Abstract
Objective.
Complex spatiotemporal neural activity encodes rich information related to behavior and cognition. Conventional research has focused on neural activity acquired using one of many different measurement modalities, each of which provides useful but incomplete assessment of the neural code. Multi-modal techniques can overcome tradeoffs in the spatial and temporal resolution of a single modality to reveal deeper and more comprehensive understanding of system-level neural mechanisms. Uncovering multi-scale dynamics is essential for a mechanistic understanding of brain function and for harnessing neuroscientific insights to develop more effective clinical treatment.
Approach.
We discuss conventional methodologies used for characterizing neural activity at different scales and review contemporary examples of how these approaches have been combined. Then we present our case for integrating activity across multiple scales to benefit from the combined strengths of each approach and elucidate a more holistic understanding of neural processes.
Main results.
We examine various combinations of neural activity at different scales and analytical techniques that can be used to integrate or illuminate information across scales, as well the technologies that enable such exciting studies. We conclude with challenges facing future multi-scale studies, and a discussion of the power and potential of these approaches.
Significance.
This roadmap will lead the readers toward a broad range of multi-scale neural decoding techniques and their benefits over single-modality analyses. This Review article highlights the importance of multi-scale analyses for systematically interrogating complex spatiotemporal mechanisms underlying cognition and behavior.
Keywords: multi-scale analyses, neural decoding, electrophysiology, functional imaging
1. Introduction
Neural recording methodologies enable us to probe neural activity and investigate how the brain implements cognitive processes and generates behavior [1, 2]. There are many technologies—electrical, optical, and chemical—that allow us to observe and perturb neural activity at different temporal and spatial scales. At any given scale, neural activity encodes rich information related to behavior and cognition [3–18]. Work to date has conventionally focused on neural activity acquired using a single modality and, thus, at a single scale. Decoding and modeling neural dynamics using measurements from one modality limits investigations to dynamics within the same level. However, complex spatiotemporal activity supports behavior and cognition, and cross-scale dynamics can reveal a deeper and more comprehensive understanding of system-level neural mechanisms [19, 20]. Since the brain exhibits functional structure across a range of disparate scales, from single neurons (micro-scale) to networks of brain areas (macro-scale), multi-scale and multi-modal techniques can overcome tradeoffs in the spatial and temporal resolution of a single modality and scale. Uncovering multi-scale dynamics is essential for illuminating the mechanistic understanding of brain function and to harness scientific insight for neuroscientifically grounded clinical treatment. A paradigm shift, enabled by state-of-the-art neurotechnologies, is currently underway to analyze neural activity across multiple scales.
Often, we are interested in activity from individual neurons and use electrodes positioned within neural tissue to record single-unit (SUA) or multiunit activity (MUA). This activity reflects action potentials (‘spikes’) from one or a small number of neurons in the brain and can be used, for example, to determine how neuronal firing rates co-vary with behavioral variables. Additionally, we commonly examine oscillatory dynamics of neural activity, which can be obtained from local field potential (LFP), electrocorticography (ECoG), and electroencephalography (EEG) signals. While each of these signals reflect summed electrical activity which manifest as neural oscillations in the brain [21], they are recorded at different scales using different recording modalities. In addition to electrophysiological recording techniques, functional brain imaging techniques such as functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS) offer a different perspective on brain function [22]. Instead of investigating electrical signals, they capture the hemodynamic consequences of the underlying neural activity. Positron emission tomography (PET) is another functional imaging technique that uses radiotracers to measure metabolic changes, as well as blood flow and neurochemical activity. Although these markers are indirect measurements of brain activity, fMRI, fNIRS, and PET are valuable techniques due to their spatial coverage of the whole brain, non-invasiveness and clinical availability [23]. These techniques all have their own strengths and challenges, and the combination of these modalities has the potential to further clarify the neural mechanisms underlying cognitive processes and behavior.
Many cognitive processes have been investigated across multiple modalities independently, including movements [24–29] and decision-making [7, 9, 10, 12, 18], reward [14, 16, 17], memory [6, 8], and arousal [3, 4], and each modality provides a different perspective on the underlying neural activity. One significant outcome from this large body of impressive scientific work is that the same cognitive process can be observed at different spatial scales. This supports the notion that the heterogeneity of different techniques can provide researchers with different perspectives that, when combined to capture the interplay between scales, can provide a more comprehensive picture of neural function. Thus, it is increasingly imperative to conduct multi-scale analyses.
In addition to creating a richer understanding of neural processes, applying multiple modalities in the same experimental setting may overcome disadvantages from each individual modality, such as the compromise between temporal and spatial resolutions [30]. For example, electrophysiological techniques have high temporal resolution and spatial specificity increases with invasiveness, but typically comes at the cost of decreasing spatial coverage. On the other hand, functional brain imaging techniques have more complete spatial coverage, but suffer from temporal delay and lower temporal resolution due to the data acquisition process and sluggishness of the hemodynamic signal being measured [31]. The combination of these electrophysiological and functional imaging techniques therefore offers both high temporal and high spatial resolutions. In addition to acquiring high-quality complementary data, multi-scale analyses help us quantify the coupling between scales and across data types (see section 3.3.2). These merits may be the key to unlocking transformative progress in behavioral and cognitive neuroscience [32].
Combining multiple modalities in a single experimental inquiry has allowed researchers to achieve better performance in brain–machine interfaces (BMIs) and to gain new mechanistic insights. For example, mathematical models combining spiking activity with LFPs improved neural prosthetic prediction accuracy [25, 33–36]. Synergistic signals from multiple neural scales also minimized electrical and computational power requirements and increased the longevity of a BMI devices [34]. Furthermore, more recent studies demonstrated that measuring multiscale activity enhanced decoding performance and provided information about connectivity or causality between different neural modalities in brain networks [35–38]. Finally, other studies incorporated information from multiple non-invasive methods to form a space- and time-resolved views of object recognition in human’s vision [39–42] and to predict fMRI blood oxygen level-dependent (BOLD) activity in the amygdala from scalp-level EEG signals [43]. As highlighted by these select examples, multi-scale analyses have already significantly improved neuroscientific understanding and neural engineering applications, and merit more in-depth studies.
In this paper, we describe typical methodologies used for characterizing neural activity at different temporal and spatial scales. For each method, we describe the state-of-the-art analysis that can be applied to this data type. We then present our case for integrating activity across multiple scales to benefit from the combined strengths of each approach and elucidate a more holistic understanding of neural processes as well as more robust strategies for decoding information from the brain. We examine various combinations of neural activity at different scales and analytical techniques that can be used to integrate or illuminate information across scales, as well the technologies that enable such studies. We support our case with examples of exciting works that have leveraged multi-scale analysis to push forward both scientific and clinical frontiers. Finally, we conclude with our perspective on the challenges and potential for future multi-scale studies, and a discussion of the power of these approaches.
2. Scales of neural interrogation and decoding
2.1. Spikes
Action potentials of individual neurons induce stereotypical extracellular ionic fluctuations, which can be measured as consistent waveforms (‘spikes’), detectable with microelectrodes or microelectrode arrays, for high temporal resolution decoding on a millisecond timescale (figure 1). Due to the sharp decay of such signals with distance, an electrode tip must be at most 20 µm to 100 µm from a neuron to reliably resolve its activity [44]. Therefore, a large number (10 s to 100 s or more) of electrodes need to be implanted in parallel or integrated into a single probe in order to extract enough information for meaningful spike-based decoding. This idea defines the next frontier of high-throughput recordings of spiking activity and spike-based neural decoding of behavioral variables or stimuli [45]. Decoders using a Kalman filter (KF) and related variations, such as the recalibrated feedback intention–trained Kalman filter (also known as ReFIT-KF), are most commonly used for spike-based decoding [24, 46]. Other novel closed-loop filters and techniques are also being developed, such as the closed-loop decoder adaptation (CLDA) [47, 48] and the closed-loop point process filter [49].
Figure 1.
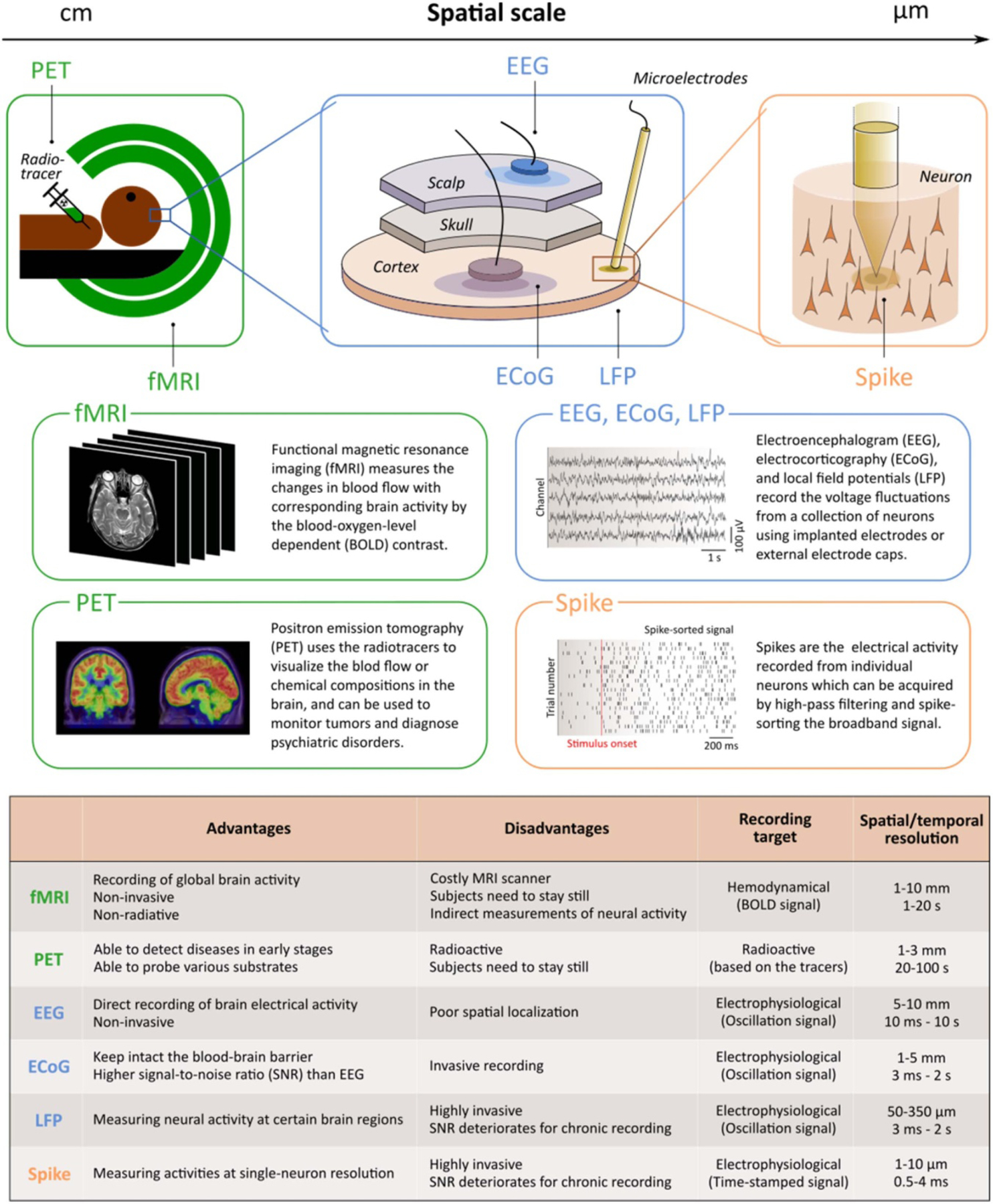
Description and comparison of different modalities. The diagram on the top depicts the general spatial scales of the signal acquisition techniques, ranging from cm to μm levels. The boxes in the middle demonstrate the raw data formats of each class of modality. The table on the bottom shows the strengths, weaknesses, and the primary targets. Spatial and temporal resolutions of each modality are also included.
Recent advances in high density, high channel count electrophysiology technology have the potential to greatly accelerate such processes [50]. However, large-scale spike-based decoding is still in its infancy, likely owing to the only recent availability of relevant recording technology, such as Neuropixels probes, and associated publicly available datasets [51, 52]. Recent work using this technology found that a deep neural network can decode natural image stimuli from hundreds of neurons recorded in the mice visual cortex [53, 54]. In another recent study [55], behavioral video data of a head fixed mouse were decoded from a Neuropixels dataset with 2688 neurons. The researchers effectively reduced the high dimensional input video with convolutional autoencoders and demonstrated that Bayesian models and dense neural networks both outperformed their linear counterparts for neural decoding. Large-scale recording with Neuropixels probes also facilitates the discovery of distributed cognitive and behavioral processes including choice and movements from signals across the mouse brain [56, 57]. Neuropixels recordings also revolutionized traditionally used spike sorting algorithms [58]. Thus, these modern techniques have already advanced the field.
In primate research, there have also been efforts to apply high channel count electrodes or arrays to decode behaviors from spiking activity [24, 26, 58, 59]. Berger and colleagues [60] reported the first use of 192 channel wireless recording for freely moving non-human primates. Their support vector machine (SVM) model was adequate for decoding multiple walk-and-reach targets. Clinically, there are also promising outcomes that successfully implemented high channel count BMIs [61–66]. Specifically, Hochberg and colleagues [61] demonstrated the first clinical BMI application where a 96-channel microelectrode array was used to detect neural ensemble activity in primary motor cortex. Most recently, using 192 channels and threshold crossing spike rates as input to a recurrent neural network decoder, Willett and colleagues [67] decoded imagined handwritten characters in humans with over 99% accuracy at a decoding speed comparable to the typing speed of age-matched controls, significantly improving the efficiency of brain–machine interface based communication.
