Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger that releases Ca2+ from endosomes and lysosomes by activating ion channels called two-pore channels (TPCs). However, no NAADP-binding site has been identified on TPCs. Rather, NAADP activates TPCs indirectly by engaging NAADP-binding proteins (NAADP-BPs) that form part of the TPC complex. After a decade of searching, two different NAADP-BPs were recently identified: Jupiter microtubule associated homolog 2 (JPT2) and like-Sm protein 12 (LSM12). These discoveries bridge the gap between NAADP generation and NAADP activation of TPCs, providing new opportunity to understand and manipulate the NAADP-signaling pathway. The unmasking of these NAADP-BPs will catalyze future studies to define the molecular choreography of NAADP action.
NAADP-mediated Ca2+ signaling via TPCs: bridging the gap
NAADP (see Glossary) is a second messenger that releases Ca2+ from acidic organelles in numerous cell types, acting in the nanomolar range to regulate many diverse cellular processes [1]. NAADP exerts these effects by activating TPCs, an ancient family of eukaryotic intracellular ligand- and/or voltage-gated ion channels localized within the endolysosomal system [2]. TPCs are Ca2+- and Na+-permeable channels, with TPC1 and TPC2 expressed in human cells [3,4]. They control subcellular trafficking events through the endolysosomal system [5], regulating the uptake of physiological substrates, as well as pathogen internalization. They also play a key role in environmental sensing [6]. TPC activity is controlled through the coordinated action of several regulatory inputs (Figure 1A). Activators include both NAADP, which releases endolysosomal Ca2+, and phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2), which evokes a highly selective Na+ current, as well as membrane voltage [3,7–9]. The behavior of the channel likely switches between these modalities with specific functional outcomes being keyed to the nature of the activating stimulus [10].
Figure 1. The action of nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins (BPs) and emerging questions enabled by their identification.
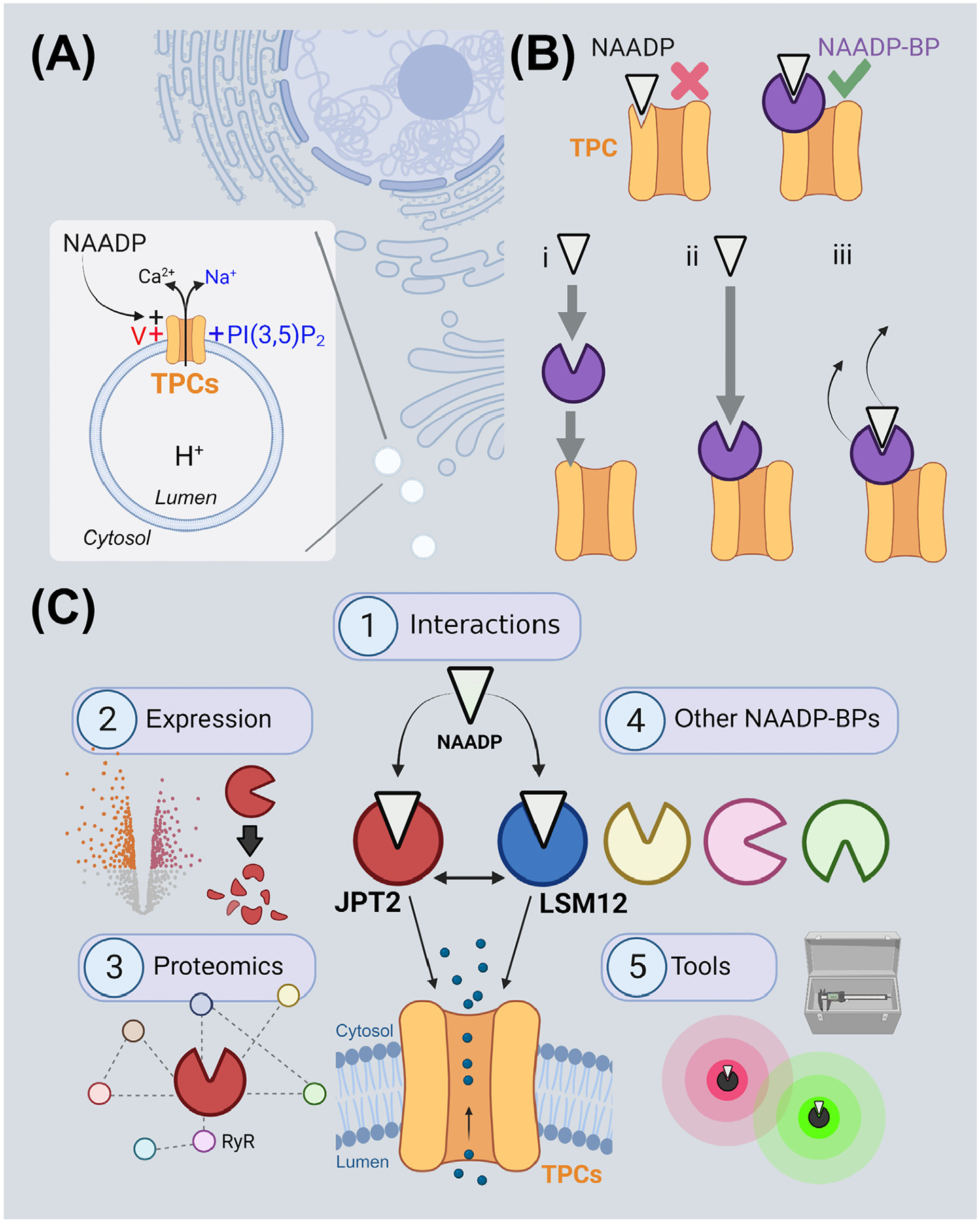
(A) Two-pore channels (TPCs) are ion channels expressed on endolysosomes. They are subject to polymodal activation by NAADP, PI(3,5)P2, and/or voltage (V). (B) Models for TPC activation by NAADP. Top: the originally envisioned model that TPCs are directly activated by the binding of NAADP (white) has not received experimental support. Rather, NAADP has been proposed to activate TPCs indirectly by engaging NAADP-BPs (purple) that are essential components for NAADP-triggered Ca2+ release. Bottom, this may occur in several ways, for example, by (i) NAADP association with the NAADP-BP causing a translocation of NAADP-liganded NAADP-BPs to the TPC complex or (ii) by NAADP engaging NAADP-BPs already associated with the TPC complex. (iii) Dissociation of the NAADP-BP from the channel complex, or dissociation of NAADP from the NAADP-BP, could serve to terminate NAADP action. (C) Future areas for NAADP-BP research enabled by the recent identification of two NAADP-BPs, JPT2 and LSM12. These include (1) the mechanism of JPT2 interaction of NAADP and JPT2 association with different TPC isoforms, (2) regulation of JPT2 expression levels, for example, through degradation, to set cellular NAADP sensitivity, (3) characterization of other functions of JPT2 and the broader JPT2 interactome as a roadmap for understanding new facets of NAADP biology, (4) identification and characterization of additional vertebrate and invertebrate NAADP-BPs, and (5) development of novel tools to manipulate this signaling pathway. Each of these areas is discussed in the main text. This figure was created at BioRender.com. Abbreviations: JPT2, Jupiter microtubule associated homolog 2; LSM12, ‘like-Sm’ protein 12; PI(3,5)P2, phosphatidylinositol 3,5-bisphosphate; RyR, ryanodine receptor.
One of the several challenges related to our understanding of these versatile ion channels, and the progression of TPCs as druggable entities, has been a fundamental gap in our knowledge of how TPCs are activated by NAADP. We know TPCs are directly gated by PI(3,5)P2 through an atomically resolved binding site [11–13]. But, by stark contrast, no NAADP-binding site has been resolved on the TPC itself. Rather, it was thought NAADP activates TPCs indirectly by binding to an unidentified NAADP-BP associated with the TPC complex (Figure 1B).
The molecular identity of this NAADP-BP has remained unknown for a decade. However, recent breakthroughs now provide the field with a surfeit of riches, as two unique NAADP-BP candidates have been identified within the past year. These two proteins, JPT2 and LSM12, both bind NAADP, interact with TPCs, and are necessary to support endogenous NAADP-evoked Ca2+ signals [14–16]. They provide considerable new impetus for understanding NAADP action. Therefore, it is timely to review how identification of these NAADP-BPs defines new questions and trajectories for research that will further our mechanistic understanding of NAADP signaling. In this review, we focus predominantly on JPT2, given our role in identifying this NAADP-BP [14]. After introducing how JPT2 was discovered, five areas will be explored (Figure 1C) relating to (1) the mechanisms by which JPT2 interacts with NAADP and TPCs; (2) the landscape of JPT2 expression and how this is regulated to determine cellular NAADP sensitivity, (3) how a broader understanding of JPT2 interactors and their cellular physiology will expand our knowledge of the cellular functions of NAADP, (4) the extended family of NAADP-BPs, including the recent identification of LSM12 [16], and finally (5) how this knowledge will catalyze development of new tools and drugs to manipulate this signaling pathway. While JPT2 is the core focus of this review, many of the themes are also applicable to the study of LSM12 [16], which is highlighted separately in its own section.
