Abstract
This study aimed to evaluate the antioxidant activity and total phenolic content (TPC) and total flavonoid content (TFC) of crude extracts obtained from three Asclepiadaceae species, namely, Calotropis procera L., Peruglaria tomentosa L., and Pentatropis spiralis (Forsk.) Decne. Both butanol and aq. methanol extracts of the three species showed the highest amount of phenol and flavonoid contents, which exhibited the greatest antioxidant activity in the scavenging of 2,2-diphenyl-2-picrylhydrazyl free radical (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical cation (ABTS), ferrous chelating effect (FIC), and hydroxyl radical (HDR) assays. Phytochemical screening of the extracts revealed the presence of alkaloids, tannins, sponins, flavonoids, terpenoids, and glycosides. LC-MS analysis was carried out to identify the major compounds from each crude extract. A total of 12 phenolic compounds in the extracts of the 3 species were identified and quantified, including 9 flavonoids, 2 hydroxybenzoic acids, and 3 hydroxycinnamic acids. The current study also revealed a good correlation between total phenolic contents and the observed antioxidant activity of the crude extracts.
Keywords: total phenolic, flavonoid, antioxidant properties, Calotropis procera, Peruglaria tomentosa, Pentatropis spiralis, Aclepiadaceae
1. Introduction
Medicinal plant species, which have recently grown in popularity, are abundant in different places around the world. They have a high content of bioactive compounds, such as flavonoids, phenolics, anthocyanins, phenolic acids, and non-nutritive and nutritive compounds such as essential oils, vitamins, minerals, etc. Recent research has shown that crude plant species have excellent medicinal value and healthcare functions, something which is also apparent from their familiar usage since ancient times for therapeutic, religious, cosmetic, nutritional, and beautification purposes [1,2,3,4].
Apocynaceae, Asclepiadoideae includes approximately 3000 species in 172 genera and has a worldwide distribution [5]. Many of these species produce cardiac glycosides which have been used for folk medicine and many traditional medical treatments, such as for mental illness and cancer [6,7]. Several species of the family are known as sources of bioactive substances [8] such as alkaloids, terpenoids, and iridoids [9].
Although the physiological process of oxidative metabolism is considered to play a significant role in cell survival, it has noted side effects due to the formation of free radicals and reactive oxygen species (ROS) that can cause damage to DNA, RNA, and protein. However, the human body has a protective mechanism in the form of antioxidants which interact with these products [10].
By definition, antioxidants are chemicals which are used to control oxidative reactions and decrease the adverse effects of reactive species in the biological system due to the low level of natural antioxidants in the body. Therefore, the current research focuses on the potential role of antioxidants and their enzymes in the treatment and prevention of atherosclerosis, heart failure, neurodegenerative disorders, aging, cancer, diabetes mellitus, and several other diseases and/or conditions [10]. Many medicinal plants are known to possess large amounts of polyphenols and potent antioxidant capacity or free radical scavenging activity [11,12,13].
Calotropis procera L. is colloquially known as Ishar in certain regions. It grows wild in the Dead Sea and Wadi Araba regions [14]. Additionally, different parts such as root, stem, leaves, flowers, and seeds of C. procera are traditionally used to cure several diseases and tackle various symptoms, such as fever, rheumatism, indigestion, cough, cold, eczema, asthma, elephantiasis, nausea, vomiting, leprosy, and diarrhea [15,16]. Phytochemical investigation of this plant has revealed the presence of triterpenes, triterpenoids, phytosterols, saponins, alkaloids and cardiac glycosides [17,18,19,20] in it, and it has also been found to have antioxidant, antimicrobial, and cytostatic properties [21,22,23].
Pentatropis spiralis (Forsk.) Decne is distributed in the tropical regions of Asia, Africa, and Australia. In Jordan, it is located in Ghor Al Mazraa [14]. The species contains biological active ingredient compounds such as triterpenes [24,25], which have been used as a remedy for gonorrhea and as a purgative [26,27].
Other poisonous species of the crude plant known as Peruglaria tomentosa L are distributed in Saharan and sub-Saharan countries of North Africa [27], including Algeria, Niger, and Egypt [28], and are also common in the Middle East region, including Saudi Arabia and Jordan [14]. This plant has been used extensively in traditional medicine as a depilatory, laxative, and anthelmintic and for skin diseases [29]. Previous phytochemical investigations have focused on checking, isolating, and characterizing several cardenolides, glycosides, flavonoid glycosides, and alkaloids, and β-sitosteryl glucoside [28,29,30,31,32,33,34].
Little information is available regarding the phytochemical constituents and antioxidant activity of C. procera, P. spiralis, and P. tomentosa (Figure 1). Therefore, the present study aimed to evaluate the total phenolic content (TPC) and total flavonoid content (TFC) of butanol, water, and aq. methanol extracts for the three species and then to identify these components using the LC-MS technique. In addition, the study evaluates the antioxidant activity of the three species.
Figure 1.
Plants used in this study.
