Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel human pathogen causing coronavirus disease 2019 (COVID-19). Rare cases of COVID-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) after the ChAdOx1 nCoV-19 (AstraZeneca) vaccination have been reported. We performed a test for anti-heparin/ platelet factor 4 (PF4) antibodies and functional assay using flow cytometry.
Method
A healthy woman presented to the emergency department with chest pain, headache, and abdominal pain after the first vaccination with AstraZeneca. Polymerase chain reaction (PCR) test for SARS-CoV-2 was negative. Chest computed tomography (CT) showed pulmonary artery embolism and brain magnetic resonance imaging (MRI) revealed cerebral sinus-venous thrombosis. Abdominal CT demonstrated the thrombosis with occlusion in her right hepatic vein. Laboratory studies revealed decreased platelet counts, and high D-dimer level. Finally, laboratory results indicated high PF4 antibodies level high and a positive platelet activation test, confirming the diagnosis of VITT.
Results
Treatments including intravenous immunoglobulin, methylprednisolone and direct oral anticoagulant were administered. The results of a follow-up platelet count and D-dimer were normal. In addition, the titer of PF4 antibodies (optical density: 0.425; normal ≤ 0.4, enzyme-linked immunosorbent assay) fell. After a 3-month follow-up, her general condition improved gradually.
Conclusions
The use of COVID-19 vaccines to prevent SARS-CoV-2 infections and complications is considered the most practicable policy for controlling the COVID-19 pandemic and is being forcefully pursued in the global area. Appropriate laboratory diagnosis facilitates the accurate and rapid diagnosis. Early recognizing and appropriate strategies for VITT are required and can provide these patients with more favorable patient outcomes. This report also elected to make comparisons of clinical manifestation, laboratory diagnosis, and management in patients with VITT.
Keywords: COVID-19 vaccine, ChAdOx1 COVID-19 (AstraZeneca) vaccine, Anti-platelet factor 4 antibodies, Platelet activation test, Vaccine-induced immune thrombotic thrombocytopenia, Thrombosis with thrombocytopenia syndrome
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS–CoV–2) is a new human pathogen which can cause fulminant respiratory syndrome that was first identified as a cluster of cases with serious pneumonia in Wuhan, China [1]. In March 2020, the World Health Organization (WHO) declared a worldwide pandemic and designated the disease taxonomy as coronavirus disease 2019 (COVID-19). COVID-19 is primarily a disease of the lungs with acute respiratory manifestations; the disease may cause systemic complications and increase mortality [2], [3]. Developing an effective and reliable vaccine was urgently pursued to control the outbreak of the global pandemic. In general, the vaccine development progresses through pre-clinical and clinical stages occurring consecutively and each may take a substantial time for completion. Inactivated or live-attenuated viruses as well as recombinant proteins and vectors technologies have been deployed to develop the COVID-19 vaccine. Other new RNA and DNA vaccines are also used for the first time in a licensed vaccine [4]. COVID-19 vaccine remains critical to control the SARS–CoV–2, the side effects of vaccination including cardiovascular, neurological, gastrointestinal, musculoskeletal, and thromboembolic events have been reported [5], [6], [7], [8], [9], [10]. Therefore, early recognition and management for COVID-19 vaccine-associated adverse reactions are imperative. Herein, we report a case of vaccine-induced immune thrombotic thrombocytopenia (VITT) after the first dose of the ChAdOx1 nCoV-19 (AstraZeneca) vaccination. This review also provided an update on the clinical manifestation, laboratory diagnosis, and management in VITT.
