Abstract
Aim
Awake prone positioning (PP) in patients with coronavirus disease 2019 (COVID‐19) can improve oxygenation. However, evidence showing that it can prevent intubation is lacking. This study investigated the efficacy of awake PP in patients with COVID‐19 who received remdesivir, dexamethasone, and anticoagulant therapy.
Methods
This was a two‐center cohort study. Patients admitted to the severe COVID‐19 patient unit were included. The primary outcome was the intubation rate and secondary outcome was length of stay in the severe COVID‐19 unit. After propensity score adjustment, we undertook multivariable regression to calculate the estimates of outcomes between patients who received awake PP and those who did not.
Results
Overall, 108 patients were included (54 [50.0%] patients each who did and did not undergo awake PP), of whom 25 (23.2%) were intubated (with awake PP, 5 [9.3%] vs. without awake PP, 20 [37.0%]; P < 0.01). The median length of stay in the severe COVID‐19 unit did not significantly differ (with awake PP, 5 days vs. without awake PP, 5.5 days; P = 0.68). After propensity score adjustment, those who received awake PP had a lower intubation rate than those who did not (odds ratio, 0.22; 95% confidence interval, 0.06–0.85; P = 0.03). Length of stay in the severe COVID‐19 patient unit did not differ significantly (adjusted percentage difference, −24.4%; 95% confidence interval, −56.3% to 30.8%; P = 0.32).
Conclusion
Awake PP could be correlated with intubation rate in patients with COVID‐19 who are receiving remdesivir, dexamethasone, and anticoagulant therapy.
Keywords: Coronavirus disease 2019, dexamethasone, intubation, prone positioning, respiratory failure
This study aimed to investigate the efficacy of awake prone positioning (PP) for patients with coronavirus disease 2019 (COVID‐19) using remdesivir, dexamethasone, and anticoagulation therapy. We undertook a two‐center propensity score‐adjusted cohort study in Japan from July 2020 to February 2021. We showed that awake PP could be correlated with intubation rate in patients with acute respiratory failure and COVID‐19.
1. Introduction
In late 2019, infection with a novel beta coronavirus, subsequently named the severe acute respiratory syndrome coronavirus 2, was reported in individuals who had visited a wet market in Wuhan, China. Since then, the virus has spread rapidly, which has led to the coronavirus disease 2019 (COVID‐19) pandemic. 1 After being infected with the virus, some individuals may remain asymptomatic or present with only mild upper respiratory symptoms. However, nearly 20% of patients experience hypoxemia. 2 In many patients with severe COVID‐19, progressive respiratory failure develops soon after the onset of dyspnea and hypoxemia. These patients commonly meet the criteria for acute respiratory distress syndrome (ARDS), which is defined as the acute onset of bilateral infiltrates, severe hypoxia, and lung edema that cannot be completely explained by cardiac failure or fluid overload. 3 A mortality rate of 49.0% has been reported among critically ill patients. 4
Importance
Prone positioning (PP) is a traditional treatment for ARDS. 5 , 6 The physiological rationale behind PP in typical ARDS is to reduce ventilation/perfusion mismatch, hypoxemia, and shunting. 7 This mechanism can be applied in nonintubated patients with ARDS and is referred to as awake PP. 8 However, information on the efficacy of awake PP for patients with comorbid respiratory failure and COVID‐19 is limited. 9 , 10
One of the difficulties in verifying the efficacy of awake PP for COVID‐19 is the discrepancy in treatment. COVID‐19 is an emerging infectious disease, and no treatment has currently proved to be remarkably effective in treating COVID‐19. However, the classes of drugs that are mainly used include antiviral agents, anti‐inflammatory agents, anticoagulant therapy, plasma, and hyperimmune immunoglobulins. Based on the pathological characteristics and different clinical stages of patients with COVID‐19, clinical researchers are testing a variety of possible treatments. 11 Thus, pharmacotherapy for COVID‐19 infections differs based on time and place. In their meta‐analysis, Cardona et al. highlighted the inherent difficulty in obtaining precise results of awake PP for patients with COVID‐19 in the novel and continually changing environment of COVID‐19. 10
Goals of this investigation
The aim of this study was to evaluate the effects of awake PP on clinical outcomes in severe illness COVID‐19 patients using the same pharmacotherapy.
