Abstract
Although opioid agonist therapy (OAT) is associated with positive health outcomes, including improved HIV management, long-term retention in OAT remains low among patients with opioid use disorder (OUD). Using data from the Veterans Aging Cohort Study (VACS), we identify variables independently associated with OAT retention overall and by HIV status. Among 7,334 patients with OUD, 13.7% initiated OAT, and 27.8% were retained 12-months later. Likelihood of initiation and retention did not vary by HIV status. Variables associated with improved likelihood of retention included receiving buprenorphine (relative to methadone), receiving both buprenorphine and methadone at some point over the 12-month period, or diagnosis of HCV. History of homelessness was associated with a lower likelihood of retention. Predictors of retention were largely distinct between patients with HIV and patients without HIV. Findings highlight the need for clinical, systems, and research initiatives to better understand and improve OAT retention.
Keywords: opioid-related disorders, buprenorphine, methadone, HIV, Veteran health
INTRODUCTION
Formulations of methadone or buprenorphine—opioid agonist therapy (OAT)—are the recommended treatment of opioid use disorder (OUD) (1,2). Retention in OAT is considered a crucial indicator of treatment success and is associated with improvements in disease outcomes, including hepatitis C (HCV) and HIV, reductions in opioid use, hospital-based care, and all-cause and overdose mortality (3–7). This is particularly relevant among people with HIV (PWH), who experience a high burden of OUD (8–10), as the health benefits of OAT extend to key HIV-related health behaviors and outcomes, including antiretroviral initiation and adherence, and improved CD4 lymphocyte counts (11,12). Moreover, OAT retention is associated with decreased injection-related HIV risk behaviors and long-term virologic success (5,13–14).
While there is no consensus regarding the optimal length of time to remain on OAT, like other chronic conditions, research supports longer duration of treatment episodes (15). Among patients diagnosed with OUD, negative outcomes frequently resurface upon discontinuation, even following lengthy periods of treatment (4,16). Research documenting superior outcomes among patients retained in OAT for longer than 12 months, relative to shorter periods, suggests that 12 months may indicate a minimum threshold for quality treatment (4,15).
Studies document wide variability in the extent to which patients meet this 12-month threshold, as well as differences by medication-type. One systematic review reported rates of 12-month retention ranging from 37–91% among randomized controlled trials (n=6), and 26–85% among non-randomized studies (n=12) (17). The review also found that methadone retention was generally superior to buprenorphine retention, with three studies reporting greater six-month retention among patients receiving methadone relative to buprenorphine, and two studies finding no difference (17). More recently, a systematic review of both randomized controlled trials and observational studies reported median 12-month retention across studies of 61% (range 20–94%) for methadone (n=24) and 45% (range 12%–62%) for buprenorphine (n=6) (18). Both reviews included studies diverse in geographic and clinical setting, patient population and treatment approach, which may explain the variance in rates of retention identified. However, neither study explicitly addressed the question of why rates of retention differed across studies.
Few consistent predictors of OAT retention have been identified. One study, analyzing predictors of buprenorphine retention within the US Veterans Health Administration (VHA), identified African-American race/ethnicity, medical comorbidity (Charlson Index), and emergency department visits in the initial 12 months of treatment as predictive of lower OAT retention (19). Another study, examining buprenorphine retention in a large urban safety net clinic, found female gender, psychiatric diagnosis and older age were associated with higher odds of retention, while unemployment, HCV diagnosis and African-American and Hispanic/Latino race/ethnicity were associated with lower odds of retention (20). Despite the high burden of OUD among PWH, and the importance of OAT receipt for key health outcomes, the association between HIV status and OAT retention remains unknown. PWH face high rates of HCV diagnoses and other comorbidities and may interact with the health system through infectious disease rather than general medicine clinics. Thus, investigating HIV status as a predictor of OAT retention may have important implications for clinical practice, as well as more generally for public health knowledge regarding a vulnerable population.
In this study, we utilize a large national cohort of PWH and patients without HIV (PWOH) to identify predictors of 12-month retention in OAT among patients treated within the VHA. Results from this study can be used to identify patients at risk of premature OAT discontinuation, and inform clinical interventions intended to support greater retention in treatment.
METHODS
Data Source
We used data from the Veterans Aging Cohort Study (VACS) (21), a national cohort of VHA patients including PWH (55,984) and 1:2 matched PWOH (116,705) who received care within VHA from October 1996-September 2017. PWH and PWOH are matched, along age, race, gender, geographic region, and fiscal year of cohort entry. Electronic medical record data is drawn from the Corporate Data Warehouse, which include International Classification of Disease (ICD) 9/10 codes, pharmacy and laboratory data, and codes representing clinic visits (stop codes). Information regarding HIV infection is determined by ICD-9-CM codes 042–044 (AIDS) and V08 (asymptomatic HIV) and ICD-10 codes (B20, Z21).
