Abstract
Background
Renal cell carcinoma (RCC) is one of the most common malignancies worldwide. Noninvasive imaging techniques, such as magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET), have been involved in increasing evolution to detect RCC. This meta-analysis aims to compare to compare the performance of MRI, SPECT, and PET in the detection of RCC in humans, and to provide evidence for decision-making in terms of further research and clinical settings.
Methods
Electronic databases including PubMed, Web of Science, Embase, and Cochrane Library were systemically searched. The keywords such as “magnetic resonance imaging”, “MRI”, “single-photon emission computed tomography”, “SPECT”, “positron emission tomography”, “PET”, “renal cell carcinoma” were used for the search. Studies concerning MRI, SPECT, and PET for the detection of RCC were included. Pooled sensitivity, specificity, and the area under the summary receiver operating characteristic (SROC) curve (AUC), etc. were calculated.
Results
A total of 44 articles were finally detected for inclusion in this study. The pooled sensitivities of MRI, 18F-FDG PET and 18F-FDG PET/CT were 0.80, 0.83, and 0.89, respectively. Their respective overall specificities were 0.90, 0.86, and 0.88. The pooled sensitivity and specificity of MRI studies at 1.5 T were 0.86 and 0.94, respectively. With respect to prospective PET studies, the pooled sensitivity, specificity and AUC were 0.90, 0.93 and 0.97, respectively. In the detection of primary RCC, PET studies manifested a pooled sensitivity, specificity, and AUC of 0.77, 0.80, and 0.84, respectively. The pooled sensitivity, specificity, and AUC of PET/CT studies in detecting primary RCC were 0.80, 0.85, and 0.89.
Conclusion
Our study manifests that MRI and PET/CT present better diagnostic value for the detection of RCC in comparison with PET. MRI is superior in the diagnosis of primary RCC.
Keywords: MRI, SPECT, PET, Renal cell carcinoma, Diagnostic performance, Meta-analysis
Introduction
Renal cancer is one of the most frequently diagnosed cancers worldwide, which ranks the 6th most frequently confirmed malignant tumor in men and the 8th in women [1]. 90% of all renal malignant tumors tend to be renal cell carcinoma (RCC) on a histopathological basis [2, 3]. There are three major histological subtypes of renal cell carcinoma: clear cell RCC, papillary RCC, and chromophobe RCC [4]. It is manifested that over one-half of patients with renal cell carcinoma are asymptomatic [5]. Approximately 33 to 50% of suspected patients are diagnosed with metastatic diseases at the time of initial detection, furthermore, 20 to 40% of patients with confirmed RCC progress to metastatic diseases even after surgical resection [6, 7]. Consequently, timely and accurate detection of the early stage and advanced stage of the disease is of great significance. Partial or radical nephrectomy is still the gold standard for the treatment of renal tumors, no significant benefit have been proved regarding RCC adjuvant therapies [5].
Biopsy diagnosis is still the gold standard for confirmation of RCC although it is an invasive modality that may result in unnecessary adverse outcomes [8]. Various noninvasive imaging approaches are commonly employed in the detection of RCC [9]. For decades, ultrasound (US) has been used as one of the first-line modalities for diagnostic imaging of patients with renal lesions due to its cost-effective nature, however, the efficacy of US is not satisfactory especially in patients with suspected malignancies [9]. Although computed tomography (CT) has been utilized as the confirmative standard for RCC imaging for decades, it manifested poor performance in differentiation among solid masses, fat-poor angiomyolipoma (AML), and oncocytoma [10, 11]. Compared to CT, magnetic resonance imaging (MRI) plays an increasingly important role in the diagnosis and restaging of RCC, particularly in patients with unclear results, allergic reactions, pregnancies, as it has no ionizing radiation exposure and superior soft tissue resolution [12, 13]. Although contrast-enhanced MRI performed better than diffusion-weighted (DW) MRI for the diagnosis of RCC, patients who have renal dysfunction are at risk for nephrogenic systemic fibrosis or contrast material–induced nephropathy [14]. In recent years, targeted imaging approaches have made great progression in the diagnosis of RCC. Single photon emission computed tomography-computed tomography (SPECT) imaging is used to differentiate RCC and detect metastases in renal cancer [15, 16]. Furthermore, positron emission tomography (PET) imaging utilizing 18F-fluoro-deoxy-glucose (FDG) and other tracers (124I-girentuximab, 68Ga-DOTATOC, 11C-acetate, 18F-fluoride) has been studied as diagnostic biomarkers in RCC [17–22]. Especially, PET plays an important role in the detection of recurrent or metastatic RCC [22, 23]. Furthermore, specific European Association of Nuclear Medicine (EANM) procedure guidelines have been intended to assist practitioners in performing, interpreting and reporting the results of FDG PET/CT for imaging of patients [24].
A large number of studies have assessed the diagnostic performance of non-invasive approaches in terms of RCC, nevertheless, the results are heterogeneous [15, 18, 22, 23, 25–27]. This study aimed to generate a more comprehensive comparison of the diagnostic performance of MRI, SPECT, and PET in the detection of RCC by conducting a meta-analysis, and subsequently to guide the diagnosis and differentiation of RCC in the field of scientific research and clinical application.
