Abstract
Background
Over million people have been infected with SARS-CoV-2 virus worldwide, with around 3% reported deaths till date. A few conventional antiviral treatments have been tried to mitigate the coronavirus. However, many alternative therapeutics are being evaluated worldwide. In the present study, we investigated traditional Indian medicinal compounds antiviral potencies as an effective drug for targeting SARS-CoV-2E. SARS-CoV-2 E protein plays a key role in coronavirus life cycle and is an interesting target for the development of anti-SARS-CoV-2 E drugs.
Methods
Molecular docking studies of medicinal compounds possessing wide range of pharmacological and antiviral activities against enveloped viruses were evaluated with the computer-aided drug design screening software; PyRx. Twelve medicinal compounds isolated from plants were screened and visualized on Biovia Discovery-Studio. Moreover, SARS-CoV-2 E protein's secondary structural insights were deciphered using Swiss Model and ProFunc web server.
Results
Glycyrrhizic acid, triterpene glycoside isolated from plants of Glycyrrhiza (licorice) showed interactions with envelope protein at chain A: Arg 61, chain B: Phe 23, chain B: Tyr 57, and chain C: Val 25. β- boswellic acid, an ayurvedic herb (pentacyclic terpenoid are produced by Boswellia) represented direct interactions and indirect binding with chain C. Their pharmacological aspects and drug-likeness properties were deduced by DruLiTo. Toxicological assessment, along with their ADME profiling, was validated using vNNADMET. The findings showed that ligands, β-boswellic acid, and glycyrrhizic acid possessed the best bindings, with the target having binding affinity (-9.1 kcal/mol) amongst compounds tested against SARS-CoV-2 E. In-vitro studies reveals the promising effect as potent SARS-CoV-2 E inhibitors. Functionality loss and structural disruptions with ∼90% were observed by UV-spectra and fluorescent based analyses.
Conclusion
The study demonstrated that β-boswellic acid, and glycyrrhizic acid are strong SARS-CoV-2 E protein inhibitors. In addition, the work linked GA antiviral activity to its effect on SARS-CoV- 2 E protein that can pave the way for designing antiviral therapeutics.
Keywords: Coronavirus, SARS-CoV-2 E, Envelope protein, Molecular docking, Indian medicinal plants, Inhibitors
Introduction
The disease produced by SARS-CoV-2 virus continued to rise, and on March 11, 2020, the World Health Organization declared COVID-19 as a pandemic (WHO, 2020). The novel Coronavirus is of zoonotic origin, mutated, and then inflicted humans with devastating outcomes (Collins, 2020, Mousavizadeh and Ghasemi, 2021). The structure of SARS-CoV-2 consists of spike (S), membrane (M), envelope (E), and nucleocapsid (N) four main structural proteins (A. Wu and Chiwaya, 2020). Moreover, there are three to four viral proteins in the coronavirus membrane (Mousavizadeh and Ghasemi, 2021). The SARS-CoV-2 E protein is a minor but essential component of the SARS-CoV-2 virus. It is a small integral membrane, pentameric (A-E chains) that forms the ion channel viroporin (Alam et al., 2020; Mandala et al., 2020; Sarkar and Saha, 2020). It is composed of 75–109 amino acid integral membrane protein, hydrophilic N-terminal domain (7–12 residues). It also has a long hydrophilic C-terminal domain, is mostly α-helical. It features a single hydrophobic transmembrane domain (HD) made up of 25 residues, targeted to Golgi membranes for virion release and secretory pathway cascade (Day, 2020; Surya et al., 2018).
While S protein was the focus of much research efforts, recently, other proteins including E proteins are also thoroughly investigated (Bojadzic et al., 2020; Caliebe et al., 2021; Li et al., 2021; Yu et al., 2021). The work by Caliebe et al. (2021) reported bioinformatics analysis of three major Boswellia resin constituents and discovered that they bind to functional proteins viz. the replicase polyprotein P0DTD1, the spike glycoprotein P0DTC2, and the nucleoprotein P0DTC9 of the SARS-CoV-2 virus. Moreover, Li et al. (2021) demonstrated by computational simulation data that glycyrrhizic acid interacted with S protein for its antiviral activity, thereby blocking SARS-CoV-2 infection. Moreover, the significance of SARS-CoV-2 E protein is to maintain a conserved Golgi complex-targeting signal being essential for the development of virus life cycle, (Day, 2020) including the processes of assembly, budding, envelope formation, and pathogenesis (Alam et al., 2020; Collins, 2020; Sarkar and Saha, 2020). It has been discovered to be a virulence factor and to play a role in virus pathogenesis (Javorsky et al., 2021). The cationic activity viroporins of CoV-2 E protein are the key driving factor that substantially impacts the secretory pathway governing viral productivity at the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). (Collins, 2020; Day, 2020). The E protein is in a region where interactions with other CoV proteins and host cell proteins occur (Sarkar and Saha, 2020). Hence, it stands as a crucial link in COVID-19 transmission (Roy and Menon, 2021).