Identification of JPT2 as an NAADP-BP
Sea urchin eggs are an indispensable model system for studying NAADP action because it is a simple experimental preparation that exhibits robust, highly reproducible Ca2+ responses to NAADP, as well as other Ca2+-mobilizing second messengers [17,18]. Soon after the Ca2+-mobilizing properties of NAADP were first discovered in this invertebrate preparation, radiolabeled NAADP (32P-NAADP; see Figure I in Box 1) was used to identify stereoselective binding sites for NAADP [19,20]. This approach was subsequently extended to mammalian tissues [21,22]. However, it took another decade to identify TPCs as NAADP targets [3,4,23]. The subsequent realization that TPCs do not bind NAADP grew from this work but was directly evidenced from photolabeling studies using NAADP-based photoprobes. Specifically, use of a photoreactive analog of 32P-NAADP (32P-5-azido-NAADP; Box 1) to resolve NAADP-binding targets revealed specific photolabeling of a ~23-kDa protein band considerably smaller than the TPCs [24–26]. The photolabeling characteristics of this low-molecular-weight NAADP-BP revealed (i) a diagnostic pharmacology of NAADP-evoked Ca2+ release, (ii) the expected selectivity for NAADP versus NADP, and (iii) irreversible binding in high K+, all features of NAADP messenger action established from earlier work [24,25]. Observed affinities of NAADP-binding sites, measured by photoaffinity labeling (PAL) or 32P-NAADP binding, were also identical [24,25]. These findings were subsequently shown to be consistent across various mammalian cell lines, primary cells, and tissues [9,27]. Further, the ~23-kDa NAADP-BP was definitively shown to be distinct from TPCs as photolabeling persisted in TPC-knockout mice [9,25].
Box 1. A chemical toolbox for probing NAADP action.
Figure I.
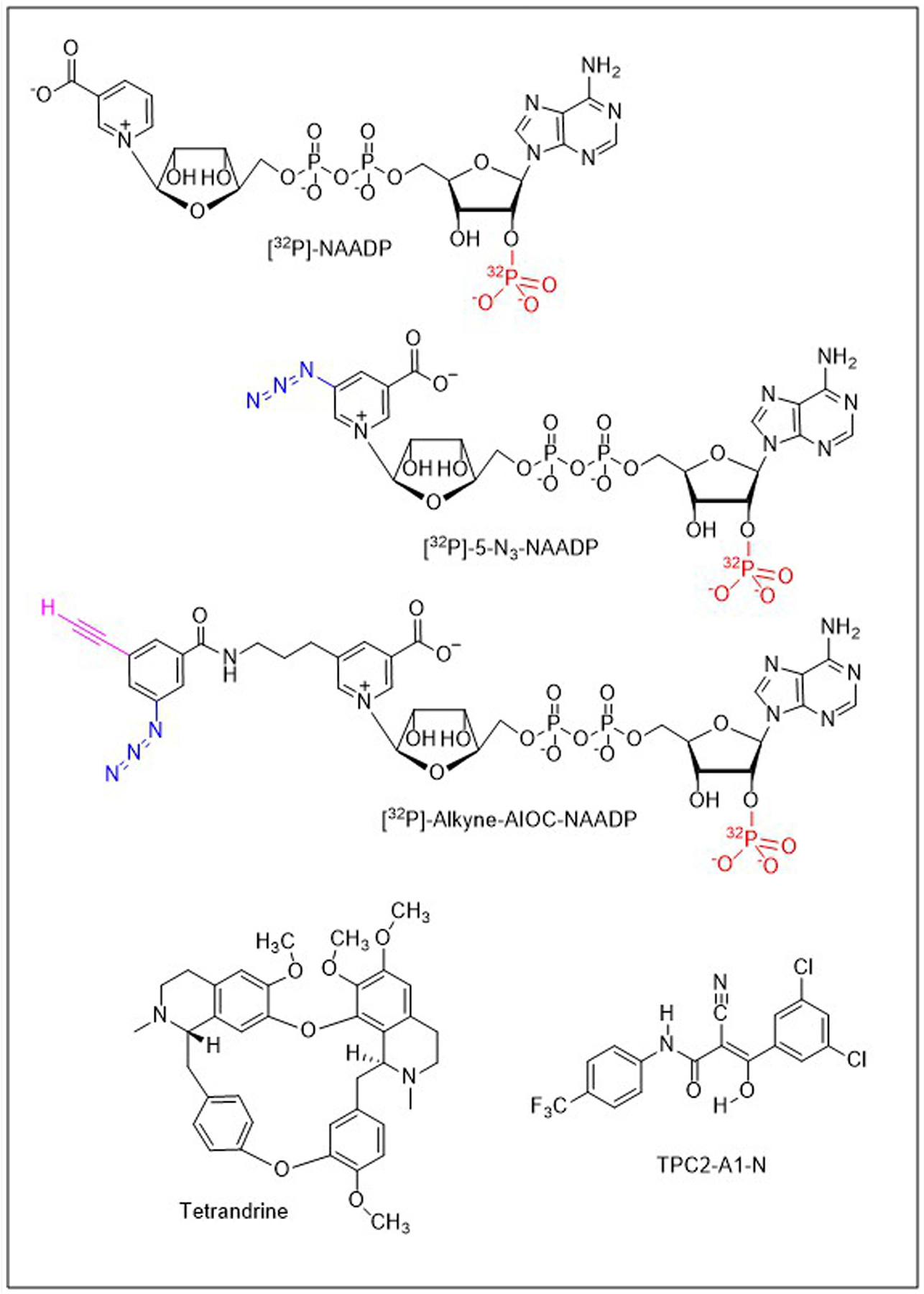
[32P]-Nicotinic acid adenine dinucleotide phosphate (NAADP; top left) represents one version of a radioisotope (red) used to study NAADP binding in cells and tissues. [32P]-5-N3-NAADP was the original photoaffinity probe used to characterize NAADP targets [24,25]. The probe incorporates a photoreactive azide group (blue) to crosslink binding partners following ultraviolet irradiation. [32P]-alkyne-’all-in-one-click’ (AIOC)-NAADP is a bifunctional probe that incorporates an additional clickable alkynyl moiety (magenta) to couple photolabeling to NAADP-BP enrichment. For example, to isolate JPT2 [14], photoprobe-bound NAADP-BPs were biotinylated via a copper-catalyzed alkyne–azide cycloaddition (CuAAC) using ‘click chemistry’ such that the biotinylated proteins could then be captured using neutravidin. Tetrandrine is a bisbenzylisoquinoline alkaloid originally shown to block two-pore channels (TPCs) [57], with other derivatives of this chemotype also blocking TPC function [58,87]. TPC2-A1-N is a novel chemotype recently identified in a drug screen profiling TPC2 that mimics NAADP-evoked Ca2+ signals and NAADP-evoked TPC currents [10].
Despite considerable efforts with the initial photoprobe, as well as synthesis and testing of iterated versions [28,29], it proved challenging to enrich this elusive NAADP-BP sufficiently to narrow down the long list of candidates collated from mass spectrometry data sets [30,31]. It was the development of a second-generation bifunctional photoprobe [32P-alkyne-’all-in-one-click’ (AIOC)-NAADP; Box 1], which retained the photoactivatable azide group but incorporated an additional ‘clickable’ alkynyl moiety to couple PAL with an enrichment strategy, that broke the status quo. This probe, when utilized in erythrocytes – a cell type with strong, selective photolabeling of the ~23-kDa NAADP-BP – resulted in our identification of JPT2 [14]. Further support that JPT2 acts as an NAADP-BP includes (i) knockdown of endogenous JPT2 reducing the intensity of the photolabeled NAADP-BP in mammalian cell lines and strongly inhibiting endogenous NAADP-evoked Ca2+ signals mediated by TPCs and (ii) coimmunoprecipitation studies demonstrating that JPT2 interaction was biased toward TPC1 compared with TPC2 [14]. JPT2 was also isolated from another blood cell (Jurkat T cells) by Roggenkamp et al. using a sequential purification protocol to track radioactivity associated with the photolabeled NAADP-BP targets [15,32].
Human JPT2 exists as three splice isoforms that differ in their N-termini (Figure 2A) [33]. Their predicted molecular weights match well the 22–23-kDa species labeled in photolabeling experiments. Additionally, JPT1 is a related protein displaying ~30% sequence identity [33]. Figure 2B displays an updated phylogeny of JPT homologs in animals [33]. JPT2 appears to be a vertebrate invention present in basal vertebrates such as Petromyzon marinus (sea lamprey). JPT1 is also present in major vertebrate classes as well as in Anneissia japonica, an echinoderm (subphylum, Crinozoa). However, JPT homologs in other echinoderms such as sea urchins and a number of additional chordate subphyla and protostomes group as an independent clade. Whether these homologs represent ancestral forms of JPT1 or JPT2 remains to be established. This is particularly pertinent in echinoderms where the Ca2+-mobilizing effects of NAADP were originally documented.
