Abstract
For people living with HIV (PLWH), patient transfers may affect engagement in care. We followed a cohort of PLWH in Cape Town, South Africa who tested positive for HIV in 2012–2013 from ART initiation in 2012–2016 through December 2016. Patient transfers were defined as moving from one healthcare facility to another on a different day, considering all healthcare visits and recorded HIV-visits only. We estimated incidence rates (IR) for transfers by time since ART initiation, overall and by gender, and associations between transfers and gaps of > 180 days in clinical care. Overall, 4,176 PLWH were followed for a median of 32 months, and 8% (HIV visits)—17% (all healthcare visits) of visits were patient transfers. Including all healthcare visits, transfers were highest through 3 months on ART (IR 20.2 transfers per 100 visits, 95% CI 19.2–21.2), but increased through 36 months on ART when only HIV visits were included (IR 9.7, 95% CI 8.8–10.8). Overall, women were more likely to transfer than men, and transfers were associated with gaps in care (IR ratio [IRR] 3.06 95% CI 2.83–3.32; HIV visits only). In this cohort, patient transfers were frequent, more common among women, and associated with gaps in care.
Keywords: HIV, ART, Patient transfer, retention in HIV care, Clinic transfer
Introduction
Achieving UNAIDS’ 90–90–90 goals to end the HIV epidemic will require sustained engagement in HIV care and ongoing viral suppression among people living with HIV (PLWH) [1]. As universal access to ART scales up globally and PLWH engage in lifetime care and treatment, how and where PLWH access care and treatment is becoming increasingly important. Over time, PLWH may choose to transfer their HIV care to different care facilities for a variety of reasons, including migration, stigma, changes in their health status, employment, or residence [2–4]. Patient transfers may increase the likelihood of gaps in ART treatment access or disengagement from HIV care [5]. However, few data exist quantifying the frequency of patient transfers over time and examining their impact on clinical care outcomes in large cohorts of PLWH on ART.
Among PLWH, there is reason to hypothesize that the frequency and impact of patient transfers may differ by gender. For example, among women, pregnancy and the postpartum period are well-documented to be high-risk periods for disengagement from HIV care [5–8]. In South Africa, high rates of postpartum mobility and patient transfers have been reported as critical challenges to sustained retention in HIV care through the perinatal period [9–11]. However, relative to women outside of perinatal period, men living with HIV are more likely to initiate ART with more advanced disease, to be lost to follow up (LTFU) from HIV care, and to have higher rates of mortality [12–17]. In order to understand how patient transfers influence sustained engagement in HIV care, differences by gender need to be taken into account.
The goal of the present analysis is to characterize patient transfers over time in a large cohort of PLWH who initiated ART between 2012 and 2016 in Cape Town, South Africa. In addition, we examine whether patient transfers are associated with gaps in clinical care of six months or more. We describe trends in patient transfers and associations with gaps in clinical care among all patients and stratified by gender.
Methods
Study Setting and Data Source
Data come from the Western Cape Province of South Africa. The overall prevalence of HIV in the Western Cape is 6.6%, but considerable variation exists in the region [18, 19]. Over time, eligibility for ART in South Africa has changed. Between 2012 and 2015, PLWH in South Africa with a CD4 count < 350 cells/mm3 were eligible to initiate lifelong ART [20]. In 2015, South Africa adopted universal ART for all pregnant and breastfeeding women, known as Option B + , and in 2016 extended universal ART access to all PLWH, regardless of CD4 count or clinical stage [21, 22]. At ART initiation, PLWH are typically registered in a paper-based or electronic medical record system. PLWH are expected to receive follow-up care at the clinic where they initiate ART or, once they are stable on ART, through decentralized adherence clubs facilitated by community health workers linked to a clinic [23].
The Western Cape Provincial Health Data Centre (PHDC) links clinical, pharmacy, laboratory, and available vital status data from all public-sector healthcare facilities within the province using unique patient identifiers, as part of ongoing efforts to support patient engagement in HIV care [24, 25]. Linked data were used to identify a cohort of PLWH in a sub-district of the Western Cape who were at least 15 years of age at their first positive HIV test between 2012 and 2013, received a CD4 cell count measure at enrollment, and had a civil identification number. Data on cohort participants were available through December 2016. Fifteen health facilities within the sub-district provide HIV testing, and several non-governmental organizations promote and perform testing, referring those who test positive for HIV to public clinics for follow-up care. Information on all healthcare visits (not just HIV visits) at public clinics throughout the Western Cape Province was linked with clinical and demographic data for all cohort participants by the Western Cape Department of Health. The primary purpose of this original cohort was to examine associations between gender, HIV testing, and subsequent HIV service uptake and outcomes [17]. South Africa has a national death registry; however, data for cohort members were not authorized for linkage to the national death registry. Ethical approval and a waiver of informed consent for the use of de-identified routine data were provided by the University of Cape Town (Protocol number 320/2015).
Study Population
Participants from the original cohort of those testing positive in 2012 and 2013 were included in the present analysis if they initiated ART between 2012 and 2016 (n = 4184) and had a healthcare visit after ART initiation (n = 4176; Fig. 1). Participants were followed from ART initiation through their last recorded healthcare encounter prior to death or administrative censoring on December 21, 2016.
Fig. 1.
Study Population of adults living with HIV initiating antiretroviral therapy (ART) in a sub-district of Cape Town, South Africa 2012–2016
Measures
A patient transfer was defined as any switch from one health facility to another that did not occur on the same day. When a participant visited multiple health facilities on the same day, we used the visit at the most frequented facility over the follow-up period in order to identify patient transfers between the preceding or subsequent healthcare encounters. Multiple transfers between the same two facilities were counted individually. Hospital admission records were excluded, since it is unlikely for ART to be given at this type of healthcare visit. Healthcare visits were categorized by encounter type; however, when participants attend a health facility for more than one reason (e.g. for prenatal care and ART), administrative systems do not always capture the visit as related to HIV care, leading to an undercount of HIV-related visits. Thus, to provide a range of estimates, we defined patient transfers in two ways, first including all available healthcare visit information (e.g. “all visits analysis”) and second including only recorded HIV visits (e.g. “recorded HIV visits analysis”), defined as encounters related to “ART visits,” “dispensing visits,” or a CD4 count or viral load lab test. Our data did not contain information on the reason for transfer, thus we were unable to distinguish between “official” transfers (e.g. when the original facility was aware of the transfer) and “silent” transfers (e.g. when the facility was not aware of the transfer).
