Abstract
Dengue virus (DV) was detected early in infected mosquito C6/36 cells by using indirect immunofluorescence (IF) in conjunction with flow cytometry. Three fixation-permeabilization methods and three DV serotype 1 (DEN-1)-specific monoclonal antibodies, 8-8 (anti-E), 16-4 (anti-NS1), and 15F3-1 (anti-NS1), were evaluated for the detection of DEN-1 in infected C6/36 cells. We found that these three monoclonal antibodies were capable of detecting DV in C6/36 cells as early as 24 h postinoculation by using a conventional indirect IF stain. Both 8-8 and 16-4 detected DV earlier and showed a greater number of DV-positive cells than 15F3-1. In flow cytometry, 3% paraformaldehyde plus 0.1% Triton X-100 with 16-4, the best fixation-permeabilization method for testing DV, showed higher sensitivity (up to 1 PFU) than indirect IF stain. The higher sensitivity of 16-4 in detecting DEN-1 was found with both IF and flow cytometry. Flow cytometry, which had a sensitivity similar to that of nested reverse transcription-PCR, was more sensitive in detecting DV in the infected mosquito cells 10 h earlier than the conventional IF stain. When clinical specimens were amplified in mosquito C6/36 cells and then assayed for DV using flow cytometry and conventional virus isolation at day 7 postinfection, both methods had 97.22% (35 out of 36) agreement. Moreover, among 12 positive samples which were detected by conventional culture method, the flow cytometry assay could detect DV in 58.33% (7 out of 12) of samples even at day 3 postinfection. In conclusion, both monoclonal antibodies 8-8 and 16-4 can be used for the early detection of DEN-1-infected C6/36 cells, with 16-4 (anti-NS1) being the best choice for the rapid diagnosis of DV by both the IF staining and flow cytometry methods.
Dengue virus, a member of the family Flaviviridae, genus Flavivirus, is an enveloped, single-stranded RNA virus. The clinical manifestations of dengue infections range from asymptomatic, undifferentiated fever, classic dengue fever, to severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (13, 19). Approximately 100 million dengue cases occur worldwide annually (13, 26). Therefore, accurate laboratory diagnosis of the dengue virus infections would be very helpful in understanding the epidemiology of this infection and disease.
Laboratory diagnosis of dengue virus infection has mainly involved techniques such as infectious virus isolation, virus-specific antibody determination, and viral RNA direct detection in clinical specimens (16). Serological diagnosis is complicated by the existence of cross-reactive antigenic determinants shared by all four dengue virus serotypes and other members of the flavivirus family. The detection of the virus-specific immunoglobulin M (IgM) antibody in a single serum sample has been used for rapid diagnosis in suspected dengue cases. However, this IgM antibody appears primarily on day 6 after infection (6, 34) and usually appears later than the first 5 days after onset, according to the virus isolation method. Direct detection and rapid typing of the dengue virus in serum using reverse transcription-PCR (RT-PCR) has two major disadvantages: (i) it cannot provide a live virus for further biological characterization, and (ii) its sensitivity varies between serotypes (14, 31). Therefore, isolating and typing this virus has remained the “gold standard” and provides advantages over the other methods in the laboratory diagnosis of dengue virus infections.
The detection of dengue virus in a cell culture is usually performed using indirect immunofluorescence (IF) stain with virus-specific monoclonal antibodies (MAbs) or polyclonal antibodies. IF staining has traditionally been performed between 6 and 10 days postinfection (11, 34). Recently, flow cytometry has been used to study virus-cell interaction (18), and it can also serve in the rapid detection of virus-infected cells, particularly in patients infected with cytomegalovirus or human immunodeficiency virus (3, 4, 9, 10). To date, MAbs and recently developed permeabilization techniques in flow cytometry have never been reported for the rapid detection of dengue virus-infected cells. In this study, the newly developed MAbs 8-8 and 16-4, which recognize E and NS1 of dengue virus serotype 1 (DEN-1), respectively, were used in conjunction with fixation-permeabilization techniques to examine the possible application of these antibodies in the early detection of the dengue virus in infected mosquito C6/36 cells by flow cytometry.
MATERIALS AND METHODS
Virus strains.