On the basis of studies gained from SARS and MERS infections, reports on the use of antiviral drugs, hypertensive medicines, or even anti-inflammatory drugs or angiotensin-converting enzyme (ACE) inhibitors along with angiotensin-receptor blockers (ARBs). Based on research on SARS, MERS, and Ebola outbreaks, scientists predict that conventional antiviral drugs like Remdesivir (nucleoside analogs), Carprofen, and Celecoxib (anti-inflammatory drugs) or Lopinavir/Ritonavir (antiretrovirals) could benefit from curing COVID-19 as well.
The urgency is to come up with an alternative solution to control and break the chain of COVID-19 transmission. Traditional Indian medicinal plants have shown to be promising source of compounds to cope COVID-19 disease (Alam et al., 2020; Saravanan et al., 2020). To discover the potent plant metabolites/molecules for the treatment and prevention of COVID-19, traditional plant secondary metabolites' screening is highly recommended. Our previous work based on screening the novel medicinal metabolites that could be a target for SARS-CoV-2 E protein (Alam et al., 2020).
The novelty of the present study was to find compounds that are components of some Indian medicinal plants that could inhibit SARS-CoV-2 E protein by molecular docking, along with in-vitro assays.
Hence, this study proves to provide a promising approach for preventing COVID-19 that remains to be deciphered further. Understanding the role of the SARS-CoV-2 E protein in viruses is thus a paramount prerequisite for identifying novel antiviral therapeutics.
Materials and methods
Chemicals
SARS-CoV-2 Envelope protein, His-and Avi tag (Cat No. GTX01547-pro) was purchased from GeneTex, Inc., CA, USA. All other reagents used were of analytical grade.
Selection of compounds that are present in Indian medicinal plants
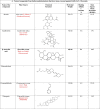
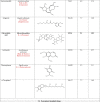
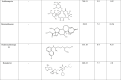
Twelve compounds from different Indian medicinal plants (Table 1 ) were selected from a library of Indian plants named the IMPPAT database, and the compounds and their structures were taken from PubChem (http://pubchem.ncbi.nlm.nih.gov). All the structures were converted from Smiles strings to PDB file format using Chimera software tool 12.1 (Pettersen et al., 2004).
Table 1.
Natural compounds from Indian medicinal plants and standard antiviral drugs that have been screened against SARS-CoV-2 E protein.
 |
 |
 |
Structural insights of the target SARS-CoV-2 E protein
SARS-CoV-2 E protein was used as a template (PDB ID-7K3G) (Mandala et al., 2020). Ramachandran plot was analyzed and showed the energetically favoured regions for backbone dihedral angles against amino acid residues in protein structure. ProFunc webserver was used to decipher the target protein's overall structure and function (http://www.ebi.ac.uk/thornton-srv/databases/ProFunc).
Docking studies of SARS-CoV-2 E protein
Molecular docking was performed on SARS-CoV-2 E of SARS-CoV-2 imported from Protein Data Bank (https://www.rcsb.org/), as PDB ID-7K3G (Mandala et al., 2020)". The ligand structures were obtained from PubChem.Significant pockets and groves present in the target proteins were visualized using Caver Analyst 2.0 beta. Docking studies of target molecules with envelope proteins of SARS-CoV-2 were performed using the PyRX virtual screening tool (Dallakyan and Olson, 2015; Trott and Olson, 2020). The input file format was a PDB file. The binding affinity of the ligand with target proteins was studied, and compounds with better binding affinity were determined. Docking results and interaction of critical amino acid residues were shown and visualized using Biovia Discovery Studio
Pharmacological profiling of medicinal compounds
The drug likeliness of the twelve selected compounds was checked with DruLiTo1 (Westerbeck and Machamer, 2019). ADMET prediction, toxicity, and recommended dose of screened herbal compounds were estimated with the vNN ADMET prediction tool.
Inhibition of SARS-CoV-2 by the twelve selected natural compounds from indian medicinal plants monitored with UV–visible spectra
The inhibitory effects of the twelve selected natural compounds on the activity of SARS-CoV-2 were tested. To monitor the structural and functional changes, UV–vis scans in the range 200–700 nm were obtained with a Shimadzu UV-1800 spectrophotometer double beam. The absorbance values were obtained by keeping the SARS-CoV-2 E protein concentration constant while varying concentrations of β-boswellic and glycyrrhizic acids (from 50 to 100 µg/ml at pH=7.4) were successively added.
Fluorescence spectroscopic measurements
The fluorescence emission spectra were acquired on Perkin Elmer LS50 fluorospectrometer (Perkin Elmer, Boston, MA, USA) equipped with 1.0 cm path length quartz cells at 25 °C. The emission spectra were recorded between 310 and 450 nm after excitation at 295 nm and slit widths were kept at 7 nm and 10 nm for excitation and emission respectively. The spectra were recorded in triplicate and normalized for buffer contributions.