In South Africa, PLWH typically receive a three- to six-month supply of ART at each visit. Therefore, we defined a gap in clinical care as no healthcare visits for greater than 180 days (6 months). Covariate information available in our data was limited to gender, age at ART initiation, year of ART initiation (2015 and 2016 were collapsed into one category for statistical models due to the small number who initiated ART in 2016 in this cohort), and CD4 count at enrollment, defined as a CD4 count within three months of first testing positive for HIV during the study period.
Statistical Analysis
The goal of the analysis was to describe patient transfers over time in a large cohort of PLWH on ART and examine whether visits with a patient transfer were associated with gaps in clinical care of > 180 days. All relationships were examined overall and stratified by gender. First, we quantified patient transfers among all healthcare visits and among recorded HIV visits only. Patient transfers were quantified among visits (all healthcare or HIV only) as well as among individual participants. Next, we used Poisson regression to estimate crude incidence rates (IR) for patient transfers by time since ART initiation and by ART treatment era (2012–2014 CD4 count < 350, 2015 Option B + , 2016 universal ART), overall and by gender for all visits and for recorded HIV visits only. Incidence rate ratios (IRR) were estimated to compare the rate of patient transfers between men and women by time since ART initiation and ART treatment era. Finally, to investigate the association between all healthcare visits or only recorded HIV visits with a patient transfer and a gap in clinical care of > 180 days, we used Poisson models with generalized estimating equations to estimate IR ratios (IRR) and to account for the correlation between multiple visits per participant. IRR were estimated overall and by gender and adjusted for year of ART initiation, age, and gender (non-stratified estimates only). Gaps in clinical care were defined using only person-time when a participant was in care (e.g. had a recorded healthcare or HIV visit) and prior to recorded mortality dates for participants who died. All analyses were performed using Stata 13 (StataCorp, College Station, TX).
Results
We included 4,176 adults with at least one follow-up visit who initiated ART between 2012 and 2016 in a sub-district of the Western Cape (Fig. 1). When analyses were restricted to recorded HIV visits only, 3,821 participants had data available and were included. The majority of the study population were women (67.7%; Table 1). Nearly three-quarters of participants were between 20 and 39 years of age at the time of their HIV positive test in 2012 or 2013. Nearly all (99%) participants initiated ART prior to the availability of universal ART in South Africa. Over half the cohort (55%) had a CD4 count ≤ 350 cells/mm3 when they tested positive for HIV.
Table 1.
Demographic and clinical characteristics of HIV-infected adults who initiated ART at their first test positive for HIV between 2012 and 2013 in Cape Town, South Africa, overall and by patient transfer status
| Characteristic at first HIV positive test | Never transferred | Transferred ≥ 1 time | All ART-initiators |
|---|---|---|---|
| 1446 (34.6) | 2730 (65.4) | N = 4176 | |
| N (%) | N (%) | N (%) | |
| Gender | |||
| Female | 849 (58.7) | 1,979 (72.5) | 2828 (67.7) |
| Male | 597 (41.3) | 751 (27.5) | 1348 (32.3) |
| Age, years | |||
| 15–19 | 21 (1.5) | 69 (2.6) | 90 (2.2) |
| 20–29 | 401 (28.0) | 1059 (39.7) | 1460 (35.6) |
| 30–39 | 583 (40.7) | 980 (36.7) | 1563 (38.1) |
| 40–49 | 293 (20.5) | 371 (13.9) | 664 (16.2) |
| 50–59 | 101 (7.0) | 161 (6.0) | 262 (6.4) |
| 60 + | 33 (2.3) | 29 (1.1) | 62 (1.5) |
| Year, first HIV + test | |||
| 2012 | 708 (49.0) | 1410 (51.7) | 2118 (50.7) |
| 2013 | 737 (51.0) | 1318 (48.3) | 2055 (49.2) |
| 2014 | 1 (0.07) | 0 (0.0) | 1 (0.02) |
| 2015 | 0 (0.0) | 2 (0.07) | 2 (0.05) |
| Year, ART initiation | |||
| 2012 | 468 (32.4) | 843 (30.9) | 1311 (31.4) |
| 2013 | 549 (38.0) | 1222 (44.8) | 1771 (42.4) |
| 2014 | 182 (12.6) | 379 (13.9) | 561 (13.4) |
| 2015 | 210 (14.5) | 267 (9.8) | 477 (11.4) |
| 2016 | 37 (2.5) | 19 (0.7) | 56 (1.3) |
| CD4 counta | |||
| ≤ 200 | 385 (29.3) | 720 (29.4) | 1105 (29.4) |
| > 200–350 | 343 (26.1) | 609 (24.9) | 952 (25.3) |
| > 350–500 | 249 (19.0) | 478 (19.5) | 727 (19.3) |
| > 500 | 337 (25.6) | 639 (26.1) | 976 (26.0) |
Defined as within 3 months of first testing positive for HIV during the study period. Missing data among all participants: age n = 258 (3%), CD4 count n = 856 (10%); missing data among ART initiators: age n = 75 (2%), CD4 count n = 416 (10%)
Among 83,060 total healthcare visits, 17% (n = 13,819) met the definition of a patient transfer (18% for women vs. 14% for men). When analyses were restricted to HIV visits only, among 38,436 recorded HIV visits, 8% met the definition of a patient transfer (8% for women vs. 6% for men). When all healthcare visits were included, participants transferred a median of two times (range 0–48) during follow-up and had a median of 17 days (IQR 6, 39) between a transfer and a subsequent healthcare visit. When only recorded HIV visits were included, participants transferred a median of 0 times (range 0–26) and had a median of 34 days (IQR 13, 120) between HIV visits. Over a median of 32 months (IQR 14–40) of follow-up on ART, 65% of participants transferred healthcare facilities at least once, including 70% of women and 56% of men. When HIV visits only were included, 31% of participants transferred at least once (34% of women and 26% of men) (Table 2).