Despite the promising outlook of decoding with high channel count spiking activities, the simultaneously increased power consumption, communication bandwidth, and computational delay for collecting, transmitting, and analyzing large volume spike data, have greatly prevented the field from advancing into real-time investigations [68] and the ultimate clinically translatable BMI applications [69] where low power, wireless, and low latency decoding systems are desired. In addition, the signal quality of spikes is prone to deterioration over time [70–73]. Spike sorting, the process of using spike waveform features to cluster detected spikes into putative individual neuron signals, has so far been identified as one of the most time- and power-consuming decoding steps. Innovations on this traditional feature extraction process, such as fully automated approaches [74] or the use of spike-related metrics such as spiking-band power (SBP) [69], could greatly advance real-time large-scale spiking-based decoding applications. This highlights the potential benefit of cross-scale data acquisition and analysis, whereby data that does not have these typically computationally expensive limitations (e.g. LFP) can be used to complement spike-based approaches. For example, Mehring et al showed that LFPs can be regarded as an additional source for decoding brain activity [25]; LFP activity, along with spikes, can be leveraged in a multi-scale model to decode a 3D reach task [35]. In addition, some material-based strategies are being developed to mitigate the problem of signal degradation over time [75]. For example, the surface coating of the electrodes [76] can be integrated with conductive polymers [77], shape-memory polymers [78], carbon nanotubes [79], flexible polymer [80], flexible mesh electronics [81], or nanoelectronic threads [82, 83] to improve the performance and reduce the interfacial mechanical mismatch. In summary, the next generation of spike-based neural decoding will likely be fueled by the synergy of high-channel count recording, advanced electrode materials, and multi-scale methods.
2.2. LFP
LFPs reflect electrical neural activity that is typically recorded extracellularly in neural tissue using invasive microelectrodes (figure 1). LFPs are thought to be generated by synchronized synaptic currents arising from hundreds of neurons and to capture key integrative synaptic processes that cannot be captured in spiking activity [21]. This signal is generally dominated by low frequency oscillations, with typical analysis focusing on activity below 200–300 Hz. Both time domain and frequency domain analyses are common with LFP. In the time domain, features of the neural signal may be used to determine location in the brain, for example changes in activity may indicate in which cortical layer [84] or hippocampal region the probe is located [85]. This is important for linking layer-specific or regional activity within a brain area to a particular behavior or process. It is also common to use a stimulus-triggered average of the LFP signal to determine the strength or properties of LFP caused by the stimulus [86, 87]. In the frequency domain, conventional analyses include computing power spectra or spectrograms, coherence, and phase synchrony. Power in different biologically-relevant frequency bands can be used to predict or decode a variety of behavioral variables [25, 35, 36, 88–90], from the goals of saccadic eye movements [91] to the value of stimuli [92] using classification techniques such as logistic regression, linear discriminant analysis (LDA), SVM, and other linear decoders. In addition, LFP has been shown to encode movement-related variables, such as the position and velocity of the subject’s hand [25, 27]. In addition to being inferred solely from LFPs, decoding of movement kinematics can be complemented by information from other modalities [25, 33–36, 93].
2.3. ECoG
ECoG measurements are extracellular field-potentials measured from electrodes placed on the surface of the brain (figure 1). ECoG electrodes can be placed directly on the pial surface (sub-dural) or on top of the dura but beneath the skull (epidural). Similarly, ECoG measurements are often divided into categories based on the size of the electrodes: µECoG refers to measurements made with electrodes on the micron scale (20–200 µm), while measurements with larger electrodes are macro-scale ECoG, typically referred to simply as ECoG. All ECoG measurements capture meso-scale field-potential signals reflecting aggregate dynamics of nearby circuit activity [21, 94] and therefore are typically analyzed in similar ways to other field potentials (e.g. LFP, EEG). The spatial resolution of ECoG signals, however, increases with decreasing electrode size and proximity to the nervous tissue. Similarly, the frequency ranges considered for ECoG analyses will vary depending on the electrode properties, with smaller electrodes able to capture higher frequency oscillations. At an extreme, sub-dural µECoG with 20 µm contacts can resolve MUA from superficial layers of the brain [95]. High-frequency ECoG activity (high-gamma, 80–200 Hz) has also been shown to be closely related to MUA [96–99].
As a meso-scale invasive measurement, ECoG has been most widely used for studying and decoding neural activity spanning multiple brain areas at the millisecond timescales relevant for behavior in clinical settings and in animals [21, 94, 100, 101]. As recording channel counts increase, ECoG recordings have proven useful for both high-density, high resolution spatial mapping of centimeter-sized brain areas [102–104] and to monitor highly distributed processing spanning nearly a full brain hemisphere [105]. Recent decoding and analysis techniques correspondingly aim to leverage the population-level dynamics and spatiotemporal characteristics of ECoG. For instance, ECoG can capture travelling waves of cortical activity that may influence ongoing neuronal processing [106–109]. One challenge in decoding and analyzing ECoG signals is their high dimensionality due to broad spectral feature spaces (multiple frequency ranges of interest) and large channel counts. Data-driven machine learning algorithms like recurrent neural networks [110] and unsupervised hierarchical clustering of neural dynamics [111] have proven valuable for decoding high-dimensional data. Another strategy is to leverage low-dimensional projections to capture spatiotemporal dynamics. Low-dimensional embedding has enabled development of state-space based decoding algorithms for ECoG [112, 113] and may be particularly useful for decoding ECoG population dynamics on a single-trial basis [114].
One reason ECoG is thought to hold promise for neural interface applications is its strong decoding performance combined with increased stability compared to other recording modalities (e.g. spiking activity) [115]. Several recent papers have demonstrated reliable real-time motor BMI control with ECoG [28, 29]. Understanding how to model potential longer-term dynamics and non-stationarities within ECoG signals is a recent area of work [116] and may be critical for enabling longitudinal studies and clinical neural interfaces.
2.4. EEG
EEG is a non-invasive technique for linking human neural activity to behavior which was first described almost 100 years ago [117]. This technique was first used to record large-scale patterns of ‘alpha’ oscillations, rhythmic ~10 Hz oscillations dominant over parietal and occipital electrodes, with subsequent studies identifying activity in adjacent frequency bands (e.g. beta, delta, etc [118]). However, discrepancies in the precise frequency boundaries between studies [119], along with considerable inter- and intra-subject variability in oscillation frequencies [120, 121] poses challenges to linking oscillations to behavior. Sidestepping such frequency differences by analyzing time-domain signals, early work focused on analyzing EEGs locked to particular stimuli or cognitive events by averaging data over many trials, so called event-related potentials [122]. Different ERP components have been identified for numerous cognitive operations spanning the breadth of study in cognitive neuroscience and have been useful in decoding cognitive operations in health and disease [123].
Subsequent research has emphasized time-frequency analysis of EEG signals to investigate spectral signatures of cognitive functions [124], with influential work showing that alpha laterality indexes spatial attention [125] and working memory capacity [126]. Building upon these findings, recent work has used inverted encoding models (IEMs) which flip the direction of inference. As the name implies, rather than asking how experimental manipulations modulate neural responses, IEMs attempt to predict neural responses using explicit models of neural activity and aim to determine how neural responses underlie stimulus representations [127]. This approach has been used to infer or ‘reconstruct’ the contents of memory or the focus of attention based on EEG patterns on single trials [128–131]. Because IEMs focus on inferring mental representations from neural activity, they are well suited for multiple measurement modalities with varying temporal or spatial scales. Indeed, since IEMs have been successfully used with fMRI [127, 132] and with EEG [133] we anticipate that IEMs will continue to grow with the increase in multi-modal imaging (see section 3.3.2).
2.5. fMRI
Traditionally, fMRI studies interrogate the blood oxygenation-level dependent (BOLD) contrast in a univariate (voxel-by-voxel) fashion to characterize task-induced neural activity in particular regions or throughout the brain. This imaging approach is non-invasive and allows whole-brain BOLD activity to be recorded simultaneously, providing the most comprehensive spatial coverage of all methods discussed so far (figure 1). This technique has enabled insights in deep brain function. For example, univariate fMRI studies showed that the striatum and the medial orbitofrontal cortex are consistently involved in processing subjective value during both decisions concerning value and receipt of valuable outcomes, irrespective of whether these outcomes are money or primary reinforcers [134], but with stronger involvement of the striatum in actual behavior [17]. Moreover, the striatum consistently processes reward prediction errors [135]. These findings may also have clinical relevance; as compared to healthy controls, patients with disorders such as depression [136] or psychosis [137] show reduced striatal activity during the receipt of rewards.
Univariate analyses depend on a uniform relation between neuronal activity and the investigated function, in both individual voxels and across participants. Multivariate methods can overcome this limitation of univariate analyses and decode heterogeneous relations between function and neuronal activity. They typically converge with univariate methods for homogeneous function-activity relations (for value functions, see [138]). Multivariate methods train classifiers on unsmoothed, voxel-wise, patterns of activity in different conditions and apply the trained classifier to independent test data. These classifiers exploit biased sampling at the level of fMRI voxels: the signal in a given voxel is thought to reflect the specific proportion of intermixed neuronal populations [139]. For example, one voxel and its underlying vasculature may sample more strongly from negative coding neurons whereas another voxel may sample more strongly from positive coding neurons and together the pattern of activity would differ between high and low value conditions.
Relatively recently developed multivariate methods provide insights not only on patterns of local activation but also on patterns of connectivity between regions and networks [140]. One variant of this approach uses condition-specific connectivity estimates from psychophysiological interaction models to train cross-validated SVMs. This approach revealed reward identity specific connectivity patterns of the central orbitofrontal cortex [141]. Specifically, the predictions of rewarding sweet and savory odors were differentially related to olfactory (i.e. piriform) cortex. Thus, the central OFC may extract sensory features of a reward from primary sensory cortices and endow them with identity-specific value. Moreover, integration of multivariate information appears to take place also in the visual domain [142], with anterior temporal lobe integrating shape information from lateral occipital cortex and color information from V4. Importantly, multivariate approaches to connectivity go beyond parallel analyses of activity patterns in different regions and may eventually capture not only interactions and information synchronicity but also transformations of information from region to region [140].
Combining fMRI with pharmacological interventions can help characterize the role of functional systems or the nature of represented information more precisely. For example, the dopamine D2 receptor antagonist amisulpride changes the whole-brain connectivity of medial and central but not of posterior-lateral orbitofrontal regions [143]. In particular, during rest it enhances multivariate connectivity of medial orbitofrontal cortex with more dorsal regions of frontal cortex and reduces connectivity of central orbitofrontal cortex with the midbrain and higher-order sensory cortices, suggesting that dopamine contributes to reconfiguring functional circuits. Moreover, dopamine D2-receptor blockade increases decoding of multivariate reward signals in medial orbitofrontal cortex, multivariate effector signals in motor and premotor cortex, and univariate reward signals in ventral striatum [144]. These effects could explain how antipsychotic drugs achieve their therapeutic benefits and suggest that network representations of specific types of information are under dopaminergic control.
Combining fMRI with transcranially applied electrical or magnetic brain stimulation can clarify whether the effects of stimulation on behavior arise through local effects at the stimulation site, through effects elsewhere, or through connectivity. For example, striatal connectivity and central orbitofrontal activity reflecting value are respectively reduced by downregulation of the temporoparietal junction [145] or the lateral prefrontal cortex [146] with transcranial magnetic stimulation (TMS). Moreover, determining stimulation sites based on resting state connectivity can allow stimulation to affect activity in regions that are not directly accessible to TMS, by virtue of their connections with the stimulated regions [147, 148].
2.6. PET
While fMRI has good spatial resolution, it lacks neurochemical specificity. PET overcomes this limitation and, at the expense of temporal resolution, can target a variety of neurotransmitter systems in healthy humans, both statically (e.g. receptor density) and dynamically (neurotransmitter release; for review, e.g. see [149]). Molecular PET can quantify the targets (receptors and transporters) of specific neurotransmitters by imaging radioligands in vivo. Other methods such as voltammetry and microdialysis are limited to intra-operative use in patients while PET can be used in healthy humans. Of particular functional relevance is the displacement of radioligands (which results in a change in binding potential) by endogenous transmitter release. This method has first been established for dopamine but more recently extended to serotonin, noradrenaline, acetylcholine and other neurotransmitters [150]. PET studies showed that neurochemical makeup varies between individuals of different ages [86, 151], and individual differences in this makeup are behaviorally and clinically relevant. For example, humans with higher capacity to synthesize dopamine in the dorsal striatum are more willing to exert cognitive effort than humans with lower synthesis capacity [152]. Moreover, patients with schizophrenia who respond to treatment show a negative correlation between prefrontal cortex grey matter volume and the striatally measured capacity to synthesize dopamine [153]. More generally, PET may contribute to tailoring therapeutic interventions to subtypes of patients and improving clinical outcomes.
Radioligands differ in their affinity to the natural receptors. For example, [11C]raclopride acts as relatively low-affinity antagonist on dopamine D2 receptors and has been used to show dopamine release in the receptor-rich striatum induced by engaging activities, such as playing a video game [154], by unexpected receipt of monetary reward but not unexpected monetary loss [155] and by drug challenges such as amphetamine (e.g. [156]) or alcohol [157]. D2 receptor antagonists with higher affinity than raclopride (e.g. [11C]/[18F]fallypride) and agonists (e.g. [11C]PHNO) can reveal dopamine release in regions with fewer dopamine receptors and have been combined with dynamic models of endogenous dopamine release. One such study showed that a reward learning task induces stronger striatal dopamine release than a non-rewarded control task and that individuals with better performance in the learning task also show stronger dopamine release as well as reward-related increase in engaging in everyday activities [158]. Thus, the role of dopamine in reward learning appears to extend to real life.
Traditionally, PET assesses net regional radioligand binding, which requires that endogenous changes of neurotransmitter release are relatively long-lasting. Recent research combining PET with voltammetry indicates that PET may also be sensitive to minute-by-minute fluctuations in radioligand binding, making it possible to detect phasic dopamine release with raclopride [159]. This research suggests that phasic and tonic dopamine levels may be more strongly related than sometimes assumed. Because this method measures change rather than absolute levels of dopamine, it is also applicable to regions outside the striatum. One application of this method compared the ingestion of milkshake against tasteless solution [160]. Immediately after ingestion, regions such as the medial and lateral orbitofrontal cortex, anterior insula, and dopaminergic midbrain showed an enhancing effect of milk-shake on dopamine release, in line with value coding. In contrast, twenty minutes later, dopamine release occurred in regions such as the pallidum and frontal pole. Resolving these separate peaks would not have been possible with conventional PET. Future efforts to validate this method for measuring the release of dopamine and other neurotransmitters will benefit from a multi-methodology and multi-scale approach.
