Abstract
We report the results of a study of the prevalence of Ehrlichia and Borrelia species in 341 questing Ixodes ricinus ticks from two locations in southern Norway. The prevalences of Borrelia burgdorferi sensu lato and Ehrlichia spp. were, respectively, 16 and 11.5% at site 1 and 17 and 6% at site 2. Prevalence and species composition of Borrelia and Ehrlichia varied with location and date of collection. The dominant Borrelia species at both sites was Borrelia afzelii, followed by Borrelia burgdorferi sensu stricto. Borrelia garinii was found in only a single tick. The dominant member of the Ehrlichia group was a recently described Ehrlichia-like organism related to the monocytic ehrlichiae. Variants of Ehrlichia phagocytophila and the agent of human granulocytic ehrlichiosis were also found. The highest prevalences for B. afzelii, B. burgdorferi sensu stricto, and the Ehrlichia-like organism were observed in May. B. afzelii was most prevalent in females, less prevalent in nymphs, and least prevalent in males, while the prevalence of Ehrlichia was highest in nymphs, lower in females, and least in males. Double infections with B. afzelii and B. burgdorferi sensu stricto and with B. afzelii and the Ehrlichia-like organism were significantly overrepresented. Tick densities were highest in May, when densities of more than 200 ticks/100 m2 were observed, and declined during the summer months to densities as low as 20 ticks/100 m2. We conclude that estimates of the prevalence of tick-borne bacteria are sensitive to the choice of date and site for collection of ticks. This is the first study of tick-borne Borrelia and Ehrlichia in Norway and the lowest reported B. garinii prevalence in Northern Europe. The prevalence of the Ehrlichia-like organism is described for the first time in questing ticks.
Tick-borne Borrelia and Ehrlichia species cause disease both in humans and animals (13). In northwest Europe these bacteria are transmitted predominantly by the bite of the hard tick Ixodes ricinus. Ticks are infected when they feed on an infected animal, and the bacteria persist in the tissues of the tick through metamorphosis and can be transmitted to a new host when the tick again feeds. Transovarial transmission is not considered to be important for Borrelia burgdorferi sensu lato (19). I. ricinus feeds widely on terrestrial vertebrates (31), which gives it the potential to support the enzootic cycles of diseases with many different host reservoirs.
Of the 10 Borrelia species known collectively as Borrelia burgdorferi sensu lato, 4 (B. burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana) are known to occur in northwestern Europe. The complex of diseases caused by B. burgdorferi sensu lato is known as Lyme borreliosis. Symptoms include arthritis, carditis, dermal symptoms, and neurological symptoms, usually preceded by erythema migrans, a characteristic rash that spreads from the bite site. Arthritis and carditis are preferentially associated with B. burgdorferi sensu stricto, B. garinii infection predisposes to neuroborreliosis, and the degenerative skin disorder acrodermatitis chronica et atrophicans (ACA) is specifically associated with B. afzelii. The implied tissue tropisms are not absolute, and the clinical symptoms of infection with the different B. burgdorferi sensu lato species overlap. B. valaisiana's status as a pathogen has yet to be confirmed (38). Symptoms of Lyme borreliosis have been recognized in Europe since the early 1900s, and B. burgdorferi sensu lato has been detected in archival ticks dating back more than 100 years (15, 22). The bacterial etiology of Lyme disease was first elucidated in the United States in 1982 by Burgdorfer et al. (5).
On the basis of 16S rRNA gene sequences, the ehrlichiae appear to fall into three clades: the monocytic Ehrlichia species, including E. canis, E. chaffeensis, E. ewingii, and E. muris; the granulocytic Ehrlichia species, including E. bovis, E. platys, E. phagocytophila, E. equi, and the human granulocytic ehrlichiosis (HGE) agent; and the E. risticii-E. sennetsu group. In addition, several species not traditionally classified as Ehrlichia fall within this clade. Cowdria ruminantium clusters with the monocytic ehrlichiae, Anaplasma marginale clusters with the granulocytic ehrlichiae, and Neorickettsia helminthoeca clusters with E. risticii and E. sennetsu (30). A review of the DNA sequence databases reveals a number of other Ehrlichia 16S rRNA gene sequences which have yet to be classified taxonomically. Among these are two 16S rRNA variants of the E. phagocytophila group and an Ehrlichia-like organism related to the monocytic group (30). Ehrlichia infection in humans characteristically causes an acute fever, often accompanied by myalgia, headache, rigors, and gastrointestinal problems, but without symptoms of upper respiratory infection, while leukopenia, thrombocytopenia, and elevated serum transaminases are typical laboratory findings (37); veterinary Ehrlichia infections are apparently similar. Leukocytes are the primary targets of infection, and these may become severely depleted, facilitating secondary infection (13). Ehrlichia infections may be quite severe, and fatalities occur both in humans and in animals.