2. Results and Discussion
2.1. Qualitative Phytochemical Analysis
Qualitative chemical analysis was carried out to ensure the presence of major phytochemical constituents in accordance with the procedures described in the Materials and Methods section of this paper [35]. The results associated with the crude extracts of C. procera and P. Spiralis (Table 1) indicated that the water fraction was present in most of the tested groups except glycosides and anthraquinones. Alkaloids and terpenes were also observed in all fractions of polar solvent, while flavonoids were absent in the aq. methanol fraction. This pattern is similar to that found in the water fraction of C. procera. Except for saponins, where the butanol extract was free of all tested groups. Phytochemical screening tests on crude extract fractions of P. tomentosa (Table 1) found that the constituents of the various fractions of P. tomentosa were different in their pattern compared with C. procera and P. spiralis. For instance, P. tomentosa was the only plant that contained glycosides, in the form of a water fraction. Moreover, alkaloids were only observed in the aq. methanol fraction of P. tomentosa.
Table 1.
Major phytochemical groups detected in crude extract fractions of C. procera, P. spiralis and P. tomentosa.
| Groups | C. procera | P. spiralis | P. tomentosa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | A | W | B | A | W | B | A | W | |
| Alkaloids | + | + | + | - | + | + | - | + | - |
| Tannins | + | - | + | - | + | + | + | - | + |
| Flavonoids | + | - | + | - | - | + | + | + | - |
| Saponins | - | + | + | + | + | + | - | + | - |
| Anthraquinone | - | - | - | - | - | - | - | - | - |
| Glycosides | - | - | - | - | - | - | - | - | + |
| Terpenoids | - | + | + | + | - | - | |||
B = butanol extract, A = aq. MeOH extract, W = water extract.
2.2. LC-MS/MS Analysis of Phytochemicals
LC-MS/MS is a confirmed hyphenated and accurate tool for rapid analysis, and it was used for the identification of a total of 12 phenolic compounds in the C. procera, P. spiralis, and P. tomentosa extracts. In all, 9 flavonoids, 2 hydroxybenzoic acids, and 3 hydroxycinnamic acids were identified by comparing their retention times and mass fragmentation pattern with data obtained from commercial standards, and then each individual compound was quantified by comparing its peak area with the calibration curve obtained for the corresponding standard (Table 2).
Table 2.
LC-QTOF-MS/MS analysis data of detected metabolites from the crude extracts of C. procera, P. spiralis, and P. tomentosa extracts.
| No. | Rt Min | Compound | Relative Percentage Amounts (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. procera | P. spiralis | P. tomentosa | |||||||||
| B | A | W | B | A | W | B | A | W | |||
| 1 | 2.57 | 4-Hydroxybenzoic acid | 2.31 | 11.23 | - | - | - | - | 3.67 | 13.62 | - |
| 2 | 3.58 | Syringic acid | - | 5.36 | - | - | - | 0.29 | - | - | - |
| 3 | 4.45 | p-Coumaric acid | 5.21 | 15.83 | 0.14 | 0.82 | 2.38 | - | 4.26 | - | 0.29 |
| 4 | 5.15 | Ferulic acid | 6.42 | - | 0.78 | 10.20 | - | - | 2.31 | - | - |
| 5 | 5.16 | 3-Glu-7-Rha Quercetin | 2.24 | - | 8.64 | 7.36 | 3.12 | 10.88 | - | - | - |
| 6 | 5.44 | 3-Hydroxy-4-methoxycinnamic acid | - | - | 5.88 | - | - | - | 3.61 | 6.13 | 10.88 |
| 7 | 5.77 | Spiraeoside | 1.45 | 9.01 | - | 3.92 | 6.41 | - | - | - | - |
| 8 | 6.26 | Luteolin 7-O-glucoside | 6.51 | 1.45 | - | - | - | 4.13 | 11.91 | 2.45 | - |
| 9 | 6.54 | Kaempferol-3-O-glucoside | 20.36 | 5.12 | 4.13 | 1.11 | 2.59 | - | 21.63 | - | 4.13 |
| 10 | 6.99 | Kaempferol-7-O-glucoside | 8.41 | - | 0.29 | - | - | - | 16.90 | - | 0 |
| 11 | 8.53 | Quercetin | - | 3.85 | - | 0.60 | 9.60 | - | - | 4.76 | - |
| 12 | 10.08 | Kaempferol | - | - | - | 0.31 | 24.79 | - | - | - | - |
| 13 | 10.47 | Isorhamnetin | 9.14 | - | - | 2.64 | 7.77 | - | - | - | - |
| 14 | 13.89 | Kaempferide | - | - | - | 8.46 | 7.56 | - | - | - | - |
B = butanol extract, A = aq. MeOH extract, W = water extract.
Kaempferol-3-O-glucoside appeared as the major phenolic subclass found in the butanol extract of C. procera (20.36%), whereas 15.83% of p-coumaric acid was found in the aq. methanol extract of the same plant. Quercetin, kaempferide, ferulic acid, and 3-glu-7-Rha Quercetin appeared as the predominant compounds in the P. spiralis extracts. Kaempferol-3-O-glucoside, kaempferol-7-O-glucoside, luteolin 7-O-glucoside, and 3-hydroxy-4-methoxycinnamic acid were the major compounds in the P. tomentosa extracts. The final findings of this study showed that the profiles and contents of phenolic compounds vary depending on the crude extract of the plant.