2.7. Beyond single-scale analyses
While tremendous neuroscientific progress has been made using the above methods in isolation, each method has tradeoffs which must be considered. These tradeoffs limit our ability to understand behaviors which likely arise from activity across spatial and temporal scales, such as human vision and memory which are thought to occur via local and interareal neural activity patterns. For example, prominent theories of human episodic memory argue that it occurs through local changes in synaptic weight between neurons combined with distributed interactions between the hippocampus and neocortex [161]. It follows that with the limitations of any particular method, no single technique holds a privileged position in fully understanding memory systems, and that combinatorial methodological approaches should provide a more complete picture as compared to any method in isolation. Below, we discuss progress towards combining recording modalities to gain a richer and more complete view of how neural activity patterns across spatial and temporal scales give rise to behavior.
3. Multi-scale analyses
3.1. Advantages of multi-scale approaches
One of the primary advantages of multi-scale and multi-modal analyses is the formation of a more complete picture of the neural processes giving rise to behavior. Unimodal methods each have a unique set of strengths and weaknesses (figure 1). For example, noninvasive electrophysiological methods like EEG and MEG have high temporal resolution but poor spatial resolution, and the inverse is true for fMRI, which has relatively poor temporal resolution but relatively high spatial resolution. Even an invasive method like ECoG, which has high temporal resolution and good spatial specificity, suffers from limited spatial coverage. However, high resolution in both the spatial and temporal domains is essential for building a more complete understanding of the neural processes underlying cognition. Therefore, one way around these limitations is to combine data from multiple recording modalities and scales of measurement, either collected simultaneously (e.g. EEG-fMRI) or collected separately but combined analytically, to effectively exploit the strengths of each method.
For example, recent work has advanced a new method for analytically combining (or ‘fusing’) the high temporal resolution of magnetoencephalography (MEG)/EEG data with the high spatial resolution of fMRI data [39–42]. This approach does not require the data from different modalities to be collected simultaneously, allowing the combination of techniques like MEG and fMRI, which cannot be collected at the same time. Briefly, this approach uses a technique related to representational similarity analysis [162] to link (or ‘fuse’) multivariate patterns of brain activity from single modalities to each other [42]. This method can be restricted to a priori regions of interest or it can be conducted in an exploratory fashion throughout the brain [40]. For example, this ‘fusion’ approach has been applied to asynchronously collected MEG and fMRI data to identify temporally and spatially precise signatures of human object recognition [39]. The improved spatiotemporal resolution achieved by fusing MEG and fMRI data allowed a more complete picture of the neural processes underlying human visual object processing, which evolves rapidly over time and involves distinct contributions from a hierarchy of brain regions.
A second crucial benefit of combining data across scales and modalities is the opportunity to understand one data type with the addition of information from another. For instance, EEG has some key advantages over fMRI in cost and portability, making it an attractive option for clinical and translational settings. However, its limited spatial resolution and poor source localization, particularly for subcortical regions like the amygdala, can limit its usefulness. Recent work creatively circumvented this limitation by using simultaneous EEG-fMRI to build a multivariate model linking data-driven features of the EEG signal (termed a ‘fingerprint’) to fMRI BOLD activity in the amygdala [43, 163]. This EEG ‘finger-print’ enabled the prediction of amygdala fMRI activity in held-out data, demonstrating improved spatial specificity over conventional EEG-only approaches [163]. This technique was exploited for EEG-based neurofeedback training in soldiers facing stressful military combat training [43]. Soldiers were successfully trained using an EEG fingerprint to down-regulate their amygdala activity and reduce their emotional reactivity. This provides promising evidence for the utility of such a multi-modal approach in creating low cost, portable, and practical clinical applications (see section 5).
3.2. Necessary tools for multi-scale analysis
To successfully implement multi-scale analyses, essential experimental tools (i.e. neurotechnologies) and computational tools (i.e. analytical methodologies) are required based on the intended modalities. From the technology perspective, the main challenges for simultaneous acquisition of multi-scale recordings lie in making a single device capable of recording or manipulating neural activity at different scales, or in developing devices for different recording modalities that are compatible with each other. For example, in order to work simultaneously with MRI, the electrical and magnetic properties of recording electrode materials have to be considered. In this case, copper, gold, silver, and carbon are preferred considering their electromagnetic properties as well as biocompatibility [164]. Furthermore, researchers have designed cutting-edge materials [165, 166], such as Opto-E-Dura, a stretchable ECoG array that is compatible with additional wide-field calcium imaging, two-photon calcium imaging, and acutely inserted microelectrode arrays [165].
In terms of analytical methods, computational and statistical tools, like the fusion approach described above, are needed to relate different types of data that inherently have disparate spatiotemporal dynamics and underlying properties. Correlational or coherence analyses are usually performed to study the interaction between spikes and LFP. Forward and inverse models have been applied across different types of field potential signals to identify large-scale brain dynamics [94, 167–173]. Mean-while, machine learning approaches are frequently used to decode experimental tasks and to predict future movements or cognitive processes based on the recorded signals [174, 175]. The development of these tools is critical and will provide us with new insights into the interplay between different modalities. In the following sections, we present discussions of powerful new multi-scale approaches that combine neural data from two or more modalities/scales.
3.3. Multi-scale examples and case studies
To have a clear visualization of current progress and efforts on single or multi-modal analysis, we customized a MATLAB script to extract the numbers of research articles corresponding to each single modality or each pair of multiple modalities from PubMed. The counts of the articles satisfying each query are returned from PubMed and illustrated as a heat-map (figure 2). The keywords we used include fMRI, PET, EEG, ECoG, LFP and spikes. We also include the expanded terms such as ‘functional magnetic resonance imaging’ for fMRI. The detailed protocol is provided in the caption of figure 2.
Figure 2.
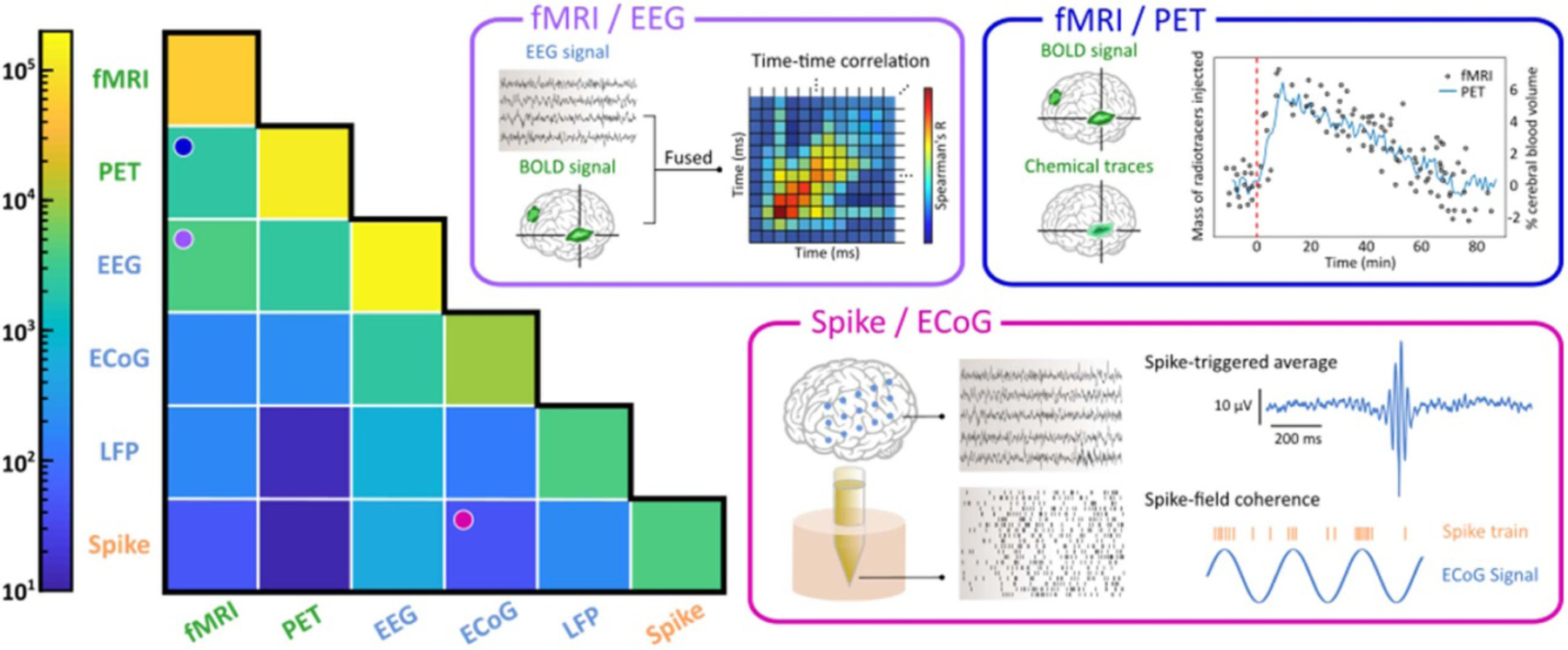
The heat map on the left shows the numbers of PubMed search results (see Supplementary Materials available online at stacks.iop.org/JNE/18/045013/mmedia) for each recording modality (shown along the diagonal) or combination of recording techniques (upper off diagonal) from 1938 (the first article on EEG retrieved from PubMed) to 2021. That is, the diagonal elements indicate the numbers of unimodal publications, while the off-diagonal elements show the numbers of publications that investigated two modalities in one study. The keywords we used for the six modalities include the acronyms and the expanded forms (e.g. (‘fMRI’ OR ‘functional magnetic resonance imaging’) for fMRI). However, we only used the keyword ‘unit activity’ for spikes, single- and multi-unit activity because ‘spikes’ itself would include irrelevant results such as viral spike proteins. For entries that involve two modalities, we used the AND operator in PubMed, such as (‘PET’ OR ‘positron emission tomography’) AND (‘EEG’ OR ‘electroencephalography’) for PET-EEG studies. Data is shown using a logarithmic color axis. Three representations of typical analyses in the combined modalities are shown in the boxes on the right, including fMRI-EEG, fMRI-PET, and spike-ECoG (e.g. see analyses in [42, 218, 277, 278]). While we have limited this heatmap to pairwise combinations, examples of work simultaneously spanning three or more modalities and/or scales are briefly discussed in sections 3.3.1 and 3.3.5.
3.3.1. Multi-scale intracortical measurements
Intracortical measurements include spikes, LFP, and ECoG activity, which span a range of temporal and spatial scales. The combinations of these types of signals can thus provide rich information about neural function. For example, spikes represent activity from individual neurons at a fast temporal scale while LFPs are believed to represented local synaptic activity generated from a population of hundreds of neurons [21] and contain oscillatory activity that may play a key role in coordinating activity across brain regions [176–178]. Analyzing the relationship between single neuron and population-level activity can reveal the integrative properties of neurons, as well as the organization of cortical circuits [179]. One notable difference with the combination of spikes and LFP compared to other signals is that they can be recorded using the same modality—implanted extracellular microelectrodes. For every electrode channel on which spikes are recorded, LFP signals can also be simultaneously acquired. This means that the joint analysis of simultaneously recorded spikes and LFPs provides an opportunity to assess the relationship between spikes and the strength of synaptic activity measured co-locally or at different locations.
A standard analysis used to characterize interactions between spikes and LFPs is the spike-triggered average of the LFP (figure 2). This time-domain metric may be used to quantify postsynaptic responses to spiking at a particular location (where LFP is recorded) [180] and determine latency between spikes and events in the LFP [94]. Alternatively, spikefield coherence is a spectral-domain metric that can be used to characterize synchronization of spike timing at particular phases of LFP activity [181] and is indicative that a local neuron contributes to the field potential signal [88, 182]. Similarly, a spike-triggered spectrum of LFP activity can be used to determine how power in different frequency bands relates to local spiking activity. Other metrics used to characterize interactions include spike-field Granger causality [183], Volterra models of LFP-spike timing relationships [179], and multiscale causality estimation [184].
Aside from analyses that characterize relationships between these two scales of activity, there are also efforts to leverage information from both scales in unison to learn something about behavior or cognition. Two distinct modeling approaches have been developed to interpret neural activity principally from spikes and LFPs, including statistical modeling and biophysical modeling. With regard to statistical modeling, earlier methods leveraged spiking and LFP activities from similar timescales in the same models [25, 33, 34]. For instance, Khorasani et al [185] proposed an adaptive filtering method using weighted common average reference filter, and decoded kinematic force information based on both LFP and MUA with a partial least square model. The decoding accuracy using the LFP and MUA outperformed models that relied solely on either LFP or MUA. The authors suggested that LFP and MUA may have information that is different or supplementary in terms of the covariates they investigated. As another example, Stavisky et al [34] recorded signals from the motor cortex when macaques performed a reaching task. They concluded that the performance of hybrid decoders from LFPs and spikes outperformed unimodal models, especially when the signal quality of spiking measurements was mediocre to poor. Sub-sequent works modeled combining activity from different spatial and temporal aspects [35, 36, 38]. Specifically, Hsieh et al developed a model that regarded spikes as a point process at fast millisecond timescale but treated LFP at slower timescale, which collaboratively improved the decoding of movement when using activity from motor cortex [35]. Their model can adaptively and separately update parameters at different rates for LFPs and spikes in closed-loop simulations. On the other hand, some studies modeled spikes and LFPs in a biophysical manner, where they aimed to identify the neural sources that contribute to the recording patterns in spikes or LFPs [21, 94, 180]. For example, the integrate-and-fire neuron model [186, 187] and its derivative, the leaky integrate- and-fire (LIF) model [188], are commonly used to describe spiking neurons and study brain functions. Moreover, Mazzoni et al successfully predicted LFPs from the LIF model and provided a simple formula that could quantitatively link neural models and LFP measurements [189].
In another recent work, spikes and LFP were used by an LDA classifier to improve accuracy of decoding value representations in a choice task with stimuli of differing values [92]. Other work has used an SVM with both spike and LFP data from lateral prefrontal cortex to decode information related to cue position, visually attended location, and saccade direction [190]. Using this approach, the authors were able to quantify how much additional information spikes carried about these variables by examining the decoding performance improvement from combined spike and LFP data, compared to using LFP signals alone. A similar approach has been used to demonstrate how anterior cingulate cortex spike and LFP activity can be used to decode intensity of pain responses to different noxious stimuli, indicating that single units play a role in the representation of pain and that LFP signal can predict nociceptive intensity [191].