Methods
Study design and setting
This was a two‐center propensity score‐adjusted cohort study undertaken in Japan from July 2020 to February 2021. We selected St Marianna University School of Medicine and Kawasaki Municipal Hospital in Kanagawa Prefecture, Japan to carry out this study. St Marianna University School of Medicine and Kawasaki Municipal Hospital are tertiary facilities that are equipped with more than 500 beds and began treating patients with COVID‐19 who had travelled on the Diamond Princess cruise ship. 12 Both hospitals have a severe COVID‐19 patient unit for patients with COVID‐19 who are suspected or confirmed to require intensive care (St Marianna University School of Medicine, 17 beds; Kawasaki Municipal Hospital, 13 beds). Beginning in July 2020, both hospitals started to prescribe remdesivir, dexamethasone, and anticoagulants as the main treatment for patients with COVID‐19. 13 , 14 , 15
The National Institutes of Health classified COVID‐19 into five groups: asymptomatic or presymptomatic, mild illness, moderate illness, severe illness, and critical illness. 16 Critical illness COVID‐19 patients have respiratory failure, septic shock, and/or multiple organ dysfunction. We included all severe illness COVID‐19 patients (pulse oximetry oxygen saturation [SpO2] <94% on room air at sea level [SpO2/fraction of inspired oxygen (FiO2) <448], ratio of arterial partial pressure of oxygen [PaO2]/FiO2 <300 mmHg, respiratory rate > 30 breaths/min, or lung infiltrates >50%) who were admitted to the severe COVID‐19 patient unit at these hospitals. The exclusion criteria were patients admitted to the severe COVID‐19 patient unit for another reason (such as surgery), intubated before admission, age less than 18 years, do‐not‐resuscitate or do‐not‐intubate orders, immunocompromised (e.g., undergoing chemotherapy, HIV infection), chronic disease/illness with an expected life span of less than 30 days (based on the opinion of the site investigator) unrelated to the current COVID‐19 diagnosis (e.g., stage IV malignancy, neurodegenerative disease), and pregnancy. The primary outcome was intubation rate and the secondary outcome was the length of stay in the severe COVID‐19 patient unit. We compared these outcomes between those who received awake PP and those who did not. The study was approved by the ethics committees of St Marianna University School of Medicine Hospital (approval no. 5246) and Kawasaki Municipal Hospital (approval no. 20217).
Awake PP
Awake PP was carried out in patients with oxygen demand at the discretion of the physicians who were in charge of the severe COVID‐19 units, given their experience in pronation maneuvers. Awake PP was initiated soon after admission. Patients were asked to remain in the prone position for as long as they could at least three times a day and for at least 6 h per day. During the procedure, the medical staff constantly monitored the patients’ awake PP. The procedure was terminated when patients were intubated, had an oxygen demand of 2 L (nasal cannula), or were discharged from the severe COVID‐19 unit. 17 , 18 In cases where patients could not tolerate awake PP due to a respiratory rate greater than 40 breaths/min, severe dyspnea, or a Glasgow Coma Scale score of 13 or lower, we discontinued awake PP and considered invasive ventilation.
Standard care for COVID‐19
The standard care for patients with COVID‐19 was remdesivir (100 mg/day for 10 days), dexamethasone (6.6 mg/day for 10 days), and anticoagulants (heparin adjusted to maintain the activated partial thromboplastin time at 1.5–2.0 times the control value or novel oral anticoagulants) at both hospitals. Ceftriaxone (2 g/day for 10 days) was prescribed when a bacterial respiratory superinfection was suspected.
