Abstract
Background
Preclinical and epidemiological studies indicate a potential chemopreventive role of low-density lipoprotein cholesterol (LDL-C) -lowering drugs in the risks of breast cancer and prostate cancer, but the causality remains unclear. We aimed to evaluate the association of genetically proxied inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, Niemann-Pick C1-Like 1 (NPC1L1), and proprotein convertase subtilisin/kexin type 9 (PCSK9) with risks of breast cancer and prostate cancer using a two-sample Mendelian randomization (MR) method.
Methods
Single-nucleotide polymorphisms (SNPs) in HMGCR, NPC1L1, and PCSK9 associated with LDL-C in a genome-wide association study (GWAS) meta-analysis from the Global Lipids Genetics Consortium (GLGC; up to 188,577 European individuals) were used to proxy inhibition of HMG-CoA reductase, NPC1L1, and PCSK9. Summary statistics with outcomes were obtained from a GWAS meta-analysis of the Breast Cancer Association Consortium (BCAC; 228,951 European females) and a Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL; 140,254 European males) consortium. SNPs were combined into multiallelic models and MR estimates representing lifelong inhibition of targets were generated using the inverse-variance weighted method.
Results
Genetically proxied inhibition of HMG-CoA reductase (OR: 0.84; 95% CI 0.74–0.95; P = 0.005) and NPC1L1 (OR: 0.72; 95% CI 0.58–0.90; P = 0.005) equivalent to a 1-mmol/L (38.7 mg/dL) reduction in LDL-C was associated with reduced breast cancer risk. There were no significant associations of genetically proxied inhibition of PCSK9 with breast cancer. In contrast, genetically proxied inhibition of PCSK9 (OR: 0.81; 95% CI 0.73–0.90; P < 0.001) but not HMG-CoA reductase and NPC1L1 was negatively associated with prostate cancer. In the secondary analysis, genetically proxied inhibition of HMG-CoA reductase (OR: 0.82; 95% CI 0.71–0.95; P = 0.008) and NPC1L1 (OR: 0.66; 95% CI 0.50–0.86; P = 0.002) was associated with estrogen receptor-positive breast cancer, whereas there was no association of HMG-CoA reductase and NPC1L1 with estrogen receptor-negative breast cancer.
Conclusions
Genetically proxied inhibition of HMG-CoA reductase and NPC1L1 was significantly associated with lower odds of breast cancer, while genetically proxied inhibition of PCSK9 was associated with reduced risk of prostate cancer. Further randomized controlled trials are needed to confirm the respective roles of these LDL-C-lowering drugs in breast cancer and prostate cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-022-01508-0.
Keywords: LDL-C-lowering drug, HMG-CoA reductase, NPC1L1, PCSK9, Breast cancer, Prostate cancer
Introduction
There were 19.3 million new cancer cases and almost 10.0 million cancer deaths worldwide in 2020, which caused an enormous economic burden for patients and society [1]. Breast cancer was the most common cancer among females, while prostate cancer was the second most frequently occurring cancer among males [1]. Although these two cancers arise in different organs in terms of anatomy and physiological function, they are typically hormone-dependent and have remarkable underlying biological similarities [2], because both organs require gonadal steroids for their development. With the limited treatment measures (e.g., surgery, radiation and hormone therapy) and poor prognosis of breast cancer and prostate cancer [2–4], primary prevention potentially offers the most cost-effective strategy for breast cancer and prostate cancer control and would effectively reduce the disease burden.
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA; target of statins) reductase, Niemann-Pick C1-Like 1 (NPC1L1; target of ezetimibe) and proprotein convertase subtilisin/kexin type 9 (PCSK9; target of PCSK9 inhibitors) are common targets of low-density lipoprotein cholesterol (LDL-C)-lowering drugs for the prevention of cardiovascular disease [5]. Previous observational studies have shown that statins, ezetimibe, and PCSK9 inhibitors have protective effects against breast cancer and prostate cancer [6–8]. For example, Cauley et al.’s prospective cohort study found that women who were using statins or other lipid-lowering drugs had a 72% and 63% reduced risk of breast cancer compared with those who were not using, respectively [6]. In addition, a meta-analysis of 27 observational studies found that statin use significantly reduced the risk of prostate cancer [7]. However, there have been some studies reporting contradictory results to the above studies with no protective effects of them for the two cancers [9–11]. Both breast cancer and prostate cancer are multigenic, multifactorial and complex trait diseases, and there may be some factors confounding the study results in observational studies. Therefore, some confounding factors may lead to inconsistent results in some observational studies on associations of HMG-CoA reductase, NPC1L1 and PCSK9 with the risks of breast cancer and prostate cancer.
Mendelian randomization (MR) method may investigate the potential causal effect of an exposure of interest on the risk of disease by utilizing genetic variants as a proxy [12, 13]. Potential unmeasured confounders and reverse causation can be minimized in MR study due to random inheritance of parental genetic variants at conception [12, 13], while two-sample MR has better methodological advantages with two separate population studies [14]. Herein, we conducted a two-sample MR study to assess the causal associations of several LDL-C-lowering drug targets (HMG-CoA reductase, NPC1L1, and PCSK9) with breast cancer and prostate cancer.