Table 2.
Patient transfers among adult living with HIV who initiated antiretroviral therapy in Cape Town, South Africa
| All visits |
Recorded HIV visits only |
|||||
|---|---|---|---|---|---|---|
| Overall N = 4176 | Women N = 2828 | Men N = 1348 | Overall N = 3821 | Women N = 2620 | Men N = 1201 | |
| Number of healthcare visits | 83,060 | 55,418 | 22,642 | 38,436 | 26,542 | 12,894 |
| Number of visits where a transfer occurred | 13,819 | 9995 | 3824 | 2987 | 2151 | 836 |
| % of visits where a transfer occurred | 17% | 18% | 14% | 8% | 8% | 6% |
| Number of patient transfers per person | ||||||
| Median IQR | 2 (0,4) | 2 (0,5) | 1 (0,4) | 0 (0,1) | 0 (0,1) | 0 (0,1) |
| Range | 0–48 | 0–32 | 0–48 | 0–26 | 0–26 | 0–24 |
| % who transferred at least once | 65% | 70% | 56% | 31% | 34% | 26% |
| Days between most recent clinic visit and transfer | ||||||
| Median IQR | 17 (6, 39) | 18 (7, 41) | 14 (6, 33) | 34 (13, 120) | 35 (14, 128) | 29 (11, 112) |
| Range | 1–1411 | 1–1411 | 1–1370 | 1–1415 | 1–1411 | 1–1415 |
| Days between transfer and next clinic visit | ||||||
| Median IQR | 18 (7, 38) | 20 (7, 41) | 15 (5, 31) | 28 (14, 59) | 29 (15, 59) | 28 (11, 58) |
| Range | 1–1411 | 1–1411 | 1–1187 | 1–1411 | 1–1411 | 1–888 |
When all healthcare visits were included, the rate of patient transfers was highest through the first three months on ART (IR 20.2 transfers per 100 visits, 95% CI 19.2, 21.2) and declined as time on ART increased (IR 15.7 transfers per 100 visits through 36 months on ART, 95% CI 15.1, 16.4; Fig. 2a). Within three months of starting ART, women had 1.22 times the rate of patient transfers compared to men. As time on ART increased, women were increasingly more likely to transfer than men (IRR 1.52, 95% CI 1.33, 1.72 through 36 months after ART initiation; Fig. 2b).
Fig. 2.
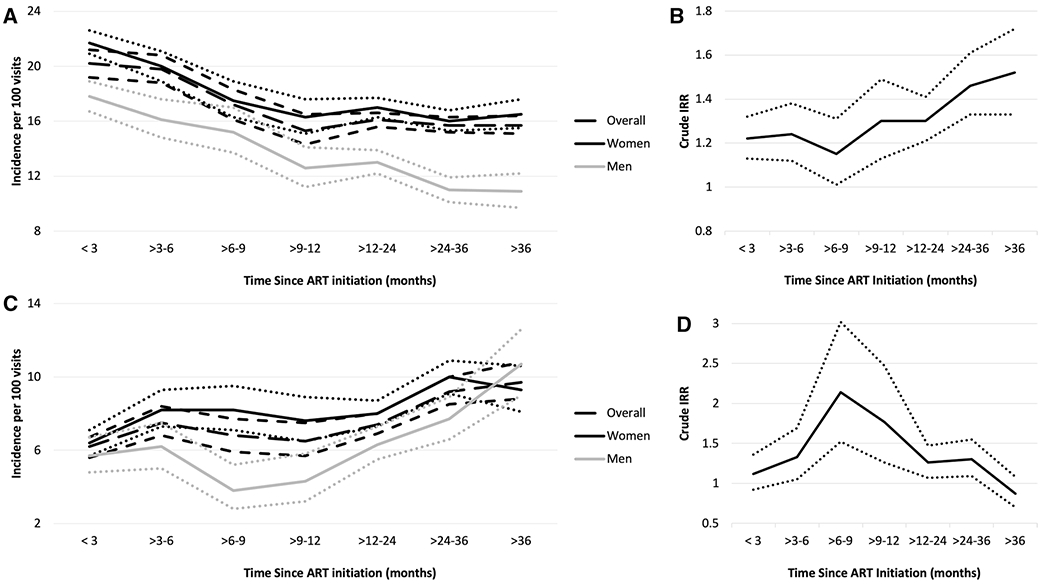
Panels A and C: Crude incidence rate1,3 of patient transfers by time from ART initiation, overall and by gender. Panels B and D: Crude IRR and 95% CIs2,4 of patient transfers comparing women to men. Panels A and B include all visits, whereas panels C and D include recorded HIV visits only. Dotted or short dashed lines represent 95% CIs. 1 Number of patient transfers over number of healthcare visits by time from ART initiation (all visits): < 3 months: 1,478/7,329; > 3–6 months: 1,446/7,316; > 6–9 months: 919/5,355; > 9–12 months: 770/5,022; > 12–24 months: 3,432/21,323 > 24–36 months: 3,296/20,976; > 36 months: 2,478/15,739. 2 Crude IRR, women vs. men (95% CI) by time from ART initiation (all visits): < 3 months: 1.22 (1.13, 1.32); > 3–6 months: 1.24 (1.12, 1.38); > 6–9 months: 1.15 (1.01, 1.31); > 9–12 months: 1.30 (1.13, 1.49); > 12–24 months: 1.30 (1.21, 1.41); > 24–36 months: 1.46 (1.33, 1.61); > 36 months: 1.52 (1.33, 1.72). 3 Number of patient transfers over number of healthcare visits by time from ART initiation (recorded HIV visits only): < 3 months: 495/8,045; > 3–6 months: 355/4,706; > 6–9 months: 213/3,154; > 9–12 months: 206/3,148; > 12–24 months: 736/9,930; > 24–36 months: 628/6,821; > 36 months: 354/3,632. 4 Crude IRR, women vs. men (95% CI) by time from ART initiation (recorded HIV visits only): < 3 months: 1.12 (0.92, 1.36); > 3–6 months: 1.33 (1.05, 1.69); > 6–9 months: 2.14 (1.52, 3.02); > 9–12 months: 1.77 (1.26, 2.47); > 12–24 months: 1.26 (1.07, 1.47); > 24–36 months: 1.30 (1.09, 1.55); > 36 months: 0.87 (0.70, 1.08)
When analyses were restricted to recorded HIV visits only, the overall rate of patient transfers was lower than when all healthcare visits were included, but tended to increase with time on ART. For example, patient transfers increased through three to six months on ART (IR 7.5 transfers per 100 visits, 95% CI 6.8, 8.4), declined slightly through 12 to 24 months on ART, and peaked at 36 months after ART initiation (IR 9.7 per 100 visits, 95% CI 8.8, 10.8;Fig. 2c). In general, women remained more likely to transfer than men (Fig. 2d).