Analytic Sample
From the larger cohort, we first identified patients with a new OUD-related behavioral treatment encounter from 10/2008–09/2016. We selected 2008 as the starting date for this study as it closely follows widespread operational efforts to expand veteran access to buprenorphine for the treatment of OUD (22). We used 9/2016 as the encounter end-date to allow for one full year of observation (up to 9/2017) following the initial encounter. All data were extracted from the VHA Corporate Data Warehouse. Patients were eligible for inclusion if they had at least 12 months of observation time within the VHA system preceding their initial OUD treatment episode. New OUD diagnosis was defined as absence of any past year OUD treatment or diagnosis, while new OUD treatment episodes were defined by the presence of inpatient or outpatient encounters documenting a primary or secondary diagnosis of opioid abuse or dependence (ICD-9-CM codes 304.0x, 305.5x and 304.7x; ICD-10 codes F11.1x and F11.2x) and either a substance use disorder (SUD) treatment specialty code (inpatient or outpatient), mental health procedure code or outpatient current procedural terminology (CPT) code (23). From this sample, we then identified patients who initiated OAT within one year of their initial OUD-related behavioral treatment encounter. The study protocol was approved by the Institutional Review Boards of the University of Pittsburgh, VA Pittsburgh Healthcare System and VA Connecticut Health System.
Measures
Retention
We examined 12-month retention in any OAT (methadone or buprenorphine), methadone alone, and buprenorphine alone. Methadone OAT receipt was identified by patient visits to VHA opioid treatment programs (OTPs) through stop code 523 designations. VHA data do not capture outpatient methadone dispensation, only patient visits to an OTP, which is a limitation of the data. Inpatient methadone prescriptions were counted towards retention if patients had initiated methadone within an OTP prior to hospitalization. We classified patients as retained on methadone if they had an OTP encounter recorded 4+ times/month in the first month of treatment and 1+ visits/month in months 2–12. While less frequent than called for by federal attendance requirements (24), VHA data record all OTP visits consisting solely of methadone dispensation as a single weekly encounter. For a visual display of the four most common patterns of OTP visits we identified through trajectory analysis, see Appendix 1. Buprenorphine OAT was defined by prescription dispensation determined through VHA Pharmacy Benefits Management records of any formulations of buprenorphine or buprenorphine/naloxone indicated for treatment of OUD; formulations of buprenorphine with a primary indication for pain were excluded. To identify buprenorphine retention, we classified patients who had ≥80% of days covered over the period of interest as retained (25,26). Patients were required to have buprenorphine prescription coverage for 28+ days in their first month of treatment, 170 days in the first six months of treatment, and 292 days over 12 months to be considered retained.
To construct our primary outcome of retention in any OAT, we included patients retained under both the methadone only and buprenorphine only retention definitions, as well as patients who met either medication-specific retention definitions but had switched between medications over the course of their treatment. However, patients who switched between medications but did not meet either medication-specific retention criteria were not counted as retained.
Covariates
We incorporated individual-level variables commonly associated with OUD and SUD treatment outcomes in prior literature including age, gender, race/ethnicity, HIV-status, HCV-status (identified by positive HCV viral load, positive antibody test, or clinical diagnosis as based on ICD-9/10 codes), urban versus rural residence (defined by rural urban commuting area (RUCA) codes), alcohol related diagnosis (ICD-9 diagnostic codes 303.01–303.03, 303.90–303.93, 305.01–305.03); non-SUD mental health diagnoses (major depression, anxiety, bipolar disorder, post-traumatic stress disorder, schizophrenia) by ICD-9 codes, year of OAT initiation (<2011 vs. ≥2011) and history of homelessness (ICD-9 code V60.0) (27,28). In analyses utilizing just the PWH sample, we included CD4 count and receipt of ART; HIV was stratified by detectable (VL>=500 copies/mL) versus non-detectable viral load (VL<500 copies/mL). Values closest to the date of the OUD behavioral treatment index visit were used for analysis (up to one year prior).
Analysis
We used chi-square and t-tests to compare differences in socio-demographic and clinical characteristics between PWH and PWOH and between those who were and were not retained in OAT at 12-months. Variables that were significantly (p <0.05) associated with retention in the univariate analyses were included in the multivariable logistic regression models. Model outcomes included 12-month retention in any OAT, methadone only, and buprenorphine only using our primary retention definitions. Models were run overall and stratified by HIV status. We reported the adjusted odds ratios with 95% confidence intervals.
As secondary outcomes, we examined retention in any OAT, methadone only, and buprenorphine only at one and six months to investigate the potential for differences in predictors of OAT discontinuation earlier in the treatment period. Analyses were conducted on the full sample and stratified by HIV. Stata version 14 was used to conduct all analyses (29).
RESULTS
Participants
Between the period 10/2008 and 9/2016, we identified 2,523 PWH and 4,811 PWOH who had a new OUD behavioral treatment episode and 12 months of follow-up data. Of these, we identified 356 PWH and 647 PWOH who initiated OAT within the following 12 months, representing 13.7% of eligible patients (14.1% for PWH and 13.5% for PWOH; z=0.61, p=0.43). Of patients who initiated OAT, 57.6% initiated methadone and 42.4% initiated buprenorphine. Of patients who initiated methadone, 6.3% later switched to buprenorphine. Of those who initiated buprenorphine, 6.7% later switched to methadone.
Bivariate comparisons
Although PWH were older on average than PWOH (55.6 versus 53.8, t=−3.50, p<0.001), no other socio-demographic characteristics differed significantly between PWH and PWOH (Table I). Overall, 59% of the sample were African-American, 30% were white, 98% were male, 86% were living in urban areas, and 61% had a history of homelessness. PWH were more likely to be diagnosed with HCV relative to PWOH (76.4% vs 53.2%, χ2=52.32, p<0.001), and PWH were less likely to have a mental health comorbidity relative to PWOH (33.2 % versus 41.9%, χ2=7.39, p=0.01).