Figure 2. JPT2 structure and evolution.
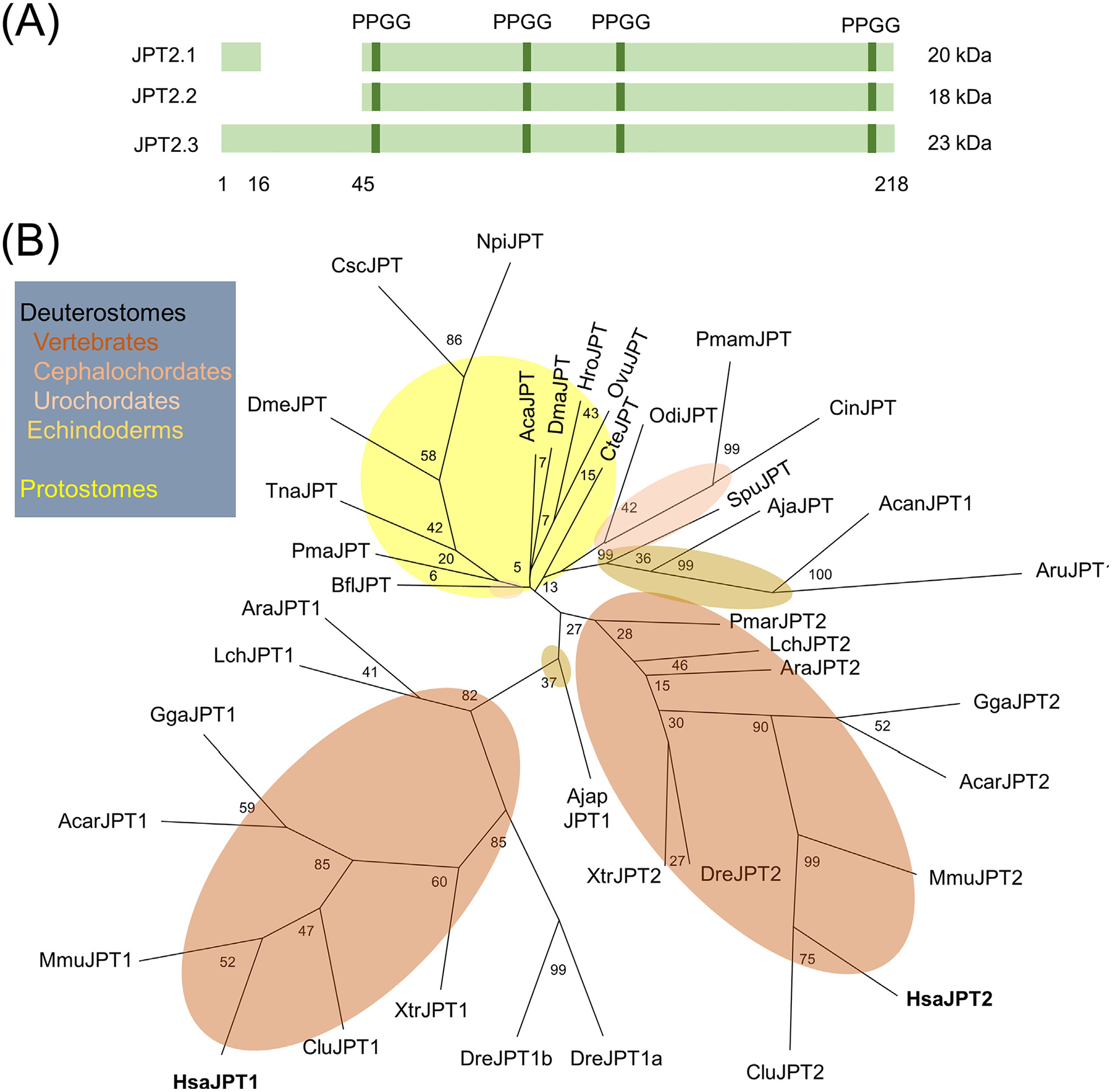
(A) Schematic representation of human JPT2 splice isoforms. Length in amino acids for JPT2.3 is shown. (B) Cladogram of JPT sequences from animals with bootstrap values shown. Phlylogenetics was performed as described in [97] using PHYML (version 3.1) with the JTT amino acid substitution model, estimated proportion of invariable sites, and the four-category discrete gamma model (JTT + I + G) selected by ProtTest (version 3.4.2). Deuterostome branches are shaded in brown, and protostome branches are shaded in yellow. Abbreviations: CteJPT (ELU08900.1), Capitella teleta; HroJPT (XP_009029441.1), Helobdella robusta; AcaJPT (XP_005102887.1), Aplysia californica; PmaJPT (XP_033742783.1), Pecten maximus; OvuJPT (XP_029648722.1), Octopus vulgaris; TnaJPT (OUC50045.1), Trichinella nativa; DmeJPT, (Q9I7K0) Drosophila melanogaster; NpiJPT, (AII97726.1) Nephila pilipes; CscJPT (XP_023222178.1), Centruroides sculpturatus; DmaJPT (XP_032791280.1), Daphnia magna; AruJPT (XP_033631490.1), Asterias rubens; AcanJPT (XP_022098988.1), Acanthaster planci; SpuJPT (XP_011684190.1), Strongylocentrotus purpuratus; AjaJPT (PIK56568.1), Apostichopus japonicus; AjapJPT (XP_033109709.1), Anneissia japonica; BflJPT (XP_002611678.1), Branchiostoma floridae; CinJPT (XP_009861954.1), Ciona intestinalis; PmamJPT (CAB3257823.1), Phallusia mammillata; OdiJPT (CBY20678.1), Oikopleura dioica; PmarJPT (XP_032826119.1), Petromyzon marinus; AraJPT1 (XP_032900329.1), AraJPT2 (XP_032896233.1), Amblyraja radiata; DreJPT1a (NP_001082982.1), DreJPT1b (NP_991176.1), DreJPT2 (NP_955869.2), Danio rerio; LchJPT1 (XP_005998303.1), LchJPT2 (XP_005997850.1), Latimeria chalumnae; XtrJPT1 (NP_001139220.1), XtrJPT2 (XP_012825626.1), Xenopus tropicalis; AcarJPT1 (XP_003217263.1), AcarJPT2 (XP_008120328.1), Anolis carolinensis; GgaJPT1 (XP_015150886.1), GgaJPT2 (NP_001265082.1), Gallus gallus; CluJPT1 (NP_001093413.1), CluJPT2 (XP_022275981.1), Canis lupus familiaris; MmuJPT1 (NP_032284.1), MmuJPT2 (NP_945175.1), Mus musculus; HsaJPT1 (NP_001002032.1), HsaJPT2 (NP_653171.1), Homo sapiens; JPT2, Jupiter microtubule associated homolog 2.
JPT2 and its interaction with NAADP and TPCs
Two key questions emerge from the identification of JPT2 as an NAADP-BP and TPC accessory protein. First, how does NAADP bind to JPT2? Second, how does JPT2 bind TPCs?
Toward the first question, the JPT2 sequence does not possess any consensus nucleotide-binding domains. Instead, it has a repeat structure (much like TPCs), and it is predicted to be a disordered protein. It is also predicted to be modified by a variety of post-translational modifications in mammalian systems, such as phosphorylation. Some of these sites have been validated experimentally and associated with the activity of specific kinases [34]. Thus, NAADP binding may be subject to phosphoregulation. Further work to analyze the properties of JPT2 in vitro is needed to define these properties and their impact on NAADP association.
Regarding the second question, understanding how JPT2 associates with TPCs is critical to establish as it is key to understanding how NAADP-evoked Ca2+ signals initiate. A variety of models can be envisaged. For example, increases in cellular NAADP levels may cause a translocation of NAADP-liganded JPT2 to TPCs to trigger activation (Figure 1Bi). Alternatively, (a fraction of) JPT2 may be prebound to TPCs, with NAADP binding causing a conformational change that opens TPCs (Figure 1Bii). That NAADP can gate TPCs in excised patches [35] and in lipid bilayers (following incorporation of purified TPCs or TPC-expressing vesicular preparations) [36,37] supports a tight association of JPT2 with TPCs. If so, dissociation of JPT2 upon NAADP binding may inactivate the complex (Figure 1Biii). Could such dissociation underpin the biphasic NAADP concentration-dependence relationship whereby micromolar concentrations of NAADP decrease channel activation [38]?