When all healthcare visits were included, the rate of patient transfers was relatively consistent across ART treatment eras (Fig. 3a). Women were more likely to transfer, including under Option B + (IRR 1.29, 95% CI 1.21, 1.37) and universal ART treatment (IRR 1.43, 95% CI 1.32, 1.55; Fig. 3b). When analyses were restricted to recorded HIV visits only, the rate of patient transfers increased in more recent ART treatment eras, although limited data was available from the universal test and treat era, leading to wide confidence intervals (Fig. 3c). Women remained more likely to transfer across all ART treatment eras, relative to men, although effect estimates were attenuated relative to when all healthcare visits were included (Fig. 3d).
Fig. 3.
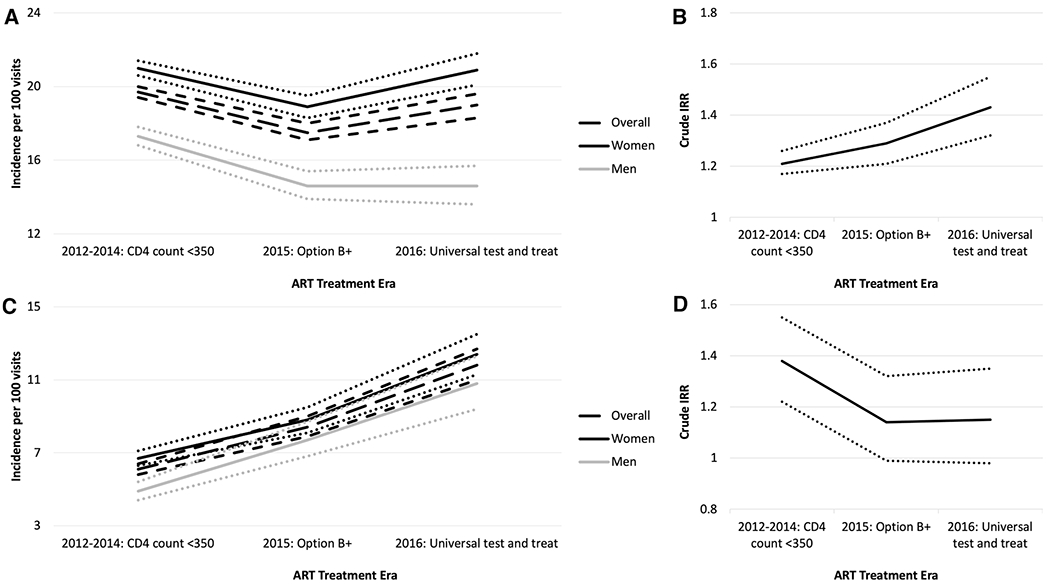
Panels A and C: Crude incidence rate1,3 of patient transfers by ART treatment area, overall and by gender. Panels B and D: Crude IRR and 95% CIs2,4 comparing women to men by ART treatment era. Panels A and B include all visits, whereas panels C and D include recorded HIV visits only. Dotted or short dashed lines represent 95% CIs. 1 Overall number of patient transfers over number of healthcare visits by ART treatment era (all visits): 2012–2014: 15,975/81,169; 2015: 5,217/29,775; 2016: 3,224/16,990. 2 Crude IRR, women vs. men (95% CI) by ART treatment era (all visits): 2012–2014: 1.21 (1.17, 1.26); 2015: 1.29 (1.21, 1.37); 2016: 1.43 (1.32, 1.55). 3 Overall number of patient transfers over number of healthcare visits by ART treatment era (recorded HIV visits only): 2012–2014: 1,383/22,783; 2015: 909/10,777; 2016: 695/5,876. 4 Crude IRR, women vs. men (95% CI) by ART treatment era (recorded HIV visits only): 2012–2014: 1.38 (1.22, 1.55); 2015: 1.14 (0.99, 1.32); 2016: 1.15 (0.98, 1.35)
Among all participants and all healthcare visits, 37% had at least one gap in care of > 180 days during the follow-up period (Table 3). The proportion of participants with a gap in clinical care increased to 51% when analyses were restricted to recorded HIV visits only. Gaps in clinical care did not vary meaningfully by gender (38% among women vs. 36% among men for all visits and 52% among women vs. 50% among for recorded HIV visits only). Approximately three-quarters of participants with at least one gap returned to care, regardless of whether all visits or only HIV visits were included. Among all return visits after a gap in care (n = 1499), 77% (n = 1155; 89% among HIV visits only) were to the same clinic attended prior to the gap, whereas 23% (11% among HIV visits only) were to a clinic different from the one previously visited, with no meaningful differences by gender.