2.3. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
The results of TPC and TFC of C. procera, P. spiralis, and P. tomentosa extracts are listed in Table 3. They show concentrated phenols in the polar fractions of the extracts, and the highest levels of phenols and flavonoids were observed in the butanol fraction, followed by the aq. methanol fraction. About 50% of the overall phenols extracted by all solvents was contained in the butanol fraction. Flavonoids showed an almost similar behavior to that of phenols, and most of them were predominant in the butanol extract, followed by the aq. methanol and the water extracts. Uncharacteristically, the content of flavonoids in the butanol extract was extremely high, representing about 86% of the overall flavonoids extracted from all solvents.
Table 3.
TPC and TFC of all extracts of C. procera, P. spiralis and P. tomentosa.
| Extracts | TPC (mg/g Gallic Acid) | TFC (mg/g Quercetin) | ||||
|---|---|---|---|---|---|---|
| C. procera | P. spiralis | P. tomentosa | C. procera | P. spiralis | P. tomentosa | |
| B | 377.2 ± 2.6 | 113.2 ± 2.3 | 311.50 ± 3.04 | 82.7 ± 1.3 | 168.5 ± 0.9 | 107.7 ± 1.5 |
| A | 126.4 ± 4.5 | 59.2 ± 1.9 | 213.00 ± 1.32 | 52.3 ± 1.8 | 112.1 ± 1.1 | 69.9 ± 1.6 |
| W | 181.5 ± 2.5 | 30.8 ± 1.6 | 62.00 ± 1.80 | 28.2 ± 2.8 | 104.3 ± 0.8 | 21.0 ± 0.8 |
Values expressed are means ± S.D. of three parallel measurements: B = butanol extract, A = aq. MeOH extract, W = water extract.
2.4. Antioxidant Activity
Various methods have been used to evaluate the free radical scavenging activity of plant extracts [36,37,38,39,40,41,42,43,44,45,46,47,48]. In the present study, four methods were used to evaluate antioxidant activity for the aq. methanol, butanol, and water extracts of three plants: DPPH, ABTS radical scavenging, hydrogen peroxide scavenging (HDR), and ferrous ion chelating activity (FIC) assays (Table 4). The results obtained using the DPPH and ATBS methods clearly indicate a dose-dependent antioxidant activity of the crude extract (Figure 2 and Figure 3).
Table 4.
IC50 (mg/mL) values of crude extracts from C. procera, P. spiralis, and P. tomentosa and standards using the DPPH, ABTS, HDR, and FIC methods.
| Plant | Crude | DPPH | ABTS | HDR | FIC |
|---|---|---|---|---|---|
| C. procera | Butanol | 0.26 ± 0.02 | 0.10 ± 0.01 | 0.52 ± 0.01 | 1.31 ± 0.07 |
| Aq. methanol | 0.35 ± 0.01 | 0.25 ± 3.0 × 10−3 | 0.86 ±0.02 | 0.85 ± 0.03 | |
| Water | 0.40 ± 0.01 | 0.58 ± 0.02 | 0.06± 0.01 | 0.09 ± 0.01 | |
| P. spiralis | Butanol | 0.15 ± 0.02 | 8.60 × 10−5 ± 1.0 × 10−5 | 0.29 ± 0.03 | 0.72 ± 0.02 |
| Aq. methanol | 0.54 ± 0.04 | 0.07 ± 3.0 × 10−3 | 0.37 ± 0.02 | 1.77 ± 0.13 | |
| Water | 1.16 ± 0.01 | 0.48 ± 0.01 | 1.16 ± 0.03 | 1.72 ± 0.02 | |
| P. tomentosa | Butanol | 0.35 ± 0.04 | 0.11 ± 0.01 | 0.88 ± 0.02 | 2.67 ±0.23 |
| Aq. methanol | 0.54 ± 0.01 | 0.15 ± 0.01 | 0.43 ± 0.09 | 0.26 ± 0.03 | |
| Water | 0.83 ± 0.04 | 0.49 ± 0.02 | 0.72 ± 0.04 | 0.44 ± 0.01 | |
| Ascorbic acid (Vit. C) | 1.78 × 10−3 ± 6.0 × 10−5 | 1.58 × 10−3 ± 3.0 × 10−5 | 2.6 × 10−3 ± 3.0 × 10−5 | 1.89 × 10−3 ± 2.00 × 10−5 | |
| α-tocopherol | 2.33 × 10−3 ± 4.0 × 10−5 | 1.79 × 10−3 ± 1.0 × 10−5 | - | - | |
| EDTA | - | - | 0.013 ± 1.5 × 10−5 | 0.02 ± 1.1 × 10−5 | |
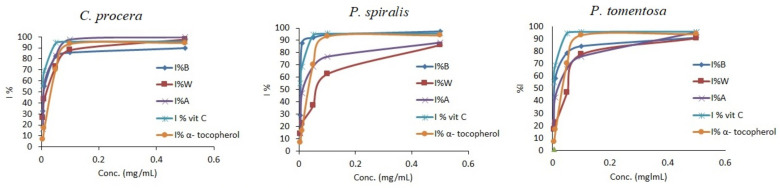
Figure 2.
Antioxidant activity of crude extracts from C. procera, P. spiralis, and P. tomentosa and standards using the DPPH method. B = butanol extract, A = aq. MeOH extract, W = water extract.