From our work, JPT2 seems to preferentially interact with TPC1 over TPC2 [14]. TPC1 expression is biased endosomally, and TPC2 is considered more lysosomal in human cells. NAADP-BP specificity for TPC isoforms could therefore serve to regionalize NAADP action to a subset of acidic Ca2+ stores, providing a route for JPT2 to selectively modify endosomal function and trafficking. Looking beyond TPCs as NAADP-BP targets, Roggenkamp et al. show that JPT2 also interacts with ryanodine receptors (RyR1) in T lymphocytes during the initial stages of T cell activation [15]. These data suggest a broader promiscuity of JPT2 beyond the canonical channel (TPC) and organellar target (acidic Ca2+ stores), such that JPT2 may be competent to confer NAADP sensitivity to multiple families of intracellular Ca2+ channels [26,39]. It should be noted that TPC1 and RyR1 are highly dissimilar proteins; therefore, this highlights an issue of whether these associations are mediated directly or through a common intermediary. Elucidation of the diversity of Ca2+ channel targets of JPT2 will be necessary for comprehensively decoding NAADP action on intracellular Ca2+ dynamics.
JPT2 expression and cellular NAADP sensitivity
Some cell types show strong responses to NAADP, some do not. Some preparations respond robustly to NAADP (intact cells), some (broken cell preparations) do not [26,40–42]. If NAADP-BPs are essential for NAADP action on intracellular Ca2+ channels, then the presence and properties of NAADP-BPs within a cell at any given point in time will determine cell sensitivity to NAADP. Consequently, regulation of pathways that control expression, properties, and local concentration of NAADP-BPs in the vicinity of TPCs will control when and where NAADP triggers endogenous Ca2+ signals. What do we know about JPT2 expression, and how JPT2 expression levels are controlled?
RNA sequencing (RNA-seq) and protein expression databases evidence that full-length JPT2 is broadly expressed in human tissues and cell lines [14], consistent with prior profiling of JPT2 expression [33,43]. The gene nomenclature for JPT2 (JPT2/HN1L) derives for shared molecular homology with JPT1 (JPT1/HN1, hematopoietic- and neurological-expressed sequence 1), named based on a high level of expression in hematopoietic cells and fetal brain tissue [44]. Like JPT1, JPT2/HN1L mRNA is also present in multiple brain regions, with highest levels in the spinal cord [45]. These observations are of interest given a documented role for NAADP in maturation of spinal neuronal circuitry [46]. Another study identified JPT2 as a highly expressed gene in neuronal stem cells and progenitors [43], which is again intriguing given the observed role of NAADP in neuronal differentiation [47,48]. How JPT2 levels change during development and how such changes correlate with the timing and localization of endogenous NAADP-evoked Ca2+ signals merits resolution. Toward this understanding, transcriptomic analyses of JPT2 expression (Figure 3A) show (with the usual caveats of RNA-to-protein conversion [49]) that JPT2 is not an abundant cellular protein [median range of 4–129 transcripts per million (TPM) for JPT2] [45]. JPT2 expression is, however, higher in cell types (SKBR3 and U2OS cells) commonly used to study NAADP-evoked Ca2+ signals [14]. Further, expression profiling suggests the abundance and variation in JPT2 levels better correlate with the expression profile of TPC1 than TPC2 (Figure 3B), consistent with observations that JPT2 preferentially interacts with TPC1, as described earlier [14].
Figure 3. NAADP-BP expression.
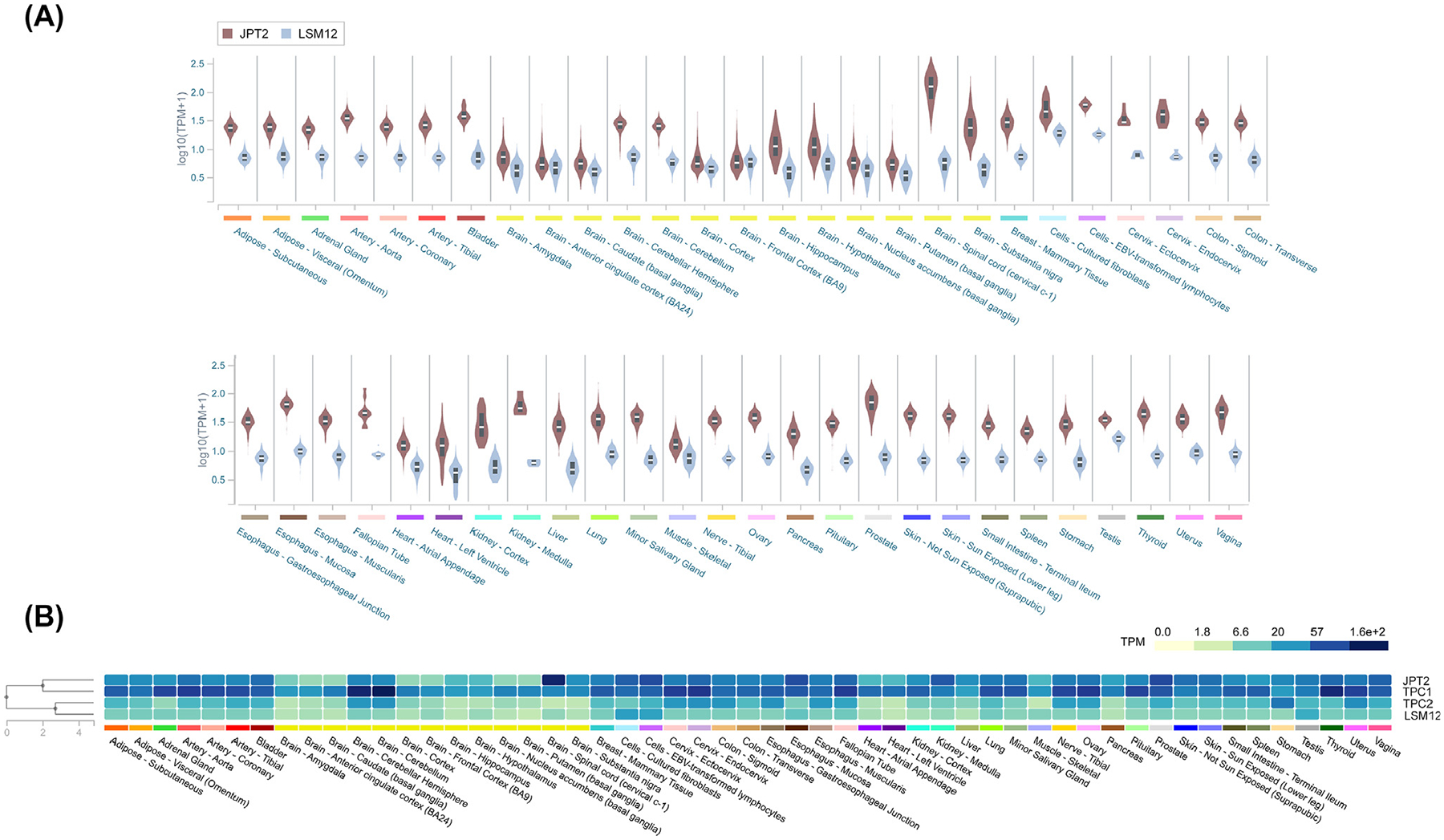
(A) Violin plot showing expression values [transcripts per million (TPM)] of JPT2 (brown) and LSM12 (blue) across a variety of tissues, calculated from a gene model where all isoforms are collapsed to a single gene. Box plots show median as well as 25th and 75th percentiles. TPM values were produced with RNA-SeQC v1.1.9. Noticeably, expression of both NAADP-BPs shows a broad tissue distribution, with increased JPT2 expression in the spinal cord, as described in the main text. (B) Heatmap depicting median TPMs for NAADP-BPs (JPT2, LSM12) as well as two-pore channels (TPCs; TPC1 and TPC2). Tissues are arrayed alphabetically. These data show JPT2 expression is better correlated with TPC1 and LSM12 expression is better correlated with TPC2. Data for Figure 3 are reproduced from the Genotype-Tissue Expression project (GTEx, v8 [45]). Abbreviations: JPT2, Jupiter microtubule associated homolog 2; LSM12, ‘like-Sm’ protein 12; NAADP-BP, NAADP-binding protein.
The unmasking of JPT2 as an NAADP-BP permits the relationship between JPT2 expression and cellular NAADP sensitivity to be assessed experimentally. In cell types where JPT2 expression is low, can JPT2 overexpression enhance NAADP responsiveness? Reciprocally, does knockdown/knockout of JPT2 impair NAADP action in different cell types and tissues? It has been challenging to demonstrate NAADP sensitivity in permeabilized cells, or by using organelle-based electrophysiological approaches, due to the potential loss of NAADP-BPs from the experimental assay systems. This roadblock has hampered study of TPC properties. If the explanation for the loss of NAADP sensitivity in broken cell preparations is simply the loss of NAADP-BPs required for NAADP action, then tethering NAADP-BPs to TPCs through molecular linkers designed into recombinant NAADP-BP:TPC expression constructs could test this possibility and facilitate the study of NAADP action on TPCs [50].