Table 3.
Gaps in care > 180 days among adult living with HIV who initiated ART in Cape Town, South Africa, including all healthcare visits and recorded HIV visits only
| All visits |
Recorded HIV visits only |
|||||
|---|---|---|---|---|---|---|
| Overall N = 4176 | Women N = 2838 | Men N = 1348 | Overall N = 3821 | Women N = 2620 | Men N = 1201 | |
| Participants with at least one treatment gap, n (%) | 1560 (37%) | 1081 (38%) | 479 (36%) | 1966 (51%) | 1366 (52%) | 600 (50%) |
| Among participants with at least one treatment gap | ||||||
| Median number of treatment gaps (IQR) | 1 (1,1) | 1 (1,1) | 1 (1,1) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Range | 1–4 | 1–4 | 1–4 | 1–4 | 1–4 | 1–5 |
| Number (%) who return at least once | 1180 (76%) | 812 (75%) | 368 (77%) | 1409 (72%) | 978 (72%) | 431 (72%) |
| Total number of returns after a treatment gap | 1499 | 1038 | 461 | 1938 | 1,344 | 594 |
| To the same clinic | 1155 (77%) | 783 (75%) | 372 (81%) | 1725 (89%) | 1,190 (89%) | 535 (90%) |
| To a different clinic | 344 (23%) | 255 (25%) | 89 (19%) | 213 (11%) | 154 (11%) | 59 (10%) |
Among all healthcare visits (n = 83,060), 2% (n = 1984) met the definition for a gap in care of > 180 days. Among visits when a patient transfer occurred, 5% of visits had a gap in care of > 180 days, compared to 2% of visits without a patient transfer. On average, at visits where a patient transfer occurred, participants were nearly three times as likely to have a gap in care of > 180 days (IRR 2.88; 95% CI 2.63, 3.16; (Table 4). Effect estimates for the association between patient transfers and gaps in care of > 180 days were stronger when analyses were restricted to recorded HIV visits only (IRR 3.06, 95% CI 2.83, 3.32). Regardless of whether all healthcare visits or only HIV visits were included, associations between patient transfers and gaps in care did not vary meaningfully by gender (All visits: IRR 2.90; 95% CI 2.61, 3.24 among women vs. IRR 2.82; 95% CI 2.36, 3.37 among men; HIV visits only: IRR 3.07; 95% CI 2.80, 3.37 among women vs. IRR 3.00; 95% CI 2.55, 3.52 among men).
Table 4.
Associations between healthcare visits with a patient transfer and a gap in clinical care of > 180 days among adults living with HIV who initiated ART, overall and by gender
| All visits |
Recorded HIV visits only |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Overall adjusted IRR (95% CI)a | Women adjusted IRR (95% CI)a | Men adjusted IRR (95% CI)a | Overall adjusted IRR (95% CI)a | Women adjusted IRR (95% CI)a | Men adjusted IRR (95% CI)a |
| Healthcare visits | ||||||
| Among visits with no patient transfer | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Among visits with a patient transfer | 2.88 (2.63, 3.16) | 2.90 (2.61, 3.24) | 2.82 (2.36, 3.37) | 3.06 (2.83, 3.32) | 3.07 (2.80, 3.37) | 3.00 (2.55, 3.52) |
| Year of ART initiation | ||||||
| 2012 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2013 | 0.83 (0.75, 0.93) | 0.85 (0.74, 0.97) | 0.78 (0.65, 0.95) | 0.85 (0.77, 0.94) | 0.88 (0.78 0.98) | 0.79 (0.66, 0.94) |
| 2014 | 0.61 (0.52, 0.73) | 0.65 (0.52, 0.79) | 0.56 (0.42, 0.74) | 0.82 (0.72, 0.94) | 0.82 (0.70, 0.98) | 0.82 (0.65, 1.04) |
| 2015 or 2016 | 0.40 (0.31, 0.51) | 0.40 (0.31, 0.53) | 0.40 (0.24, 0.66) | 0.45 (0.37, 0.55) | 0.44 (0.35, 0.54) | 0.51 (0.36, 0.73) |
| Age group, years | ||||||
| 20–29 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 16–19 | 0.52 (0.29, 0.95) | 0.51 (0.27, 0.95) | 0.67 (0.11, 4.20) | 0.31 (0.14, 0.68) | 0.24 (0.10, 0.62) | 1.69 (0.91, 3.12) |
| 30–39 | 0.91 (0.81, 1.03) | 0.90 (0.79, 1.03) | 1.06 (0.79, 1.41) | 1.29 (1.16, 1.44) | 1.31 (1.17, 1.46) | 1.27 (0.94, 1.72) |
| 40–49 | 0.87 (0.75, 1.01) | 0.81 (0.67, 0.98) | 1.07 (0.80, 1.45) | 1.53 (1.35, 1.74) | 1.51 (1.30, 1.76) | 1.56 (1.17, 2.15) |
| 50–59 | 0.71 (0.58, 0.87) | 0.65 (0.50, 0.85) | 0.89 (0.62, 1.28) | 1.30 (1.09, 1.55) | 1.23 (0.98, 1.55) | 1.41 (0.99, 2.00) |
| 60 + | 0.41 (0.24, 0.69) | 0.33 (0.16, 0.67) | 0.57 (0.27, 1.22) | 1.44 (1.06, 1.94) | 1.17 (0.76, 1.82) | 1.79 (1.13, 2.83) |
| Gender | ||||||
| Female | 1.00 | – | – | 1.00 | – | – |
| Male | 0.96 (0.86, 1.07) | – | – | 0.83 (0.76, 0.92) | – | – |
IRR incidence rate ratio
Adjusted for: age (modeled as a categorical variable), year of ART initiation, and gender (for overall estimate only)
Discussion
In a large HIV treatment cohort in South Africa, between one- and two-thirds of participants had at least one patient transfer over a median of nearly three years of follow-up, depending on whether all healthcare visits or only recorded HIV visits were included. When all healthcare visits were considered, patient transfers were more frequent than when only recorded HIV visits were considered and were highest at the time of ART initiation. When only recorded HIV visits were included, patient transfers tended to increase with time on ART. Regardless of whether all visits or recorded HIV visits were considered, patient transfers were typically more common among women than men. When all healthcare visits were considered, over a third of all participants experienced a gap in clinical care of over 180 days during the follow-up period, and this increased to over half when only recorded HIV visits were included. In both analyses, gaps in clinical care did not vary meaningfully by gender. Across analyses and, among all patients with a gap in care, over three quarters or more returned to care during the follow-up period. Visits in which patient transfers occurred were strongly associated with an increased likelihood of a gap in clinical care, for both women and men, regardless of whether all healthcare visits or recorded HIV visits were considered.