Materials and methods
The entire process of this study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [28].
Search strategy and selection criteria
The electronic databases of PubMed, Web of Science, Embase, and Cochrane Library were comprehensively searched with a publication date from inception to January 31, 2021. Articles in the English language were considered. The following key terms were used for the database research: “magnetic resonance imaging”, “MRI”, “single-photon emission computed tomography”, “SPECT”, “positron emission tomography”, “PET”, “renal cell carcinoma”. Besides, we manually screened the references of the articles included for more potentially eligible studies. The inclusion criteria of studies were as follows: 1) MRI, SPECT, and/or PET were used for the detection of RCC in patients with suspected or confirmed RCC; 2) a reference standard was utilized to assess diagnostic performance; 3) absolute numbers of patients with true positive (TP), false positive (FP), true negative (TN) and false negative (FN) results can be retrieved in the published articles or recalculated based on other parameters (accuracy rate, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), number of all participants) presented in the manuscripts. In case that the studies were undertaken by the same research group, those with the largest sample size or the most complete information were included to avoid duplicates. Articles were excluded if they were case reports, reviews, letters, news, conference abstracts, animal studies, or studies with insufficient data.
Two independent investigators (QY and HX) conducted the process of literature search and study inclusion. Discrepancies were resolved by discussion. If no consensus was reached, a third author (JN) was involved.
Data extraction and quality assessments
Two researchers (QY and YZ) independently performed the title and abstract screening according to the inclusion criteria. A full-text reading of the literature was conducted for the final inclusion. The following information was extracted from each study: first author’s name, year of publication, study design, type of RCC (primary or recurrent/metastatic), number of patients analyzed, percentage of the male, age of the participants, reference standard, imaging modality and type of radiotracers used in the study, absolute numbers of patients with TP, TN, FP, and FN numbers.
To evaluate the methodological quality of the enrolled studies, we used the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. This method contains four main components in terms of participant selection, index test, reference standard, as well as flow and timing, all the components are assessed in terms of risk of bias, and the first three components are also evaluated the concerns of applicability [29].
Statistical analysis
We calculated pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the 95% confidence intervals (CIs) and the area under the summary receiver operating characteristic (SROC) curve (AUC). A Cochran Q value and the I2 statistic were used to detect the heterogeneity of studies included. I2 statistics in the range of 0–25%, 25–50%, 50–75%, and 75–100% were considered to be of insignificant, low, moderate, and high heterogeneity between studies, respectively [30]. Meta-regression was performed to investigate the possible source of heterogeneity between the included studies. A Deeks’ method was introduced to statistically test the asymmetry of the funnel plot and detect publication bias. We conducted sensitivity analysis to evaluate the impacts of one single study on the overall outcomes. All statistical analyses were processed on the study basis using the Stata 15.0 software and Review Manager 5.3 software. A p value < 0.05 was considered to be statistically significant.
Results
Study selection and characteristics
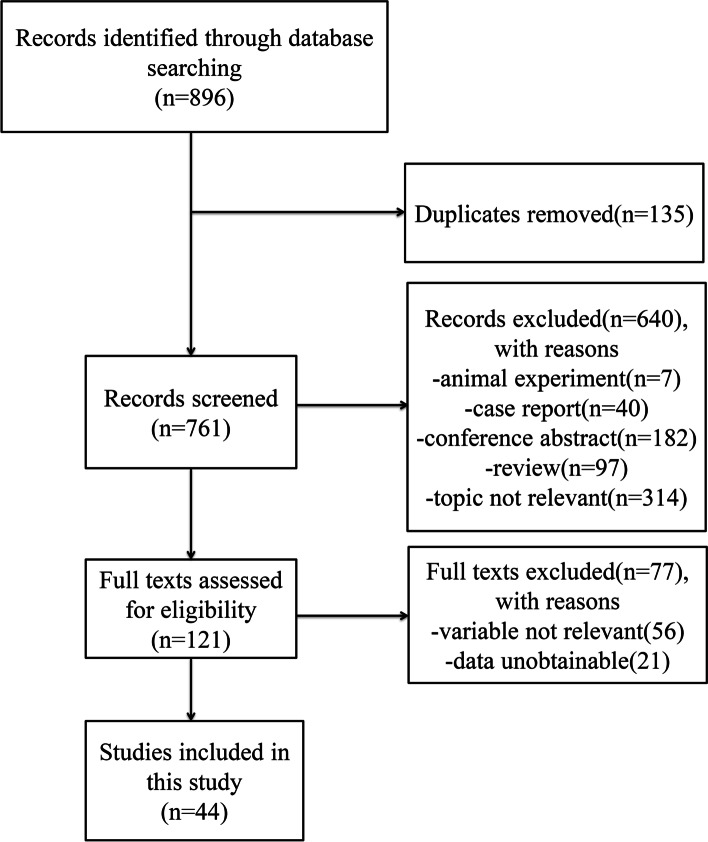
A total number of 896 articles were identified from the online databases. Among them, we excluded 135 duplicates and 640 irrespective studies based on an initial screening of titles and abstracts. After the full text confirmation for eligibility of the remaining 121 articles, 44 articles with 50 studies and 2545 patients were identified for final inclusion in this study. No additional studies were found through reference screening of the included papers. Figure 1 shows the flow of the database search and literature selection process. Detailed characteristics of studies included were shown in Table 1. The results of the quality evaluation of the included studies manifested that the high quality of the included studies (Fig. 2).
