Abstract
Bcl-2-associated X protein (BAX) is a critical executioner of mitochondrial-regulated cell death through its lethal activity of permeabilizing the outer mitochondrial membrane. While physiological function of BAX ensures tissue homeostasis, dysregulation of BAX leads to aberrant cell death. Despite BAX being a promising therapeutic target for human diseases, historically, development of drugs has focused on anti-apoptotic BCL-2 proteins, due to challenges in elucidating the mechanism of BAX activation and identifying druggable surfaces of BAX. Here, we discuss recent studies that have provided structure-function insights and identified regulatory surfaces which control BAX activation. Moreover, we emphasize the development of small molecule orthosteric, allosteric, and oligomerization modulators that provide novel opportunities for biological investigation and progress towards drugging BAX.
Keywords: BAX, BCL-2 family, apoptosis, mitochondria, BAX activators, BAX inhibitors
Targeting BAX activation in cell death
The BCL-2 family of proteins are well established as key regulators of apoptotic cell death [1–4]. The key step of apoptosis is mitochondrial outer membrane (MOM) permeabilization (MOMP) which irreversibly commits a cell to death [5,6]. Pro-apoptotic effector BCL-2 proteins (See glossary) BAX and BAK play a central role by forming the MOM embedded oligomeric pores which execute MOMP [4–7]. BAX and BAK exist primarily in inactive states maintained in part by interaction with anti-apoptotic BCL-2 proteins such as BCL-2, BCL-xL, or MCL-1 [8–12]. Under cellular stress BH3-only proteins such as BIM, BID, and PUMA utilize their BCL-2 homology 3 (BH3) domain to both inhibit anti-apoptotic BCL-2 proteins as well as directly activate pro-apoptotic BAX and BAK by initiating a series of conformational changes that results in the formation of MOMP inducing oligomeric pores [8,13–15]. The mechanism by which BAX is transformed from an inactive cytosolic monomer to a lethal mitochondrial oligomer is detailed in Box 1 and Figure 1.
Text Box 1: BAX activation mechanism.
In healthy cells BAX primarily exists as an inactive cytosolic monomer or auto-inhibited homodimer (Figure 1a) [10,31]. Under cellular stress BH3-only proteins such as BIM use their BH3-domain to bind the BAX N-terminal trigger site formed by helices α1 and α6 which initiates the BAX activation cascade (Figure 1a) [32,39,41,58,77]. BH3-only binding induces a conformational change of loop α1–α2 from a closed conformation, where the loop is closely associated with α1 and α6, to an open conformation, where the loop is dissociated and solvent exposed [10,31,32,39]. Following this step, additional conformational changes occur such as exposure of the 6A7-epitope (a marker of BAX activation) and exposure of the BAX-BH3 domain (α2) (Figure 1C) [21,39]. Most critically, BAX transmembrane helix α9 is mobilized from the C-terminal canonical site (formed by helices α3–α5) allowing BAX to translocate to the mitochondrial outer membrane (MOM) [39,78]. Once anchored to the MOM via helix α9, BAX can undergo further conformational change by BH3-only binding to the now unoccupied canonical site allowing BAX to separate into latch domain (helix α6–α8), the core domain (helix α3–α5) and the dislodgement its BH3 domain (helix α2) (Figure 1d) [53,54,79]. Moreover, BAX can propagate cytosolic inactive BAX monomers to the MOM through its BH3 domain (α2) and the trigger site of inactive BAX monomers, consistent with the BAX autoactivation mechanism [39]. The core domain maintains the overall structure of the canonical site, allowing it to bind to BH3-domains, specifically another BAX-BH3, allowing for formation of symmetrical dimers via the BH3-domain of one BAX protomer binding to the canonical site of another BAX protomer (Figure 1e) [53,54,79]. These symmetrical BAX dimers are assumed to be the building block of the homo-oligomeric pores which permeabilize the MOM (Figure 1f) [80–84]. Although the precise structure of the BAX oligomeric pore remains unresolved, likely owing to its large size and heterogeneity, interactions have been reported between adjacent BAX dimers via core, latch, and transmembrane domains which likely contribute to its formation and stability [80–84]. Moreover, certain lipids can be involved in the regulation of BAX activation, integration into the MOM and formation of BAX oligomers [85]. Recent data suggest that lipids are instrumental in mediating spontaneous BAX activation and membrane translocation through the association of BAX α9 and the MOM without direct interaction of BAX and BH3-only proteins [86]. MOM permeabilization by BAX oligomers most notably allow for the release of low molecular weight apoptogens such as cytochrome c (12 kDa), which bind to APAF1 to form the heptameric apoptosome which in turn activates caspases and begins the process of apoptosis [87]. It should however be noted that BAX pores are also responsible for release of mitochondrial DNA (104 kDa) into the cytosol during apoptosis [88]. The precise details of how BAX forms higher order oligomers at the MOM remains unknown and is a significant gap in our understanding of the overall BAX activation mechanism.
Figure 1: BAX activation pathway.
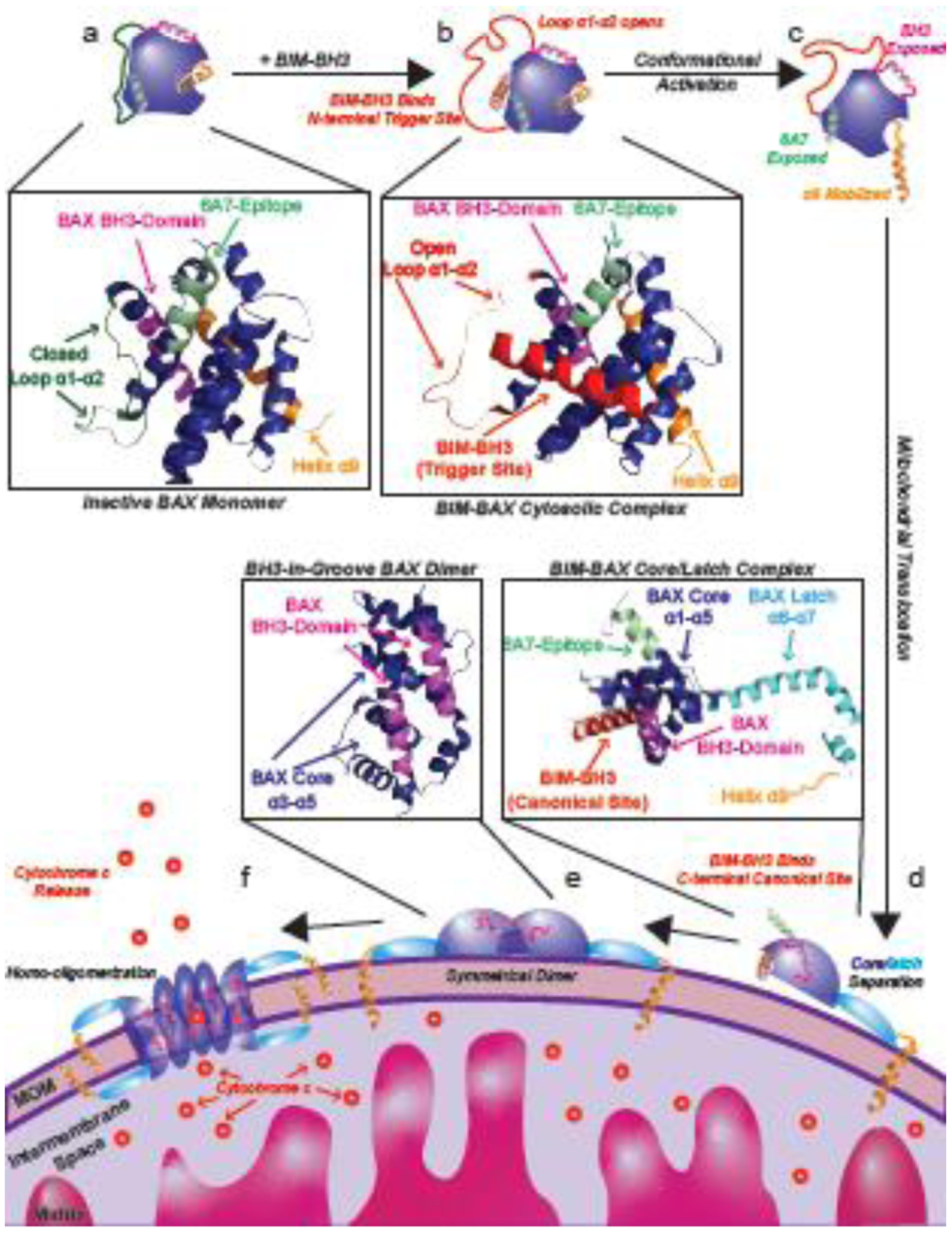
Cytosolic BAX (a, PDB: 1F16) is activated when a BH3-only protein such as BIM binds with its BH3 domain to the N-terminal BAX trigger site (b, PDB: 2K7W) initiating several conformational changes (c). Upon BH3 triggering, a conformational change of loop α1–α2 from a closed conformation, to an open conformation, contributes to exposure of the 6A7 epitope in α1 and BAX BH3-domain (α2) and subsequent dissociation of α9 from the C-terminal canonical pocket. Conformationally active BAX then translocates to the outer mitochondrial outer membrane where it can also bind to BH3-only proteins via its canonical site and separate its structure into a core (α1–α5) and latch (α6–α8) domains (d). BH3-in-groove symmetrical BAX homodimers in which the BH3-domain of one BAX protomer is bound by the canonical site of the other BAX protomer is believed to form the building block of the oligomeric BAX pore and (e) together with other BAX regions are then capable of forming higher oligomer pores which permeabilize the mitochondrial outer membrane to release cytochrome c and other apoptogens (f) which commit the cell to apoptosis.