Further examples come from the motor control literature, where researchers found that between spiking and LFP activity, spiking encodes direction more strongly than LFP, while high frequency (100–300 Hz) LFP best encodes speed and low frequency (10–40 Hz) LFP best encodes movement initiation [192]. These findings indicate that distinct information is represented by each signal type, and BMI performance may therefore be optimized by strategically using direction, speed, and go/no-go information from spikes and LFP. Furthermore, even in cases in which spikes and LFP encode similar information [25, 34, 193], combining these two modalities can still improve the decoding performance. For example, Abbaspourazad et al [36] used a multi-scale low-dimensional model to describe the predicted behavior and spike-LFP activity characterized by some principal modes. They found that combined spike-LFP activity can learn multi-scale predictive modes more effectively and more accurately predict naturalistic movements. In addition to representing distinct information in both scales, this study demonstrated that adding these two signals with similar contents also improved decoding performance by acting as a form of denoising. Additionally, the use of high-frequency LFP (on the order of~102–103 Hz) as a proxy for threshold crossing-detected action potentials is gaining attention. This LFP band was hypothesized [194] to contain information similar to low-pass-filtered spikes and had more robust chronic performance than threshold crossing spike rates. Nason et al [69] defined SBP as the power in the 300–1000 Hz band. They found a robust correlation between SBP and single-unit firing rate, across changes in firing rate or recording noise level. SBP produced more accurate predictions of macaque finger movements than the threshold crossing rate feature when both inputs entered SVM decoders. These works demonstrate the power of leveraging both spikes and field potentials to yield neuroscientific insights and develop robust BMI paradigms that can translate to the clinical domain.
Another form of multi-scale electrophysiology is integrating intracortical spike and/or LFP recordings with ECoG (figure 2). Integrating these modalities synchronously requires addressing physical compatibility of the two recording modalities, which has seen considerable progress over the past decade (e.g. [195]). Existing strategies for integration include using ECoG arrays fabricated with holes through which intracortical probes can be inserted [195–197], performing sparse ECoG measurements without a monolithic array that provide space to insert probes [198], placing multi-unit arrays underneath ECoG arrays [199], and custom devices with mixed electrode formats [200]. Spatial registration of the two measurements may be critical for analysis and interpretation of signals and is therefore an important consideration in methodological design.
Multi-scale analysis methods for ECoG and intra-cortical recordings are similar to those of spike-LFP. This includes both examining temporal relationships between the signals (trigger-based analyses, coherence) and using mixed neural features as inputs for decoders. Comparison of signals suggests that ECoG may capture aspects of neural processing that are distinct from spikes and LFPs [196, 200, 201]. For example, classification of spoken vowels from combined intracortical and ECoG recordings was highest when decoders used a mixture of spikes, LFP, and ECoG features, with spiking combined with ECoG providing the largest benefits of any two pairs [200]. Interestingly, simultaneous macro ECoG and multi-electrode intracortical array recordings suggest that for a matched number of electrodes, ECoG recordings in visual cortex provided higher decoding accuracy of visual stimuli than LFP or spiking activity [201]. The difference in brain area coverage may partly contribute to these differences and highlights the potential utility of multi-scale measurements. Fully leveraging multi-scale measurements for decoding algorithms will require new mathematical approaches that can handle the statistical and temporal differences between spiking activity and field potentials, which is an emerging area of work [35].
3.3.2. EEG and fMRI
Beginning in the mid-1990s (e.g. [202]), simultaneous collection of EEG and fMRI data has enabled the direct linking of electrical brain activity and hemodynamic responses. For example, during resting periods, the BOLD signal in the occipital cortex has been found to correlate negatively with alpha activity [203], that is, cortical inactivity measured with fMRI is associated with large-scale synchronous activity measured with EEG. This demonstrates that simultaneous EEG-fMRI does not merely localize the neural sources of EEG signals, but can identify distinct neural signatures across the two modalities that correspond to the same underlying mental state. Furthermore, EEG and fMRI have complementary strengths in temporal resolution (milliseconds vs seconds) and spatial resolution (centimeters vs millimeters). Exploiting their combined spatiotemporal resolution overcomes the limitations of each and allows for the characterization of the temporal dynamics of cognition in precise neural circuits [204].
The key advantage of simultaneous collection of EEG and fMRI data, as opposed to post-hoc combination of data collected separately (e.g. [42, 205]), is to guarantee identical sensory stimulation, perception, and behavior which makes it possible to investigate how intrinsic brain states interact with extrinsic processing (figure 2). Acquiring these data involves the use of specialized EEG hardware that is safe and compatible with the MR environment, although the data are highly sensitive to artifacts and methods like independent component analysis (ICA) must be used to identify and remove artifactual signals [206]. A bulk of the simultaneous EEG-fMRI studies conducted thus far have focused on healthy subjects: based on the scientific articles published in PubMed since 2014 using the key word ‘simultaneous EEG-fMRI’, nearly 60% of all studies have focused on healthy control subjects rather than on patients with neurological impairments [207].
The simultaneous acquisition of EEG and fMRI data has been used to study the brain at rest and during the execution of specific tasks, yielding insights that would not have been possible within a single modality [208]. Linking electrical activity to specific neural circuits provides more mechanistic explanations of cognition. For example, low-frequency theta-alpha oscillations (4–13 Hz) during a recognition memory test have been shown to emanate from a canonical memory circuit consisting of the hippocampus, the striatum, and the prefrontal cortex [209]. High-frequency gamma-band oscillations (30–100 Hz), on the other hand, have been linked to occipito-parietal network activation during tests of visual perception [210]. Furthermore, studies of decision making have shown that modulations of power in beta (12.5–30 Hz) and theta bands, associated with positive and negative feedback, are linked to activation in the subcortical reward network and in fronto-parietal areas, respectively [211].
Finally, EEG and fMRI have been combined in neurofeedback studies which allow for targeted manipulation of brain activity. In healthy adults, modulation of the thalamic nuclei during the retrieval of happy autobiographical memories produced changes in posterior alpha power that correlated with changes in the thalamic BOLD signal [212]. In conclusion, the simultaneous combination of EEG and fMRI offers the opportunity to characterize the relationship between temporally precise electrical brain activity and spatially specific regional hemodynamic activity to better understand the neural dynamics of behavior and cognition.
3.3.3. Multiple imaging modalities: fMRI and PET
The combination of fMRI with PET (e.g. [149, 213]; figure 2), which has been developed over the past 15 years [214] enhances and informs insights gained with single-modality methods. While both imaging modalities provide functional measures of neural activity, they offer distinct advantages. fMRI measures BOLD signals which are thought to reflect regional time-varying changes in neural activity and is able to achieve a higher temporal resolution than PET [215], whereas PET is able to visualize metabolic processes and provide information on neurotransmitter-specific activity [216]. Combining these two modalities enables the study of how BOLD activation relates to neurotransmitter release, which is particularly powerful when linking these measures to a particular behavior or investigating an intervention [14, 149, 217–221].
One line of research in this domain showed that striatal BOLD responses correlate with relative dopamine D2/D3 receptor occupancy in the striatum of non-human primates [218]. In humans, BOLD activity elicited by reward anticipation in the dopamine producing substantia nigra and ventral tegmental area correlates with reward-related striatal dopamine release as measured with PET [14]. Relatedly, striatal BOLD activity induced by reward anticipation correlates with dopamine transporter [219] and dopamine D2 receptor [220, 221] availability in the dopamine producing regions and with dopamine release in the striatum [222]. Thus, combining PET with fMRI can elucidate neurochemical systems which synergistically implement cognitive and motivational functions. Using combined fMRI-PET machines together with time-resolved PET [159] will allow future investigations of behavior and cognition to control for sensory stimulation and more tightly integrate neurochemical information with BOLD responses.
3.3.4. Functional imaging (fMRI or PET) with intracortical measurements
Combining a non-invasive imaging modality with invasive intracortical methods can provide complementary information about network-level and cellular-level neural activity. While functional imaging methods such as fMRI and PET enjoy large spatial coverage of the brain, they provide only indirect measurements of neural activity. The combination of one of these modalities with extracellular recording techniques using penetrating electrodes, can both provide multi-scale spatial information regarding neural activity as well as help inform the relationship of the imaging metrics (i.e. BOLD or PET signal) to cellular function (similar to what was described in section 3.3.2 for EEG-fMRI). For example, it has been demonstrated in parietal cortex of macaques that small clusters of task-selective neurons can facilitate large fMRI activation [223]. Additionally, in Parkinsonian patients it was shown that spiking in the globus pallidus internus correlated with thalamic glucose metabolism, as measured through PET, and that variations in spiking rates are associated with individual differences in glucose metabolic activity [224].
Although there is power in combining these multi-scale techniques, scientific and technical challenges have limited their combined popularity in the research and clinical communities. The first simultaneous recordings of LFP and spiking activity with fMRI reported that LFP signals had a stronger contribution to and higher correlation with BOLD responses compared to spiking activity [225]. Evidence supports that this is likely due to the spatial specificity of spiking activity, in contrast to LFP signals which integrate activity over a large spatial region [225]. This weak relationship between BOLD fMRI and spikes may explain the low number of fMRI-spike studies (n = 48, see figure 2).
Other challenges include (a) the need for MR-compatible electrodes which do not cause significant artifacts in MR images and (b) the appropriateness for both modalities in a given subject. To the first point, there is active work to develop extracellular recording methods to acquire LFP, spikes, or both, that minimize susceptibility artifacts during fMRI. These methods generally leverage new signal acquisition and processing paradigms [225, 226], and/or novel electrode designs and materials [227–229]. To the second point, invasive intracortical methods are not typically used in human subjects unless clinically motivated. For this reason, non-invasive methods (EEG, fMRI, PET) are typically leveraged in studies with healthy human subjects. On the other hand, intracortical methods are more easily implemented in animal models. However, fMRI and PET are both sensitive to movement, which introduces additional challenges with these functional imaging methods in awake animals. For this reason, many fMRI-spike and PET-spike studies are performed on anesthetized animals (e.g. [225, 227–230]), although some studies utilize specialized MR-compatible restraint systems to limit movement during imaging (e.g. [223, 231]).
3.3.5. Combining three or more modalities/scales
While more limited, there are notable examples that have simultaneously acquired data across three or more scales and/or recording modalities. This is most feasible for multi-scale electrophysiological approaches. For example, as discussed in section 3.3.1, decoding of speech production from ventral sensorimotor cortex benefits from simultaneous information from spike, LFP, and ECoG recordings [200]. In addition, Moosmann et al investigated the correlation between fNIRS, fMRI, and EEG [232], and Golkowski et al simultaneously measured EEG, PET, and fMRI in patients suffering from disorders of consciousness [233]. These combinations have also been explored by other research groups, highlighting the potential for incorporating three or more modalities in one experimental setting [234, 235]. Combining across three or more scales in this way can be particularly beneficial, both for characterizing the types of information carried at different scales [201], and for capitalizing on complementary sources of information to build more robust decoders for applications like BMI [236]. Finally, simultaneous recordings across more than two scales/modalities have proven indispensable for understanding the underlying neural source(s) of the signals measured with noninvasive modalities like EEG [237] and fMRI [225].
4. Challenges for future multi-scale studies
While we have highlighted many exciting and impressive works (figure 2) that have combined multi-scale neural activity for a variety of neuroscientific, clinical, and engineering goals, there are still challenges ahead to promote the advancement of multi-scale efforts. One major challenge is the need for multi-scale modeling and analytical methods to provide fundamental infrastructure for understanding neurological processes, disease, cognition, and behavior. New, versatile frameworks to elucidate multi-scale activity at a mechanistic level will enable this challenge to be addressed. Another major challenge is the need for harmonious technologies to enable cross-scale interrogation of neural activity. Although significant insights can be made with data that is not acquired synchronously, neurotechnologies that allow activity recorded using different modalities simultaneously will serve to broaden our toolkit for making consequential advancements.
The two core challenges presented above are affected by several related factors. Firstly, there is a lack of software options that can operate across multiple modalities. While leading electro-physiology companies, such as Plexon Inc. and Black-rock Microsystems, already provide solutions for recording activity such as spikes, LFP, ECoG, and EEG simultaneously, there are limited commercial options for simultaneous acquisition of other combinations of data types, such as electrophysiological with functional imaging data. Simultaneous acquisition of EEG and fNIRS can be completed with the same head montage, but the data itself must be acquired with different systems. This need for cohesive software options goes hand-in-hand with hardware consolidation to support recording neural activity synchronously at different scales from different modalities. Secondly, funding mechanisms that encourage collaborations between researchers with specializations in different modalities would fuel progress across all fronts: analysis, neurotechnology advancement, and software and hardware development. These efforts cannot thrive without the investment of resources from funding agencies and labs. Thirdly, scientific meetings that promote cross-dialogue and discussion about insights and challenges across modalities can play a role in bringing together teams of researchers with a range of expertise. Existing conferences in the neural engineering and neuroscience fields can promote this objective with special sessions, forums, or satellite meetings focused on multi-scale and multi-modal methods. Fourthly, to facilitate this paradigm of integration of different types of neural activity, there is also a need for a common lexicon to communicate across modalities. Altogether, addressing these challenges and considerations will enable an exciting future in multi-scale studies.
5. Outlook and translational applications
Understanding multi-scale interactions can facilitate not only our knowledge of neural function, but also the development of rehabilitative therapies that may leverage information across scales to create more effective and potentially restorative treatments. Brain imaging in the context of rehabilitative interventions is often utilized through neurofeedback or BMI platforms. Multimodal neurofeedback approaches take advantage of the different measurement scales provided by each imaging modality. For example, the high spatial resolution of fMRI can be combined with the high temporal resolution of EEG. This can lead to stronger therapeutic effects by providing patients with more detailed information to develop better neural strategies compared to a single modality alone [238]. Young et al [239] demonstrated the first implementation of simultaneous neurofeedback using fMRI and EEG data to train healthy participants to self-regulate neural activity in an emotion regulation task. The addition of EEG recordings allowed examination of the electrophysiological correlates of fMRI neurofeedback training, including frontal EEG asymmetry and EEG coherence, that could be translated to patients with major depressive disorder [240] and post-traumatic stress disorder [241].
BMIs exploit an individual’s ability to modulate a target brain region and translate this to control of an external device. These platforms have been widely investigated as a method to assist impaired individuals’ ability to interact with the world around them. However, certain challenges exist that limit the usability of BMIs, such as the requirement to optimize control features and identify successful mental strategies to properly control a device [242]. Additionally, a phenomenon known as ‘BMI illiteracy’ exists, in which around 15%–30% of individuals are unable to learn to control a BMI [243]. Multimodal approaches have been suggested to address these challenges by providing the BMI with more detailed information from the temporal dynamics of electrical activity and the hemodynamic fluctuations in the cortex [242]. This can improve a BMI’s ability to decode the user’s intention which leads to better usability and control [244, 245].