Fig. 1.
Search results and flow chart of the meta-analysis
Table 1.
Study characteristics
| Name of the first author | Year of publication | Study design | Population | Type of RCC | Reference test | No. of Patients analysed | Male, No. (%) | Age (SD or IQR) | Modalities | Image analysis | Tracers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams | 2019 | Prospective | Suspected or known RCC | Primary | Histopathology | 27 | 70 | 61 (14) | MRI | Quantitative | – |
| Aide | 2003 | Prospective | Suspected RCC | Primary | Histopathology | 35 | 60 | 60 (14) | PET | Qualitative | 18F-FDG |
| Alongi | 2016 | Prospective | Suspected or known RCC | Primary | Histopathology and/or other imaging | 104 | 69 | 63 (13) | PET/CT | Qualitative | 18F-FDG |
| Aslan | 2018 | Retrospective | Known RCC | Primary | Histopathology | 49 | 52 | 62 (14) | MRI | Qualitative | – |
| Bertagna | 2013 | Retrospective | Known RCC | Primary | Histopathology | 68 | 72 | 68 (4) | PET/CT | Qualitative | 18F-FDG |
| Chang | 2003 | Retrospective | Known RCC | Primary | Histopathology | 15 | 47 | 56 (15) | PET | Qualitative | 18F-FDG |
| Chen | 2014 | Retrospective | Suspected RCC | Primary | Histopathology | 35 | 57 | 57 (29–77) | MRI | Qualitative | – |
| Chen | 2015 | Prospective | Suspected RCC | Primary | Histopathology | 71 | 75 | 50 (21–78) | MRI | Qualitative | – |
| Choi | 2021 | Retrospective | Known RCC | Primary | Histopathology | 110 | 67 | NR | MRI | Qualitative | – |
| Dilhuydy | 2006 | Retrospective | Suspected or known RCC | Recurrent or metastatic | Histopathology | 18 | NR | 57 (13) | PET/CT | Qualitative | 18F-FDG |
| Ding | 2016 | Retrospective | Suspected or known RCC | Primary | Histopathology | 35 | 58 | 52 (12) | MRI | Qualitative | – |
| Divgi | 2013 | Prospective | Suspected or known RCC | Primary | Histopathology | 204 | 63 | 56 (12) | PET/CT | Qualitative | 124I-girentuximab |
| Fisher | 2008 | Prospective | Suspected or known RCC | Primary | Histopathology | 122 | 64 | 58 (32–80) | SPECT | Qualitative | 99mTc-EC20 |
| Fuccio | 2014 | Retrospective | Known RCC | Primary | Histopathology | 69 | NR | 62 (36–86) | PET/CT | Qualitative | 18F-FDG |
| Goldberg | 1997 | Prospective | Suspected or known RCC | Primary | Histopathology | 10 | 57 | 49 (37–76) | PET | Qualitative | 18F-FDG |
| Jadvar | 2003 | Retrospective | Suspected or known RCC | Primary | Histopathology and clinical follow-up | 25 | 72 | NR | PET | Qualitative | 18F-FDG |
| Johnson | 2019 | Retrospective | Suspected or known RCC | Primary | Histopathology | 57 | NR | 62 (15) | MRI | Qualitative | – |
| Kang | 2004 | Prospective | Suspected or known RCC | Primary | Histopathology | 17 | 74 | 59 (28–79) | PET | Qualitative | 18F-FDG |
| Kumar | 2010 | Retrospective | Known RCC | Primary | Histopathology and clinical follow-up and conventional imaging finding | 103 | 87 | 57 (12) | PET/CT | Qualitative | 18F-FDG |
| Kwon | 2015 | Prospective | Suspected or known RCC | Primary | Histopathology and clinical follow-up | 73 | 60 | 52 (28–71) | MRI | Qualitative | – |
| Li | 2020 | Retrospective | Known RCC | Primary | Histopathology | 127 | 78 | 56 (12) | MRI | Quantitative | – |
| Lima | 2020 | Retrospective | Known RCC | Primary | Histopathology | 47 | 55 | 61 (12) | MRI | Qualitative | – |
| Lyu | 2018 | Retrospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 35 | 54 | 55 (15) | MRI | Qualitative | – |
| Majhail | 2003 | Retrospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 36 | 79 | 63 (45–82) | PET | Qualitative | 18F-FDG |
| Miyakita | 2002 | Retrospective | Known RCC | Primary | Histopathology | 19 | 79 | 57 (10) | PET | Qualitative | 18F-FDG |
| Muselaers | 2013 | Retrospective | Suspected RCC | Primary | Histopathology | 29 | 41 | 64 (8) | SPECT | Qualitative | 111In-Girentuximab |
| Nakamoto | 2019 | Retrospective | Suspected or known recurrent RCC | Primary | Histopathology | 25 | 76 | 64 (38–86) | PET/CT | Qualitative | 68Ga-DOTATOC/18F-FDG |
