In patients with COVID-19, we recommend inpatient thromboprophylaxis only over inpatient plus extended thromboprophylaxis after hospital discharge |
| VAS-European Independent Foundation in Angiology/Vascular Medicine8 (September 13, 2020) | NA | Routine thromboprophylaxis with weight-adjusted intermediate doses of LMWH (unless contraindication) | Routine thromboprophylaxis with weight-adjusted intermediate doses of LMWH (unless contraindication) | Evaluation of the risk of VTE before hospital discharge using the IMPROVE-D-dimer score and prolonged postdischarge thromboprophylaxis with rivaroxaban, betrixaban, or LMWH |
| World Health Organization5 (January 25, 2021) | NA | In hospitalized patients with COVID-19, without an established indication for higher dose anticoagulation, we suggest administering standard thromboprophylaxis dosing of anticoagulation rather than therapeutic or intermediate dosing | In hospitalized patients with COVID-19, without an established indication for higher dose anticoagulation, we suggest administering standard thromboprophylaxis dosing of anticoagulation rather than therapeutic or intermediate dosing | NA |
| American Society of Hematology7 (February 8, 2021) | NA | Prophylactic-intensity over intermediate-intensity or therapeutic-intensity anticoagulation for patients with COVID-19-related acute illness who do not have suspected or confirmed VTE | Prophylactic-intensity over intermediate-intensity or therapeutic-intensity anticoagulation for patients with COVID-19-related critical illness who do not have suspected or confirmed VTE | NA |
| National Institutes of Health9 (February 11, 2021) | For nonhospitalized patients with COVID-19, anticoagulants and antiplatelet therapy should not be initiated for the prevention of VTE or arterial thrombosis unless the patient has other indications for the therapy or is participating in a clinical trial | Hospitalized nonpregnant adults with COVID-19 should receive prophylactic dose anticoagulation | Hospitalized nonpregnant adults with COVID-19 should receive prophylactic dose anticoagulation | Hospitalized patients with COVID-19 should not routinely be discharged from the hospital while on VTE prophylaxis. Continuing anticoagulation with a US Food and Drug Administration-approved regimen for extended VTE prophylaxis after hospital discharge can be considered for patients who are at low risk for bleeding and high risk for VTE |
| National Institute for Health and Care Excellence4 (March 6, 2021) | Consider pharmacologic prophylaxis if the risk of VTE outweighs the risk of bleeding | Consider a treatment dose of an LMWH, unless contraindicated, for young people and adults with COVID-19 who: (1) are likely to be in the hospital for the next 3 d; (2) need supplemental oxygen and who are not yet receiving high-flow oxygen, CPAP, noninvasive ventilation, or invasive mechanical ventilation | For young people and adults who are already receiving high-flow oxygen, CPAP, noninvasive ventilation, or invasive mechanical ventilation and are on a standard prophylactic dose of an LMWH for VTE prophylaxis: (1) consider increasing anticoagulation to an intermediate dose; (2) reassess VTE and bleeding risks daily | Treatment should be for a minimum of 14 d or until discharge |
LMWH = low-molecular-weight heparin; NA = not available; UFH = unfractionated heparin; VAS = Angiology Vascular Medicine.
Since then, our understanding of the underlying pathophysiologic mechanisms of this prothrombotic state has advanced. While it is beyond the scope of this manuscript to describe these mechanisms in detail, it should be noted that there are two distinct but related processes: a hypercoagulable state that leads to large vessel macrothrombosis and a primary endotheliopathy that results in extensive in situ, immunothrombosis. A more detailed review of these mechanisms has recently been published.11 Over the past 15 months, we have also seen the emergence of randomized controlled trials focusing on the optimal dosing for thromboprophylaxis in both moderately ill hospitalized (non-ICU) and critically ill (ICU) patients. Here we provide updated guidance focused on prevention of thrombosis in hospitalized patients. We are not updating any prior guidance related to pre- or posthospital prophylaxis, diagnosis, or treatment.