Direct pharmacological modulation of BAX has long been an attractive target due to the prominent role of BAX in human diseases [16–18]. Cancer cells often evade apoptosis by overexpressing anti-apoptotic inhibitors of BAX, however small molecule activators of BAX have been shown to overcome this effect and inhibit cancer growth in animal models [2,19–23]. Pharmacological inhibition of BAX has been shown to inhibit neuronal and cardiomyocyte cell death and is a promising target for the treatment of heart disease, neurodegeneration, and stroke [3,24–30]. Beyond the therapeutic implications of pharmacologic BAX modulation, studying how small molecules, peptides, and antibodies interact with BAX and modulate its activity have provided invaluable insight into how BAX is transformed from an inactive monomer or dimer to a deadly oligomeric MOM embedded pore. Furthermore, these studies have identified numerous binding sites and hot spots on the surface of BAX capable of modulating BAX activity. Remarkably, it will be seen that the majority of the surface of BAX has been identified as capable of modulating BAX activity. This review will discuss examples of both physiological and pharmacological modulation of BAX with a focus on binding site characteristics and structure activity relationship (SAR) of various BAX modulators as well as their potential therapeutic utility when applicable (Table 1).
Table 1.
Characteristics of BAX activators and inhibitors.
| Proposed Binding Site | BAX Ligand | Method of Identification | Activity | Supporting Evidence for Binding Site | PDB | Critical Contacts | Binding Affinity | Cellular and In Vivo Activity | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Trigger Site | BIM-BH3 | N/A* | Activator | NMR | 2K7W | K21 | 283 nM† | Endogenous BAX activator, stapled BIM-BH3 domain induce apoptosis in leukemia xenografts | *Endogenous BAX activator |
| BID-BH3 | N/A* | Activator | Disulfide linking | 885 nM† | Endogenous BAX activator, stapled BID-BH3 domain induce apoptosis in leukemia xenografts | *Endogenous BAX activator | |||
| BAM7 | Computational screen | Activator | NMR | K21 | 2.6 μM* | Induces BAX dependent apoptosis in mouse embryonic fibroblasts (MEFs) | *Ki calculated based on competitive fluorescence polarization IC50 | ||
| BTSA1 | Pharmacophore enhancement of BAM7 | Activator | NMR | K21 | 144 nM* | Induces apoptosis in various leukemia cell lines and in leukemia xenografts | *EC50 for BAX and fluorescently labeled BTSA1 in fluorescence polarization assay | ||
| Compound 8 | Structure based optimization of BAM7 | Activator | K21*, E17*, D142* | 800 nM** | Induces apoptosis in various solid and hematologic malignancy cell lines and in lung cancer xenografts | *Proposed contacts, not-confirmed by mutagenesis **Ki calculated based on competitive fluorescence polarization IC50 |
|||
| Eltrombopag* | Similarity screen of BAM7 | Inhibitor | NMR | R145, R134 | 145 nM** | Inhibits apoptosis induced by staurosporine (STS) and BH3-mimetics in MEFs | *FDA-Approved thrombopoietin receptor agonist, BAX activity in vivo has not been confirmed **KD determined by microscale thermophoresis |
||
| 3G11 | Phage Display | Inhibitor | NMR | R134, K21 | 38 nM* | *KD determined by biolayer interferometry | |||
| 3C10 | Commercially available | Activator/inhibitor* | Crystal Structure** | 5W5Z | R34, R37, M38 | *Inhibits WT BAX, activates BAX S184L **Crystal Structure Solved for 3C10:BAXP168G Complex |
|||
| Canonical Site | BIM-BH3 | N/A* | Activator | Crystal Structure | 4ZIE | BAX R109 - BIM D157 | 283 nM† | Endogenous BAX activator, stapled BIM-BH3 domain induce apoptosis in leukemia xenografts | *Endogenous BAX activator |
| BID-BH3 | N/A* | Activator | Crystal Structure | 4BD2 | BAX R109 - BID D95 | 885 nM† | Endogenous BAX activator, stapled BID-BH3 domain induce apoptosis in leukemia xenografts | *Endogenous BAX activator | |
| SMBA1 | Computational Screen | Activator | S184* | 43 nM** | Induces apoptosis in lung cancer cell lines and xenografts | *Proposed contacts, not-confirmed by mutagenesis **Ki calculated based on competitive fluorescence polarization IC50 |
|||
| CYD-4–61, GL0388 | SAR of SMBA1 | Activator | S184* | Induces apoptosis in breast cancer cell lines and xenografts | *Proposed contacts, not-confirmed by mutagenesis | ||||
| Compound 106 | Computational Screen | Activator | Induces apoptosis in various cell lines and in lung cancer xenografts | ||||||
| MSN-125, MSN-50, DAN004* | Liposome screen | Inhibitor** | Inhibits apoptosis induced by STS or actinomycin D in BMK cells, protects neurons from excitotoxic cell death | *DAN004 exhibited cellular toxicity **Inhibitors of BAX and BAK |
|||||
| Non-Canonical Site | vMIA | N/A* | Inhibitor | NMR | 2LR1 | D84, D86 | 22 nM** | Expressed by cytomegalovirus to evade host cell apoptosis | *Cytomegalovirus expressed protein **KD determined by direct binding fluorescence anisotropy assay |
| BIF-44 | Ligand based NMR screen | Sensitizor | NMR | 37 μM* | *KD by isothermal titration calorimetry | ||||
| BCL-2-BH4 | N/A* | Inhibitor | NMR | BCL-2 I19, L23 | 177 nM** | *BCL-2 inhibition of BAX is well established, but the in vivo role of BCL-2-BH4 has not been definitively shown in vivo **EC50 for BAX and fluorescently labeled BCL-2-BH4 stappled peptide in fluorescence polarization assay |
|||
| BAI1 | Liposome Screen | Inhibitor | NMR | D84, K123 | 15 μM* | Inhibits apoptosis induced by TNF-α+cycloheximide in MEFs, BTSA1 in leukemia cells, and doxorubicin in primary cardiomyocytes; prevents doxorubicin induced cardiomyopathy in vivo | *KD determined by microscale thermophoresis | ||
| OICR77A | Liposome Screen | Activator | C126A | C126 | 255 nM* | Induces apoptosis in BMK cells** | *KD by isothermal titration calorimetry **OICR77A was shown to induce apoptosis independent of BAX expression |
||
| Miscellaneous* | Bci1, Bci2 | Liposome Screen | Inhibitor | Inhibits apoptosis induced by STS in HeLa cells and MEFs; prevents hippocampal damage after global brain ischemia in vivo | *No binding site has been proposed for Bci1 and Bci2 |
Affinities for BIM and BID to BAX are EC50 data reported from wild-type BAX and fluorescently labeled BIM-BH3 or BID-BH3 stapled peptides. It should be noted that these assays cannot differentiate trigger site versus canonical site binding and therefore the affinity for each site. Furthermore, these assays use truncated BIM and BID constructs which likely do not fully recapitulate all interactions between BAX and BIM and BID in the cellular environment.
Trigger site modulators of BAX
The BAX N-terminal trigger site is characterized by the shallow hydrophobic surface of BAX formed by α1, α6, and loop α1–α2 [31,32]. The trigger site was identified using NMR chemical shift perturbations and paramagnetic relaxation enhancement data of BAX in complex with a stapled helix of the BH3-domain of BIM. This enabled determination of a NMR-based model structure of BAX in complex with the BH3-domain of BIM [32]. Binding of the BH3 domain of BIM to this previously unknown site distinguishes BAX from other BCL-2 family members, which primarily interact with BH3 domains via the canonical site formed by α2, α3, α4, and α5 [33–38]. The BIM-BH3–BAX complex exhibits several stabilizing ionic interactions between trigger site residues and the charged residues of BH3-domains, most notably a critical interaction between BAX K21 and BIM-BH3 D158 [32] (Figure 2a) The interaction is further stabilized by the four conserved hydrophobic residues of BH3-domains [32]. Trigger site binding of BIM induces an “opening” of loop α1–α2 potentially via contact with two bulky tyrosine residues of BIM-BH3(Y162/Y163) [32].
Figure 2: Trigger site modulators of BAX.