| Nakatani | 2011 | Retrospective | Suspected recurrent RCC | Primary | Histopathology and clinical follow-up | 28 | 75 | 63 (45–78) | PET | Qualitative | 18F-FDG |
| Oyama | 2014 | Prospective | Suspected RCC | Primary | Histopathology | 34 | NR | 67 (38–87) | PET | Qualitative | 18F-FDG/11C-acetate |
| Ozturk | 2016 | Retrospective | Suspected recurrent or metastatic lesions | Recurrent or metastatic | Histopathology or clinical follow-up | 132 | 68 | 61 (12) | PET | Qualitative | 18F-FDG |
| Ozulker | 2011 | Prospective | Suspected RCC | Primary | Histopathology | 18 | 44 | 57 (11) | PET/CT | Qualitative | 18F-FDG |
| Park | 2009 | Retrospective | Suspected recurrent or metastatic lesions | Recurrent or metastatic | Histopathology or by clinical follow-up | 63 | 75 | 54 (31–76) | PET/CT | Qualitative | 18F-FDG |
| Purkayastha | 2020 | Retrospective | Known RCC | Primary | Histopathology | 43 | 82 | 64 (38–85) | MRI | Quantitative | – |
| Ramdave | 2001 | Prospective | Suspected or known primary RCC | Primary | Histopathology | 17 | 56 | 61 (32–79) | PET | Qualitative | 18F-FDG |
| Safaei | 2002 | Prospective | Suspected RCC | Primary | Histopathology | 36 | 78 | 54 (11) | PET | Qualitative | 18F-FDG |
| Sharma | 2014 | Prospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 16 | 75 | 53 (14) | PET/CT | Qualitative | 18F-Fluoride |
| Sheikhbahaei | 2017 | Prospective | Suspected RCC | Primary | Histopathology | 48 | 73 | 59 (40–81) | SPECT/CT | Qualitative | 99mTc-MIBI |
| Sistani | 2020 | Prospective | Known RCC | Primary | Histopathology | 31 | 79 | 60 (30–83) | SPECT/CT | Qualitative | 99mTc-MIBI |
| Sohaib | 2009 | Prospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 47 | 70 | 62 (29–79) | SPECT/MRI | Qualitative | 99mTc-MDP |
| Sun | 2020 | Retrospective | Known RCC | Primary | Histopathology | 45 | 60 | 57 (12) | MRI | Quantitative | – |
| Win | 2015 | Retrospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 315 | 85 | 47.5 | PET/CT | Qualitative | 18F-FDG |
| Wu | 2002 | Prospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 52 | 67 | (46–73) | SPECT/PET | Qualitative | 99mTc-MDP/18F-FDG |
| Youssef | 2018 | Prospective | Suspected metastatic RCC | Recurrent or metastatic | Histopathology | 20 | 65 | NR | PET/CT | Qualitative | 18F-FDG |
| Zhao | 2020 | Retrospective | Known RCC | Primary | Histopathology | 35 | 69 | 61 | MRI | Quantitative | – |
RCC renal cell carcinoma, SD standard deviation, IQR interquartile range, MRI magnetic resonance imaging, SPECT single photon emission computed tomography, PET positron emission tomography, NR not reported
Fig. 2.
Methodological assessment of studies included on the QUADAS-2 tool
Diagnostic performance of imaging modalities
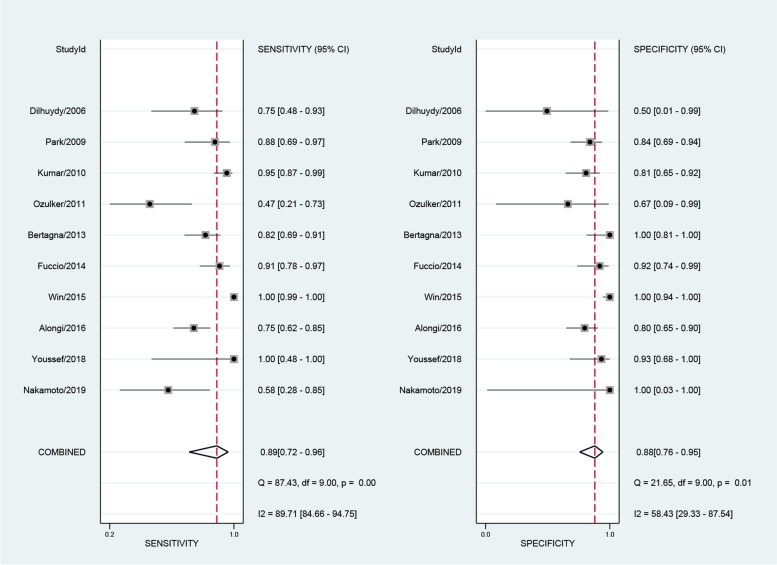
The numbers of SPECT or SPECT/CT studies utilizing 99mTc-EC (ethylenecysteine), 111In-Girentuximab, 99mTc-sestamibi (99mTc-MIBI), and 99mTc-methylene diphosphonate (99mTc-MDP) were 1, 1, 2, and 3, respectively. The sensitivities of these studies ranged from 0.29 to 0.95 and the specificities ranged from 0 to 0.94. The numbers of PET or PET/CT studies using 18F-FDG (18F-fluorodeoxyglucose), 18F-fluoride, 124I-girentuximab, and 11C-acetate as radiopharmaceuticals were 20, 1, 1, and 1, respectively. We performed the sensitivity analysis to assess the impacts of single study on the overall outcomes. No study was identified as outliers. The final numbers of studies in terms of the meta-analysis of MRI, 18F-FDG PET and 18F-FDG PET/CT were 16, 13, and 10, respectively. The pooled sensitivity of MRI, FDG PET and FDG PET/CT were 0.80 [0.70,0.88], 0.83 [0.64, 0.93] and 0.89 [0.72, 0.96], respectively. The overall specificities were 0.90 [0.84,0.94], 0.86 [0.75, 0.92] and 0.88 [0.76, 0.95] for MRI, FDG PET and FDG PET/CT. The AUC values of MRI, FDG PET and FDG PET/CT were 0.93 [0.90, 0.95], 0.88 [0.85, 0.90] and 0.94 [0.92, 0.96] (see Figs. 3, 4, 5 and 6).
