Abstract
Background
Evidence on the association between adverse childhood experiences (ACEs) and handgrip strength (HGS) in later life was limited and inconclusive. We aimed to explore the impact of ACEs on HGS among middle-aged and older Chinese adults.
Methods
We conducted a cross-sectional study with data extracted from the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative survey with respondents recruited from 450 villages/urban communities of 28 provinces. Participants aged 45 years or older were drawn from the CHARLS 2014 life history survey and the 2015 health survey. Twelve ACE indicators before the age of 17 years were collected. HGS was measured with a dynamometer and the maximum value of HGS obtained from both hands was used in the analyses. Low muscle strength (LMS) was defined according to the recommendation of European Working Group on Sarcopenia in Older People (EWGSOP). Multivariate linear and logistic regression models were constructed to evaluate the association of ACEs with continuous HGS and LMS, with adjustment for age, sex, marital status, ethnicity, area of residence, smoking and drinking status, body mass index, hypertension, dyslipidaemia, diabetes mellitus, cardiovascular disease, arthritis, hip fracture, and memory-related disease.
Results
Of the 7209 eligible participants, 2258 (31.3%) had experienced three or more ACEs. Compared to individuals without ACEs, exposure to ≥ 3 ACEs was negatively associated with continuous HGS in kilogram (β = -0.93, 95% CI: -1.37, -0.49) and positively associated with the risk of LMS (OR = 1.34, 95% CI: 1.12, 1.61). Such associations were consistently found both in men and women who had experienced three or more ACEs. Significant dose–response relationship between the number of ACEs and outcomes was also observed in the overall population and different sex groups.
Conclusion
Exposure to ACEs was associated with lower HGS and increased risk of LMS among middle-aged and older Chinese adults, indicating the importance of intervention in individuals with experience of ACEs in order to mitigate its detrimental impact on HGS and promote healthy ageing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-02796-z.
Keywords: Adverse childhood experiences, Handgrip strength, Middle-aged and older adults, Chinese
Introduction
As a reliable indicator to reflect muscle strength and muscle mass, handgrip strength (HGS) has been widely used to diagnose sarcopenia, a condition characterized with gradual loss of muscle mass and strength [1]. Participants with low muscle strength (LMS) were at higher risk of developing a range of adverse health outcomes, including cognitive impairment, mobility decline, cardiovascular disease, and even mortality [2–5]. Ageing is one of the major risk factors for reduced HGS, with higher prevalence of LMS in older populations [6]. Data from the Korean Longitudinal Study of Aging (KLoSA) demonstrated that the prevalence of LMS in people aged below 65 years was 13.0%, while this figure went up to 67.5% in those aged ≥ 65 years [7]. In addition, low socio-economic status, physical inactivity, malnutrition, and morbidity have also been demonstrated to be risk factors of LMS in later life [8–11].
Adverse childhood experiences (ACEs) are defined as exposure to a string of potentially stressful events during childhood [12]. It is usually comprised of emotional and physical neglect and abuse, household challenges, and other stressful experiences, such as economic hardship, community violence, unsafe neighbourhood, and bullying [12, 13]. Exposure to ACEs has been demonstrated to be a risk factor of adverse health conditions in later life, such as ischemic heart disease, cancer, and chronic lung disease [12, 14]. There is limited research that has also investigated the link between ACEs and LMS in later life, however, with mixed results [8, 15–17]. For example, a cross-sectional study of 24,179 adults aged between 50–96 years from the Survey of Health Ageing and Retirement in Europe (SHARE) found that disadvantaged socioeconomic circumstances in early life were associated with lower muscle strength in later life, especially in women [8]. Data from the same survey further confirmed that compared to women without any ACE exposure, those exposed to two or more ACEs had significantly increased risk of muscle weakness, while such association was not observed in men [15]. On the contrary, the Health and Retirement Study (HRS) showed that experience of childhood misfortune predicted steeper declines in HGS only in men, but not in women [16]. Furthermore, a cross-sectional study of 3732 Chinese adults aged ≥ 65 years even showed that exposure to famine during childhood was not significantly associated with HGS in later life [17]. These limited and inconsistent findings suggested the need for further studies.
In this study, we aimed to assess the association of ACEs with continuous HGS and LMS among middle-aged and older adults using data from the China Health and Retirement Longitudinal Study (CHARLS). Subgroup analysis was also conducted to evaluate the sex-specific associations.
Methods
Study design and population
This cross-sectional study used data from the CHARLS, a nationally representative survey of people aged 45 years or older [18]. The baseline survey was conducted from June 2011 to March 2012. A multistage, probability-proportional-to-size (PPS) sampling strategy was used to recruit eligible participants. At first, 150 county-level units were randomly chosen from 28 provinces across China stratified by region, rural/urban residence, and per capita statistics on gross domestic product (GDP). Three neighbourhood communities or administrative villages were then randomly chosen from each county-level unit in urban or rural areas, respectively. In the next step, random sampling was applied to select households from the neighbourhood communities or administrative villages. Collective dwellings, such as military bases, schools, dormitories, nursing homes, and domestic helpers living in the homes of employers were excluded. Then, within each selected household, one person aged 45 years or above was assigned as the main respondent, and her or his spouse was interviewed as well [18]. Participants were followed-up every two years with a small share of new respondents recruited each time. Up to now, three follow-up surveys were conducted in 2013, 2015, and 2018, respectively. Information on life history were additionally collected in 2014 among all live respondents in the first two surveys (i.e., the 2011 and 2013 surveys). In the current study, we used data from the 2014 and 2015 CHARLS surveys, as the most updated data on HGS were collected in 2015.