Study Design and Methods
The panel followed standard CHEST process for the development of rapid guidance statements, as detailed in the first version of this guideline.3 Conflict of interest declarations were reviewed for all panelists by the Professional Standards Committee. No panelist required any management for the topic areas being updated. The panel was aware of recently published or studies expected to be published soon regarding optimal thromboprophylaxis and thus chose to limit this update to guidance statements in this topic area. The original Population, Intervention, Comparator, Outcome questions were used to guide the search, using MEDLINE via PubMed. Screening and full text selection were performed in duplicate by pairs of panel members. Additional studies were identified by panel members as they were published. Included studies12, 13, 14, 15, 16, 17, 18, 19, 20 are outlined in Table 2 .
Table 2.
Included Studies
| Study Characteristics | mpRCT (critically ill)13 | mpRCT (noncritically ill)12 | ACTION16 | RAPID19 | INSPIRATION18 | HEP-COVID20 | HESACOVID15 | Perepu et al17 |
|---|---|---|---|---|---|---|---|---|
| Design | Adaptive, multinational, open-label RCT | Adaptive, multinational, open-label RCT | Multicenter (Brazil), open-label RCT | Multinational, open-label RCT | Multicenter (Iran), open-label RCT with 2 × 2 factorial designa | Multicenter (US), open-label RCT | Single-center (Brazil), open-label RCT | Multicenter (US), open-label RCT |
| Intervention | Therapeutic dose heparin until discharge or day 14 | Therapeutic dose heparin until discharge or day 14 | Rivaroxaban 20 mg daily for 30 db | Therapeutic dose heparin until discharge or day 28 | Intermediate dose heparin for 30 d | Therapeutic dose heparin until discharge | Therapeutic dose heparin for ≥ 4 to 14 d | Intermediate dose enoxaparin until discharge |
| Comparator | Usual care pharmacologic thromboprophylaxis (up to intermediate dose heparin) until discharge | Usual care pharmacologic thromboprophylaxis (up to intermediate dose heparin) until discharge | BMI-adjusted prophylactic dose heparin until dischargec | BMI-adjusted prophylactic dose heparin until discharge or day 28 | Weight- and BMI-adjusted prophylactic dose heparin for 30 d | Usual care pharmacologic thromboprophylaxis (up to intermediate dose heparin) until discharge | Weight- adjusted prophylactic dose heparin | BMI-adjusted prophylactic dose enoxaparin until discharge |
| Primary outcome | Organ support-free days up to day 21 | Organ support-free days up to day 21 | Hierarchical composite of time to death, duration of hospitalization, or duration of supplemental oxygen use through 30 d | Composite of ICU admission, noninvasive or invasive mechanical ventilation, or death up to 28 d | Composite of acute VTE, arterial thrombosis, treatment with ECMO, or mortality within 30 d | Composite of VTE, ATE, or death from any cause within 30 ± 2 d | Change in Pao2/ Fio2 ratio from baseline to day 14 | Mortality at 30 d |
| Major bleeding criteria | ISTH | ISTH | ISTH | ISTH | BARC (type 3 or 5) | ISTH | TIMI | ISTH |
| Screening ultrasound for DVT | No | No | No | No | No | 10 +4 d or at discharge | No | No |
| Eligibility based on D-dimer | No | No | >ULN | ≥ 2× ULN or > ULN and oxygen saturation ≤ 93% | No | > 4× ULN | > 1,000 μg/L | No |
| No. of randomized participants | 1,207d | 2,244e | 615 | 465 | 598 | 257 | 20 | 176 |
| No. of participants included in primary analysis | 1,103 | 2,219 | 614 | 465 | 562 | 253 | 20 | 173 |
| Age (y) | 61 (mean) | 59 (mean) | 57 (mean) | 60 (mean) | 62 (median) | 67 (mean) | 57 (mean) | 64 (median) |