Prevalence data for Borrelia and Ehrlichia in ticks provide a guide to the health risk associated with a tick bite and are therefore of public health interest. This question has been addressed for Borrelia, and to a lesser extent for Ehrlichia, in the United States, Japan, and a number of European countries (3, 6, 17, 18, 21, 25, 27, 36). Both prevalence and species distribution are subject to geographical variation.
In Norway, Lyme arthritis, neuroborreliosis, and ACA are endemic. HGE has recently been reported (4), and symptoms of tick-borne fever caused by E. phagocytophila have been recorded in sheep, goats, and cattle since 1780 (33, 34). I. ricinus reaches its northern limit about the 66th parallel on the northwest coast of Norway. North of this limit, Lyme borreliosis is a very uncommon import disease in humans and tick-borne fever in livestock is not reported (33). In 1999 there were 146 notified cases of serious Lyme borreliosis in Norway, an incidence rate of 3.4/100,000. Erythema migrans is not included as this is not a notifiable disease. Of the reported cases, 62% were from the southern counties of Telemark, Aust-Agder, and Vest-Agder; 49% of the reported cases were neuroborreliosis, 33% were Lyme arthritis, and 6% were ACA; the remaining 12% were unclassified (10). The prevalence of Borrelia and Ehrlichia in ticks in Norway has not hitherto been studied.
In order to establish an epidemiological context for the prevalence of tick-borne diseases in Norway, we have surveyed the prevalence of Ehrlichia and Borrelia in ticks from two locations in southeastern Norway—one chosen for its very high tick density and the other for its association with a case of E. phagocytophila infection in a moose (16). In order to determine to what extent the results of prevalence surveys may be extrapolated beyond the date and vicinity of collection and also to provide data on the epizootology of tick-borne diseases, we report the differences in Ehrlichia and Borrelia prevalence between the two localities and their variation through a season. In addition, we investigate the association of Ehrlichia and Borrelia infection with developmental stage and the correlation between species in multiple infections.
MATERIALS AND METHODS
Localities.
Ticks were collected from two sites. Site 1, Langøya, is a limestone island (9°47′E, 59°0′N) close to the mainland coast of southeastern Norway. Although previously used as pasturage for cattle and sheep, the island is no longer used for agriculture and is uninhabited. Local residents report that ticks became troublesome on the island after a series of dry summers in the mid-1980s. The island supports a flock of roe deer as well as a range of smaller mammals. Vegetation is mixed oak woodland with varied undergrowth interspersed with areas of open grassland. The collection area is one such area, an overgrown meadow. Site 2, Marka (9°48′E, 59°8′N), is a mainland area of mixed woodland on alkaline igneous rock in the vicinity of sheep pasture. A moose suffering from an E. phagocytophila infection was found in the area in July 1999 and tick-borne fever is endemic in local sheep flocks (16).
Collection of ticks.
Questing ticks were collected by flagging undergrowth with a 70-by-120-cm white towel (40). Ticks attaching themselves to the towel were picked with tweezers and immersed in 70% ethanol. Collection at site 1 was carried out at approximately monthly intervals during the summer months of 1998 and 1999. To measure the tick density, collection was carried out in the same 10-by-10-m square marked area. For Borrelia and Ehrlichia prevalence studies, we aimed to collect 25 adult males, 25 adult females, and 50 nymphs. Larvae were not included in the study. If an insufficient number of ticks was found in the 100-m2 collection area, supplementary ticks were collected by flagging randomly around the collection area, concentrating on paths and animal tracks. Collection at site 2 was conducted in July 1999 solely by flagging along paths and tracks.
For site 1, PCR analysis was conducted on 25 adult females, 24 to 26 adult males, and 47 to 50 nymphs for each collection date. For site 2, all 46 ticks collected were analyzed. Only ticks collected in 1999 were subjected to PCR analysis.
Extraction of DNA from ticks.
After removal of excess ethanol, ticks were cut in half longitudinally with a flame-sterilized scalpel blade and transferred to a pellet-pestle tube (Kontes Scientific Glassware/Instruments, Vineland, N.J.) containing 60 μl of proteinase K (40 μg/ml)–1 mM Tris-HCl (pH 8.0)–2 mM EDTA–0.01% Tween 20 and crushed using a pellet pestle until release of abdominal contents was visible. After incubation at 65°C for 1 h and 100°C for 10 min, tick extracts were stored at −20°C until use for PCR.
Detection of Borrelia and Ehrlichia by PCR.