How are JPT2 expression levels controlled? JPT2 expression is likely determined by mechanisms controlling gene transcription, mRNA stability, and degradation. Our knowledge of these processes for JPT2 is currently quite limited. We do know that microRNA (miR)-212–5p, which is implicated in cancer progression [51] and Parkinson’s disease [52], binds within the 3′ untranslated region (UTR) of JPT2, with JPT2 expression being inversely correlated with miR-212–5p levels in hepatic cancer [53]. JPT2 is also predicted to be modified by various post-translational modifications, and interaction with binding partners may provide another way to regulate NAADP-BP levels. Understanding these processes may afford new opportunity for therapeutic approaches to control NAADP action.
Other JPT2 functions
Identification of JPT2 as an NAADP-BP establishes an unexpected new functionality to this protein. The existing literature surrounding JPT2 is currently small but highlights roles in viral infection and cancer. This association is intriguing given the growing evidence of a role for TPCs in both these areas [14,54–59], summarized in Box 2. Do the known cellular roles of JPT2 extend our understanding of NAADP action into novel aspects of cellular physiology?
Box 2. TPCs in viral infection and cancer.
There is growing evidence that TPCs control viral trafficking through the endolysosomal system [14,57–59,88,89]. This role of TPCs was first shown by Sakurai et al. who validated TPCs as druggable targets that blocked Ebola virus infection [57]. Their work identified the natural product tetrandrine (a bisbenzylisoquinoline alkaloid) as a TPC antagonist that inhibited Ebola virus infectivity in vitro and in a mouse model [57]. Genetic knockdown, or knockout of TPC1 or TPC2, also prevented Ebola virus entry in vitro [57]. Mechanistically, these effects relate to the role of TPCs in regulating vesicular fusion events between different compartments of the endolysosomal system, through which diverse pathogens traverse to gain cytoplasmic access [5,57,74,90]. Subsequent work using a related bisbenzylisoquinoline (fangchinoline) demonstrated that TPC inhibition blocked infectivity of a pseudotyped Middle East respiratory syndrome coronavirus (MERS-CoV) [58,60]. The potency of various blockers at inhibiting NAADP-evoked Ca2+ release correlated well with the extent of inhibition of viral infectivity [60]. Knockdown or chemical blockade of TPCs also blocked infection of a SARS-CoV-2 pseudovirus, an outcome also seen in assays using wild-type, replication-competent SARS-CoV-2 [59,88]. In silico analyses suggest that several known antiviral agents may act as TPC ligands [91]. Genome-wide CRISPR screening found that knockout of TPC1 inhibited infectivity of authentic SARS-CoV-2 in human alveolar epithelial cells [92].
Emerging data implicate TPCs in multiple aspects of tumorigenesis [87,93–95]. In early stages of tumorigenesis, TPCs promote tumor growth, the secretion of enzymes that degrade extracellular components, cell migration, and invasiveness. TPCs act as nutrient sensors, regulating autophagy and energy metabolism: TPC2 biases pathways of cellular energy usage to promote proliferation [87] while also enhancing tumor growth by stimulating new blood vessel formation [93,94]. Knockdown, or pharmacological blockade, of either TPC1 or TPC2 reduces cell attachment and migration in several human cell lines by disrupting β1-integrin trafficking to the leading edge of migrating cells [95]. Knockout of TPC2 is sufficient to impair proliferation and migration of RIL175 cells (a mouse hepatocellular carcinoma cell line) in vitro and block tumor growth in vivo [87]. Antitumor effects are also seen after pharmacological inhibition of TPC2: treatment of mice with a new TPC2 blocker (SG-094, a smaller derivative of the same bisbenzylisoquinoline chemotype) inhibited tumor growth [87]. Importantly, the role of TPCs may depend on the stage of tumor advancement. In late-stage metastatic melanoma, TPC2 knockout increased invasiveness and enzyme secretion [96]. Understanding the role of TPCs throughout the tumorigenic process and in multiple tumor types will be the focus of much future work.
Collectively, these studies highlight the promise of TPCs as druggable targets for both antivirals and chemotherapeutics. Antiviral and anticancer effects are seen with several of the same compounds that target TPC function.
Viral infection
The first area of functional convergence concerns viral infection. TPCs regulate viral internalization pathways into cells, as shown by experiments using spike-pseudotyped coronaviruses [14,58,60] that report translocation through the endolysosomal system. Indeed, knockdown of JPT2 inhibited cellular infectivity of a SARS-CoV-2 pseudovirus, an effect phenocopied by either genetic (siRNA) or pharmacological inhibition of TPCs [14]. Interestingly, this outcome was not mimicked by JPT1 siRNA, which may imply that JPT1 is unlikely to be an NAADP receptor [14]. A role of JPT2 in curtailing apoptosis in response to viral infection has also been demonstrated [43]. Further, JPT2 may regulate viral egress from cells as it has been shown to localize to virus-like particles during their exit from host cells [61]. The role of JPT2 in host responses to viral infection and immune surveillance will be an area of increasing focus [6,62].
Cancer
The second area of functional convergence between JPT2 and TPC function is in cancer. JPT2/HN1L has been implicated in cancer progression in breast cancer [63–65], non-small cell lung cancer [66,67], hepatocellular carcinoma [53], and adenocarcinoma [68], and bioinformatic analyses confirm JPT2 mRNA is upregulated in many cancers. Further, RNA expression data from 33 different cancer types, compiled in the GEPIA atlas [69,70], show significantly elevated JPT2 mRNA levels in the majority of tumor types (Figure 4A). One of the highest expression levels in noncancerous cells occurs in blood cells, which is noteworthy given the two photolabeling studies that identified JPT2 employed different types of blood cells [14,15].
Figure 4. JPT2 expression in cancer.
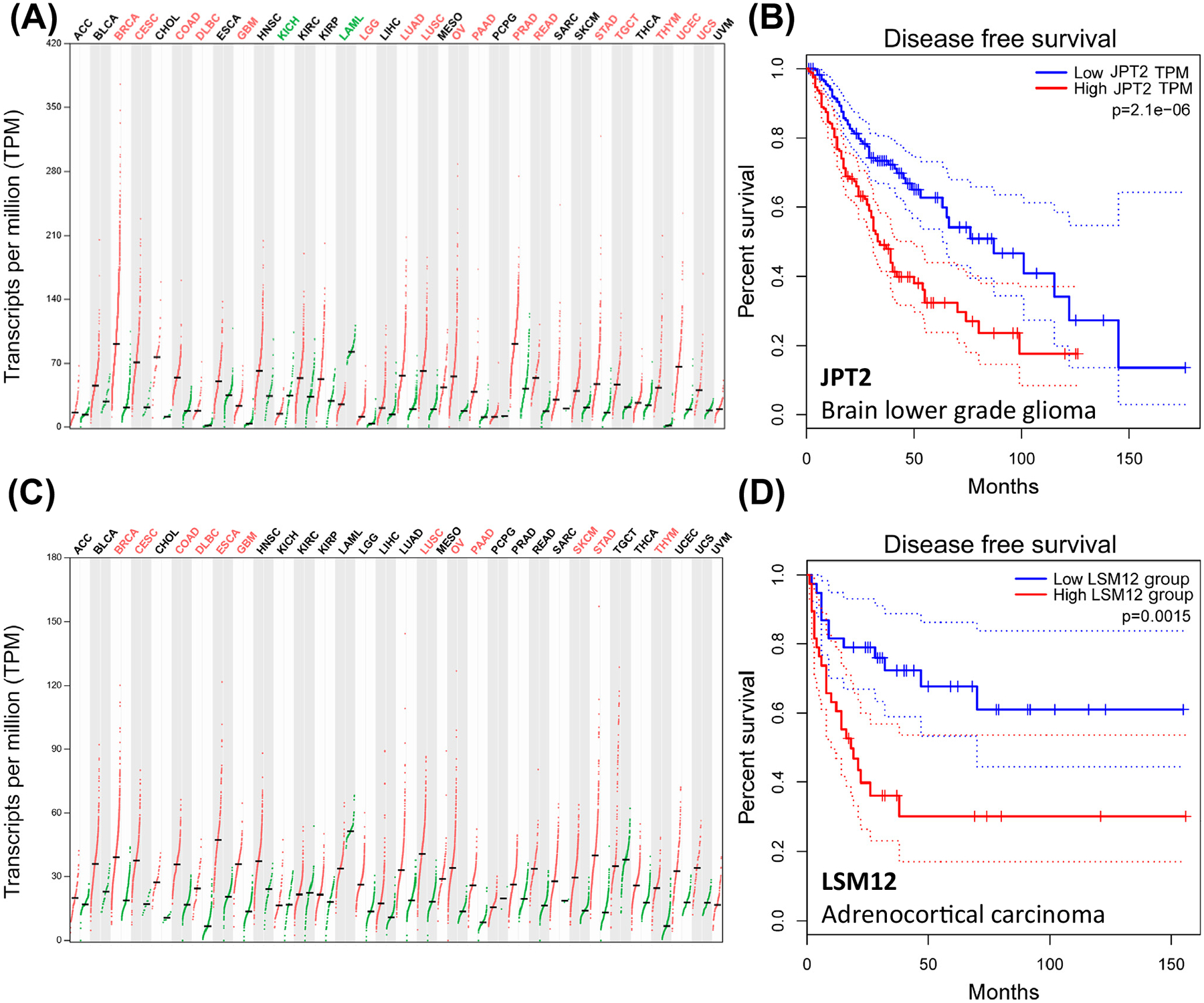
(A) Expression plot detailing JPT2 transcript levels in 33 different tumors (red) compared with controls (green). Different cancers are color coded to indicate statistically higher JPT2 expression in cancerous (red) or normal tissue (green) or no statistical difference (black). Mean expression (bar). Cancer abbreviations: ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma, endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma. (B) Disease-free survival plot for high versus low JPT2 expression levels (median ± 50% cutoffs) in LGG (n = 257). Dotted lines (95% confidence). (C and D) Similar analyses for (C) LSM12 expression and (D) outcomes in ACC (n = 38). Expression and survival data for Figure 4 are reproduced from GEPIA2 [69,70]. Abbreviations: JPT2, Jupiter microtubule associated homolog 2; LSM12, ‘like-Sm’ protein 12.
