Abstract
Since the beginning of the COVID-19 pandemic in 2019–2020, Cytokine & Growth Factor Reviews has published several Special Issues focused on the biology, pathogenesis and therapeutic options in the treatment of COVID-19 infection, including articles on the involvement of the chemokine system in the cytokine storm in COVID-19, intervention in the early stages of COVID-19 pneumonia, the therapeutic value of corticosteroid treatment, early clinical intervention with type 1 interferons, progress in vaccine development, and organ specific complications of COVID-19. By 2022, multiple highly efficacious vaccines are available and are being administered in countries around the world, therapeutic options have been clinically evaluated and approved, and SARS-CoV-2 has arguably become the most thoroughly studied virus in history. But, with progress has also come unanticipated problems – misinformation, anti-vaxxers, opposition to protective masks, and politically motivated interference disguised as knowledge. With this issue of CGFR, we continue to document the global coronavirus pandemic and provide an update on the emergence of viral variants, the global effort to administer vaccines and the impediments to progress posed by misinformation and anti-vaccine sentiment.
1. Introduction
In June of 2020, Cytokine & Growth Factor Reviews published a Special Issue of the journal entitled The Coronavirus Pandemic 2020 (volume 53), featuring reviews from China, Italy, Russia and America, early ground zero countries hit by the pandemic; this issue reflected our early understanding of SARS-CoV-2 biology, pathogenesis, immune response and treatment strategies. Subsequent Special Issues of CGFR - August 2020, volume 54; April 2021 vol 58 and this issue February 2022, volume 63 – continue to highlight reviews focusing on the global spread of the COVID-19 pandemic. Along the way, CGFR has published articles on the involvement of the chemokine system in the cytokine storm in COVID-19 [1], [2], [3], intervention in the early stages of COVID-19 pneumonia [4], the therapeutic value of corticosteroid treatment [5] early clinical intervention with type 1 interferons [6], [7], [8], [9], progress in vaccine development [10] and organ specific complications of COVID-19 [11].
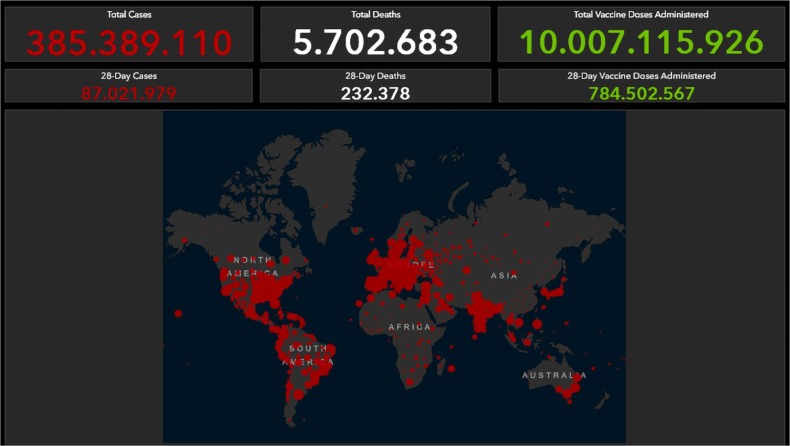
By now, we all recognize that the origins of the COVID-19 pandemic began in December 2019, with the first known cases detected in Wuhan, China. By March 2020, the global spread of the virus prompted the World Health Organization (WHO) to declare the outbreak a pandemic [12]. As of February 3, 2022, more than 385 million cases have been identified and almost 6 million deaths reported, both numbers likely a gross underestimate of the extent of SARS-CoV-2 spread. Throughout the pandemic, the Johns Hopkins University Coronavirus Resource Center has provided a daily update on cases, deaths, and global hotspots during the unfolding pandemic tragedy ( Fig. 1) (https://coronavirus.jhu.edu/map.html).
Fig. 1.
Daily update on cases, deaths and global hotspots by the Johns Hopkins University Coronavirus Resource Center.
Today, multiple highly efficacious vaccines have been generated and are being administered in countries around the world, therapeutic options have been clinically evaluated and approved, and SARS-CoV-2 has arguably become one of the most thoroughly studied viruses in history. But with progress has also come unanticipated problems - misinformation, anti-vaxxers, opposition to protective masks, and political interference disguised as knowledge. This Introduction to our Special Issue provides an update on the emergence of viral variants, the global effort to administer vaccines and the impediments to progress posed by misinformation and anti-vaccine sentiment.
2. Viral variants
The origins of SARS-CoV-2 have been extensively debated since the beginning of the pandemic, with two main theories proposing either a laboratory escape or a natural emergence of the virus. While no evidence of a laboratory origin has been reported so far, accumulating genetic and epidemiological data indicate SARS-CoV-2 outbreak as the result of a zoonotic event [13], [14], [15], [16].
In January 2020, the first whole-genome sequence of SARS-CoV-2 was published on GISAID which helped to start the production of diagnostic tests against COVID-19 and the formulation of the first vaccines. The two main public repositories of SARS-CoV-2 sequences, GISAID and NCBI/NLM, facilitate an organized, rapid, open data sharing. (see: www.gisaid.org and www.ncbi.nlm.nih.gov/sars-cov-2/). As of February 3, 2022, 7,795,318 genome sequence submissions of SARS-CoV-2 data were shared via GISAID.