Methods
Study design
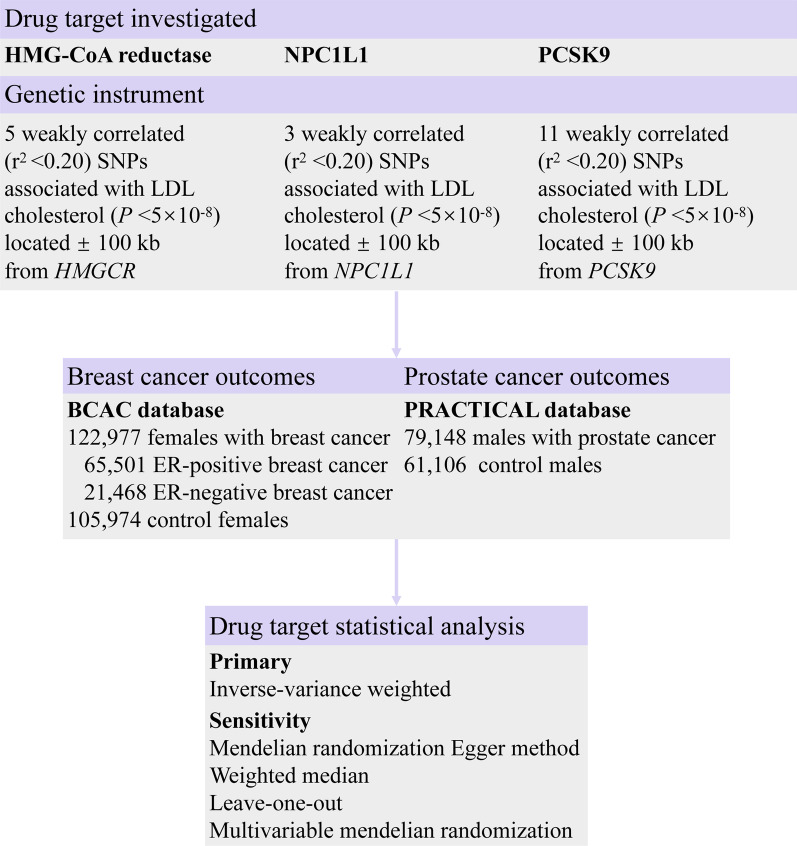
As illustrated in Fig. 1, we designed an MR study to systematically investigate associations between inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 and the risks of breast cancer and prostate cancer. Summary-level data of SNPs as genetic instruments for HMG-CoA reductase, NPC1L1, and PCSK9 were obtained from previously large-scale GWASs of European ancestry [15]. The protocol and data collection were approved by the ethics committee of the original GWASs, and written informed consent was obtained from each participant before data collection.
Fig. 1.
Genetic instrument construction, data sources, and analysis plan in a study of associations about the inhibition of HMG-CoA reductase, NPC1L1 and PCSK9 on breast cancer and prostate cancer. HMG-CoA reductase, 3-hydroxy-3-methylglutaryl coenzyme A; NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; BCAC database, The Breast Cancer Association Consortium; PRACTICAL database, Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome Consortium; ER, estrogen receptor
Genetic instruments of LDL-C-lowering drug targets
To generate genetic instruments to proxy HMG-CoA reductase, NPC1L1, and PCSK9, summary genetic association data were obtained from a GWAS meta-analysis of LDL-C levels in the Global Lipids Genetics Consortium (GLGC) [15]. The GLGC (up to 188,577 European individuals) included 93,982 from 37 studies genotyped with the Metabochip array and 94,595 from 23 GWAS cohorts excluding overlap with Metabochip cohorts [15]. In most of the included studies, blood lipid concentrations were measured in fasting blood specimens (after at least 8 h of fasting), and individuals undergoing lipid-lowering treatment were excluded when possible.
To proxy HMG-CoA reductase, 5 SNPs associated with LDL-C at the genome-wide significance level (P < 5.0 × 10−8) and within ± 100 kb windows of the gene region encoding HMG-CoA reductase were obtained. Similarly, 3 LDL-C related SNPs within ± 100 kb from NPC1L1 and 11 LDL-C related SNPs within ± 100 kb from PCSK9 were used to proxy NPC1L1 and PCSK9, respectively. To maximize instrument strength, SNPs used as proxies for each drug target were permitted to be in low linkage disequilibrium (r2 < 0.20) with each other to increase the proportion of variance in each respective drug target explained by the instrument. The characteristics of genetic variants genetically proxied HMG-CoA reductase, NPC1L1 and PCSK9 are shown in Table 1.
Table 1.