2. Report of a clinical scenario
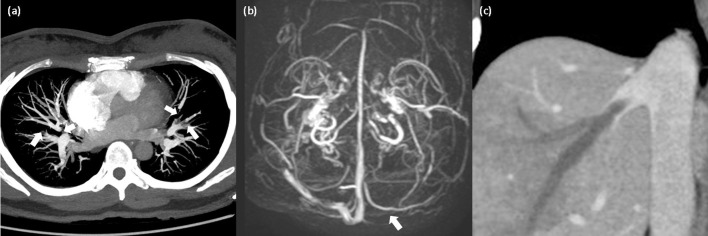
A 40-year-old woman presented to the emergency department with a 1-day history of chest pain, headache, and abdominal pain. She was healthy in the past and just received the first vaccination with AstraZeneca 6-day prior. The family history was unremarkable. Her temperature was 37.2 °C, blood pressure was in the normal range, and no heart murmur was found. On initial assessment, skin petechiae over bilateral upper and lower extremities were found. Pregnancy test result was negative and urinalysis result was normal. Polymerase chain reaction (PCR) test for SARS-CoV-2 was negative. Blood tests indicated decreased platelet count (31 × 109/L; normal ≥ 150 × 109/L) and high D-dimer level (>10,000 ng/mL; normal ≤ 250 ng/mL, latex enhanced immunoturbidimetric immunoassay). The results of screening tests for autoimmune antibodies were negative and no schistocytes were found in peripheral blood smears. Coagulation tests results including plasma fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin, protein S and protein C were all in normal range. Chest computed tomography (CT) showed pulmonary embolism (Fig. 1 a) and brain magnetic resonance venography (MRV) revealed cerebral sinus venous thrombosis (Fig. 1b). In addition, abdominal CT demonstrated the thrombosis with obstruction in her right hepatic vein (Fig. 1c). The level of blood platelet factor 4 (PF4) antibodies using enzyme-linked immunosorbent assay (ELISA) of Lifecodes PF4 IgG assay (Immucor) was high (110.76 ng/ml; normal ≤ 40 ng/ml) and result of platelet activation test (Fig. 2 ) was positive, confirming the diagnosis of VITT. Medical treatments including intravenous immunoglobulin (1 g/kg daily for 2 days), methylprednisolone (40 mg/day for 4 days) and anticoagulation with the direct oral anticoagulant dabigatran were administered. After a 3-month follow-up, the platelet count (263 × 109/L; normal ≥ 150 × 109/L) and D-dimer level (234.51 ng/mL; normal ≤ 250 ng/mL) were in normal range. In addition, the titer of PF4 antibodies (optical density: 0.425; normal ≤ 0.4, ELISA) fell compared with initial presentation. Her general condition completely recovered after a 6-month follow-up.
Fig. 1.
(1a) Maximum intensity projection reconstruction of chest computed tomography (CT) showed pulmonary artery embolism. (1b) Brain magnetic resonance venography (MRV) revealed cerebral sinus venous thrombosis. (1c) Abdominal computed tomography (CT) showed the thrombosis with occlusion in her right hepatic vein.
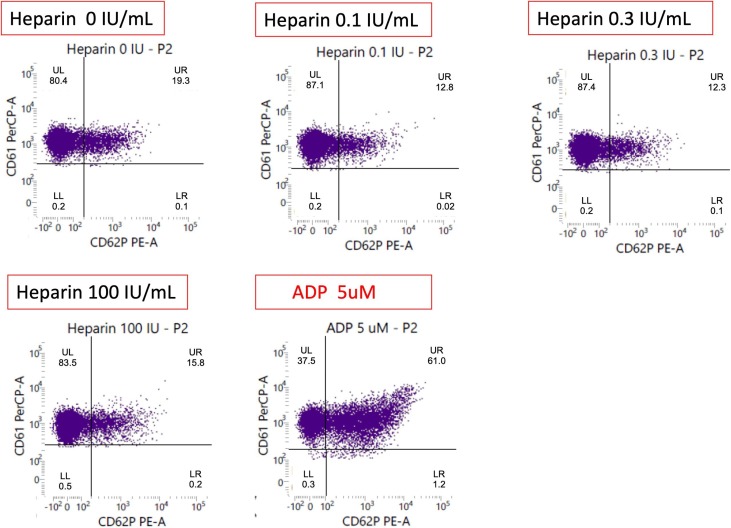
Fig. 2.