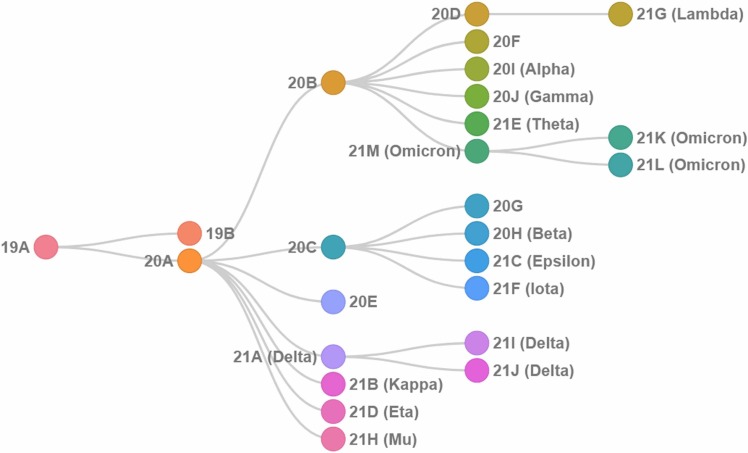
Genetic alterations in the viral genome have been cataloged as it multiplies, spreads and mutates within the human population ( Fig. 2) [17], [18]. The emergence of new variants continues to fuel the pandemic - since the genetic differences in viral properties, particularly in the Spike protein, has altered pathogenicity, infectivity, transmissibility, antigenicity and/or vaccine efficacy [19], [20]. WHO classified emerging variants as Variants Being Monitored (VBM), Variants of Interest (VOIs), Variants of Concern (VOCs) and Variants of High Consequence (VOHC) (see: www.who.int) [12].
Fig. 2.
Phylogenetic relationships among the main viral variants adapted from Nextstrain [21], [22] (see nextstrain.org).
2.1. Delta variant
The Delta variant (21A Delta or B.1.617.2) was first detected in India in late 2020 and by February 2022, had spread to over 193 countries, becoming the dominant strain globally [12]. The Delta variant possesses significantly increased transmissibility and is between 40% and 60% more transmissible compared to Alpha, the previous dominant variant, and around 225% more transmissible than the original strain from Wuhan [23]. A study from the University of Toronto highlighted that Delta variant was responsible for a 108% rise in hospitalization, 235% increase of intensive care unit admission and a 133% surge in death compared to other variants [24]. Delta remained the dominant variant until the second half of December 2021, accounting for 77% of cases; however, with the emergence of the Omicron in South Africa, more than 22.5% of the global cases were caused by Omicron. Strikingly, at the end of December, Delta cases decreased to 41%, while Omicron cases rose to 58.6% and Omicron became the new dominant global variant [12], [25].
The Delta variant has accumulated several mutations in the gene encoding the Spike protein. The mutations causing substitutions at the positions P681R, L452R and T478K affect the transmissibility, pathogenicity and immune escape of the Delta variant. Moreover, additional mutations have been identified at the positions T19R, R158G, T478K, D950N and deletions at the positions E156-, F157-, A28271-, ORF8:D119- and ORF8:F120- [26], [27], [28].
2.2. Omicron variant
The Omicron variant (B.1.1.529) was first reported to the WHO on 24 November 2021 and was detected in specimens collected in Botswana and South Africa. Genome sequencing demonstrated that Omicron contained 60 genetic mutations compared with the original SARS-CoV-2 isolates [29]. Omicron was found to be highly transmissible, with estimates that it can multiply in the lung cells 70 times faster than the Delta variant [30], [31], while the overall binding of Omicron Spike to ACE2 receptor remained comparable to Delta. The combination of two mutations in the Spike protein – Q498 and N501Y – have been reported to increase the binding affinity to ACE2 in in-vitro studies [32], but other Omicron mutations seem to have the opposite effect, most notably the K417N substitution. Mutations that are likely responsible for enhanced Omicron transmissibility act by increasing the evasion of the antibody response to a greater degree, thus partly explaining the number of "breakthrough" infections in previously vaccinated individuals. Enhanced immune evasion was linked with the acquisition of an N-linked glycan in RBD residue N370, which drives neutralization escape as it sits in the binding footprint common to many antibodies [33], [34].
Accumulated data now demonstrate that Omicron does not cause the same level of disease severity as previous variants, particularly when compared with the Delta variant. The COVID-19 Response Team at Imperial College London enumerated this difference, reporting a 40% lower risk of hospitalization with Omicron, compared to the Delta variant [35]. Other studies indicated that infection with Omicron was 90% less fatal than the Delta variant, with 51% decrease in the risk of severe disease requiring hospitalization [36].
2.3. COVID-19 complications
Symptoms of COVID-19 range from mild ‘cold & fever’ symptoms to severe respiratory distress, requiring hospitalization and therapeutic intervention. Most often, symptoms include fatigue, headache, fever, cough, nasal congestion, breathing difficulties, anosmia (loss of smell), ageusia (loss of taste), muscle pain and digestive symptoms with abdominal pain [37], [38]. 40.5% of the people infected by the virus do not develop any symptoms (asymptomatic carriers) [39], [40]. Noticeable symptoms usually develop one to five days after infection with the virus. Of symptomatic patients, 81% develop mild to moderate symptoms. 14% suffer from severe symptoms and 5% of patients develop critical symptoms. Severe and critical symptoms include multiorgan dysfunction, respiratory failure, chronic kidney impairment, heart complications, stroke, Guillain-Barre syndrome, and others [37], [41].
The majority of patients recover from the acute phase of the disease. Nevertheless, some patients continue to suffer from a range of symptoms months after recovery, a recognized medical condition that is now termed ‘Long Covid’. Long Covid is characterized by long term complications or persisting symptoms after the typical COVID-19 recovery period. Nearly every organ system can be affected by Long Covid - most common effects are observed in the respiratory, nervous and cardiovascular systems [42]. Symptoms include fatigue, malaise, musculoskeletal pain, headaches, shortness of breath, parosmia, anosmia, low fever, anemia and cognitive dysfunction. Some patients experience multisystem inflammatory syndrome, where severe damage to organs has been observed [37], [43]. WHO released a clinical case definition in October 2021: “Post COVID-19 condition occurs in individuals with a history of probable or confirmed SARS CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis” [12].