Table I:
Characteristics of participants with at least one Opioid Use Disorder (OUD) diagnosis who initiated opioid agonist treatment for OUD from 2008–2016, stratified by HIV status (N =1,003)
| PWH (n =356) | PWOH (n =647) | t-statistic or χ2 | p-value | |
|---|---|---|---|---|
| Age, mean years (SD) | 55.6 (6.9) | 53.8 (8.3) | −3.49 | <0.001 |
| Race/Ethnicity, n (%) | 2.45 | 0.49 | ||
| White | 99 (27.8) | 205 (31.7) | ||
| African-American | 215 (60.4) | 375 (58.0) | ||
| Latino/other | 42 (11.8) | 67 (10.4) | ||
| Male gender | 348 (97.8) | 638 (98.6) | 1.01 | 0.32 |
| Rurality, n (%) | 21 (5.9) | 47 (7.3) | 3.91 | 0.14 |
| History of Homelessness, n (%) | 226 (63.5) | 385 (59.5) | 1.53 | 0.22 |
| Hepatitis C, n (%) | 272 (76.4) | 344 (53.2) | 52.32 | <0.001 |
| Any mental health diagnosis, n (%) | 118 (33.2) | 271 (41.9) | 7.39 | 0.01 |
| Major depression | 38 (10.7) | 88 (13.6) | 1.79 | 0.18 |
| Anxiety | 16 (4.5) | 57 (8.8) | 6.34 | 0.01 |
| PTSD | 31 (8.7) | 85 (13.1) | 4.41 | 0.04 |
| Bipolar disorder | 26 (7.3) | 61 (9.4) | 1.31 | 0.25 |
| Schizophrenia | 11 (3.1) | 43 (6.7) | 5.70 | 0.02 |
| Unhealthy alcohol use (AUDIT-C), n (%) | 98 (28.2) | 178 (28.2) | 0.00 | 0.99 |
| Alcohol related diagnosis, n (%) | 111 (31.2) | 225 (34.8) | 1.33 | 0.25 |
| Initiated OUD treatment 2011–2016, n (%) | 187 (52.5%) | 355 (54.9%) | 0.51 | 0.48 |
| Viral load suppressed (<500 copies/mL), n (%) | 224 (74.0) | |||
| CD4 ≥ 200 cells/mm3, n (%) | 248 (82.0)a | -- | -- | |
| Antiretroviral therapy receipt, n (%) | 296 (83.2) | -- | -- |
Abbreviations: SD, standard deviation; PTSD, post-traumatic stress disorder; AUDIT-C, Alcohol Use
Disorders Identification Test - Consumption.
All data cells are listed as n (%) except where mean (SD) is indicated.
T-statistic is reported for age; χ2 is reported for all other patient characteristics.
52 patients were missing data on this outcome.
Among patients who initiated any OAT, 27.8% were retained for 12 months (29.8% for PWH and 26.7% for PWOH), and likelihood of retention did not vary by HIV status (χ2=1.05, p=0.30). (Table II) Likewise, year of initiation did not affect likelihood of retention (28.4% for 2008–2010 and 27.3% for 2011–2016; χ2=0.15, p=0.70). The 12-month retention rate was 31.2% among those initiated on buprenorphine and 20.3% among those initiated on methadone. Likelihood of retention did not differ by HIV status for either methadone (χ2=1.90, p= 0.17) or buprenorphine (χ2=0.30, p= 0.58). We then identified 12-month retention among patients who had received a combination of methadone and buprenorphine at some point over the year (these patients were included in prior retention numbers based on the medication that they had initiated). Of these patients, 35.6% were retained at 12 months. (See Table II for rates of retention at one and six months.)
Table II:
Retention of patients in buprenorphine, methadone or any OAT following treatment initiation (n=1,003) (2008–2017), stratified by HIV status, bivariate comparisons
| Retention in OAT, n (%) | |||
|---|---|---|---|
| Buprenorphine (n=488) | Methadone (n=675) | Any OAT (n=1,003) | |
| One month | |||
| PWH | 120 (71.4) | 124 (51.2) | 223 (62.6) |
| PWOH | 234 (73.1) | 224 (51.7) | 425 (65.7) |
| Total | 354 (72.5) | 348 (51.6) | 648 (64.6) |
| Six months | |||
| PWH | 82 (48.8) | 74 (30.6) | 145 (40.7) |
| PWOH | 153 (47.8) | 131 (30.3) | 272 (42.0) |
| Total | 235 (48.2) | 205 (30.4) | 417 (41.6) |
| 12 months | |||
| PWH | 55 (32.7) | 56 (23.1) | 106 (29.8) |
| PWOH | 97 (30.3) | 81 (18.7) | 173 (26.7) |
| Total | 152 (31.2) | 137 (20.3) | 279 (27.8) |
No statistically significant differences were identified.
Patients who switched between methadone and buprenorphine over the course of the year were included in both individual medication retention calculations.