Experimental analyses consistently show elevated JPT2 expression is associated with increased cancer cell invasiveness, metastasis, and poorer survival [63,68]. For example, in non-small cell lung cancer, overexpression of JPT2 was detected in the majority of patient tumor samples compared with nontumor controls, and elevated expression of JPT2 positively correlated with tumor size and poor prognosis [66]. Reciprocally, knockdown of JPT2 in several models inhibited cell proliferation, migration, and tumor growth – again an effect seen in several different cancers [63,64,68]. For example, knockdown of JPT2 was associated with decreased tumorigenesis and metastasis of hepatocellular carcinoma cells in vivo [53]. Altogether, higher JPT2 expression often correlates with poorer clinical prognosis. Figure 4B provides an example of a disease-free survival plot for low-grade glioma for different JPT2 expression backgrounds.
More than 75 JPT2 mutants are detailed in the Cancer Genome Atlas, with approximately a quarter of breast cancer patients harboring JPT2 mutations [64]. How different JPT2 mutants [65] and JPT2 isoforms impact NAADP binding, TPC association, and cellular growth phenotypes merits exploration. While all this evidence implies similar associations of JPT2 and TPCs in tumorigenesis, some caution is needed. TPCs can function independently from any involvement of NAADP: they are activated by PI(3,5)P2 to evoke endolysosomal Na+ currents [7]. Equally, while JPT2 is implicated in multiple pathways of cell growth and division, it is not yet known whether NAADP binding is needed for JPT2 functionality in these pathways [63,64,66].
Defining the JPT2 interactome
Finally, identification of JPT2 lifts the veil on a broader JPT2 interactome. Multiple JPT2 interactors are predicted from various screening analyses [71,72], although each of these candidates requires further validation. The role of these JPT2-interacting proteins could be critical for controlling TPC activity, for example, by regulating the NAADP-binding affinity of JPT2 or by controlling the subcellular localization of JPT2. In addition, interactors could sequester JPT2 away from TPCs until appropriate physiological stimuli are sensed. Again, this will be a key area to explore.
A growing family of NAADP-BPs
Further excitement surrounds the identification of a second NAADP-BP, LSM12, which is a member of the ‘like-Sm’ RNA-binding protein family [16,73]. LSM12 was resolved as the only shared candidate between the TPC and NAADP interactomes and was shown to bind NAADP with high affinity (Kd for NAADP, ~30 nM) and selectivity over NADP [16]. Additional support for LSM12 as an NAADP-BP includes (i) NAADP conjugated to agarose beads was unable to interact with either TPC1 or TPC2 in the absence of LSM12, (ii) NAADP-evoked Na+ currents and NAADP-evoked Ca2+ signals were absent in the LSM12-knockout HEK cell line overexpressing TPC2, but responses could be restored by reconstituting LSM12 expression or injection of recombinant protein, and (iii) LSM12 functionality required its LSM domain and endogenous NAADP-evoked Ca2+ release was compromised in embryonic fibroblasts derived from transgenic mice, where a short sequence in the LSM domain was deleted [16]. Expression of LSM12 mRNA, like JPT2, shows low tissue specificity (Figure 3) [45]. LSM12 mRNA is also upregulated in many different tumors (Figure 4C), often in the same cancer types that exhibit increased JPT2 expression [69,70]. As a result, LSM12 upregulation is also correlated with poor clinical outcomes (Figure 4D).
With these two NAADP-BPs now identified, it merits comment that neither JPT2 nor LSM12 featured in previously published TPC proteomic data sets [30,31,74]. Conditions under which these prior proteomic studies were performed may not have been optimal to capture the dynamics of NAADP-BP association with TPCs. Alternatively, other necessary components of the interacting complex may yet to be revealed. Equally unresolved is the nature of relationship between JPT2 and LSM12. Do they physically interact? Knockdown of either NAADP-BP individually, even though the other remains, appears sufficient to block endogenous NAADP-evoked Ca2+ signals, and both independently bind NAADP [14,16], but the presence of both seems to be necessary to support NAADP action. Does this imply they partner in a complex, or do they function epistatically in pathways of TPC activation or inactivation? If they do not physically interact, how do they interact functionally? How does the presence of both NAADP-BPs shape cellular responsiveness to NAADP?
The discovery of JPT2 and LSM12 further begs the question of whether there are more NAADP-BPs to be unmasked. Beyond enzymes that metabolize NAADP, the existence of additional NAADP-BPs that function as signal transducers seems likely. In support of an expanded family of NAADP-BPs, photolabeling analyses in sea urchin egg homogenates [17,75] have resolved invertebrate NAADP-BPs with molecular weights (45 kDa, 40 kDa, and 30 kDa) distinct from the ~23-kDa NAADP-BPs identified in mammalian cells [24]. These sea urchin NAADP-BPs also bind NAADP with high affinity and selectivity, exhibit the known pharmacology of NAADP-evoked Ca2+ release and NAADP binding properties (irreversibility in high K+), and immunoprecipitate with sea urchin TPCs [24]. The identity of these urchin NAADP-BPs, and consequently any relationship to JPT2 and LSM12, is currently unknown.
Therefore, it seems plausible that more NAADP-BPs will be discovered, such that the appropriate question may not be how many, but what are their functional niches? While the currently accepted paradigm of NAADP action culminates in TPC activation, TPCs may be just one type of effector engaged by NAADP-liganded NAADP-BPs. This is shown by the work of Roggenkamp et al., which implicates an interaction between JPT2 and RyR1 [15,32]. However, the roles of the NAADP-BPs may extend beyond regulation of Ca2+ dynamics such that other functional outputs, yet to be appreciated, will emerge. For example, in Drosophila, which lacks TPCs, JPT already has its own identity as a microtubule-binding protein [76]. Does Drosophila JPT bind NAADP? If so, to what effect? Additionally, Gunaratne et al. [14] isolated JPT2 from erythrocytes, a cell type where organelles and intracellular Ca2+ channels are absent, but NAADP is present [77]. What is the function of JPT2 in red blood cells? By analogy with another family of second messenger-binding proteins – inositol polyphosphate-binding proteins [78] or to the STIM family of Ca2+ sensors that bind multiple types of Ca2+ channels [79] – a broader family of NAADP-BPs may fulfill pleiotropic messenger roles beyond engagement of TPCs. This will be an important area of study now that the identity of these NAADP-BPs is established.
New tools
Identification of both NAADP-BPs provides new opportunities to monitor and manipulate NAADP signaling. For example, the NAADP binding modules in each NAADP-BP can potentially be engineered into NAADP sensors able to report NAADP dynamics in intact cells. This approach has been successfully realized for other second messengers, including inositol trisphosphate (IP3) and cAMP through the development of fluorescence resonance energy transfer (FRET)-based reporters [80,81]. Such tools could resolve the spatiotemporal dynamics of NAADP in intact cells and monitor the spatiotemporal relationship between NAADP and Ca2+ dynamics. Fluorescence-based NAADP reporters would also enable higher-throughput screening applications with the goal of identifying agonists coupled to NAADP generation. Therefore, the development of reporters for NAADP will be a powerful new approach to enable a transition away from the traditional radioligand-based approaches. The identification of the NAADP-BPs also affords opportunity to develop new ligands to manipulate this signaling pathway. This includes ligands that interact with the NAADP-binding site(s) on each NAADP-BP, as well as ligands that act at the binding interfaces between NAADP-BPs and TPCs. The identification of the NAADP-BPs provides a molecular framework to explore these druggable interfaces through modeling and screening activities.