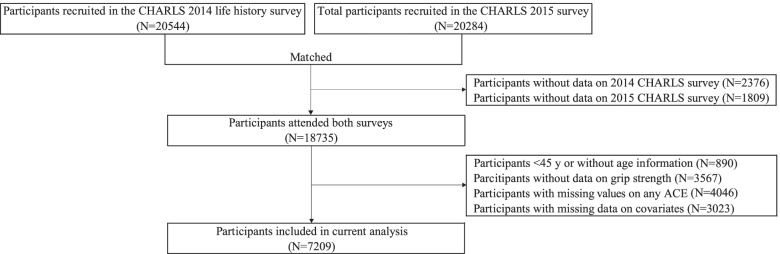
A total of 20,544 and 20,284 individuals were recruited in the CHARLS 2014 and 2015 surveys, respectively (Fig. 1). We identified 18,735 respondents who had attended both surveys. Participants aged < 45 years or without age information (N = 890), without data on HGS (N = 3567), ACE indicators (N = 4046) or covariates (N = 3023) were excluded. Finally, 7209 eligible participants aged between 45 and 95 years were included for further analysis on the association between ACEs and HGS.
Fig. 1.
Study flowchart of participant selection
Definition of adverse childhood experiences
All ACE indicators were assessed by dichotomous or multiple-choice questions in the CHARLS life history survey (see Supplementary Table 1). Based on previous literatures [13, 19, 20], a total of 12 ACEs before the age of 17 years old were considered in our study, i.e., physical abuse, emotional neglect, household substance abuse, household mental illness, domestic violence, incarcerated household member, parental separation or divorce, unsafe neighbourhood, bullying, parental death, sibling death, and parental disability.
In line with previous studies, participants were further categorized into four groups based on the total number of ACEs they had ever experienced (i.e., 0, 1, 2, and ≥ 3 ACEs) [21, 22].
Measurement of handgrip strength
HGS was measured by a standardized dynamometer (Yuejian WL-1000, China) in kilograms [23]. Participants were asked to stay in a standing position, hold the dynamometer at a right angle (90°), and squeeze the handle with their maximum effort for a few seconds [23]. Each hand was measured twice and the largest HGS reading obtained from both hands was used in the analysis [24].
We adopted the recommendation of European Working Group on Sarcopenia in Older People (EWGSOP) to define LMS based on sex and body mass index (BMI), which was commonly used in research literatures [15, 25, 26]. In detail, participants were first stratified based on quartiles of BMI in men and women respectively. Then, the lowest 20% of HGS in each stratum were established as the cut-off values for LMS. In our study population, the cut-off points for LMS in men were 28.0, 30.8, 32.0, and 34.0 kg for those with a BMI ≤ 20.8, 20.9–23.1, 23.2–25.7, and > 25.7 kg/m2, respectively. The cut-off points for LMS in women were 18.0, 20.5, 21.0, and 21.0 kg for those with a BMI ≤ 21.8, 21.9–24.2, 24.3–26.6, and > 26.6 kg/m2, respectively.
Covariates
Demographic and lifestyle characteristics were collected through face-to-face interviews, including age, sex (men and women), marital status (married/cohabitated and unmarried), ethnicity (Han ethnicity and ethnic minority population), area of residence (rural and urban), smoking (never smoker, ever smoker, and current smoker) and drinking status (never drinker, ever drinker, and current drinker).
BMI was calculated as weight (kg) divided by the square of height (m2). Blood pressure was measured three times using an Omron™ HEM-7200 sphygmomanometer [18]. Physician-diagnosed chronic diseases, including hypertension, dyslipidaemia, diabetes mellitus (DM), cardiovascular disease (CVD, including chronic heart disease and stroke), arthritis (including rheumatism), hip fracture, and memory-related disease (including Alzheimer’s disease, Parkinson’s disease, and cerebral atrophy) were self-reported by each participant. Hypertension was confirmed if the participant had physician-diagnosed hypertension, and/or with a mean systolic blood pressure (BP) ≥ 140 mmHg, and/or mean diastolic BP ≥ 90 mmHg, and/or on anti-hypertensive drugs [27]. According to the American Diabetes Association criteria, a participant was defined as having DM if he/she had fasting plasma glucose ≥ 7.0 mmol/L, and/or random plasma glucose ≥ 11.1 mmol/L, and/or glycated hemoglobin (HbA1c) ≥ 6.5%, and/or self-reported DM diagnosed by a physician, and/or on glucose-lowering drugs or insulin treatment [28, 29].
Statistical analysis
The normality of continuous variable was evaluated by Kolmogorov–Smirnov test combined with Q-Q plot. Comparison of characteristics according to the four categories of ACEs was evaluated by one-way analysis of variance (ANOVA) and Chi-square tests for continuous and categorical variables, respectively. To assess the trend of characteristics across different ACEs groups, ANOVA trend analyses using polynomial contrasts were conducted for continuous data and Mantel–Haenszel test of trend was performed for categorical data.
Multivariate linear and logistic regression models were used to assess the impact of ACEs on continuous HGS (kg) and LMS, respectively. Model 1 was adjusted for age and sex. Model 2 was additionally controlled for marital status, ethnicity, and area of residence. To determine the mediating role of health behaviours and chronic conditions, we further included smoking and drinking status, BMI, hypertension, dyslipidaemia, DM, CVD, arthritis, hip fracture, and memory-related disease in Model 3. Assumptions of linear regression models, including linearity, normality, homoscedasticity and absence of multicollinearity, were checked for all linear regression models. To graphically depict the predicted odds of LMS at different age in the four ACEs categories, we constructed quadratic fitting curves in men and women, respectively, with adjustment for covariates listed in Model 3 [30, 31]. Subgroup analysis was conducted by sex, as sex-specific associations between ACEs and HGS have been reported previously [15]. In consideration of potential survivorship bias, we conducted sensitivity analysis by re-evaluating such association among participants aged < 70 years and those aged ≥ 70 years, respectively.