As access to universal ART scales up, PLWH are increasingly being asked to engage in lifelong HIV care in order to remain on ART and virally suppressed. To maintain sustained engagement in HIV care, PLWH need to be able to access HIV services at a healthcare facility that is convenient and accessible to them [26]. However, migration, patient preferences, stigma, poor treatment by staff, and health status may all play a role in patients choosing to transfer to a new facility [2, 3]. Our findings demonstrates that patient transfers between healthcare facilities among PLWH are common–in less than three years of follow-up, 31 to 65% of PLWH in this cohort transferred at least one time, with a median of two transfers among all participants when all healthcare visits were included. Estimates of patient transfers in our analysis include both formal and informal transfers for all healthcare visits and thus are higher than estimates from studies examining formal transfers for HIV care only [27–29]. In a separate study of PLWH engaged in care between 2002 and 2009, over a median follow-up of 2.5 years, only 13.2% of patients transferred formally, with the proportion of transfers increasing with more recent calendar year of enrollment.[29] Including both formal and informal transfers for all healthcare visits may provide a more comprehensive picture of patient transfers over time for PLWH.
The time around ART initiation is well-documented to be a high-risk period for disengagement from HIV care [30–38]. Our findings including all healthcare visits indicated a higher rate of patient transfers close to the time of ART initiation and may suggest that participants initiating ART may also be at high-risk for transferring to a different facility. Patient transfers may be particularly high around the time of ART initiation among persons who have previously initiated ART, those seeking care further from their home to minimize potential stigma, those with transportation barriers, and among women transitioning to receiving ART outside of antenatal services postpartum [10, 29, 39–41]. When analyses were restricted to recorded HIV visits only, patient transfers increased slightly through three to six months on ART, again possibly indicating a high-risk period for patient transfers, and were highest through 36 months on ART. The high rate of patient transfers with more time on ART may reflect the increased likelihood of transferring over time (not just around the time of ART initiation) for persons living with HIV. Unfortunately, we have limited covariate information on PLWH in this cohort to examine differences in patient transfers by patient characteristics, including transitioning to ART care postpartum. However, in general, women were more likely than men to transfer through 36 months after ART initiation, regardless of whether all healthcare visits or only HIV visits were considered.
In this cohort, trends in patient transfers overall and by gender did not differ meaningfully across different ART treatment eras. However, very few PLWH in this cohort initiated ART in 2015 or 2016, when universal ART was being scaled up in South Africa [21, 22]. Thus, additional data on patient transfers from the era of universal ART is needed to understand how access to lifelong ART for all PLWH influences patient transfers over time.
Our findings suggest that patient transfers may be an important contributor to gaps in clinical care among PLWH on ART. In this cohort, 30–50% of all participants experienced at least one gap of over 180 days (6 months) in clinical care, with gaps in care being more likely when only HIV visits were considered. Three quarters of participants with a gap in care returned to care, with most returning to the clinic they visited prior to the gap in care. However, on average, visits where patient transfers occurred were associated with an approximately threefold increase in the likelihood of a gap in clinical care for both men and women in analyses including all healthcare visits and HIV visits only. These findings suggest that patient transfers may be a useful indicator of PLWH at high risk for disengagement from clinical care or gaps in ART access.
Our findings have a number of important strengths and limitations. Strengths include using comprehensive, linked electronic medical record data to quantify patient transfers by time from ART initiation and across changes in ART treatment guidelines. This analysis is also among the first to quantify patient transfers, including both formal and informal transfers, over time in a large cohort of PLWH and to examine whether visits with a patient transfer correlate with gaps in clinical care, both among all healthcare visits and recorded HIV visits only. Unfortunately, in these data we were unable to examine whether gaps in clinical care correlated with a higher risk of unsuppressed viral load or mortality in PLWH. We acknowledge as a limitation that analyses including all healthcare visits likely overestimate patient transfers and that analyses restricted to recorded HIV visits may underestimate patient transfers (due to not all HIV-related visits being indicated as such in administrative records). By including estimates including all healthcare visits and restricted to recorded HIV visits only, our analyses provide a range of plausible estimates for patient transfers over time and their association with gaps in clinical care. Additional limitations include the inability to stratify findings by official versus silent transfers or to identify indicated transfers, such as transfers among women from an antenatal clinical to an HIV clinic for ART services postpartum. We also had limited covariate information to control for potential confounders; however, our goal was not to estimate causal effects, but rather to describe patient transfers over time and examine if patient transfers are a useful indicator of gaps in clinical care. Given that we included both silent and official patient transfers across all healthcare visits, our estimates of patient transfers should be considered an upper limit. In addition, our data were not linked to the national population registry in South Africa and thus underestimate mortality. However, when estimating patient transfers and gaps in clinical care, we only examined time periods when participants were in care and thus alive. Finally, while PLWH typically receive a three to 6-month supply of ART, in some instances ART may be dispensed for longer, and we were unable to account for this in our analysis.