Statistical analysis
All the experiments were performed in triplicate, and data were expressed as mean ± standard deviation (SD) from replicate runs. Analysis of variance (ANOVA) using Origin 2020 (9.7) (OriginLab Corporation, Inc., Northampton, MA, USA) was considered to indicate a statistically significant differences between samples (p < 0.05).
Results and discussion
Selection of compounds from Indian medicinal plants for SARS-CoV-2 E inhibitors
In our study, the twelve compounds detailed in Table 1 were selected from traditional Indian medicinal plants. These compounds were screened based on their reported antiviral and anti-inflammatory properties (Benarba and Pandiella, 2020) and thus, they can be promising antiviral drugs against SARS-CoV-2. These compounds are used in the treatment of various respiratory diseases, which are the primary targets in COVID-19 infections (Ahmad et al., 2021; Patel et al., 2021; Verma et al., 2021). The conventionally used commercial antiviral drugs in the clinics were also considered relevant to the present study (Table 1) (Bojadzic et al., 2020).
Structure-based pharmacophore modeling
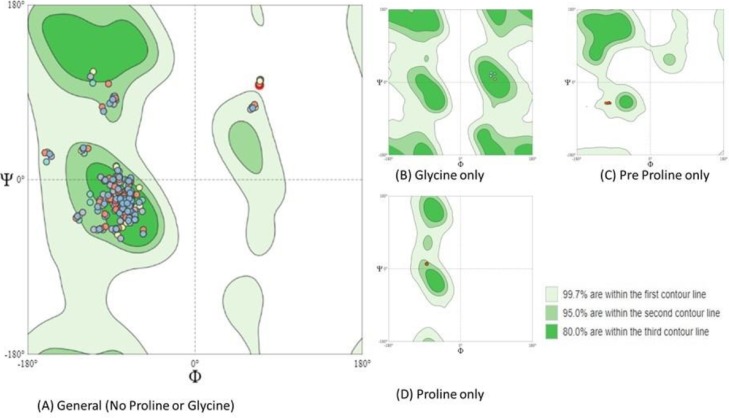
The detailed structure elucidation of the target E protein, assessing backbone was studied. Histogram of Ramachandran plot used to count Φ (Phi; C—N-CA-C) / Ψ (Psi; N—CA-C-N) occurrences displayed categories A to D in Fig. 1 .
Fig. 1.
Histograms of Ramachandran plot showing Φ (Phi; C—N-CA-C) / Ψ (Psi; N—CA-C-N).
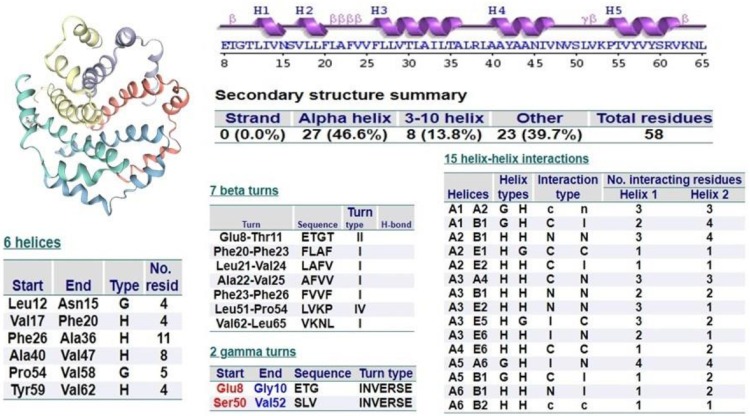
The plot distribution favored 88.93% amino acid with 16.8% rotamer outliers and 2.14% Ramachandran outliers. ProFunc webserver was used to examine the overall structure and functions of the E protein. Secondary structure prediction of single-chain revealed α-helix (46.6%), 310 helix (13.8%) and others (39.7%). It also depicted secondary structural elements like fifteen helix-helix interaction, six helices, seven β turns and two γ turns. Major secondary structures are highlighted in Fig. 2 .
Fig. 2.
Secondary structural assessment of target protein (SARS-CoV-2 E protein).