Characteristics of LDL-C-lowering genetic variants proxied for inhibition of HMG-CoA reductase, NPC1L1, and PCSK9
| SNPa | Nearest gene | Position (build 37) | EA | NEA | EAF | Beta | Se | P value |
|---|---|---|---|---|---|---|---|---|
| HMG-CoA reductase | ||||||||
| rs12916 | HMGCR | chr5:74656539 | T | C | 0.57 | − 0.073 | 0.004 | 7.8e−78 |
| rs10515198 | HMGCR | chr5:74641560 | G | A | 0.90 | − 0.060 | 0.006 | 6.0e−22 |
| rs12173076 | CERT1 | chr5:74697050 | T | G | 0.88 | − 0.065 | 0.006 | 2.3e−27 |
| rs3857388 | HMGCR | chr5:74620377 | T | C | 0.87 | − 0.042 | 0.006 | 2.2e−11 |
| rs7711235 | ANKRD31 | chr5:74540397 | A | G | 0.73 | − 0.038 | 0.006 | 5.0e−10 |
| NPC1L1 | ||||||||
| rs2073547 | NPC1L1 | chr7:44582331 | A | G | 0.81 | − 0.049 | 0.005 | 1.9e−21 |
| rs217386 | DDX56 | chr7:44600695 | A | G | 0.41 | − 0.036 | 0.004 | 1.2e−19 |
| rs7791240 | DDX56 | chr7:44602589 | T | C | 0.91 | − 0.043 | 0.007 | 1.8e−10 |
| PCSK9 | ||||||||
| rs11591147 | PCSK9 | chr1:55505647 | T | G | 0.02 | − 0.497 | 0.018 | 8.6e−143 |
| rs11206510 | PCSK9 | chr1:55496039 | C | T | 0.15 | − 0.083 | 0.005 | 2.4e−53 |
| rs2479409 | PCSK9 | chr1:55504650 | A | G | 0.67 | − 0.064 | 0.004 | 2.5e−50 |
| rs585131 | PCSK9 | chr1:55524116 | C | T | 0.18 | − 0.064 | 0.005 | 2.7e−35 |
| rs11206514 | PCSK9 | chr1:55516004 | C | A | 0.39 | − 0.051 | 0.004 | 1.0e−32 |
| rs2495477 | PCSK9 | chr1:55518467 | G | A | 0.40 | − 0.064 | 0.005 | 7.3e−30 |
| rs572512 | PCSK9 | chr1:55517344 | C | T | 0.65 | − 0.048 | 0.005 | 5.3e−26 |
| rs2479394 | BSND | chr1:55486064 | A | G | 0.72 | − 0.039 | 0.004 | 1.6e-19 |
| rs12067569 | PCSK9 | chr1:55528629 | G | A | 0.97 | − 0.089 | 0.010 | 2.0e−17 |
| rs10493176 | USP24 | chr1:55538552 | G | T | 0.11 | − 0.078 | 0.010 | 2.5e−14 |
| rs11583974 | USP24 | chr1:55551718 | G | A | 0.97 | − 0.065 | 0.012 | 4.0e−09 |
HMG-CoA reductase (HMGCR), 3-hydroxy-3-methylglutaryl coenzyme A; NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; SNP, Single-Nucleotide Polymorphism; EA, Effect Allele; NEA, Non-effect Allele; EAF, Effect Allele Frequency
aLDL-C associated SNPs (P < 5.0 × 10–8) in low linkage disequilibrium (r2 < 0.20) with each other and within ± 100 kb windows of gene region encoding HMG-CoA reductase, NPC1L1, and PCSK9 were used as genetic instruments (adapted from Willer et al. [15])
Data sources for outcomes
Genetic association data of breast cancer, ER-positive breast cancer, and ER-negative breast cancer were obtained from a GWAS in the Breast Cancer Association Consortium (BCAC). The BCAC is an international collaboration initiated in 2005 to study genetic susceptibility to breast cancer, including 122,977 female cases and 105,974 female controls of European ancestry [16]. In BCAC, breast cancer cases (i.e., ER-positive breast cancer, ER-negative breast cancer, etc.) were mostly from the hospital and Cancer Registry [17]. The SNP-prostate cancer risk estimates were obtained from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium, which included genomic data of 79,148 European prostate cancer cases and 61,106 European controls [18]. Our analysis includes overall prostate cancer, which can be classified into several clinically relevant strata (e.g., T1, T2, T3, and M1) with the Gleason score and prostate specific antigen [18]. In the present study, the primary outcomes were breast cancer and prostate cancer, and the secondary outcomes included ER-positive breast cancer and ER-negative breast cancer.
Statistical analysis
The strength of the genetic instruments of LDL-C-lowering drug targets was evaluated using the F-statistic [19], calculated by the equation F = (N − K − 1) × R2/K × (1 − R2), where R2, calculated by using the package gtx in R (version 4.1.0; R Development Core Team), was the proportion of variation in HMG-CoA reductase, NPC1L1, or PCSK9 explained by the SNPs, N was the sample size, and K was the number of SNPs in genetically proxied inhibition of HMG-CoA reductase, NPC1L1, or PCSK9. Conventionally, an F statistic of at least 10 was indicative of evidence against weak instrument bias [19]. In addition, an online web tool named mRnd (https://shiny.cnsgenomics.com/mRnd/) was used to calculate the statistical power in this MR study [20].