In a recent study from the UK Office for National Statistics, it was concluded that approximately 14% of people who tested positive for SARS-CoV-2 suffered from one or more symptoms consistent with Long Covid for longer than three months [44] . Another study from the University of Oxford showed that 37% of COVID-19 patients experienced at least one symptom three to six months after diagnosis [45] . Long Covid risk factors include: age (especially those above 50 years old), obesity and asthma, coupled with the development of more than five symptoms during the first week of acute SARS-CoV-2 infection [46], [47], [48], [49] . Moreover, other studies suggested that women are more likely to develop Long Covid than men, even though they are less likely to develop severe acute COVID-19 [49].
Numerous hospitals and clinics have initiated special multidisciplinary programs, with specialists from a wide range of departments in order to offer comprehensive care and help with Long Covid recovery [50]. The treatment is individualized according to the grouping of clinical signs and symptoms and includes numerous clinical, functional, cognitive, psychological, and nutritional aspects.
3. Vaccine development
The development, manufacture, testing and distribution of vaccines against SARS-CoV-2, essentially in the space of a year, will surely be viewed as one of the most remarkable accomplishments in the history of modern medicine. Prior to 2020–2021, the pharmacological ‘dogma’ stated that several years of effort were required to develop a vaccine against an infectious disease; however, an unprecedented collaboration between government, academia and the pharmaceutical industry accelerated the scope of vaccine development [51], [52].
The rapid development of COVID-19 vaccines was not a consequence of hastily conducted research, inadequate clinical trials or blind luck, but rather the result of decades of public and private vaccine research that for the most part flew ‘under the radar’ of the general public. During the latter half of the 20th century, vaccine development was guided by new laboratory methods that established cell and virus culture systems enabling the production of vaccines that protected against several devastating diseases; poliomyelitis, measles, mumps, yellow fever and rubella are all examples of viral diseases that have been drastically reduced or completely eradicated in the global population by vaccine development. New 21st century state-of-the-art techniques – next generation sequencing platforms, mRNA delivery & expression techniques, and novel recombinant vector systems – all contributed to the ‘next gen’ acceleration of vaccine research, particularly in relation to the development of COVID-19 vaccines [53], [54], [55], [56].
Pharmaceutical research also placed a focus on several vaccines targeting animal diseases caused by canine coronavirus, feline coronavirus and infectious bronchitis virus in birds, but up until 2020, no vaccine was available that protected human against coronavirus infection [57]. Efforts to create vaccines against SARS (severe acute respiratory syndrome) [58] after the 2002 SARS epidemic or the 2012 MERS epidemic (Middle East respiratory syndrome) [59] failed to identify effective candidates; however, the accumulated experience further propelled the public private partnerships in their efforts to identify prophylactic vaccines against SARS-CoV-2.
By November 2020, vaccine candidates targeting the viral Spike protein were being tested in large phase 3 clinical trials. Just nine months after the COVID-19 pandemic began, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK, granted emergency approval to the world’s first COVID-19 vaccine, Pfizer/BioNTech’s BNT162b2 vaccine using mRNA delivery technology [60]. The Moderna mRNA vaccine followed shortly thereafter, as did Johnson & Johnson recombinant adenovector vaccine, and the Oxford–AstraZeneca adenovector vaccine, sold under the brand names Covishield and Vaxzevria [12], [37], [38], [61]. As of February 3, 2022, it is estimated that 61,2% of the world population had been vaccinated with at least one dose of a vaccine, and in total 10.14 billion doses had been administrated globally. Each day 20,72 million doses are delivered. (see: ourworldindata.org/covid-vaccinations).
3.1. Vaccine types
Currently, there are more than eight advanced technology platforms used to develop effective vaccines against COVID‑19. These platforms include nucleic acid technologies (genetically modified DNA and mRNA), protein subunits, peptides and recombinant proteins, non-replicating viral vectors, replicating viral vectors, virus-like particles, inactivated viruses, and attenuated viruses ( Fig. 3). Moreover, other vaccine technology platforms are under investigation in pre-clinical and clinical trials, including multiple DNA plasmid, lentiviral vectors, VSV display of SARS‑CoV‑2 Spike protein and others [62], [63].
Fig. 3.
Technological platforms used to develop vaccines against COVID-19 [64], [65].
Over 30 different vaccines have been granted emergency use authorization or have been made available for use outside of clinical trials by at least one country in the world. Ten vaccines against COVID-19 have been approved for use by WHO ( Table 1), only 5 are approved by EMA for use within the EU and only 3 COVID-19 vaccines are FDA authorized or approved for use in the United States [12], [38], [66].
Table 1.