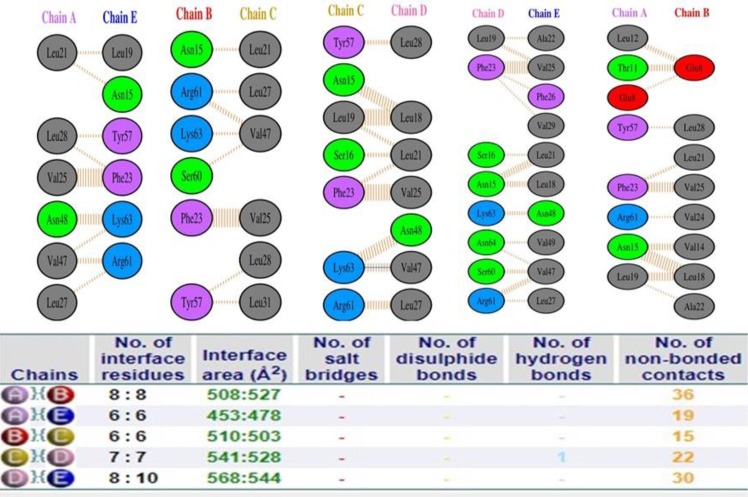
Further to it, the interactions among subunits are deciphered. The critical amino acid residues showing interaction among chains were shown in Fig. 3 .
Fig. 3.
Intramolecular interaction among pentameric chains of target protein (SARS-CoV-2 E protein).
Such interactions were found relevant during ligand interaction with the E protein. To gain insights into the structure of the SARS-CoV-2 E protein, the cavity/pocket required for catalytic sites was studied using Caver Analyst 2.0 beta. The SARS-CoV-2 E protein had cavities/pockets that were found to be essential for catalysis. A ligand-binding site was observed on the site present on the cavity. Out of twelve screened compounds, we have demonstrated the best bindings were showed by β-boswellic and glycyrrhicic acids
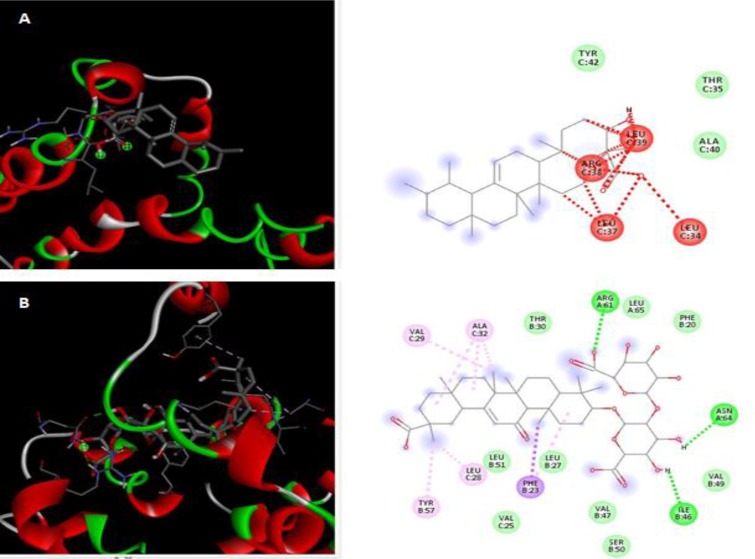
Cavities and pockets of the proteins, as demonstrated, was targeted by these two ligands. Furthermore, structural guided studies were carried out to predict the orthosteric or allosteric sites present in the target molecules. As per our findings, the binding had taken place at the allosteric site. Binding at the allosteric site provides information about the nature of ligand binding. For the allosteric binding, ligands will induce conformational changes in the protein functionality and overall protein structure (J. Wu and Chiwaya, 2020). The study also revealed that various critical residues were involved in interacting with the ligand. Various interactions such as van der waals, hydrophobic, alkyl, hydrogen bonding was visualised using Biovia Discovery Studio 2020. (Fig. 4 A and B).
Fig. 4.
Docking and interaction of best-fit ligands with proteins showing critical residues and nature of interactions (A) β-boswellic acid, (B) Glycyrrhizic acid.
Molecular docking-based virtual screening
To further examine and visualize the binding mode of SARS-CoV-2 E protein with the screened compounds, molecular docking was performed in E protein to analyze the binding details. We analyzed the ligand's docking with the selected molecules i.e., E protein using Autodock Vina to obtain a better overview of binding affinity when the outcome was established along with root square mean deviation values (Trott and Olson, 2010). The binding affinity should be higher than 6.0. Contrary to expressing high values by binding affinity, root mean square deviation (RMSD) values need to be lowest, proving the best fit result. Table 1 shows the binding affinity of the selected plant compounds. The docking and binding affinity of the compounds with the SARS-CoV-2 E protein. β-boswellic acid present in the Indian plant Boswellia serrata Roxb. ex Colebr. (Burseraceae) (Fig. 4A) and glycyrrhizic acid present in Glycyrrhiza glabra L. (Poacaeae) (Fig. 4B) were found to be the best antiviral compound among the screened ligands for drug targeting of the E protein. These two unconventional metabolites of medicinal compounds showed intermolecular interaction with the SARS-CoV-2 E protein. Glycyrrhizic acid showed interactions with E protein at chain A: Arg 61, chain B: Phe 23, chain B: Tyr 57, and chain C: Val 25. A direct hit at the key residues forming helices and turns led to E protein structure disruption.