The target oxygenation value at both hospitals was SpO2 greater than 90% with oxygen inhalation or high‐flow nasal cannula. We considered mechanical ventilation if the patient’s PaO2/FiO2 was less than 150 mmHg, respiratory rate higher than 40 breaths/min, or Glasgow Coma Scale score was 13 or lower.
Data collection
We collected data on the patients’ age, sex, height, weight, comorbidities (hypertension, cardiovascular disease [history of acute myocardial infarction and angina], chronic heart failure, cerebrovascular disease, diabetes mellitus, current cancer, chronic obstructive pulmonary disease or asthma, chronic kidney disease [none, with, or without dialysis], and dementia), admission from another hospital, and time from symptom onset to admission. We also obtained clinical information, including vital signs (systolic blood pressure, diastolic blood pressure, heart rate, respiration rate, body temperature), SpO2/FiO2, chest X‐ray (bilateral opacities, unilateral opacities, none), chest computed tomography (bilateral opacities, unilateral opacities, none), white blood cell count, C‐reactive protein, D‐dimer, and ferritin, because these markers have been reported to be associated with the risk for clinical deterioration in COVID‐19. 19 We collected information on treatment, including awake PP, remdesivir, dexamethasone, anticoagulant therapy, high‐flow nasal oxygen, and continuous renal replacement therapy, and included only those treatments that were given before intubation. Prognosis data, including the intubation rate, and the duration of stay in the severe COVID‐19 patient unit, were also obtained. We extracted all data from the electronic medical charts.
Statistical analyses
First, we compared patient characteristics and primary and secondary outcomes between those who received awake PP and those who did not using Fisher’s exact test and the Wilcoxon rank‐sum test. We then undertook a propensity score adjustment for being assigned to the awake PP group.
The propensity score was calculated using a logistic regression analysis with forward stepwise selection (significance levels for model exit and entry were 0.1 and 0.05, respectively), including more than 40 variables, including patients’ demographic characteristics, vital signs, and laboratory measures. We selected iterative model‐building algorithms using forward stepwise regression because these are designed to create good predictive models of exposure. 20 , 21
After propensity score adjustment, we undertook multivariable regression to calculate the estimates of outcomes between patients who received awake PP and those who did not. For the primary outcome, we performed a logistic regression analysis. For the secondary outcome, we used generalized linear models with a negative binomial distribution and log‐link function. We also undertook subgroup analysis for the length of stay in the severe COVID‐19 unit among patients without intubation.
All analyses were performed using STATA/MP version 15.1 (StataCorp).
Results
We included 108 patients (66 patients from St Marianna University School of Medicine and 42 patients from Kawasaki Municipal Hospital) in the study. Table 1 compares the patients’ characteristics between those who received awake PP and those who did not. The SpO2/FiO2 ratio was significantly lower in those who received awake PP than in those who did not (median SpO2/FiO2 ratio: 240.0 for those who received awake PP and 321.4 for those who did not; P < 0.01). Table 2 shows the results of the bivariate analysis of the primary and secondary outcomes. For the primary outcome, 25 (23.2%) patients were intubated, and patients who received awake PP had a significantly lower intubation rate than those who did not (9.3% who received awake PP versus 37.0% who did not; P < 0.01). For the secondary outcome, the length of stay in the severe COVID‐19 unit among all patients was 5.0 days (interquartile range [IQR], 3.0–12.0 days) and did not significantly differ (median length of stay in the severe COVID‐19 unit was 5.0 days for those who received awake PP and 5.5 days for those who did not; P = 0.68).
Table 1.