10 Vaccines Approved for Use by WHO [67]. (Last updated 2 February 2022).
| Vaccine type | Manufacturer | Vaccine Name | Approved |
|---|---|---|---|
| mRNA | Pfizer/BioNTech | BNT162b2 | 131 countries, WHO, EMA, FDA |
| Moderna | mRNA-1273 | 85 countries, WHO, EMA, FDA | |
| Protein Subunit | Novavax | NVX-CoV2373 | 30 countries, WHO, EMA |
| Serum Institute of India | Covovax (Novavax formulation) | 3 countries, WHO | |
| Inactivated virus | Bharat Biotech, India | COVAXIN | 13 countries, WHO |
| SinoPharm (Beijing) | Covilo / BBIBP-CorV | 85 countries, WHO | |
| Sinovac | CoronaVac | 51 countries, WHO | |
| Non replicating viral vector | Janssen (Johnson&Johnson) | Ad26. COV | 100 countries, WHO, EMA, FDA |
| Oxford/AstraZeneca | AZD1222 | 134 countries, WHO, EMA | |
| Serum Institute of India | Covishield (Oxford/AstraZeneca formulation) | 47 countries, WHO |
3.2. Mix and match
Due to the shortage of vaccines because of high demand, questions emerged as to whether the combination of two different COVID-19 vaccines, so called mix and match or heterologous vaccination, could contribute to a stronger immune response, resulting in protection equal or superior to a homologous vaccination based on a double dose of mRNA vaccines. Available data now indicate that two rounds of either homologous or heterologous immunization do protect against severe illness in fully vaccinated individuals [68], [69], [70]. Likewise, in those individuals previously infected with SARS-CoV-2, immunization provides strong protection against disease (up to 90% protection). Because antibody titer and immunity decrease over time, especially in individuals aged 65 years or older, booster vaccine shots are strongly recommended, including the mix and match method. Fully vaccinated status prevents serious COVID-19 illness, hospitalization and death [71], [72]. While the protection level decreases with different viral variants, the approved vaccines still appear to provide sufficient protection. In the US, where the vaccination rate is virtually stagnant at 64%, data on hospitalizations demonstrated that the monthly rate of COVID-19-associated hospitalizations was 46 times higher in unvaccinated adults ages 50–64 years and 52x higher in unvaccinated adults ages 65 years and older ( Fig. 4), compared to fully vaccinated individuals with additional or booster doses in each age group [37]. Each new variant poses distinct challenges; at the time of writing, Pfizer/BioNTech has announced clinical testing of an Omicron-specific mRNA vaccine [73].
Fig. 4.
Rates of COVID-19 associated hospitalizations by vaccination status in adults ages 50–64 years, November-December 2021 [37].
3.3. Post-vaccination complications
Mild side effects, like soreness, redness, rash, and inflammation at the injection site have been reported for all the vaccines and they are related with the introduction of a foreign compound into the body. Other reported side effects, that last only a few days, include headache, fatigue, myalgia and arthralgia [74], [75]. A very rare event is hypersensitivity to one or more ingredients of the vaccines that in even rarer cases can lead to anaphylaxis [76], [77], [78] . A few rare blood clotting syndromes had been reported in small numbers of individuals who had received the AZD1222 – Oxford/AstraZeneca or the Janssen vaccines (Johnson & Johnson). These vaccines were suspended in some countries until their safety profile had been reassessed, finally demonstrating that the rate of blood clotting problems was not higher in vaccinated individuals than the whole population. Currently, both vaccines are approved for use by WHO and EMA [79], [80]. An association between mRNA vaccine and the onset of myocarditis and pericarditis has also been reported; the highest incidence was detected in young male patients, with mild to moderate disease severity and symptoms resolution in most of the cases [81], [82], [83], [84]. There is a multitude of factors that can influence the immune response after vaccination or after exposure to the virus, including the infection history of each individual, the severity of disease, age and others. Nevertheless, vaccination is recommended even after natural infection, since it offers stronger immunological protection [85], [86].
4. Misinformation
One of the most unexpected and bewildering consequences of the SARS-CoV-2 pandemic has been the extreme level of misinformation emanating from the individuals, groups and media. Misleading information, ranging from conspiracy theories that the virus does not exist, to theories that vaccines can alter human DNA have spread almost as rapidly as the virus [87]. WHO proposed the term ‘Infodemic’ to describe the overabundance of information including false or misleading information in digital and physical environments during a disease outbreak [12]. Misinformation has led to mistrust in health authorities that threatens the public health response and has undoubtedly contributed to low vaccination rates in some areas of the world [88].
To address this problem, governments, universities and private organizations have taken measures to disrupt the spread of misinformation. More than 130 UN member states signed a cross regional statement on the COVID-19 ‘infodemic’ and the UN task force initiated the “Verified” campaign to deliver life-saving information on COVID-19 [89], [90]. Additional efforts have included a collaboration between WHO and the UK government in an awareness campaign named “Stop the Spread” to counteract the consequences of false information. Cambridge University partnered with the UK Cabinet Office and developed an online game called “Go Viral!” [12]. European Commission in the Joint Communication to the European Parliament titled ‘Communication on the Global EU response to COVID-19′ highlighted all the EU actions against COVID-19 disinformation and mobilized significant resources to fund projects regarding information veracity [91].
Moreover, leading social media platforms also stepped up to introduce new policies against posting alarmist rhetoric or false narratives. Twitter announced that will block repeat fake news offenders from social media, while Facebook and Instagram launched a fact-checking initiative; by April 2020, Google announced a $6.5 million investment to fight misinformation, with an urgent focus on coronavirus. Many other platforms like TikTok and Snapchat have also introduced new policies to counter the spread of misinformation [92], [93].
4.1. Vaccine hesitancy
The reluctance of a portion of the population to get vaccinated – vaccine hesitancy – was a major health concern long before the COVID-19 pandemic; in the age of COVID-19, vaccine hesitancy has been characterized as “a leading global health threat” by WHO [12]. The basis for these low rates of vaccine acceptance within a specific cultural and/or geographic group is usually multi-factorial, and often linked with misinformation or lack of sufficient information regarding vaccines and vaccination, issues related to vaccine efficacy and safety, distrust in government and health organizations, or the sense that personal freedom supersedes the societal need to respond to a global threat [94], [95], [96].
Promoting vaccination, especially against COVID-19, requires an understanding of the reasons contributing to such hesitancy. Leading drivers of acceptance of an approved COVID-19 vaccine include individual health concerns, as well as trust in the national health authorities and scientists [97]. Vaccine hesitancy remains a particularly intractable problem and further evaluation, education and research on vaccine acceptance needs to be prioritized, together with efforts to maintain trust in health and political authorities.