Methadone retention is defined as 4+ visits to an OTP in the first month of treatment and 1+ times in months 2–12. Buprenorphine retention is defined as buprenorphine prescription coverage for 28+ days in month 1, 170 days in the first six months of treatment, and 292 days over a year, or ≥80% of days covered. Patients retained under either the methadone only or buprenorphine only retention definitions met our criteria for retention in any OAT.
To rule out the possibility that buprenorphine initiated within an OTP may explain the observed low rate of methadone retention, we excluded patients with a visit to an OTP who had received buprenorphine at any point, and found similarly low rates of methadone retention.
Multivariable analyses for one-year retention in OAT– full sample
Adjusted analyses utilizing the full sample revealed that, relative to receiving methadone alone, receiving buprenorphine (adjusted odds ratio [aOR] =1.42; 95% CI: 1.02–1.98), or receiving both methadone and buprenorphine at some point during the year (aOR=1.85; 95% CI 1.24–2.76) was associated with a higher likelihood of 12-month retention (Table III). Patients with a diagnosis of HCV were also more likely to be retained (aOR = 1.47; 95% CI 1.06–2.03). In contrast, history of homelessness (aOR=0.49; 95% CI 0.36–0.66) was associated with a lower likelihood of 12-month retention.
Table III.
Multivariable logistic regression, predictors of 12-month retention in any OAT, overall and by HIV
| Predictors of 12-month retention in OAT | |||
|---|---|---|---|
| Full Sample | PWH | PWOH | |
| aOR (95% CI) (n=1003) | aOR (95% CI) (n=356) | aOR (95% CI) (n=647) | |
| Age at initiation | 1.00 (0.98– 1.02) | 1.00 (0.96– 1.03) | 1.00 (0.98– 1.03) |
| Race/Ethnicity (ref = white) | |||
| African-American | 0.77 (0.54–1.07) | 0.66 (0.38–1.16) | 0.83 (0.54–1.28) |
| Latino/other | 0.84 (0.51–1.39) | 0.61 (0.27–1.40) | 1.02 (0.54–1.93) |
| Mental health diagnosis (ref = none) | 0.81 (0.59–1.11) | 1.07 (0.63–1.82) | 0.70 (0.47–1.04) |
| Alcohol related diagnosis | 0.86 (0.61–1.20) | 0.96 (0.56–1.65) | 0.85 (0.55–1.31) |
| Buprenorphine (ref=methadone) | 1.42 (1.02–1.98) | 1.48 (0.87–2.51) | 1.40 (0.91–2.15) |
| Buprenorphine and methadone (ref=methadone) | 1.85 (1.24–2.76) | 1.75 (0.89–3.41) | 1.98 (1.19–3.30) |
| HIV Status | 1.10 (0.81–1.49) | ||
| HIV viral load suppressed (ref=detectable) | 1.17 (0.65–2.12) | ||
| Hepatitis C | 1.47 (1.06–2.03) | 1.99 (1.08–3.68) | 1.31 (0.88–1.94) |
| Rural location (ref = urban) | 1.02 (0.97–1.09) | 1.05 (0.96–1.15) | 1.03 (0.95–1.12) |
| History of homelessness | 0.49 (0.36–0.66) | 0.80 (0.48–1.33) | 0.37 (0.25–0.54) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval
Any OAT is defined as either buprenorphine or methadone.
In models stratified by HIV status, some notable differences emerged. Among PWH, only HCV diagnosis remained significantly associated with retention. Receiving buprenorphine, or a combination of buprenorphine and methadone, and history of homelessness were not significantly associated with retention in the PWH only model. Among PWOH, receiving buprenorphine-relative to methadone alone-was not significantly associated with retention, nor was HCV diagnosis.
Buprenorphine Retention
Among all patients who filled prescriptions for buprenorphine (n=488), only history of homelessness (aOR=0.50; 95% CI 0.33–0.77), was associated with a decreased likelihood of 12-month retention. (Table IV). Among PWH, rurality was positively associated with retention (aOR=1.15; 95% CI 1.00–1.33), but history of homelessness was not. Akin to the main model, among PWOH only homelessness was associated with a lower likelihood of retention (aOR=0.33; 95% CI 0.19–0.57).
Table IV.