All analyses were performed using STATA 15.0 (StataCorp. College Station, TX, USA). All tests were two-sided and a p-value of less than 0.05 was considered to be statistically significant.
Results
Of the 7209 participants included in our analysis, 19.8% experienced no ACE and 31.3% reported the experience of three or more ACEs (Table 1). More than half (52.9%) of the participants were women and the mean age was 60.8 (SD: 8.9) years. Compared to participants without ACE exposure, those with experience of three or more ACEs were statistically older, more likely to be men, rural residents, current smokers and drinkers. They also had significantly higher prevalence of CVD, arthritis, and memory-related disease. With increase in the number of ACEs, the mean HGS decreased from 38.6 (SD: 7.7) kg to 36.5 (SD: 8.4) kg in men and from 26.2 (SD: 6.3) kg to 24.5 (SD: 6.6) kg in women. In contrast, the prevalence of LMS increased in both sexes with the number of ACEs increased.
Table 1.
Characteristics of participants by number of ACEs
| Characteristicsa | Total | No. of ACEs | p for difference | p for trend | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥ 3 | ||||
| n | 7209 | 1424 (19.8%) | 1950 (27.0%) | 1577 (21.9%) | 2258 (31.3%) | ||
| Demographic and lifestyle factors | |||||||
| Mean age (years) | 60.8 (8.9) | 60.0 (8.6) | 60.4 (8.9) | 60.6 (8.8) | 61.8 (9.1) | < 0.001 | < 0.001 |
| Sex, n (%) | < 0.001 | < 0.001 | |||||
| Men | 3396 (47.1%) | 580 (40.7%) | 883 (45.3%) | 772 (49.0%) | 1161 (51.4%) | ||
| Women | 3813 (52.9%) | 844 (59.3%) | 1067 (54.7%) | 805 (51.0%) | 1097 (48.6%) | ||
| Marital status, n (%) | 0.041 | 0.015 | |||||
| Married or cohabitated | 6353 (88.1%) | 1279 (89.8%) | 1715 (87.9%) | 1399 (88.7%) | 1960 (86.8%) | ||
| Unmarried | 856 (11.9%) | 145 (10.2%) | 235 (12.1%) | 178 (11.3%) | 298 (13.2%) | ||
| Ethnicity, n (%) | 0.918 | 0.499 | |||||
| Han ethnicity | 6683 (92.7%) | 1316 (92.4%) | 1806 (92.6%) | 1461 (92.6%) | 2100 (93.0%) | ||
| Ethnic minority population | 526 (7.3%) | 108 (7.6%) | 144 (7.4%) | 116 (7.4%) | 158 (7.0%) | ||
| Area of residence, n (%) | 0.004 | 0.001 | |||||
| Rural | 4660 (64.6%) | 897 (63.0%) | 1224 (62.8%) | 1013 (64.2%) | 1526 (67.6%) | ||
| Urban | 2549 (35.4%) | 527 (37.0%) | 726 (37.2%) | 564 (35.8%) | 732 (32.4%) | ||
| Smoking status, n (%) | < 0.001 | < 0.001 | |||||
| Never smoker | 4110 (57.0%) | 906 (63.6%) | 1146 (58.8%) | 854 (54.2%) | 1204 (53.3%) | ||
| Ever smoker | 999 (13.9%) | 172 (12.1%) | 260 (13.3%) | 239 (15.2%) | 328 (14.5%) | ||
| Current smoker | 2100 (29.1%) | 346 (24.3%) | 544 (27.9%) | 484 (30.7%) | 726 (32.2%) | ||
| Drinking status, n (%) | < 0.001 | < 0.001 | |||||
| Never drinker | 3884 (53.9%) | 880 (61.8%) | 1100 (56.4%) | 788 (50.0%) | 1116 (49.4%) | ||
| Ever drinker | 809 (11.2%) | 125 (8.8%) | 211 (10.8%) | 180 (11.4%) | 293 (13.0%) | ||
| Current drinker | 2516 (34.9%) | 419 (29.4%) | 639 (32.8%) | 609 (38.6%) | 849 (37.6%) | ||
| Health assessment | |||||||
| BMI (kg/m2) | 23.9 (3.8) | 24.2 (3.8) | 24.0 (3.7) | 24.1 (3.8) | 23.6 (3.8) | < 0.001 | < 0.001 |
| Systolic BP (mmHg) | 128.1 (19.9) | 127.7 (19.7) | 128.5 (19.4) | 129.2 (20.6) | 127.4 (20.1) | 0.037 | 0.520 |
| Diastolic BP (mmHg) | 75.6 (11.8) | 75.5 (11.9) | 75.5 (11.4) | 76.5 (12.0) | 74.9 (11.9) | < 0.001 | 0.289 |
| History of chronic diseases, n (%) | |||||||
| Hypertension | 3109 (43.1%) | 598 (42.0%) | 832 (42.7%) | 698 (44.3%) | 981 (43.4%) | 0.608 | 0.303 |
| Dyslipidaemia | 1172 (16.3%) | 217 (15.2%) | 298 (15.3%) | 270 (17.1%) | 387 (17.1%) | 0.203 | 0.052 |
| DM | 1265 (17.5%) | 248 (17.4%) | 320 (16.4%) | 280 (17.8%) | 417 (18.5%) | 0.372 | 0.196 |
| CVD | 1314 (18.2%) | 252 (17.7%) | 318 (16.3%) | 279 (17.7%) | 465 (20.6%) | 0.003 | 0.004 |
| Arthritis | 2704 (37.5%) | 425 (29.8%) | 634 (32.5%) | 612 (38.8%) | 1033 (45.7%) | < 0.001 | < 0.001 |
| Hip fracture | 149 (2.1%) | 25 (1.8%) | 40 (2.1%) | 26 (1.6%) | 58 (2.6%) | 0.183 | 0.129 |
| Memory-related disease | 168 (2.3%) | 29 (2.0%) | 34 (1.7%) | 37 (2.3%) | 68 (3.0%) | 0.044 | 0.014 |
| HGS (kg) | |||||||
| Men | 37.7 (8.5) | 38.6 (7.7) | 38.4 (8.9) | 38.1 (8.5) | 36.5 (8.4) | < 0.001 | < 0.001 |