4.2. Anti-vaxxers
Activists against vaccinations, known as anti-vaxxers, have spread a range of conspiracy theories and fake news about vaccines and vaccination, based on distorted information, prior prejudices, lack of scientific knowledge or understanding, and even religion. These theories include misguided claims about lethal side effects of vaccination, spread of COVID-19 by 5G networks, implantation of ‘microchips’ during vaccination, or vaccines used as tracking devices. Since anti-vaxxer theories lack any scientific support, protests in many places (Los Angeles, New York, London, Paris, Zagreb, Madrid, Canada and several US states) have ended with physical intimidation and violence. In Los Angeles, a man was stabbed and a reporter attacked at a protest against COVID-19 vaccines; in Canada, Prime Minister Trudeau was hit with gravel thrown by an anti-vaccine protester at a campaign event [98]. Similarly, in Germany and other European countries, doctors have experienced increased abuse in clinics, as well as threats of violence by mail. As a consequence, healthcare workers all over the world are addressing the need for more protection against harassment and threats to their unions [95], [99].
4.3. Vaccine mandates
Many countries that struggle with low rates of vaccination or vocal anti-vaccination movements have introduced laws that enforce mandatory vaccination. Italy, Hungary, France, Greece, UK, New Zealand, and other countries announced that all health workers need to be vaccinated, and whoever refuses will be suspended. In countries such as Austria, Greece, Italy and Indonesia, vaccination for older people became mandatory and whoever refuses will receive a monetary fine from the local governments. In Singapore, the government will no longer cover the COVID-19 medical bills of unvaccinated people. The Canadian government announced that unvaccinated workers in the public service and transportation sectors will be placed on administrative leave without pay. In the US vaccination became mandatory for health care workers (to be vaccinated by 4 January 2022), federal government employees (to be vaccinated by 22 November 2021) and military personnel [100], [101]. Fig. 5 depicts the global distribution of vaccinations throughout the world – the total number of people who are completely vaccinated as described in the initial protocols, divided by the total population of the country. A major discrepancy between developed wealthy Western countries and underdeveloped nations becomes clearly evident.
Fig. 5.
Share of the population fully vaccinated against COVID-19, January 30, 2022 [104].
5. The end of the pandemic
Two years into a pandemic that has caused almost 6 million deaths and globally affected all 7.9 billion individuals on earth, we all wonder when this pandemic is going to end? There is cause for optimism. On the one hand, the UN health agency chief said that ‘2022 maybe the year the world ends the acute stage of the COVID-19 pandemic’. Dr. Anthony Fauci, Director of NIAID and Special Advisor to US Pres. Joe Biden, said during the Davos World Economic Forum that ‘since the Omicron variant is highly transmissible, but according to existing data is less likely to cause as severe disease as some previous variants, it could mark a transition from this COVID-19 pandemic chapter to an endemic phase’. On the other hand, the transition from a pandemic to an endemic phase can only be achieved if global vaccination campaigns continue and another variant does not emerge with resistance to current immunization strategies.
Paradoxically, the Omicron wave of December 2021-January 2022 has produced a small sense of optimism that the pandemic end game may be approaching, with the spring-summer of 2022 providing a respite from the worst of the global spread. While Omicron has led to new record-breaking levels of infection, disrupted businesses and societal activities again, and left many health care workers sickened or exhausted, the number of Omicron-related deaths has been smaller than anticipated. The record levels of infection in most countries have likely boosted overall immunity (herd immunity) at the population level [102]. Another point of optimism centers around the fact that new vaccine technologies, particularly the mRNA delivery approaches, can be rapidly incorporated to respond to changes in viral genetics; clinical evaluation of a new Omicron-specific mRNA vaccine has already been announced by Pfizer/BioNTech [73].
Optimism for the future must be tempered however with the realization that a new SARS-CoV-2 variant could appear that evades host immunity to B cell generated antibodies and T cell-directed adaptive responses. Such a scenario would be devastating to an exhausted population, but a sufficiently high level of global immunization could intervene to mute the emergence and the degree of severity of such a novel variant. At the individual level, two-shot immunity has been shown to protect against Omicron to the level of 68%; recent work indicates that a third booster shot provides maximal protection (>90%) against the risk of severe illness [103]; as illustrated by Fig. 4, it is unvaccinated individuals that make up the vast majority of current hospitalizations.
5.1. Vaccine equity – Access to vaccination
Despite the availability of safe and effective vaccines against COVID-19, access to these vaccines remains unequal. High income countries, although representing only 14% of the global population, had purchased 51% of all pre-sold doses by the end of 2020. Some countries bought more vaccine doses than necessary to inoculate 100% of their population. According to the WHO, if the doses were distributed in an equal way, there would be enough vaccine to protect the elderly and health workers worldwide [12].
WHO had set a goal for all countries to vaccinate at least 10% of their populations by September 2021. Altogether, 56 countries - most of them in Africa - were not able to reach this target. Likewise, many countries remain at risk of missing the WHO target of vaccinating 70% of the population of each country by the middle of 2022. In late November 2021 the WHO published that ‘it is vitally important that inequities in access to COVID-19 vaccines are urgently addressed to ensure that vulnerable groups everywhere, including health workers and older persons, receive their first and second doses, alongside equitable access to treatment and diagnostics [12]. Inequalities in vaccine distribution facilitate the emergence of new variants like SARS-CoV-2 Omicron variant’. As noted countless times in the media over the past two years, vaccination remains the one sure way out of this pandemic. Health care workers, activists, politicians and individuals all need to continue to convey this vital information. At the time of writing, some European countries were beginning to loosen or abandon their lockdown restrictions, with many other countries to follow soon. The sense that we are reaching an endpoint in the pandemic is unmistakable, although it remains to be determined if this is the final curtain or simply an intermission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the students of INITIATE, the Marie Curie International Training Network INITIATE for their support during the preparation of this article. This work was supported by the Marie Skłodowska-Curie Actions (MSCA) Innovative Training Networks (ITN): H2020-MSCA-ITN-2019 Grant agreement No 813343.