NAADP is a biased agonist and evokes a significant Ca2+ permeability through TPCs, while PI (3,5)P2 activation results in a monovalent Na+ flux [10]. These dual agonists therefore trigger unique responses from the same ion channel target [82,83], analogous to the phenomenon of biased signaling that is well elaborated at G protein-coupled receptors (GPCRs) [84]. Are NAADP-BPs the critical effectors of this signaling bias by transducing the effects of their engaged ligands to stabilize a specific conformation of TPC subunits and pore architecture that supports the characteristic Ca2+ permeability diagnostic of NAADP action. Of interest is recent work identifying novel TPC2 chemotypes (e.g., TPC2-A1-N; Box 1) that mimic NAADP action [10,85], though it remains to be determined how TPC2-A1-N functions as a NAADP mimetic. It may act like NAADP and bind NAADP-BPs that interact with TPC2, or it may act as an NAADP-BP mimetic engaging TPC2 at the NAADP-BP interaction site. Alternatively, it could work through another undescribed mechanism. Irrespective of these answers, these ligands provide a clear example of progress in developing agents to manipulate the function of the TPC complex, and the identification of NAADP-BPs spurs additional possibilities for tool development. This effort may realize therapeutic benefit in diseases where NAADP dysfunction is established [86].
Concluding remarks
The recent identification of two mammalian NAADP-BPs, after a decade-long search, is an exciting development for the NAADP signaling field. These discoveries provide new impetus to understand and manipulate NAADP action in different cells and tissues and to resolve how NAADP signaling is perturbed in disease states. With these NAADP-BPs finally yielding their identity, many new questions are accessible about structural mechanism, regulatory control, functional impact, and cell biology of both NAADP-BPs (see Outstanding questions). These questions have become open to interrogation now that the identity of the NAADP-BP candidates is known. It will be an exciting journey to explore wherever these discoveries lead.
Outstanding questions.
How do the NAADP-BPs JPT2 and LSM12 interact with NAADP and TPCs? What is the structural basis for high-affinity, selective NAADP binding? How do the different NAADP-BPs associate with TPCs and how is this association regulated by NAADP?
How is JPT2 and LSM12 expression controlled? What regulatory mechanisms determine NAADP-BP abundance? How do these mechanisms set cellular NAADP sensitivity?
Is NAADP binding integral to known functions of JPT2 and LSM12? Are established functions of JPT2 and LSM12 regulated by NAADP-evoked Ca2+ signaling, thereby expanding the cellular roles of this signaling pathway?
Are there other NAADP-BPs out there? Do JPT2 and LSM12 fully account for NAADP action or are there even more relatives in the NAADP-BP family tree? If so, what are their relationships?
Can we generate JPT2- and LSM12-based probes to further interrogate NAADP action? How will increased understanding of the NAADP-BPs and their interaction with TPCs reveal new opportunities for tool and drug development? Can we use these to better manipulate this important signaling pathway?
Highlights.
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger that releases Ca2+ by activating ion channels known as two-pore channels (TPCs), resident on endosomes and lysosomes.
The mechanism of TPC activation by NAADP is unclear as no binding site(s) for NAADP has been identified on the TPC complex.
It has long been postulated that NAADP activates vertebrate TPCs indirectly via unidentified ~23-kDa NAADP-binding proteins (NAADP-BPs) associated with the TPC complex.
Recent work has revealed the identity of these elusive NAADP-BPs as Jupiter microtubule associated homolog 2 (JPT2) and ‘like-Sm’ protein 12 (LSM12).
These discoveries enable new insight into this signaling pathway and the role of NAADP in cellular physiology and disease.
Acknowledgments
Work was supported by NIH-GM088790 and NSF-2027748 (to J.S.M.) and R15-GM131329 (to J.T.S.). S.P. was supported by BBSRC grants (BB/N01524X/1; BB/T015853/1). X.C. was supported in part by the UCLA Specialty Training and Advanced Research (STAR) fellowship program and the NIH training grant T32HL007895.
Glossary
- Biased agonist
a ligand that induces a receptor conformation that preferentially couples to a specific signaling outcome. Typically used for GPCRs in the context of G protein versus β-arrestin coupling, but also applicable to ion channel signaling outcomes. Here, NAADP acts as a biased agonist as it evokes a Ca2+ flux through two-pore channels (TPCs)
- Bifunctional photoprobe
a chemical probe with dual functional groups used in photolabeling studies. The first functionalized substituent is photoreactive to enable light-evoked crosslinking with specific targets. The second functionalized substituent is a moiety that enables an enrichment strategy to isolate the photolabeled target
- Jupiter microtubule associated homolog 2 (JPT2)
one of the two members of the Jupiter gene family (JPT1/HN1 and JPT2/HN1L) that has been shown to regulate cell proliferation and survival. Recently identified as a NAADP-binding protein and TPC accessory protein required for endogenous NAADP-evoked Ca2+ release and viral trafficking
- ‘Like-Sm’ protein 12 (LSM12)
one member of the larger LSM protein family, representatives of which are conserved evolutionarily from prokaryotes to humans. LSM family members are traditionally viewed as having roles in post-transcriptional regulation of RNA expression. Also recently identified as a NAADP-binding protein and TPC accessory protein required for NAADP-evoked Ca2+ release
- NAADP-binding protein (NAADP-BP)
in mammalian cells, unidentified ~23-kDa proteins resolved in photolabeling studies shown to possess properties and behavior that mimic the characteristics of the NAADP Ca2+ release pathway. These NAADP-BPs are postulated to confer NAADP sensitivity to TPCs by acting as TPC accessory proteins necessary for NAADP-evoked Ca2+ release
- Nicotinic acid adenine dinucleotide phosphate (NAADP)
a potent Ca2+-releasing second messenger produced in response to cell stimulation. NAADP releases Ca2+ from endosomes and lysosomes in many different organisms through activation of a family of ion channels known as TPCs
- Two-pore channel (TPC)
a class of voltage- and/or ligand-gated (activated by both NAADP and PI(3,5)P2) ion channels that is found intracellularly in endosomes and lysosomes
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Lee HC (2005) Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J. Biol. Chem 280, 33693–33696 [DOI] [PubMed] [Google Scholar]
- 2.Patel S (2015) Function and dysfunction of two-pore channels. Sci. Signal 8, re7. [DOI] [PubMed] [Google Scholar]
- 3.Calcraft PJ et al. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brailoiu E et al. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchant JS and Patel S (2015) Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem. Soc. Trans 43, 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman SA et al. (2020) Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. Science 367, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X et al. (2012) TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cang C et al. (2013) mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 152, 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruas M et al. (2015) Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 34, 1743–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerndt S et al. (2020) Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. eLife 9, e54712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.She J et al. (2018) Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 556, 130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.She J et al. (2019) Structural mechanisms of phospholipid activation of the human TPC2 channel. eLife 8, e45222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S (2018) Two-pore channels open up. Nature 556, 38–40 [DOI] [PubMed] [Google Scholar]
- 14.Gunaratne GS et al. (2021) Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling. Sci. Signal 14, eabd5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roggenkamp HG et al. (2021) HN1L/JPT2: a signaling protein that connects NAADP generation to Ca2+ microdomain formation. Sci. Signal 14, eabd5647. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J et al. (2021) Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat. Commun 12, 4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galione A et al. (2014) Preparation and use of sea urchin egg homogenates for studying NAADP-mediated Ca2+ release. Cold Spring Harb Protoc 2014, 988–992 [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y et al. (2019) Probing Ca2+ release mechanisms using sea urchin egg homogenates. Methods Cell Biol. 151, 445–458 [DOI] [PubMed] [Google Scholar]
- 19.Aarhus R et al. (1996) Activation and inactivation of Ca2+ release by NAADP+. J. Biol. Chem 271, 8513–8516 [DOI] [PubMed] [Google Scholar]
- 20.Berridge G et al. (2002) Solubilization of receptors for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate. J. Biol. Chem 277, 43717–43723 [DOI] [PubMed] [Google Scholar]
- 21.Patel S et al. (2000) Widespread distribution of binding sites for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate, in the brain. J. Biol. Chem 275, 36495–36497 [DOI] [PubMed] [Google Scholar]
- 22.Bak J et al. (2001) NAADP receptors are present and functional in the heart. Curr. Biol 11, 987–990 [DOI] [PubMed] [Google Scholar]
- 23.Zong X et al. (2009) The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walseth TF et al. (2012) Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J. Biol. Chem 287, 2308–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin-Moshier Y et al. (2012) Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem 287, 2296–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchant JS et al. (2012) The molecuar basis for Ca(2+) signalling by NAADP: two-pore channels in a complex? Messenger (Los Angel) 1, 63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walseth TF et al. (2012) Nicotinic acid adenine dinucleotide 2’-phosphate (NAADP) binding proteins in T-lymphocytes. Messenger (Los Angel) 1, 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunaratne GS et al. (2019) 5-Azido-8-ethynyl-NAADP: a bifunctional, clickable photoaffinity probe for the identification of NAADP receptors. Biochim. Biophys. Acta Mol. Cell Res 1866, 1180–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asfaha TY et al. (2019) The synthesis and characterization of a clickable-photoactive NAADP analog active in human cells. Cell Calcium 83, 102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin-Moshier Y et al. (2014) The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. U. S. A 111, 13087–13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krogsaeter EK et al. (2019) The protein interaction networks of mucolipins and two-pore channels. Biochim. Biophys. Acta Mol. Cell Res 1866, 1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walseth TF and Guse AH (2021) NAADP: from discovery to mechanism. Front. Immunol 12, 703326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou G et al. (2004) Cloning, expression and subcellular localization of HN1 and HN1L genes, as well as characterization of their orthologs, defining an evolutionarily conserved gene family. Gene 331, 115–123 [DOI] [PubMed] [Google Scholar]