Conclusions
In this cohort of PLWH on ART in South Africa, one-third to two-thirds of participants transferred healthcare facilities at least once over a median of approximately three years of follow-up when only HIV visits and all healthcare visits were considered. In general, patient transfers were more common among women and around the time of ART initiation when all healthcare visits were considered, but tended to increase with time on ART in analyses restricted to HIV visits only. Throughout the follow-up period and across analyses including all or HIV-only visits, over a third of participants experienced a gap in clinical care of more than 180 days, and three-quarters of participants with a gap returned to care. Visits in which a transfer occurred were strongly associated with having a gap in clinical care, suggesting that patient transfers may be a useful clinical indicator of PLWH at high-risk for disengagement. These findings demonstrate that patient transfers among PLWH are common and may influence continuity in HIV care. More flexible strategies that accommodate patients moving between facilities, including digital health passports and strategies to make it easier to access care across a range of clinics, are urgently needed to improve continuity in HIV care and treatment outcomes for PLWH.
Acknowledgements
We thank and acknowledge the Provincial Health Data Centre of the Western Cape Department of Health, and Mariette Smith in particular, for the data linkage, preparation and anonymization for the parent study, and Andrew Boulle for assistance in understanding and interpreting the various data sources. We thank the South African Medical Research Council, the National Institute of Mental Health (R01MH106600, D43 TW011308 and R00MH112413), the Fogarty International Center (D43 TW011308) and the National Institute of Child Health and Development (1R24HD077976) for grant support. The content of this paper is solely the responsibility of the author and does not necessarily represent the official views of the US National Institutes of Health or the South African Medical Research Council.
Funding
This work was supported by grants awarded jointly by the National Institute of Mental Health and the South African Medical Research Council (grant number R01 MH106600), the National Institute of Mental Health (R00MH112413), the Fogarty International Center and the National Institute of Mental Health (D43 TW011308) and the National Institute of Child Health and Development (1R24HD077976).
Footnotes
Conferences: A portion of this was presented at the International AIDS Society Meeting, 22 July 2019 in Mexico City, Mexico.
Conflict of interest The author declares that they have no conflict of interest.
Data Availability
Data for the present analysis come from the Provincial Health Data Centre of the Western Cape Department of Health and cannot be passed on to third parties without prior approval from the Provincial Health Data Center (https://www.westerncape.gov.za/general-publication/provincial-health-data-centre). Code for all analyses is available from Angela Bengtson (angela_bengtson@brown.edu).
References
- 1.UNAIDS. 90–90–90: Treatment for All 2018 [January 5, 2018]. http://www.unaids.org/en/resources/909090.
- 2.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS. 2015;10(6):430–8. [DOI] [PubMed] [Google Scholar]
- 3.Fox MP, Bor J, Brennan AT, MacLeod WB, Maskew M, Stevens WS, et al. Estimating retention in HIV care accounting for patient transfers: a national laboratory cohort study in South Africa. PLoS Med. 2018;15(6):e1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengtson AM, Go V, Kumwenda W, Lurie M, Kutengule A, Owino M, et al. “A way of escaping”: a qualitative study exploring reasons for clinic transferring and its impact on engagement in care among women in Option B. AIDS Care. 2020;32(1):72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambia J, Kabudula C, Risher K, Xavier Gómez-Olivé F, Rice BD, Etoori D, et al. Outcomes of patients lost to follow-up after antiretroviral therapy initiation in rural north-eastern South Africa. Trop Med Int Health. 2019;24(6):747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etoori D, Gomez-Olive FX, Reniers G, Rice B, Renju J, Kabudula CW, et al. Outcomes after being lost to follow-up differ for pregnant and postpartum women when compared to the general HIV treatment population in rural South Africa. J Acquir Immune Defic Syndr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV. 2016;3(4):e175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. Aids. 2014;28(4):589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clouse K, Fox MP, Mongwenyana C, Motlhatlhedi M, Buthelezi S, Bokaba D, et al. “I will leave the baby with my mother”: Long-distance travel and follow-up care among HIV-positive pregnant and postpartum women in South Africa. J Int AIDS Soc. 2018;21(Suppl 4):e25121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips TK, Clouse K, Zerbe A, Orrell C, Abrams EJ, Myer L. Linkage to care, mobility and retention of HIV-positive postpartum women in antiretroviral therapy services in South Africa. J Int AIDS Soc. 2018;21(Suppl 4):e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tweya H, Gugsa S, Hosseinipour M, Speight C, Ng’ambi W, Bokosi M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe. Malawi Trop Med Int Health. 2014;19(11):1360–6. [DOI] [PubMed] [Google Scholar]
- 12.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkinson LM, Abwalaba RA, Arudo J, Barry M. Ten-year survival with analysis of gender difference, risk factors, and causes of death during 13 years of public antiretroviral therapy in rural Kenya. Medicine. 2020;99(21):e20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kranzer K, Lewis JJ, Ford N, Zeinecker J, Orrell C, Lawn SD, et al. Treatment interruption in a primary care antiretroviral therapy program in South Africa: cohort analysis of trends and risk factors. J Acquir Immune Defic Syndr. 2010;55(3):e17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88(9):681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osler M, Cornell M, Ford N, Hilderbrand K, Goemaere E, Boulle A. Population-wide differentials in HIV service access and outcomes in the Western Cape for men as compared to women, South Africa: 2008 to 2018: a cohort analysis. J Int AIDS Soc. 2020;23(Suppl 2):e25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurie MN, Kirwa K, Callaway J, Cornell M, Boulle A, Bengtson AM, et al. Quantifying the HIV treatment cascade in a South African health sub-district by gender: retrospective cohort study. Trop Med Int Health. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistics South Africa. Mid-Year Population Estimates: 2017. Pretoria, South Africa: Statistics South Africa; 2017. [Google Scholar]