DEN-1 (Hawaii strain), DEN-2 (New Guinea C strain), DEN-3 (H87), and DEN-4 (H241) were obtained from D. Gubler, Centers for Disease Control and Prevention, Atlanta, Ga. Two DEN-1 Taiwan local isolates (766733 and 066267) were kindly provided by The Division of Epidemiology, National Institute of Preventive Medicine, Taipei, Taiwan, Republic of China. All virus strains were inoculated into mosquito C6/36 cells with growth medium containing 50% Mitsumashi and Maramorsch Insect Medium (MMIM; Sigma) plus 50% Dulbecco's modified Eagle's minimal essential medium (DMEM; GIBCO) and incubated at 28°C for 7 to 9 days. The virus was harvested from supernatants, aliquoted, stored at −80°C, and titrated in BHK cells by plaque assay (27).
Clinical samples.
Acute-phase serum samples, including 12 dengue-positive and 24 dengue-negative specimens (confirmed by conventional virus isolation in C6/36 mosquito cells by the National Institute of Preventive Medicine), were collected for culturing in the same kind of mosquito cells. The diluted serum samples (1:100; 100-μl aliquots) were inoculated into 6-well plates and incubated at 28°C. The cells were harvested at both 3 and 7 days postinoculation and then examined using flow cytometry.
MAbs.
The hybridoma cell lines that secreted dengue E or NS1 protein-specific MAbs were identified by enzyme-linked immunosorbent assay and immunoprecipitation assay with dengue virus-infected C6/36 cell lysates as previously described (5, 21). 8-8 (IgG2a) and 16-4 (IgG2b), which reacted with E and NS-1 proteins of DEN-1, respectively, were used in this study. Five other MAbs, 15F3-1 (anti-NS1 of DEN-1; ATCC HB47; IgG1), 3H5-1 (anti-DEN-2; ATCC HB46; IgG1), 5D4-11 (anti-DEN-3; ATCC HB49; IgG1), and 1H10-6 (anti-DEN-4; ATCC HB48; IgG1) were also employed for comparison.
Detection of virus antigen in infected cells at different postinoculation times using indirect IF.
After confluent growth of C6/36 cells in 96-well plates (Costar), DEN-1 (Hawaii strain) and two local DEN-1 isolates (766733 and 066267) were inoculated and incubated at 28°C. All three virus strains were inoculated at different multiplicities of infection (MOI; the number of PFU of the tested virus per cell) under the same conditions. At different postinoculation time intervals, dengue virus antigens in the infected cells were detected with indirect IF (15). The mock-infected C6/36 cells were run in parallel as virus-infected cells and served as negative controls.
Flow cytometry.
C6/36 mosquito cell monolayers were infected with dengue viruses or clinical samples and then were removed from the flask, washed with phosphate-buffered saline (PBS) (pH 7.2) twice, fixed, and permeabilized simultaneously using three different methods, the paraformaldehyde-Tween method (17), the paraformaldehyde-methanol method (17), and the paraformaldehyde-Triton method (29), for comparison. The permeabilized cells were washed and incubated with DEN-1 MAb for 45 min at 37°C. Cells were then washed and incubated with fluorescein isothiocyanate (FITC)-labeled affinity-purified goat anti-mouse IgG (Kirkegaard & Perry Laboratories) for 30 min at 37°C. After incubation, cells were washed twice with PBS (pH 7.2), suspended in PBS, and then analyzed using a FACScan flow cytometer (Becton Dickinson Immunocytometry System, San Jose, Calif.). The mock-infected C6/36 cells were run in parallel and served as negative controls. At least 10,000 cells were gated by light scatter and collected in a list mode manner. Data analysis was performed with Cell Quest software (Becton Dickinson). The percentage of positive cells and the mean fluorescence intensities were determined on FITC fluorescence histograms using a region defined according to mock-infected cell control analysis.
RT-nPCR of DEN-1.
