Abstract
Introduction:
Mechanisms underlying sex differences in HFpEF are poorly understood. We sought to examine sex differences in measures of arterial stiffness and the association of arterial stiffness measures with left ventricular hemodynamic responses to exercise in men and women.
Methods:
We studied 83 men (mean age 62 years) and 107 women (mean age 59 years) with HFpEF who underwent CPET with invasive hemodynamic monitoring and arterial stiffness measurement (augmentation pressure [AP], augmentation index [AIx], and aortic pulse pressure [AoPP]). Sex differences were compared using multivariable linear regression. We examined the association of arterial stiffness with abnormal left ventricular diastolic response to exercise, defined as a rise in pulmonary capillary wedge pressure relative to cardiac output (ΔPCWP/ΔCO) ≥2 mmHg/L/min, using logistic regression models.
Results:
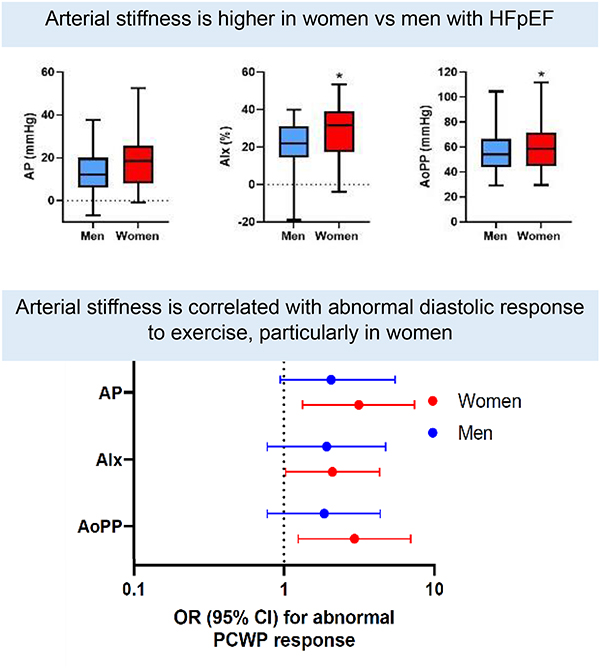
Women with HFpEF had increased arterial stiffness compared with men. AP was nearly 10mmHg higher, and AIx was more than 10% higher among women compared with men (P<0.0001 for both). Arterial stiffness measures were associated with a greater PCWP response to exercise, particularly among women. A 1-SD higher AP was associated with a >3-fold increased odds of abnormal diastolic exercise response (AP: OR 3.16, 95% CI 1.34–7.42, p=0.008 [women] vs OR 2.07, 95% CI 0.95–5.49, p=0.15 [men]) with similar findings for AIx and AoPP.
Conclusions:
Arterial stiffness measures are significantly higher in women with HFpEF than men, and associated with abnormally steep increases in PCWP with exercise, particularly among women. Arterial stiffness may preferentially contribute to abnormal diastolic function during exercise in women with HFpEF compared with men.
Keywords: sex, arterial stiffness, heart failure with preserved ejection fraction
SUMMARY
We measured heart and lung function as well as blood pressure in men and women with heart failure who underwent specialized bicycle exercise stress testing.
Women with heart failure had stiffer blood vessels compared with men.
Stiffer blood vessels were associated with greater difficulty exercising, especially in women with heart failure.
VISUAL ABSTRACT
INTRODUCTION
The prevalence of heart failure with preserved ejection fraction (HFpEF) is rising, with tremendous associated morbidity and mortality. Despite its high prevalence, uncertainties remain regarding its pathophysiology, and to date, there are few effective medical therapies.1 Epidemiologic studies consistently demonstrate that the majority of HFpEF patients are women.1 What accounts for the female preponderance of HFpEF is incompletely understood,2 but sex differences in cardiovascular structure and remodeling have been previously described. Compared with men, women develop more concentric LV hypertrophy in response to hypertension (HTN) and aging.3, 4 Concomitantly, vascular stiffness is worse in women compared with men in the general population.5 Increased vascular stiffness has been implicated in the underlying pathophysiology of HFpEF via worse ventricular-vascular coupling and diastolic function, and may in part explain why women are more vulnerable to developing HFpEF than men.6, 7
Among patients with HFpEF, increased arterial stiffness and abnormal arterial loading have been demonstrated during exercise.6 Moreover, increased arterial stiffness has been associated with abnormal LV hemodynamic measurements and diastolic dysfunction in the HFpEF population overall.8, 9 We and others recently demonstrated that among patients with HFpEF, women appear to exhibit worse hemodynamic profiles compared with men with HFpEF including greater deficits in systolic and diastolic reserve and peripheral oxygen extraction.10 Whether sex differences in arterial stiffness and loading exist among patients with HFpEF is not known.