Ten-microliter aliquots of the tick extract were amplified in 100-μl PCRs using granulocytic Ehrlichia-specific primers EHR521-EHR747 (24). Five-microliter aliquots of tick extract were amplified in 50-μl multiplex PCRs using species-specific Borrelia primers GI-R–GI-L (B. burgdorferi sensu stricto), GII-R–GII-L (B. garinii), and GIII-R–GIII-L (B. afzelii) (8) as previously described (16). PCR products were detected on 2% agarose gels stained with SYBR-gold (Molecular Probes, Eugene, Oreg.) and photographed under 302-nm UV transillumination.
Samples positive using the EHR521-EHR747 primer set were reamplified using the primer set 16S8FE–B-GA1B, and Ehrlichia species were determined using the reverse line blot assay as previously described (30). Where this assay was negative, the sequence of the EHR521-EHR747 amplicon was determined (MWG Biotech, Ebersburg, Germany) and compared with public-domain databases using the BLAST software (Swiss Institute for Bioinformatics [http://www.ch.embnet.org]).
Statistical methods.
Statistical significance was calculated using the χ2 test.
RESULTS
Density and population structure of ticks.
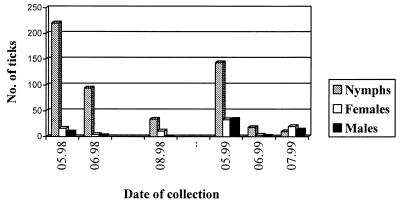
The density of ticks at site 1 was monitored over a period of 2 years (Fig. 1). Densities varied during the season, reaching a maximum of 245 ticks/100 m2 in May 1998 and showing a general tendency to decline over summer, reaching a minimum of 20 ticks/100 m2 in June 1999.
FIG. 1.
Density and stage of ticks per 100 m2 at site 1, May 1998 to July 1999.
Nymphs were generally the most abundant stage found. The ratio of nymphal to adult ticks varied between 15.6:1 in June 1998 and 0.3:1 in July 1999 and was higher in 1998 (8.3:1) than in 1999 (1.6:1). Female ticks were more abundant than males in both years (3:1 in 1998, 1.2:1 in 1999).
Prevalence of Borrelia in ticks.
The prevalence of Borrelia species is shown in Table 1. The overall prevalence of Borrelia in ticks at site 1 (May to July 1999) was 16% (46 of 294 ticks) and was significantly higher (P < 0.0005) in May (32% [31 of 96 ticks]) than in June (7% [7 of 98 ticks]) or July (8% [8 of 98 ticks]). The prevalence of Borrelia at site 2 in July was 17% (8 of 47 ticks).
TABLE 1.
Results of Borrelia and Ehrlichia species determination for ticks from site 1 and site 2
| Location and date | Instar (no.) of ticks | No. of ticks infected witha:
|
Total no. of ticks that wereb:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Borrelia
|
Ehrlichia
|
Borrelia + Ehrlichia
|
||||||||||||||
| Afz | SS | Gar | Afz + SS | ELO | HGE | Ph | Esp | ELO + Afz | ELO + Afz + SS | Esp + Afz + SS | Borr Pos | Ehr Pos | Borr + Ehr Pos | Neg | ||
| Site 1 | ||||||||||||||||
| May 1999 | Nymph (47) | 4 | 1 | 3 | 1 | 5 | 2 | 1 | 8 | 1 | 8 | 30 | ||||
| Male (24) | 1 | 1 | 1 | 1 | 3 | 1 | 20 | |||||||||
| Female (25) | 7 | 2 | 2 | 1 | 1 | 1 | 9 | 3 | 2 | 11 | ||||||
| Total (96) | 12 | 4 | 0 | 4 | 2 | 0 | 1 | 1 | 6 | 4 | 1 | 20 | 4 | 11 | 61 | |
| June 1999 | Nymph (47) | 3 | 1 | 2 | 1 | 3 | 4 | 40 | ||||||||
| Male (26) | 1 | 1 | 1 | 1 | 24 | |||||||||||
| Female (25) | 3 | 1 | 1 | 1 | 3 | 3 | 19 | |||||||||
| Total (98) | 7 | 0 | 0 | 0 | 2 | 1 | 3 | 2 | 0 | 0 | 0 | 7 | 8 | 0 | 83 | |
| July 1999 | Nymph (50) | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 5 | 42 | ||||||
| Male (25) | 25 | |||||||||||||||
| Female (25) | 4 | 1 | 1 | 1 | 5 | 2 | 18 | |||||||||
| Total (100) | 6 | 1 | 1 | 3 | 1 | 1 | 2 | 0 | 0 | 0 | 8 | 7 | 0 | 85 | ||
| Total for site 1 (294) | 25 | 4 | 1 | 5 | 7 | 2 | 5 | 5 | 6 | 4 | 1 | 35 | 19 | 11 | 229 | |
| Site 2 (July 1999) | Nymph (41) | 6 | 1 | 2 | 6 | 1 | 2 | 32 | ||||||||
| Male (6) | 6 | |||||||||||||||
| Female (0) | ||||||||||||||||
| Total (47) | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 6 | 1 | 2 | 38 | |
Abbreviations: Afz, B. afzelii; SS, B. burgdorferi sensu stricto; Gar, B. garinii; ELO, Ehrlichia-like organism; HGE, HGE agent variant; Ph, E. phagocytophila variant; Esp, Ehrlichia, species not determined.