Moreover, on account of linkages between the chains, indirect effects were also noticed. For example, the ligand, glycyrrhizic acid interacted with chain B: Tyr 57, chain C: Leu 28, and chain C: Leu 31, which promoted disorganization among protein integrity. Further validation was taken up with β-boswellic acid, which revealed similar interaction with chain C of the protein. It pronounced indirect effect, showing interaction and the unfavorable binding that led to the protein's disruption.
Apart from these compounds, commercially available drugs such as azithromycin, remdesivir, dexamethasone, thymoquinone, and hydroxychloroquine, which have been proposed as remedies against COVID-19, were also taken up as reference/control and compared with the twelve selected compounds screened in this work (Khan and Al-Balushi, 2021; Sharma et al., 2020). The present study showed that two medicinal compounds viz. glycyrrhizic acid and β-boswellic acid had greater efficacy and far better binding affinity than the five currently employed drugs for inhibiting COVID-19 (Yu et al., 2021). The binding affinity of presently used drugs in the treatment of COVID-19 are as follows; azithromycin (−5.9 kcal/mol), hydroxychloroquine (−6.4 kcal/mol), remdesivir (−7.7 kcal/mol), dexamethasone (−7.2 kcal/mol) and thymoquinone (−5.3 kcal/mol). Whereas our findings highlighted that out of the proposed medicinal metabolites, two were the most favourable bioactive compounds, i.e., β-boswellic and glycyrrhizic acids (Alam et al., 2020; Roy and Menon, 2021). These compounds showed a binding affinity of −9.1 kcal/mol, which performed far better in docking studies.
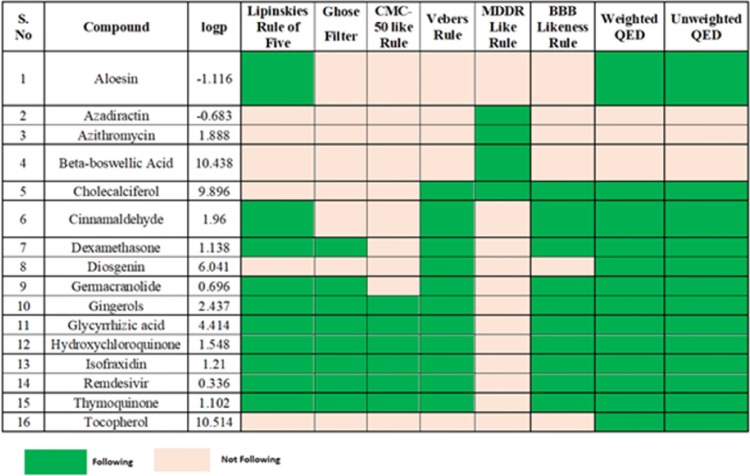
Moreover, apart from the structural insights, we delved into the pharmacological aspects of these compounds. Assessment of these compounds as drug formulation was done by DruLiTo 1.0. Various pharmacological aspects were studied for each compound, and their potential as a drug molecule was examined. The evaluation based on the parameters is not absolute, but it will be used to calculate ligand molecules for drug-like properties quickly. Parameters that defined the drug-likeliness are judged on the following criteria such as Lipinski's Rule (ClogP ≤5), MDDR like rules (No. of rings ≥3; No. of rigid bonds ≥18; No. of rotatable bonds ≥6), Veber Rule (Rotatable bond count ≤10; PSA ≤140), Ghose Filter (logP {−0.4∼5.6}; MR (Molar Refractivity 40∼); molecular weight (160∼480); Number of Atoms (20∼70); Polar Surface Area < 140), BBR rule (H-bond {8–10}; Molecular weight (400–500); No. of acids), CMC-50 like rule (AlogP {1.3∼4.1}; Molar refractivity (70–110); molecular weight (230–390); Number of Atoms (30–55)) and QED (Quantitative estimation of drug-likeness). Fig. 5 presents the drug-likeness of these compounds based on the parameters as mentioned earlier.
Fig. 5.
Validation of screened compounds possessing drug-likeness properties.