Fig. 3.
Forest plot for the detection performance of MRI
Fig. 4.
Forest plot for the detection performance of 18F-FDG PET
Fig. 5.
Forest plot for the detection performance of 18F-FDG PET/CT
Fig. 6.
SROC curves for diagnostic performance of MRI, 18F-FDG PET and 18F-FDG PET/CT. A: SROC curve for diagnostic performance of MRI. B: SROC curve for diagnostic performance of 18F-FDG PET. C: SROC curve for diagnostic performance of 18F-FDG PET/CT
Subgroup analysis of the performance of MRI
The pooled sensitivity and specificity of MRI studies at 1.5 T were 0.86 [0.64, 0.96] and 0.94 [0.76, 0.99], respectively. The AUC of MRI studies at 1.5 T was 0.96 [0.94, 0.98]. With respect to prospective MRI studies, the pooled sensitivity, specificity and AUC were 0.88 [0.81, 0.93], 0.91 [0.71, 0.98] and 0.90 [0.88, 0.93]. In the detection of primary RCC, MRI studies revealed a pooled sensitivity, specificity, and AUC of 0.76 [0.65, 0.85], 0.88 [0.81, 0.93], and 0.90 [0.87, 0.92] respectively. More details were shown in Table 2.
Table 2.
Results of Subgroup analysis
| Subgroups | Sensitivity | I2 of sensitivity (%) | Q value of sensitivity | p of sensitivity | Specificity | I2 of specificity (%) | Q value of specificity | p of specificity | PLR | NLR | DOR | SROC Curve AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI at 1.5 T | 0.86 [0.64, 0.96] | 92.11 | 63.39 | < 0.001 | 0.94 [0.76, 0.99] | 75.77 | 20.63 | < 0.001 | 15.2 [3.3, 70.0] | 0.15 [0.05, 0.42] | 104 [18, 604] | 0.96 [0.94, 0.98] |
| Prospective studies | ||||||||||||
| MRI | 0.88 [0.81, 0.93] | 26.43 | 5.44 | 0.25 | 0.91 [0.71, 0.98] | 80.70 | 20.72 | < 0.001 | 10.2 [2.7, 38.4] | 0.13 [0.08, 0.22] | 79 [15, 403] | 0.90 [0.88, 0.93] |
| PET | 0.90 [0.56, 0.98] | 96.19 | 157.33 | < 0.001 | 0.93 [0.54, 0.99] | 64.60 | 16.95 | 0.01 | 12.6 [1.4, 117.3] | 0.11 [0.02, 0.63] | 119 [5, 2597] | 0.97 [0.95, 0.98] |
| Primary RCC | ||||||||||||
| MRI | 0.76 [0.65, 0.85] | 79.90 | 59.70 | < 0.001 | 0.88 [0.81, 0.93] | 66.50 | 35.82 | < 0.001 | 6.4 [4.3, 9.7] | 0.27 [0.18, 0.39] | 24 [16, 37] | 0.90 [0.87, 0.92] |
| PET | 0.77 [0.55, 0.91] | 89.86 | 88.73 | < 0.001 | 0.80 [0.60, 0.91] | 0 | 5.37 | 0.80 | 3.8 [1.8, 7.9] | 0.28 [0.13, 0.61] | 13 [4, 45] | 0.84 [0.80, 0.87] |
| PET/CT | 0.80 [0.64, 0.90] | 82.35 | 28.33 | < 0.001 | 0.85 [0.73, 0.93] | 20.38 | 6.28 | 0.28 | 5.5 [2.8, 10.8] | 0.23 [0.11, 0.46] | 24 [7, 78] | 0.89 [0.85, 0.91] |
RCC renal cell carcinoma, MRI magnetic resonance imaging, PET positron emission tomography, CT computerized tomography, PLR positive likelihood ratio, NLR negative likelihood ratio, DOR diagnostic odds ratio, SROC summary receiver operating characteristic, AUC area under the SROC curve
Subgroup analysis of the performance of PET and PET/CT
With respect to prospective PET studies, the pooled sensitivity, specificity and AUC were 0.90 [0.56, 0.98], 0.93 [0.54, 0.99] and 0.97 [0.95, 0.98], respectively. In the detection of primary RCC, PET studies revealed a pooled sensitivity, specificity, and AUC of 0.77 [0.55, 0.91], 0.80 [0.60, 0.91], and 0.84 [0.80, 0.87], respectively. In addition, the pooled sensitivity, specificity, and AUC of PET/CT studies in detecting primary RCC were 0.80 [0.64, 0.90], 0.85 [0.73, 0.93], and 0.89 [0.85, 0.91], respectively. More details were shown in Table 2.