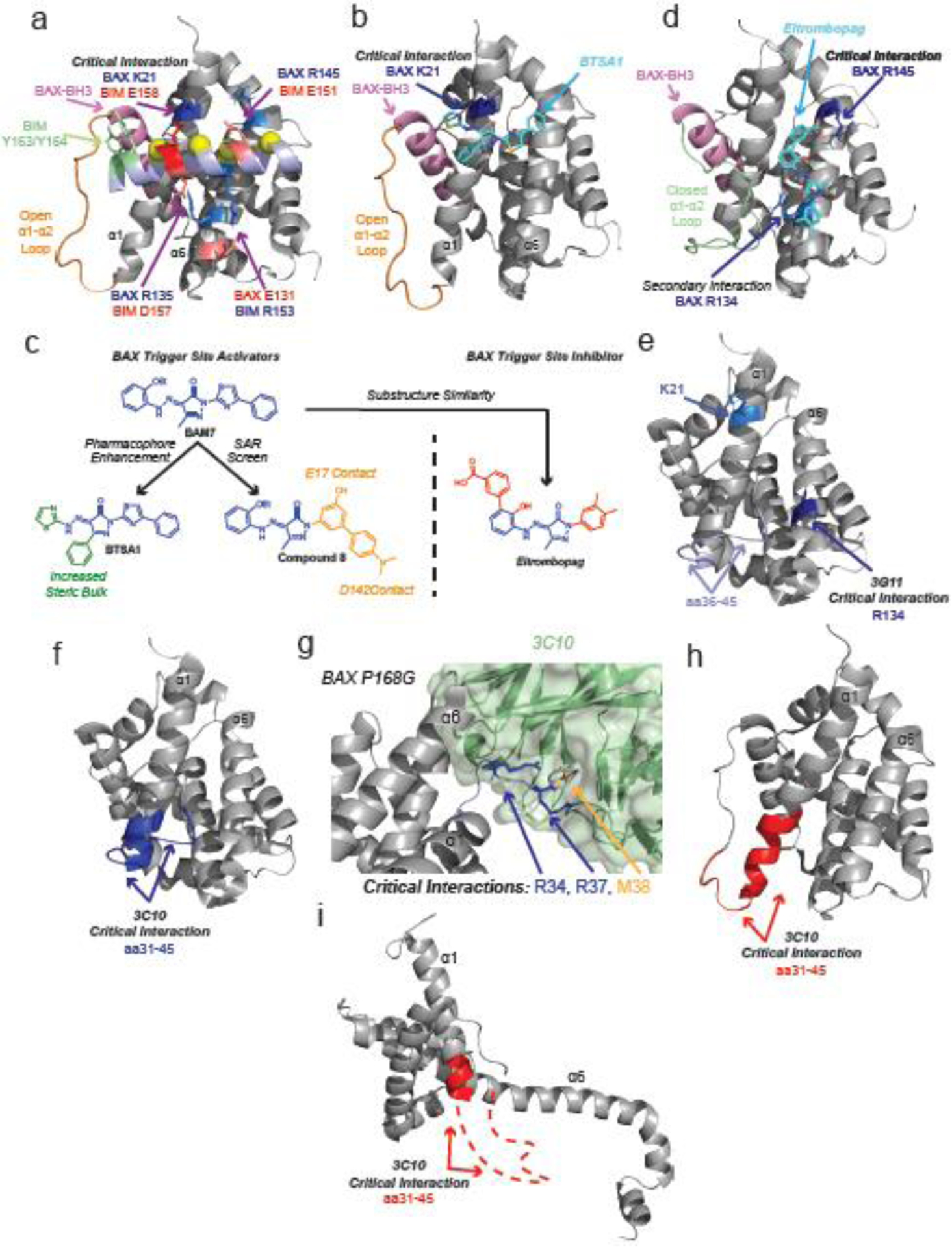
a, Detailed diagram of the BAX/BIM-BH3 complex (PDB: 2K7W). BIM-BH3 forms hydrophobic contacts with α1 and α6 via h1–h4 (yellow). Ionic interactions between BIM and BAX are labeled red and blue with the critical BAX K21/BIM E158 interaction noted. Loop α1–α2 (orange), directly coupled to the BAX BH3-domain (pink), is maintained in an open conformation by tyrosine residues on BIM (green). b, Modelled structure of BTSA1 (light blue) bound to BAX trigger site, forming a critical hydrogen bond at K21 (blue) and using thiazole and phenyl group to open loop α1–α2 (orange). c, BAX activators (BAM7, BTSA1, and compound 8) and inhibitor (Eltrombopag) possess a similar core (blue). Substitution of a methyl for phenyl and thiazole group in BTSA1 (green) likely contributes to greater potency of BAX activation in vitro and in vivo. The biphenyl of compound 8 is proposed to make contacts with E17 and D142. d, Modelled structure of Eltrombopag (light blue) bound to BAX trigger such that loop α1–α2 remains in a closed conformation (green). Eltrombopag forms a critical interaction with R145 and a secondary interaction with R134. e, 3G11 inhibits BAX via a critical interaction with R134 (dark blue) and a secondary interaction with K21 (light blue) on the trigger site. HDX-MS shows 3G11 protects residues 36–45 on loop α1–α2 (lavender) from solvent exposure. (PDB: 1F16) f, 3C10 binds to residues 35–41 on cytosolic BAX and inhibits BAX activation. (PDB: 1F16) g, Co-crystal structure of BAX P168G (grey) and antibody 3C10 variable region (green). (PDB: 5W5X) Residues R35, R37 (blue), and M38 (orange) bind to the variable region. h–i, 3C10 activates membrane localized BAX mutant S184L by binding to residues 35–41(red). Potential conformations of BAX S184L include BAX with open loop α1–α2 (h, PDB: 2K7W) or core latch separated BAX (i, PDB: 4ZIE). Red dashed line corresponds to BAX residues to which 3C10 binds that are not resolved in PDB: 4ZIE.
Mobilization of loop α1–α2 contributes to exposure of the BAX BH3-domain (α2) and subsequent activation steps [39]. Indeed binding of BIM-BH3 is unable to promote conformational activation and oligomerization to an oxidized cysteine mutant of BAX (L45C/M137C) in which loop α1–α2 is “locked” in a closed position by a disulfide bond [39]. Analysis of BAX chemical shift perturbations distal to the BIM binding site highlights changes in the BAX BH3-domain (α2) as well loop α4–α5/α7 and the α4/α6 interfaces which could allosterically couple N-terminal trigger site binding to mobilize the C-terminal canonical site and transmembrane α9 [39]. Crosslinking studies of full-length BIM and BAX showed that the C-terminal region of BIM may in fact directly interact with the α4–α5/α7 interface [39]. Crystal structures of inactive trigger site mutants have also identified these regions as potential sites of allosteric communication between the trigger site and canonical site [40,41]. Although no structure has been solved, it is likely that both BID-BH3 and PUMA-BH3 can initiate BAX activation at the trigger site via similar contacts to that of BIM-BH3. NMR and photocrosslinking data using stapled helices of the BH3-domains of PUMA and BID evidenced interactions with the trigger site [43–44]. Moreover, cysteine containing mutants of BID-BH3 and BAX form disulfide bonds at the trigger site [41]. Taken together, this data suggests the trigger site couples to the canonical site and controls BAX activation via multiple allosteric routes.
Small molecules BAM7 and BTSA1 are also capable of directly activating BAX via interaction at the trigger site (Figure 2b) [21,45]. Both small molecules feature a hydrazone substituted pyrazalone core, which can form a hydrogen bond with K21 [21,45] Although close structural analogs, BTSA1 differs significantly from BAM7 by exchanging a small methyl group for a bulky phenyl substitution to the pyrazalone core [21]. This addition of a bulky group may act to mimic the bulky tyrosines of BIM and aid in displacing loop α1–α2, which could explain the greatly enhanced activity of BTSA1 [21,45] (Figure 2c). Indeed molecular dynamics simulations suggest that BTSA1 may promote opening of BAX loop α1–α2 [21]. BTSA1 has demonstrated great therapeutic potential in as a single agent in acute myeloid leukemia (AML) xenograft models [21]. Furthermore, BTSA1 exhibits synergy in combination with the already FDA approved BCL-2 selective inhibitor Venetoclax (ABT-199) [21]. Structure-based lead optimization of BAM7 also lead to the identification of compound 8 (Figure 2c) [46]. This compound also makes a critical contact with K21 but substitutes the phenylthiazole of BAM7 and BTSA1 with a functionalized biphenyl group. Newly added hydroxy- and dimethylamino-groups are proposed to form additional contacts with E17 and D142, respectively [46].
Another close analog of BAM7 is the FDA approved thrombopoietin receptor Eltrombopag [47,48] Despite its structural similarity to trigger site activators of BAX, Eltrombopag inhibits BAX activation by binding to the trigger site in a distinct conformation, forming a critical ionic interaction between a carboxylate and R145 as well as a secondary hydrogen bond between the pyrazolone core and R134 (Figure 2d) [49]. This unique binding mode allows Eltrombopag to block activator binding without mobilizing loop α1–α2 and activating BAX. Furthermore, structural studies and molecular dynamic simulations strongly suggest Eltrombopag stabilizes the inactive BAX structure via previously reported allosteric mechanisms via the α4–α5/α7 and the α4/α6 interfaces [41,42,49] The fact that Eltrombopag is a well-tolerated orally available FDA approved drug raises the unique possibility of repurposing Eltrombopag to inhibit pathologic cell death.