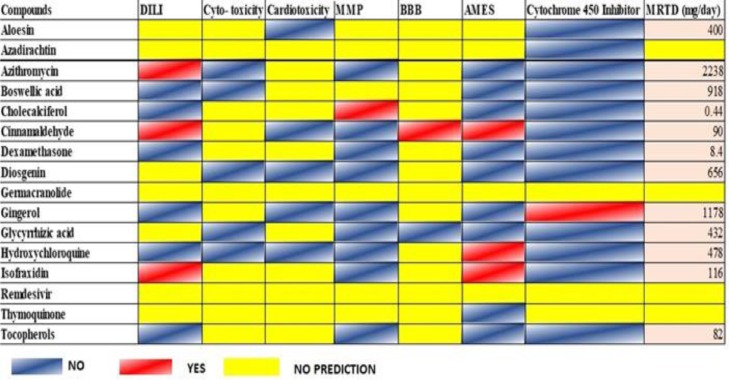
ADMET properties assessment: In-silico ADMET screening of the selected compounds
Further, toxicological assessment of these compounds and their ADME (Absorption, distribution, metabolism, and excretion) profile was inferred using vNN-ADMET prediction tool. This tool has a database of chemical compounds and their toxicological effects on the human body. With analysis, it gave a prediction of our target compounds. It examined their various toxicological properties like drug-induced liver injury (DILI), cytotoxicity, cardiotoxicity, mitochondrial toxicity (MMP), Blood-brain barrier (BBB), mutagenicity (AMES Test), Cytochrome P450 enzyme inhibition, and maximum recommended therapeutic dose (MRTD mg/day). Such evaluation explained the significance of tested compounds and their safe use for therapy. Fig. 6 showed a toxicological assessment of the screened compounds along with MRTD.
Fig. 6.
Toxicological assessment of various parameters shown by tested compounds.
After validation, it was found that β-boswellic and glycyrrhizic acids were nontoxic. Considering the potency of β-boswellic and glycyrrhizic acids to mitigate efficiently in the present work, it seemed pertinent to study their in-vitro antiviral effects on SARS-CoV-2 E protein as well.
Effects of β-boswellic and glycyrrhizic on the structure of SARS-CoV-2 E protein
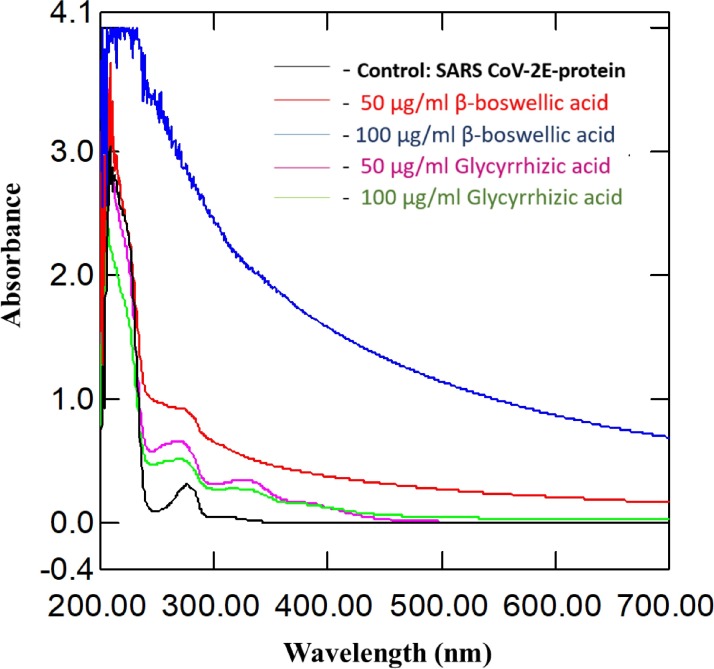
UV-visible spectral studies
Scans acquired from the range 200–700 nm led to prominent loss in the structural integrity of protein. The disruption was observed at 280 nm after treatment with both β-boswellic and glycyrrhizic acids in Fig 7 .
Fig. 7.
Functionality loss and structural disruptions revealed by UV–vis spectra.
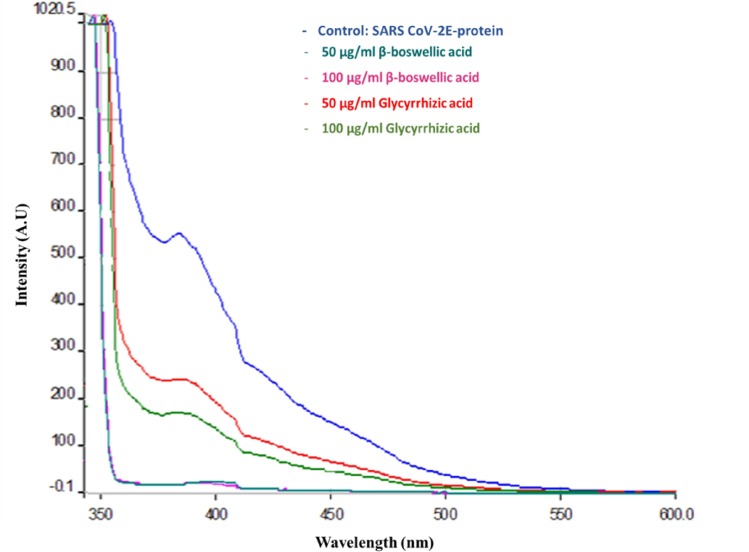
Fluorescence spectroscopy
Fluorescence spectroscopy was applied to analyze drug–protein interactions by measuring the change of fluorescence emission intensity to decipher the quenching mechanism. The native α-helix structure was seen to be destabilized in the presence of both β-boswellic and glycyrrhicic acids. The structural changes were notable on reaction with glycyrrhizic acid at 50 µg/ml. Moreover, the E protein structure was completely disrupted by 100 µg/ml β-boswellic acid. The tertiary structural changes monitored by intrinsic fluorescence spectroscopy showed no emission spectra suggesting disruption in the microenvironment of the E protein.