- 19.Poolman M, Walt Nvd, Luwaca B. Annual Progress Report 2015/16. Western Cape, South Africa: Western Cape Providincial Council on AIDS. 2017. [Google Scholar]
- 20.Meintjes G, Maartens G, Boulle A, Conradie F, Goemaere E, Hefer E, et al. Guidelines for antiretroviral therapy in adults by the Southern African HIV clinicians society. South African J HIV Med. 2012;13(3):114–33. [Google Scholar]
- 21.Meintjes G, Black J, Conradie F, Dlamini S, Maartens G, Manzini TC, et al. Southern African HIV Clinicians Society adult antiretroviral therapy guidelines: Update on when to initiate antiretroviral therapy. South Afr J HIV Med. 2015;16(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Department of Health SA. National Consolidated Guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: Department of Health; 2015. [Google Scholar]
- 23.Wilkinson L, Harley B, Sharp J, Solomon S, Jacobs S, Cragg C, et al. Expansion of the adherence club model for stable antiretroviral therapy patients in the cape metro, South Africa 2011–2015. Trop Med Int Health. 2016;21(6):743–9. [DOI] [PubMed] [Google Scholar]
- 24.Beck EJ, Shields JM, Tanna G, Henning G, de Vega I, Andrews G, et al. Developing and implementing national health identifiers in resource limited countries: why, what, who, when and how? Global Health Action. 2018;11(1):1440782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulle A, Heekes A, Tiffin N, Smith M, Mutemaringa T, Zinyakatira N, et al. Data centre profile: the provincial health data centre of the western cape province, South Africa. Int J Popul Data Sci. 2019;4(2):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Consolidated guidelines on person-centred HIV patient monitoring and case surveillance Geneva, Switzerland: World Health Organization; 2017. http://apps.who.int/iris/bitstream/10665/255702/1/9789241512633-eng.pdf.. [Google Scholar]
- 27.Giuliano M, Liotta G, Andreotti M, Mancinelli S, Buonomo E, Scarcella P, et al. Retention, transfer out and loss to follow-up two years after delivery in a cohort of HIV+ pregnant women in Malawi. Int J STD AIDS. 2016;27(6):462–8. [DOI] [PubMed] [Google Scholar]
- 28.Hickey MD, Omollo D, Salmen CR, Mattah B, Blat C, Ouma GB, et al. Movement between facilities for HIV care among a mobile population in Kenya: transfer, loss to follow-up, and reengagement. AIDS Care. 2016;28(11):1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nglazi MD, Kaplan R, Orrell C, Myer L, Wood R, Bekker LG, et al. Increasing transfers-out from an antiretroviral treatment service in South Africa: patient characteristics and rates of virological non-suppression. PLoS ONE. 2013;8(3):e57907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeke CE, Nabitaka V, Rowan A, Guerra K, Kabbale A, Asire B, et al. Assessing linkage to and retention in care among HIV patients in Uganda and identifying opportunities for health systems strengthening: a descriptive study. BMC Infect Dis. 2018;18(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bor J, Fox MP, Rosen S, Venkataramani A, Tanser F, Pillay D, et al. Treatment eligibility and retention in clinical HIV care: a regression discontinuity study in South Africa. PLoS Med. 2017;14(11):e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung NC, Bolton-Moore C, Chilengi R, Kasaro MP, Stringer JS, Chi BH. Patient engagement in HIV care and treatment in Zambia, 2004–2014. Trop Med Int Health. 2017;22(3):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosset A, Protopopescu C, Larmarange J, Orne-Gliemann J, McGrath N, Pillay D, et al. Retention in care trajectories of HIV-Positive individuals participating in a universal test-and-treat program in Rural South Africa (ANRS 12249 TasP Trial). J Acquir Immune Defic Syndr. 2019;80(4):375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascoe SJ, Fox MP, Huber AN, Murphy J, Phokojoe M, Gorgens M, et al. Differentiated HIV care in South Africa: the effect of fast-track treatment initiation counselling on ART initiation and viral suppression as partial results of an impact evaluation on the impact of a package of services to improve HIV treatment adherence. J Int AIDS Soc. 2019;22(11):e25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens WS, Gous NM, MacLeod WB, Long LC, Variava E, Martinson NA, et al. Multidisciplinary Point-of-Care Testing in South African Primary Health Care Clinics Accelerates HIV ART Initiation but Does Not Alter Retention in Care. J Acquir Immune Defic Syndr. 2017;76(1):65–73. [DOI] [PubMed] [Google Scholar]
- 36.Ugoji C, Okere N, Dakum P, Ake-Uzoigwe R, Igboelina D, Ndembi N, et al. Correlates of patient retention in hiv care and treatment programs in Nigeria. Curr HIV Res. 2015;13(4):300–7. [DOI] [PubMed] [Google Scholar]
- 37.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8(7):e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas AD, Zaniewski E, Anderegg N, Ford N, Fox MP, Vinikoor M, et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellowski JA, Weber AZ, Phillips TK, Brittain K, Zerbe A, Abrams EJ, et al. “You must leave but I didn’t want to leave”: qualitative evaluation of the integration of ART into postnatal maternal and child health services in Cape Town. South Africa AIDS Care. 2020;32(4):480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in eastern africa: application of a sampling-based approach. Clin Infect Dis. 2016;62(7):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips T, McNairy ML, Zerbe A, Myer L, Abrams EJ. Implementation and operational research: postpartum transfer of care among HIV-infected women initiating antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2015;70(3):e102–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for the present analysis come from the Provincial Health Data Centre of the Western Cape Department of Health and cannot be passed on to third parties without prior approval from the Provincial Health Data Center (https://www.westerncape.gov.za/general-publication/provincial-health-data-centre). Code for all analyses is available from Angela Bengtson (angela_bengtson@brown.edu).