Several antibodies have been identified which also bind to the BAX trigger site and exhibit variable BAX modulating activity. The antibody 3G11 binds to the BAX trigger site forming contacts with α1, α6, and loop α1–α2 [50]. Like Eltrombopag, 3G11 serves as an inhibitor of BAX, preventing membrane translocation and permeabilization [49,50]. Interestingly, although the 3G11-BAX interaction is partially mediated by K21, mutagenesis showed that R134 contributed significantly more to inhibition (Figure 2e). Furthermore, hydrogen deuterium exchange mass spectrometry (HDX-MS) indicated protection of the N-terminal of loop α1–α2 and residues underneath it, suggesting close association between loop α1–α2, BAX α1 and α6, and the antibody. The inhibitory activity of Eltrombopag and 3G11 highlight how different specific interactions at the same overall binding site might modulate BAX activity in opposite ways [21,45,49,50]. Bifunctional sites of modulation have also been observed in BAK. Inhibition of BAK at the canonical site was achieved by substitution of an unnatural amino acid to a BIM-BH3 peptide, converting it from an activator to an inhibitor [51].
Another antibody, 3C10, was identified to bind to residues 31–45 encompassing part of α1 and loop α1–α2 [52]. (Figure 2f) Surprisingly, 3C10 was shown to activate a BAX mutant (S184L), which is constitutively located on the MOM while inhibiting the mitochondrial translocation of wild type cytosolic BAX [52]. Using HDX-MS, 3C10 was later shown to inhibit mobilization of transmembrane α9 in a manner similar to that of the inactive BAX mutant, P168G [42]. A co-crystal structure of 3C10 bound to an inactive BAX mutant P168G showed contacts predominantly with BAX R34, R37, and M38 (Figure 2g) [42]. Unfortunately much of loop α1–α2 was not resolved leaving uncertainty to its overall conformation, particularly given the absence of a wild type BAX-3C10 co-crystal structure [42]. The structure of the BAX S184L mutant has not been solved leaving uncertainty as to the exact conformation of loop α1–α2, but the fact that 3C10 exhibits the opposite activity in S184L suggests it may be in an open and disordered conformation, such as that of activated soluble BAX or the core/latch separated BAX (Figure 2h–i) [32,53]. The lack of conclusive structural information for inactive mitochondrial BAX mutants such as S184L represents a significant gap in our understanding of the BAX activation mechanism. Notably several other antibodies to loop α1–α2 were also identified as inhibitors of cytosolic BAX while activators of mitochondrial BAX S184L, highlighting the bifunctional nature of this region [42]. Furthermore, the fact that a mutation at the C-terminal α9 (S184L) is capable of drastically changing the activity of an antibody which binds to residues at the N-terminal trigger site suggests that allosteric communication between the two sites can occur in either direction. Despite promising in vitro data it remains to be seen whether these antibodies will be able to target intracellular BAX.
Canonical site modulators of BAX
Multidomain pro-apoptotic (BAX and BAK) and anti-apoptotic BCL-2 proteins share a structurally similar hydrophobic groove formed by α2, α3, α4, and α5 known as the C-terminal canonical site [4,5]. Extensive structural and biochemical data has shown that anti-apoptotic BCL-2 proteins use these grooves to potently bind to BH3-domains of the pro-apoptotic BCL-2 family of proteins [33–37]. Furthermore, the NMR structure of the pro-apoptotic effector BAK showed that it too binds to BH3-domains at the canonical site to induce activation and MOMP [38]. BAX however, is unique among the BCL-2 proteins because its amphipathic α9 transmembrane helix is sequestered in its own canonical groove while in the inactive cytosolic state [10,31] (Figure 1a). However, once BAX is activated at the trigger site, as discussed above, it translocates to the MOM to undergo further activation steps and conformational changes that involve the canonical site [39,42,53].
Several dimeric crystal structures of truncated BAX lacking its C-terminal α9 have been solved featuring BAX bound to both BIM and BID BH3-domains [53,54]. These structures feature a domain-swapped dimer in which the core of BAX (α1–α5) separates from the latch (α6–α8) [53,54]. The core of one protomer binds to the latch of the other to form “core/latch domain-swapped dimer” with the canonical site of each protomer bound to a BH3 domain [53,54]. The structures show that BIM and BID utilize the four conserved hydrophobic BH3-residues (h1–h4) as well an additional hydrophobic contact formed the N-terminal end of the BH3-domain known as h0 to bind the BAX canonical site [53,54] (Figure 3a–b). BIM and BID also both form a critical ionic interaction by their conserved BH3 aspartate (BID D95, BIM D157) with BAX R109 as well as a secondary ionic interaction at BAX R94 [53,54]. However, BIM R153 is capable of forming an additional ionic interaction by its close proximity to BAX D98/D102 [54]. Instead of an arginine at this position, BID has an alanine (A91) [53] (Figure 3c). These crystal structures provide useful evidence that may explain why BIM has been shown to more potently activate BAX compared to BID [55]. This has been shown to be clinically relevant, as cancer cells lacking BAK are resistant to chemotherapeutic agents that act through BID to induce apoptosis [55].
Figure 3: Canonical site modulators of BAX.
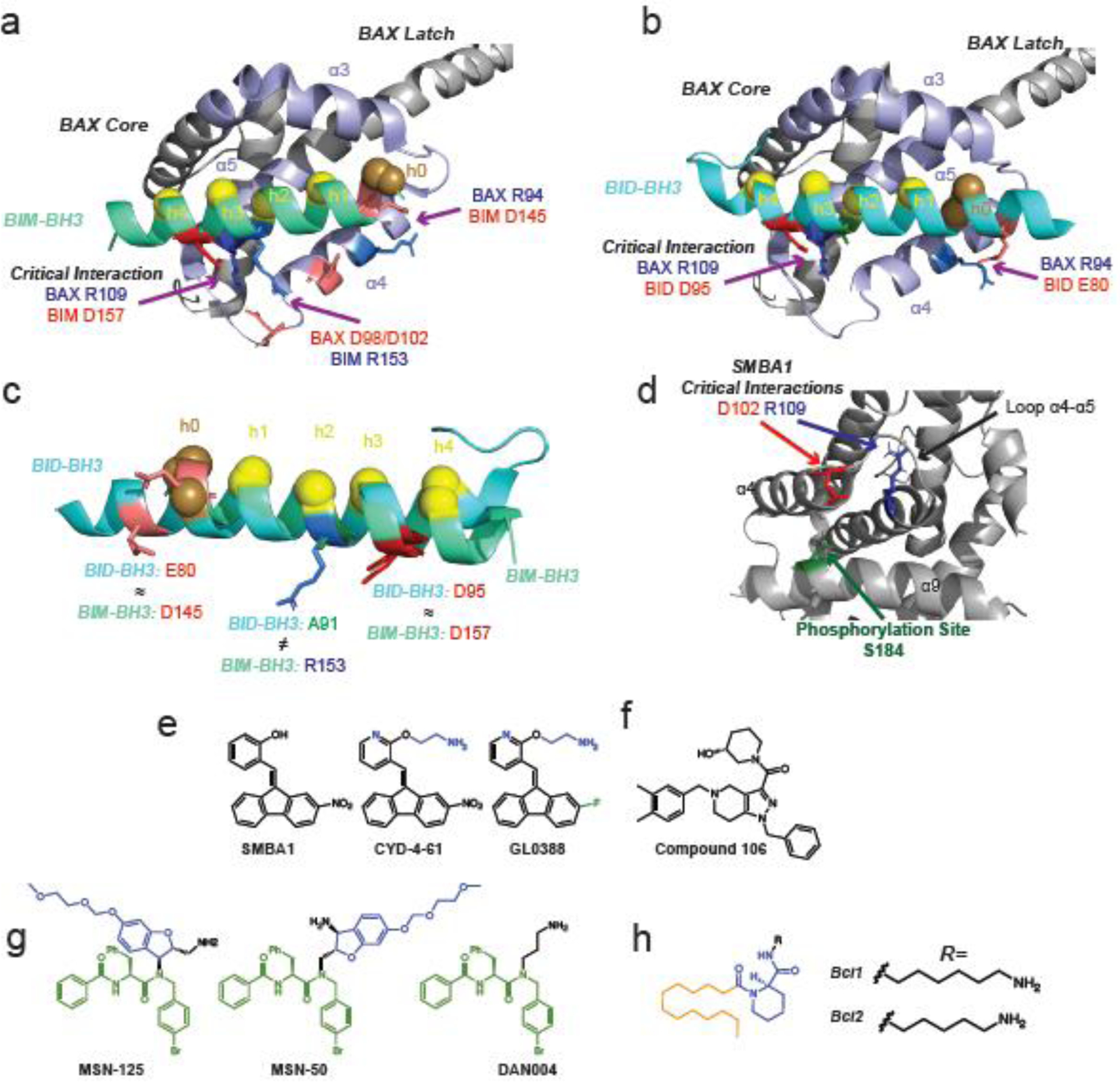
a-b, Crystal structures of BAX bound to BIM-BH3 (a, light green, PDB: 4ZIE) and BID-BH3 (b, light blue, PDB: 4BD2). Canonical site helices are highlighted in lavender. Conserved hydrophobic BH3-domain residues h1–h4 are highlighted in yellow with secondary h0 residues highlighted in brown. Ionic interactions between BH3-domains and BAX are labeled red (anion) and blue (cation) with the critical BAX R109 interaction with conserved BH3-aspartate noted. c, Overlay of BID-BH3 (light blue) and BIM-BH3 (light green) highlighting similarities and differences in interaction with BAX canonical site. Notably, BIM-BH3 is capable of an additional ionic interaction with BAX D98/D102 via BIM R153. d, Proposed binding site of SMBA1, CYD-4-61, and GL0388 centered about S184 (green) on α9 featuring critical contacts with D102 (red) on α4 and R109 (blue) on α5. (PDB: 1F16) e, Structure of canonical site BAX activators SMBA1, CYD-4-61, and GL0388. Structural differences enhancing activity or reducing toxicity of CYD-4-61 and GL0388 compared to SMBA1 are highlighted in blue and green, respectively. f, Structure of proposed canonical site BAX activator compound 106. g, Small molecule inhibitors of BAX activation MSN-125, MSN-50, and DAN004 are predicted to bind to the BAX and BAK canonical site. The peptide like region and methoxyethyl methyl ether protected phenoxy group are highlighted in green and blue respectively. DAN004 features only a primary alkyl amine. h, BAX channel inhibitors Bci1 and Bci2 have an unknown binding site and feature a C12 lipid like tail (orange), a chiral core with two amide bonds (blue), and either C6 or C5 primary alkyl amines (black).