The functional result of platelet activation was positive in our patient: the positive functional result of platelet activation was presented in serum without heparin, the percentage of platelets activated for both anti-CD61 and anti-CD62p conjugated antibodies was 19.19% to indicate that platelet activation was triggered (data were shown in the upper right quadrant of each plot). In the presence of patient serum plus heparin (0.1 IU/mL, 0.3 IU/mL, and 100 IU/mL), donor platelets show the reduced reactivity with 12.8%, 12.3% and 15.8% of activated platelets. The definition of positive flow cytometric assay was with percentage of activated platelets more than 11%. Abbreviation: CD61 (glycoprotein IIIa): a marker of platelet identification; CD62p (p-selectin): a marker of platelet activation; Adenosine diphosphate (ADP): a positive control of normal platelet activation.
3. Discussion
3.1. The pathogenesis and diagnosis of Vaccine-induced immune thrombotic thrombocytopenia (VITT)
VITT has been reported as a very rare complication and typically after the first-dose AstraZeneca vaccination. This phenomenon is similar to type II heparin-induced thrombocytopenia (HIT) and lead to the platelet activation through the FcγRIIA receptor. VITT is characteristically associated with thrombocytopenia, thrombosis, raised D-dimer, and high levels of immunoglobulin G (IgG) class anti-PF4 antibodies. A complete blood cell count investigation could be performed for anyone with a clinical suspicion of VITT. Not only AstraZeneca vaccines but other COVID-19 vaccines (especially adenoviral vaccines) have been reported to be associated with rare cases of VITT, which can cause multiple systemic involvements in different parts of the body, such as the brain, lung, abdomen, and limbs [10], [11], [12], [13], [14]. Furthermore, several different polymorphisms in exon 4 of the FcγRIIA region could be implicated in the modulation of the immune-complexdependent thrombotic risk in patients with VITT.
3.2. The Epidemiology, clinical Manifestations, and management of Vaccine-induced immune thrombotic thrombocytopenia (VITT)
To date, the incidence is still unclear but it appears to be extremely rare [5], [6], [7]. Initial reports demonstrated a female preponderance, with female sex accounting for 9 of 11 cases in one study and 4 of 5 cases in another series [10], [11]. Younger age is proposed as possible risk factors, but these associations may be skewed by the initial demographics of the populations [10], [11]. According to UK Parliament, the total risk of VITT following a dose of the University of Oxford/AstraZeneca vaccine was around 10.9 per million doses. This varied based on the age groups and it was estimated to be about 1 in 100,000 for people more than 50-year old and 1 in 50,000 for people aged between 18 and 49 year-old [12]. The pathophysiology of VITT is not fully understood and presumably the development of antibodies against PF4, further resulting in platelet depletion with thrombosis [6]. Until November 24, 2021, 74 cases of VITT were reported to be associated with AstraZeneca vaccinations from the Taiwan Centers for Disease Control, the estimated incidence rate was around 5.1 per million doses [13]. Clinical manifestations should be considered for the diagnosis of VITT including new onset severe headache; double or blurred vision; chest, abdominal, back or a leg pain; dyspnea; sensation changes or unilateral weakness; or a leg edema; and unknown cause of skin petechial rashes 5–14 days later after the first-dose AstraZeneca vaccination. Medical treatment includes intravenous immunoglobulin, methylprednisolone, direct oral anticoagulants, and even plasma exchange could be considered in some serious cases of VITT. During the hospital stay, we performed a follow-up PCR test for SARS-CoV-2 in our patient and the results were all negative. Other causes of thrombosis and/or thrombocytopenia disorders including thrombotic thrombocytopenic purpura (TTP) should be also kept in mind, especially in persons with negative testing of PF4 antibody. Clinical presentation of TTP has also been reported to be associated with the complication of COVID-19 vaccination [14], [15].