Characteristics of patients with severe COVID‐19 who received awake prone positioning and those who did not
| With awake prone positioning (n = 54) | Without awake prone positioning (n = 54) | P value | |
|---|---|---|---|
| Patient demographics | |||
| Age, years | 68.0 (58.0–76.0) | 70.0 (59.0–79.0) | 0.35 |
| Female sex | 17 (31.5) | 31 (57.4) | <0.01 |
| Body mass index | 23.9 (22.6–26.4) | 24.2 (22.1–28.2) | 0.21 |
| Comorbidities | |||
| Hypertension | 34 (63.0) | 38 (70.4) | 0.54 |
| Cardiovascular disease | 6 (11.1) | 9 (16.7) | 0.58 |
| Chronic heart failure | 7 (13.0) | 5 (9.3) | 0.76 |
| Cerebrovascular disease | 6 (11.1) | 11 (20.4) | 0.29 |
| Diabetes mellitus | 27 (50.0) | 25 (46.3) | 0.85 |
| Current cancer | 1 (1.9) | 2 (3.7) | 1.00 |
| Chronic obstructive pulmonary disease or asthma | 7 (13.0) | 3 (5.6) | 0.32 |
| Chronic kidney disease | |||
| Without dialysis | 4 (7.4) | 7 (13.0) | 0.73 |
| With dialysis | 4 (7.4) | 3 (5.6) | |
| Dementia | 1 (1.8) | 11 (20.4) | <0.01 |
| Vital signs | |||
| Heart rate, b.p.m. | 83 (70–102) | 86.5 (76–94) | 0.65 |
| Systolic blood pressure, mmHg | 136 (127.0–148.0) | 137.5 (123.0–153.0) | 0.81 |
| Diastolic blood pressure, mmHg | 78.0 (68.0–86.0) | 78.5 (66.0–90.0) | 0.68 |
| Respiration rate, breaths/min | 24.0 (20.0–26.0) | 22.5 (20.0–25.0) | 0.14 |
| Body temperature, °C | 36.7 (36.5–37.5) | 37.6 (37.0– 38.3) | <0.01 |
| Pulse oximetry oxygen saturation/fraction of inspired oxygen | 240.0 (202.2–335.7) | 321.4 (247.5–391.7) | <0.01 |
| Blood test results | |||
| White blood cell count, 103/μL | 7000 (4710–8650) | 5180 (4480–6900) | 0.03 |
| C‐reactive protein, mg/dL | 8.2 (4.9–12.4) | 6.6 (4.2–10.0) | 0.25 |
| D‐dimer, μg/mL | 1.0 (0.7–1.9) | 1.3 (0.8–2.4) | 0.08 |
| Ferritin, ng/mL | 604.0 (346.5–953.5) | 468.0 (222.0–870.8) | 0.06 |
| Imaging technique | |||
| Chest X‐ray | |||
| None | 1 (1.9) | 7 (13.0) | 0.01 |
| Unilateral opacities | 5 (9.3) | 9 (16.7) | |
| Bilateral opacities | 48 (88.9) | 38 (70.4) | |
| Chest computed tomography | |||
| None | 0 (0.0) | 0 (0.0) | 0.11 |
| Unilateral opacities | 1 (2.0) | 4 (9.2) | |
| Bilateral opacities | 50 (98.0) | 38 (86.4) | |
| Treatment | |||
| Antibiotic | 45 (83.3) | 23 (42.6) | <0.01 |
| Remdesivir | 49 (90.7) | 32 (59.3) | <0.01 |
| Dexamethasone | 53 (98.2) | 49 (90.7) | 0.09 |
| Anticoagulant therapy | 54 (100.0) | 48 (88.9) | 0.01 |
| Renal replacement treatment | 5 (8.9) | 3 (5.6) | 0.46 |
| Nasal high flow | 18 (32.1) | 2 (3.7) | <0.01 |
| Clinical information | |||
| Time from symptom onset to admission, days | 8.0 (4.5–9.5) | 5.0 (3.0–7.0) | <0.01 |
| Admission from another hospital | 22 (40.7) | 14 (25.9) | 0.10 |
Data are shown as n (%) or median (interquartile range).
Table 2.