Although separation of the core and latch domains of BAX is accepted to be a crucial part of activation, the dimer itself is however believed to be an “off-pathway” product and not part of the apoptotic pore responsible for MOMP. Instead, the “BAX BH3-in-groove” symmetrical dimer in which the BH3-domain of one BAX protomer is bound by the canonical site of the other protomer, is believed to form the building block of the oligomeric BAX pore [56,57] (Figure 1e). While there may seem to be contradictions between BAX activation models with regard to activation at the trigger site versus canonical site, the models are not necessarily mutually exclusive. A holistic view of the data suggests that activation of cytosolic BAX is initiated at the trigger site, allowing for translocation to the MOM and exposure of the canonical site, where further BH3-binding interactions can promote the formation of symmetrical BAX homodimers and ultimately oligomers, which can induce MOMP and apoptosis [32,39,41,53,56, 58].
Small molecule activators and inhibitors of BAX have been identified which reportedly interact with the BAX canonical site. Several small molecule BAX agonists (SMBAs) have been identified from a computational screen which target a pocket centered about S184, a phosphorylation site which inhibits BAX activation [23,59,60] (Figure 3d). The proposed binding model of all SMBAs feature α9 sequestered in the canonical site and the small molecules binding to a surface formed by α4, α9, and the hairpin loop α4–α5, which has been previously implicated in transmitting the BH3-binding information from the trigger site to the canonical site [23,59,60]. SAR studies based on SMBA1 identified an analog, CYD-4-61, with significantly higher potency [60]. Further SAR studies identified GL0388, which replaces a nitro-group with a fluorine to reduce non-specific toxicity [23] (Figure 3e). Based on molecular docking, CYD-4-61 and GL0388 are predicted to form hydrogen bond contacts at BAX R109 and E102 [23,60] (Figure 3d). Although there is a lack of structural data (e.g. NMR, crystallography, mutagenesis) to directly support this binding site, SMBA1, CYD-4-61, and GL0388 have shown promise in inducing BAX dependent apoptosis in several cancers including in vivo xenografts [23,59,60]. An independent computational screen targeting the BAX canonical site also identified compound 106, which was found to activate BAX and induce BAX dependent cell death in vitro as well as reduce tumor volume in an in vivo xenograft model (Figure 3f) [61]. Unfortunately there is minimal structural data on compound 106 and it bears no obvious resemblance to other reported canonical site activators making it difficult to predict it’s binding characteristics.
An in vitro screen of small-molecule has also identified two molecules (MSN-125, MSN-50) that inhibit BAX activation [29] (Figure 3g). Interestingly these inhibitors block both BAX and BAK dependent MOMP and cell death [29]. These compounds do not inhibit BAX translocation but only oligomerization [29]. Crosslinking mutagenesis studies revealed that these molecules likely interact with the canonical site of BAX and BAK in a way that inhibits formation of the BH3-in-groove symmetrical dimer as well as blocking sites necessary for higher order oligomerization, perhaps trapping activated BAX in the state after translocation and core/latch separation [29]. These compounds are close analogs, featuring two identical fragments. The predicted active pharmacophore of these molecules resemble peptides, featuring two amide bonds flanking a phenylalanine [29] (Figure 3g). They also feature a methoxyethyl chloromethyl ether protected phenoxy group, presumably a remnant of compound synthesis.
A third compound, DAN004, which mimics only the peptide like region with a primary alkyl amine was the most potent inhibitor of BAX activation in vitro but exhibited significant cellular toxicity that was not studied further [29]. Interestingly, DAN004 bears a slight resemblance two earlier reported “BAX channel inhibitors”, Bci1 and Bci2 [29, 30] (Figure 3h) These molecules, like MSN-125 and MSN-50, feature a core with two amide bonds and a chiral center. Furthermore, Bci1 and Bci2 feature primary alkyl amines, much like the potent DAN004, although the alkyl amine of DAN004 is considerably shorter. Bci1/2 do differ significantly from MSN-125/50 and DAN004 by the presence of a lipid like alkyl tail which likely interacts with membranes. Despite only minor structural similarity, the mechanisms for both classes of compounds are similar in that both inhibit BAX after translocation [29, 30]. Bci1 and Bci2 have been shown to prevent BAX mediated membrane permeabilization but, unlike MSN-125/50, do not prevent BAX oligomerization [29]. It is also surprising that Bci1 and Bci2 were capable of inhibiting cell death in a stroke model of brain ischemia while DAN004 was toxic [30]. On the other hand, MSN-125 and MSN-50 protected primary cortical neurons from excitotoxic stimuli [29]. Unfortunately the mechanism of Bci1 and Bci2 is not fully described and no binding site has been proposed. Further exploration of these molecules could help better elucidate the structural changes BAX undergoes after translocation but before oligomerization.
Non-canonical modulators of BAX
Several peptides and small molecules have been reported to bind to regions outside of both the canonical and trigger site here referred to as the non-canonical site. Interestingly these non-canonical BAX modulators bind in a clustered region while forming unique contacts and having unique effects on BAX function. (Figure 4a) The first reported of these non-canonical modulators is the cytomegalovirus protein vMIA (viral mitochondria-localized inhibitor of apoptosis) [62]. This peptide binds potently binds to a site formed by the hairpins of loop α3–α4 and loop α5–α6 utilizing two arginines (R139 and R146) to make important electrostatic contacts with D84 and D86 on BAX (Figure 4b). Interestingly, vMIA recruits BAX to the MOM but inhibits BAX activation [62,63]. The NMR structure of the vMIA-BAX complex highlights how intracellular pathogens use unique mechanisms to evade host cell apoptosis in order to replicate and avoid immune response [62–64].
Figure 4: Non-canonical modulators of BAX.