Heterogeneity and publication bias
Deek’s tests for publication bias yielded p values of 0.94, 0.02, and 0.08 for MRI, FDG PET, and FDG PET/CT, which revealed that there was a possible publication bias in the pooled analysis of FDG PET studies.
Discussion
Renal cell carcinoma is the most commonly diagnosed subtype of kidney cancers and accounts for approximately 2–3% of all malignancies [21]. The research of Motzer et al. demonstrated that the average 5-year survival rates for patients with RCC decreased with the disease stages (I to IV), from 96 to 23% [31]. Moreover, the early signs and symptoms of RRC are not specific which introduces difficulties for the early detection of this disease in primary or metastatic sites [32]. Renal biopsy is an accurate method to establish a histological diagnosis for RRC, however, it is may induce a risk of procedural adverse events [33]. Noninvasive approaches namely MRI, SPECT, and PET have been in evolution during the past decades [15, 34, 35]. Based on various studies of the diagnostic value of noninvasive modalities in the detection of RCC, we carry out a meta-analysis to compare the diagnostic efficacy of these approaches.
The meta-analysis was processed on the basis of study design, type of imaging modalities, type of radiotracers, type of RCC. To our knowledge, some of these dimensions have not been discussed in relevant meta-analyses [36–38]. Results revealed that the pooled sensitivity of PET/CT (0.89 [0.72, 0.96]) was the highest. MRI demonstrated the highest overall specificity (0.90 [0.84,0.94]). MRI and PET/CT showed high diagnostic performance in detecting RCC. Results of subgroup analysis manifested that PET/CT imaging had better performance than PET. Sensitivity and specificity of PET-CT are higher compared with PET alone. Furthermore, our research indicated that PET/CT and MRI revealed better performance in detecting primary RCC compared with PET alone. Due to the limited number of studies regarding recurrent or metastatic RCC, we didn’t conduct meta-analysis of this subgroup, this is one of the limitations of our study.
In this meta-analysis, we conducted a detailed literature search to improve the probability of searching as many related studies as possible. Two independent investigators completed the whole process of data extraction using standardized electronic forms. Furthermore, we evaluated the heterogeneity between the studies included. There were significant heterogeneities among studies. Distinctions in the year of publication, study methodology, patient characteristics, reference standard, and radiotracers may be the source of heterogeneity. Unfortunately, meta regression was not able to be performed to investigate the likely cause of heterogeneity due to limited number of covariates extracted from the enrolled studies. Subgroup analysis was undertaken to explore the possible source of heterogeneity. For the analysis of PET, the source of heterogeneity may be attributed to the type of radiotracers, type of RCC, and study design. However, not all potential source of heterogeneity was analyzed because of the insufficient number of studies in different subgroups. On account of this limitation, the efficacy of heterogeneity assessment in the study may be biased. Besides, publication bias was detected through the Deeks’ funnel plot asymmetry test in the analysis of PET studies. The publication bias may be attributed to the strict exclusion criteria of this meta-analysis. Although there is heterogeneity among studies included and publication bias, the findings of this analysis may introduce evidence and assistances concerning scientific research and clinical practice in the detection of RCC. In regard to further research, novel radiotracers with higher uptake ratios between tumor tissues to normal tissues and lower levels of renal excretion need to be further investigated on account of the results of this meta-analysis. In terms of application in the clinical setting, MRI is recommended as the favorable imaging method to help detect RCC due to lack of radiation exposure and high soft-tissue resolution. PET/CT shows better performance than PET alone for the diagnosis of RCC under the current development of functional imaging modalities. Of note, combined employment of various detection techniques is may be of assistance to increase the overall diagnostic accuracy. Interestingly, the hybrid PET/MRI, which provides combined anatomical and metabolic information, has drawn much attention in recent years, results of the study of PET/MRI in the detection of RCC is promising, and recent prospective studies are in progress [39, 40].
Acknowledgements
Not applicable.
Abbreviations
- RCC
Renal cell carcinoma
- MRI
Magnetic resonance imaging
- DW
Diffusion-weighted
- SPECT
Single photon emission computed tomography
- PET
Positron emission tomography
- CT
Computerized tomography
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- TP
True positive
- FP
False positive
- TN
True negative
- FN
False negative
- PLR
Positive likelihood ratio
- NLR
Negative likelihood ratio
- DOR
Diagnostic odds ratio
- CI
Confidence interval
- SROC
Summary receiver operating characteristic
- AUC
Area under the SROC curve
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies-2
Authors’ contributions
QY conceived and designed this study. HX and YZ were responsible for the collection, extraction, and analysis of the data. QY was responsible for data analysis and writing the paper. JN and SH performed the quality evaluation of the writing and polished the English language. All authors reviewed the paper and reached an agreement to approve the final manuscript.