As shown in Fig. 8 , SARS-CoV-2 E showed a characteristic emission band at around 370 nm, whereas treated test samples i.e., treated with β-boswellic and glycyrrhicic acids emitted almost no emission spectrum at the highest concentration. The fluorescence intensity of SARS-CoV-2 E was decreased progressively with the addition of increasing β-boswellic and glycyrrhicic acids concentrations (50 to 100 µg/ml), which indicated that these two medicinal metabolites interacted with SARS-CoV-2 E.
Fig. 8.
Fluorescence spectra depicting the effect pronounced by various concentrations of Glycyrrhizic acid and β-boswellic acid on SARS-CoV-2 E protein.
The presence of 100 µg/ml β-boswellic acid led to a maximum reduction in the fluorescence intensity. On the other hand, glycyrrhizic acid led to less changes in the tertiary structure of the SARS-CoV-2 E accompanied by blue shifts. Eventually with increasing concentration from 50 to 100 µg/ml noticeable structural loss was observed by both these β-boswellic and glycyrrhicic acids. This might be the possible reason for the loss of activity in SARS-CoV-2 E. Effect on the active site of the SARS-CoV-2 E after binding by these acids was noticed (Gomaa et al., 2021; Sun et al., 2021; van de Sand et al., 2021). Hence the fluorescence quenching in both β-boswellic and glycyrrhicic acids indicated major changes in the immediate microenvironment of the SARS-CoV-2 E protein.
Conclusions
The present study was carried out to look for potential antiviral agents to combat COVID-19. Molecular docking studies were conducted to identify binding of medicinal metabolites with SARS-CoV-2 E protein. Out of screened compounds, β-boswellic acid (B. serrata) was found to be most suitable, along with glycyrrhizic acid (G. glabra). With the advantage of being a natural compound and certain pharmacological studies showed no harmful side effects, these novel compounds could be a good choice to be used for the treatment of COVID-19 patients. This study is in concordance to important antecedents with boswellic and glycyrrhinic acids against SARS-CoV2 and could be a probable treatment of COVID-19 (Caliebe et al., 2021; Gomaa et al., 2021; Li et al., 2021; Roy and Menon, 2021; Sun et al., 2021; van de Sand et al., 2021; Yu et al., 2021).
Hence, further investigations on their clinical use must be undertaken to validate β-boswellic and glycyrrhicic acids curative effects to address the pandemic caused by SARS-CoV-2. A structural understanding of their effect on SARS-CoV-2 E, was achieved by UV and intrinsic fluorescence spectroscopy analyses. The UV and fluorescence spectra revealed that interaction with boswellic and glycyrrhicic acids caused microenvironmental and structural changes in E protein. In-vitro studies revealed the promising effect as potent SARS-CoV-2 E protein inhibitors. These findings provide a rational basis for understanding drugs interaction with SARS-CoV-2 E for finding new compounds to cope with COVID-19 pandemic.
Declaration of Competing interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
SWF gratefully appreciates the financial assistance provided by Department of Science and Technology (DST), Government of India (INSPIRE Fellowship (no. IF160621)). SA gratefully appreciates the financial support provided by Indian Institute of Technology Delhi (IIT Delhi).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phyplu.2022.100241.