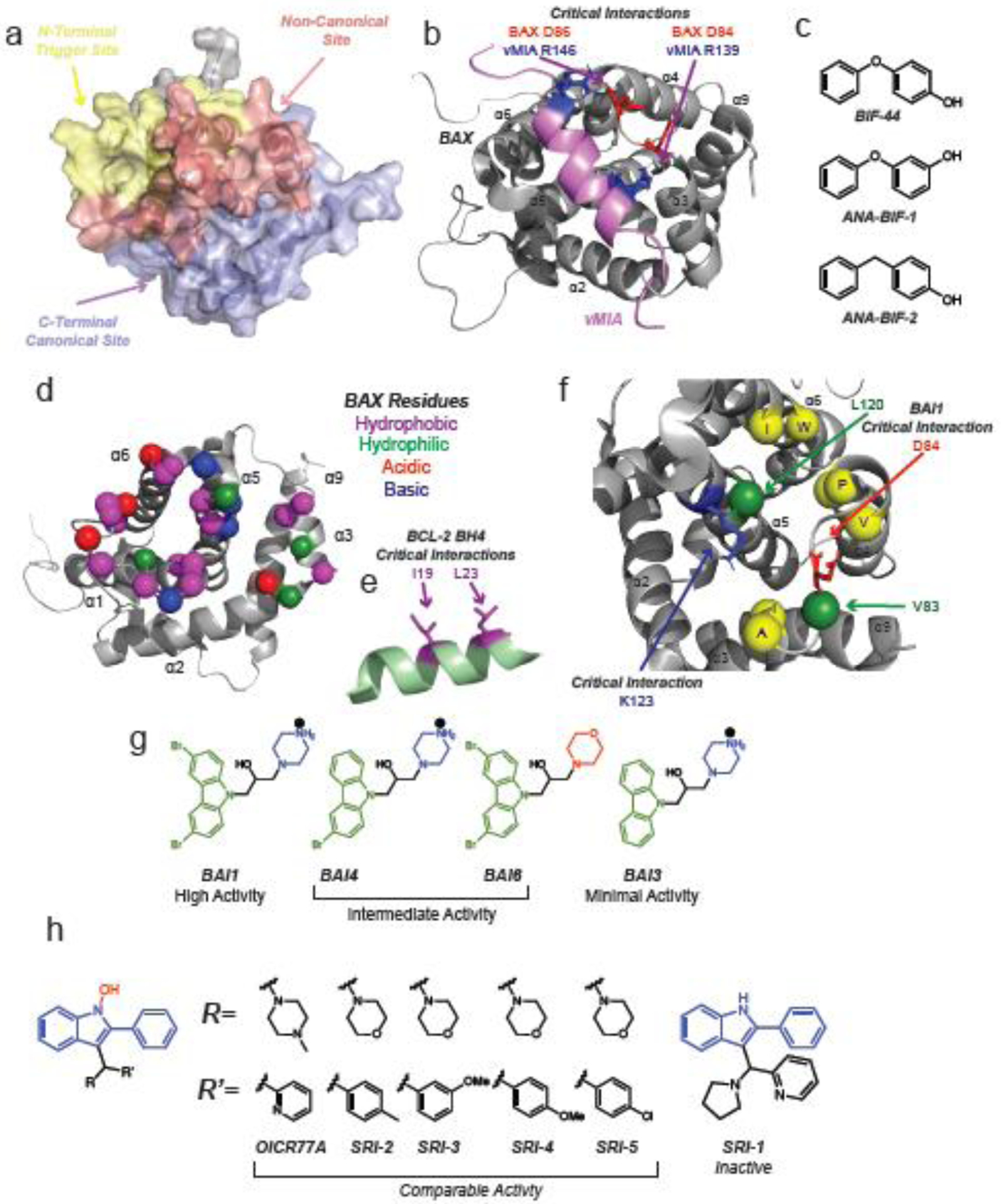
a, BAX surface highlighting the N-terminal trigger site (yellow), C-terminal canonical site (lavender), and the non-canonical region (red). The non-canonical site can loosely be defined as including the hairpins of α3–α4 and α5–α6 as well as the N-terminal of loop α1–α2. b, NMR solution structure of BAX (grey) and BAX inhibiting peptide viral mitochondrial localized inhibitor of apoptosis (vMIA, pink) (PDB: 2LR1). Critical ionic interactions between BAX D84 and D86 and vMIA R139 and R146 are highlighted. c, Small molecule fragments BIF-44 and analogs bind to the vMIA binding site and sensitize BAX to activation by BH3-only activators. d, Binding site of the BCL-2 BH4 domain to inactive BAX (PDB: 1F16). Hydrophobic, hydrophilic, acidic, and basic residues are labeled as described in the figure. e, The BCL-2 BH4 domain (green) forms critical interactions with BAX via the hydrophobic residues I19 and L23 (purple). f, Allosteric BAX inhibitors (BAIs) bind to a site formed by α3, α5, α6 and loop α3–α4 making contacts with several hydrophobic residues highlighted in yellow. BAI1 makes critical contacts with K123 and D44 via its secondary alcohol and cationic piperazine, respectively. Obstructive mutagenesis of V83W/L120W (green) inhibited BAI1 binding mediated BAX inhibition. g, Structure-activity relationship studies of BAI1 highlighted the importance of steric bulk of the dibromo-carbazole (green) as well as the cationic piperazine (blue). h, BAX activators OICR77A and select analogs activate BAX at an unknown site but require the presence of BAX C126, which lies in the non-canonical region. The common phenyl substituted N-hydroxyindole core is highlighted in blue with various R and R’ groups displayed in black. The absence of the N-hydroxy group (red) in SR-1 (right) eliminates BAX activation activity.
A ligand based fragment screen using BAX identified a small molecule fragment, BIF-44, which was capable of sensitizing BAX to activation with only minimal ability to activate BAX directly [65] (Figure 4c). Several close analogs of BIF-44 (ANA-BIFs) exhibited similar sensitizing ability [65]. Surprisingly, NMR analysis and competitive binding experiments showed that BIF-44 and vMIA in fact share a binding site and make similar contacts including D84, however specific interactions are not explored further [62,65]. Molecular dynamics simulations and HDXMS indicate that BIF-44 binding induces increased conformational flexibility in loop α1–α2 [65]. No such experiments have been performed for the vMIA peptide, it is however notable that the NMR analysis of vMIA bound to BAX shows chemical shift perturbations on α9 which are absent in the analysis of BIF-44 bound to BAX [62,65]. Overall the data suggest that this site is capable of allosterically modulating the N-terminal trigger site, as in the case of BIF-44, or the canonical site, in the case of vMIA.
Another adjacent but distinct binding site is that of the BCL-2 BH4 domain which is capable of inhibiting BAX activation via a non-canonical mechanism [66]. Rather than sequestering the exposed BAX-BH3 domain after activation, the BCL-2 BH4 domain is capable of inhibiting BAX in its apo inactive form at a surface formed by the α1 C-terminus, loop α1–α2, loop α2–α3, and loop α5–α6 [66] (Figure 4d). Binding of the BCL-2 BH4 peptide induced protection of the N-terminal 6A7-epitope as well as loop α1–α2 as assessed by HDXMS [66]. Mutagenesis data is not available for BAX, but mutagenesis of the BCL-2 BH4 domain identified I19 and L23 as critical residues to maintain the interaction with BAX (Figure 4e). Notably, a small molecule BCL-2 BH4 antagonist, BDA-366, was proposed to bind to BCL-2 and was to shown to inhibit lung cancer growth in vivo, but was later shown to induce apoptosis independent of BCL-2 [67,68]. BCL-2 BH4 has been shown to have anti-apoptotic activity in cells and in vivo [69,70].
The small molecule inhibitor of BAX activation, BAI1, also binds to a site adjacent to that of vMIA [71] (Figure 4f). BAI1 was initially reported as an inhibitor of MOMP however its mechanism was unknown [72,73]. Structural and biochemical studies revealed that BAI1 was indeed a direct inhibitor of BAX activation [71]. Similarly to BIF-44, BAI1 induces conformational changes at sites distal to its binding pocket, notably loop α1–α2, α7, α8, and α9 as determined by NMR and HDXMS [71]. BAI1 forms specific contacts with D84 and K123 via its cationic piperazine and alcohol groups, respectively [71] (Figure 4f). Obstructive mutagenesis of binding site residues V83 and L120 to tryptophan (V83W/L120W) as well as charge reversal mutants of D84K/D86K exhibited significantly weaker inhibition by BAI1 [71]. Interestingly, BAX V83W/L120W also exhibited weaker activation by tBID and BIM [71]. It is possible that the large aromatic tryptophan residue side chains behave similarly to the large aromatic dibromo-carbazole of BAI1 in reducing the sensitivity of BAX to BH3-only activators. Indeed SAR studies of BAI1 and analogs highlights the importance of steric bulk in the carbazole substitution [71]. The absence of one or both of the carbazole bromines, reduces activity by a factor of 2.5 and 6 respectively. Furthermore, replacement of the piperazine with a morpholine reduces activity by a factor 2.5 as well, supporting the ionic interaction at D84 (Figure 4g). BAI1 shows great therapeutic potential by inhibiting cardiomyocyte cell death in in vivo models of doxorubicin-induced cardiotoxicity [24].
The small molecule OICR77A and several analogs (SRI2 through SRI5) were found to directly activate BAX with a critical contact at BAX C126, which lies in the non-canonical region of BAX [74] (Figure 4h). Beyond the generation of the BAX C126A mutant, which does not bind to OICR77A and analogs, there is little structural data to predict or describe the binding mode. However, SAR revealed that the presence of N-hydroxyindole is critical to activity as evidence by the inactive SRI-1 [74]. The authors do note the possibility of covalent modification of C126 given the reactivity of N-hydroxyindoles and the fact that OICR77A induces apoptosis in cells independent of BAX expression [74]. However the binding site is likely specific as the compounds were capable of activating C62A. Covalent modification of C126 by lipids has been shown to enhance BIM induced BAX activation [75]. However, covalent modification of C126 with fluorophores has not been shown to enhance BAX activation [76]. Whether covalent or non-covalent, the data suggest that BAX modulation is possible via a binding site centered about C126 [74,75]. Although by no means close analogs, the BAI compounds and OICR77A share the presence of large aromatic groups and a 6-membered heterocycle [71,74]. Given the proximity of their putative binding sites and the variable activity of non-canonical BAX binders, it seems plausible that BAI1 and OICR77A share similar binding characteristics given their shared aromatic and piperazine groups, although further structural data such as NMR would be necessary to confirm this.
Concluding Remarks and Future Perspectives
Although at a glance the inactive BAX structure may appear to lack any distinct binding sites, we have seen that numerous peptides, antibodies, and small molecules are capable of binding to BAX [10,31]. Once considered undruggabe, BAX has proven to be druggable by both activators and inhibitors at nearly every point on its surface. These studies indicate that there are several ways to modulate the surface and activity of BAX. Studying BAX activation and inhibition in the context of protein-protein and protein-small molecule interactions highlights the allosteric communication that occurs throughout the surface and core of BAX and has furthered our understanding of the complex structural changes that BAX must undergo to translocate, dimerize, oligomerize, and induce MOMP [39,42,49,58,65,71,77]. It is tempting to suggest that these findings may inform similar physiologic and pharmacologic mechanisms for other BCL-2 proteins and particularly pro-apoptotic BAK and BOK. Additionally, small molecule modulators of BAX not only can serve as valuable research tools but hold excellent promise as potential therapeutics for cancer and other diseases [21,23,24,49,71]. Future investigations should focus on deeper understanding of BAX structure-function relationships along the BAX activation pathway and BAX pore at MOM, the delineation of what is the most effective targeting BAX surface or conformation to activate or inhibit BAX, the use of BAX modulators in understanding the role of BAX in homeostatic and pathologic conditions, and ultimately the translation of BAX therapeutics to patients (see Outstanding Questions).