| Women, No./total No. (%) | 331/1,103 (30) | 921/2,231 (41) | 247/615 (40) | 201/465 (43) | 237/562 (42) | 117/253 (46) | 4/20 (20) | 76/173 (44) |
| BMI ≥ 30 kg/m2, No./total No. (%) | Median 30 kg/m2 | Median 30 kg/m2 | 264/615 (43) | 191/455 (42) | 123/535 (23) | Mean 31 kg/m2 | Mean 34 kg/m2 | 106/173 (61) |
| D-dimer ≥ 2× ULN, No./total No. (%) | 207/433 (48) | 630/1705 (37) | ≥ 3 ULN: 167/615 (27) | 227/465 (49) | 94/188 (50) | 253/253 (100) | NR | NR |
| Oxygen support at baseline | ||||||||
| None | 0 | 279/2,231 (13)f | 155/615 (25) | g | 0 | 9/253 (3.6) | 0 | NRh |
| Low-flow nasal cannula or mask, No./total No. (%) | 15/1,103 (1.4) | 1485/2,231 (67)f | 369/615 (60) | g | 256/562 (46) | 192/253 (76) | 0 | NRh |
| High-flow nasal cannula, No./total No. (%) | 358/1,103 (32) | 53/2231 (2.4) | 48/615 (7.8) | 27/465 (5.8) | 15/562 (2.7) | i | 0 | NRh |
| Noninvasive positive pressure ventilation, No./total No. (%) | 415/1,103 (38) | 45/2,231 (2.0) | 5/615 (0.1) | 0 | 178/562 (32) | i | 0 | NRh |
| Invasive ventilation, No./total No. (%) | 315/1,103 (29) | 0 | 38/615 (6.2) | 0 | 113/562 (20) | 13/253 (5.1) | 20/20 (100) | 40/173 (23) |
| Cotreatment at baseline | ||||||||
| Antiplatelet agent, No./total No. (%) | 75/979 (7.7)j | 259/2153 (12)k | 48/615 (7.8) | 53/465 (11) | 172/562 (31) | 64/253 (25) | 0 | NR |
| Glucocorticoids, No./total No. (%) | 884/1,077 (82) | 894/1,447 (62) | 510/615 (83) | 323/465(69) | 524/562 (93) | 204/250 (82) | 14/20 (70) | 130/173 (75)l |
| Remdesivir, No./total No. (%) | 346/1,096 (32) | 811/2226 (36) | NR | 0 | 338/562 (60) | 178/253 (70) | 0 | 105/173 (61)l |
| Tocilizumab, No./total No. (%) | 20/1,096 (32) | 13/2,148 (0.6) | NR | 0 | 74/562 (13) | NR | 0 | NR |
ATE = arterial thromboembolism; BARC = Bleeding Academic Research Consortium; ECMO = extracorporeal membrane oxygenation; ISTH = International Society on Thrombosis and Haemostasis; mpRCT = multiplatform randomized controlled trial; NR = not reported; RCT = randomized controlled trial; TIMI = Thrombolysis In Myocardial Infarction; ULN = upper limit of normal.
INSPIRATION was an RCT with a 2 × 2 factorial design comparing intermediate dose vs prophylactic dose anticoagulation and statin therapy vs matching placebo.
In patients with a creatinine clearance of 30 to 49 mL/min or those taking azithromycin, rivaroxaban 15 mg daily was used (66 of 280 patients taking rivaroxaban, 24%). Unstable patients received enoxaparin 1 mg/kg subcutaneous bid or therapeutic dose IV unfractionated heparin (30 of 311 patients, 9.6%).
Extended prophylaxis beyond hospital discharge was prescribed in 38 of 304 (13%) patients allocated to the comparator group.
A total of 81 patients were excluded, because they did not have confirmed COVID-19.
A total of 12 patients were excluded, because they did not have confirmed COVID-19.
In REMAP-CAP, levels of oxygen support (including no support) below the level of high-flow nasal cannula were not reported.
Levels of oxygen support below the level of high-flow nasal cannula were not reported.
Levels of oxygen support other than mechanical ventilation were not reported. At baseline, 107 (62%) patients were admitted to an ICU.
A total of 45 of 253 (18%) patients were on either high-flow or noninvasive positive pressure ventilation.
Not listed are 113 patients who were co-enrolled in the REMAP-CAP Antiplatelet Domain (47 in the therapeutic dose anticoagulation group and 66 in the usual care pharmacologic thromboprophylaxis group).
Not listed are 74 patients who were co-enrolled in the REMAP-CAP Antiplatelet Domain (39 in the therapeutic dose anticoagulation group and 35 in the usual-care pharmacologic thromboprophylaxis group).
Treatment during trial period.