| Women | 25.5 (6.5) | 26.2 (6.3) | 25.8 (6.5) | 25.7 (6.5) | 24.5 (6.6) | < 0.001 | < 0.001 |
| LMS, n (%) | |||||||
| Men | 695 (20.5%) | 94 (16.2%) | 168 (19.0%) | 145 (18.8%) | 288 (24.8%) | < 0.001 | < 0.001 |
| Women | 788 (20.7%) | 150 (17.8%) | 205 (19.2%) | 158 (19.6%) | 275 (25.1%) | < 0.001 | < 0.001 |
ACE adverse childhood experience, BMI body mass index, BP blood pressure, CVD cardiovascular disease, DM diabetes mellitus, HGS handgrip strength, LMS low muscle strength
aContinuous data were reported as mean (SD) and categorical data were reported as number (percentage)
The associations between ACEs with continuous HGS were presented in Table 2. In the overall population, compared to participants with no ACE exposure, those experienced three or more ACEs had significantly lower HGS in Model 1 (β = -1.26, 95% CI: -1.71, -0.81). We also observed a significantly decreasing trend in HGS as the number of ACEs increased (p value for trend < 0.001). Similar associations were found in the adjusted Model 2. After further adjustment for health behaviours and chronic diseases in Model 3, the effect size of the association between ACEs and HGS was attenuated but still significant among adults with exposure to three or more ACEs (β = -0.93, 95% CI: -1.37, -0.49). Similar findings were also observed for the ACE ≥ 3 group in both men (Model 3, β = -1.04, 95% CI: -1.75, -0.33; p value for trend = 0.001) and women (Model 3, β = -0.93, 95% CI: -1.46, -0.39; p value for trend = 0.001).
Table 2.
Association between ACEs and continuous HGS (kg) in the overall population and different sex groups in linear regression models
|
Overalld N = 7209 |
Mend N = 3396 |
Womend N = 3813 |
|
|---|---|---|---|
| Model 1a | β (95% CI) | ||
| ACEs | |||
| 0 | ref | ref | ref |
| 1 | -0.16 (-0.62, 0.30) | -0.19 (-0.97, 0.58) | -0.22 (-0.76, 0.32) |
| 2 | -0.34 (-0.82, 0.14) | -0.39 (-1.18, 0.40) | -0.36 (-0.94, 0.21) |
| ≥ 3 | -1.26 (-1.71, -0.81) | -1.39 (-2.12, -0.65) | -1.17 (-1.71, -0.64) |
| p for trend | < 0.001 | < 0.001 | < 0.001 |
| Model 2b | |||
| ACEs | |||
| 0 | ref | ref | ref |
| 1 | -0.16 (-0.61, 0.30) | -0.20 (-0.96, 0.56) | -0.21 (-0.75, 0.32) |
| 2 | -0.32 (-0.80, 0.16) | -0.39 (-1.17, 0.39) | -0.35 (-0.93, 0.22) |
| ≥ 3 | -1.18 (-1.62, -0.73) | -1.33 (-2.06, -0.61) | -1.11 (-1.65, -0.57) |
| p for trend | < 0.001 | < 0.001 | < 0.001 |
| Model 3c | |||
| ACEs | |||
| 0 | ref | ref | ref |
| 1 | -0.12 (-0.57, 0.32) | -0.22 (-0.96, 0.51) | -0.18 (-0.71, 0.35) |
| 2 | -0.28 (-0.75, 0.19) | -0.40 (-1.16, 0.36) | -0.29 (-0.87, 0.28) |
| ≥ 3 | -0.93 (-1.37, -0.49) | -1.04 (-1.75, -0.33) | -0.93 (-1.46, -0.39) |
| p for trend | < 0.001 | 0.001 | 0.001 |
ACE adverse childhood experience, CI confidence interval, HGS handgrip strength
aModel 1: Adjusted for age and sex in the overall population, and adjusted for age in different sex groups
bModle 2: Additionally adjusted for marital status, ethnicity, and area of residence
cModel 3: Additionally adjusted for smoking and drinking status, BMI, hypertension, dyslipidaemia, DM, CVD, arthritis, hip fracture, and memory-related disease
dIn the overall population, the average HGS were 31.2, 31.5, 31.7, and 30.7 kg in the groups with 0, 1, 2, and ≥ 3 ACEs. In men, the average HGS were 38.6, 38.4, 38.1, and 36.5 kg in the groups with 0, 1, 2, and ≥ 3 ACEs. In women, the average HGS were 26.2, 25.8, 25.7, and 24.5 kg in the groups with 0, 1, 2, and ≥ 3 ACEs
We further assessed the associations between ACEs and the prevalence of LMS in the overall population and by sex (Table 3). Compared to participants without any exposure to ACE, those with three or more ACEs had significantly increased risk of LMS in all models (OR = 1.44, 95% CI: 1.21, 1.72 in Model 1, OR = 1.41, 95% CI: 1.18, 1.68 in Model 2, and OR = 1.34, 95% CI: 1.12, 1.61 in Model 3). However, exposure to one or two ACEs was not significantly associated with the prevalence of LMS in any of the models. Additionally, compared to the reference group, both men and women with three or more ACE exposures had increased likelihood of having LMS even in the fully adjusted model (Model 3, OR = 1.48, 95% CI: 1.11, 1.96 in men, and OR = 1.30, 95% CI: 1.02, 1.64 in women).