In this context, we sought to investigate differences in arterial stiffness and arterial load among men and women with HFpEF. The goals of the present study are twofold. Using a unique sample of patients with invasive arterial waveforms and hemodynamic measurements at rest and throughout incremental exercise, we first compared baseline measures of arterial stiffness and load in men and women with physiologically defined HFpEF. Second, we sought to investigate whether sex differences in arterial stiffness may relate to LV responses to exercise.
METHODS
Study Sample
We included patients with HFpEF, chronic NYHA class II-III symptoms, and no evidence of active ischemia during exercise who underwent clinically indicated CPET for evaluation of dyspnea with invasive hemodynamic monitoring and invasive arterial stiffness measurements at Massachusetts General Hospital between 2009 to 2017. HFpEF was defined as preserved LVEF ≥ 50% with evidence of elevated LV filling pressures at rest (rPCWP≥15 mmHg), or during exercise with elevated PCWP relative to cardiac output (ΔPCWP/ΔCO > 2.0 mmHg/L/min, as defined previously) with a peak exPCWP ≥ 15mmHg, and percent predicted peak VO2 < 80% during a maximal effort study (defined as a respiratory exchange ratio [RER] of >1.0).11, 12
From this sample (n=270), we excluded participants with clinical diagnosis of pulmonary arterial hypertension (n=8), history of heart or lung transplantation (n=10), complex adult congenital heart disease (n=10), mitochondrial disorder (n=4), undergoing evaluation for lung transplantation (n=1), significant valvular disease (n=39), and oxygen-dependent lung disease (n=8). The final patient sample included 190 patients (83 men and 107 women, Figure 1). Informed consent was obtained for all participants and the study was approved by the Massachusetts General Hospital Institutional Review Board.
Figure 1.
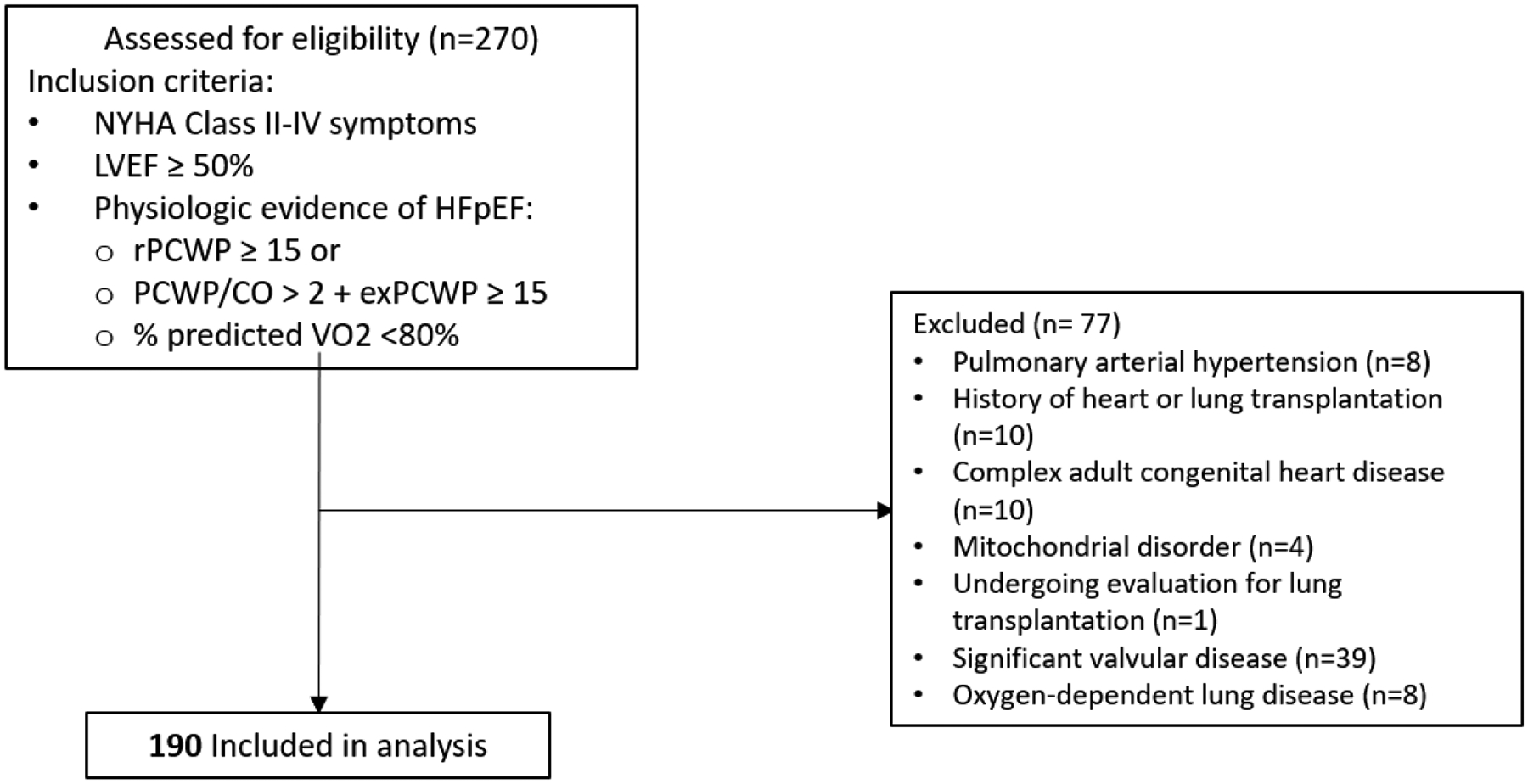
Consort diagram. Flow chart shows the patients assessed for eligibility, excluded, and included in the final analysis.
Clinical Variables and Biomarkers
Participants underwent history and physical examination including vital signs, body mass index (BMI), and fasting blood draw at the time of CPET. Medical history was ascertained via review of medical records. Fasting blood samples were immediately processed and stored at −80°C. High-sensitivity C-reactive protein (Roche, hsCRP, intra-assay coefficient of variation 0.4–8.4%) was ascertained using an immunoturbidimetric assay, and plasma N-terminal pro-B type natriuretic peptide (Roche, NT-proBNP, intra-assay coefficient of variation 2.4–3.8%) was assayed using an electrochemiluminescence immunoassay. Echocardiography obtained within 1 year of CPET was reviewed. Echocardiography parameters of interest included LVEF, LV hypertrophy, left atrial (LA) enlargement, and diastolic dysfunction as assessed by transmitral flow and mitral annular tissue velocities.