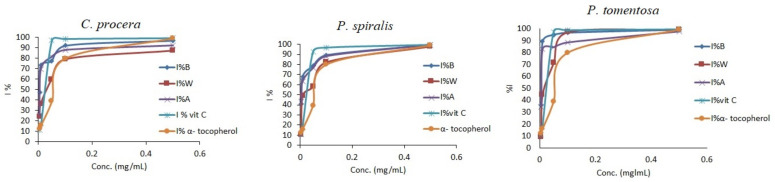
Figure 3.
Antioxidant activity of crude extracts from C. procera, P. spiralis, and P. tomentosa and standards using the ABTS method. B = butanol extract, A = aq. MeOH extract, W = water extract.
Furthermore, in Table 4, the results indicate the order of radical scavenging power—for instance, butanol > aq. methanol > water in three species. The DPPH and ABTS scavenging activities of the butanol extracts from C. procera (IC50 = 0.26 ± 0.02 mg/mL and 0.10 ± 0.01 mg/mL, respectively), P. spiralis (IC50 = 0.15 ± 0.02 mg/mL and 8.60 × 10−5 ± 1.0 × 10−5 mg/mL, respectively), and P. tomentosa (IC50 = 0.35 ± 0.04 mg/mL and 0.11 ± 0.01 mg/mL, respectively) are also shown. These values demonstrate the strong antioxidant activity of the butanol extracts compared with those of α-tocopherol and Vit. C (Table 4), which may be attributed to butanol extracts’ high TPC and TFC.
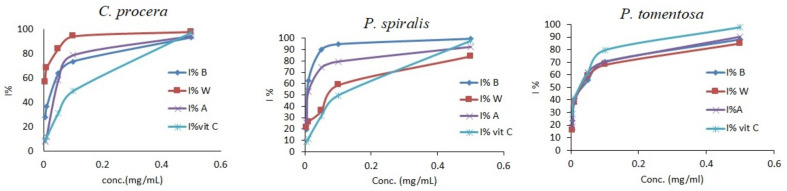
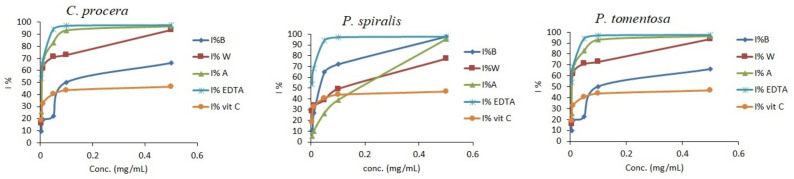
Because of the highly reactive species of hydroxyl radicals toward proteins, lipids, and DNA, with their markedly harmful effects on cell survival when overproduced [49], removal of these types of radicals is particularly important for living systems in order to maintain redox homeostasis. Figure 4 shows the scavenging activities of extracts obtained from C. procera, P. spiralis, and P. tomentosa. It reveals that HDR activity was also concentration-dependent, and again, the W fraction obtained from C. procera, B extract obtained from P. spiralis, and A extract obtained from P. tomentosa were the most active fractions (Figure 4, Table 4). The chelating activities of all crude extracts of C. procera, P. spiralis, and P. tomentosa were also investigated using the FIC method (Figure 5), and the results showed that increases took place in a dose-dependent manner. Based on the IC50 values, the water fractions in the three plants had the highest chelating activity (Table 4).
Figure 4.
Antioxidant activity of crude extracts from C. procera, P. spiralis, and P. tomentosa and standards using the HDR method. B = butanol extract, A = aq. MeOH extract, W = water extract.
Figure 5.
Antioxidant activity of crude extracts from C. procera, P. spiralis, and P. tomentosa and standards using the FIC method. B = butanol extract, A = aq. MeOH extract, W = water extract.
3. Materials and Methods
3.1. Plant Materials
Fresh aerial parts of C. procera, P. spiralis, and P. tomentosa were collected from Jordan Valley and Aqaba in June 2014. The plants were identified by Jamil N. Lahham, Department of Biology, Faculty of Science, Yarmouk University (C. procera (Aiton) Aiton fil (YU/01/AC/1001), P. spiralis (Forskal) Decne (YU/09/AP/1001), and P. tomentosa L. (YU/10/AP/1001)). All plants were air-dried in shade.
3.2. Materials and Equipment
UV spectra were measured using a Biochrom WPA Wave light II UV–visible spectrophotometer. All chemicals used in this investigation were purchased from Sigma-Aldrich (Buchs, Switzerland), including DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzoline-6-sulfonic acid) diammonium salt), Folin & Ciocalteu’s phenol reagent, ferrozin(3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p’-disulfonic acid monosodium salt hydrate), FeCl2 (VWR, Randor, PA, USA), NaOH, AlCl3, Na2CO3, NaNO2, H2O2, FeSO4, and salicylic acid (Sigma-Aldrich, Buchs, Switzerland). K2S2O8 was a product of Fluka (Steinheim, Germany).
3.3. Extraction and Partitioning
Air-dried and ground plant materials obtained from all plants were extracted using a Soxhlet extractor with petroleum ether to remove fatty acids. After drying, plant residue was extracted using the same apparatus with methanol. The obtained alcoholic gummy residue was then partitioned between CHCl3 and H2O (1:1) in accordance with the procedures mentioned in the literature [36,37,38,39,40,41,42,43,44,45,46,47,48]. The dried chloroform residue was then subjected to partitioning between 10% aqueous methanol and hexane. Polar organic compounds were extracted from water using n-butanol. Thereafter, all fractions obtained were screened for their phytochemical constituents and assayed for their total phenol, total flavonoids, and in vitro antioxidant activities.