Table 3.
Association between ACEs and LMS in the overall population and different sex groups in logistic regression models
|
Overalld N = 7209 |
Mend N = 3396 |
Womend N = 3813 |
|
|---|---|---|---|
| Model 1a | OR (95% CI) | ||
| ACEs | |||
| 0 | ref | ref | ref |
| 1 | 1.11 (0.92, 1.34) | 1.24 (0.93, 1.67) | 1.04 (0.81, 1.32) |
| 2 | 1.11 (0.91, 1.35) | 1.18 (0.87, 1.60) | 1.07 (0.83, 1.39) |
| ≥ 3 | 1.44 (1.21, 1.72) | 1.56 (1.18, 2.05) | 1.37 (1.09, 1.73) |
| p for trend | < 0.001 | 0.002 | 0.004 |
| Model 2b | |||
| ACEs | |||
| 0 | ref | ref | ref |
| 1 | 1.10 (0.91, 1.33) | 1.24 (0.92, 1.67) | 1.03 (0.81, 1.32) |
| 2 | 1.10 (0.90, 1.34) | 1.18 (0.87, 1.61) | 1.07 (0.83, 1.38) |
| ≥ 3 | 1.41 (1.18, 1.68) | 1.54 (1.17, 2.03) | 1.35 (1.07, 1.70) |
| p for trend | < 0.001 | 0.002 | 0.007 |
| Model 3c | |||
| ACEs | |||
| 0 | ref | ref | ref |
| 1 | 1.10 (0.91, 1.33) | 1.27 (0.94, 1.72) | 1.03 (0.80, 1.31) |
| 2 | 1.08 (0.89, 1.32) | 1.17 (0.86, 1.60) | 1.05 (0.81, 1.36) |
| ≥ 3 | 1.34 (1.12, 1.61) | 1.48 (1.11, 1.96) | 1.30 (1.02, 1.64) |
| p for trend | 0.001 | 0.011 | 0.023 |
ACE adverse childhood experience, CI confidence interval, LMS low muscle strength, OR odds ratio
aModel 1: Adjusted for age and sex in the overall population, and adjusted for age in different sex groups
bModel 2: Additionally adjusted for marital status, ethnicity, and area of residence
cModel 3: Additionally adjusted for smoking and drinking status, BMI, hypertension, dyslipidaemia, DM, CVD, arthritis, hip fracture, and memory-related disease
dIn the overall population, the prevalence of LMS was 17.1%, 19.1%, 19.2%, and 24.9% in the groups with 0, 1, 2, and ≥ 3 ACEs. In men, the prevalence of LMS was 16.2%, 19.0%, 18.8%, and 24.8% in the groups with 0, 1, 2, and ≥ 3 ACEs. In women, the prevalence of LMS was 17.8%, 19.2%, 19.6%, and 25.1% in the groups with 0, 1, 2, and ≥ 3 ACEs
Figure 2 presented the sex-specific predicted probability of LMS at different age in four levels of ACEs. The predicted probability of LMS increased with age in both sexes. Compared to those reported no experience of ACEs, the odds of LMS increased in those with ACE exposures and was the highest in the group with exposure to three or more ACEs.
Fig. 2.
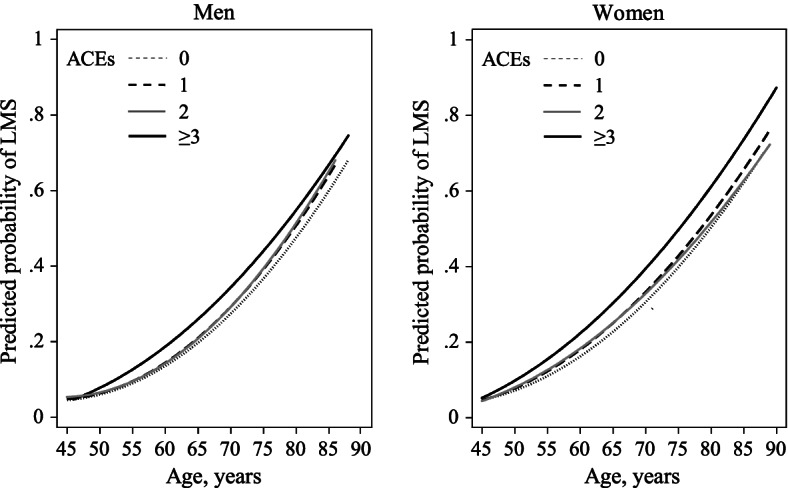
Predicted probability of LMS by number of ACEs and sex. ACE, adverse childhood experience; LMS, low muscle strength
In sensitivity analysis, the impact of ACEs on HGS remained evident among those aged < 70 years, whereas it was attenuated to statistically insignificant among participants aged ≥ 70 years (Supplementary Table 2 and 3).
Discussion
In this cross-sectional study, we found that exposure to three or more ACEs was associated with lower HGS and increased risk of LMS among middle-aged and older adults. The associations were statistically significant in both men and women. In addition, there was a significant dose–response relationship between the number of ACEs and risk of LMS. We further observed that the risk of LMS increased with age, in both sex groups.
The associations of ACEs with HGS have not been well documented before [8, 15–17]. One comparable cross-sectional analysis of the SHARE study was conducted among participants aged between 50–96 years from 14 European countries [15]. It suggested that exposure to two or more ACEs was positively associated with the risk of muscle weakness in women, but not in men [15]. Similar findings were also reported in the association between disadvantaged early-life socioeconomic circumstances and later-life muscle strength from the same study [8]. In contrast, a study conducted in the United States found that childhood misfortune was linked to lower HGS in both sexes in the cross-sectional settings, while the longitudinal analysis revealed that childhood misfortune was associated with steeper declines in HGS only in men, but not in women [16]. A Chinese study even showed that famine during childhood was not significantly associated with lower HGS among older people ≥ 65 years [17].