Cardiopulmonary Exercise Testing
All patients underwent insertion of a pulmonary artery catheter via the internal jugular vein and placement of a systemic arterial catheter via the radial artery. Subjects subsequently performed maximal upright cycle ergometry with a previously described ramp protocol (3-minute initial period of unloaded exercise followed by a 5 to 15 W/min continuous incremental ramp). Breath-by-breath gas exchange (MedGraphics, St. Paul, MN) and minute-by-minute hemodynamic measures were obtained at rest and exercise. Key gas exchange variables obtained included peak oxygen uptake (VO2) values taken as the highest 30-sec average over the final 90-sec of exercise, peak respiratory exchange ratio (RER), and arterial-venous O2 content difference (C(a-v)O2). Hemodynamic parameters included pulmonary artery capillary wedge pressure (PCWP), PA pressure, and Fick cardiac output (CO). All measurements obtained from the pulmonary artery catheter were assessed at end-expiration. Abnormal diastolic response to exercise was defined as a rise in pulmonary capillary wedge pressure relative to cardiac output (ΔPCWP/ΔCO) of ≥ 2 mmHg/L/min).11
Arterial Stiffness and Load Measures
Invasive radial artery pressure tracings were obtained and digitized at 240 Hz for offline analysis (SphygmoCor, AtCor, NSW, AUS). Using a generalized transfer function, central pressure waveforms were derived from continuous invasive radial arterial catheter-based pressure waveforms. Pressure waveforms were further reviewed manually for quality control. Arterial stiffness parameters were derived from pulse wave analysis and included augmentation pressure (AP), augmentation index (AIx), and aortic pulse pressure (AoPP). Higher AP, AIx, and AoPP are surrogates for greater arterial stiffness.13 The subendocardial viability ratio (SEVR) is calculated as the ratio of the diastolic-pressure-time index (DPTI) to tension-time index (TTI) with lower values indicating unfavorable ischemic predisposition.14
Invasive hemodynamic parameters and direct Fick cardiac output were used to calculate integrated measures of arterial load including systemic vascular resistance index (SVRi), pulse pressure amplification (PPA), effective arterial elastance index (EaI), and total arterial compliance index (TACI). SVRi is defined as (80•[mean central blood pressure (BP) – RA pressure]•BSA/CO. PPA is the ratio of peripheral to central PP. EaI is calculated as the ratio between ventricular end systolic pressure and stroke volume index (SVi) and is an integrated measure of arterial stiffness of the entire arterial system. TACI is calculated as SVI/central PP and reflects the overall reservoir function of the arterial system. Higher EaI and PP and lower TACI and PPA indicate greater arterial load. While arterial load is comprised of both a steady resistive component and a pulsatile component, we refer to SVRi, PPA, EaI, and TACI as measures of arterial load, acknowledging that these parameters are strictly measures of steady state flow resistance.15