RT-nested PCR (RT-nPCR) was performed using modified procedures and primers developed by Lanciotti et al. (20). Dengue virus RNA was extracted from virus-infected mosquito cells using a QIAamp blood kit (QIAGEN, Hilden, Germany). A one-step RT-PCR (Life Technologies) was performed in a 50-μl reaction volume containing RT/Taq, 1.2 mM MgSO4, 0.2 mM concentrations of each deoxynucleoside triphosphate, and 12.5 pmol (each) of primers D1, D2 (20), and D2′ (5′-TTGCACCAACAATCTATGTCTTCTGGTTC-3′; capsid/precursor M [C/prM] region downstream primer). The D2′ primer was used to cover more dengue virus strains of DEN-1 with mutations in recent years in Taiwan. The mixture was incubated at 50°C for 45 min, inactivated at 95°C for 3 min, and amplified for 30 cycles (model 480; Perkin-Elmer Cetus) under the following conditions: 94°C for 60 s, 50°C for 60 s, 72°C for 60 s, and a final extension at 72°C for 9 min. The diluted (1:500) 2.5 μl of the first-run PCR product was further amplified with the inner pair of primers in a 25-μl reaction mixture containing a 5 mM concentration of each deoxynucleoside triphosphate, 25 mM MgCl2, 12.5 pmol of each primer (D1 and TS1) (20), and 1.25 U of Taq DNA polymerase (Promega). After denaturation at 94°C, the second amplification run was performed for 35 cycles (94°C for 30 s, 58°C for 30 s, 72°C for 45 s, and a final extension at 72°C for 10 min). The DEN-1-specific PCR products (482 bp) were analyzed by electrophoresis on 2% agarose gels and were visualized by staining the gels in an ethidium bromide solution.
RESULTS
Detection of DEN-1 by indirect IF.
With MAbs and indirect IF stain, DEN-1 was detected by all three DEN-1 serotype-specific MAbs (15F3-1 [anti-NS1], 8-8 [anti-E], and 16-4 [anti-NS1]) at an MOI of 0.1 on day 1 postinoculation (Table 1). Only 8-8 and 16-4 detected DEN-1 at an MOI of 0.01 on the same postinoculation day. The sensitivity of these three MAbs in the detection of DEN-1 virus was different, with 16-4 being more sensitive than 8-8 and 8-8 being more sensitive than 15F3-1. Even at an MOI of 0.001, 16-4 (anti-NS1) was found to detect DEN-1 at the earliest time, which was 1 day earlier than that for the other two MAbs.
TABLE 1.
Detection of DEN-1 in C6/36 cells infected at different MOIs on day 1 postinoculation using indirect IF stain
| MAb | Virus strain | No. of positive cells detected at an MOI of:
|
|||
|---|---|---|---|---|---|
| 0.1 | 0.01 | 0.001 | 0.0001 | ||
| 15F3-1 (anti-NS1) | Hawaii | <1a (2+)b | —c | — | — |
| 766733 | <1 (2+) | — | — | — | |
| 066267 | <1 (2+) | — | — | — | |
| 8-8 (anti-E) | Hawaii | 4 (2+) | <1 (1+) | — | — |
| 766733 | 78 (3+) | 5 (1+) | — | — | |
| 066267 | 80 (3+) | 6 (1+) | — | — | |
| 16-4 (anti-NS1) | Hawaii | 12 (4+) | 5 (1+) | <1 (1+) | +/—d |
| 766733 | 78 (3+) | 5 (1+) | <1 (1+) | +/— | |
| 066267 | 80 (3+) | 7 (1+) | <1 (1+) | +/— | |
Mean number of dengue virus-positive cells under observation in four different fields. Magnification, ×400.
Numbers in parentheses represent a rating of fluorescence intensity assigned arbitrarily.
—, negative result.
+/−, equivocal result.
A difference in the sensitivity of these MAbs in detecting DEN-1 was also found on days 2 and 3 postinoculation (MOI = 0.01), although greater numbers of dengue virus-infected cells were observed on days 2 and 3 than on day 1 postinfection. 8-8 and 16-4 were much better than 15F3-1 at detecting the DEN-1 antigen at two lower MOIs (0.001 and 0.0001) (data not shown). These results indicate that both type-specific MAbs, 8-8 and 16-4, can be used in the early detection of dengue virus-infected C6/36 mosquito cells, with 16-4 (anti-NS1) being the most sensitive.
Comparison of three fixation-permeabilization methods for dengue virus-infected C6/36 cells by using flow cytometry.