- 34.Chi Y et al. (2008) Identification of CDK2 substrates in human cell lysates. Genome Biol. 9, R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brailoiu E et al. (2010) An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem 285, 38511–38516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitt SJ et al. (2010) TPC2 i a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J. Biol. Chem 285, 35039–35046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybalchenko V et al. (2012) Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J. Biol. Chem 287, 20407–20416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg I et al. (2000) Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+-signaling. J. Cell Biol 150, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guse AH (2012) Linking NAADP to ion channel activity: a unifying hypothesis. Sci. Signal 5, pe18. [DOI] [PubMed] [Google Scholar]
- 40.Galione A (2015) A primer of NAADP-mediated Ca2+ signalling: from sea urchin eggs to mammalian cells. Cell Calcium 58, 27–47 [DOI] [PubMed] [Google Scholar]
- 41.Marchant JS and Patel S (2013) Questioning regulation of two-pore channels by NAADP. Messenger (Los Angel) 2, 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan AJ et al. (2015) TPC: the NAADP discovery channel? Biochem. Soc. Trans 43, 384–389 [DOI] [PubMed] [Google Scholar]
- 43.Kumada T et al. (2010) Ttyh1, a Ca2+-binding protein localized to the endoplasmic reticulum, is required for early embryonic development. Dev. Dyn 239, 2233–2245 [DOI] [PubMed] [Google Scholar]
- 44.Tang W et al. (1997) Murine Hn1 on chromosome 11 is expressed in hemopoietic and brain tissues. Mamm. Genome 8, 695–696 [DOI] [PubMed] [Google Scholar]
- 45.Consortium GTEx (2015) Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelu JJ et al. (2018) TPC2-mediated Ca2+ signaling is required for the establishment of synchronized activity in developing zebrafish primary motor neurons. Dev. Biol 438, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brailoiu E et al. (2006) Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J. Biol. Chem 281, 15923–15928 [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z-H et al. (2013) Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells. PLoS One 8, e66077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edfors F et al. (2016) Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol 12, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J et al. (2020) Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat. Commun 12, 4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W et al. (2020) The functions and targets of miR-212 as a potential biomarker of cancer diagnosis and therapy. J. Cell. Mol. Med 24, 2392–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun S et al. (2018) MicroRNA-212–5p prevents dopaminergic neuron death by inhibiting SIRT2 in MPTP-induced mouse model of Parkinson’s disease. Front. Mol. Neurosci 11, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L et al. (2019) HN1L-mediated transcriptional axis AP-2γ/METTL13/TCF3-ZEB1 drives tumor growth and metastasis in hepatocellular carcinoma. Cell Death Differ. 26, 2268–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm C et al. (2018) Endolysosomal cation channels and cancer—a link with great potential. Pharmaceuticals (Basel) 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faris P et al. (2018) Endolysosomal Ca2+ signalling and cancer hallmarks: two-pore channels on the move, TRPML1 lags behind! Cancers (Basel) 11, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alharbi AF and Parrington J (2019) Endolysosomal Ca2+ signaling in cancer: the role of TPC2, from tumorigenesis to metastasis. Front. Cell Dev. Biol 7, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakurai Y et al. (2015) Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347, 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunaratne GS et al. (2018) NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium 75, 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ou X et al. (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun 11, 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunaratne GS et al. (2018) A screening campaign in sea urchin egg homogenate as a platform for discovering modulators of NAADP-dependent Ca2+ signaling in human cells. Cell Calcium 75, 42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston GP et al. (2019) Nipah virus-like particle egress is modulated by cytoskeletal and vesicular trafficking pathways: a validated particle proteomics analysis. mSystems 4, e00194–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei J et al. (2019) HN1L is essential for cell growth and survival during nucleopolyhedrovirus infection in silkworm, Bombyx mori. PLoS One 14, e0216719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiao D et al. (2021) HN1L promotes migration and invasion of breast cancer by up-regulating the expression of HMGB1. J. Cell. Mol. Med 25, 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y et al. (2018) HN1L promotes triple-negative breast cancer stem cells through LEPR-STAT3 pathway. Stem Cell Rep. 10, 212–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z-B et al. (2019) Detection of breast cancer stem cell gene mutations in circulating free DNA during the evolution of metastases. Breast Cancer Res. Treat 178, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L et al. (2017) Overexpression of HN1L promotes cell malignant proliferation in non-small cell lung cancer. Cancer Biol. Ther 18, 904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petroziello J et al. (2004) Suppression subtractive hybridization and expression profiling identifies a unique set of genes overexpressed in non-small-cell lung cancer. Oncogene 23, 7734–7745 [DOI] [PubMed] [Google Scholar]
- 68.Wang ZY et al. (2021) HN1L promotes invasion and metastasis of the esophagogastric junction adenocarcinoma. Thorac. Cancer 12, 650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang Z et al. (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang Z et al. (2019) GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oughtred R et al. (2021) The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murali T et al. (2011) DroID 2011: a comprehensive, integrated resource for protein, transcription factor, RNA and gene interactions for Drosophila. Nucleic Acids Res. 39, D736–D743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lekontseva NV et al. (2021) Diversity of LSM family proteins: similarities and differences. Biochemistry (Mosc.) 86, S38–S49 [DOI] [PubMed] [Google Scholar]
- 74.Grimm C et al. (2014) High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun 5, 4699. [DOI] [PubMed] [Google Scholar]
- 75.May JM (2012) Vitamin C transport and its role in the central nervous system. Subcell. Biochem 56, 85–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karpova N et al. (2006) Jupiter, a new Drosophila protein associated with microtubules. Cell Motil. Cytoskeleton 63, 301–312 [DOI] [PubMed] [Google Scholar]
- 77.Churamani D et al. (2004) Determination of cellular nicotinic acid-adenine dinucleotide phosphate (NAADP) levels. Biochem. J 380, 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu M et al. (2016) Inositol polyphosphates intersect with signaling and metabolic networks via two distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A 113, E6757–E6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cahalan MD (2010) Cell biology. How to STIMulate calcium channels. Science 330, 43–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyamoto A and Mikoshiba K (2017) Probes for manipulating and monitoring IP3. Cell Calcium 64, 57–64 [DOI] [PubMed] [Google Scholar]
- 81.Kim N et al. (2021) cAMP biosensors based on genetically encoded fluorescent/luminescent proteins. Biosensors (Basel) 11, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung M-K et al. (2008) TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci 11, 555–564 [DOI] [PubMed] [Google Scholar]
- 83.Herrington J and Arey BJ (2014) Conformational mechanisms of signaling bias of ion channels. In Biased Signaling in Physiology, Pharmacology and Therapeutics (1st edn) (Arey BJ, ed.), pp. 173–207, Academic Press [Google Scholar]
- 84.Wootten D et al. (2018) Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol 19, 638–653 [DOI] [PubMed] [Google Scholar]
- 85.Gerndt S et al. (2020) Discovery of lipophilic two-pore channel agonists. FEBS J. 287, 5284–5293 [DOI] [PubMed] [Google Scholar]
- 86.Patel S and Kilpatrick BS (2018) Two-pore channels and disease. Biochim. Biophys. Acta Mol. Cell Res 1865, 1678–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muller M et al. (2021) Gene editing and synthetically accessible inhibitors reveal role for TPC2 in HCC cell proliferation and tumor growth. Cell Chem. Biol 28, 1119–1131.e27 [DOI] [PubMed] [Google Scholar]
- 88.Clementi N et al. (2021) Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res 163, 105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan N et al. (2020) Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J. 34, 4147–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruas M et al. (2010) Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr. Biol 20, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Penny CJ et al. (2019) Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers. Biochim. Biophys. Acta Mol. Cell Res 1866, 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daniloski Z et al. (2021) Identification of required host factors for SARS-CoV-2 infection in human cells. Cell 184, 92–105.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pafumi I et al. (2017) Naringenin impairs two-pore channel 2 activity and inhibits VEGF-induced angiogenesis. Sci. Rep 7, 5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Favia A et al. (2014) VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc. Natl. Acad. Sci. U. S. A 111, E4706–E4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen ONP et al. (2017) Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res. 77, 1427–1438 [DOI] [PubMed] [Google Scholar]
- 96.D’Amore A et al. (2020) Loss of two-pore channel 2 (TPC2) expression increases the metastatic traits of melanoma cells by a mechanism involving the Hippo signalling pathway and store-operated calcium entry. Cancers (Basel) 12, 2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z-H et al. (2021) A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii. Nat. Commun 12, 5802. [DOI] [PMC free article] [PubMed] [Google Scholar]