3.3. Diagnostic assays for Anti-platelet factor 4 antibodies
Laboratory investigations have been demonstrated using the Zymutest HIA IgG ELISA, the Lifecodes PF4 IgG ELISA, and the Asserachrom HPIA IgG ELISA to successfully detect anti-PF4 in patients with VITT. Rapid methods revealed poor sensitivity for anti-PF4 antibodies in VITT [16], [17], [18]. Sean Platton, et al. recommended that none of the rapid assays tested, which may be suitable for the exclusion of HIT, is suitable for the exclusion of VITT. Rapid assays are used for the diagnosis of HIT should confirm that requests for diagnosis of VITT so that the correct tests are performed [16]. In addition, physicians should be aware that ELISA assays are usually not widely available in the diagnostic laboratories. A very small number of laboratories in the world are able to perform platelet activation assays. To date, no single ELISA method can detect all cases of VITT. When a single ELISA test is negative in patients with symptoms suspicious of VITT, a second ELISA or platelet activation assay should be considered when there is a strong clinical suspicion [16], [17], [18]. Recent data demonstrated that ELISA HIT assays have the most appropriate sensitivity for all pathophysiologically relevant anti-PF4 antibodies. The positive rate has been reported to be around ninety-five percent in patients with VITT [9]. In our patient, the serum sample was presented with Lifecodes PF4 IgG ELISA (Immucor) of anti-human heparin platelet factor 4 (H-PF4) antibody kit. Absorbance was measured in an ELISA reader at 450 nm (Synergy HTX multi-mode reader, Biotek, VT, USA).
3.4. Diagnostic assays for platelet activation tests
Regarding the laboratory aspects, apart from thrombocytopenia, the key diagnostic feature is the positivity of the ELISA test for VITT, which identifies antibodies to PF4 and still needs to be confirmed by a functional heparin-induced platelet activation assay (HIPA) or serotonin-release assay (SRA) [9], [19], [20].
The flow cytometry (FC) method of HIPA in our patient was performed in-house method with some modifications as the follows: (a) use of normal donor platelets regardless of blood group type; (b) use of CD61 (glycoprotein IIIa) and CD62p (p-selectin) as indicators of the identification and activation of platelet, individually; (c) use of adenosine diphosphate to check the standard platelet activation; (d) all processes were performed at the temperature (20–25 °C); (e) use of phosphate-buffered saline as the buffer solution for the entire procedure; and (f) analysis of 10,000 platelets per sample [20], [21]. This patient's plasma was incubated without unfractionated heparin (UFH) and with 0.1 IU/ml, 0.3 IU/ml and 100 IU/ml UFH (Fig. 2). The positive result of platelet activation tests was presented in serum without UFH, the percentage of activated platelets was 19.2% to indicate that platelet activation was triggered. In the presence of this patient’s serum plus UFH, donor platelets showed the reduced reactivity with of platelet activation. VITT had unique pattern of platelet reactivity in vitro, which presented activated platelets without UFH and did not exhibit any dependence on UFH for platelet activation [6], [21], [22].
4. Conclusion
VITT is a very rare disease but may lead to fatal thrombotic complications. Healthcare providers should keep in mind this serious adverse reaction of AstraZeneca vaccine. The current therapeutic management of confirmed VITT is based on the administration of intravenous immunoglobulin and non-heparin anticoagulants. Early diagnosis and appropriate management can improve clinical outcomes. Further experimental studies could be performed to clarify the precise mechanism through which AstraZeneca COVID-19 vaccine activates the generation of anti-PF4 autoantibodies. More clinical researches are needed to evaluate the incidence of VITT, its pathogenesis, more rapid, accurate, available laboratory testing, and the best management. Finally, future study could be performed to identify individuals at increased risk of developing VITT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the staff and researchers at the Taoyuan Armed Forces General Hospital, and Medical Affairs Bureau, Ministry of National Defense, Taiwan; in particular, Dr. Jiann-Torng Chen, Dr. Chien-Sung Tsai, Dr. and Jhih-Ying Lin who responded fast and commanded properly during the nosocomial infection of COVID-19 in June 2021. This study was supported by grants from the Research Fund of the Taoyuan Armed Forces General Hospital (TYAFGH-D-111038). Finally, the authors thank the patient’s consenting to publish the clinical information.