Predictors of 12-month retention in buprenorphine, full sample and stratified sample
| Predictors of 12-month retention in buprenorphine | |||
|---|---|---|---|
| Full Sample (n=488) | PWH (n=168) | PWOH (n=320) | |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age at initiation | 0.99 (0.97–1.02) | 0.98 (0.93–1.03) | 1.00 (0.97–1.03) |
| Race/Ethnicity (ref = white) | |||
| African-American | 0.69 (0.44–1.07) | 0.49 (0.22–1.11) | 0.76 (0.44–1.33) |
| Latino/other | 1.09 (0.56–2.13) | 1.12 (0.36–3.43) | 1.10 (0.46–2.62) |
| Mental health diagnosis (ref = none) | 1.17 (0.77–1.78) | 1.24 (0.57–2.71) | 1.16 (0.69–1.94) |
| Alcohol related diagnosis | 0.97 (0.61–1.54) | 1.19 (0.53–2.69) | 1.02 (0.56–1.86) |
| HIV Status | 1.20 (0.79–1.84) | ||
| HIV viral load suppressed (ref=detectable) | 1.46 (0.57–3.73) | ||
| Hepatitis C | 1.33 (0.86–2.08) | 2.25 (0.96–5.30) | 1.08 (0.62–1.88) |
| Rural location (ref = urban) | 1.05 (0.96–1.13) | 1.15 (1.00–1.33) | 1.01 (0.91–1.13) |
| History of homelessness | 0.50 (0.33–0.77) | 1.13 (0.53–2.43) | 0.33 (0.19–0.57) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval
Methadone Retention
Among all patients with methadone visits to an OTP (n=675), diagnosis of HCV (aOR=1.58; 95% CI 1.01–2.48) was associated with a higher likelihood of 12-month retention, while mental health diagnosis (aOR=0.61; 95% CI 0.38– 0.96) and history of homelessness (aOR=0.53; 95% CI 0.35–0.79) were associated with a lower likelihood of retention. (Table V). Among PWH, only Latino/other race/ethnicity was associated with a lower likelihood of retention (aOR=0.21; 95% CI 0.05– 0.83). Among PWOH, those with a mental health diagnosis, (aOR=0.38; 95% CI 0.20–0.70), and those with a history of homelessness (aOR=0.45; 95% CI 0.26–0.76) were less likely to be retained at 12 months.
Table V.
Predictors of 12-month retention in methadone, full sample and stratified sample
| Predictors of 12-month retention in methadone | |||
|---|---|---|---|
| Full Sample (n=675) | PWH (n=242) | PWOH (n=433) | |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age at initiation | 1.01 (0.98–1.04) | 1.03 (0.98–1.08) | 1.01 (0.97–1.04) |
| Race/Ethnicity (ref = white) | |||
| African-American | 0.94 (0.57–1.54) | 0.67 (0.31–1.45) | 1.22 (0.61–2.41) |
| Latino/other | 0.71 (0.34–1.49) | 0.21 (0.05–0.83) | 1.52 (0.59–3.91) |
| Mental health diagnosis (ref = none) | 0.61 (0.38–0.96) | 1.23 (0.60–2.51) | 0.38 (0.20–0.70) |
| Alcohol related diagnosis | 0.76 (0.48–1.20) | 0.72 (0.35–1.46) | 0.83 (0.45–1.53) |
| HIV Status | 1.14 (0.75–1.71) | ||
| HIV viral load suppressed (ref=detectable) | 0.93 (0.43–2.00) | ||
| Hepatitis C | 1.58 (1.01–2.48) | 1.46 (0.63–3.38) | 1.70 (0.98–2.95) |
| Rural location (ref = urban) | 0.99 (0.91–1.08) | 0.97 (0.85–1.11) | 1.05 (0.94–1.18) |
| History of homelessness | 0.53 (0.35–0.79) | 0.68 (0.34–1.33) | 0.45 (0.26–0.76) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval
Secondary outcomes: Multivariate retention in OAT, methadone only and buprenorphine only at one and six months, overall and by HIV status
In analyses examining predictors of one and six-month retention, there were a few notable differences from the primary models. Those of African-American race/ethnicity were less likely to be retained in several specifications - e.g., retention in any OAT at six months among the full sample, (aOR=0.67; 95% CI 0.49–0.92) and PWOH, (aOR=0.63; 95% CI 0.43–0.94), as well as buprenorphine retention at six months, (aOR=0.56; 95% CI 0.37–0.84), and one month, (aOR=0.51; 95% CI 0.32–0.81). HCV diagnosis was positively associated with retention in any OAT among PWOH at one month (aOR=2.07; 95% CI 1.43–3.00), and six months, (aOR=1.52; 95% CI 1.07–2.16) and retention in methadone among PWOH at one month, (aOR=2.18; 95% CI 1.43–3.31) and six months, (aOR=1.76; 95% CI 1.11–2.80). Unlike the 12-month retention model, receiving buprenorphine, relative to methadone, was predictive of retention on any OAT among PWOH at one month, (aOR=2.48; 95% CI 1.64–3.73) and six months, (aOR=1.72; 95% CI 1.17–2.52), as well as PWH at one month, (aOR=2.91; 95% CI 1.71–4.95) and six months, (aOR=2.17; 95% CI 1.31–3.61). Receiving a combination of methadone and buprenorphine was also predictive of retention on any OAT among PWH at one month, (aOR=10.39; 95% CI 4.11–26.24) and six months, (aOR=3.66; 95% CI 1.90–7.05).
DISCUSSION
We identified low rates of initiation and 12-month retention in OAT among PWH and PWOH, with no difference in the likelihood of retention by HIV status. Less than 14% of patients with OUD initiated OAT, six months following initiation less than half were retained, and by 12 months just 28% were retained. In models utilizing the full sample, retention was higher among patients initiating buprenorphine relative to methadone, as well as among patients who had received a combination of methadone and buprenorphine sequentially. HCV diagnosis also predicted higher likelihood of retention, while history of homelessness was associated with a lower likelihood retention. Akin to prior research, our primary models identified relatively few individual characteristics predictive of OAT retention (19). It should be noted, however, that, consistent with some prior research, some secondary model specifications revealed lower odds of retention among African-American patients, suggesting the need for further efforts to reduce disparities in OAT retention (30). Future research may yield greater insights if analyses distinguish between patients whose OAT discontinuation reflects a decision made by the clinician versus those whose decision to discontinue was made by the patient, as grouping these distinct populations together may obscure important differences in predictors.