In the main analysis, we used the inverse-variance weighted (IVW) MR method to estimate the effect of genetically proxied HMG-CoA reductase, NPC1L1, and PCSK9 on breast cancer and prostate cancer [21]. The heterogeneity between SNPs was evaluated by Cochran’s Q test [22]. If heterogeneity existed, random-effect IVW models were used and otherwise fixed-effect IVW models. To assess the robustness of the findings in the main analysis, we also performed a series of sensitivity analyses due to their resilience to violations of certain assumptions underlying the MR study. Firstly, we conducted MR-Egger regression analysis, of which the intercept term could be interpreted as pleiotropy across all variants [23, 24]. Secondly, we employed the weighted median approach to provide reliable estimates, in which analysis of the MR estimates was robust when < 50% of genetic variants were invalid [25]. Thirdly, iterative leave-one-out analysis was used to explore outlying or pleiotropic genetic variants by leaving each of them out of the MR analysis in turn [24, 26]. Finally, given the associations of LDL-C-lowering target instruments with body mass index and age at menarche [27], we further performed MR analysis with multivariable adjustment to estimate the relatively direct effects of LDL-C-lowering target on the risk of breast cancer (adjusting for body mass index and age at menarche) and prostate cancer (adjusting for body mass index) [28–31].
All MR estimates were presented as odds ratios (ORs) and were scaled up from individual SNP-level effects on LDL-C levels to reflect the equivalent of a 1-mmol/L (38.7-mg/dL) reduction in LDL-C levels. For the two primary outcomes (breast cancer and prostate cancer), all statistical tests were 2-sided, and a significance threshold was set at P < 0.008 (Bonferroni-correction significance threshold calculated as 0.05 divided by 6 [3 drug targets against 2 outcomes]). For the secondary outcome (ER-positive breast cancer and ER-negative breast cancer), an observed 2-sided P < 0.05 was considered to be statistically significant because these analyses were only exploratory analyses. All analyses were conducted with packages named TwoSampleMR, MendelianRandomization, and gtx in R software (version 4.1.0; R Development Core Team).
Results
The strength of genetic instruments for LDL-C-lowering drug targets (HMG-CoA reductase, NPC1L1, and PCSK9) and the statistical power of this MR analysis are presented in Additional file 1: Table S1. The F statistics for the association of genetic instruments with HMG-CoA reductase, NPC1L1, and PCSK9 ranged from 71.63 to 195.81, suggesting that there is little instrument bias in the present MR study.
Associations of LDL-C-lowering drug targets with breast cancer
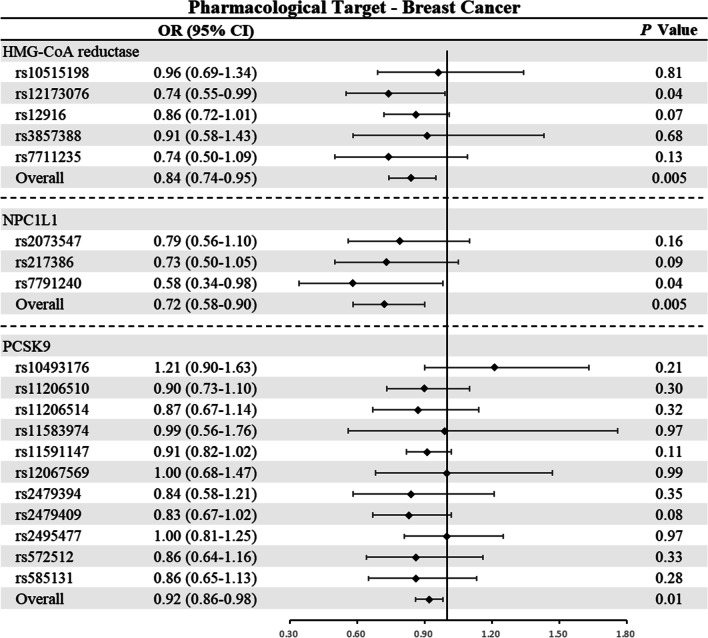
In light of Cochran’s Q test, the fixed-effect IVW models were used in the main analysis of breast cancer due to the lack of heterogeneity (Additional file 1: Table S2). As shown in Table 2 and Fig. 2, genetically proxied inhibition of HMG-CoA reductase (OR: 0.84; 95% CI 0.74–0.95; P = 0.005) and NPC1L1 (OR: 0.72; 95% CI 0.58–0.90; P = 0.005) equivalent to a 1-mmol/L (38.7 mg/dL) reduction in LDL-C was significantly associated with a reduced risk of breast cancer, respectively. However, the results showed no significant relationship between genetically determined inhibition of PCSK9 and the risk of breast cancer, although there was a trend of reduced risk of breast cancer (OR: 0.92; 95% CI 0.86–0.98; P = 0.01). In addition, further analysis showed that genetically proxied inhibition of HMG-CoA reductase and NPC1L1 were mainly associated with lower risk of ER-positive breast cancer (HMG-CoA reductase: OR: 0.82, P = 0.008; NPC1L1: OR: 0.66, P = 0.002) but not ER-negative breast cancer (Additional file 1: Table S3).