Biographies

Magdalini Alexandridi is currently a Ph.D. student and Early Stage Researcher (ESR) in the Istituto Pasteur Italia (Rome,Italy) under the supervision of Prof. John Hiscott, funded by the Marie Sklodowska-Curie Actions ITN INITIATE (Innate Immunometabolism as antiviral target). Her research is focused on the effect of virus infection on metabolic pathways and the activation of the oxidative stress response. She earned her B.Sc. in Biochemistry at the University of Thessaly (Greece) and her M.Sc. on Developmental Biology at the International Master Program of the Sorbonne University (Paris, France). She has completed internships at the Institut de Biologie de l′École Normale Supérieure (IBENS, Paris, France), at the Charite Medical University of Berlin (Berlin, Germany) and at the Biomedical Research Foundation of Academy of Athens (BRFAA, Athens, Greece).

Julija Mazej is a Ph.D. student in the Department of Molecular Medicine at the University of Sapienza in Rome, Italy. She works in the laboratory of Prof. Giuseppe Sciumè and Prof. Angela Santoni on the research project centering around innate lymphoid cells and their responses in the context of viral infections. She is also an early-stage researcher in the innovative training network INITIATE (innate-immunometabolism as antiviral target) funded by Marie Skłodowska-Curie Actions.

Enrico Palermo is a post-doctoral fellow at Istituto Pasteur Italia – Fondazione Cenci Bolognetti, in Rome. His expertize covers diagnosis and research in the field of viral infectious diseases. He obtained his Ph.D. from Sapienza University of Rome, working on novel therapeutic approaches to clear latent HIV-1 infection, based on innate immune stimulation and epigenetic reprogramming. He is currently studying molecular processes involved in host-pathogens interactions, with a focus on antiviral innate immunity and mechanisms of viral immune evasion.

John Hiscott, Ph.D. is Professor & Director of the Pasteur Laboratories, Istituto Pasteur-Fondazione Cenci Bolognetti in Rome Italy. Dr. Hiscott’s research is internationally recognized for his contributions to the field of antiviral innate immunity and the host response to emerging viral pathogens such as influenza, dengue and HIV-1. Dr. Hiscott also investigates the use of oncolytic viruses as novel immunotherapeutics for cancer. Dr. Hiscott earned his doctorate in medical sciences from New York University Medical Center and completed post-doctoral training at the Roche Institute in New Jersey, and the University of Zurich in Switzerland. Prior to joining Istituto Pasteur Italia, Dr. Hiscott was Professor & Director of the Molecular Oncology Group at Jewish General Hospital, McGill University in Montreal & Program Director at the Vaccine & Gene Therapy Institute of Florida. Dr. Hiscott has earned numerous awards and distinctions, including the Milstein Award from the International Society of Interferon and Cytokine Research, Scientist and Senior Scientist scholarships from the Canadian Institutes of Health Research, and the Beijerinck Professorship in Virology from Leiden University Medical Center, The Netherlands.
References
- 1.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine and Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine and Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine and Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X., et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine and Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solinas C., Perra L., Aiello M., Migliori E., Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine & Growth Factor Rev. 2020;54:8–23. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aricò E., Bracci L., Castiello L., Gessani S., Belardelli F. Are we fully exploiting type I Interferons in today’s fight against COVID-19 pandemic? Cytokine and Growth Factor Rev. 2020;54:43–50. doi: 10.1016/j.cytogfr.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Asmi F., McManus F.P., Thibault P., Chelbi-Alix M.K. Interferon, restriction factors and SUMO pathways. Cytokine and Growth Factor Rev. 2020;55:37–47. doi: 10.1016/j.cytogfr.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Quarleri J., Delpino M.V. Type I and III IFN-mediated antiviral actions counteracted by SARS-CoV-2 proteins and host inherited factors. Cytokine and Growth Factor Rev. 2021;58:55–65. doi: 10.1016/j.cytogfr.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opdenakker G., van Damme J. Interferons and other cytokines, genetics and beyond in COVID-19 and autoimmunity. Cytokine and Growth Factor Rev. 2021;58:134–140. doi: 10.1016/j.cytogfr.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakhiet M., Taurin S. SARS-CoV-2: targeted managements and vaccine development. Cytokine & Growth Factor Rev. 2021;58:16–29. doi: 10.1016/j.cytogfr.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dariya B., Nagaraju G.P. Understanding novel COVID-19: Its impact on organ failure and risk assessment for diabetic and cancer patients. Cytokine and Growth Factor Rev. 2020;53:43–52. doi: 10.1016/j.cytogfr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(WHO), “World Health Organization.” 〈www.who.int〉 (accessed Feb. 02, 2022).
- 13.Rasmussen A.L. On the origins of SARS-CoV-2. Nat. Med. 2021;27(1):9. doi: 10.1038/s41591-020-01205-5. [DOI] [PubMed] [Google Scholar]
- 14.Holmes E.C., et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184(19):4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H., et al. Identification of novel bat coronaviruses sheds light on the evolutionary origins of SARS-CoV-2 and related viruses. Cell. 2021;184(17):4380–4391. doi: 10.1016/j.cell.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey W.T., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton M.I., MacGowan S.A., Kutuzov M.A., Dushek O., Barton G.J., van der Merwe P.A. Effects of common mutations in the SARS-CoV-2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. eLife. 2021;10 doi: 10.7554/eLife.70658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadfield J., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.“Nextstrain.” 〈https://nextstrain.org〉 (accessed Feb. 02, 2022).