Borr Pos, positive for B. burgdorferi sensu lato; Ehr Pos, positive for Ehrlichia; Borr+Ehr Pos, positive for both B. burgdorferi sensu lato and Ehrlichia; Neg, negative for both Borrelia and Ehrlichia.
B. afzelii was the dominant species at both sites and at all time points. Its prevalence at site 1 varied between 29% (27 of 96 ticks) in May and 7% (7 of 98 ticks) in June and July. The prevalence of B. burgdorferi sensu stricto at site 1 was 14% (13 of 96 ticks) in May; in subsequent months only a single B. burgdorferi sensu stricto-positive tick was detected. B. garinii was observed only in a single tick collected at site 1 in July. The prevalence of B. afzelii at site 2 in July was 17% (8 of 47 ticks); this was the only Borrelia species detected at this site.
Ehrlichia prevalence in ticks.
Screening of ticks by PCR with primers EHR521-EHR747 gave positive results with 37 of 341 ticks. Results of species determination by reverse line blot and DNA sequencing are shown in Table 1. Four positive results could be attributed to sequences with 98% homology to species of Wolbachia (12, 28, 29). The remaining 33 positive results are attributed to the presence of Ehrlichia, although in six cases species determination could not be completed. A total of 30 of 294 ticks (11.5%) collected at site 1 in the period May to July 1999 and 3 of 44 ticks (7%) collected at site 2 in July 1999 contained Ehrlichia. The predominant Ehrlichia species was an Ehrlichia-like organism previously described in ticks from Holland (20 of 33 ticks); variants of E. phagocytophila (5 of 33 ticks) and HGE agent (2 of 33 ticks) were also detected.
The prevalence of the Ehrlichia-like organism at site 1 declined from 13% (12 of 96 ticks) in May to 2% (2 of 98 ticks) in June and 3% (3 of 98 ticks) in July (χ2 = 11.73; P = 0.003). The prevalence of other Ehrlichia species was too low to allow assessment of monthly variation.
Interstadial variation in Borrelia and Ehrlichia prevalence.
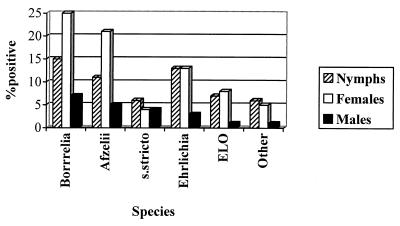
Figure 2 shows interstadial variation in the prevalence of Borrelia and Ehrlichia species (see also Table 1). The rates of prevalence of Borrelia in nymphs, females, and males were, respectively, 15% (22 of 144 ticks), 25% (19 of 75 ticks), and 7% (5 of 75 ticks), these differences being significant (χ2=9.93; P = 0.007) and largely due to differences in the prevalence of B. afzelii. Ehrlichia was most prevalent in nymphs (13% [18 of 144 ticks]) and females (13% [10 of 75 ticks]) and least prevalent in males (3% [2 of 75 ticks]). This pattern is seen both for the Ehrlichia-like organism and for other Ehrlichia species. The reduced prevalence of Ehrlichia in male ticks is statistically significant (χ2 = 6.28; P = 0.043).
FIG. 2.
Interstadial variation in prevalence of B. burgdorferi sensu lato (Borrelia), B. afzelii (Afzelii), B. burgdorferi sensu stricto (s.stricto), Ehrlichia species (Ehrlichia), the Ehrlichia-like organism (ELO), and other Ehrlichia species. Data are for site 1, May to July 1999, and include 144 nymphs, 75 females, and 75 males divided equally between three collection dates. All occurrences of ticks containing the species indicated, including multiply positive ticks, are counted.
Double infections.
Eleven ticks from site 1 (11 of 294 ticks [3.7%]) contained both Borrelia and Ehrlichia (Table 1). All these ticks contained B. afzelii, and, with a single exception, where the Ehrlichia species was not identified, all contained the Ehrlichia-like organism. All of these doubly infected ticks were collected in May. This is an approximately fourfold excess of double infections over that expected from a random association of these two organisms (0.8%) and is statistically significant both for the entire sample (χ2 = 27.66; P < 0.005) and for May (χ2 = 20.68; P < 0.005). Of these 10 double infections, 7 were in nymphal ticks.