Noninvasive brain stimulation, such as TMS and transcranial direct current stimulation (tDCS), has also been investigated as a treatment for major depressive disorder, schizophrenia, epilepsy, phobia, and stroke rehabilitation [246]. However, many behavioral manifestations of neurological and psychiatric disease are the result of alterations in distributed brain networks, and neuroimaging technology can be utilized to determine the optimal electrode placements for multichannel tDCS [247]. Computational models have also been built for tDCS [248] and TMS [249] to better estimate current flow patterns in the brain, to design new electrode montages, or to improve dosimetry. The simultaneous acquisition of neuronal and hemodynamic responses along with stimulation can provide an objective form of feedback to guide the stimulation procedure, quantify the stimulation effects, and investigate the underlying neural dynamics [246, 250–252]. Furthermore, the combination of fMRI and EEG with concurrent stimulation can further elucidate the effects of stimulation on connected brain regions [145, 253]. However, a major challenge with simultaneous stimulation and measurement is the introduction of strong artifacts in the obtained signal. In the case of concurrent TMS and EEG, the electromagnetic field generated by the stimulation pulse can induce a large measurement artifact that is much stronger than the typical electro-physiological brain activity measured by EEG [254]. Additional care must be given to compensate for these artifacts by using recently developed EEG amplifiers to characterize the stimulus pulse and filter it from the measured signal [255] or to measure activity only after the stimulation artifact has subsided [256]. Despite these challenges, many studies have already leveraged the power of combining TMS/tDCS with electrophysiological recordings or functional imaging techniques for various applications. For example, the combination of offline repetitive TMS (rTMS) and fMRI shows causal relationship between neural computations and strategic social behavior [257]; simultaneous MEG/EEG and TMS can identify the causal influence of oscillatory entrainment on working memory performance [258]; and offline rTMS can provide lasting disruption of the hemodynamic activity in the prefrontal cortex measured with fMRI [259]. In addition to overcoming technological barriers to bridge different techniques, computational models also play a critical role in integrating information across modalities. For example, a model that characterizes both fMRI activation and electrical field mapping using tDCS can address inter-subject and within-subject variabilities [260]; a biophysical model has also been demonstrated to assist with personalizing TMS protocols in conjunction with resting-state fMRI [261]. These promising results highlight the synergy of noninvasive modulatory approaches such as TMS/tDCS and noninvasive neural recording techniques such as fMRI/EEG.
Capturing multi-scale interactions can also help elucidate the neural dynamics underlying healthy neurophysiological processes. A combination of electrophysiological and hemodynamic imaging can be beneficial to understand how neuronal changes relate to neurovascular coupling [262]. For example, a multimodal approach of EEG and fNIRS revealed an inverse relationship between oxyhemoglobin concentration and alpha and beta power during a motor task [263]. This can be beneficial to clarifying the neural dynamics during motor imagery, a popular target for BMIs and neurofeedback for its potential applications for stroke rehabilitation [264].
There is considerable evidence across neuroscientific disciplines that the brain integrates and represents information across spatial and temporal scales. In perception, information is integrated within 300–600 µm cortical columns and more than 30 distinct brain regions have been identified which together support our understanding of the visual world [265, 266]. Similarly, cascading molecular changes can modulate synaptic strength to encode memories [267]. Regarding memory, it has been recognized since the stimulation studies of Wilder Penfield that memories are widely distributed throughout the brain [268]. Contemporary theories of episodic memory argue for the integration and segregation of information distributed between the hippocampus and neocortex as central to memory organization [161, 269]. Neural oscillations exert an influence on both local and distributed neural populations and may subserve integrative functions (reviewed in [21, 270, 271]). It follows that interrogation of oscillations should be informative for understanding behaviors which require integration over scales. Indeed, mounting evidence has shown that oscillations provide similar or better readout of behavior than local neuronal spiking [272–276]. These findings indicate that approaches which interrogate population-level activity measured using multiple methods will provide more insight into behavior by leveraging combinatory power when analyzing data [51] across different spatial or temporal scales of analysis.
Supplementary Material
Footnotes
Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Supplementary material for this article is available online http://doi.org/10.1088/1741-2552/ac160f
Data availability statement
No new data were created or analyzed in this study.
References
- [1].Hong G and Lieber CM 2019. Novel electrode technologies for neural recordings Nat. Rev. Neurosci 20 330–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rubin A, Sheintuch L, Brande-Eilat N, Pinchasof O, Rechavi Y, Geva N and Ziv Y 2019. Revealing neural correlates of behavior without behavioral measurements Nat. Commun 10 4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chang C, Leopold DA, Schölvinck ML, Mandelkow H, Picchioni D, Liu X, Ye FQ, Turchi JN and Duyn JH 2016. Tracking brain arousal fluctuations with fMRI Proc. Natl Acad. Sci 113 4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dos Santos Lima GZ, Lobao-Soares B, Corso G, Belchior H, Lopes SR, De Lima Prado T, Nascimento G, França AC, De Fontenele-Araújo J and Ivanov PC 2019. Hippocampal and cortical communication around micro-arousals in slow-wave sleep Sci. Rep 9 5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gründemann J, Bitterman Y, Lu T, Krabbe S, Grewe BF, Schnitzer MJ and Lüthi A 2019. Amygdala ensembles encode behavioral states Science 364 eaav8736. [DOI] [PubMed] [Google Scholar]
- [6].Günseli E, Fahrenfort JJ, Van Moorselaar D, Daoultzis KC, Meeter M and Olivers CNL 2019. EEG dynamics reveal a dissociation between storage and selective attention within working memory Sci. Rep 9 13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hampton AN and O’Doherty JP 2007. Decoding the neural substrates of reward-related decision making with functional MRI Proc. Natl Acad. Sci 104 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Holmes CD, Papadimitriou C and Snyder LH 2018. Dissociation of LFP power and tuning in the frontal cortex during memory J. Neurosci 38 8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koprinkova-Hristova PD, Bocheva N, Nedelcheva S and Stefanova M 2019. Spike timing neural model of motion perception and decision making Front. Comput. Neurosci 13 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nácher V, Ledberg A, Deco G and Romo R 2013. Coherent delta-band oscillations between cortical areas correlate with decision making Proc. Natl Acad. Sci 110 15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Onken A, Xie J, Panzeri S and Padoa-Schioppa C 2019. Categorical encoding of decision variables in orbitofrontal cortex PLoS Comput. Biol 15 e1006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pesaran B 2010. Neural correlations, decisions, and actions Cogn. Neurosci 20 166–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Santacruz SR, Rich EL, Wallis JD and Carmena JM 2017. Caudate microstimulation increases value of specific choices Curr. Biol 27 3375–3383.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schott BH. et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 2008;28:14311. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M and Harris KD 2019. Spontaneous behaviors drive multidimensional, brainwide activity Science 364 eaav7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tobler PN. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- [17].Weber SC, Kahnt T, Quednow BB and Tobler PN 2018. Frontostriatal pathways gate processing of behaviorally relevant reward dimensions PLoS Biol 16 e2005722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao F, Zeng Y and Xu B 2018. A brain-inspired decision-making spiking neural network and its application in unmanned aerial vehicle Front. Neurorobot 12 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buschman TJ and Kastner S 2015. From behavior to neural dynamics: an integrated theory of attention Neuron 88 127–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harbecke J 2020. The methodological role of mechanistic-computational models in cognitive science Synthese 1–23
- [21].Buzsáki G, Anastassiou CA and Koch C 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes Nat. Rev. Neurosci 13 407–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scarapicchia V, Brown C, Mayo C and Gawryluk JR 2017. Functional magnetic resonance imaging and functional near-infrared spectroscopy: insights from combined recording studies Front. Hum. Neurosci 11 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ulmer S and Jansen O 2013. fMRI Basics and Clinical Applications 2nd (Berlin: Springer; ) [Google Scholar]
- [24].Gilja V et al. 2012. A high-performance neural prosthesis enabled by control algorithm design Nat. Neurosci 15 1752–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mehring C, Rickert J, Vaadia E, De Oliveira S C, Aertsen A and Rotter S 2003. Inference of hand movements from local field potentials in monkey motor cortex Nat. Neurosci 6 1253–4 [DOI] [PubMed] [Google Scholar]
- [26].Schaffelhofer S, Agudelo-Toro A and Scherberger H 2015. Decoding a wide range of hand configurations from macaque motor, premotor, and parietal cortices J. Neurosci 35 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].So K, Dangi S, Orsborn AL, Gastpar MC and Carmena JM 2014. Subject-specific modulation of local field potential spectral power during brain–machine interface control in primates J. Neural Eng 11 026002. [DOI] [PubMed] [Google Scholar]
- [28].Benabid AL et al. 2019. An exoskeleton controlled by an epidural wireless brain–machine interface in a tetraplegic patient: a proof-of-concept demonstration Lancet Neurol 18 1112–22 [DOI] [PubMed] [Google Scholar]
- [29].Silversmith DB, Abiri R, Hardy NF, Natraj N, Tu-Chan A, Chang EF and Ganguly K 2021. Plug-and-play control of a brain–computer interface through neural map stabilization Nat. Biotechnol 39 326–35 [DOI] [PubMed] [Google Scholar]
- [30].Sitaram R et al. 2017. Closed-loop brain training: the science of neurofeedback Nat. Rev. Neurosci 18 86–100 [DOI] [PubMed] [Google Scholar]
- [31].Liao CH, Worsley KJ, Poline J-B, Aston JAD, Duncan GH and Evans AC 2002. Estimating the delay of the fMRI response Neuroimage 16 593–606 [DOI] [PubMed] [Google Scholar]
- [32].Kowalczyk MA, Omidvarnia A, Abbott DF, Tailby C, Vaughan DN and Jackson GD 2020. Clinical benefit of presurgical EEG-fMRI in difficult-to-localize focal epilepsy: a single-institution retrospective review Epilepsia 61 49–60 [DOI] [PubMed] [Google Scholar]
- [33].Bansal AK, Truccolo W, Vargas-Irwin CE and Donoghue JP 2011. Decoding 3D reach and grasp from hybrid signals in motor and premotor cortices: spikes, multiunit activity, and local field potentials J. Neurophysiol 107 1337–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stavisky SD, Kao JC, Nuyujukian P, Ryu SI and Shenoy KV 2015. A high performing brain-machine interface driven by low-frequency local field potentials alone and together with spikes J. Neural Eng 12 036009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hsieh H-L, Wong YT, Pesaran B and Shanechi MM 2018. Multiscale modeling and decoding algorithms for spike-field activity J. Neural Eng 16 016018. [DOI] [PubMed] [Google Scholar]
- [36].Abbaspourazad H, Choudhury M, Wong YT, Pesaran B and Shanechi MM 2021. Multiscale low-dimensional motor cortical state dynamics predict naturalistic reach-and-grasp behavior Nat. Commun 12 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bighamian R, Wong YT, Pesaran B and Shanechi MM 2019. Sparse model-based estimation of functional dependence in high-dimensional field and spike multiscale networks J. Neural Eng 16 056022. [DOI] [PubMed] [Google Scholar]
- [38].Abbaspourazad H, Hsieh H-L and Shanechi MM 2019. A multiscale dynamical modeling and identification framework for spike-field activity IEEE Trans. Neural Syst.Rehabil. Eng 27 1128–38 [DOI] [PubMed] [Google Scholar]
- [39].Cichy RM, Pantazis D and Oliva A 2014. Resolving human object recognition in space and time Nat. Neurosci 17 455–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cichy RM, Pantazis D and Oliva A 2016. Similarity-based fusion of MEG and fMRI reveals spatio-temporal dynamics in human cortex during visual object recognition Cereb. Cortex 26 3563–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mohsenzadeh Y, Mullin C, Lahner B, Cichy RM and Oliva A 2019. Reliability and generalizability of similarity-based fusion of MEG and fMRI data in human ventral and dorsal visual streams Vision (Basel) 3 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cichy RM and Oliva A 2020. A M/EEG-fMRI fusion primer: resolving human brain responses in space and time Neuron 107 772–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Keynan JN et al. 2019. Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience Nat. Hum. Behav 3 63–73 [DOI] [PubMed] [Google Scholar]
- [44].Kleinfeld D, Luan L, Mitra PP, Robinson JT, Sarpeshkar R, Shepard K, Xie C and Harris TD 2019. Can one concurrently record electrical spikes from every neuron in a mammalian brain? Neuron 103 1005–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nurmikko AV 2018. Approaches to large scale neural recording by chronic implants for mobile BCIs 2018 6th Int. Conf. on Brain-Computer Interface (BCI) pp 1–2
- [46].Ajiboye AB et al. 2017. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration Lancet 389 1821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Orsborn AL, Moorman HG, Overduin SA, Shanechi MM, Dimitrov DF and Carmena JM 2014. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control Neuron 82 1380–93 [DOI] [PubMed] [Google Scholar]
- [48].Orsborn AL, Dangi S, Moorman HG and Carmena JM 2012. Closed-loop decoder adaptation on intermediate time-scales facilitates rapid BMI performance improvements independent of decoder initialization conditions IEEE Trans. Neural Syst. Rehabil. Eng 20 468–77 [DOI] [PubMed] [Google Scholar]
- [49].Shanechi MM, Orsborn AL, Moorman HG, Gowda S, Dangi S and Carmena JM 2017. Rapid control and feedback rates enhance neuroprosthetic control Nat. Commun 8 13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Steinmetz NA, Koch C, Harris KD and Carandini M 2018. Challenges and opportunities for large-scale electrophysiology with Neuropixels probes Curr. Opin. Neurobiol 50 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Allen Institute for Brain Science. Allen Brain Atlas SDK: Visual Coding-Neuropixels 2019. (available at: https://allensdk.readthedocs.io/en/latest/visual_coding_neuropixels.html)