Appendix. Supplementary materials
References
- Ahmad S., Zahiruddin S., Parveen B., Basist P., Parveen A., Parveen R., Ahmad M. Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Front. Pharmacol. 2021;11:2470. doi: 10.3389/fphar.2020.578970. 10.3389/fphar.2020.578970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S., Fatima S.W., Khare S.K. Unravelling promise of Indian herbal compounds as potential COVID-19 therapeutic agent. Res. Sq. 2020;1:1–15. 10.21203/rs.3.rs-41688/v1. [Google Scholar]
- Benarba B., Pandiella A. Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 2020;11:1189. doi: 10.3389/fphar.2020.01189. 10.3389/fphar.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOVIA . Dassault Systèmes; San Diego: 2020. Dassault Systèmes, BIOVIA Workbook, Release 2020; BIOVIA Pipeline Pilot, Release. [Google Scholar]
- Bojadzic D., Alcazar O., Buchwald P. Methylene blue inhibits the SARS-CoV-2 spike–ACE2 protein-protein interaction–a mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2020;11:2255. doi: 10.3389/fphar.2020.600372. 10.3389/fphar.2020.600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliebe R.H., Scior T., Ammon H.P. Binding of boswellic acids to functional proteins of the SARS-CoV-2 virus: bioinformatic studies. Arch. Pharm. 2021;354 doi: 10.1002/ardp.202100160. e2100160. 10.1002/ardp.202100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. Bringing needed structure to COVID-19 drug development. USA: National Institutes of Health. 2020 https://directorsblog.nih.gov/2020/04/02/bringing-needed-structure-to-covid-19-drug-development/. Accessed 16 December 2021. [Google Scholar]
- Dallakyan S., Olson A.J. Small-Molecule library screening by docking with PyRx. Chem. Biol. Humana Press, New York. 2015:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. British Med. J. 2020;368:1086. doi: 10.1136/bmj.m1086. 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- Gomaa A.A., Mohamed H.S., Abd-Ellatief R.B., Gomaa M.A. Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology. 2021;29:1033–1048. doi: 10.1007/s10787-021-00841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javorsky A., Humbert P.O., Kvansakul M. Structural basis of coronavirus E protein interactions with human PALS1 PDZ domain. Commun. Biol. 2021;4:1–8. doi: 10.1038/s42003-021-02250-7. 10.1038/s42003-021-02250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., Al-Balushi K. Combating COVID-19: the role of drug repurposing and medicinal plants. J. Infect. Public Health. 2021;14:495–503. doi: 10.1016/j.jiph.2020.10.012. 10.1016/j.jiph.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu D., Wang L., Zhang M., Zhang G., Li E., He S. Glycyrrhizic acid inhibits SARS-CoV-2 infection by blocking spike protein-mediated cell attachment. Molecules. 2021;26:6090. doi: 10.3390/molecules26206090. 10.3390/molecules26206090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala V.S., McKay M.J., Shcherbakov A.A., Dregni A.J., Kolocouris A., Hong M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020;27:1202–1208. doi: 10.1038/s41594-020-00536-8. 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021;54:159–163. doi: 10.1016/j.jmii.2020.03.022. 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B., Sharma S., Nair N., Majeed J., Goyal R.K., Dhobi M. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol. Cell Biochem. 2021;476:2345–2364. doi: 10.1007/s11010-021-04084-7. 10.1007/s11010-021-04084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Roy A., Menon T. Evaluation of bioactive compounds from Boswellia serrata against SARS CoV 2. Vegetos. 2021;17:1–11. doi: 10.1007/s42535-021-00318-7. 10.1007/s42535-021-00318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan K.M., Zhang H., Senthil R., Kumar Vijayakumar K., Sounderrajan V., Wei Y., Shakila H. Structural basis for the inhibition of SARS-CoV2 main protease by Indian medicinal plant-derived antiviral compounds. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1834457. 10.1080/07391102.2020.183445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M., Saha S. Structural insight into the role of novel SARS-CoV-2 E protein: a potential target for vaccine development and other therapeutic strategies. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0237300. 10.1371/journal.pone.0237300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Tiwari S., Deb M.K., Marty J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106054. 10.1016%2Fj.ijantimicag.2020.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., He G., Huang N., Thilakavathy K., Lim J.C.W., Kumar S.S., Xiong C. Glycyrrhizic acid: a natural plant ingredient as a drug candidate to treat COVID-19. Front. Pharmacol. 2021;12:1740. doi: 10.3389/fphar.2021.707205. 10.3389/fphar.2021.707205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surya W., Li Y., Torres J. Structural model of the SARS coronavirus E channel in LMPG micelles. Biochim. Biophys. Acta Biomembr. 2018;1860:1309–1317. doi: 10.1016/j.bbamem.2018.02.017. 10.1016/j.bbamem.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sand L., Bormann M., Alt M., Schipper L., Heilingloh C.S., Steinmann E., Todt D., Dittmer U., Elsner C., Witzke O., Krawczyk A. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13:609. doi: 10.3390/v13040609. 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A.K., Kumar V., Singh S., Goswami B.C., Camps I., Sekar A., Yoon S., Lee K.W. Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS-CoV-2 associated target proteins revealed by molecular docking, molecular dynamics and MM-PBSA studies. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111356. 10.1016/j.biopha.2021.111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerbeck J.W., Machamer C.E. The infectious bronchitis coronavirus envelope protein alters Golgi pH to protect the spike protein and promote the release of infectious virus. J. Virol. 2019;93:15–19. doi: 10.1128/JVI.00015-19. 10.1128/JVI.00015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID 19-11 March 2020.
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chiwaya N. Coronavirus map: the COVID-19 virus is spreading across the world. Here’s where cases have been confirmed. USA: NBC News. 2020 https://www.nbcnews.com/health/health-news/coronavirus-map-confirmed-cases-2020-n1120686. Accessed 8 January 2022. [Google Scholar]
- Yu S., Zhu Y., Xu J., Yao G., Zhang P., Wang M., Zhao Y., Lin G., Chen H., Chen L., Zhang J. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153364. 10.1016/j.phymed.2020.153364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.