Risk of bias was assessed by the methodologist using Version 2 of the Cochrane risk-of bias tool for randomized trials (RoB 2).21 Data abstraction was done in duplicate. Any discrepancies were resolved through consensus. Primary outcomes included VTE (pulmonary embolism [PE] and DVT), fatal PE, major bleeding, fatal bleeding, mortality, and organ support-free days (OSFDs). As our original guideline focused on VTE, we did not include arterial thrombosis as an outcome. A meta-analysis, using a random effects model, was performed. The panel then created summaries using the GRADE Evidence-to-Decision framework.22 These summaries were discussed by the entire group, and updated guidance statements were suggested and voted upon using a modified Delphi approach. This approach utilized several rounds of anonymous voting, with survey results and comments presented to the panel after each round until consensus was achieved. Per CHEST policy, consensus was defined as at least 80% agreement for each recommendation with at least 75% voting participation rate from the panel. Recommendation 1 was controversial and required four rounds of voting (see context comments below). Recommendation 2 reached consensus in three rounds of voting. Recommendations 3 and 4 each reached consensus after two rounds of voting.
Results and Recommendations
As in the first version of the guideline, we chose to provide separate guidance statements for VTE prevention for hospitalized patients with acute illness (also described as moderately ill, or non-ICU patients) and critically ill patients either hospitalized in the ICU or receiving ICU level care. However, we divided each original Population, Intervention, Comparator, Outcome question and resulting recommendation into two questions: standard prophylaxis vs therapeutic (or escalated dose) prophylaxis and standard prophylaxis vs intermediate dose prophylaxis.
Hospitalized Patients With Acute Illness
Question 1: Should patients with acute illness with COVID-19 be treated with therapeutic anticoagulation or thromboprophylaxis for prevention of VTE?
There were four studies reporting on 3,475 patients addressing this question, the largest being the multiplatform ATTACC, ACTIV-4a, and REMAP-CAP study12 that enrolled 2,244 patients. Additional studies included the ACTION,16 RAPID,19 and HEP-COVID20 trials. For both the multiplatform12 and HEP-COVID20 trials, we were only able to include PE as DVT rates were not mutually exclusive from PE. There was a reduction in any VTE in the therapeutic anticoagulation group (risk ratio [RR], 0.48 [95% CI, 0.30-0.78]), at the expense of increased major bleeding (RR, 1.79 [95% CI, 1.01-3.16]) (Figs 1A, 1B ). No data regarding fatal PE could be extracted. Fatal bleeding was extremely rare and occurred in 0.3% (3/1,180) of the therapeutic anticoagulation group compared with 0.1% (1/1,047) in the thromboprophylaxis group in the multiplatform trial. The RAPID19 trial reported no fatal bleeding in either group. There was no statistically significant difference in mortality between therapeutic and prophylactic anticoagulation (RR, 0.75 [95% CI, 0.41-1.37]) (Fig 1C). Therapeutic anticoagulation increased OSFDs compared with usual care with an OR of 1.27 (95% CI, 1.03-1.58) in the multiplatform trial.12 The RAPID19 trial noted a statistically insignificant increased odds of OSFDs in the therapeutic group with an OR of 1.41 (95% CI, 0.90-2.20), and the HEP-COVID20 trial reported 7/84 (8.3%) in the therapeutic group required mechanical ventilation compared with 13/86 (15.1%) in the thromboprophylaxis group. After considerable discussion and four rounds of voting, the panel reached consensus on the following recommendation.
Figure 1.
Outcomes in moderately ill hospitalized patients receiving therapeutic anticoagulation vs standard thromboprophylaxis.
1. In hospitalized patients with acute illness with COVID-19 who have low risk of bleeding, with consideration for the remarks below, we suggest therapeutic dose heparin (UFH or LMWH) over current standard dose anticoagulant thromboprophylaxis (Conditional Recommendation, Ungraded Consensus-Based Statement).
Remarks: Providers should carefully weigh the risks of thrombosis and bleeding in making this decision. Patients with a significantly elevated D-dimer level (studies have previously defined this as 2-4 × the upper limit of normal), those with prior VTE, or other comorbidities known to be associated with VTE may be at increased risk of thrombosis. Patients with high risk of bleeding include, but are not limited to, those with known bleeding within the last 30 days requiring ED presentation or hospitalization, known history of an inherited or acquired bleeding disorder, active dual antiplatelet therapy, recent ischemic stroke, intracranial malignancy, history of bleeding diatheses (eg, hemophilia), history of GI bleeding within previous 3 months, thrombolysis within the previous 7 days, presence of an epidural or spinal catheter, recent major surgery < 14 days, or uncontrolled hypertension (systolic BP > 200 mm Hg, diastolic BP > 120 mm Hg).