Statistical Analysis
Baseline clinical characteristics and arterial stiffness measures were summarized for men and women. Results are reported as means ± standard deviation (SD) or medians and inter-quartile range (IQR) for continuous variables and percentages for dichotomous variables and differences between men and women were tested using Chi square, Student’s t-test or Wilcoxon rank sum test as appropriate. We examined sex differences in arterial stiffness parameters using multivariable linear regression models, adjusting for age, body mass index (BMI), hypertension (HTN), diabetes (DM), smoking status, and previous myocardial infarction (MI). SphygmoCor-derived measures (AP, AIx, AoPP, and SEVR) were additionally adjusted for heart rate. To account for differences due to heart rate, we examined arterial stiffness parameters stratified by resting heart rate (above and below median heart rate). In exploratory analyses, we adjusted arterial stiffness parameters for height and weight in lieu of BMI. Finally, we evaluated the association of arterial stiffness parameters with LV diastolic response to exercise (ΔPCWP/ΔCO) using partial correlation coefficients and multivariable logistic regressions adjusting for age. Again, Sphygmocor-derived measures (AP, Aix, AoPP, and SEVR) were adjusted for both age and heart rate. Finally, we performed sensitivity analyses reclassifying HFpEF as preserved LVEF ≥ 50% with evidence of elevated LV filling pressures at rest (rPCWP≥15 mmHg), or during exercise with elevated PCWP relative to cardiac output (ΔPCWP/ΔCO > 2.0 mmHg/L/min) with a peak exPCWP ≥ 25mmHg, and percent predicted peak VO2 < 80.16 All skewed data were natural log-transformed to achieve normal distribution, and regression models were standardized, expressing beta-coefficients in standard deviation units for continuous variables.
Analyses were conducted using STATA v.15.1 (College Station, TX). All tests were two-sided and a p-value of <0.05 was considered significant.
RESULTS
A total of 190 patients with HFpEF, LVEF ≥ 50%, and NYHA Class II-III symptoms were included in this analysis, and 56% (n=107) were women (Table 1). Women and men were of similar age (59±14 vs 62±13 years, respectively, p=0.07) and BMI (31.3±7.8 vs 30.9±4.9 kg/m2, respectively). Women had fewer comorbidities than men including a lower prevalence of hypertension (53% vs 75%, respectively, p<0.0001) and diabetes (13% vs 30%, respectively, p=0.004). NT-pro BNP levels were similar in women and men (108 [51–260] vs 89 [39–404], p=0.65, Supplemental Table 1). For subset of participants with available echocardiograms, women had higher LVEF and lower LA dimension compared with men (LVEF: 66±7 vs 64±8, p=0.02, LA dimension: 36±6 vs 40±6, p=0.004, Supplemental Table 2).
Table 1.