Ten ticks from site 1 contained both B. afzelii and B. burgdorferi sensu stricto. This represents 71% of ticks (10 of 14) positive for B. burgdorferi sensu stricto and 3.4% of all ticks collected at site 1. Nine of the ten doubly positive ticks were collected in May. This is an approximately fivefold excess over that expected by chance association (0.7%) and is significant for the entire sample (χ2 = 40.47; P < 0.05) and for May (χ2 = 12.57; P < 0.05). Eight of the doubly infected ticks were nymphs.
Comparison of the two geographical locations.
Comparison of Borrelia and Ehrlichia prevalence data for site 2 on 13 July 1999 with those for site 1 at the two bracketing dates, 23 June and 27 July (Table 1), suggests marked differences in the burden of tick-borne microflora. In nymphal ticks, the prevalence of B. afzelii was 15% (6 of 41 ticks) at site 2 and 6% (6 of 97 ticks) at site 1, while the prevalence of Ehrlichia was 6% (3 of 41 ticks) at site 2, including only the Ehrlichia-like organism, and 9% (9 of 97 ticks) at site 1, including a wider range of Ehrlichia species, of which the Ehrlichia-like organism comprised only one-third. However, these differences are not statistically significant.
DISCUSSION
We have investigated the prevalence of B. burgdorferi sensu lato and Ehrlichia in questing ticks at two sites in southeastern Norway. At site 1, the prevalence of Borrelia was 16%, comprising 14% B. afzelii, 5% B. burgdorferi sensu stricto, <1% B. garinii, and 3.4% B. burgdorferi-B. afzelii double infections, and the prevalence of Ehrlichia was 11.5%, comprising 6% Ehrlichia-like organism, 1.7% E. phagocytophila variant, and 0.6% HGE agent variant. At site 2, the prevalence of Borrelia was 17% and the prevalence of Ehrlichia was 6%. Only B. afzelii and the Ehrlichia-like organism were detected at site 2. The dominant Ehrlichia species detected here, the Ehrlichia-like organism, was first detected in ticks feeding on deer in Holland (30). The 16S rRNA gene sequence indicates that this species belongs to the monocytic Ehrlichia group, but unfortunately nothing is known about its biology.
Aside from the predominant Ehrlichia-like organism, we note the presence of variants of E. phagocytophila and the HGE agent previously identified in ticks in Holland (30) and Sweden (36). The E. phagocytophila variant has also been found in white-tailed deer in Wisconsin (2), and HGE agent variants have been found in connection with human disease in Scandinavia (4). Ticks carrying bacteria with prototype HGE agent or E. phagocytophila 16S rRNA sequences were not found, in spite of the facts that prototype E. phagocytophila is known to be endemic in southern Norway and that a moose calf with prototype E. phagocytophila infection was found at site 2 prior (2 weeks) to the date of collection of ticks (16).
The clinical picture of Lyme borreliosis in Norway, where neuroborreliosis dominates, might lead one to expect that the dominant Borrelia species would be B. garinii, which is preferentially associated with neurological symptoms. However, our results show B. garinii to be very uncommon and B. afzelii to be dominant. Indeed, the B. garinii prevalence is, as far as we are aware, the lowest observed in Northern Europe. However, our sample of ticks is not necessarily representative for the whole of Norway (see below).
Comparable studies have been performed in Ireland (18), Switzerland (3, 27), Slovenia (25), Sweden (36), Finland (17), Wisconsin (24), Delaware (6), and Holland (30), although only in the last two of these studies, which investigated ticks collected from animals, were both Ehrlichia and Borrelia detected. The reported prevalences of Ehrlichia vary from 1.3% in Switzerland (E. phagocytophila genogroup) (27) to 50% in Connecticut (HGE agent) (21), and B. burgdorferi sensu lato prevalences varying from 1.3% in Switzerland (3) to 55% in areas of Finland (17) have been reported. Species composition for Borrelia varies, with B. afzelii being dominant in Holland (30) and Finland (17), as in this study, and B. valaisiana (not detected in this study) being dominant in Ireland (18) and Switzerland (3). In Holland, Schouls et al. (30) found members of the E. phagocytophila genogroup in more than 60% of Ehrlichia-positive ticks and Ehrlichia-like organism in 15%, whereas the E. phagocytophila genogroup and Ehrlichia-like organism were present in, respectively, 25 and 50% of positive ticks in this study. Geographical differences in the proportions of different tick-borne organisms might reflect local variation in the availability of host organisms with differential susceptibilities, self-perpetuating random differences in abundance, or cyclic fluctuations caused by transient population immunity.
At comparable dates, there was no obvious similarity between the two locations in the frequency and species composition for Borrelia and Ehrlichia species, except that B. afzelii was the dominant Borrelia species. The two localities are only 15 km apart, although they are separated by open water.