3.4. Qualitative Phytochemical Analysis
Crude extracts obtained from C. procera, P. spiralis, and P. tomentosa were tested for the presence of flavonoids, alkaloids, tannins, terpenes, saponins, and glycosides in accordance with the procedures described in the literature [35]. Qualitative results expressed as (+) for the presence and (−) for the absence of the indicated phytochemical classes are summarized in Table 1.
3.5. LC-MS Analysis of Phytochemicals
Metabolite profiling of the crude extracts was carried out using an A Bruker Daltonik (Bremen, Germany) Impact II ESI-Q-TOF system equipped with a Bruker Daltonik Elute UHPLC system (Bremen, Germany) in both positive (M + H) and negative (M − H) electrospray ionization modes (Table 2). Chromatographic separation was performed on a C18 reversed phase column (100 × 2.1 mm, 1.8 µm, 120 Å) from Bruker Daltonik (Bremen, Germany) at 30 °C, with an autosampler temperature of 8 °C and a total run time of 20 min using water/methanol (90:10%) with 5 mM ammonium formate and 0.1% formic acid as an eluent. Plant samples were dissolved with 2.0 mL DMSO; the volume was completed to 50 mL using acetonitrile, then each sample was centrifuged at 4000 rpm for 2 min, and 3.0 µL was injected. The composition of the samples was determined based on the identification of the m/z ratio with a reference to the retention time of the used standards.
3.6. Total Phenolic (TPC) and Total Flavonoid Contents (TFC)
TPC was determined using the Folin–Ciocalteu method in accordance with the procedures described in the literature [44,46,48], with slight modifications. Briefly, 0.5 mL of each extract was treated with 2.5 mL of Folin–Ciocalteu reagent (2N) (diluted ten-fold) and 2 mL of Na2CO3 (75 g/L). The mixture was allowed to stand at room temperature for 15 min, and absorbance was then recorded at 765 nm. Methanol was used as a blank solution. TPC in different extracts of both plants was expressed as mg/g gallic acid equivalent. All measurements were performed in triplicate.
TFC in the different extracts obtained from C. procera, P. spiralis, and P. tomentosa was determined calorimetrically using the aluminum chloride assay method [47,48]. A 1.0 mL aliquot from the stock solution (1 mg/mL) of each extract, diluted in 4.0 mL distilled water, was introduced into a 10.0 mL volumetric flask, to which 0.30 mL of sodium nitrite solution (5% NaNO2, w/v) was added. The resulting mixture was allowed to stand for 5 min; then, 0.30 mL of aluminum chloride solution (10% AlCl3, w/v) was added. The resulting solution was incubated for another 6 min, after which 2.0 mL of 1.0 M NaOH solution was added, and the final volume was adjusted to 10.0 mL with distilled water. After 15 min, absorbance was measured at 510 nm. Methanol was used as a blank solution. TFC was measured and performed in triplicate and expressed as mg quercetin/g of dry extract.
3.7. Antioxidant and Radical Scavenging Activity
The antioxidant activity of different extracts of C. procera, P. spiralis, and P. tomentosa was screened using the 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS), ferrous ion chelating (FIC), and hydroxyl radical assay (HR) methods in accordance with the procedures mentioned in the literature [46,47,48]. Alpha-tocopherol, EDTA, and ascorbic acid were used as positive controls. The IC50 of the extracts and positive controls, expressed as mean ± SD, is shown in Table 4. All determinations of the IC50 using the three assay methods were conducted in triplicate.
3.8. Statistical Analysis
All experiments were conducted independently at least three times. Statistical analysis was performed using PLS Toolbox 4.0 (Eigenvector Research, Inc., Manson, WA, USA) under MATLAB 7.0.4. (MathWorks, Newton, MA, USA). P values were determined through one-way ANOVA followed by LSD, and differences were considered significant if p < 0.05. Data were expressed as means ± SEM. All data were reported as mean ± SD values.
4. Conclusions
This study reports the results obtained from phytochemical analysis performed via LC-MS on crude extracts retrieved from three Asclepiadaceae species, namely, C. procera, P. tomentosa, and P. spiralis. Several flavonoids and phenolic compounds were identified in the extracts, explaining the well-known biological activities of these species. The present study confirmed that the aerial parts of these species from Jordan contain a large amount of kaempferol-3-O-glucoside, p-coumaric acid, and luteolin-7-O-glucoside. In addition, the study revealed that the antioxidant activity of the butanol and aq. methanol extracts of the three species correlates with the highest amount of phenolic and flavonoid contents, which exhibited the greatest antioxidant activity in the scavenging of DPPH free radical, ABTS radical cation, ferrous chelating effect, and hydroxyl radical assays. These results indicate that the butanol extracts of these species may be useful as biologically active agents in food and pharmaceutical formulations since they are rich in phenolic and flavonoid compounds.
Acknowledgments
We would like to thank the Deanship of Scientific Research and Graduate Studies at Yarmouk University for funding this research project (Grant no. 9/2016).