The results from the three different fixation-permeabilization methods used to detect DEN-1 in infected C6/36 cells by flow cytometry are shown in Table 2. Paraformaldehyde-Triton X-100 detected the greatest number of positive DEN-1-infected cells by the different MAbs (16-4 and 15F3-1). Similar results were also found for DEN-2, -3, and -4 viruses. Due to its higher sensitivity and the shorter time required for detecting dengue virus-positive cells, the paraformaldehyde-Triton X-100 method was used to prepare cells for the following flow cytometry analysis.
TABLE 2.
Comparison of three fixation-permeabilization methods for the detection of dengue virus in C6/36 cells using flow cytometrya
| Fixation-permeabilization method | Mean percentage of positive cellsb
|
||||
|---|---|---|---|---|---|
| DEN-1 MAb (15F3-1) | DEN-1 MAb (16-4) | DEN-2 MAb (3451) | DEN-3 MAb (504-11) | DEN-4 MAb (IH10-6) | |
| Paraformaldehyde-Tween-20 | 49.45 | 54.40 | 88.92 | 71.30 | 66.72 |
| Paraformaldehyde-methanol | 56.78 | 70.68 | 47.70 | 88.99 | 78.72 |
| Paraformaldehyde-Triton X-100 | 67.43 | 72.36 | 86.92 | 93.65 | 95.10 |
C6/36 cells were infected with DEN-1 (Hawaii), DEN-2 (New Guinea C), DEN-3 (H87) and DEN-4 (H241) at an MOI of 0.1 and incubated at 28°C for 7 days.
Mean percentage of positive cells by two measurements.
Comparison of three MAbs in detecting DEN-1 virus antigen in C6/36 cells by flow cytometry.
16-4 (anti-NS1) was the most sensitive MAb against NS1 in detecting DEN-1-infected C6/36 cells using flow cytometry (Table 3). 15F3-1 (anti-NS1) was the least sensitive MAb in detecting the virus-infected mosquito cells using the same method. Although the lowest percentage of DEN-1-infected cells was detected by 8-8 (anti-E) only at day 1 postinoculation, the number of positive cells increased rapidly at day 2 postinoculation and at later time points. Flow cytometry was able to detect the dengue virus at day 1 postinoculation using all three serotype-specific MAbs, 16-4, 8-8, and 15F3-1.
TABLE 3.
Comparison of three MAbs used for the detection of DEN-1 virus (Hawaii) antigen in C6/36 cells using flow cytometrya
| MAb | Mean percentage of positive cells at day postinoculationb:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | |
| 15F3-1 (anti-NS1) | 5.12 | 18.11 | 30.96 | 44.52 | 47.56 |
| 16-4 (anti-NS1) | 7.51 | 23.92 | 33.56 | 50.16 | 82.10 |
| 8-8 (anti-E) | 3.25 | 21.67 | 32.34 | 48.88 | 81.32 |
C6/36 cells were infected with DEN-1 (Hawaii) at an MOI of 0.1 and incubated at 28°C.
Mean percentage of positive cells by two measurements.
Comparison of the timing and sensitivity in detecting DEN-1 virus in infected mosquito cells using indirect IF, flow cytometry, and RT-nPCR.