Site 1 was sampled in May, June, and July 1999. We found that the prevalences of B. afzelii, B. burgdorferi sensu stricto, and the Ehrlichia-like organism were much higher in May than in June and July. It is not possible at this point to determine whether these changes reflect seasonal effects in the prevalence of Borrelia and Ehrlichia species in ticks or merely random changes over time. A peak of Borrelia prevalence in spring and early summer has been reported from Sweden (35), while Stafford et al. (32) found no significant seasonal trend in the prevalence of B. burgdorferi in nymphal ticks in Connecticut over a 9-year period. We are currently working on a more complete time series from the same location in 2000 which should cast light on the question of seasonal trends in Borrelia and Ehrlichia prevalence.
Our results show that samples taken at different time points and from different locations may have very different prevalences of Borrelia and Ehrlichia. This would suggest that estimates based on spot studies such as this study may have only local and temporary applicability, which would limit their value in forming public health policy.
Double infections with B. afzelii and the Ehrlichia-like organism and with B. afzelii and B. burgdorferi occurred at a level four to five times that expected by chance association. Most occurred in ticks collected in May, when the prevalence of tick-borne bacteria and the density of ticks were highest, and they involved the three most prevalent tick-borne bacteria detected. Most doubly infected ticks were nymphs. As a nymphal tick has taken only one blood meal, these double infections must have been acquired from the same animal. We suggest that the excess of double infections may be the result of very high tick densities in the previous year. Under such conditions, host animals will be highly infested with ticks and thus prone to acquire multiple infections. Doubly infected animals have been shown to transmit double infections to feeding ticks (20). Borrelia-Ehrlichia coinfections and two-species Borrelia coinfections have been reported by a number of authors (9, 26). A seroepidemiological study in Norway (1) showed that 10% of patients seropositive for B. burgdorferi also had antibodies to Ehrlichia.
There was evidence of interstadial variation in the prevalence of B. afzelii, of the Ehrlichia-like organism, and of other Ehrlichia species. B. afzelii was most prevalent in adult females, less so in nymphs, and least so in males, while for Ehrlichia, the prevalence was similar in nymphs and females and low in males. The low prevalence of Borrelia and Ehrlichia in males might be explained in three ways: differential feeding (male and female immature ticks selecting different hosts), differential survival of the bacteria in males and females, or differential survival of infected ticks. Other studies have reported the prevalence of Borrelia to be greater in adults (18), greater in nymphs (22), or equal in both stages (23). We are not aware of any previous reports of interstadial variation in Ehrlichia prevalence.
Measurement of tick density over 2 years indicates a seasonal peak in May or earlier. This is consistent with previous findings of maximum nymphal tick activity in spring and early summer (14). This is likely to be a consequence of day length, which affects emergence from diapause, and humidity. The low-humidity conditions which are likely to be encountered in the later summer months inhibit both emergence from diapause and survival of questing ticks (7). The overall predominance of nymphal ticks is probably partly a consequence of the natural tendency of all populations to be dominated by young individuals but may also be a consequence of the fact that our collection period does not extend into autumn, when adults are predicted to be most abundant (11).
The PCR primers (EHR521-EHR747) used in this study were designed to be specific for the E. phagocytophila genogroup (24). However, these primers also detect the Ehrlichia-like organism, a member of the monocytic Ehrlichia group, and sequences related to Wolbachia. Thus, although these primers are useful for screening ticks prior to the application of more-precise species-specific methods, they might seriously overestimate the prevalence of the E. phagocytophila group if used alone. Wolbachia persicus has been isolated from a number of tick species and is known to be pathogenic for the soft tick Ornithodorus moubata (39). Wolbachia symbionts modulate reproductive function in arthropods, causing, among other effects, cytoplasmic incompatibility and parthenogenesis and distorted sex ratios (12, 28, 29). This might explain the high ratio of females to males (3:1) observed in 1998.
This is the first study of Ehrlichia and Borrelia in ticks from Norway. We observe the lowest hitherto reported Northern European prevalence of B. garinii, though a more widespread survey will be needed to determine if this is related to the fact that I. ricinus reaches the northwestern limit of its distribution in Norway. This is also the first study in questing ticks of the prevalence of a recently described Ehrlichia-like organism, related to the monocytic ehrlichiae, and shows this organism to be the dominant Ehrlichia species in the locality. Time series results are consistent with a peak prevalence of this organism in spring and early summer, though further data are needed to confirm this trend. Temporal variations in the prevalence of Ehrlichia species have not to our knowledge been reported previously.
ACKNOWLEDGMENTS
We thank Pia M. Øistad, Katrine Pedersen, and Jannicke F. Remme. for assistance with the collection of ticks. We also acknowledge the advice of Reidar Mehl in the selection of a collection site and methods of collection, Tone Grande for help with statistical analyses, and Joe Bunnell for teaching us his method for extracting DNA from ticks.