Author Contributions
Conceptualization, M.A.A.-Q. and J.N.L.; methodology, M.A.A.-Q., S.S.A.-B., N.A.-B. and Y.A.-D.; software, A.S.M. and I.F.A.-M.; validation, M.A.A.-Q., S.T.A.O. and M.S.A.-S.; formal analysis, N.A.-B., I.F.A.-M. and M.S.A.-S.; investigation, M.A.A.-Q. and J.N.L.; resources, M.A.A.-Q. and J.N.L.; data curation, N.A.-B., I.F.A.-M. and S.S.A.-B.; writing—original draft preparation, M.A.A.-Q., A.S.M., S.T.A.O. and M.S.A.-S.; writing—review and editing, M.A.A.-Q., Y.A.-D., A.S.M., S.T.A.O., I.F.A.-M., N.A.-B. and M.S.A.-S.; visualization, Y.A.-D. and M.A.A.-Q.; supervision, M.A.A.-Q. and I.F.A.-M.; project administration, M.A.A.-Q.; funding acquisition, M.A.A.-Q. and S.T.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deanship of Scientific Research and Graduate Studies at Yarmouk University, grant number [9/2016].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sengul M., Ercisli S., Yildiz H., Gungor N., Kavaz A., Cetin B. Antioxidant, antimicrobial activity and total phenolic content within the aerial parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iran. J. Pharm. Res. 2011;10:49–55. [PMC free article] [PubMed] [Google Scholar]
- 2.Zia-Ul-Haq M., Ahmad S., Qayum M., Ercisli S. Compositional studies and antioxidant potential of Albizia lebbeck (L.) Benth. Pods and seeds. Turk. J. Biol. 2013;37:25–32. [Google Scholar]
- 3.Mollova S., Fidan H., Antonova D., Bozhilov D., Stanev S., Kostova I., Stoyanova A. Chemical composition and antimicrobial and antioxidant activity of Helichrysum italicum (Roth) G. Don subspecies essential oils. Turk. J. Agric. For. 2020;44:371–378. doi: 10.3906/tar-1909-34. [DOI] [Google Scholar]
- 4.Subasi I. Seed fatty acid compositions and chemotaxonomy of wild Crambe (Brassicaceae) taxa in Turkey. Turk. J. Agric. For. 2020;44:662–670. doi: 10.3906/tar-1912-76. [DOI] [Google Scholar]
- 5.Endress M.E., Liede-Schumann S., Meve U. Advances in Apocynaceae: The enlightment, an introduction. Ann. Mo. Bot. Gard. 2007;94:259–267. doi: 10.3417/0026-6493(2007)94[259:AIATEA]2.0.CO;2. [DOI] [Google Scholar]
- 6.Ahmed O.M., Fahim H.I., Boules M.W., Ahmed H.Y. Cardiac and testicular toxicity effects of the latex and ethanolic leaf extract of Calotropis procera on male albino rats in comparison to abamectin. SpringerPlus. 2016;5:1–21. doi: 10.1186/s40064-016-3326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prabha M.R., Vasantha K. Antioxidant, cytotoxicity and polyphenolic content of Calotropis procera (Ait.) R. Br. Flowers. J. Appl. Pharm. Sci. 2011;1:136–140. [Google Scholar]
- 8.Barbosa-Fiho J.M., Medeiros K.C.P., Diniz M.F.F.M., Batista L.M., Athayde-Filho P.F., Silva M.S., Cunha E.V.L., Almeida J.R.G.S., Quintans-Junior L.J. Natural products inhibitors of the enzime acetylcholinesterase. Rev. Bras. Farmacogn. 2006;16:258–285. doi: 10.1590/S0102-695X2006000200021. [DOI] [Google Scholar]
- 9.Carvalho M.G., Alves C.F., Werli A.A., Braz-Filho R. Metabolitos especiais isolados de Laseguia erecta (Apocynaceae) Rev. Bras. Farmacogn. 2006;16:497–500. doi: 10.1590/S0102-695X2006000400010. [DOI] [Google Scholar]
- 10.Ajitha M., Rajnarayana K. Role of oxygen free radicals in human disease. Indian Drug. 2001;38:545–554. [Google Scholar]
- 11.Phuyal N., Jha P.K., Raturi P.P., Rajbhandary S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci. World J. 2020;2020:8780704. doi: 10.1155/2020/8780704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdulhafiz F., Mohammed A., Kayat F., Bhaskar M., Hamzah Z., Podapati S.K., Reddy L.V. Xanthine oxidase inhibitory activity, chemical composition, antioxidant properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq) Molecules. 2020;25:2658. doi: 10.3390/molecules25112658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulhafiz F., Mohammed A., Kayat F., Zakaria S., Hamzah Z., Reddy Pamuru R., Reduan M.F.H. Micropropagation of Alocasia longiloba Miq and comparative antioxidant properties of ethanolic extracts of the field-grown plant, in vitro propagated and in vitro-derived callus. Plants. 2020;9:816. doi: 10.3390/plants9070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Eisawi D. List of Jordan vascular plants. Mitt. Bot. Munchen. 1982;18:79–182. [Google Scholar]