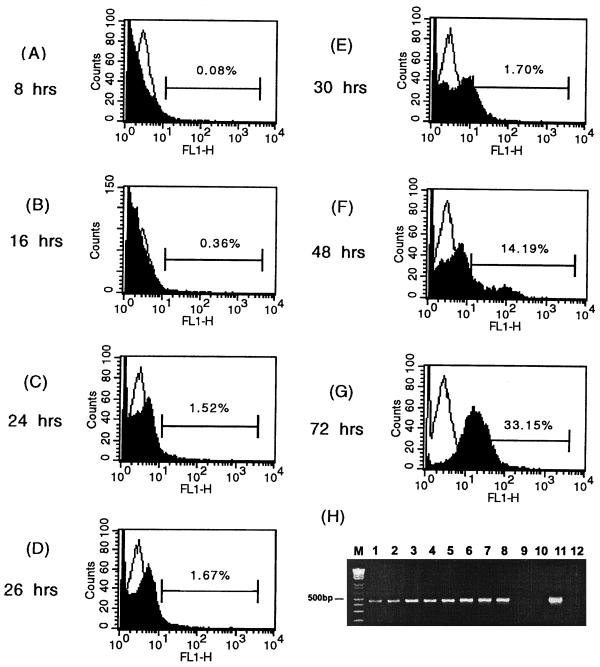
Flow cytometry was found to detect DEN-1 virus antigen as early as 16 h postinoculation (MOI = 0.01), whereas conventional IF staining using 16-4 was found to detect the virus antigen at 26 h postinoculation. However, the RT-nPCR method detected DEN-1 at 8 h postinoculation of C6/36 cells, much earlier than the flow cytometry method (Fig. 1). The sensitivity of flow cytometry in detecting dengue virus at day 3 postinfection is shown in Table 4. When used in the flow cytometry, both 15F3-1 and 16-4 detected DEN-1 virus in C6/36 cells infected with 1 PFU of the virus. In contrast, conventional IF staining by using these two MAbs could detect DEN-1 only at levels of 10 to 100 PFU or more. The sensitivity results obtained using flow cytometry were also comparable with those for RT-nPCR (data not shown).
FIG. 1.
Flow cytometric histograms of DEN-1 (Hawaii strain)-infected C6/36 cells stained with DEN-1 MAb 16-4 (anti-NS1) (black) overlaid with histograms of mock-infected cells (white). C6/36 mosquito cells were infected with DEN-1 (Hawaii) at an MOI of 0.01. (A through G) Different time points postinfection. FL1-H, FITC fluorescence intensity. The bars represent the proportion of positive cells. (H) Detection of DEN-1 viral RNA in infected C6/36 cells collected at different time points postinfection using RT-nPCR. Lanes 1 to 8 show results at 8, 12, 16, 24, 26, 30, 48, and 72 h postinfection, respectively. Lane 9, mock-infected C6/36 cells; lane 10, culture medium only; lane 11, DEN-1 (Hawaii) virus; lane 12, reagent control; lane M, molecular size markers.
TABLE 4.
Comparison of the sensitivity in detection of DEN-1 virus (Hawaii)-infected C6/36 cells by conventional IF staining and flow cytometrya
| Virus inoculation (PFU) | Results of detection of dengue virus antigen in C6/36 cells at 3 days postinoculation
|
|||
|---|---|---|---|---|
| IF staining
|
Flow cytometryb
|
|||
| 15F3-1 | 16-4 | 15F3-1 | 16-4 | |
| 1,000 | + | + | + (1.16) | + (3.23) |
| 100 | + | + | + (0.98) | + (2.40) |
| 10 | − | + | + (0.88) | + (1.87) |
| 1 | − | − | + (0.37) | + (0.96) |
| 0 | − | − | − (≤0.1) | − (≦0.1) |
C6/36 cells were infected with DEN-1 (Hawaii) in 6-well plates and incubated at 28°C for 3 days.
Percentages in parentheses represent mean percentages of positive cells reacted with MAb 16-4 by two measurements.
Application of flow cytometry to detect dengue virus in C6/36 cells infected with serum samples collected from patients suspected to be infected with dengue during the epidemic season in Taiwan.
Among 12 clinical acute-phase serum samples positive for DEN-1 virus, confirmed by conventional culture and the IF stain method during the 1987 to l988 dengue fever epidemic in Southern Taiwan, all (12 out of 12, or 100%) were detected as positive samples by both RT-nPCR and our developed flow cytometry method at 7 days postinoculation. However, 7 out of these 12 tested samples (58.33%) were observed to be positive using flow cytometry at 3 days postinoculation, which is less than the 7 days required by conventional IF staining. Because there was not a sufficient volume of these 1987 to 1988 samples, only the flow cytometry rather than the IF method was used to retest on day 3 postinoculation. Another 24 clinical acute-phase serum samples were proven negative for dengue virus isolation by using conventional culture and IF staining. Three of these cultures were positive by RT-nPCR. Among these three, one showed positive results at 7 days postinoculation using our flow cytometry method.