Outstanding Questions.
What is the structure of mitochondrial BAX before and after oligomerization and what comprises a minimal BAX pore?
What cytosolic or mitochondrial BAX surface or conformation is the most effective target pharmacologically?
Is there a therapeutic advantage to pre-translocation versus post-translocation inhibition or activation of BAX?
Is there a role for dual BAX-BAK activators or inhibitors and how could these be identified?
What pathological conditions can be benefited by BAX activators or inhibitors and are there long-term effects of pharmacologic BAX inhibition?
Can pre-clinical BAX activators and inhibitors be safely transitioned to clinical trials
Highlights.
A growing number of peptides, antibodies, and structurally diverse small molecules have been reported as direct modulators of BAX
BAX can be activated or inhibited via multiple distinct binding sites and mechanisms
Mechanistic studies of physiologic and synthetic BAX modulators have provided important insights into the BAX activation pathway and apoptosis
BAX activators and inhibitors have shown promising activity in in vivo models of cancer, cardiomyopathy, and neurodegenerative disease
A thorough understanding of BAX binding sites and key features of active pharmacophores will allow further development of clinically useful BAX modulators
Acknowledgements
This work is supported by R01CA178394, PR191593P1, P30CA013330, P30AG038072, P01AG031782, the Irma T. Hirschl Trust Career Award and a Pershing Square Sohn Cancer Research Alliance Award to E.G. A.Z.S is supported by F30CA228453 and T32GM007288 training grants.
Glossary:
- Anti-Apoptotic BCL-2 Proteins
Includes BCL-2, BCL-xL, BCL-w, MCL-1 and BFL-1/A1. Proteins capable of directly inhibiting effector BCL-2 proteins and BH3-only proteins by sequestering their BH3-domains.
- BH3-Only Proteins
Includes activator proteins (i.e. BIM, BID, PUMA) which are capable of inhibiting anti-apoptotic proteins as well as directly activating effector proteins as well as sensitizer proteins (i.e. BAD, BMF, HRK) inhibit anti-apoptotic proteins but cannot directly activate effectors.
- C-Terminal Canonical Site
Groove formed by α2–α5. Helix α9 is sequestered in this site in the inactive cytosolic BAX conformation. BH3-only proteins can interact at this site after release of α9 and translocation to the mitochondrial outer membrane. Interface by which activated symmetrical BAX dimers are formed.
- Effector BCL-2 Proteins
Includes BAX, BAK, and BOK which are capable of forming oligomeric pores in the mitochondrial outer membrane.
- N-Terminal Trigger Site
Surface formed by α1, α6, and loop α1–α2. Site of BAX activation initiation upon activator binding.
- Non-Canonical Site
Can be loosely defined as the hairpins of α3–α4 and α5–α6 as well as the N-terminal portion of loop α1–α2.
- Off-Pathway Dimer
Crystal structures of BIM BH3 and BID BH3 bound to the canonical site of BAX form core-latch domain swapped dimers. Although BAX is known to dimerized after activation, the core-latch domain swapped dimers are not part of this pathway. Instead, it is believed that the BH3-in-groove dimer (Figure 1e) is the building block of higher order BAX oligomers which form the apoptotic pore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh R, Letai A & Sarosiek K Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20, 175–193, doi: 10.1038/s41580-018-0089-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delbridge AR, Grabow S, Strasser A & Vaux DL Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer 16, 99–109, doi: 10.1038/nrc.2015.17 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Del Re DP, Amgalan D, Linkermann A, Liu Q & Kitsis RN Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol Rev 99, 1765–1817, doi: 10.1152/physrev.00022.2018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kale J, Osterlund EJ & Andrews DW BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ 25, 65–80, doi: 10.1038/cdd.2017.186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luna-Vargas MP & Chipuk JE The deadly landscape of pro-apoptotic BCL-2 proteins in the outer mitochondrial membrane. Febs j 283, 2676–2689, doi: 10.1111/febs.13624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait SW & Green DR Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11, 621–632, doi: 10.1038/nrm2952 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Kalkavan H, and Green DR (2018). MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HC et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat Cell Biol 17, 1270–1281, doi: 10.1038/ncb3236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edlich F et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116, doi: 10.1016/j.cell.2011.02.034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner TP et al. An Autoinhibited Dimeric Form of BAX Regulates the BAX Activation Pathway. Mol Cell 63, 485–497, doi: 10.1016/j.molcel.2016.06.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moldoveanu T et al. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell 24, 677–688, doi: 10.1016/j.molcel.2006.10.014 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Sattler M et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986, doi: 10.1126/science.275.5302.983 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Chen L et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17, 393–403, doi: 10.1016/j.molcel.2004.12.030 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Ren D et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 330, 1390–1393, doi: 10.1126/science.1190217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glab JA, Mbogo GW, and Puthalakath H (2017). BH3-Only Proteins in Health and Disease. Int Rev Cell Mol Biol 328, 163–196. [DOI] [PubMed] [Google Scholar]
- 16.Garner TP, Lopez A, Reyna DE, Spitz AZ & Gavathiotis E Progress in targeting the BCL-2 family of proteins. Curr Opin Chem Biol 39, 133–142, doi: 10.1016/j.cbpa.2017.06.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogmore JP, Uehling D & Andrews DW Pharmacological Targeting of Executioner Proteins: Controlling Life and Death. J Med Chem 64, 5276–5290, doi: 10.1021/acs.jmedchem.0c02200 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Walensky LD Targeting BAX to drug death directly. Nat Chem Biol 15, 657–665, doi: 10.1038/s41589-019-0306-6 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Carneiro BA & El-Deiry WS Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17, 395–417, doi: 10.1038/s41571-020-0341-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cory S, Roberts AW, Colman PM, and Adams JM (2016). Targeting BCL-2-like Proteins to Kill Cancer Cells. Trends Cancer 2, 443–460. [DOI] [PubMed] [Google Scholar]
- 21.Reyna DE et al. Direct Activation of BAX by BTSA1 Overcomes Apoptosis Resistance in Acute Myeloid Leukemia. Cancer Cell 32, 490–505.e410, doi: 10.1016/j.ccell.2017.09.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Ding C, Zhang J, Xie M, Park D, Ding Y, Chen G, Zhang G, Gilbert-Ross M, Zhou W, et al. (2017). Modulation of Bax and mTOR for Cancer Therapeutics. Cancer Res 77, 3001–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G et al. Further lead optimization on Bax activators: Design, synthesis and pharmacological evaluation of 2-fluoro-fluorene derivatives for the treatment of breast cancer. Eur J Med Chem 219, 113427, doi: 10.1016/j.ejmech.2021.113427 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Amgalan D et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat Cancer 1, 315–328, doi: 10.1038/s43018-020-0039-1 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelan RS et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA 109, 6566–6571, doi: 10.1073/pnas.1201608109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo W, Lee HP, Smith MA, Zhu X, Matsuyama S, and Lee HG (2012). Inhibition of Bax protects neuronal cells from oligomeric Abeta neurotoxicity. Cell Death Dis 3, e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki K, Yanagihara T, Yokoyama T, Maeyama T, Ogata-Suetsugu S, Arimura-Omori M, Mikumo H, Hamada N, Harada E, Kuwano K, et al. (2017). Bax-inhibiting peptide attenuates bleomycin-induced lung injury in mice. Biol Open 6, 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun MY, Cui KJ, Yu MM, Zhang H, Peng XL, and Jiang H (2015). Bax inhibiting peptide reduces apoptosis in neonatal rat hypoxic-ischemic brain damage. Int J Clin Exp Pathol 8, 14701–14708. [PMC free article] [PubMed] [Google Scholar]
- 29.Niu X et al. A Small-Molecule Inhibitor of Bax and Bak Oligomerization Prevents Genotoxic Cell Death and Promotes Neuroprotection. Cell Chem Biol 24, 493–506.e495, doi: 10.1016/j.chembiol.2017.03.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetz C et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem 280, 42960–42970, doi: 10.1074/jbc.M505843200 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Youle RJ & Tjandra N Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645–654, doi: 10.1016/s0092-8674(00)00167-7 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Gavathiotis E et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081, doi: 10.1038/nature07396 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EF et al. Physiological restraint of Bak by Bcl-xL is essential for cell survival. Genes Dev 30, 1240–1250, doi: 10.1101/gad.279414.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku B, Liang C, Jung JU & Oh BH Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res 21, 627–641, doi: 10.1038/cr.2010.149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czabotar PE et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci USA 104, 6217–6222, doi: 10.1073/pnas.0701297104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Dai S, Zhu Y, Marrack P & Kappler JW The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity 19, 341–352, doi: 10.1016/s1074-7613(03)00234-6 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Denisov AY et al. Structural model of the BCL-w-BID peptide complex and its interactions with phospholipid micelles. Biochemistry 45, 2250–2256, doi: 10.1021/bi052332s (2006). [DOI] [PubMed] [Google Scholar]