Table 2.
Associations between genetically proxied inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 and breast cancer and prostate cancer
| Outcome | Case, No | OR (95% CI) | P valuea |
|---|---|---|---|
| HMG-CoA reductase | |||
| Breast cancer | 122,977 | 0.84 (0.74–0.95) | 0.005 |
| Prostate cancer | 79,148 | 0.85 (0.73–1.00) | 0.05 |
| NPC1L1 | |||
| Breast cancer | 122,977 | 0.72 (0.58–0.90) | 0.005 |
| Prostate cancer | 79,148 | 1.23 (0.92–1.63) | 0.16 |
| PCSK9 | |||
| Breast cancer | 122,977 | 0.92 (0.86–0.98) | 0.01 |
| Prostate cancer | 79,148 | 0.81 (0.73–0.90) | 4.52e−05 |
HMG-CoA reductase, 3-hydroxy-3-methylglutaryl coenzyme A; NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; OR, Odds Ratio; CI, confidence interval
aSignificance threshold was set at P < 0.008 (Bonferroni-correction significance threshold calculated as 0.05 divided by 6 [3 drug targets against 2 outcomes])
Fig. 2.
Mendelian randomization estimates of the associations between inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 with breast cancer among women. HMG-CoA reductase, 3-hydroxy-3-methylglutaryl coenzyme A Reductase; NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; CI, confidence interval. An observed 2-sided P < 0.008 (Bonferroni-correction significance threshold calculated as 0.05 divided by 6 [3 drug targets against 2 outcomes]) was considered to be statistically significant
In the sensitivity analyses, MR-Egger showed no evidence of directional pleiotropy for the associations between inhibition of HMG-CoA reductase (odds [intercept]: 1.00; P = 0.77), NPC1L1 (odds [intercept]: 0.99; P = 0.84), and PCSK9 (odds [intercept]: 1.00; P = 0.72) and breast cancer (Additional file 1: Table S4). The results of leave-one-out sensitivity analyses showed that no individual SNP substantially drove the associations of HMG-CoA reductase and NPC1L1 with breast cancer (Additional file 1: Table S5).
Associations of LDL-C-lowering drug targets with prostate cancer
In the main analysis of prostate cancer, fixed-effect IVW models were used for HMG-CoA reductase and NPC1L1 because of no heterogeneity, while random effect IVW model was also used for PCSK9 (Additional file 1: Table S2). As shown in Table 2 and Fig. 3, genetically proxied PCSK9 inhibition was associated with a reduced risk of prostate cancer (OR: 0.81; 95% CI 0.73–0.90; P = 4.52 × 10–5). In contrast, genetically proxied inhibition of HMG-CoA reductase (OR: 0.85; 95% CI 0.73–1.00; P = 0.05) or NPC1L1 (OR: 1.23; 95% CI 0.92–1.63; P = 0.16) was not significantly associated with prostate cancer.
Fig. 3.
Mendelian randomization estimates of the associations between inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 with prostate cancer among men. HMG-CoA reductase, 3-hydroxy-3-methylglutaryl coenzyme A Reductase; NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; CI, confidence interval. An observed 2-sided P < 0.008 (Bonferroni-correction significance threshold calculated as 0.05 divided by 6 [3 drug targets against 2 outcomes]) was considered to be statistically significant
In light of the MR-Egger regression findings (Table 2), we found no evidence against directional pleiotropy for the associations between genetically determined inhibition of HMG-CoA reductase (odds [intercept]: 1.00; P = 0.52), NPC1L1 (odds [intercept]: 1.03; P = 0.64), and PCSK9 (odds [intercept]: 0.99; P = 0.09) and the risk of prostate cancer (Additional file 1: Table S4). In the sensitivity analysis applying the weighted median method, the association of genetically proxied PCSK9 inhibition with prostate cancer (OR: 0.85; 95% CI 0.76–0.95; P = 0.005; Additional file 1: Table S6) remained significant. Leave-one-out sensitivity analysis showed that the association between PCSK9 inhibition and prostate cancer was not substantially driven by any individual SNP (Additional file 1: Table S7).
Multivariable MR analyses
The results of multivariable MR analysis on the associations of LDL-C-lowering drug targets with breast cancer and prostate cancer are presented in Table S8. In terms of breast cancer, multivariable MR analyses adjusting for body mass index and age at menarche suggested that genetically proxied inhibition of HMG-CoA reductase was associated with a reduced risk of breast cancer (OR: 0.85; 95% CI 0.75–0.95; P = 0.007). For prostate cancer, significant associations were observed for genetically proxied PCSK9 inhibition and low odds of prostate cancer (OR: 0.81; 95% CI 0.71–0.92; P = 0.002).