The inconsistent findings across different studies could be explained by discrepancies in ACE definitions. Our study covered a relatively wider range of adverse events during childhood and further categorized the 12 ACEs into one of four cumulative-score groups, while other studies captured different domains of childhood adversities, such as childhood disease and impairment [16], or only evaluated the impact of a single ACE indicator on HGS [8, 17]. Nevertheless, measuring a single ACE component might overestimate its impact on HGS [16], as previous evidence suggested that ACE indicators were interrelated and usually happened in clusters [32, 33]. Therefore, evaluating associations between the cumulative number of ACEs experienced and HGS might be more clinically significant. Furthermore, HGS measurement was also diverse across different studies. Our study used the maximum value of HGS obtained from two hands in the analyses based on a well-established protocol for measuring HGS in large epidemiological studies [24], while some studies used the mean value of HGS collected from both hands [16, 17]. Another explanation for the discrepancies across studies might be the different socio-cultural backgrounds, as individual’s perception and reaction of ACEs could be distinct in different cultures [34]. Future studies could further compare the impact of ACEs on health outcomes across different cultures. Therefore, it was reasonable for the inconsistent findings in different studies.
The exact mechanisms underlying the association between ACE exposure and low HGS are so far not fully understood. Individuals with exposure to ACEs had chronic stress and elevated allostatic load [35–37], which could further lead to increased inflammation levels and dysfunction in the immune system [35, 38], and subsequently cause damages in skeletal muscle and lower HGS [39]. Previous evidence has also suggested a positive association between ACE exposures and telomere length shortening [40], which could lead to cellular dysfunction in tissues and organs, and ultimately result in declines in HGS [41]. Furthermore, stress caused by ACEs might impair the development of brain and nervous system, which could further lead to problems in coping, planning, learning, and self-management [36, 42, 43]. This provided some explanation for the associations between ACEs and several behavioural problems in adulthood, such as smoking, drinking, physical inactivity, and sleep disorders [12, 44–46]. These behavioural problems could consequently deteriorate musculoskeletal health and cause LMS [47–49]. In our study, we also observed a positive trend between the number of ACEs experienced and the prevalence of smoking and drinking. However, after further adjustment for the lifestyle factors and chronic diseases, the significant association between ACEs and HGS was still present, suggesting that ACEs was an independent risk factor for declines in HGS.
When explored such association by age, the deleterious impact of ACEs on HGS among participants aged ≥ 70 years was attenuated to statistical insignificance. This might be explained by the potential survivorship bias, as the vulnerable population exposed to greater ACEs might have died earlier than those without ACE exposure. Therefore, individuals aged ≥ 70 years in our study could be derived from the most resilient survivors who were less likely to be affected by ACEs. Nevertheless, future longitudinal studies could reduce such bias by addressing censored data appropriately.
To our best knowledge, this was among the first studies to evaluate the association between the cumulative adversities during childhood and HGS in later life among middle-aged and older Chinese. We not only assessed the impact of ACEs on continuous HGS, but also defined LMS based on age- and BMI-specific cut-off points according to well-accepted recommendations [25]. We have also included different domains of ACEs, which all have been proven to be significant risk factors for several health outcomes [12, 20]. However, our study has some limitations. First, information on ACEs was retrospectively collected through self-report, which was subjective to recall bias. Previous literatures have indicated that prospective and retrospective measures of ACEs could identify different groups of individuals [50, 51]. In addition, retrospective measures might underestimate the impact of ACEs on objectively evaluated health outcomes, like HGS [51]. Nevertheless, retrospective reports of major and easily defined ACEs are considered to have acceptable psychometric properties, and might provide some unique and complementary information compared to prospective measurement of ACEs [51, 52]. Therefore, retrospective studies on ACEs still have a worthwhile place in epidemiological research and clinical practice [50, 52]. Second, although we have adjusted for several confounders in the multivariate analyses, some reported risk factors of LMS, such as physical activity and diet [53, 54], were not included due to data unavailability. Third, resilience was found to be an effect modifier in the association between ACEs and multiple health outcomes in adulthood [55, 56]. Nonetheless, we are unable to explore the modifying role of resilience, since no relevant information was collected in CHARLS. Future studies should confirm the possible role of resilience in reducing the burden of LMS among individuals with exposure to ACEs.
Conclusion
This study demonstrated the significant associations of exposure to ACEs with lower HGS and increased likelihood of LMS among middle-aged and older Chinese adults, without sex differences. Significant dose–response relationship was observed between the number of ACE exposures and the outcomes. Our findings add fuel to the ongoing research about the lifelong adverse effects of ACEs on health outcomes. It also emphasized the importance of intervening individuals with ACEs, in order to mitigate its detrimental impact on HGS and promote healthy ageing. Nonetheless, future longitudinal studies and biomolecular research are required to confirm such associations and underlying mechanisms.
Supplementary Information
Additional file 1: Table 1. Definition of adverse childhood experiences. Table 2. Association between ACEs and continuous HGS (kg) by age in the overall population and different sex groups in linear regression models. Table 3. Association between ACEs and LMS by age in the overall population and different sex groups in logistic regression models.
Acknowledgements
We thank the China Health and Retirement Longitudinal Study team for providing data and training in using the datasets.