- [52].Steinmetz N, Pachitariu M, Stringer C, Carandini M and Harris K 2019. (available at: https://janelia.figshare.com/articles/dataset/Eight-probe_Neuropixels_recordings_during_spontaneous_behaviors/7739750)
- [53].Iqbal A, Dong P, Kim C and Jang H 2019. Decoding neural responses in mouse visual cortex through a deep neural network 2019 Int. Joint Conf. on Neural Networks (IJCNN) pp 1–7
- [54].Jun JJ et al. 2017. Fully integrated silicon probes for high-density recording of neural activity Nature 551 232–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Batty E et al. 2019. BehaveNet: nonlinear embedding and Bayesian neural decoding of behavioral videos Dec. 8–14, 2019 Vancouver, Canada Advances in Neural Information Processing Systems 32 pp 15706–17 [Google Scholar]
- [56].Steinmetz NA, Zatka-Haas P, Carandini M and Harris KD 2019. Distributed coding of choice, action and engagement across the mouse brain Nature 576 266–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Musall S, Kaufman MT, Juavinett AL, Gluf S and Churchland AK 2019. Single-trial neural dynamics are dominated by richly varied movements Nat. Neurosci 22 1677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Trautmann EM et al. 2019. Accurate estimation of neural population dynamics without spike sorting Neuron 103 292–308.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schwarz DA et al. 2014. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys Nat. Methods 11 670–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Berger M, Agha NS and Gail A 2020. Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex eLife 9 e51322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD and Donoghue JP 2006. Neuronal ensemble control of prosthetic devices by a human with tetraplegia Nature 442 164–71 [DOI] [PubMed] [Google Scholar]
- [62].Hochberg LR et al. 2012. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm Nature 485 372–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gilja V et al. 2015. Clinical translation of a high-performance neural prosthesis Nat. Med 21 1142–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nuyujukian P. et al. Cortical control of a tablet computer by people with paralysis. PLoS One. 2018;13:e0204566. doi: 10.1371/journal.pone.0204566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jarosiewicz B. et al. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci. Transl. Med. 2015;7:313ra179. doi: 10.1126/scitranslmed.aac7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pandarinath C, Nuyujukian P, Blabe CH, Sorice BL, Saab J, Willett FR, Hochberg LR, Shenoy KV and Henderson JM 2017. High performance communication by people with paralysis using an intracortical brain-computer interface eLife 6 e18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Willett FR, Avansino DT, Hochberg LR, Henderson JM and Shenoy KV 2021. High-performance brain-to-text communication via handwriting Nature 593 249–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hu S, Ciliberti D, Grosmark AD, Michon F, Ji D, Penagos H, Buzsáki G, Wilson MA, Kloosterman F and Chen Z 2018. Real-time readout of large-scale unsorted neural ensemble place codes Cell Rep 25 2635–2642.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nason SR et al. 2020. A low-power band of neuronal spiking activity dominated by local single units improves the performance of brain–machine interfaces Nat. Biomed. Eng 4 973–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Engel AK, Moll CKE, Fried I and Ojemann GA 2005. Invasive recordings from the human brain: clinical insights and beyond Nat. Rev. Neurosci 6 35–47 [DOI] [PubMed] [Google Scholar]
- [71].Nuyujukian P, Kao JC, Fan JM, Stavisky SD, Ryu SI and Shenoy KV 2014. Performance sustaining intracortical neural prostheses J. Neural Eng 11 066003. [DOI] [PubMed] [Google Scholar]
- [72].Kashkoush AI, Gaunt RA, Fisher LE, Bruns TM and Weber DJ 2019. Recording single- and multi-unit neuronal action potentials from the surface of the dorsal root ganglion Sci. Rep 9 2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Debnath S, Bauman MJ, Fisher LE, Weber DJ and Gaunt RA 2014. Microelectrode array recordings from the ventral roots in chronically implanted cats Front. Neurol 5 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chung JE, Magland JF, Barnett AH, Tolosa VM, Tooker AC, Lee KY, Shah KG, Felix SH, Frank LM and Greengard LF 2017. A fully automated approach to spike sorting Neuron 95 1381–1394.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gulino M, Kim D, Pané S, Santos SD and Pˆego AP 2019. Tissue response to neural implants: the use of model systems toward new design solutions of implantable microelectrodes Front. Neurosci 13 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ludwig KA, Langhals NB, Joseph MD, Richardson-Burns SM, Hendricks JL and Kipke DR 2011. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes J. Neural Eng 8 014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kim S-M et al. 2018. High-performance, polymer-based direct cellular interfaces for electrical stimulation and recording NPG Asia Mater 10 255–65 [Google Scholar]
- [78].Shoffstall AJ. et al. Characterization of the neuroinflammatory response to thiol-ene shape memory polymer coated intracortical microelectrodes. Micromachines. 2018;9:486. doi: 10.3390/mi9100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hanein Y and Bareket-Keren L 2013. Carbon nanotube-based multi electrode arrays for neuronal interfacing: progress and prospects Front. Neural Circuits 6 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chung JE et al. 2019. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays Neuron 101 21–31.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fu T-M, Hong G, Zhou T, Schuhmann TG, Viveros RD and Lieber CM 2016. Stable long-term chronic brain mapping at the single-neuron level Nat. Methods 13 875–82 [DOI] [PubMed] [Google Scholar]
- [82].Luan L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017;3:e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].He F, Sullender CT, Zhu H, Williamson MR, Li X, Zhao Z, Jones TA, Xie C, Dunn AK and Luan L 2020. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes Sci. Adv 6 eaba1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xing D, Yeh C-I and Shapley RM 2009. Spatial spread of the local field potential and its laminar variation in visual cortex J. Neurosci 29 11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Buzsáki G 2002. Theta oscillations in the hippocampus Neuron 33 325–40 [DOI] [PubMed] [Google Scholar]
- [86].De Boer L, Axelsson J, Riklund K, Nyberg L, Dayan P, Bäckman L and Guitart-Masip M 2017. Attenuation of dopamine-modulated prefrontal value signals underlies probabilistic reward learning deficits in old age eLife 6 e26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Simoncelli EP, Pillow JW, Paninski L and Schwartz O 2004. Characterization of neural responses with stochastic stimuli (Chapter 23) The cognitive neurosciences 3rd Gazzaniga M (Cambridge, MA: MIT Press; ) pp 327–38 [Google Scholar]
- [88].Pesaran B, Pezaris JS, Sahani M, Mitra PP and Andersen RA 2002. Temporal structure in neuronal activity during working memory in macaque parietal cortex Nat. Neurosci 5 805–11 [DOI] [PubMed] [Google Scholar]
- [89].Scherberger H, Jarvis MR and Andersen RA 2005. Cortical local field potential encodes movement intentions in the posterior parietal cortex Neuron 46 347–54 [DOI] [PubMed] [Google Scholar]
- [90].Heldman DA, Wang W, Chan SS and Moran DW 2006. Local field potential spectral tuning in motor cortex during reaching IEEE Trans. Neural Syst. Rehabil. Eng 14 180–3 [DOI] [PubMed] [Google Scholar]
- [91].Markowitz DA, Wong YT, Gray CM and Pesaran B 2011. Optimizing the decoding of movement goals from local field potentials in macaque cortex J. Neurosci 31 18412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rich EL and Wallis JD 2016. Decoding subjective decisions from orbitofrontal cortex Nat. Neurosci 19 973–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bansal AK, Vargas-Irwin CE, Truccolo W and Donoghue JP 2011. Relationships among low-frequency local field potentials, spiking activity, and three-dimensional reach and grasp kinematics in primary motor and ventral premotor cortices J. Neurophysiol 105 1603–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pesaran B, Vinck M, Einevoll GT, Sirota A, Fries P, Siegel M, Truccolo W, Schroeder CE and Srinivasan R 2018. Investigating large-scale brain dynamics using field potential recordings: analysis and interpretation Nat. Neurosci 21 903–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG and Buzsáki G 2015. NeuroGrid: recording action potentials from the surface of the brain Nat. Neurosci 18 310–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dubey A and Ray S 2020. Comparison of tuning properties of gamma and high-gamma power in local field potential (LFP) versus electrocorticogram (ECoG) in visual cortex Sci. Rep 10 5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ray S, Crone NE, Niebur E, Franaszczuk PJ and Hsiao SS 2008. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography J. Neurosci 28 11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Manning V, Best DW, Faulkner N and Titherington E 2009. New estimates of the number of children living with substance misusing parents: results from UK national household surveys BMC Public Health 9 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Miller KJ, Honey CJ, Hermes D, Rao RPN, denNijs M and Ojemann JG 2014. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations Neuroimage 85 711–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fukushima M, Chao ZC and Fujii N 2015. Studying brain functions with mesoscopic measurements: advances in electrocorticography for non-human primates Curr. Opin. Neurobiol 32 124–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Parvizi J and Kastner S 2018. Promises and limitations of human intracranial electroencephalography Nat. Neurosci 21 474–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chiang C-H. et al. Development of a neural interface for high-definition, long-term recording in rodents and nonhuman primates. Sci. Transl. Med. 2020;12:eaay4682. doi: 10.1126/scitranslmed.aay4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Viventi J et al. 2011. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo Nat. Neurosci 14 1599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kaiju T, Inoue M, Hirata M and Suzuki T 2021. High-density mapping of primate digit representations with a 1152-channel µECoG array J. Neural Eng 18 036025. [DOI] [PubMed] [Google Scholar]
- [105].Chao ZC, Takaura K, Wang L, Fujii N and Dehaene S 2018. Large-scale cortical networks for hierarchical prediction and prediction error in the primate brain Neuron 100 1252–1266.e3 [DOI] [PubMed] [Google Scholar]
- [106].Davis ZW, Muller L, Martinez-Trujillo J, Sejnowski T and Reynolds JH 2020. Spontaneous travelling cortical waves gate perception in behaving primates Nature 587 432–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Muller L, Chavane F, Reynolds J and Sejnowski TJ 2018. Cortical travelling waves: mechanisms and computational principles Nat. Rev. Neurosci 19 255–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhang H, Watrous AJ, Patel A and Jacobs J 2018. Theta and alpha oscillations are traveling waves in the human neocortex Neuron 98 1269–1281.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang H and Jacobs J 2015. Traveling theta waves in the human hippocampus J. Neurosci 35 12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Anumanchipalli GK, Chartier J and Chang EF 2019. Speech synthesis from neural decoding of spoken sentences Nature 568 493–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang NXR, Olson JD, Ojemann JG, Rao RPN and Brunton BW 2016. Unsupervised decoding of long-term, naturalistic human neural recordings with automated video and audio annotations Front. Hum. Neurosci 10 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sani OG, Yang Y, Lee MB, Dawes HE, Chang EF and Shanechi MM 2018. Mood variations decoded from multi-site intracranial human brain activity Nat. Biotechnol 36 954–61 [DOI] [PubMed] [Google Scholar]
- [113].Yang Y, Sani OG, Chang EF and Shanechi MM 2019. Dynamic network modeling and dimensionality reduction for human ECoG activity J. Neural Eng 16 056014. [DOI] [PubMed] [Google Scholar]
- [114].Flint RD, Tate MC, Li K, Templer JW, Rosenow JM, Pandarinath C and Slutzky MW 2020. The representation of finger movement and force in human motor and premotor cortices eNeuro 7 ENEURO.0063–20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chao Z, Nagasaka Y and Fujii N 2010. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkey Front. Neuroeng 3 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ahmadipour P, Yang Y, Chang EF and Shanechi MM 2020. Adaptive tracking of human ECoG network dynamics J. Neural Eng 18 016011. [DOI] [PubMed] [Google Scholar]
- [117].Berger H 1929. Über das Elektrenkephalogramm des Menschen Arch. Für Psychiatr. Nervenkrankh 87 527–70 [Google Scholar]
- [118].Brazier MAB 1961. A History of the Electrical Activity of the Brain: The First Half-Century (London: Macmillan; ) [Google Scholar]
- [119].Newson JJ and Thiagarajan TC 2019. EEG frequency bands in psychiatric disorders: a review of resting state studies Front. Hum. Neurosci 12 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Haegens S, Cousijn H, Wallis G, Harrison PJ and Nobre AC 2014. Inter- and intra-individual variability in alpha peak frequency Neuroimage 92 46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Watrous AJ and Buchanan RJ 2020. the oscillatory reconstruction algorithm (ORCA) adaptively identifies frequency bands to improve spectral decomposition in human and rodent neural recordings J. Neurophysiol 124 1914–22 [DOI] [PubMed] [Google Scholar]
- [122].Luck S 2014. An Introduction to the Event-Related Potential Technique 2nd (Cambridge, MA: MIT Press; ) [Google Scholar]
- [123].Kappenman ES and Luck SJ 2012. ERP components: the ups and downs of brainwave recordings The Oxford Handbook of Event-Related Potential Components. Oxford Library of Psychology (Oxford: Oxford University Press; ) pp 3–30 [Google Scholar]
- [124].Roach BJ and Mathalon DH 2008. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia Schizophr. Bull 34 907–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Worden MS, Foxe JJ, Wang N and Simpson GV 2000. Anticipatory biasing of visuospatial attention indexed by retinotopically specific α-bank electroencephalography increases over occipital cortex J. Neurosci 20 RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vogel EK and Machizawa MG 2004. Neural activity predicts individual differences in visual working memory capacity Nature 428 748–51 [DOI] [PubMed] [Google Scholar]