Results of bivariate analysis for intubation rate and length of stay in a severe COVID‐19 patient unit
| Total (n = 108) | With awake prone positioning (n = 54) | Without awake prone positioning (n = 54) | P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Intubation, n (%) | 25 (23.2) | 5 (9.3) | 20 (37.0) | <0.01 |
| Secondary outcome | ||||
| Length of stay in the severe COVID‐19 patient unit, day, median (IQR) | 5.0 (3.0 to 12.0) | 5.0 (3.0–9.0) | 5.5 (3.0–14.0) | 0.68 |
IQR, interquartile range.
For calculation of the propensity score, the variables identified by the propensity score were weight, history of hypertension, chronic heart failure, chronic kidney disease (without or with dialysis), dementia, prescribed antibiotic, respiration rate, heart rate, and SpO2/FiO2 ratio. To assess the prediction performance, we constructed a receiver operating characteristic (ROC) curve (Fig. 1); the area under the ROC curve was 0.88 (95% confidence interval [CI], 0.82–0.95).
Fig. 1.
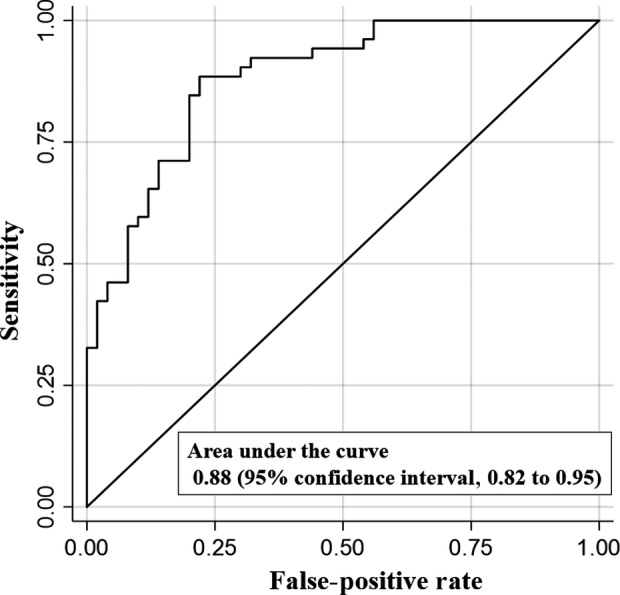
Receiver operating characteristic (ROC) curve for the prediction of awake prone positioning in patients with severe COVID‐19 according to propensity score.
Table 3 shows the results of the propensity score‐adjusted analysis for intubation rate and length of stay in the severe COVID‐19 unit. Those who received awake PP had significantly lower odds of intubation than those who did not receive awake PP (odds ratio, 0.22; 95% CI, 0.06–0.85; P = 0.03); however, no between‐group difference was detected in the length of stay in the severe COVID‐19 unit (adjusted percentage difference, −24.5%; 95% CI, −56.3% to 30.8%; P = 0.32).
Table 3.
Results of propensity score‐adjusted analysis for intubation rate and length of stay in a severe COVID‐19 patient unit
| Adjusted OR | 95% CI of OR | Adjusted percentage difference | 95% CI of adjusted percentage difference | P value | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Intubation | 0.22 | 0.06–0.85 | 0.03 | ||
| Secondary outcome | |||||
| Length of stay in the severe COVID‐19 patient unit | −24.4% | −56.3% to 30.8% | 0.32 | ||
CI, confidence interval; OR, odds ratio.
In terms of subgroup analysis, Table 4 presents the results of the bivariate and propensity score‐adjusted analysis for the length of stay in the severe COVID‐19 unit among patients without intubation (median length of stay in the severe COVID‐19 unit among patients without intubation was 4.0 days; IQR, 2.0–7.0 days). The length of stay in the severe COVID‐19 unit was significantly longer among those who received awake PP than among those who did not (median length of stay in the severe COVID‐19 unit was 5.0 days for those who received awake PP and 3.0 days for those who did not; P = 0.02). However, we did not detect a significant between‐group difference in the length of stay in the severe COVID‐19 patient unit among patients without intubation after propensity score‐adjusted analysis (adjusted percentage difference, 15.3%; 95% CI, −39.4% to 119.4%; P = 0.66).