Discussion
In this large-scale MR analysis of 122,977 cases and 105,974 controls for breast cancer and 79,148 cases and 61,106 controls for prostate cancer, we investigated the effect of three common LDL-C-lowering drug targets (HMG-CoA reductase, NPC1L1, and PCSK9) on the risks of breast cancer and prostate cancer. We found that genetically proxied inhibition of HMG-CoA reductase and NPC1L1 but not PCSK9 were significantly associated with reduced risk of breast cancer (mainly ER-positive breast cancer), while only genetically determined PCSK9 inhibition was associated with low odds of prostate cancer. These findings suggested that there were possible mechanism-specific protective effects of statins (targeting HMG-CoA reductase) and ezetimibe (targeting NPC1L1) on breast cancer, whereas PCSK9 inhibitors might have protective effect on prostate cancer.
In recent decades, observational studies investigating the effects of statins and alternate LDL-C-lowering drugs (ezetimibe and PCSK9 inhibitors) on the risks of breast cancer and prostate cancer have yielded inconsistent results [6, 7, 9, 10, 32]. In an analysis based on a multicenter cohort study involving 7,528 Caucasian females in the United States, the participants who used lipid-lowering drugs had a significantly reduced risk of developing breast cancer compared with those who did not use [6]. A meta-analysis of 27 observational studies showed that statins use significantly reduced the risk of prostate cancer by 7% [7]. The above two findings seemed to reveal the potential application possibility of lipid-lowering drugs in the prevention of breast cancer and prostate cancer. However, in an analysis based on the Cancer Prevention Study II Nutrition Cohort with a 10-year follow-up period, long-term use of cholesterol-lowering drugs were not associated with the risk of breast cancer and prostate cancer [10]. Lipid-lowering drugs have been widely used to prevent hyperlipidemia, atherosclerosis and cardiovascular disease in population. Clarifying the relationship between lipid-lowering drugs and breast cancer and prostate cancer has not only scientific significance, but also practical implications for the prevention of breast cancer and prostate cancer. The inconsistent results of the above studies may be due to the reason why observational study and conventional analysis are incapable of completely overcoming the confounding because of the polygenic and multifactorial characteristics of breast cancer and prostate cancer with complex traits.
With regard to the inherent bias of residual confounding and reverse causation in observational studies, MR is an increasingly used method to draw definitive conclusions regarding the causality of the association between exposures and diseases via considering genetic variants as instrumental variables [12–14]. MR leverages the random allocation of exposure-influencing genetic alleles and can avoid reverse-causality bias and minimize confounding by other determinants in a similar pattern as randomized controlled trials [33]. Recently, genetically proxied inhibition of HMG-CoA reductase but not NPC1L1 and PCSK9 was associated with lower odds of epithelial ovarian cancer in MR study, while no such MR study was currently available for breast cancer and prostate cancer [27]. In the present study, we first found that genetically proxied inhibition of HMG-CoA reductase and NPC1L1 were significantly associated with lower odds of breast cancer (especially ER-positive breast cancer), while the inhibition of PCSK9 was associated with reduced risk of prostate cancer.
There exists numerous important public health significances and clinical implications. From our findings, the problems that lipid lowering drugs maybe prevent breast cancer and prostate cancer in population deserve to be further studied, and even statins and ezetimibe may be recommended for the prevention of breast cancer in the population, especially ER-positive breast cancer, among females with hyperlipidemia. In contrast, PCSK9 inhibitors may be the preferred LDL-C-lowering drug among males with hyperlipidemia to prevent prostate cancer. Some randomized controlled trials for preventing cardiovascular disease have suggested that statins have provocative and unexpected benefits for reducing colorectal cancer and melanoma [34–36]. However, there have not been randomized controlled trials designed to study the effects of statins and ezetimibe on breast cancer and PCSK9 inhibitors on prostate cancer. Therefore, further well-designed randomized trials are warranted to verify the protective effects of statins and ezetimibe on breast cancer and PCSK9 inhibitors on prostate cancer. The validated findings will promote precise prevention and develop personalized treatment strategies for breast cancer and prostate cancer.
Several biological mechanisms may underlie the beneficial effect of LDL-C-lowering drugs on breast cancer and prostate cancer. HMG-CoA reductase inhibition may reduce intratumoural autocrine hormone production by lowering intracellular cholesterol, promote apoptosis breast cancer cells through inducing nitric oxide synthase expression [37, 38]. As the target of ezetimibe, inhibition of NPC1L1 can decrease the level of bile-derived cholesterol to inhibit the angiogenesis of breast tumors [39]. PCSK9 inhibition appeared to be implicated in prostate cancer by regulating the expression of squalene monooxygenase and lectin-like oxidized low-density lipoprotein receptor-1 [40–42]. In addition, the influence of hormones, sex differences in immunity, and complexity of the regulatory mechanism in tumor angiogenesis may also contribute to the different roles of stains, ezetimibe and PCSk9 inhibition in breast cancer and prostate cancer [39, 40, 43]. Further functional studies are warranted to investigate the mechanisms underlying the specific protective effects of certain lipid-lowering drugs on the breast cancer and prostate cancer.