References
- 1.Zhu N.a., Zhang D., Wang W., Li X., Yang B.o., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y.-D., Chi W.-Y., Su J.-H., Ferrall L., Hung C.-F., Wu T.-C. Coronavirus vaccine development: from SARS and MERS to COVID-19. J. Biomed. Sci. 2020;27:104. doi: 10.1186/s12929-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dakay K., Cooper J., Bloomfield J., Overby P., Mayer S.A., Nuoman R., Sahni R., Gulko E., Kaur G., Santarelli J., Gandhi C.D., Al-Mufti F. Cerebral venous sinus thrombosis in covid-19 infection: a case series and review of the literature. J Stroke Cerebrovasc Dis. 2021;30(1):105434. doi: 10.1016/j.jstrokecerebrovasdis.2020.105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., Goldblatt D., Kotoucek P., Thomas W., Lester W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R.-L., Chiang W.-F., Shyu H.-Y., Chen M.-H., Lin C.-I., Wu K.-A., Yang C.-C., Huang L.-Y., Hsiao P.-J. COVID-19 vaccine-associated acute cerebral venous thrombosis and pulmonary artery embolism. QJM. 2021;114(7):506–507. doi: 10.1093/qjmed/hcab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sholzberg M., Arnold D.M., Laupacis A. Recognizing, managing and reporting vaccine-induced immune thrombotic thrombocytopenia. CMAJ. 2021;193(24):E913–E915. doi: 10.1503/cmaj.210882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M., Liumbruno G.M., Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur. J. Haemato. 2021;107(2):173–180. doi: 10.1111/ejh.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.-H., Skattør T.H., Tjønnfjord G.E., Holme P.A. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-19 vaccines safety and blood clots. 19 May, 2021; https://post.parliament.uk/covid-19-vaccines-safety-and-blood-clots/.
- 13.Notification of the adverse events after COVID-19 vaccination. Taiwan Centers for Disease Control; https://www.cdc.gov.tw/Category/MPage/Q8n9n-Q4aBpRrGnKVGFkng.
- 14.Hwang J., Lee S.B., Lee S.W., Lee M.H., Koyanagi A.i., Jacob L., Tizaoui K., Yon D.K., Shin J.I., Smith L. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J. Autoimmun. 2021;122:102681. doi: 10.1016/j.jaut.2021.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadoff J., Davis K., Douoguih M. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination - Response from the Manufacturer. N. Engl. J. Med. 2021;384(20):1965–1966. doi: 10.1056/NEJMc2106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platton S., Bartlett A., MacCallum P., Makris M., McDonald V., Singh D., Scully M., Pavord S. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J. Thromb. Haemost. 2021;19(8):2007–2013. doi: 10.1111/jth.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly‐Stitt C., Kitchen S., Jennings I., Horner K., Jones R., Makris M., Walker I.D. Anti-PF4 testing for vaccine-induced immune thrombocytopenia and thrombosis and heparin induced thrombocytopenia: Results from a UK National External Quality Assessment Scheme exercise April 2021. J. Thromb. Haemost. 2021;19(9):2263–2267. doi: 10.1111/jth.15423. [DOI] [PubMed] [Google Scholar]
- 18.Sachs U.J., Cooper N., Czwalinna A., Müller J., Pötzsch B., Tiede A., et al. PF4-Dependent Immunoassays in Patients with Vaccine-Induced Immune Thrombotic Thrombocytopenia: Results of an Interlaboratory Comparison. Thromb. Haemost. 2021 Jun 24 doi: 10.1055/a-1535-9002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Alam W. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A review of the potential mechanisms and proposed management. Sci. Prog. 2021;104(2) doi: 10.1177/00368504211025927. 003685042110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favaloro E.J. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021;43(4):559–570. doi: 10.1111/ijlh.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y.-C., Lin C.-Y., Tsai C.-S. The frequency of heparin-induced thrombocytopenia in Taiwanese patients undergoing cardiopulmonary bypass surgery. J. Formos. Med. Assoc. 2015;114(10):981–987. doi: 10.1016/j.jfma.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Hogan M., Berger J.S. Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc. Med. 2020;25(2):160–173. doi: 10.1177/1358863X19898253. [DOI] [PubMed] [Google Scholar]