Authors’ contributions
Conceptualization, L.L. and V.Y.G.; data curation, L.L. and W.S.; formal analysis, L.L. and W.S.; funding acquisition, V.Y.G.; methodology, V.Y.G., C.L. and W.C.; supervision, C.L. and V.Y.G.; writing—original draft, L.L. and W.S.; writing—review and editing, C.L., W.C. and V.Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the start‐up fund from the Sun Yat-sen University [grant number 51000–18841211]; and Guangdong Basic and Applied Basic Research Foundation [grant number 2019A1515110571]. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Availability of data and materials
The data that support the findings of this study are available on the website of the China Health and Retirement Longitudinal Study (CHARLS) at http://charls.pku.edu.cn/index/en.html. To access and use this survey data for research purpose, an approval should be obtained from the CHARLS team at Peking University.
Declarations
Ethics approval and consent to participates
Ethical approval for this study was granted from the Institutional Review Board at Peking University (IRB approval number: IRB00001052-11015 and IRB00001052-11014. Each respondent who agreed to participate in the survey has signed a written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67(1):66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae EJ, Park NJ, Sohn HS, and Kim YH, Handgrip Strength and All-Cause Mortality in Middle-Aged and Older Koreans. Int J Environ Res Public Health, 2019. 16(5). 10.3390/ijerph16050740. [DOI] [PMC free article] [PubMed]
- 4.McGrath R, Vincent BM, Hackney KJ, Robinson-Lane SG, Downer B, Clark BC. The Longitudinal Associations of Handgrip Strength and Cognitive Function in Aging Americans. J Am Med Dir Assoc. 2020;21(5):634–639.e1. doi: 10.1016/j.jamda.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/s0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 6.Ramírez-Vélez R, Correa-Bautista JE, García-Hermoso A, Cano CA, Izquierdo M. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J Cachexia Sarcopenia Muscle. 2019;10(2):278–286. doi: 10.1002/jcsm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang SK, Kim JH, Lee Y. Effect of relative handgrip strength on cardiovascular disease among Korean adults aged 45 years and older: Results from the Korean Longitudinal Study of Aging (2006–2016) Arch Gerontol Geriatr. 2020;86:103937. doi: 10.1016/j.archger.2019.103937. [DOI] [PubMed] [Google Scholar]
- 8.Cheval B, Boisgontier MP, Orsholits D, Sieber S, Guessous I, Gabriel R, et al. Association of early- and adult-life socioeconomic circumstances with muscle strength in older age. Age Ageing. 2018;47(3):398–407. doi: 10.1093/ageing/afy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lino VT, Rodrigues NC, O’Dwyer G, Andrade MK, Mattos IE, Portela MC. Handgrip Strength and Factors Associated in Poor Elderly Assisted at a Primary Care Unit in Rio de Janeiro, Brazil. PLoS ONE. 2016;11(11): e0166373. 10.1371/journal.pone.0166373. [DOI] [PMC free article] [PubMed]
- 10.de Lima TR, Silva DAS, de Castro JAC, Christofaro DGD. Handgrip strength and associated sociodemographic and lifestyle factors: A systematic review of the adult population. J Bodyw Mov Ther. 2017;21(2):401–413. doi: 10.1016/j.jbmt.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Riviati N, Setiati S, Laksmi PW, Abdullah M. Factors Related with Handgrip Strength in Elderly Patients. Acta Med Indones. 2017;49(3):215–219. [PubMed] [Google Scholar]
- 12.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 13.Cronholm PF, Forke CM, Wade R, Bair-Merritt MH, Davis M, Harkins-Schwarz M, et al. Adverse Childhood Experiences: Expanding the Concept of Adversity. Am J Prev Med. 2015;49(3):354–361. doi: 10.1016/j.amepre.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Wang HH, Lu C, Chen W, Guo VY. Adverse Childhood Experiences and Subsequent Chronic Diseases Among Middle-aged or Older Adults in China and Associations With Demographic and Socioeconomic Characteristics. JAMA Netw Open. 2021;4(10):e2130143. doi: 10.1001/jamanetworkopen.2021.30143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheval B, Chabert C, Sieber S, Orsholits D, Cooper R, Guessous I, et al. Association between Adverse Childhood Experiences and Muscle Strength in Older Age. Gerontology. 2019;65(5):474–484. doi: 10.1159/000494972. [DOI] [PubMed] [Google Scholar]
- 16.Smith NR, Ferraro KF, Kemp BR, Morton PM, Mustillo SA, Angel JL. Childhood Misfortune and Handgrip Strength Among Black, White, and Hispanic Americans. J Gerontol B Psychol Sci Soc Sci. 2019;74(3):526–535. doi: 10.1093/geronb/gbw147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo J, Leung JC, Wong SY. Impact of childhood experience of famine on late life health. J Nutr Health Aging. 2010;14(2):91–95. doi: 10.1007/s12603-009-0193-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, et al. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282(17):1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 20.Rod NH, Bengtsson J, Budtz-Jørgensen E, Clipet-Jensen C, Taylor-Robinson D, Andersen A-MN, et al. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. The Lancet. 2020;396(10249):489–497. doi: 10.1016/s0140-6736(20)30621-8. [DOI] [PubMed] [Google Scholar]
- 21.Tani Y, Fujiwara T, Kondo K. Association Between Adverse Childhood Experiences and Dementia in Older Japanese Adults. JAMA Netw Open. 2020;3(2):e1920740. doi: 10.1001/jamanetworkopen.2019.20740. [DOI] [PubMed] [Google Scholar]
- 22.Bjorkenstam C, Kosidou K, Bjorkenstam E. Childhood adversity and risk of suicide: cohort study of 548 721 adolescents and young adults in Sweden. BMJ. 2017;357:j1334. doi: 10.1136/bmj.j1334. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Li X, Xu M, Zhang Z, He L, Li Y. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China Health and Retirement Longitudinal Study. PLoS ONE. 2021;16(3):e0247617. doi: 10.1371/journal.pone.0247617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 25.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costanzo L, De Vincentis A, Di Iorio A, Bandinelli S, Ferrucci L, AntonelliIncalzi R, et al. Impact of Low Muscle Mass and Low Muscle Strength According to EWGSOP2 and EWGSOP1 in Community-Dwelling Older People. J Gerontol A Biol Sci Med Sci. 2020;75(7):1324–1330. doi: 10.1093/gerona/glaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr. et al., The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-72. https://doi.org/10.1001/jama.289.19.2560. [DOI] [PubMed]