- 15.Mudi S.Y., Bukar A. Anti-plasmodia activity of leaf extracts of Calotropis procera Linn. Biokemistri. 2011;23:29–34. [Google Scholar]
- 16.Parihar G., Sharma A., Ghule S., Sharma P., Deshmukh P., Srivastava D. Anti-inflammatory effect of Calotropis procera root bark extract. Asian J. Pharm. Life Sci. 2011;1:29–44. [Google Scholar]
- 17.Falguni S. Range of Seasonal Phytochemical Variations in Calotropis procera. Int. J. Med. Arom Plants. 2011;1:180–183. [Google Scholar]
- 18.Gupta A., Singh R., Purwar C., Chauhan D., Singh J. Two pentacyclic triterpenes from the stem of Calotropis procera. Indian J. Chem. 2003;42B:2030. [Google Scholar]
- 19.Ansari S.H., Ali M. New oleanene triterpenes from root bark of Calotropis procera (Ait.) R.Br. Indian J. Chem. 2000;39B:287. [Google Scholar]
- 20.Samy R.P., Chow V.T.K. Pilot study with regard to the wound healing activity of protein from Calotropis procera (Ait.) R. Br. Evid.-Based Complement. Altern. Med. 2012;2012:294528. doi: 10.1155/2012/294528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moronkola D.O., Ogukwe C., Awokoya K.N. Chemical compositions of leaf and stem essential oils of Calotropis procera Ait R.Br [Asclepiadaceae] Chem. Sin. 2011;2:255–260. [Google Scholar]
- 22.Al-Robal A.A., Abo-Khatwa A.N., Danish E.Y. Toxicological studies on the latex of the usher plants Calotropis procera (Ait). R. Br. in Saudi Arabia 111. Effects of usher latex on the fine structures, Oxygen consumption and Na+/k+- transporting ATPase activity of albino rat kidneys. Arab-Gulf J. Sci. Res. 1993;11:441–445. [Google Scholar]
- 23.Mueen A.K.K., Rana A.C., Dixit V.K. Calotropis species (Asclepediaceae)-A comprehensive review. Pharmacogn. Mag. 2005;1:4852. [Google Scholar]
- 24.Rasool N., Ahmad V.U., Malik A. Two new triterpenoids from Pentatropis spiralis. Fitoterapia. 1992;63:156–159. doi: 10.1016/j.fitote.2010.06.007. [DOI] [Google Scholar]
- 25.Rasool N., Khan A.Q., Viqar A., Abdul M. New cycloartane-type triterpene from Pentatropis spiralis. J. Nat. Prod. 1991;54:889–892. doi: 10.1021/np50075a027. [DOI] [Google Scholar]
- 26.Al-Qura’n S. Ethnobotanical survey of folk toxic plants in southern part of Jordan. Toxicon. 2005;46:119–129. doi: 10.1016/j.toxicon.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Shabana M., Mirhom Y., Genenah A., Aboutabl E., Amer H. Study into wild Egyptian plants of potential medicinal activity. ninth communication: Hypoglycaemic activity of some selected plants in normal fasting and alloxanised rats. Arch. Exp. Vet. 1990;44:389–394. [PubMed] [Google Scholar]
- 28.Gohar A., El-Olemy M., Abdel-Sattar E., El-Said M., Niwa M. Cardenolides and β-sitosterol glucoside from Pergularia tomentosa L. J. Nat. Prod. Sci. 2000;63:142–146. [Google Scholar]
- 29.Hassan S.W., Umar R.A., Ladan M.J., Nyemike P., Wasagu R.S.U., Lawal M., Ebbo A.A. Nutritive value, phytochemical and Antifungal Properties of Pergularia tomentosa L. (Asclepiadaceae) Int. J. Pharmacol. 2007;3:334–340. doi: 10.3923/ijp.2007.334.340. [DOI] [Google Scholar]
- 30.Bellakhdar J., La P., Marocaine T. Me’decine Arabe Ancienne et Savoirs Populaires. Ibis Press; Paris, France: 1998. [Google Scholar]
- 31.Benchelah A.C., Bouziane H., Maka M., Ouahes C., Monod T. Fleurs du Sahara: Voyage Ethnobotanique Avec Les Touaregs du Tassili. Ibis Press; Paris, France: 2011. [Google Scholar]
- 32.Piacente S., Masullo M., De Neve N., Dewelle J., Hamed A., Kiss R., Mijatovic T. Cardenolides from Pergularia tomentosa display cytotoxic activity resulting from their potent inhibition of Na+/K+- ATPase. J. Nat. Prod. 2009;72:1087–1091. doi: 10.1021/np800810f. [DOI] [PubMed] [Google Scholar]
- 33.Heneidak S., Grayer R.J., Kite G.C., Simmonds M.S.J. Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae) Biochem. Syst. Ecol. 2006;34:575–584. doi: 10.1016/j.bse.2006.03.001. [DOI] [Google Scholar]
- 34.Achenk F., Doumad-Mitiche B. Insecticidal activity of alkaloids extract of Pergularia tomentosa (Asclepiadaceae) against fifth instar Larvae of Locusta migratoria cinerascens. Int. J. Sci. Adv. Technol. 2013;3:8–13. [Google Scholar]