Context
The panel struggled to come to consensus on this recommendation. From a pure VTE perspective, these trials are consistent with historical trials—therapeutic heparin, either intravenous UFH or full dose LMWH, reduces VTE at the cost of increased bleeding, without any benefit in overall mortality. The reporting of decreased OSFDs in the multiplatform trial12 deserves notice but is not a typical outcome used in VTE studies. That said, in balance, the benefits of therapeutic vs prophylactic dosing appear to favor the former. Several nuanced issues beyond this were discussed. The ACTION16 trial, which was the only one to use therapeutic doses of rivaroxaban as opposed to heparin, showed no overall benefit. To explain this, we might invoke that there are additional pleiotropic and/or antiinflammatory effects of heparin that are beneficial beyond the benefits of thromboprophylaxis. While plausible, studies in similarly ill patients without COVID-19 have been inconclusive. The panel also noted the inconsistency in effect between hospitalized patients with acute (moderate) illness and critically ill patients, with the OSFD benefit only being seen in the former. This invokes a hypothesis regarding timing of the initiation. Perhaps early administration of therapeutic heparin does indeed affect the underlying pathophysiologic mechanisms in a way that reduces macrothrombosis and microthrombosis, but once patients develop more severe end-organ damage, the harmful effects outweigh any benefit. This is also plausible but not fully studied. Another concern raised was the likelihood of ascertainment bias (patients in the therapeutic arm of an open-label trial may be less likely to undergo diagnostic testing for VTE). Finally, the panel raised concern that the extremely low rate of bleeding in these trials does not match real-world rates, perhaps because patients with high risk of bleeding were excluded, and thus assessment of bleeding risk is paramount in decision-making. Panel members also pointed out the heterogeneous populations included in the trials and the known changes in standard management over time. Given all of this, the panel voted to make a conditional recommendation in favor of therapeutic anticoagulation, while noting in the remarks that the decision should be based upon the risk of thrombosis (those with higher D-dimer levels or other risks for VTE may be at higher risk) and the risk of bleeding (see Table 3 for a more extensive list of factors associated with an increased risk of bleeding). Although consensus was reached, one panel member strongly disagreed with this recommendation.
Table 3.
High Bleeding Risk Patients
| Bleeding within last 30 d needing acute care setting |
| History of inherited or acquired bleeding disorder |
| Recent ischemic stroke |
| History of intracranial hemorrhage |
| Presence of epidural or spinal catheter |
| Intracranial malignancy |
| History of bleeding diathesis (ie, hemophilia) |
| Recent GI bleeding (within 3 mo) |
| Thrombolysis in previous 7 d |
| Recent major surgery (within 14 d) |
| Uncontrolled hypertension (sBP > 200 mm Hg or dBP > 120 mm Hg) |
| Baseline INR > 2.0 or aPTT > 50 s |
| Hemoglobin < 8 g/dL |
| Platelet count < 50 × 109/L |
| Dual antiplatelet agents |
Bleeding risk should be individualized and discussed on case-by-case basis. aPTT = activated partial thromboplastin time; dBP = diastolic BP; INR = international normalized ratio; sBP = systolic BP.
Question 2: Should patients with COVID-19 hospitalized in the ward setting be treated with intermediate dose anticoagulation or thromboprophylaxis?
There were no randomized trials addressing this question. The only study to inform this question was an observational cohort that reported on rates of VTE stratified by thromboprophylaxis received.14 The rate of VTE in the intermediate dosing group was 7/33 (21%) and in the prophylactic dose group, 20/67 (30%). Bleeding estimates were not reported. Given our original recommendation against intermediate dosing in these patients, the evidence already presented above for consideration of therapeutic dosing in this cohort, and the lack of any evidence suggesting clear benefit of this approach, the panel voted to endorse the prior recommendation, with consensus reached after one round of voting.