Table 4.
Results of bivariate and propensity score‐adjusted analyses for length of stay in a severe COVID‐19 patient unit among patients without intubation
| Bivariate analysis | Total (n = 83) | With awake prone positioning (n = 49) | Without awake prone positioning (n = 34) | P value |
|---|---|---|---|---|
| Length of stay in the severe COVID‐19 patient unit, days; median among patients without intubation (IQR) | 4.0 (2.0–7.0) | 5.0 (3.0–9.0) | 3.0 (2.0–5.0) | 0.02 |
| Propensity score‐adjusted analysis | Adjusted percentage difference | 95% CI of adjusted percentage difference | P value |
|---|---|---|---|
| Length of stay in the severe COVID‐19 patient unit among patients without intubation | 15.3% | −39.4% to 119.4% | 0.66 |
CI, confidence interval; IQR, interquartile range.
Discussion
We hypothesized that awake PP might contribute to reducing the intubation rate. There are three reasons why avoiding intubation is essential. First, intubation is associated with certain complications such as dysphagia, aspiration, and aspiration pneumonia. 22 , 23 Avoiding intubation is an important clinical factor that contributes to the prognosis of the patient. Second, intubation is an aerosol‐generating procedure, and it poses a risk of COVID‐19 infection for medical staff who intubate patients with COVID‐19. 24 Third, COVID‐19 has led to resource limitations. In the United States, perhaps the earliest example was the near‐immediate understanding that there were insufficient high‐filtration N‐95 masks for health care worker use, prompting contingency guidance on how to reuse masks designed for single use. Physicians in Italy have proposed directing crucial resources, such as intensive care beds and ventilators, to patients with COVID‐19 who can benefit most from treatment. 25 , 26 Thus, a decrease in the intubation rate might be beneficial not only for patients but also for reducing the contamination risk for medical personnel as well as for resource‐limited scenarios.
Several studies have reported the positive effects of awake PP on oxygenation parameters in awake, nonintubated patients with COVID‐19‐associated ARDS. 27 , 28 Awake PP reduces ventilation/perfusion mismatching, hypoxemia, and shunting. Because of gravitational effects and conformational shape matching of the lung with the chest cavity, awake PP decreases the pleural pressure gradient between the dependent and nondependent lung regions. This is believed to generate more homogeneous lung aeration and strain distribution, thereby enhancing the recruitment of the dorsal lung unit. 29 This might decrease the respiratory effort, which in turn could decrease the incidence of self‐induced lung injury. Therefore, the use of awake PP might allow patients to postpone or avoid tracheal intubation. Ehrmann et al. also reported that awake PP appeared to be safe and had a favorable effect on the primary composite outcome of intubation. 30 In our study, the results obtained on circumventing intubation were relatively better than those reported by Ehrmann et al. There could be two reasons for this. First, the median daily duration of awake PP in the study by Ehrmann et al. was 5.0 h (IQR, 1.6–8.8). By contrast, the protocol used in our study encouraged the use of awake PP for at least 6 h/day. Second, Ehrmann et al. did not mention any details regarding standard care. Thus, the standard care might have differed between our study and that by Ehrmann et al., thus resulting in the discrepancy between the findings.
