Feelings of low energy, common in older adults,1 are multifactorial but may portend a neurodegenerative disorder in some individuals. Parkinson’s disease (PD) neuropathology begins years before clinical diagnosis2 and involves several structures implicated in energy.3 Fatigue and sleepiness are associated with increased risk of PD,2 but the broader construct of self-reported energy level (SEL), which relates to the subjective capacity for activity, has not been studied in the PD prodrome. Toward this end, we examined changes in SEL in older adults who developed PD versus those who did not. We hypothesized that SEL declines more in the former, and that the rate of decline accelerates in the time leading up to PD diagnosis.
This was a case–control analysis (see Supplement S1 for details) using data from the Health, Aging and Body Composition Study, a prospective cohort study of community-dwelling older adults.4 SEL was assessed annually for up to 8 years with an interviewer-administered question: “Choose the category that best describes your usual energy level in the past month on a scale of 0 to 10”; higher scores indicate greater SEL. SEL assessed with this question is associated with objective measures of energy expenditure.5 Depression was assessed with 10-item Center for Epidemiologic Studies Depression (CES-D-10). PD cases were adjudicated by an expert team.6 The study was performed in accordance with Declaration of Helsinki. All participants provided written informed consent.
Linear mixed models (LMMs) compared change in SEL in PD versus non-PD, adjusting for age, sex, and depression. To test differences in SEL trajectory, a quadratic term for SEL was introduced.
The analytic sample consisted of 2638 participants (Figure S1, Table S1), mean age at baseline was 74.61 years, and 48% were male. A total of 27 incident PD cases were identified. Mean time from baseline to PD was 4.07 years. See Table S2 for SEL mean values longitudinally. In the LMM predicting SEL change, the linear relationship between PD status and slope of SEL was not statistically significant (β = −0.086, P = 0.078). However, the LMM that included a quadratic term indicated that over time the PD group had a greater annual decline in SEL compared with the non-PD group (β = −0.037, P = 0.041; Figure 1/Supplement S1).
FIG. 1.
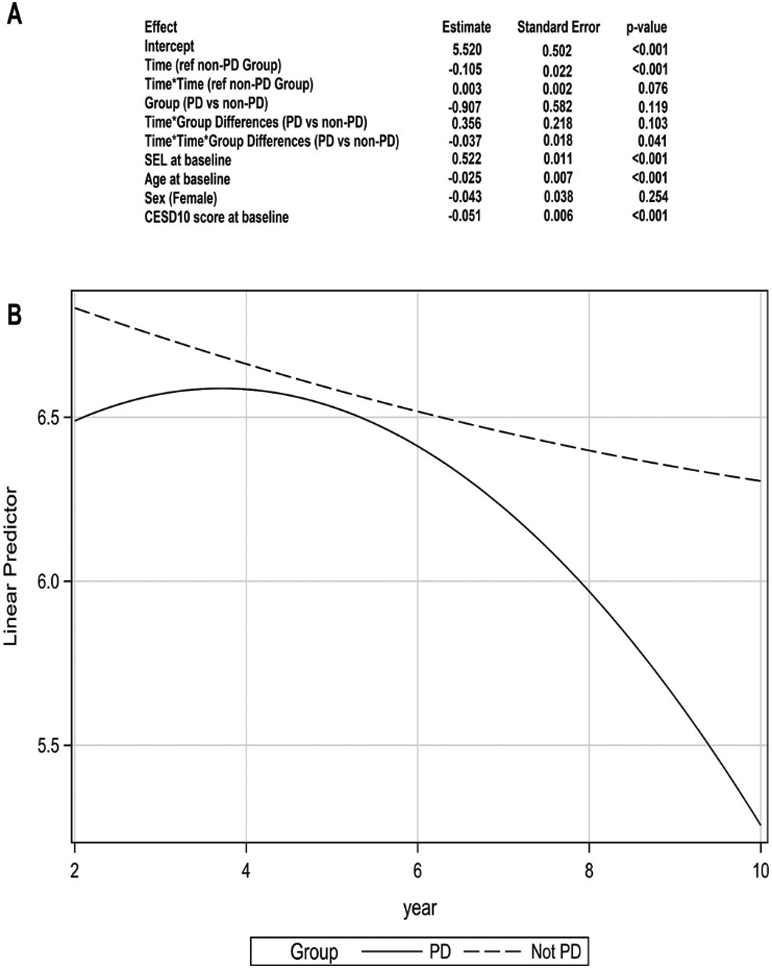
(A) Modeling of trajectory of subjective energy level (SEL): linear mixed model with quadratic term (B) Graphical representation of predicted values of SEL in PD group (solid line) versus non-PD group (dashed line). Fit computed at baseline SEL, 6.742; age, 74.48 years; baseline CESD-D-10, 2.91; male sex. See Supplementary Material S1 for what defines baseline for each variable specified.
In individuals who developed PD, SEL declined in the years prior to PD diagnosis and in a pattern significantly different from older adults who did not develop PD.
Changes in monoaminergic pathways play a role in anergia.3 Dopaminergic abnormalities begin years before the motor manifestations of PD emerge,2 and the putative trajectory of dopamine cell loss mirrors the changes in SEL preceding PD that we report. Reductions in SEL could also reflect changes in “energetics”—metabolic processes that mediate energy on a cellular level. Indeed, peripheral bioenergetic defects have been demonstrated in individuals with idiopathic REM sleep behavior disorder,7 a highly specific feature of prodromal PD.
Limitations of this study include retrospective PD case ascertainment and small number of PD cases.
Declining energy may be a characteristic of the PD prodrome. Future work is needed to understand what SEL represents in prodromal PD and what factors contribute to it its decline.
Supplementary Material
Funding agencies:
The HABC study was supported by the National Institute on Aging (NIA) contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Relevant conflicts of interest/financial disclosures: Nothing to report.
References
- 1.Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc 2000;48(4):426–430. [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB. Prodromal Parkinson disease: do we miss the signs? Nat Rev Neurol 2019;15(8):437–438. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004;363(9413):978–988. [DOI] [PubMed] [Google Scholar]
- 4.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the health ABC study. J Gerontol A Biol Sci Med Sci 2001;56(10):M644–M649. [DOI] [PubMed] [Google Scholar]
- 5.Tian Q, Glynn NW, Ehrenkranz RC, Sprague BN, Rosso AL, Rosano C. Perception of energy and objective measures of physical activity in older adults. J Am Geriatr Soc 2020;68(8):1876–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Shrestha S, Huang X, et al. Olfaction and incident parkinson disease in US white and black older adults. Neurology. 2017;89(14):1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AM, Depp C, Ryan BJ, et al. Mitochondrial dysfunction and increased glycolysis in prodromal and early parkinson’s blood cells. Mov Disord 2018;33(10):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