2. In hospitalized patients with acute illness with COVID-19 who are not receiving therapeutic dose heparin (UFH or LMWH), we recommend current standard dose anticoagulant thromboprophylaxis over intermediate dose anticoagulation (defined as LMWH bid or increased weight-based dosing that is less than recommended therapeutic doses) (Strong Recommendation, Ungraded Consensus-Based Statement).
Context
Given the lack of randomized controlled trials to address this question, there was unanimous agreement regarding this recommendation. Panel members also noted that “intermediate dose anticoagulation” leaves too much room for error and confusion in clinical practice.
Critically Ill Patients
Question 3: Should critically ill patients with COVID-19 be treated with therapeutic anticoagulation or thromboprophylaxis for prevention of VTE?
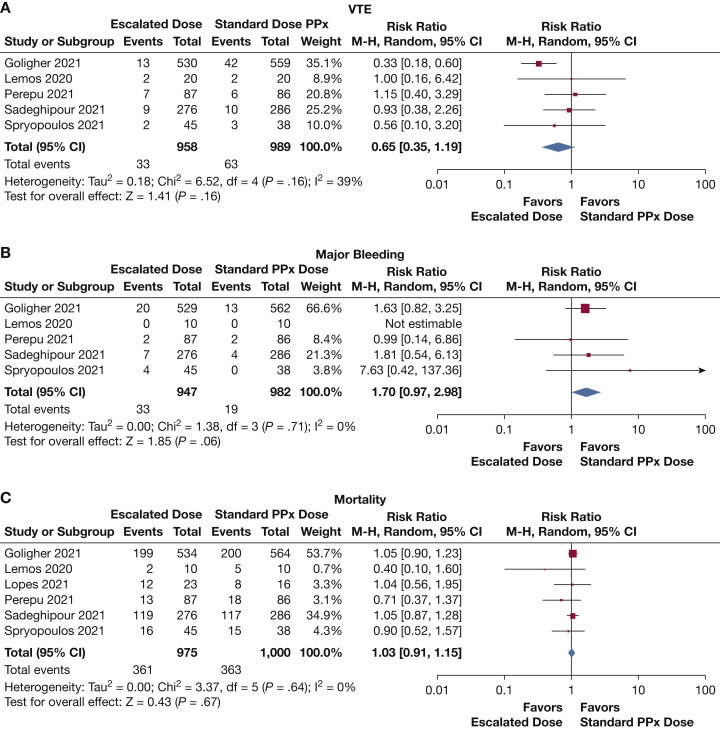
For this analysis, we chose to include all studies that compared standard thromboprophylaxis dosing vs “escalated” dosing (intermediate or therapeutic). This was done because the panel recognized that common practice in many ICU settings includes varying escalated dosing protocols. There were five studies to inform this question, which included a total of 1,947 patients. Again, the largest was the multiplatform ATTACC, ACTIV-4a, and REMAP-CAP13 trial, which accounted for 1,089 patients. Additional studies included the HESACOVID,15 INSPIRATION,18 HEP-COVID,20 and Perepu et al17 studies. It should be noted that the multiplatform trial and the HEP-COVID trial reflect PE data only. There were additional patients with DVT in each group, but we could not determine if these patients overlapped those with PE, and thus they were excluded from analysis. Perepu et al17 and Sadeghipour et al18 used intermediate dose anticoagulation in the “therapeutic anticoagulation” group. These were included in this analysis as this was a higher than standard dosing. There was a nonsignificant reduction in VTE (RR, 0.65 [95% CI, 0.35-1.19]) (Fig 2 A). None of these studies reported fatal PE. There was a nonsignificant increase in major bleeding (RR, 1.70 [95% CI, 0.97-2.98]) (Fig 2B). The INSPIRATION18 trial had 2/276 patients with fatal bleeding in the intermediate anticoagulation group compared with 0/286 in the thromboprophylaxis group, and the HESACOVID15 had no fatal bleeding in either group. The multiplatform trial reported a nonsignificant reduction in OSFDs (OR, 0.83 [95% CI, 0.67-1.03]). The HESACOVID15 trial reported 15 (6-16) ventilator-free days in the therapeutic dose cohort vs 0 (0-11) ventilator-free days in the prophylactic group, which was significant (P = .028). In the HEP-COVID20 trial, 10/38 (26.3%) in the therapeutic group required mechanical ventilation compared with 8/35 (22.9%) in the thromboprophylaxis group. Six trials were included in the mortality analysis. In addition to the trials mentioned above, the ACTION16 trial also included a small number of critically ill patients (n = 39) who were incorporated in the analysis. There was no significant difference in mortality between the two groups (RR, 1.03 [95% CI, 0.91-1.15]) (Fig 2C).