- 38.Moldoveanu T et al. BID-induced structural changes in BAK promote apoptosis. Nat Struct Mol Biol 20, 589–597, doi: 10.1038/nsmb.2563 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavathiotis E, Reyna DE, Davis ML, Bird GH & Walensky LD BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell 40, 481–492, doi: 10.1016/j.molcel.2010.10.019 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chi X et al. The carboxyl-terminal sequence of bim enables bax activation and killing of unprimed cells. Elife 9, doi: 10.7554/eLife.44525 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dengler MA et al. BAX Activation: Mutations Near Its Proposed Non-canonical BH3 Binding Site Reveal Allosteric Changes Controlling Mitochondrial Association. Cell Rep 27, 359–373.e356, doi: 10.1016/j.celrep.2019.03.040 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Robin AY et al. Ensemble Properties of Bax Determine Its Function. Structure 26, 1346–1359.e1345, doi: 10.1016/j.str.2018.07.006 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Edwards AL, Gavathiotis E, LaBelle JL, Braun CR, Opoku-Nsiah KA, Bird GH, and Walensky LD (2013). Multimodal interaction with BCL-2 family proteins underlies the proapoptotic activity of PUMA BH3. Chem Biol 20, 888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leshchiner ES, Braun CR, Bird GH, and Walensky LD (2013). Direct activation of full-length proapoptotic BAK. Proc Natl Acad Sci USA 110, E986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES & Walensky LD Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol 8, 639–645, doi: 10.1038/nchembio.995 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stornaiuolo M et al. Structure-Based Lead Optimization and Biological Evaluation of BAX Direct Activators as Novel Potential Anticancer Agents. Journal of Medicinal Chemistry 58, 2135–2148, doi: 10.1021/jm501123r (2015) [DOI] [PubMed] [Google Scholar]
- 47.Bussel JB et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 357, 2237–2247, doi: 10.1056/NEJMoa073275 (2007). [DOI] [PubMed] [Google Scholar]
- 48.McHutchison JG et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med 357, 2227–2236, doi: 10.1056/NEJMoa073255 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Spitz AZ, Zacharioudakis E, Reyna DE, Garner TP & Gavathiotis E Eltrombopag directly inhibits BAX and prevents cell death. Nat Commun 12, 1134, doi: 10.1038/s41467-021-21224-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uchime O et al. Synthetic Antibodies Inhibit Bcl-2-associated X Protein (BAX) through Blockade of the N-terminal Activation Site. J Biol Chem 291, 89–102, doi: 10.1074/jbc.M115.680918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brouwer JM et al. Conversion of Bim-BH3 from Activator to Inhibitor of Bak through Structure-Based Design. Mol Cell 68, 659–672.e659, doi: 10.1016/j.molcel.2017.11.001 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Iyer S et al. Identification of an activation site in Bak and mitochondrial Bax triggered by antibodies. Nat Commun 7, 11734, doi: 10.1038/ncomms11734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czabotar PE et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531, doi: 10.1016/j.cell.2012.12.031 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Robin AY et al. Crystal structure of Bax bound to the BH3 peptide of Bim identifies important contacts for interaction. Cell Death Dis 6, e1809, doi: 10.1038/cddis.2015.141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarosiek KA et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol Cell 51, 751–765, doi: 10.1016/j.molcel.2013.08.048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bleicken S et al. Topology of active, membrane-embedded Bax in the context of a toroidal pore. Cell Death Differ 25, 1717–1731, doi: 10.1038/s41418-018-0184-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westphal D, Kluck RM & Dewson G Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ 21, 196–205, doi: 10.1038/cdd.2013.139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dengler MA, Gibson L & Adams JM BAX mitochondrial integration is regulated allosterically by its α1–α2 loop. Cell Death Differ, doi: 10.1038/s41418-021-00815-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xin M et al. Small-molecule Bax agonists for cancer therapy. Nat Commun 5, 4935, doi: 10.1038/ncomms5935 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu G et al. Structure-activity relationship studies on Bax activator SMBA1 for the treatment of ER-positive and triple-negative breast cancer. Eur J Med Chem 178, 589–605, doi: 10.1016/j.ejmech.2019.06.004 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Zhao G et al. Activation of the Proapoptotic Bcl-2 Protein Bax by a Small Molecule Induces Tumor Cell Apoptosis. Molecular and Cellular Biology 34, 1198–1207, doi: 10.1128/mcb.00996-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma J et al. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc Natl Acad Sci USA 109, 20901–20906, doi: 10.1073/pnas.1217094110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnoult D et al. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc Natl Acad Sci USA 101, 7988–7993, doi: 10.1073/pnas.0401897101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzsimmons L & Kelly GL EBV and Apoptosis: The Viral Master Regulator of Cell Fate? Viruses 9, doi: 10.3390/v9110339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pritz JR et al. Allosteric sensitization of proapoptotic BAX. Nat Chem Biol 13, 961–967, doi: 10.1038/nchembio.2433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barclay LA et al. Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism. Mol Cell 57, 873–886, doi: 10.1016/j.molcel.2015.01.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han B et al. Small-Molecule Bcl2 BH4 Antagonist for Lung Cancer Therapy. Cancer Cell 27, 852–863, doi: 10.1016/j.ccell.2015.04.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vervloessem T et al. BDA-366, a putative Bcl-2 BH4 domain antagonist, induces apoptosis independently of Bcl-2 in a variety of cancer cell models. Cell Death Dis 11, 769, doi: 10.1038/s41419-020-02944-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vervliet T et al. BH4 domain peptides derived from Bcl-2/Bcl-XL as novel tools against acute pancreatitis. Cell Death Discov 4, 58, doi: 10.1038/s41420-018-0054-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donnini S et al. Prevention of ischemic brain injury by treatment with the membrane penetrating apoptosis inhibitor, TAT-BH4. Cell Cycle 8, 1271–1278, doi: 10.4161/cc.8.8.8301 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Garner TP et al. Small-molecule allosteric inhibitors of BAX. Nat Chem Biol 15, 322–330, doi: 10.1038/s41589-018-0223-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bombrun A et al. 3,6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J Med Chem 46, 4365–4368, doi: 10.1021/jm034107j (2003). [DOI] [PubMed] [Google Scholar]
- 73.Peixoto PM, Ryu SY, Bombrun A, Antonsson B & Kinnally KW MAC inhibitors suppress mitochondrial apoptosis. Biochem J 423, 381–387, doi: 10.1042/bj20090664 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Brahmbhatt H, Uehling D, Al-Awar R, Leber B & Andrews D Small molecules reveal an alternative mechanism of Bax activation. Biochem J 473, 1073–1083, doi: 10.1042/bcj20160118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen DT, Wales TE, McHenry MW, Engen JR & Walensky LD Site-Dependent Cysteine Lipidation Potentiates the Activation of Proapoptotic BAX. Cell Rep 30, 3229–3239.e3226, doi: 10.1016/j.celrep.2020.02.057 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lovell JF et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074–1084, doi: 10.1016/j.cell.2008.11.010 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Bloch NB et al. The conformational stability of pro-apoptotic BAX is dictated by discrete residues of the protein core. Nat Commun 12, 4932, doi: 10.1038/s41467-021-25200-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ & Cheng EH Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009; 36(3): 487–99. Epub 2009/11/18. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Westphal D, Kluck RM & Dewson G Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ 21, 196–205, doi: 10.1038/cdd.2013.139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bleicken S et al. Topology of active, membrane-embedded Bax in the context of a toroidal pore. Cell Death Differ 25, 1717–1731, doi: 10.1038/s41418-018-0184-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bleicken S et al. Structural model of active Bax at the membrane. Mol Cell 56, 496–505, doi: 10.1016/j.molcel.2014.09.022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flores-Romero H, Ros U & Garcia-Saez AJ Pore formation in regulated cell death. The EMBO journal 39, e105753–e105753, doi: 10.15252/embj.2020105753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z, Subramaniam S, Kale J, Liao C, Huang B, Brahmbhatt H, Condon SG, Lapolla SM, Hays FA, Ding J, et al. (2016). BH3-in-groove dimerization initiates and helix 9 dimerization expands Bax pore assembly in membranes. EMBO J 35, 208–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lv F, Qi F, Zhang Z, Wen M, Kale J, Piai A, Du L, Wang S, Zhou L, Yang Y, et al. (2021). An amphipathic Bax core dimer forms part of the apoptotic pore wall in the mitochondrial membrane. EMBO J 40, e106438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mignard V, Lalier L, Paris F, and Vallette FM (2014). Bioactive lipids and the control of Bax pro-apoptotic activity. Cell Death Dis 5, e1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo X, O’Neill KL, and Huang K (2020). The third model of Bax/Bak activation: a Bcl-2 family feud finally resolved? F1000Res 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan C, Scheres SH, and Shi Y (2015). Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev 29, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McArthur K et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047, doi: 10.1126/science.aao6047 (2018). [DOI] [PubMed] [Google Scholar]