REFERENCES
- 1.Bakken J S, Kreuth J, Tilden R L, Dumler J S, Kristiansen B E. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1996;15:829–832. doi: 10.1007/BF01701530. [DOI] [PubMed] [Google Scholar]
- 2.Belongia E, Reed K D, Mitchell P D, Kolbert C P, Persing D H, Gill J S, Kazmierczak J J. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J Clin Microbiol. 1997;35:1465–1468. doi: 10.1128/jcm.35.6.1465-1468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernasconi M V, Valsangiacomo C, Balmelli T, Péter O, Piffaretti J. Tick zoonoses in the southern part of Switzerland (Canton Ticino): occurrence of Borrelia burgdorferi sensu lato and Rickettsia sp. Eur J Epidemiol. 1997;13:209–215. doi: 10.1023/a:1007394901846. [DOI] [PubMed] [Google Scholar]
- 4.Bjöersdorf A, Berglund J, Kristiansen B E, Söderström C, Eliasson I. Human granulocytic ehrlichiosis: 12 Scandinavian case reports of the new tick-borne zoonosis. Svensk Vet Tidning. 1999;51:29–34. [PubMed] [Google Scholar]
- 5.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 6.Curran K L, Kidd J B, Vassallo J, Van Meter V L. Borrelia burgdorferi and the causative agent of human granulocytic ehrlichiosis in deer ticks, Delaware. Emerg Infect Dis. 2000;6:408–411. doi: 10.3201/eid0604.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel M, Dusbabek F. Micrometeorological and microhabitat factors affecting maintenance and dissemination of tick-borne diseases in the environment. In: Sonenshine D E, Mather T N, editors. Ecological dynamics of tick-borne zoonoses. Oxford, United Kingdom: Oxford University Press; 1994. pp. 68–90. [Google Scholar]
- 8.Demaerschalk I, ben Messaoud A, de Kesel M, Hoyois B, Lobet Y, Hoet P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fingerle V, Munderloh U G, Liegl G, Wilske B. Coexistence of ehrlichiae of the phagocytophila group with Borrelia burgdorferi in Ixodes ricinus from Southern Germany. Med Microbiol Immunol (Berlin) 1999;188:145–149. doi: 10.1007/s004300050117. [DOI] [PubMed] [Google Scholar]
- 10.Folkehelsa. Surveillance of communicable diseases in Norway 1999. Oslo, Norway: Statens Institutt for Folkehelsa; 1999. [Google Scholar]
- 11.Gardiner W P, Gettinby G A. A weather-based prediction model for the life cycle of the sheep tick, Ixodes ricinus L. Vet Parasitol. 1983;13:77–84. doi: 10.1016/0304-4017(83)90022-5. [DOI] [PubMed] [Google Scholar]
- 12.Giordano R, Jackson J J, Robertson H M. The role of Wolbachia bacteria in reproductive incompatibilities and hybrid zones of Diabrotica beetles and Gryllus crickets. Proc Natl Acad Sci USA. 1997;94:11439–11444. doi: 10.1073/pnas.94.21.11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granström M. Tick-borne zoonoses in Europe. Clin Microbiol Infect. 1997;3:156–169. doi: 10.1111/j.1469-0691.1997.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 14.Gray J S. The development and seasonal activity of the tick Ixodes ricinus, a vector of Lyme borreliosis. Rev Med Vet Entomol. 1991;79:323. [Google Scholar]
- 15.Hubbard M J, Baker A S, Cann K J. Distribution of Borrelia burgdorferi spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th century assessed by PCR. Med Vet Entomol. 1998;12:89–97. doi: 10.1046/j.1365-2915.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins A J, Handeland K, Stuen S, Schouls L M, Meen R T, Kristiansen B E. Granulocytic ehrlichiosis in a moose calf. J Wildl Dis. 2001;37:201–203. doi: 10.7589/0090-3558-37.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Junttila J, Peltomaa M, Soini H, Marjamäki M, Viljanen M K. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J Clin Microbiol. 1999;37:1361–1365. doi: 10.1128/jcm.37.5.1361-1365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirstein F, Rijpkema S, Mokenboer M, Gray J S. The distribution and prevalence of B. burgdorferi genomospecies in Ixodes ricinus ticks in Ireland. Eur J Epidemiol. 1997;13:67–72. doi: 10.1023/a:1007360422975. [DOI] [PubMed] [Google Scholar]
- 19.Lane R S. Competence of ticks as vectors of microbial agents with an emphasis on Borrelia burgdorferi. In: Sonenshine D E, Mather T N, editors. Ecological dynamics of tick-borne zoonoses. Oxford, United Kingdom: Oxford University Press; 1994. pp. 45–67. [Google Scholar]