- [127].Sprague TC, Saproo S and Serences JT 2015. Visual attention mitigates information loss in small- and large-scale neural codes Trends Cogn. Sci 19 215–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Foster JJ, Sutterer DW, Serences JT, Vogel EK and Awh E 2015. The topography of alpha-band activity tracks the content of spatial working memory J. Neurophysiol 115 168–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Foster JJ, Sutterer DW, Serences JT, Vogel EK and Awh E 2017. Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention Psychol. Sci 28 929–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Samaha J, Barrett JJ, Sheldon AD, LaRocque JJ and Postle BR 2016. Dissociating perceptual confidence from discrimination accuracy reveals no influence of metacognitive awareness on working memory Front. Psychol 7 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sutterer DW, Foster JJ, Serences JT, Vogel EK and Awh E 2019. Alpha-band oscillations track the retrieval of precise spatial representations from long-term memory J. Neurophysiol 122 539–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sprague TC and Serences JT 2013. Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices Nat. Neurosci 16 1879–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wan Q, Cai Y, Samaha J and Postle BR 2020. Tracking stimulus representation across a 2-back visual working memory task R. Soc. Open Sci 7 190228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bartra O, McGuire JT and Kable JW 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value Neuroimage 76 412–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chase HW, Kumar P, Eickhoff SB and Dombrovski AY 2015. Reinforcement learning models and their neural correlates: an activation likelihood estimation meta-analysis Cogn. Affect. Behav. Neurosci 15 435–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Keren H et al. 2018. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies Am. J. Psychiatry 175 1111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P and Fusar-Poli P 2015. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis JAMA Psychiatry 72 1243–51 [DOI] [PubMed] [Google Scholar]
- [138].Burke CJ, Baddeley M, Tobler PN and Schultz W 2016. Partial adaptation of obtained and observed value signals preserves information about gains and losses J. Neurosci 36 10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kahnt T 2018. A decade of decoding reward-related fMRI signals and where we go from here Neuroimage 180 324–33 [DOI] [PubMed] [Google Scholar]
- [140].Anzellotti S and Coutanche MN 2018. Beyond functional connectivity: investigating networks of multivariate representations Trends Cogn. Sci 22 258–69 [DOI] [PubMed] [Google Scholar]
- [141].Howard JD, Gottfried JA, Tobler PN and Kahnt T 2015. Identity-specific coding of future rewards in the human orbitofrontal cortex Proc. Natl Acad. Sci 112 5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Coutanche MN and Thompson-Schill SL 2015. Creating concepts from converging features in human cortex Cereb. Cortex 25 2584–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kahnt T and Tobler PN 2017. Dopamine modulates the functional organization of the orbitofrontal cortex J. Neurosci 37 2827–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kahnt T, Weber SC, Haker H, Robbins TW and Tobler PN 2015. Dopamine D2-receptor blockade enhances decoding of prefrontal signals in humans J. Neurosci 35 4104–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Soutschek A, Moisa M, Ruff CC and Tobler PN 2020. The right temporoparietal junction enables delay of gratification by allowing decision makers to focus on future events PLoS Biol 18 e3000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Howard JD and Kahnt T 2021. Causal investigations into orbitofrontal control of human decision making Curr. Opin. Behav. Sci 38 14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hermiller MS, Chen YF, Parrish TB and Voss JL 2020. Evidence for immediate enhancement of hippocampal memory encoding by network-targeted theta-burst stimulation during concurrent fMRI J. Neurosci 40 7155–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Tambini A and D’Esposito M 2020. Causal contribution of awake post-encoding processes to episodic memory consolidation Curr. Biol 30 3533–3543.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Sander CY and Hesse S 2017. News and views on in-vivo imaging of neurotransmission using PET and MRI Q J. Nucl. Med. Mol. Imaging 61 414–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Finnema SJ et al. 2015. Application of cross-species PET imaging to assess neurotransmitter release in brain Psychopharmacology 232 4129–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Larsen B, Olafsson V, Calabro F, Laymon C, Tervo-Clemmens B, Campbell E, Minhas D, Montez D, Price J and Luna B 2020. Maturation of the human striatal dopamine system revealed by PET and quantitative MRI Nat. Commun 11 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Westbrook A, Van Den Bosch R, Määttä JI, Hofmans L, Papadopetraki D, Cools R and Frank MJ 2020. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work Science 367 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].D’Ambrosio E et al. 2019. The relationship between grey matter volume and striatal dopamine function in psychosis: a multimodal 18F-DOPA PET and voxel-based morphometry study Mol. Psychiatry 26 1332–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ and Grasby PM 1998. Evidence for striatal dopamine release during a video game Nature 393 266–8 [DOI] [PubMed] [Google Scholar]
- [155].Pappata S et al. 2002. In vivo detection of striatal dopamine release during reward: a PET study with [(11)C]raclopride and a single dynamic scan approach Neuroimage 16 1015–27 [DOI] [PubMed] [Google Scholar]
- [156].Weinstein JJ et al. 2018. PET imaging of dopamine-D2 receptor internalization in schizophrenia Mol. Psychiatry 23 1506–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Urban NBL et al. 2010. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(11)C]raclopride Biol. Psychiatry 68 689–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kasanova Z, Ceccarini J, Frank MJ, Van Amelsvoort T, Booij J, Heinzel A, Mottaghy F and Myin-Germeys I 2017. Striatal dopaminergic modulation of reinforcement learning predicts reward—oriented behavior in daily life Biol. Psychol 127 1–9 [DOI] [PubMed] [Google Scholar]
- [159].Lippert RN, Cremer AL, Edwin Thanarajah S, Korn C, Jahans-Price T, Burgeno LM, Tittgemeyer M, Brüning JC, Walton ME and Backes H 2019. Time-dependent assessment of stimulus-evoked regional dopamine release Nat. Commun 10 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Thanarajah SE et al. 2019. Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans Cell Metab 29 695–706.e4 [DOI] [PubMed] [Google Scholar]
- [161].Nadel L and Moscovitch M 1997. Memory consolidation, retrograde amnesia and the hippocampal complex Curr. Opin. Neurobiol 7 217–27 [DOI] [PubMed] [Google Scholar]
- [162].Kriegeskorte N, Mur M and Bandettini P 2008. Representational similarity analysis—connecting the branches of systems neuroscience Front. Syst. Neurosci 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Meir-Hasson Y, Kinreich S, Podlipsky I, Hendler T and Intrator N 2014. An EEG finger-print of fMRI deep regional activation Neuroimage 102 128–41 [DOI] [PubMed] [Google Scholar]
- [164].Mirsattari SM, Lee DH, Jones D, Bihari F and Ives JR 2004. MRI compatible EEG electrode system for routine use in the epilepsy monitoring unit and intensive care unit Clin. Neurophysiol 115 2175–80 [DOI] [PubMed] [Google Scholar]
- [165].Renz AF, Lee J, Tybrandt K, Brzezinski M, Lorenzo DA, Cerra Cheraka M, Lee J, Helmchen F, Vörös J and Lewis CM 2020. Opto-E-Dura: a soft, stretchable ECoG array for multimodal, multiscale neuroscience Adv. Healthcare Mater 9 2000814. [DOI] [PubMed] [Google Scholar]
- [166].Tian L et al. 2019. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring Nat. Biomed. Eng 3 194–205 [DOI] [PubMed] [Google Scholar]
- [167].Cuffin BN, Cohen D, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy J and Schomer D 1991. Tests of EEG localization accuracy using implanted sources in the human brain Ann. Neurol 29 132–8 [DOI] [PubMed] [Google Scholar]
- [168].Van Veen B D, Van Drongelen W, Yuchtman M and Suzuki A 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering IEEE Trans. Biomed. Eng 44 867–80 [DOI] [PubMed] [Google Scholar]
- [169].Nunez PL and Srinivasan R 2006. Electric Fields of the Brain: The Neurophysics of EEG (Oxford: Oxford University Press; ) [Google Scholar]
- [170].Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J and Lounasmaa OV 1993. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain Rev. Mod. Phys 65 413–97 [Google Scholar]
- [171].Lindén H, Hagen E, Lęski S, Norheim ES, Pettersen KH and Einevoll GT 2014. LFPy: a tool for biophysical simulation of extracellular potentials generated by detailed model neurons Front. Neuroinformatics 7 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Michel CM and Brunet D 2019. EEG source imaging: a practical review of the analysis steps Front. Neurol 10 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Chang Y-J, Chen Y-I, Yeh H-C, Carmena JM and Santacruz SR 2020. Bi-directional modeling between cross-scale neural activity bioRxiv ( 10.1101/2020.11.30.404244) [DOI]
- [174].Tam W, Wu T, Zhao Q, Keefer E and Yang Z 2019. Human motor decoding from neural signals: a review BMC Biomed. Eng 1 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Yu BM, Santhanam G, Sahani M and Shenoy KV 2010. Chapter 7—Neural decoding for motor and communication prostheses Statistical Signal Processing for Neuroscience and Neurotechnology (Academic: New York: ) pp 219–63 [Google Scholar]
- [176].Canolty RT, Ganguly K, Kennerley SW, Cadieu CF, Koepsell K, Wallis JD and Carmena JM 2010. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies Proc. Natl Acad. Sci 107 17356–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Fries P 2005. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence Trends Cogn. Sci 9 474–80 [DOI] [PubMed] [Google Scholar]
- [178].Lakatos P, Karmos G, Mehta AD, Ulbert I and Schroeder CE 2008. Entrainment of neuronal oscillations as a mechanism of attentional selection Science 320 110. [DOI] [PubMed] [Google Scholar]
- [179].Zanos S, Zanos TP, Marmarelis VZ, Ojemann GA and Fetz EE 2012. Relationships between spike-free local field potentials and spike timing in human temporal cortex J. Neurophysiol 107 1808–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Einevoll GT, Kayser C, Logothetis NK and Panzeri S 2013. Modelling and analysis of local field potentials for studying the function of cortical circuits Nat. Rev. Neurosci 14 770–85 [DOI] [PubMed] [Google Scholar]
- [181].Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA and Farmer SF 1995. A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms Prog. Biophys. Mol. Biol 64 237–78 [DOI] [PubMed] [Google Scholar]
- [182].Wong YT, Fabiszak MM, Novikov Y, Daw ND and Pesaran B 2016. Coherent neuronal ensembles are rapidly recruited when making a look-reach decision Nat. Neurosci 19 327–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Gong X, Li W and Liang H 2019. Spike-field Granger causality for hybrid neural data analysis J. Neurophysiol 122 809–22 [DOI] [PubMed] [Google Scholar]
- [184].Wang C and Shanechi MM 2019. Estimating multiscale direct causality graphs in neural spike-field networks IEEE Trans. Neural Syst. Rehabil. Eng 27 857–66 [DOI] [PubMed] [Google Scholar]
- [185].Khorasani A, Shalchyan V and Daliri MR 2019. Adaptive artifact removal from intracortical channels for accurate decoding of a force signal in freely moving rats Front. Neurosci 13 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Burkitt AN 2006. A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input Biol. Cybern 95 1–19 [DOI] [PubMed] [Google Scholar]
- [187].Burkitt AN 2006. A review of the integrate-and-fire neuron model: II. Inhomogeneous synaptic input and network properties Biol. Cybern 95 97–112 [DOI] [PubMed] [Google Scholar]
- [188].Brunel N and Van Rossum MCW 2007. Lapicque’s 1907 paper: from frogs to integrate-and-fire Biol. Cybern 97 337–9 [DOI] [PubMed] [Google Scholar]
- [189].Mazzoni A, Lindén H, Cuntz H, Lansner A, Panzeri S and Einevoll GT 2015. Computing the local field potential (LFP) from integrate-and-fire network models PLoS Comput. Biol 11 e1004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Tremblay S, Doucet G, Pieper F, Sachs A and Martinez-Trujillo J 2015. Single-trial decoding of visual attention from local field potentials in the primate lateral prefrontal cortex is frequency-dependent J. Neurosci 35 9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Zhang Q et al. 2018. Local field potential decoding of the onset and intensity of acute pain in rats Sci. Rep 8 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Perel A. Non-invasive monitoring of oxygen delivery in acutely ill patients: new frontiers. Ann. Intensive Care. 2015;5:24. doi: 10.1186/s13613-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Flint RD, Lindberg EW, Jordan LR, Miller LE and Slutzky MW 2012. Accurate decoding of reaching movements from field potentials in the absence of spikes J. Neural Eng 9 046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Perge JA, Zhang S, Malik WQ, Homer ML, Cash S, Friehs G, Eskandar EN, Donoghue JP and Hochberg LR 2014. Reliability of directional information in unsorted spikes and local field potentials recorded in human motor cortex J. Neural Eng 11 046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Toda H, Suzuki T, Sawahata H, Majima K, Kamitani Y and Hasegawa I 2011. Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex Neuroimage 54 203–12 [DOI] [PubMed] [Google Scholar]
- [196].Miyakawa N, Majima K, Sawahata H, Kawasaki K, Matsuo T, Kotake N, Suzuki T, Kamitani Y and Hasegawa I 2018. Heterogeneous redistribution of facial subcategory information within and outside the face-selective domain in primate inferior temporal cortex Cereb. Cortex 28 1416–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Orsborn AL, Wang C, Chiang K, Maharbiz MM, Viventi J and Pesaran B 2015. Semi-chronic chamber system for simultaneous subdural electrocorticography, local field potentials, and spike recordings 2015 7th Int. IEEE/EMBS Conf. on Neural Engineering (NER) pp 398–401
- [198].Yazdan-Shahmorad A, Kipke DR and Lehmkuhle MJ 2013. High gamma power in ECoG reflects cortical electrical stimulation effects on unit activity in layers V/VI J. Neural Eng 10 066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Dubey A and Ray S 2019. Cortical electrocorticogram (ECoG) is a local signal J. Neurosci 39 4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Ibayashi K, Kunii N, Matsuo T, Ishishita Y, Shimada S, Kawai K and Saito N 2018. Decoding speech with integrated hybrid signals recorded from the human ventral motor cortex Front. Neurosci 12 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Kanth ST and Ray S 2020. Electrocorticogram (ECoG) is highly informative in primate visual cortex J. Neurosci 40 2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Huang-Hellinger FR et al. 1995. Simultaneous functional magnetic resonance imaging and electrophysiological recording Hum. Brain Mapp 3 13–23 [Google Scholar]
- [203].Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C and Krakow K 2003. EEG-correlated fMRI of human alpha activity Neuroimage 19 1463–76 [DOI] [PubMed] [Google Scholar]
- [204].Debener S, Ullsperger M, Siegel M and Engel AK 2006. Single-trial EEG–fMRI reveals the dynamics of cognitive function Trends Cogn. Sci 10 558–63 [DOI] [PubMed] [Google Scholar]