This study had a number of important strengths. First, to the best of our knowledge, this is the first MR study to detect the causal effects of lipid-lowering drugs on breast cancer, breast cancer subtypes, and prostate cancer. Second, the present study used genetic variants within genes that encoded drug targets to proxy the potential effect of commonly prescribed LDL-C-lowering therapies, which could reflect the impact of life-long exposure on breast cancer or prostate cancer and avoid the drawback of limited exposure time and follow-up time in clinical trials or observational studies. Third, the summary statistics of lipid-lowering drugs, breast cancer and prostate cancer were collected from well-designed GWAS with large sample size, which enabled us to make causal inferences with high statistical power. Fourth, our study harnessed the homogeneity of European populations with respect to genetic background and external sociocultural determinants, decreasing spurious associations due to population stratification and other confounding factors [44]. Certainly, further studies included multi-ethnicity samples should be conducted to confirm our findings.
Our study also had several limitations. First, MR estimates represented the long-term modulation of drug targets on disease risk, which might suggest larger risk reductions per unit change in drug target compared with those obtained from drug administration over a relatively shorter duration. Therefore, if lipid-lowering drug treatment could lower the risk of breast cancer or prostate cancer, the magnitude of risk lowering achieved through taking lipid-lowering drugs might not correspond to the effect size observed in this MR study. Second, although we attempted to control for pleiotropy in the MR study, pleiotropy still represented a major challenge to decipher the roles of specific lipid-based pathways. In the present study, MR-Egger regression analysis showed no pleiotropic effects, indicating that the possibility of directional pleiotropy bias may be minimal. Third, MR analysis might be biased by potential violations of standard instrumental variable assumptions [23]. However, several sensitivity analyses in the present study observed no evidence of violations and further confirmed the robustness of the results in the main analysis.
Conclusions
Genetically proxied inhibition of HMG-CoA reductase and NPC1L1 was significantly associated with lower odds of breast cancer (especially ER-positive breast cancer), while genetically proxied inhibition of PCSK9 was associated with reduced risk of prostate cancer. Further randomized controlled trials are needed to confirm the respective roles of these LDL-C-lowering drugs in breast cancer and prostate cancer.
Supplementary Information
Additional file 1. Supplementary Online Content.
Acknowledgements
The investigators of the GLGC, BCAC, and PRACTICAL consortium are thanked for making their results publicly available. Full acknowledgement and funding statements for each of these resources are available via the relevant cited reports.
Abbreviations
- LDL-C
Low-density lipoprotein cholesterol
- HMG-CoA
3-Hydroxy-3-methylglutaryl coenzyme A
- NPC1L1
Niemann-Pick C1-Like 1
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- MR
Mendelian randomization
- SNPs
Single-nucleotide polymorphisms
- GWAS
Genome-wide association study
- GLGC
Global Lipids Genetics Consortiumthe
- BCAC
Breast Cancer Association Consortium
- PRACTICAL
Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium
- ER
Estrogen receptor
- IVW
Inverse-variance weighted
Authors' contributions
LS, YZ and ZZ conceived and designed the study. LS, YZ and ZZ coordinated the study. LS, HD, YJ, MS, DG, PY, YW, FL, YZ, and ZZ oversaw and monitored gathering of data on SNPs. LS, HD, YZ and ZZ conducted the statistical analysis and prepared the paper. YZ and ZZ revised the paper. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grants: 82103917, 82103921, and 82020108028), the high level personnel project of Jiangsu Province (Grant: JSSCBS20210712), the Natural Science Research Project of Jiangsu Provincial Higher Education (Grant: 21KJB330006), the Startup Fund from Soochow University (Grant: Q413900420), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Availability of data and materials
Single-nucleotide polymorphisms (SNPs) in HMGCR, NPC1L1, and PCSK9 associated with LDL-C in a genome-wide association study (GWAS) meta-analysis from Global Lipids Genetics Consortium (GLGC; up to 188,577 European individuals) were used to proxy therapeutic inhibition of HMG-CoA reductase, NPC1L1, and PCSK9, respectively. Summary statistics for these SNPs were obtained from a GWAS meta-analysis of the Breast Cancer Association Consortium (BCAC; 228,951 European females) and from a Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL; 140,254 European males) consortium.
Declarations
Ethics approval and consent to participate
The protocol and data collection were approved by the ethics committee of the original GWASs, and written informed consent was obtained from each participant before data collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lulu Sun and Huan Ding have contributed equally to this work
Contributor Information
Yonghong Zhang, Email: yhzhang@suda.edu.cn.