DISCUSSION
Early laboratory confirmation of suspected dengue cases facilitates important action in the prevention and control of dengue outbreaks and, thus, in limiting the spread of dengue virus and reducing the incidence of DHF and DSS. Virus isolation is a very crucial method, especially in the early viremia stage (13, 34, 33), because it not only provides information concerning the dengue virus serotypes but also preserves the virus isolates derived from different clinical manifestations for future virological and molecular epidemiological studies. Detection of virus antigens is generally 2- to 10-fold more sensitive than the quantitation of infectious virions by using plaque assay (2). We have demonstrated in this study that the two new type-specific DEN-1 MAbs, 8-8 and 16-4, can successfully be used in the early detection of virus-infected C6/36 mosquito cells from virus stock preparations and patient serum samples using both the IF staining and flow cytometry methods.
Flow cytometry, which detects viral antigens either on the surface of or within infected cells, has been successfully used in the rapid detection of the herpes simplex virus and rotavirus in clinical samples after virus amplification in tissue culture. It was also more effective than the conventional IF method for virus detection (1, 25). In this study, we have also demonstrated that dengue virus in clinical samples can be rapidly detected by flow cytometry after being cultured in infected mosquito C6/36 cells. Two major factors, the permeabilization method and the selection of MAbs, are involved in the detection of intracellular virus using flow cytometry. Most of the published studies employed the paraformaldehyde-methanol method for permeabilization (24). We found that the paraformaldehyde-Triton X-100 method detected a higher percentage of dengue virus antigen-positive C6/36 cells than the paraformaldehyde-methanol method. This was consistent with the findings of the flow cytometric terminal deoxynucleotidyltransferase analysis (28).
The sensitivity of dengue virus detection in infected C6/36 cells varies when using different MAbs in IF and flow cytometry, particularly in comparison at various MOIs. Since many Chinese patients like to shop around different clinics, the use of the most sensitive MAb can shorten the detection time even when very few virus particles exist in the tested specimens. 16-4 (anti-NS1) offered the highest sensitivity, probably due to multiple antigenic determinants in the NS1 protein expressed both intracellularly and on the cell surface (23). Alternatively, the function of NS1 associated with virus maturation and/or release and enhancement of viral RNA replication, particularly at the early stage of replication, might also increase the probability of virus detection (22).
Using flow cytometry methods, the smaller number of virus- positive cells obtained by reaction with anti-E MAb (8-8) than with anti-NS1 MAb (16-4) at day 1 postinoculation might be explained by the time required for dengue virus maturation and the appearance of its E antigens on the cell surface. Greater binding by anti-NS1 had also been reported for yellow fever virus-infected cells, possibly due to the presence of larger amounts of NS1 than E proteins associated with the cell membrane (30).
MAbs have rarely been used to detect variation among different dengue virus strains as well as other flaviviruses, such as the West Nile virus (8). Henchal et al. did not find any strain variation with indirect IF when they tested the two DEN-1 strains (Hawaii strain isolated in l944 and CAREC strain isolated in l977) by MAbs of 15F3, 5C11, 9D12, and 13E7 (15). However, Gubler pointed out that 15F3 did not detect certain DEN-1 strains and suggested using 1F1 to replace 15F3 (12). DEN-1 has been the most widely distributed serotype in Taiwan and several other countries (7, 32). The two Taiwan DEN-1 local virus strains used in this study, which infected larger numbers of C6/36 mosquito cells than the Hawaii strain, might have higher replication efficiency for better detection. Our data suggest that care in choosing MAbs for the detecting of different strains of DEN-1 is required. Alternatively, other MAbs against nonstructural proteins should be used for confirmation when 15F3-1 gives negative results. Variations of two Bangkok DEN-2 virus strains isolated in l980 in Thailand were also observed by using different MAbs (35). Therefore, cocktails of different MAbs against various strains of dengue virus will increase its sensitivity for detection and can be used to search for possible different dengue virus variants cocirculating in the same epidemic.
In conclusion, the criteria for selecting the appropriate MAb used in detecting dengue virus using the IF and flow cytometry methods should include (i) the earliest time to give positive results, (ii) detection capability at the lowest MOI, and (iii) coverage for as many dengue virus strains as possible. Our new dengue virus type-specific MAbs, 8-8 and 16-4, in conjunction with flow cytometry were shown to be reliable and practical for rapid virus diagnosis in clinical patients, active surveillance of dengue, and examination of the interaction of the dengue virus with different subsets of peripheral blood leukocytes. This method is also applicable for titration virus infectivity of attenuated dengue virus vaccine and elucidating the differences in dengue virus pathogenesis in dengue fever versus DHF and DSS.