Figure 2.
Outcomes in critically ill patients receiving increased-dose anticoagulation vs standard thromboprophylaxis. PPx = prophylaxis or thromboprophylaxis.
3. In critically ill patients with COVID-19, we suggest current standard dose anticoagulant thromboprophylaxis (with UFH or LMWH) over therapeutic dose anticoagulation (Conditional Recommendation, Ungraded Consensus-Based Statement).
Context
There was no disagreement about the direction of the recommendation. The data supporting thromboprophylaxis for the critically ill are quite robust. Other than the effect on PE seen in the multiplatform trial, there was insufficient evidence to suggest deviation from standard thromboprophylaxis. When all outcomes in the multiplatform trial, and not just PE, are factored in, the case against therapeutic anticoagulation is quite strong. Furthermore, although the risk of VTE is likely lower on therapeutic anticoagulation (absolute risk reduction, approximately 5%), the risk of major bleeding is higher (absolute risk increase, approximately 1%-2%), and there is no effect on mortality. Considering the risk of ascertainment bias for VTE in these open-label trials, the incomplete reporting of VTE events (no DVTs reported) and the high probability of inferiority of therapeutic anticoagulation compared with usual thromboprophylaxis for OSFDs in the multiplatform trial, the data thus far support continued use of existing guidelines.
Question 4: Should critically ill patients with COVID-19 be treated with intermediate dose anticoagulation or thromboprophylaxis for prevention of VTE?
For this question, we chose to focus on studies primarily designed specifically to evaluate intermediate dose thromboprophylaxis, as opposed to a combination of escalated doses. There were two studies to inform this question, the INSPIRATION18 and Perepu et al17 trials. A total of 725 patients were included in the analysis. There was no difference in VTE (RR, 1.00 [95% CI, 0.51-1.96]), major bleeding (RR, 1.53 [95% CI, 0.54-4.28]), or mortality (RR, 0.98 [95% CI, 0.73-1.32]) (Fig 3 ). No data were reported regarding fatal PE. The INSPIRATION18 trial reported that there was no difference between the two groups in ventilator-free days.
Figure 3.
Outcomes in critically ill patients receiving intermediate-dose thromboprophylaxis vs standard thromboprophylaxis.
4. In critically ill patients with COVID-19, we suggest current standard dose anticoagulant thromboprophylaxis over intermediate dose anticoagulation (defined as LMWH bid or increased weight-based dosing that is less than recommended therapeutic doses) (Conditional Recommendation, Ungraded Consensus-Based Statement).
Context
There was no disagreement in the direction of the recommendation. The panel noted that the INSPIRATION18 trial, the largest, did not show a benefit in VTE reduction and yet there was a potential increase in bleeding risk (statistically not significant). Therefore, the panel considered upgrading the recommendation from conditional to strong. Some felt this was perhaps premature given some methodological issues of the study. Ultimately the panel chose to maintain the conditional recommendation in favor of standard thromboprophylaxis in this cohort.
Discussion and Limitations
This manuscript serves as a brief update to the original guidance statement.3 Although we have better quality evidence, many questions remain. Despite this, our panel felt that it was important to share our thoughts regarding the new evidence, especially as one recommendation differs in direction from the original publication, and the others have more evidence to support them. The decision to change the recommendation was not an easy one. While the new trials are higher quality evidence, the interpretation of the results is not without controversy. Progression of respiratory failure due to COVID-19 is a different end point than preventing VTE. In the end, we felt that it was an important end point.