Funding
This project is funded by Construction project of Shanghai Key Laboratory of Molecular Imaging (18DZ2260400), Shanghai Municipal Education Commission (Class II Plateau Disciplinary Construction Program of Medical Technology of SUMHS, 2018–2020), Wuxi Municipal Health Commission (Z201910) and the Taihu Lake Talent Plan (2020).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianming Ni, Email: nijianming@njmu.edu.cn.
Shudong Hu, Email: hsd2001054@163.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Gray RE, Harris GT. Renal Cell Carcinoma: Diagnosis and Management. Am Fam Physician. 2019;99(3):179–184. [PubMed] [Google Scholar]
- 3.Leibovich BC, Lohse CM, Crispen PL, Boorjian SA, Thompson RH, Blute ML, Cheville JC. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183(4):1309–1315. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8(5):484–500. doi: 10.4329/wjr.v8.i5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ (Clinical research ed) 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vig SVL, Zan E, Kang SK. Imaging for metastatic renal cell carcinoma. Urol Clin North Am. 2020;47(3):281–291. doi: 10.1016/j.ucl.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin N Am. 2003;30(4):843–852. doi: 10.1016/S0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 8.Albiges L, Fay AP, McKay RR, Kaymakcalan MD, Choueiri TK. Diagnosis of renal cell carcinoma: a Clinician's perspective. Surg Pathol Clin. 2015;8(4):657–662. doi: 10.1016/j.path.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Sankineni S, Brown A, Cieciera M, Choyke PL, Turkbey B. Imaging of renal cell carcinoma. Urol Oncol. 2016;34(3):147–155. doi: 10.1016/j.urolonc.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Kwon YS, Labib M, Foran DJ, Singer EA. Magnetic resonance imaging as a biomarker for renal cell carcinoma. Dis Markers. 2015;2015:648495. doi: 10.1155/2015/648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi SH, Prezzi D, Kelly-Morland C, Goh V. Imaging for the diagnosis and response assessment of renal tumours. World J Urol. 2018;36(12):1927–1942. doi: 10.1007/s00345-018-2342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willatt JM, Hussain HK, Chong S, Kappil M, Azar SF, Liu PS, Ruma JA, Elsayes KM. MR imaging in the characterization of small renal masses. Abdom Imaging. 2014;39(4):761–769. doi: 10.1007/s00261-014-0109-x. [DOI] [PubMed] [Google Scholar]
- 13.Hudson JM, Bailey C, Atri M, Stanisz G, Milot L, Williams R, Kiss A, Burns PN, Bjarnason GA. The prognostic and predictive value of vascular response parameters measured by dynamic contrast-enhanced-CT, −MRI and -US in patients with metastatic renal cell carcinoma receiving sunitinib. Eur Radiol. 2018;28(6):2281–2290. doi: 10.1007/s00330-017-5220-2. [DOI] [PubMed] [Google Scholar]
- 14.Taouli B, Thakur RK, Mannelli L, Babb JS, Kim S, Hecht EM, Lee VS, Israel GM. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009;251(2):398–407. doi: 10.1148/radiol.2512080880. [DOI] [PubMed] [Google Scholar]
- 15.Sistani G, Bjazevic J, Kassam Z, Romsa J, Pautler S. The value of (99m)Tc-sestamibi single-photon emission computed tomography-computed tomography in the evaluation and risk stratification of renal masses. Can Urol Assoc J. 2021;15(6):197–201. [DOI] [PMC free article] [PubMed]
- 16.Sohaib SA, Cook G, Allen SD, Hughes M, Eisen T, Gore M. Comparison of whole-body MRI and bone scintigraphy in the detection of bone metastases in renal cancer. Br J Radiol. 2009;82(980):632–639. doi: 10.1259/bjr/52773262. [DOI] [PubMed] [Google Scholar]
- 17.Divgi CR, Uzzo RG, Gatsonis C, Bartz R, Treutner S, Yu JQ, Chen D, Carrasquillo JA, Larson S, Bevan P, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol. 2013;31(2):187–194. doi: 10.1200/JCO.2011.41.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamoto Y, Ishimori T, Shimizu Y, Sano K, Togashi K. Clinical utility of (68)Ga-DOTATOC positron emission tomography/computed tomography for recurrent renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46(7):1524–1530. doi: 10.1007/s00259-019-04298-4. [DOI] [PubMed] [Google Scholar]
- 19.Oyama N, Ito H, Takahara N, Miwa Y, Akino H, Kudo T, Okazawa H, Fujibayashi Y, Komatsu K, Tsukahara K, et al. Diagnosis of complex renal cystic masses and solid renal lesions using PET imaging: comparison of 11C-acetate and 18F-FDG PET imaging. Clin Nucl Med. 2014;39(3):e208–e214. doi: 10.1097/RLU.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Karunanithi S, Chakraborty PS, Kumar R, Seth A, Julka PK, Bal C, Kumar R. 18F-fluoride PET/CT for detection of bone metastasis in patients with renal cell carcinoma: a pilot study. Nucl Med Commun. 2014;35(12):1247–1253. doi: 10.1097/MNM.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 21.Alongi P, Picchio M, Zattoni F, Spallino M, Gianolli L, Saladini G, Evangelista L. Recurrent renal cell carcinoma: clinical and prognostic value of FDG PET/CT. Eur J Nucl Med Mol Imaging. 2016;43(3):464–473. doi: 10.1007/s00259-015-3159-6. [DOI] [PubMed] [Google Scholar]