ACKNOWLEDGMENTS
This work was supported by the National Health Research Institute, Taipei, Taiwan, Republic of China (grants 85-CNT-CR-01-P, 86-CNT-CR-501-P, DD01-86IX-CR-501P, and NHRI-CN-CL8903P).
We also sincerely thank Guan-Jin Huang and Mei-Ying Liao for their technical assistance in experiments and Ai-Fen Yu, Lisa S. Chiang, Laura Lo, Hui-Ting Wang, and Hui-Chi Chen for their administrative assistance.
REFERENCES
- 1.Abad F X, Pinto R M, Bosch A. Flow cytometry detection of infectious rotaviruses in environmental and clinical samples. Appl Environ Microbiol. 1998;64:2392–2396. doi: 10.1128/aem.64.7.2392-2396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed R, King C C, Oldstone M B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987;61:1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Barrientos A, Arroyo J, Canton R, Nombela C, Sanchez-Perez M. Applications of flow cytometry to clinical microbiology. Clin Microbiol Rev. 2000;13:167–195. doi: 10.1128/cmr.13.2.167-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brussaard C P, Marie D, Bratbak G. Flow cytometric detection of viruses. J Virol Methods. 2000;85:175–182. doi: 10.1016/s0166-0934(99)00167-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen L K, Liao C L, Lin C G, Lai S C, Liu C I, Ma S H, Huang Y Y, Lin Y L. Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology. 1996;217:220–229. doi: 10.1006/viro.1996.0109. [DOI] [PubMed] [Google Scholar]
- 6.Chow L, Hsu S T. MAC-ELISA for the detection of IgM antibodies to dengue type 1 virus (rapid diagnosis of dengue type 1 virus infection) Chinese J Microbiol Immunol. 1989;22:278–285. [PubMed] [Google Scholar]
- 7.Chow L. Dengue fevers in Taiwan. Epidemiol Bull ROC. 1998;14:264–270. [Google Scholar]
- 8.Damle R G, Yeolekar L R, Rao B L. Strain analysis and epitope mapping of West Nile virus using monoclonal antibodies. Acta Virol. 1998;42:389–395. [PubMed] [Google Scholar]
- 9.Detrick B, Hooks J J, Keiser J, Tabbara I. Detection of cytomegalovirus proteins by flow cytometry in the blood of patients undergoing hematopoietic stem cell transplantation. Exp Hematol. 1999;27:569–575. doi: 10.1016/s0301-472x(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 10.Gadol N, Crutcher G J, Busch M P. Detection of intracellular HIV in lymphocytes by flow cytometry. Cytometry. 1994;15:359–370. doi: 10.1002/cyto.990150412. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson F, McBride J, Norton R. Culture-amplified detection of dengue virus from serum in an outbreak of dengue fever. J Med Virol. 1999;57:212–215. [PubMed] [Google Scholar]
- 12.Gubler D J. Application of serotype-specific monoclonal antibodies for identification of dengue viruses. In: Yunker C E, editor. Arboviruses in arthropod cells in vitro. Boca Raton, Fla: CRC Press; 1987. pp. 3–14. [Google Scholar]
- 13.Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris E, Roberts T G, Smith L, Selle J, Kramer L D, Valle S, Sandoval E, Balmaseda A. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol. 1998;36:2634–2639. doi: 10.1128/jcm.36.9.2634-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henchal E A, Gentry M K, McCown J M, Brandt W E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 16.Henchal E A, Putnak J R. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imbert-Marcille B M, Robillard N, Poirier A S, Coste-Burel M, Cantarovich D, Milpied N, Billaudel S. Development of a method for direct quantification of cytomegalovirus antigenemia by flow cytometry. J Clin Microbiol. 1997;35:2665–2669. doi: 10.1128/jcm.35.10.2665-2669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagodzinski P P, Trzeciak W H. Application of monoclonal antibodies to monitor the synthesis of a glycoprotein core of envelope glycoproteins of human immunodeficiency virus (HIV-1) Biomed Pharmacother. 2000;54:50–53. doi: 10.1016/s0753-3322(00)88641-2. [DOI] [PubMed] [Google Scholar]