- 20.Levin M L, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68:2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnarelli L A, Stafford III K C, Mather T N, Yeh M, Horn K D, Dumler J S. Hemocytic Rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–2714. doi: 10.1128/jcm.33.10.2710-2714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matuschka F R, Ohlenbusch A, Eiffert H, Richter D, Spielman A. Characteristics of Lyme disease spirochetes in archived European ticks. J Infect Dis. 1996;174:424–426. doi: 10.1093/infdis/174.2.424. [DOI] [PubMed] [Google Scholar]
- 23.Nuttall P A, Randolph S, Carey D, Craine N, Livesley A, Gern L. The ecology of Lyme borreliosis in the UK. In: Axford J S, Rees D H E, editors. Lyme borreliosis. New York, N.Y: Plenum Press; 1994. pp. 125–129. [Google Scholar]
- 24.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 25.Petrovec M, Sumner J W, Nicholson W L, Childs J E, Strle F, Barlič J, Lotrič-Furlan S, Avšič-Županc T. Identity of Ehrlichia DNA sequences derived from Ixodes ricinus ticks with those obtained from patients with human granulocytic ehrlichiosis in Slovenia. J Clin Microbiol. 1999;37:209–210. doi: 10.1128/jcm.37.1.209-210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichon B, Godfroid E, Hoyois B, Bollen A, Rodhain F, Perez-Eid C. Simultaneous infection of Ixodes ricinus nymphs by two Borrelia burgdorferi sensu lato species. Possible implications for clinical manifestations. Emerg Infect Dis. 1995;1:89–90. doi: 10.3201/eid0103.950304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusterla N, Leutenegger C M, Huder J B, Weber R, Braun U, Lutz H. Evidence of the human granulocytic ehrlichiosis agent in Ixodes ricinus ticks in Switzerland. J Clin Microbiol. 1999;37:1332–1334. doi: 10.1128/jcm.37.5.1332-1334.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousset F, Solignac M P. Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proc Natl Acad Sci USA. 1995;92:6389–6393. doi: 10.1073/pnas.92.14.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc R Soc Lond B Biol Sci. 1992;250:91–98. doi: 10.1098/rspb.1992.0135. [DOI] [PubMed] [Google Scholar]
- 30.Schouls L M, van de Pol I, Rijpkema S G T, Schott C S. Detection and identification of Ehrlichia, Borrelia Burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonenshine D E. Introduction. In: Sonenshine D E, Mather T N, editors. Ecological dynamics of tick-borne zoonoses. Oxford, United Kingdom: Oxford University Press; 1994. pp. 3–19. [Google Scholar]
- 32.Stafford K C, III, Cartter M L, Magnarelli L A, Ertel S H, Mshar P A. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuen S. The distribution of tick-borne fever in Norway. Norsk Veterinærtidskrift. 1997;109:83–87. [Google Scholar]
- 34.Stuen S. Sjodogg (tick-borne fever)—a historical review. Norsk Veterinærtidskrift. 1998;110:703–705. [Google Scholar]
- 35.Talleklint L, Jaenson T G T. Seasonal variation in density of questing Ixodes ricinus (Acari: Ixodidae) nymphs and prevalence of infection with B. burgdorferi s.l. in south central Sweden. J Med Entomol. 1996;33:592–597. doi: 10.1093/jmedent/33.4.592. [DOI] [PubMed] [Google Scholar]
- 36.Von Stedingk L V, Gürtelschmid M, Hanson H S, Gustafson R, Dotevall L, Olsson Engval E, Granström M. The human granulocytic ehrlichiosis agent in Swedish ticks. Clin Microbiol Infect Dis. 1997;3:573–574. doi: 10.1111/j.1469-0691.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 37.Walker D H the Task Force on Consensus Approach for Ehrlichiosis. Diagnosing human ehrlichioses: current status and recommendations. ASM News. 2000;66:287–290. [Google Scholar]
- 38.Wang G, van Dam A P, Schwartz I, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological and clinical implications. Clin Microbiol Rev. 1999;12:633–635. doi: 10.1128/cmr.12.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss E, Dasch G A. The family Rickettsiaceae: pathogens of domestic animals and invertebrates; nonpathogenic arthropod symbionts. In: Starr M P, Stolp H, Truper H G, Balows A, Schlegel H G, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1981. pp. 2161–2171. [Google Scholar]
- 40.Wilson M L. Population ecology of tick vectors: interaction, measurement and analysis. In: Sonenshine D E, Mather T N, editors. Ecological dynamics of tick-borne zoonoses. Oxford, United Kingdom: Oxford University Press; 1994. pp. 20–44. [Google Scholar]