- 28.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 29.Standards of medical care in diabetes--2010. Diabetes Care, 2010. 33 Suppl 1(Suppl 1): p. S11–61. DOI: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed]
- 30.Cheval B, Chabert C, Orsholits D, Sieber S, Guessous I, Blane D, et al. Disadvantaged Early-Life Socioeconomic Circumstances Are Associated With Low Respiratory Function in Older Age. J Gerontol A Biol Sci Med Sci. 2019;74(7):1134–1140. doi: 10.1093/gerona/gly177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agahi N, Shaw BA, Fors S. Social and economic conditions in childhood and the progression of functional health problems from midlife into old age. J Epidemiol Community Health. 2014;68(8):734–740. doi: 10.1136/jech-2013-203698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraro KF, Shippee TP. Aging and cumulative inequality: how does inequality get under the skin? Gerontologist. 2009;49(3):333–343. doi: 10.1093/geront/gnp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Björkenstam E, Hjern A, Mittendorfer-Rutz E, Vinnerljung B, Hallqvist J, Ljung R. Multi-exposure and clustering of adverse childhood experiences, socioeconomic differences and psychotropic medication in young adults. PLoS ONE. 2013;8(1):e53551. doi: 10.1371/journal.pone.0053551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa AC, Guerra RO, Thanh TuM, Phillips SP, Guralnik JM, Zunzunegui MV. Lifecourse adversity and physical performance across countries among men and women aged 65–74. PLoS ONE. 2014;9(8):e102299. doi: 10.1371/journal.pone.0102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Nusslock R, Miller GE. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17(10):88. doi: 10.1007/s11886-015-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27(1):8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526.e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 40.Lang J, McKie J, Smith H, McLaughlin A, Gillberg C, Shiels PG, et al. Adverse childhood experiences, epigenetics and telomere length variation in childhood and beyond: a systematic review of the literature. Eur Child Adolesc Psychiatry. 2020;29(10):1329–1338. doi: 10.1007/s00787-019-01329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baylis D, Ntani G, Edwards MH, Syddall HE, Bartlett DB, Dennison EM, et al. Inflammation, telomere length, and grip strength: a 10-year longitudinal study. Calcif Tissue Int. 2014;95(1):54–63. doi: 10.1007/s00223-014-9862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 43.Fox SE, Levitt P, Nelson CA., 3rd How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health. 2017;2(8):e356–e366. doi: 10.1016/s2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 45.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37(3):268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 46.Kajeepeta S, Gelaye B, Jackson CL, Williams MA. Adverse childhood experiences are associated with adult sleep disorders: a systematic review. Sleep Med. 2015;16(3):320–330. doi: 10.1016/j.sleep.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rapuri PB, Gallagher JC, Smith LM. Smoking is a risk factor for decreased physical performance in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62(1):93–100. doi: 10.1093/gerona/62.1.93. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Solà J, Preedy VR, Lang CH, Gonzalez-Reimers E, Arno M, Lin JC, et al. Molecular and cellular events in alcohol-induced muscle disease. Alcohol Clin Exp Res. 2007;31(12):1953–1962. doi: 10.1111/j.1530-0277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang T, Feng W, Li S, Tan Q, Zhang D, Wu Y. Impact of obesity and physical inactivity on the long-term change in grip strength among middle-aged and older European adults. J Epidemiol Community Health. 2019;73(7):619–624. doi: 10.1136/jech-2018-211601. [DOI] [PubMed] [Google Scholar]
- 50.Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement Between Prospective and Retrospective Measures of Childhood Maltreatment: A Systematic Review and Meta-analysis. JAMA Psychiat. 2019;76(6):584–593. doi: 10.1001/jamapsychiatry.2019.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, et al. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry. 2016;57(10):1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 53.Ramsey KA, Rojer AGM, D’Andrea L, Otten RHJ, Heymans MW, Trappenburg MC, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2021;67: 101266. 10.1016/j.arr.2021.101266. [DOI] [PubMed]
- 54.Gedmantaite A, Celis-Morales CA, Ho F, Pell JP, Ratkevicius A, Gray SR. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech Ageing Dev. 2020;189:111269. doi: 10.1016/j.mad.2020.111269. [DOI] [PubMed] [Google Scholar]
- 55.Logan-Greene P, Green S, Nurius PS, Longhi D. Distinct contributions of adverse childhood experiences and resilience resources: a cohort analysis of adult physical and mental health. Soc Work Health Care. 2014;53(8):776–797. doi: 10.1080/00981389.2014.944251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youssef NA, Belew D, Hao G, Wang X, Treiber FA, Stefanek M, et al. Racial/ethnic differences in the association of childhood adversities with depression and the role of resilience. J Affect Disord. 2017;208:577–581. doi: 10.1016/j.jad.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Definition of adverse childhood experiences. Table 2. Association between ACEs and continuous HGS (kg) by age in the overall population and different sex groups in linear regression models. Table 3. Association between ACEs and LMS by age in the overall population and different sex groups in logistic regression models.
Data Availability Statement
The data that support the findings of this study are available on the website of the China Health and Retirement Longitudinal Study (CHARLS) at http://charls.pku.edu.cn/index/en.html. To access and use this survey data for research purpose, an approval should be obtained from the CHARLS team at Peking University.