- 35.Siddiqui A., Ali M. Pratical Pharmaceutical Chemistry. 1st ed. CBS Publish and Distributors; New Delhi, India: 1997. pp. 126–131. [Google Scholar]
- 36.Al-Qudah M.A., Saleh A.M., Alhawsawi N.L., Al-Jaber H.I., Rizvi S.A., Afifi F.U. Composition, antioxidant and cytotoxic activities of essential oils from Fresh and air-dried aerial parts of Pallenis spinosa. Chem. Biodivers. 2017;14:e1700146. doi: 10.1002/cbdv.201700146. [DOI] [PubMed] [Google Scholar]
- 37.Al-Qudah M.A. Chemical composition of essential oil from Jordanian Lupinus varius L. Arab. J. Chem. 2013;6:225–227. doi: 10.1016/j.arabjc.2011.01.012. [DOI] [Google Scholar]
- 38.Al-Qudah M.A., Al-Ghoul A.M., Tarawneh I.N., Al-Jaber H.I., Al Shboul T.M., Abu Zarga M.H., Abu-Orabi S.T. Antioxidant activity and chemical composition of essential oils from Jordanian Ononis natrix L. and Ononis Sicula Guss. J. Biol. Act. Prod. Nat. 2014;4:52–61. doi: 10.1080/22311866.2014.890069. [DOI] [Google Scholar]
- 39.Al-Qudah M.A., Obeidat S.M., Saleh A.M., El-Oqlah A.A., Al-Masaeed E., Al-Jaber H.I., Abu-Orabi S.T. Volatile components analysis, total phenolic, flavonoid contents, and antioxidant activity of Phlomis species collected from Jordan. J. Essent. Oil-Bear. Plants. 2018;21:583–599. doi: 10.1080/0972060X.2018.1489739. [DOI] [Google Scholar]
- 40.Abu-Orabi S.T., Al-Qudah M.A., Saleh N.R., Bataineh T.T., Obeidat S.M., Al-Sheraideh M.S., Lahham J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020;13:6256–6266. doi: 10.1016/j.arabjc.2020.05.043. [DOI] [Google Scholar]
- 41.Al-Qudah M.A. Antioxidant activity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J. Biol. Act. Prod. Nat. 2016;6:101–111. doi: 10.1080/22311866.2016.1199286. [DOI] [Google Scholar]
- 42.Al-Qudah M.A., Obeidat S.M., Muhaidat R., Al-Trad B., Al-Jaber H.I., Lahham J.N. Intercomparative investigation of the total phenols, total flavonoids, in vitro and in vivo antioxidant activities of Capparis Cartilaginea (Decne.) maire and weiller and Capparis Ovata Desf. from Jordan. Pharmacogn. Mag. 2018;14:154. doi: 10.4103/pm.pm_356_17. [DOI] [Google Scholar]
- 43.Al-Qudah M.A., Tashtoush H.I., Khlaifat E.F., Ibrahim S.O., Saleh A.M., Al-Jaber H.I., Abu Orabi S.T. Chemical constituents of the aerial parts of Salvia judaica Boiss. from Jordan. Nat. Prod. Res. 2020;34:2981–2985. doi: 10.1080/14786419.2019.1597349. [DOI] [PubMed] [Google Scholar]
- 44.Al-Qudah M.A., Allahham F.E., Obeidat S.M., Al-Jaber H.I., Lahham J.N., Abu Orabi S.T. In vitro antioxidant activities, total phenolics and total flavonoids of the different extracts of Capparis spinosa L. and Capparis decidua Edgew (forssk.) from Jordan. Int. J. Pharm. Res. 2020;12:1226–1236. doi: 10.31838/ijpr/2020.12.03.183. [DOI] [Google Scholar]
- 45.Al-Jaber H.I., Abu Zarga M.H., Al-Aboudi A.F., Al-Qudah M.A., Al-Shawabkeh A.F., Abaza I.F., Afifi F.U. Essential oil composition and anticholinesterase activity evaluation of Achillea fragrantissima growing wild in Jordan. J. Herbs Spices Med. Plants. 2018;24:272–281. doi: 10.1080/10496475.2018.1463933. [DOI] [Google Scholar]
- 46.Al-Jaber H.I., Hammad H.M., Al-Qudah M.A., Abaza I.F., Al-Humaidi J.Y., Abu-Zarga M.H., Afifi F.U. Volatile oil composition and antiplatelet activity of Jordanian Achillea biebersteinii collected at different growth stages. J. Essent. Oil-Bear. Plants. 2014;17:584–598. doi: 10.1080/0972060X.2014.884762. [DOI] [Google Scholar]
- 47.Al-Qudah M.A., Abu Zarga M.H. Chemical composition of essential oils from aerial parts of Sisymbrium irio from Jordan. J. Chem. 2010;7:6–10. doi: 10.1155/2010/973086. [DOI] [Google Scholar]
- 48.Al-Humaidia J.Y., Al-Qudah M.A., Al-Saleema M.S., Alotaibia S.M. Antioxidant activity and chemical composition of essential oils of selected cleome species growing in Saudi Arabia. Jordan J. Chem. 2019;14:29–37. [Google Scholar]
- 49.Bedwell S., Dean R.T., Jessup W. The action of defined oxygen-centred free radicals on human low-density lipoprotein. Biochem. J. 1989;262:707–712. doi: 10.1042/bj2620707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.