Baseline clinical and CPET characteristics in men and women with HFpEF
| Men N=83 | Women N=107 | p-value | ||||
|---|---|---|---|---|---|---|
| Clinical Characteristic | ||||||
| Age, years | 62 (13) | 59 (14) | 0.07 | |||
| Body mass index, kg/m2 | 30.9 (4.9) | 31.3 (7.8) | 0.65 | |||
| Hypertension, n (%) | 85 (75) | 83 (53) | <0.0001 | |||
| Diabetes mellitus, n (%) | 25 (30) | 14 (13) | 0.004 | |||
| Prevalent MI, n (%) | 7 (8) | 3 (2) | 0.08 | |||
| Prevalent AF, n (%) | 27 (33) | 13 (12) | 0.001 | |||
| Paced rhythm, n (%) | 4 (5) | 2 (2) | 0.25 | |||
| Previous HF admission, n (%) | 7 (8) | 8 (8) | 0.81 | |||
| Current smoking, n (%) | 1 (1) | 3 (3) | 0.44 | |||
| Total cholesterol, mg/dL | 160 (37) | 175 (36) | 0.11 | |||
| Laboratory Values | ||||||
| CRP, pg/mL | 2.3 [1.2, 5.2] | 3.2 [1.2, 6.3] | 0.25 | |||
| NT-pro BNP, pg/mL | 86 [39, 404] | 108 [51, 260] | 0.65 | |||
| eGFR, ml/min/1.73m2 | 77 (22) | 83 (30) | 0.17 | |||
| Hemoglobin, g/dL | 14.1 (1.8) | 12.7 (1.5) | <0.0001 | |||
| Pharmacotherapy | ||||||
| Diuretics, n (%) | 38 (46) | 40 (37) | 0.24 | |||
| ACE inhibitor, n (%) | 20 (24) | 18 (17) | 0.21 | |||
| ARB, n (%) | 15 (18) | 10 (9) | 0.08 | |||
| ß-adrenergic blocker, n (%) | 42 (51) | 49 (38) | 0.06 | |||
| Calcium channel blocker, n (%) | 11 (13) | 19 (18) | 0.40 | |||
| Gas Exchange Parameters | ||||||
| Peak VO2, mL/kg/min | 16 (4) | 15 (4) | 0.02 | |||
| % predicted VO2, Jones | 69 (17) | 69 (14) | 0.94 | |||
| VE/VCO2 slope | 36 (9) | 37 (8) | 0.76 | |||
| Max work, watts | 113 (37) | 87 (30) | <0.0001 | |||
| RER, peak | 1.2 (0.11) | 1.2 (0.11) | 0.56 | |||
| Peak C(a-v)O2 | 12.8 (1.9) | 10.8 (1.7) | <0.0001 | |||
| Hemodynamic Parameters | Rest | Peak | Rest | Peak | Prest | Ppeak |
| SBP, mmHg | 150 (21) | 192 (36) | 153 (22) | 192 (25) | 0.44 | 0.88 |
| DBP, mmHg | 77 (13) | 76 (11) | 89 (18) | 92 (17) | 0.67 | 0.18 |
| PP, mmHg | 72 (17) | 103 (24) | 75 (18) | 100 (20) | 0.40 | 0.38 |
| HR, beats/min | 73 (15) | 125 (26) | 78 (13) | 136 (24) | 0.02 | 0.003 |
| PCWP, mmHg | 16 (6) | 25 (7) | 14 (5) | 25 (7) | 0.01 | 0.52 |
| PAP, mmHg | 25 (7) | 42 (9) | 23 (6) | 38 (9) | 0.01 | 0.002 |
| CO, L/min | 5.4 (1.7) | 11.6 (3.4) | 5.0 (1.4) | 10.5 (3.0) | 0.04 | 0.02 |
| CI, L/min/m2 | 2.5 (0.7) | 5.4 (1.3) | 2.7 (0.7) | 5.7 (1.4) | 0.17 | 0.19 |
| PCWP/CO, mmHg/L/min | 2.9 (1.7) | 3.2 (1.8) | 0.16 | |||
| PCWP/CO >2.0 mmHg/L/min, n (%) | 60 (72%) | 86 (80%) | 0.19 | |||
Values are means (standard deviations) or medians [inter-quartile range] unless otherwise noted.