- 22.Youssef MA, Elshafey MH, Moghazy KM, Elrashedy AA. Dual modality imaging of positron emission tomography-computed tomography (PET-CT) in evaluation of postoperative renal cancer patients. Egypt J Radiol Nucl Med. 2018;49(4):1083–1092. doi: 10.1016/j.ejrnm.2018.06.014. [DOI] [Google Scholar]
- 23.Win AZ, Aparici CM. Clinical effectiveness of (18)f-fluorodeoxyglucose positron emission tomography/computed tomography in management of renal cell carcinoma: a single institution experience. World J Nuclear Med. 2015;14(1):36–40. doi: 10.4103/1450-1147.150535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 20. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozturk H. Diagnostic role of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in restaging renal cell carcinoma. Minerva Urol Nefrol. 2016;68(3):263–269. [PubMed] [Google Scholar]
- 26.Sheikhbahaei S, Jones CS, Porter KK, Rowe SP, Gorin MA, Baras AS, Pierorazio PM, Ball MW, Higuchi T, Johnson PT, et al. Defining the added value of 99mTc-MIBI SPECT/CT to conventional cross-sectional imaging in the characterization of enhancing solid renal masses. Clin Nucl Med. 2017;42(4):e188–e193. doi: 10.1097/RLU.0000000000001534. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Pan L, Zha T, Xing W, Chen J, Duan S. The role of MRI texture analysis based on susceptibilityweighted imaging in predicting Fuhrman grade of clear cell renal cell carcinoma. Acta Radiol. 2021;62(8):1104–11. [DOI] [PubMed]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London, England) 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Reitsma JB, Moons KG, Bossuyt PM, Linnet K. Systematic reviews of studies quantifying the accuracy of diagnostic tests and markers. Clin Chem. 2012;58(11):1534–1545. doi: 10.1373/clinchem.2012.182568. [DOI] [PubMed] [Google Scholar]
- 30.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyu HL, Cao JX, Wang HY, Wang ZB, Hu MG, Ma L, Wang YW, Ye HY. Differentiation between pancreatic metastases from clear cell renal cell carcinoma and pancreatic neuroendocrine tumor using double-echo chemical shift imaging. Abdominal Radiol (New York) 2018;43(10):2712–2720. doi: 10.1007/s00261-018-1539-7. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Liu YJ, Dong D, Bai X, Huang QB, Guo AT, Ye HY, Tian J, Wang HY. Multiparametric MRI Radiomic model for preoperative predicting WHO/ISUP nuclear grade of clear cell renal cell carcinoma. J Magn Reson Imaging. 2020;52(5):1557–1566. doi: 10.1002/jmri.27182. [DOI] [PubMed] [Google Scholar]
- 34.Lindenberg L, Mena E, Choyke PL, Bouchelouche K. PET imaging in renal cancer. Curr Opin Oncol. 2019;31(3):216–221. doi: 10.1097/CCO.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel C, Ziegelmüller B, Ljungberg B, Bensalah K, Bex A, Canfield S, Giles RH, Hora M, Kuczyk MA, Merseburger AS, et al. Imaging in suspected renal-cell carcinoma: systematic review. Clin Genitourinary Cancer. 2019;17(2):e345–e355. doi: 10.1016/j.clgc.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MP, Katlariwala P, Murad MH, Abele J, McInnes MDF, Low G. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: a systematic review and meta-analysis. Abdominal Radiol (New York) 2020;45(8):2532–2541. doi: 10.1007/s00261-020-02469-8. [DOI] [PubMed] [Google Scholar]
- 37.Ma H, Shen G, Liu B, Yang Y, Ren P, Kuang A. Diagnostic performance of 18F-FDG PET or PET/CT in restaging renal cell carcinoma: a systematic review and meta-analysis. Nucl Med Commun. 2017;38(2):156–163. doi: 10.1097/MNM.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 38.Wang HY, Ding HJ, Chen JH, Chao CH, Lu YY, Lin WY, Kao CH. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging. 2012;12(3):464–474. doi: 10.1102/1470-7330.2012.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin Q, Hung SC, Wang L, Lin W, Fielding JR, Rathmell WK, Khandani AH, Woods ME, Milowsky MI, Brooks SA, et al. Associations between tumor vascularity, vascular endothelial growth factor expression and PET/MRI Radiomic signatures in primary clear-cell-renal-cell-carcinoma: proof-of-concept study. Sci Rep. 2017;7:43356. doi: 10.1038/srep43356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly-Morland C, Rudman S, Nathan P, Mallett S, Montana G, Cook G, Goh V. Evaluation of treatment response and resistance in metastatic renal cell cancer (mRCC) using integrated (18)F-Fluorodeoxyglucose ((18)F-FDG) positron emission tomography/magnetic resonance imaging (PET/MRI); the REMAP study. BMC Cancer. 2017;17(1):392. doi: 10.1186/s12885-017-3371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.