Although rates of retention did not vary by HIV status, stratified analyses revealed that predictors of retention were largely distinct for PWH and PWOH. Specifically, history of homelessness was negatively associated with retention among PWOH, but not PWH, while HCV diagnosis was positively associated with retention among PWH, but not PWOH. Further, among PWOH, receiving a combination of buprenorphine and methadone was associated with an increased likelihood of retention, but receiving buprenorphine alone was not, while among PWH there were no differences in retention associated with type of medication received.
While the rates of retention we identify through 2017 are relatively low, and similar between those who initiated treatment between 2008–2010 and 2011–2016, VHA has undertaken multiple national initiatives to expand OAT treatment accessibility since 2018 (e.g., expanding access to SUD treatment through telemedicine; initiatives to improve access to MOUD through primary care, mental health, and pain clinics) (31–32). Thus, we would expect to see higher rates of OAT retention moving forward, although this requires confirmation in future research.
Distinctions in predictors identified between PWH and PWOH suggest differences in care delivery and/or services available to PWOH and PWH within VHA. For instance, robust staffing models within VHA Infectious Disease clinics, which typically include social workers and case managers, may help facilitate connections to supportive housing services, which allow for stability and retention in OAT (33). This aligns with research that has shown HIV positive status to be negatively associated with homelessness among patients in VHA care (34). It may also be the case that PWH simply have more clinical encounters than do PWOH, which could allow for greater opportunities to connect with needed services. The positive association between HCV diagnosis and retention among PWH, but not PWOH, suggest that VHA’s recent extensive efforts to engage patients with HCV in treatment with direct-acting antivirals via expanded funding, patient outreach, and system redesign (35) may have specifically targeted infectious disease clinics where PWH frequently receive care and/or may have prioritized PWH. If confirmed by future research, these findings suggest that staffing models and support services provided by VHA to PWH within HIV Clinics are having important positive effects for health and may prove beneficial if expanded to PWOH, as well as patient in other healthcare organizations. Distinctions in the association between medication type by HIV status are surprising but may reflect small sample size rather than true differences. Secondary analyses support this interpretation, showing improved rates of retention at one and six months among both PWH and PWOH for both buprenorphine and the medication combination relative to methadone alone.
Patient retention identified in our analysis differs substantially from the 61.6% of VHA patients retained on buprenorphine reported by Manhapra et al (2017) (19). This is likely explained by differences in the patient populations examined (all VHA patients diagnosed with OUD as opposed to the more vulnerable, HIV infected and uninfected patients composing the VACS cohort), as well as divergent methodologies utilized. Our analysis identified continuous retention in buprenorphine using a more restrictive days-covered standard, while Manhapra calculated retention as the number of days between first and last buprenorphine prescription, which may have masked cycles in and out of care.
The fact that retention was higher among those receiving buprenorphine compared to those receiving methadone was surprising, given past research finding the opposite (36–37). It is possible that our results reflect changes in clinical practice from earlier eras of buprenorphine treatment (e.g., the use of higher and flexible doses of buprenorphine) and/or patient characteristics (e.g., increased frequency of OUD developing following exposure to prescribed opioids) that may have increased average retention in buprenorphine relative to methadone treatment over time. For instance, although medication choice reflects patient and provider preference, VHA utilizes a stepped-care approach to OUD treatment, wherein patients with mild to moderate OUD are generally prescribed buprenorphine, while patients with moderate to severe OUD, who have not been successful on buprenorphine and require a higher intensity of treatment, could be assigned to an opioid treatment program and dispensed methadone (32). Despite these potential differences in medication use associated with patient characteristics, within a given VHA facility, PWH will have the same access to specific OAT medications as PWOH. Alternatively, it could be that there is something distinct about the VHA context that explains this result. In analyses comparing retention of buprenorphine relative to methadone among VHA patients, one study found that new episodes of buprenorphine were associated with 37% decreased risk of ending relative to new episodes of methadone treatment (38). The author suggests that this may reflect unmeasured characteristics (e.g., disease severity) of patients referred to methadone relative to buprenorphine treatment, if such characteristics are negatively associated with retention. Alternatively, it is possible that the low rate of methadone retention we observe reflects patient transfer to non-VHA methadone programs, although clinical experience suggests that this is not common. As much of the drop-out in methadone treatment occurred in the first month following initiation, VHA OTPs should examine the barriers to retention patients experience in this first month of treatment and consider strategies to better engage new patients in care.
It was also notable that sequentially receiving a combination of methadone and buprenorphine at some point, compared to methadone alone, was associated with improved retention not just at 12 months, but six and one months as well. This suggests that shifting between medications to address patients’ changing needs and preferences could help retain patients in treatment over time. It also supports a stepped care approach to OUD treatment that allows patients to move between treatment settings if indicated (32).