- 23.Callaway E. The mutation that helps Delta spread like wildfire. Nature. 2021;596(7873):472–473. doi: 10.1038/d41586-021-02275-2. [DOI] [PubMed] [Google Scholar]
- 24.Fisman D.N., Tuite A.R. Progressive increase in virulence of novel SARS-CoV-2 variants in Ontario, Canada. medRxiv. 2021 doi: 10.1101/2021.07.05.21260050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlov M. How does Omicron spread so fast? A high viral load isn’t the answer. Nature. 2022 doi: 10.1038/d41586-022-00129-z. [DOI] [PubMed] [Google Scholar]
- 26.Kuzmina A., et al. SARS CoV-2 delta variant exhibits enhanced infectivity and a minor decrease in neutralization sensitivity to convalescent or post-vaccination sera. iScience. 2021;24(12) doi: 10.1016/j.isci.2021.103467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito A., et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 2021;7896:300–306. doi: 10.1038/s41586-021-04266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planas D., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 29.Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600(7887):21. doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- 30.“Lab studies, animal studies, and epidemiological data all indicate that Omicron may cause less severe disease than previous variants,” Harvard Medical School, Web Page, Jan. 06, 2022. 〈https://www.health.harvard.edu/diseases-and-conditions/coronavirus-resource-center〉 (accessed Jan. 31, 2022).
- 31.Papanikolaou V., et al. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene. 2022;814 doi: 10.1016/j.gene.2021.146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahradník J., et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat. Microbiol. 2021;6(9):1188–1198. doi: 10.1038/s41564-021-00954-4. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 34.Nabel K.G., et al. Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain. Science. 2022;375(6578) doi: 10.1126/science.abl6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.S.L. van Elsland and E. Head, Some reduction in hospitalisation for Omicron v Delta in England: early analysis, Imperial College London, website, Dec. 22, 2021. https://www.imperial.ac.uk/news/232882/some-reduction-hospitalisation-omicron-delta-england/ (accessed Jan. 31, 2022).
- 36.Lewnard J.A., Hong V.X., Patel M.M., Kahn R., Lipsitch M., Tartof S.Y. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California. medRxiv. 2022 doi: 10.1101/2022.01.11.22269045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC, Centers for disease control and prevention. 〈https://www.cdc.gov〉 (accessed Feb. 02, 2022).
- 38.FDA, U.S. food & drug administration. 〈https://www.fda.gov〉 (accessed Feb. 02, 2022).
- 39.Ma Q., et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oran D.P., Topol E.J. The proportion of sars-cov-2 infections that are asymptomatic: a systematic review. Ann. Intern. Med. 2021;174:655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N. Engl. J. Med. 2020;383(25):2451–2460. doi: 10.1056/nejmcp2009575. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Leon S., et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1/16144) doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 44.Office for National Statistics, Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK, Online, Apr. 01, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 (accessed Jan. 31, 2022).
- 45.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLOS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sudre C.H., et al. Attributes and predictors of long COVID. Nat. Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid – mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 48.Khunti K., Davies M.J., Kosiborod M.N., Nauck M.A. Long COVID – metabolic risk factors and novel therapeutic management. Nat. Rev. Endocrinol. 2021;17(7):379–380. doi: 10.1038/s41574-021-00495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27(1):28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 50.Meeting the challenge of long COVID., Nat. Med. vol. 26, no. 12, p. 1803, 2020, doi: 10.1038/s41591-020-01177-6. [DOI] [PubMed]
- 51.Moss W.J., Griffin Diane E. Measles. Lancet. 2012:153–164. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- 52.Nossal G.J. The global alliance for vaccines and immunization – a millennial challenge. Nat. Immunol. 2000;1(1):5–8. doi: 10.1038/76852. [DOI] [PubMed] [Google Scholar]
- 53.Yamada A., Sasada T., Noguchi M., Itoh K. Next-generation peptide vaccines for advanced cancer. Cancer Sci. 2013;104(1):15–21. doi: 10.1111/cas.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanekiyo M., Graham B.S. Next-generation influenza vaccines. Cold Spring Harbor Perspect. Med. 2021;11(8) doi: 10.1101/cshperspect.a038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delany I., Rappuoli R., de Gregorio E. Vaccines for the 21st century. EMBO Mol. Med. 2014;6:708–720. doi: 10.1002/emmm.201403876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang S., Lu L., Du L. Development of SARS vaccines and therapeutics is still needed. Fut. Virol. 2013;8(1):1–2. doi: 10.2217/fvl.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao W., et al. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim E., et al. Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32(45):5975–5982. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khehra N., Padda I., Jaferi U., Atwal H., Narain S., Parmar M.S. Tozinameran (BNT162b2) vaccine: the journey from preclinical research to clinical trials and authorization. AAPS PharmSciTech. 2021;22(5):172. doi: 10.1208/s12249-021-02058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livingston E.H., Malani P.N., Creech C.B. The Johnson & Johnson vaccine for COVID-19. JAMA. 2021;325(15):1575. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 62.Pushparajah D., Jimenez S., Wong S., Alattas H., Nafissi N., Slavcev R.A. Advances in gene-based vaccine platforms to address the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021;170:113–141. doi: 10.1016/j.addr.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 64.COVID-19 Vaccine tracker. 〈https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/〉 (accessed Feb. 02, 2022).
- 65.Shrotri M., Swinnen T., Kampmann B., Parker E.P.K. An interactive website tracking COVID-19 vaccine development. Lancet. 2021;9(5):e590–e592. doi: 10.1016/S2214-109X(21)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.European Medicines Agency (EMA), COVID-19 vaccines: key facts,” Online. 〈https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts#vaccine-authorisation-section〉 (accessed Jan. 31, 2022).