Zhengbao Zhu, Email: zbzhu@suda.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer-more similiar than different. Nat Rev Cancer. 2010;10(3):205–212. doi: 10.1038/nrc2795. [DOI] [PubMed] [Google Scholar]
- 3.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 4.Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 5.Banks E, Crouch SR, Korda RJ, Stavreski B, Page K, Thurber KA, Grenfell R. Absolute risk of cardiovascular disease events, and blood pressure- and lipid-lowering therapy in Australia. Med J Aust. 2016;204(8):320. doi: 10.5694/mja15.01004. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, Cummings SR, Bauer DC. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003;12(8):749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 7.Bansal D, Undela K, D'Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS ONE. 2012;7(10):e46691. doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, Schaffner CP, Kim J, Freeman MR. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174(3):1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100(11):2308–2316. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71(5):1763–1771. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 13.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, Evans DM, Smith GD. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michailidou K, Lindstrom S, Dennis J, Beesley J, Hui S, Kar S, Lemacon A, Soucy P, Glubb D, Rostamianfar A, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551(7678):92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–61, 361e351–352. [DOI] [PMC free article] [PubMed]
- 18.Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, Dadaev T, Leongamornlert D, Anokian E, Cieza-Borrella C, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–936. doi: 10.1038/s41588-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S, Thompson SG, Collaboration CCG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 20.Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julian PTH, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42. doi: 10.1097/EDE.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Smith GD, Lewis SJ, Relton CL, Martin RM. Association between genetically proxied inhibition of HMG-CoA reductase and epithelial ovarian cancer. JAMA. 2020;323(7):646–655. doi: 10.1001/jama.2020.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao C, Patel CJ, Michailidou K, Peters U, Gong J, Schildkraut J, Schumacher FR, Zheng W, Boffetta P, Stucker I, et al. Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol. 2016;45(3):896–908. doi: 10.1093/ije/dyw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Warren Andersen S, Shu XO, Michailidou K, Bolla MK, Wang Q, Garcia-Closas M, Milne RL, Schmidt MK, Chang-Claude J, et al. Genetically predicted body mass index and breast cancer risk: Mendelian randomization analyses of data from 145,000 women of European descent. PLoS Med. 2016;13(8):e1002105. doi: 10.1371/journal.pmed.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Otín C. DIAMANDIS EP: breast and prostate cancer-an analysis of common epidemiological, genetic, and biochemical features. Endocr Rev. 1998;19(4):365–396. doi: 10.1210/edrv.19.4.0337. [DOI] [PubMed] [Google Scholar]
- 31.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251–260. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 33.Bennett DA, Holmes MV. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart. 2017;103(18):1400–1407. doi: 10.1136/heartjnl-2016-310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Lisa B. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 35.Long-Term Interventionwith Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 36.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 37.Borgquist S, Bjarnadottir O, Kimbung S, Ahern TP. Statins: a role in breast cancer therapy? J Intern Med. 2018;284(4):346–357. doi: 10.1111/joim.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67(15):7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 39.Pelton K, Coticchia CM, Curatolo AS, Schaffner CP, Zurakowski D, Solomon KR, Moses MA. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am J Pathol. 2014;184(7):2099–2110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stopsack KH, Gerke TA, Andren O, Andersson SO, Giovannucci EL, Mucci LA, Rider JR. Cholesterol uptake and regulation in high-grade and lethal prostate cancers. Carcinogenesis. 2017;38(8):806–811. doi: 10.1093/carcin/bgx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Z, Liu S, Wang X, Deng X, Fan Y, Shahanawaz J, Shmookler Reis RJ, Varughese KI, Sawamura T, Mehta JL. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc Res. 2015;107(4):556–567. doi: 10.1093/cvr/cvv178. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Chavarria I, Cerro RP, Parra NP, Sandoval FA, Zuniga FA, Omazabal VA, Lamperti LI, Jimenez SP, Fernandez EA, Gutierrez NA, et al. Lectin-like oxidized LDL receptor-1 is an enhancer of tumor angiogenesis in human prostate cancer cells. PLoS ONE. 2014;9(8):e106219. doi: 10.1371/journal.pone.0106219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozdemir BC, Dotto GP. Sex hormones and anticancer immunity. Clin Cancer Res. 2019;25(15):4603–4610. doi: 10.1158/1078-0432.CCR-19-0137. [DOI] [PubMed] [Google Scholar]
- 44.Thanassoulis G, O'Donnell CJ. Mendelian randomization: nature's randomized trial in the post-genome era. JAMA. 2009;301(22):2386–2388. doi: 10.1001/jama.2009.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Online Content.
Data Availability Statement
Single-nucleotide polymorphisms (SNPs) in HMGCR, NPC1L1, and PCSK9 associated with LDL-C in a genome-wide association study (GWAS) meta-analysis from Global Lipids Genetics Consortium (GLGC; up to 188,577 European individuals) were used to proxy therapeutic inhibition of HMG-CoA reductase, NPC1L1, and PCSK9, respectively. Summary statistics for these SNPs were obtained from a GWAS meta-analysis of the Breast Cancer Association Consortium (BCAC; 228,951 European females) and from a Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL; 140,254 European males) consortium.