LIMITATIONS
Out study has several limitations. First, our definitions of retention may not capture all patients retained in OAT. For instance, patients who switched between buprenorphine and methadone across the time period of our study but did not meet either medication-specific retention criteria were not counted as retained, although they may have been receiving continuous treatment were the periods covered by both medications combined. Second, our definition of retention in methadone treatment may not accurately capture all patients who received continuous methadone treatment over time, potentially over or under-counting patients. Third, given that we are only able to identify methadone dosing via clinic stop codes and inpatient receipt, it is possible that patients classified as initiating methadone may in fact have initiated buprenorphine within the OTP. However, excluding these patients from our analysis did not substantively change the results. It is also possible that patients with recorded visits to an OTP may never have initiated OAT. Fourth, our data may not capture all OAT treatments received outside of the VHA context. Fifth, patients receiving extended-release injectable naltrexone (XRN) were not included in these analyses although, across the period of the study, XRN was rarely used as an OUD treatment within VHA (22). Sixth, our sample was composed of patients with both an OUD diagnosis as well as a documented treatment encounter in either an SUD specialty, mental health or other outpatient setting. Patients who received OAT treatment in other settings may have been excluded from our sample. However, the large majority of buprenorphine prescribing within VHA during the study period occurred within specialty SUD and mental health settings, and an initial behavioral health treatment visit would have generally occurred regardless of treatment setting (39). Finally, it is important to note that, although both based on months in treatment, our definitions of retention in methadone and buprenorphine may not be strictly comparable, given differences in prescribing/dispensation practices as well as data limitations.
CONCLUSION
Among a particularly vulnerable population of veterans receiving VHA care, less than 30% were retained in OAT at 12 months, regardless of HIV status. This study highlights significant challenges in treatment retention among this population and the need for further clinical, systems, and research initiatives to better understand and improve treatment retention (40).
Acknowledgments:
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA022886 (Kraemer) and U10 AA013566 (Justice)). Dr. Wyse’s time was supported by Career Development Award 1IK2HX003007 from the U.S. Department of Veterans Affairs Health Services Research and Development, K12HS026370 from the Agency for Healthcare Research and Quality and resources from the VA Health Services Research and Development-funded Center to Improve Veteran Involvement in Care at the VA Portland Health Care System (CIN 13-404). Dr. Korthuis’ time was supported by the National Institutes of Health, National Institute on Drug Abuse (UG3DA044831, UG1DA015815). Dr. Edelman’s time was supported by NIDA (R01DA040471). Dr. Crystal’s time was supported by NIDA (R01DA047347) and NCATS (UL1TR003017). A prior version of this research was presented at the College on Problems of Drug Dependence Annual Meeting, 2020. The authors have no relevant financial or non-financial interests to disclose. The content of this article is solely the responsibility of the authors and does not represent the official views of the Department of Veterans Affairs or the Agency for Healthcare Research and Quality.
Appendix 1. Four Trajectory Group Model: Patterns of Methadone Treatment Over 12 Months
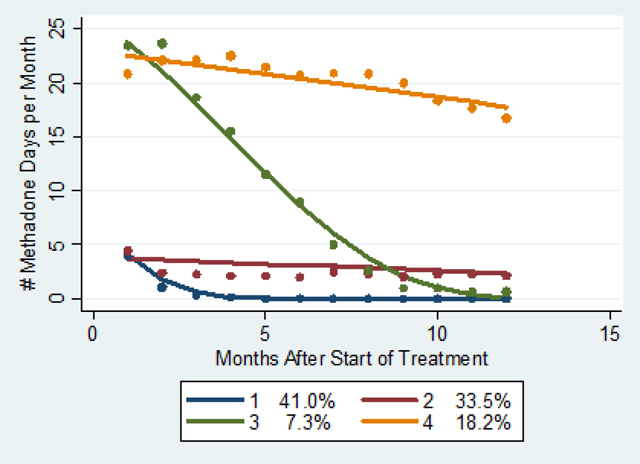
To identify distinctive pattern of methadone treatment over time, as indicated by number of visit days (OTP/stop code 523 or inpatient) per month, we utilized group-based trajectory modeling. Trajectory modeling sorts each participant’s measurements (number of visits per month) into “clusters” and estimates distinct trajectories. The procedure calculates each individual’s probability of belonging to each trajectory group and assigns the individual to the trajectory with the highest probability of membership. We used months after start of treatment as the time scale. We used a normal model and evaluated a 4-trajectory group model. With methadone retention defined as 4+ visits/month to an OTP in the first month and 1+ visits/month in months 2–12, we calculated 12-month retention within each trajectory group as follows: 0% for group 1, 17% for group 2, 7% for group 3, and 75% for group 4.
Footnotes
Declarations
Conflict of Interest/Competing interests: The authors have no relevant financial or non-financial interests to disclose. The content of this article is solely the responsibility of the authors and does not represent the official views of the Department of Veterans Affairs or the Agency for Healthcare Research and Quality.
Ethics approval: The study protocol was approved by the Institutional Review Boards of the University of Pittsburgh, VA Pittsburgh Healthcare System and VA Connecticut Health System and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate: not applicable.
Consent for publication: not applicable.
Availability of data: not applicable.
Code availability: not applicable.