Some may question our decision not to include arterial thrombotic events as a primary outcome. These are clearly important to patients and clinicians. Our original publication, however, was focused on VTE,3 and this was designed as an update to that publication. In addition, a recent meta-analysis that did include these events would not likely have changed our recommendations.23
Questions remain and should guide further research. Does the timing of heparin administration affect the ultimate outcome? Should patients admitted to the ICU during their admission continue therapeutic heparin? Does heparin indeed have antiinflammatory, antiviral, or other pleiotropic effects in COVID-19? Which mechanisms of the prothrombotic state are prominent, and does this influence the optimal approach? Are current standard therapies changing the baseline risk of VTE? Should the approach to vaccinated patients be any different than unvaccinated patients?
Conclusions and Future Directions
We have learned quite a bit regarding thrombosis in patients with COVID-19 pneumonia. Although advances in care have improved overall outcomes, current evidence supports that the rates of VTE are higher in these patients, at least in the ICU.24 , 25 At this time, we believe critically ill patients should still receive standard thromboprophylaxis for VTE, and moderately ill patients with a low bleeding risk might benefit from therapeutic heparin. We see no role for intermediate dose thromboprophylaxis in either setting. The World Health Organization plans to perform a meta-analysis that includes several small trials along with the studies included here, and their findings may inform practice (PROSPERO registration ID is CRD42020213461). In addition, results from recently published trials examining the effect of pre- and post-hospital prophylaxis may lead to additional guideline updates.
Acknowledgments
Author contributions: L. K. M. has full responsibility of the content and accuracy of the manuscript. S. B., M. C., J. C., K. D., A. H., D. J., G. L., L. K. M., P. R., T. T., P. W. played an equal role in development of the PICO questions and drafting of the recommendations. L. K. M. drafted the initial manuscript. D. J. prepared Table 1. T. T. prepared Table 2. P. R. prepared Table 3. J. I. performed the literature searches, ROB assessment, meta-analyses and evidence summaries. S. B., M. C., J. F. C., K. D., A. B. H., J. I., D. J., G. L., P. R., T. T., P. W. contributed equally to review and editing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at https://www.chestnet. org/Guidelines-and-Resources.
References
- 1.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Pan Y., Xu C., Li D. Characteristics of emergency patients with markedly elevated D-dimer levels. Sci Rep. 2020;10(1):7784. doi: 10.1038/s41598-020-64853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moores L.K., Tritschler T., Brosnahan S., et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NICE COVID-19 management. 2021. https://www.guidelines.co.uk/infection/nice-covid-19-management/455939.article
- 5.COVID-19 clinical management: living guidance. World Health Organization; 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuker A., Tseng E.K., Nieuwlaat R., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerotziafas G.T., Catalano M., Colgan M.P., et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb Haemost. 2020;120(12):1597–1628. doi: 10.1055/s-0040-1715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panel C-TG . National Institutes of Health; 2021. Coronavirus Disease 209 (COVID-19) Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov [PubMed] [Google Scholar]
- 10.Spyropoulos A.C., Levy J.H., Ageno W., et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poor H.D. Pulmonary thrombosis and thromboembolism in COVID-19. Chest. 2021;160(4):1471–1480. doi: 10.1016/j.chest.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellmunt-Montoya S., Riera C., Gil D., et al. COVID-19 infection in critically ill patients carries a high risk of venous thrombo-embolism. Eur J Vasc Endovasc Surg. 2021;61(4):628–634. doi: 10.1016/j.ejvs.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos A.C.B., do Espirito Santo D.A., Salvetti M.C., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes R.D., de Barros E.S.P.G.M., Furtado R.H.M., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perepu U.S., Chambers I., Wahab A., et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19(9):2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeghipour P., Talasaz A.H., Rashidi F., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sholzberg M., Tang G.H., Rahhal H., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spyropoulos A.C., Goldin M., Giannis D., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne J.A.C., Savovic J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Alonso-Coello P., Oxman A.D., Moberg J., et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 23.Ortega-Paz L., Galli M., Capodanno D., et al. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials [published online ahead of print September 14, 2021]. Eur Heart J Cardiovasc Pharmacother. https://doi.org/10.1093/ehjcvp/pvab070 [DOI] [PMC free article] [PubMed]
- 24.Jimenez D., Garcia-Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai V., Tan B.K., Mainbourg S., et al. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: a systematic review with meta-analysis. Vascul Pharmacol. 2021;139:106882. doi: 10.1016/j.vph.2021.106882. [DOI] [PMC free article] [PubMed] [Google Scholar]