- 19.Kalaynarooj S, Vaughn D W, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis B L, Rothman A L, Nisalak A, Ennis F A. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti R S, Calisher C H, Gubler D J, Chang G J, Vorndam A V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y L, Liao C L, Chen L K, Yeh C T, Liu C I, Ma S H, Huang Y Y, Huang Y L, Kao C L, King C C. Study of dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72:9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie J M, Jones M K, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 23.Mason P W, Zugel M U, Semproni A R, Fournier M J, Mason T L. The antigenic structure of dengue type 1 virus envelope and NS1 proteins expressed in Escherichia coli. J Gen Virol. 1990;71:2107–2114. doi: 10.1099/0022-1317-71-9-2107. [DOI] [PubMed] [Google Scholar]
- 24.McSharry J J. Uses of flow cytometry in virology. Clin Microbiol Rev. 1994;7:576–604. doi: 10.1128/cmr.7.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McSharry J J, Costantino R, McSharry M B, Venezia R A, Lehman J M. Rapid detection of herpes simplex virus in clinical samples by flow cytometry after amplification in tissue culture. J Clin Microbiol. 1990;28:1864–1866. doi: 10.1128/jcm.28.8.1864-1866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morens D M, Halstead S B, Repik P M, Putvatana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J Clin Microbiol. 1985;22:250–254. doi: 10.1128/jcm.22.2.250-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry A, Duenzl M L, Ansari M Q. Flow cytometric terminal deoxynucleotidyltransferase analysis. Evaluation of Triton X-100 and methanol permeabilization methods compared with immunofluorescence microscopy. Arch Pathol Lab Med. 1994;118:1119–1122. [PubMed] [Google Scholar]
- 29.Ronni T, Sareneva T, Pirhonen J, Julkunen I. Activation of IFN-α, IFN-γ, MxA, and IFN regulatory factor 1 genes in influenza A virus-infected human peripheral blood mononuclear cells. J Immunol. 1995;154:2764–2774. [PubMed] [Google Scholar]
- 30.Schlesinger J J, Brandriss M W, Putnak J R, Walsh E E. Cell surface expression of yellow fever virus non-structural glycoprotein NS1: consequences of interaction with antibody. J Gen Virol. 1990;71:593–599. doi: 10.1099/0022-1317-71-3-593. [DOI] [PubMed] [Google Scholar]
- 31.Sudiro T M, Ishiko H, Green S, Vaughn D W, Nisalak A, Kalayanarooj S, Rothman A L, Raengsakulrach B, Janus J, Kurane I, Ennis F A. Rapid diagnosis of dengue viremia by reverse transcriptase-polymerase chain reaction using 3′-noncoding region universal primers. Am J Trop Med Hyg. 1997;56:424–429. doi: 10.4269/ajtmh.1997.56.424. [DOI] [PubMed] [Google Scholar]
- 32.Vajpayee M, Mohankumar K, Wali J P, Dar L, Seth P, Broor S. Dengue virus infection during post-epidemic period in Delhi, India. Southeast Asian J Trop Med Public Health. 1999;30:507–510. [PubMed] [Google Scholar]
- 33.Vaughn D W, Green S, Kalayanarooj S, Innis B L, Nimmannitya S, Suntayakorn S, Rothman A L, Ennis F A, Nisalak A. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 34.Vorndam V, Kuno G. Laboratory diagnosis of dengue virus infections. In: Gubler D J, Kuno G, editors. Dengue and dengue hemorrhagic fever. New York, N.Y: CAB International; 1997. pp. 313–333. [Google Scholar]
- 35.Walker P J, Henchal E A, Blok J, Repik P M, Henchal L S, Burke D S, Robbins S J, Gorman B M. Variation in dengue type 2 viruses isolated in Bangkok during 1980. J Gen Virol. 1988;69:591–602. doi: 10.1099/0022-1317-69-3-591. [DOI] [PubMed] [Google Scholar]