- [205].Itthipuripat S, Sprague TC and Serences JT 2019. Functional MRI and EEG index complementary attentional modulations J. Neurosci 39 6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lee J-H, Oh S, Jolesz FA, Park H and Yoo S-S 2009. Application of independent component analysis for the data mining of simultaneous EEG–fMRI: preliminary experience on sleep onset Int. J. Neurosci 119 1118–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Mele G, Cavaliere C, Alfano V, Orsini M, Salvatore M and Aiello M 2019. Simultaneous EEG-fMRI for functional neurological assessment Front. Neurol 10 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Deligianni F, Centeno M, Carmichael DW and Clayden JD 2014. Relating resting-state fMRI and EEG whole-brain connectomes across frequency bands Front. Neurosci 8 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Herweg NA, Apitz T, Leicht G, Mulert C, Fuentemilla L and Bunzeck N 2016. Theta-alpha oscillations bind the hippocampus, prefrontal cortex, and striatum during recollection: evidence from simultaneous EEG–fMRI J. Neurosci 36 3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Beldzik E, Domagalik A, Beres A and Marek T 2019. Linking visual gamma to task-related brain networks—a simultaneous EEG-fMRI study Psychophysiology 56 e13462. [DOI] [PubMed] [Google Scholar]
- [211].Andreou C, Frielinghaus H, Rauh J, Mußmann M, Vauth S, Braun P, Leicht G and Mulert C 2017. Theta and high-beta networks for feedback processing: a simultaneous EEG–fMRI study in healthy male subjects Transl. Psychiatry 7 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Zotev V, Misaki M, Phillips R, Wong CK and Bodurka J 2018. Real-time fMRI neurofeedback of the mediodorsal and anterior thalamus enhances correlation between thalamic BOLD activity and alpha EEG rhythm Hum. Brain Mapp 39 1024–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Wehrl HF, Sauter AW, Judenhofer MS and Pichler BJ 2010. Combined PET/MR imaging—technology and applications Technol. Cancer Res. Treat 9 5–20 [DOI] [PubMed] [Google Scholar]
- [214].Judenhofer MS et al. 2008. Simultaneous PET-MRI: a new approach for functional and morphological imaging Nat. Med 14 459–65 [DOI] [PubMed] [Google Scholar]
- [215].Glover GH 2011. Overview of functional magnetic resonance imaging Neurosurg. Clin. N Am 22 133–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Ametamey SM, Honer M and Schubiger PA 2008. Molecular imaging with PET Chem. Rev 108 1501–16 [DOI] [PubMed] [Google Scholar]
- [217].Urban NBL, Slifstein M, Meda S, Xu X, Ayoub R, Medina O, Pearlson GD, Krystal JH and Abi-Dargham A 2012. Imaging human reward processing with positron emission tomography and functional magnetic resonance imaging Psychopharmacology 221 67–77 [DOI] [PubMed] [Google Scholar]
- [218].Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, Vanduffel W, Rosen BR and Mandeville JB 2013. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI Proc. Natl Acad. Sci 110 11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Dubol M, Trichard C, Leroy C, Sandu A-L, Rahim M, Granger B, Tzavara ET, Karila L, Martinot J-L and Artiges E 2018. Dopamine transporter and reward anticipation in a dimensional perspective: a multimodal brain imaging study Neuropsychopharmacology 43 820–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Dang LC, Samanez-Larkin GR, Castrellon JJ, Perkins SF, Cowan RL and Zald DH 2018. Individual differences in dopamine D2 receptor availability correlate with reward valuation Cogn. Affect. Behav. Neurosci 18 739–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Selvaggi P et al. 2019. Increased cerebral blood flow after single dose of antipsychotics in healthy volunteers depends on dopamine D2 receptor density profiles Neuroimage 188 774–84 [DOI] [PubMed] [Google Scholar]
- [222].Salimpoor VN, Benovoy M, Larcher K, Dagher A and Zatorre RJ 2011. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music Nat. Neurosci 14 257–62 [DOI] [PubMed] [Google Scholar]
- [223].Van Dromme ICL, Vanduffel W and Janssen P 2015. The relation between functional magnetic resonance imaging activations and single-cell selectivity in the macaque intraparietal sulcus Neuroimage 113 86–100 [DOI] [PubMed] [Google Scholar]
- [224].Eidelberg D et al. 1997. Metabolic correlates of pallidal neuronal activity in Parkinson’s disease Brain 120 1315–24 [DOI] [PubMed] [Google Scholar]
- [225].Logothetis NK, Pauls J, Augath M, Trinath T and Oeltermann A 2001. Neurophysiological investigation of the basis of the fMRI signal Nature 412 150–7 [DOI] [PubMed] [Google Scholar]
- [226].Lippert MT, Steudel T, Ohl F, Logothetis NK and Kayser C 2010. Coupling of neural activity and fMRI-BOLD in the motion area MT Magn. Reson. Imaging 28 1087–94 [DOI] [PubMed] [Google Scholar]
- [227].Duffy BA, Choy M, Chuapoco MR, Madsen M and Lee JH 2015. MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges Neuroimage 123 173–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Dunn JF, Tuor UI, Kmech J, Young NA, Henderson AK, Jackson JC, Valentine PA and Teskey GC 2009. Functional brain mapping at 9.4T using a new MRI-compatible electrode chronically implanted in rats Magn. Reson. Med 61 222–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Chuapoco MR, Choy M, Schmid F, Duffy BA, Lee HJ and Lee JH 2019. Carbon monofilament electrodes for unit recording and functional MRI in same subjects Neuroimage 186 806–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Noor MS, Yu L, Murari K and Kiss ZHT 2020. Neurovascular coupling during deep brain stimulation Brain Stimul 13 916–27 [DOI] [PubMed] [Google Scholar]
- [231].Popivanov ID, Jastorff J, Vanduffel W and Vogels R 2014. Heterogeneous single-unit selectivity in an fMRI-defined body-selective patch J. Neurosci 34 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H and Villringer A 2003. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy Neuroimage 20 145–58 [DOI] [PubMed] [Google Scholar]
- [233].Golkowski D, Merz K, Mlynarcik C, Kiel T, Schorr B, Lopez-Rolon A, Lukas M, Jordan D, Bender A and Ilg R 2017. Simultaneous EEG–PET–fMRI measurements in disorders of consciousness: an exploratory study on diagnosis and prognosis J. Neurol 264 1986–95 [DOI] [PubMed] [Google Scholar]
- [234].Rajkumar R, Rota Kops E, Mauler J, Tellmann L, Lerche C, Herzog H, Shah NJ and Neuner I 2017. Simultaneous trimodal PET-MR-EEG imaging: do EEG caps generate artefacts in PET images? PLoS One 12 e0184743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Wu R, Yang P-F and Chen LM 2017. Correlated disruption of resting-state fMRI, LFP, and spike connectivity between area 3b and S2 following spinal cord injury in monkeys J. Neurosci 37 11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Balasubramanian K, Takahashi K, Slutzky M and Hatsopoulos NG 2014. Multi-modal decoding: longitudinal coherency changes between spike trains, local field potentials and electrocorticogram signals 2014 36th Annual Int. Conf. IEEE Engineering in Medicine and Biology Society pp 5192–5 [DOI] [PMC free article] [PubMed]
- [237].Whittingstall K and Logothetis NK 2009. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex Neuron 64 281–9 [DOI] [PubMed] [Google Scholar]
- [238].Zotev V, Mayeli A, Misaki M and Bodurka J 2020. Emotion self-regulation training in major depressive disorder using simultaneous real-time fMRI and EEG neurofeedback Neuroimage Clin 27 102331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC and Bodurka J 2014. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder PLoS One 9 e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Zotev V, Yuan H, Misaki M, Phillips R, Young KD, Feldner MT and Bodurka J 2016. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression Neuroimage Clin 11 224–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zotev V, Phillips R, Misaki M, Wong CK, Wurfel BE, Krueger F, Feldner M and Bodurka J 2018. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD Neuroimage Clin 19 106–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Corsi M-C, Chavez M, Schwartz D, Hugueville L, Khambhati AN, Bassett DS and De Vico Fallani F 2019. Integrating EEG and MEG signals to improve motor imagery classification in brain–computer interface Int. J. Neural Syst 29 1850014. [DOI] [PubMed] [Google Scholar]
- [243].Vidaurre C and Blankertz B 2010. Towards a cure for BCI illiteracy Brain Topogr 23 194–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Chiarelli AM, Croce P, Merla A and Zappasodi F 2018. Deep learning for hybrid EEG-fNIRS brain–computer interface: application to motor imagery classification J. Neural Eng 15 036028. [DOI] [PubMed] [Google Scholar]
- [245].Khan MJ and Hong K-S 2017. Hybrid EEG–fNIRS-based eight-command decoding for BCI: application to quadcopter control Front. Neurorobotics 11 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Curtin A, Tong S, Sun J, Wang J, Onaral B and Ayaz H 2019. A systematic review of integrated functional near-infrared spectroscopy (fNIRS) and transcranial magnetic stimulation (TMS) studies Front. Neurosci 13 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Ruffini G, Fox MD, Ripolles O, Miranda PC and Pascual-Leone A 2014. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields Neuroimage 89 216–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Bikson M, Rahman A and Datta A 2012. Computational models of transcranial direct current stimulation Clin. EEG Neurosci 43 176–83 [DOI] [PubMed] [Google Scholar]
- [249].Gomez-Tames J, Laakso I and Hirata A 2020. Review on biophysical modelling and simulation studies for transcranial magnetic stimulation Phys. Med. Biol 65 24TR03. [DOI] [PubMed] [Google Scholar]
- [250].Dutta A, Jacob A, Chowdhury SR, Das A and Nitsche MA 2015. EEG-NIRS based assessment of neurovascular coupling during anodal transcranial direct current stimulation—a stroke case series J. Med. Syst 39 36. [DOI] [PubMed] [Google Scholar]
- [251].Otal B, Dutta A, Foerster Á, Ripolles O, Kuceyeski A, Miranda PC, Edwards DJ, Ilić TV, Nitsche MA and Ruffini G 2016. Opportunities for guided multichannel non-invasive transcranial current stimulation in poststroke rehabilitation Front. Neurol 7 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Soutschek A and Tobler PN 2020. Causal role of lateral prefrontal cortex in mental effort and fatigue Hum. Brain Mapp 41 4630–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Peters JC, Reithler J, De Graaf T A, Schuhmann T, Goebel R and Sack AT 2020. Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity Commun. Biol 3 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Tremblay S et al. 2019. Clinical utility and prospective of TMS–EEG Clin. Neurophysiol 130 802–44 [DOI] [PubMed] [Google Scholar]
- [255].Varone G, Hussain Z, Sheikh Z, Howard A, Boulila W, Mahmud M, Howard N, Morabito FC and Hussain A 2021. Real-time artifacts reduction during TMS-EEG co-registration: a comprehensive review on technologies and procedures Sensors (Basel) 21 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Honda Y, Nakamura S, Ogawa K, Yoshino R, Tobler PN, Nishimura Y and Tsutsui K-I 2021. Changes in beta and high-gamma power in resting-state electrocorticogram induced by repetitive transcranial magnetic stimulation of primary motor cortex in unanesthetized macaque monkeys Neurosci. Res S0168–0102(21) 00052–3 [DOI] [PubMed]
- [257].Hill CA, Suzuki S, Polania R, Moisa M, O’Doherty JP and Ruff CC 2017. A causal account of the brain network computations underlying strategic social behavior Nat. Neurosci 20 1142–9 [DOI] [PubMed] [Google Scholar]
- [258].Albouy P, Weiss A, Baillet S and Zatorre RJ 2017. Selective entrainment of theta oscillations in the dorsal stream causally enhances auditory working memory performance Neuron 94 193–206.e5 [DOI] [PubMed] [Google Scholar]
- [259].Lee TG and D’Esposito M 2012. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI–TMS study J. Neurosci 32 15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Esmaeilpour Z, Shereen AD, Ghobadi-Azbari P, Datta A, Woods AJ, Ironside M, O’Shea J, Kirk U, Bikson M and Ekhtiari H 2020. Methodology for tDCS integration with fMRI Hum. Brain Mapp 41 1950–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Castrillon G, Sollmann N, Kurcyus K, Razi A, Krieg SM and Riedl V 2020. The physiological effects of noninvasive brain stimulation fundamentally differ across the human cortex Sci. Adv 6 eaay2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Berger A, Horst F, Müller S, Steinberg F and Doppelmayr M 2019. Current state and future prospects of EEG and fNIRS in robot-assisted gait rehabilitation: a brief review Front. Hum. Neurosci 13 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Lachert P, Janusek D, Pulawski P, Liebert A, Milej D and Blinowska KJ 2017. Coupling of oxy- and deoxyhemoglobin concentrations with EEG rhythms during motor task Sci. Rep 7 15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Zich C, Debener S, Kranczioch C, Bleichner MG, Gutberlet I and De Vos M 2015. Real-time EEG feedback during simultaneous EEG–fMRI identifies the cortical signature of motor imagery Neuroimage 114 438–47 [DOI] [PubMed] [Google Scholar]
- [265].Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P and Friston KJ 2012. Canonical microcircuits for predictive coding Neuron 76 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Goldman-Rakic PS and Rakic P 1991. Preface: cerebral cortex has come of age Cereb. Cortex 1 11822724 [Google Scholar]
- [267].Kandel ER, Dudai Y and Mayford MR 2014. The molecular and systems biology of memory Cell 157 163–86 [DOI] [PubMed] [Google Scholar]
- [268].Penfield W. Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proc. Natl Acad. Sci. 1958;44:51. doi: 10.1073/pnas.44.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Ranganath C and Ritchey M 2012. Two cortical systems for memory-guided behaviour Nat. Rev. Neurosci 13 713–26 [DOI] [PubMed] [Google Scholar]
- [270].Fell J and Axmacher N 2011. The role of phase synchronization in memory processes Nat. Rev. Neurosci 12 105–18 [DOI] [PubMed] [Google Scholar]
- [271].Watrous AJ, Fell J, Ekstrom AD and Axmacher N 2015. More than spikes: common oscillatory mechanisms for content specific neural representations during perception and memory Curr. Opin. Neurobiol 31 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Hyman BT et al. 2012. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease Alzheimers Dement 8 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Canolty RT, Cadieu CF, Koepsell K, Knight RT and Carmena JM 2012. Multivariate phase–amplitude cross-frequency coupling in neurophysiological signals IEEE Trans. Biomed. Eng 59 8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Salazar RF, Dotson NM, Bressler SL and Gray CM 2012. Content-specific fronto-parietal synchronization during visual working memory Science 338 1097–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Belitski A, Gretton A, Magri C, Murayama Y, Montemurro MA, Logothetis NK and Panzeri S 2008. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information J Neurosci 28 5696–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Agarwal G, Stevenson IH, Berényi A, Mizuseki K, Buzsáki G and Sommer FT 2014. Spatially distributed local fields in the hippocampus encode rat position Science 344 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Fischer P, Lipski WJ, Neumann W-J, Turner RS, Fries P, Brown P and Richardson RM 2020. Movement-related coupling of human subthalamic nucleus spikes to cortical gamma eLife 9 e51956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Callahan JW and Abercrombie ED 2015. Relationship between subthalamic nucleus neuronal activity and electrocorticogram is altered in the R6/2 mouse model of Huntington’s disease J. Physiol 593 3727–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study.