In a previous study, the length of hospital stay was associated with the intubation rate. 31 However, in our study, we found that the length of stay in the severe COVID‐19 patient unit was not statistically different between patients who received awake PP and those who did not, despite the significant difference in the intubation rate. In the subgroup analysis, we found that the length of stay in the severe COVID‐19 unit was longer among patients who received awake PP than those who did not receive awake PP. We collected data pertaining to SpO2/FiO2 from patients without intubation and found a significant difference between the two groups (with awake PP: median 242.5 mmHg, IQR 202.2–290.6; without awake PP: median 332.1 mmHg, IQR 250.0–395.8; P < 0.01). As a result, patients without awake PP were discharged from the severe COVID‐19 units earlier. We could not determine the difference in the length of stay between the groups in the severe COVID‐19 patient unit after propensity score‐adjusted analysis. Previous research has shown that patients with COVID‐19 who require hospitalization or intensive care treatment experience severe functional impairments after treatment and require continuous aftercare. 32 However, the COVID‐19 pandemic has led to a shortage of beds and an increase in the number of infectious admissions, and transferring these patients to the general ward or another hospital is challenging. 33 Furthermore, some patients in our study developed another disease (e.g., urinary tract infection and aspiration pneumonia) during their stay in the severe COVID‐19 patient unit. We believe that these factors influenced the length of stay in the severe COVID‐19 patient unit.
This study had several limitations. First, the sample size was small. Despite the early exposure, dense and aging population, and minimal social distancing measures, Japan has reported low infection and mortality rates from COVID‐19. 34 We were unable to evaluate a larger cohort. The power of this study might have been inadequate.
Second, approximately 30% of the patients were transferred from another hospital. These patients may have received pharmacotherapy other than remdesivir, dexamethasone, and anticoagulant therapy and awake PP could have been carried out at the previous hospital for our study participants who did not receive awake PP at our institutions.
The third limitation was missing data. Due to the contagious nature of COVID‐19, certain examinations—such as computed tomography, body measurements, and blood gas tests—were carried out in a limited manner. In particular, arterial blood gas was not measured in most patients or was measured several hours after admission. Thus, we used the SpO2/FiO2 ratio at admission. 35 However, these deficits could have affected our results.
The fourth limitation was the retrospective nature of this study. This study design is less powerful than that of randomized control trials. However, the design of our study was still sufficiently robust because it was supplemented by propensity score adjustment. This study could serve as an innovative and novel basis for future randomized control studies.
Conclusions
Awake PP might be correlated with the intubation rate in patients with severe illness COVID‐19 who were treated with remdesivir, dexamethasone, and anticoagulant therapy.
Disclosure
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Acknowledgments
The authors would like to thank Enago for the English language review.
References
- 1. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tavernier E, McNicholas B, Pavlov I et al. Awake prone positioning of hypoxaemic patients with COVID‐19: protocol for a randomised controlled open‐label superiority meta‐trial. BMJ Open 2020; 10: e041520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N. Engl. J. Med. 2020; 383: 2451–60. [DOI] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 5. Scholten EL, Beitler JR, Prisk GK et al. Treatment of ARDS with prone positioning. Chest 2017; 151: 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guérin C, Reignier J, Richard JC et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013; 368: 2159–68. [DOI] [PubMed] [Google Scholar]
- 7. Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur. Respir. J. 2002; 20: 1017–28. [DOI] [PubMed] [Google Scholar]