References
- 1.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Syst Rev. 2014; (2). 10.1002/14651858.CD002207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine. Medications for Opioid Use Disorder Save Lives. Washington, DC: National Academies Press; 2019. [PubMed] [Google Scholar]
- 3.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samples H, Williams AR, Crystal S, Olfson M. Impact of long-term buprenorphine treatment on adverse health care outcomes in Medicaid: The impact of longer treatment on health care outcomes for opioid use disorder within a key population of Medicaid enrollees. Health Aff. 2020;39(5):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelman EJ, Chantarat T, Caffrey S, et al. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected, opioid-dependent patients. Drug Alcohol Depend. 2014;139:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roux P, Carrieri MP, Villes V, et al. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103(11):1828–1836. [DOI] [PubMed] [Google Scholar]
- 7.Norton BL, Beitin A, Glenn M, DeLuca J, Litwin AH, Cunningham CO. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss L, Netherland J, Egan JE, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr. 2011;56:S68–S75. [DOI] [PubMed] [Google Scholar]
- 9.Lesko CR, Keil AP, Moore RD, Chander G, Fojo AT, Lau B. Measurement of current substance use in a cohort of HIV-infected persons in continuity HIV care, 2007–2015. Am J Epidemiol. 2018;187(9):1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldfield BJ, Muñoz N, McGovern MP, et al. Integration of care for HIV and opioid use disorder. AIDS. 2019;33(5):873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr HIV/AIDS Rep. 2012;9(4):287–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roux P, Carrieri MP, Cohen J, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin Infect Dis. 2009;49(9):1433–1440. [DOI] [PubMed] [Google Scholar]
- 14.Adams JW, Marshall BD, Salleh NA, Barrios R, Nolan S, Milloy MJ. Receipt of opioid agonist treatment halves the risk of HIV-1 RNA viral load rebound through improved ART adherence for HIV-infected women who use illicit drugs. Drug Alcohol Depend. 2020;206:107670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo-Ciganic W, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111(5):892–902. [DOI] [PubMed] [Google Scholar]
- 16.Williams AR, Samples H, Crystal S, Olfson M. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2020;177(2):117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2016;35(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor AM, Cousins G, Durand L, Barry J, Boland F. Retention of patients in opioid substitution treatment: A systematic review. PloS One. 2020;15(5):e0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manhapra A, Petrakis I, Rosenheck R. Three-year retention in buprenorphine treatment for opioid use disorder nationally in the Veterans Health Administration. Am J Addict. 2017;26(6):572–80. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein ZM, Kim HW, Cheng DM, et al. Long-term retention in office based opioid treatment with buprenorphine. J Subst Abuse Treat. 2017;74:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006; S25–S30. [DOI] [PubMed] [Google Scholar]
- 22.Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy AJ, McGinnis KA, Merlin JS, et al. Impact of intensity of behavioral treatment, with or without medication treatment, for opioid use disorder on HIV outcomes in persons with HIV. J Subst Abuse Treat. 2021;132(108509). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Substance Abuse and Mental Health Services. Federal Guidelines for Opioid Treatment Programs. HHS Publication No. (SMA) PEP15-FEDGUIDEOTP. 2015. December Available at: https://store.samhsa.gov/sites/default/files/d7/priv/pep15-fedguideotp.pdf. Accessed August 20, 2020. [Google Scholar]
- 25.Saloner B, Daubresse M, Caleb Alexander G. Patterns of buprenorphine-naloxone treatment for opioid use disorder in a multistate population. Med Care. 2017;55(7):669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldfield BJ, McGinnis KA, Edelman EJ, et al. Predictors of initiation of and retention on medications for alcohol use disorder among people living with and without HIV. J Subst Abuse Treat. 2020;109:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGinnis KA, Skanderson M, Edelman EJ, et al. Impact of behavioral and medication treatment for alcohol use disorder on changes in HIV-related outcomes among patients with HIV: a longitudinal analysis. Drug Alcohol Depend. 2020;217:108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 14. 2015. College Station, TX: StataCorp LP. [Google Scholar]
- 30.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018;95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunet N, Moore DT, Lendvai Wischik D, Mattocks KM, Rosen MI. Increasing buprenorphine access for veterans with opioid use disorder in rural clinics using telemedicine. Subst Abuse. Epub ahead of print. 2020; 1–8. [DOI] [PubMed] [Google Scholar]
- 32.Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abuse. 2020;41(3):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Veterans Affairs. VHA DIRECTIVE 1304. National Human Immunodeficiency Virus Program. Washington, DC: Veterans Health Administration; 2019. Available from: https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3056. [Google Scholar]
- 34.Ghose T, Gordon AJ, Metraux S, et al. The association between HIV status and homelessness among veterans in care. J Community Psychol. 2015;43(2):189–98. [Google Scholar]
- 35.Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the US Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499–504. [DOI] [PubMed] [Google Scholar]
- 36.Hser Y-I, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104(7):1193–1200. [DOI] [PubMed] [Google Scholar]
- 38.Barnett PG. Comparison of costs and utilization among buprenorphine and methadone patients. Addiction. 2009;104(6):982–92. [DOI] [PubMed] [Google Scholar]
- 39.Valenstein-Mah H, Hagedorn H, Kay CL, Christopher ML, Gordon AJ. Underutilization of the current clinical capacity to provide buprenorphine treatment for opioid use disorders within the Veterans Health Administration. Subst Abuse. 2018;39(3):286–88. [DOI] [PubMed] [Google Scholar]
- 40.Chan B, Gean E, Arkhipova-Jenkins I, et al. Retention strategies for medications for opioid use disorder in adults: a rapid evidence review. J Addict Med. 2020;15(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