- 67.COVID19 Vaccine Tracker, “10 Vaccines Approved for Use by WHO.” 〈https://covid19.trackvaccines.org/agency/who/〉 (accessed Jan. 31, 2022).
- 68.Callaway E. Mix-and-match COVID vaccines ace the effectiveness test. Nature. 2021 doi: 10.1038/d41586-021-02853-4. [DOI] [PubMed] [Google Scholar]
- 69.Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21(3):346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ledford H. Could mixing COVID vaccines boost immune response? Nature. 2021;590(7846):375–376. doi: 10.1038/d41586-021-00315-5. [DOI] [PubMed] [Google Scholar]
- 71.Tenforde M.W., et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. J. Am. Med. Assoc. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallapaty S., Callaway E., Kozlov M., Ledford H., Pickrell J., van Noorden R. How COVID vaccines shaped 2021 in eight powerful charts. Nature. 2021;600:580–583. doi: 10.1038/d41586-021-03686-x. [DOI] [PubMed] [Google Scholar]
- 73.Sheridan C. COVID-19 vaccine makers chase variant-ready vaccines. Nat. Biotechnol. 2022 doi: 10.1038/d41587-022-00001-5. [DOI] [PubMed] [Google Scholar]
- 74.Pormohammad A., et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines. 2021;9(5) doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patone M., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021;27(12):2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Risma K.A., et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J. Allergy Clin. Immunol. 2021;147(6):2075–2082. doi: 10.1016/j.jaci.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Vrieze J. Pfizer’s vaccine raises allergy concerns. Science. 2021;371(6524):10–11. doi: 10.1126/science.371.6524.10. [DOI] [PubMed] [Google Scholar]
- 78.Banerji A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J. Allergy Clin. Immunol. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ledford H. COVID vaccines and blood clots: five key questions. Nature. 2021;592(7855):495–496. doi: 10.1038/d41586-021-00998-w. [DOI] [PubMed] [Google Scholar]
- 81.Oster M.E., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from december 2020 to august 2021. JAMA. 2022;327(4):331. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I. v, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Witberg G., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patone M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2021 doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agrawal B. Heterologous immunity: role in natural and vaccine-induced resistance to infections. Front. Immunol. 2019;10:2631. doi: 10.3389/fimmu.2019.02631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Callaway E. Had COVID? You’ll probably make antibodies for a lifetime. Nature. 2021 doi: 10.1038/d41586-021-01442-9. [DOI] [PubMed] [Google Scholar]
- 87.Lynas M. COVID: Top 10 current conspiracy theories. Alliance Sci. 2020 〈https://allianceforscience.cornell.edu/blog/2020/04/covid-top-10-current-conspiracy-theories/〉 accessed Jan. 31, 2022. [Google Scholar]
- 88.Briand S.C., et al. Infodemics: a new challenge for public health. Cell. 2021;184(25):6010–6014. doi: 10.1016/j.cell.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.(UN), United Nations, Online, May 21, 2020. 〈https://www.un.org/en〉 (accessed Feb. 02, 2022).
- 90.United Nations (UN), Verified Campaign. 〈https://shareverified.com〉 (accessed Jan. 31, 2022).
- 91.European Commission, Fighting disinformation, Online. 〈https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/fighting-disinformation_en〉 (accessed Jan. 31, 2022).
- 92.Donovan J. Social-media companies must flatten the curve of misinformation. Nature. 2020 doi: 10.1038/d41586-020-01107-z. [DOI] [PubMed] [Google Scholar]
- 93.Cinelli M., et al. The COVID-19 social media infodemic. Sci. Rep. 2020;10(1):16598. doi: 10.1038/s41598-020-73510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sturgis P., Brunton-Smith I., Jackson J. Trust in science, social consensus and vaccine confidence. Nat. Hum. Behav. 2021;5(11):1528–1534. doi: 10.1038/s41562-021-01115-7. [DOI] [PubMed] [Google Scholar]
- 95.Thorp H.H. Self-inflicted wounds. Science. 2021;374(6569):793. doi: 10.1126/science.abn1244. [DOI] [PubMed] [Google Scholar]
- 96.Machingaidze S., Wiysonge C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021;27(8):1338–1339. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 97.Lindholt M.F., Jørgensen F., Bor A., Petersen M.B. Public acceptance of COVID-19 vaccines: cross-national evidence on levels and individual-level predictors using observational data. BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2020-048172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.A. Coletta and B. Pietsch, Trudeau, facing ‘anti-vaxxer mobs’ on election trail, is met with flying gravel at campaign stop, Online, The Washington Post. https://www.washingtonpost.com/world/2021/09/06/canada-election-trudeau-vaccines/ (accessed Jan. 31, 2022).
- 99.Hotez P. COVID vaccines: time to confront anti-vax aggression. Nature. 2021;592(7856):661. doi: 10.1038/d41586-021-01084-x. [DOI] [PubMed] [Google Scholar]
- 100.Drew L. The case for mandatory vaccination. Nature. 2019;575(7784):S58–S60. doi: 10.1038/d41586-019-03642-w. [DOI] [PubMed] [Google Scholar]
- 101.Hagan K., Forman R., Mossialos E., Ndebele P., Hyder A.A., Nasir K. COVID-19 vaccine mandate for healthcare workers in the United States: a social justice policy. Exp. Rev. Vaccines. 2022;21(1):37–45. doi: 10.1080/14760584.2022.1999811. [DOI] [PubMed] [Google Scholar]
- 102.Adam D. Will Omicron end the pandemic? Here’s what experts say. Nature. 2022;602(7895):20–21. doi: 10.1038/d41586-022-00210-7. [DOI] [PubMed] [Google Scholar]
- 103.Bar-On Y.M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Our world in data. 〈https://ourworldindata.org〉 (accessed Feb. 02, 2022).