- 8. Ding L, Wang L, Ma W et al. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi‐center prospective cohort study. Crit. Care 2020; 24: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrando C, Mellado‐Artigas R, Gea A et al. Awake prone positioning does not reduce the risk of intubation in COVID‐19 treated with high‐flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit. Care 2020; 24: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardona S, Downing J, Alfalasi R et al. Intubation rate of patients with hypoxia due to COVID‐19 treated with awake proning: a meta‐analysis. Am. J. Emerg. Med. 2021; 43: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stasi C, Fallani S, Voller F et al. Treatment for COVID‐19: an overview. Eur. J. Pharmacol. 2020; 889: 173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moriarty LF, Plucinski MM, Marston BJ et al. Worldwide, February–March 2020. MMWR Morb. Mortal. Wkly Rep. 2020; 69: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. RECOVERY Collaborative Group , Horby P, Lim WS et al. Dexamethasone in hospitalized patients with Covid‐19 — preliminary report. N. Engl. J. Med. 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zhang D, Du G et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet 2020; 395: 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang N, Bai H, Chen X et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020; 18: 1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institutes of Health. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease (COVID‐19) Treatment Guidelines. 2019. Available from: https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 17. Bower G, He H. Protocol for awake prone positioning in COVID‐19 patients: to do it earlier, easier, and longer. Crit. Care 2020; 24: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coppo A, Bellani G, Winterton D et al. Feasibility and physiological effects of prone positioning in non‐intubated patients with acute respiratory failure due to COVID‐19 (PRON‐COVID): a prospective cohort study. Lancet Respir. Med. 2020; 8: 765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ayanian S, Reyes J, Lynn L et al. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark. Med 2020; 14: 1091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi D, Kuriyama N, Yanase F et al. Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker use prior to medical intensive care unit admission and in‐hospital mortality: propensity score‐matched cohort study. J. Nephrol. 2019; 32: 595–603. [DOI] [PubMed] [Google Scholar]
- 21. Brookhart MA, Schneeweiss S, Rothman KJ et al. Variable selection for propensity score models. Am. J. Epidemiol. 2006; 163: 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tikka T, Hilmi OJ. Upper airway tract complications of endotracheal intubation. Br. J. Hosp. Med. (Lond.) 2019; 80: 441–7. [DOI] [PubMed] [Google Scholar]
- 23. Frajkova Z, Tedla M, Tedlova E et al. Postintubation dysphagia during COVID‐19 outbreak‐contemporary review. Dysphagia 2020; 35: 549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zahoor M, Haq IU. Airway management for emergent surgeries during COVID‐19 pandemic. J. Coll. Physicians Surg. Pak. 2021; 30: S35–7. [DOI] [PubMed] [Google Scholar]
- 25. Vergano M, Bertolini G, Giannini A et al. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource‐limited circumstances: the Italian perspective during the COVID‐19 epidemic. Crit. Care 2020; 24: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emanuel EJ, Persad G, Upshur R et al. Fair allocation of scarce medical resources in the time of Covid‐19. N. Engl. J. Med. 2020; 382: 2049–55. [DOI] [PubMed] [Google Scholar]
- 27. Caputo ND, Strayer RJ, Levitan R. Early self‐proning in awake, non‐intubated patients in the emergency department: a single ED's experience during the COVID‐19 pandemic. Acad. Emerg. Med. 2020; 27: 375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elharrar X, Trigui Y, Dols A‐M et al. Use of prone positioning in nonintubated patients with COVID‐19 and hypoxemic acute respiratory failure. JAMA 2020; 323: 2336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koeckerling D, Barker J, Mudalige NL et al. Awake prone positioning in COVID‐19. Thorax 2020; 75: 833–4. [DOI] [PubMed] [Google Scholar]
- 30. Ehrmann S, Li J, Ibarra‐Estrada M et al. Awake prone positioning for COVID‐19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open‐label meta‐trial. Lancet Respir. Med. 2021; 9: 1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rees EM, Nightingale ES, Jafari Y et al. COVID‐19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020; 18: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. bij de Vaate E, KHL G, Goossens PH. Personalized recovery of severe COVID19: rehabilitation from the perspective of patient needs. Eur. J. Clin. Investig. 2020; 50: e13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayasaki E. Covid‐19: how Japan squandered its early jump on the pandemic. BMJ 2020; 369: m1625. [DOI] [PubMed] [Google Scholar]
- 34. Iwasaki A, Grubaugh ND. Why does Japan have so few cases of COVID‐19? EMBO Mol. Med. 2020; 12: e12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen W, Janz DR, Shaver CM et al. Clinical characteristics and outcomes are similar in ARDS diagnosed by oxygen saturation/Fio2 ratio compared with Pao2/Fio2 ratio. Chest 2015; 148: 1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