Abbreviations: ACE = angiotensin converting enzyme, ARB = angiotensin receptor blocker, DBP = diastolic blood pressure, MI = myocardial infarction, HF = heart failure, eGFR = estimated glomerular filtration rate, hsCRP = high sensitivity C-reactive protein, IL-6 = interleukin-6, LA = left atrium, LV = left ventricle, NT-pro BNP = N-terminal pro b-type natriuretic peptide, PAP = pulmonary artery pressure, PCWP = pulmonary capillary wedge pressure, RER = respiratory exchange ratio, SBP = systolic blood pressure, TR = tricuspid regurgitation.
We next examined sex differences in resting and exercise hemodynamic parameters obtained during invasive CPET. Resting and exercise SBP, DBP, and pulse pressure (PP) were similar in men and women (p>0.05 for all). By contrast, both resting and peak heart rates were higher in women (resting HR: 78±13 bpm [women] vs 73±15 bpm [men], p=0.02; peak HR: 136±24 bpm [women] vs 125±26 bpm [men], p=0.003). While cardiac output was lower in women compared with men (resting CO: 5.0±1.4 L/min [women] vs 5.4 ± 1.7 L/min, p=0.04; peak CO: 10.5 ± 3.0 L/min [women] vs 11.6 ± 3.4 L/min [men], p=0.02), cardiac indices were similar (resting CI: 2.7±0.7 L/min/m2 [women] vs 2.5±0.7 L/min/m2 [men], p=0.17; peak CI: 5.7±1.4 L/min/m2 [women] vs 5.4±1.3 L/min/m2 [men], p=0.19).
Arterial stiffness and pulsatile load are higher in women vs men with HFpEF
Measures of arterial stiffness and load are displayed in Table 2, Supplemental Table 3, and Figure 2. Despite similar rest and exercise blood pressures, women had greater arterial stiffness as evidenced by higher AP and AIx. Specifically, women had on average nearly 6 mmHg higher AP compared with men and >10% higher AIx (P<0.001 for both). After multivariable-adjustment, AP and AIx remained 0.72 and 0.67 SD higher in women compared with men respectively (AP: ß 0.72, SE 0.12, p<0.0001, AIx: ß 0.67, SE 0.12, p<0.001, Table 3). Aortic pulse pressure was similarly 0.5 standard deviations higher in women compared with men after multivariable adjustment (AoPP: ß 0.5, SE 0.12, P<0.0001). Of note, women also had a worse ischemic predisposition as evidenced by a lower SEVR (SEVR: ß −0.57, SE 0.13, p<0.001), which was predominantly driven by sex differences in the systolic (TTI) rather than the diastolic component of the SEVR (DPTI) (Table 3).
Table 2.
Measures of arterial wave reflection and load in men and women
| Men N=83 | Women N=107 | P-value | |
|---|---|---|---|
| Arterial Stiffness | |||
| AP, mmHg | 13.7 (10.1) | 19.1 (12.4) | 0.003 |
| AIx, % | 21.7 (11.9) | 28.9 (13.7) | 0.0002 |
| AoPP, mmHg | 56.7 (17.0) | 60.5 (18.9) | 0.16 |
| Ischemic Predisposition | |||
| SEVR, mmHg | 147.5 (30.7) | 125.1 (24.9) | 0.0001 |
| TTI, mmHg·sec / min | 2575 (497) | 2898 (579) | 0.0001 |
| DPTI, mmHg·sec / min | 3701 (663) | 3554 (735) | 0.08 |
| Arterial Load | |||
| SVRI, dynes·sec/cm5/m2 | 3740 (928) | 3393 (892) | 0.62 |
| Eal, mmHg·m2/mL | 4.2 (1.3) | 4.4 (1.3) | 0.1 |
| TACI, mL/mmHg·m2 | 0.65 (0.20) | 0.62 (0.23) | 0.09 |
| PPA | 1.36 (0.20) | 1.32 (0.21) | 0.15 |
Values are means (standard deviations). Abbreviations: AP = augmentation pressure, AIx = augmentation index, AoPP = aortic pulse pressure, SEVR = subendocardial viability ratio, TTI = tension time index, DPTI = diastolic perfusion time index, SVRI = systemic vascular resistance index, EaI = effective arterial elastance index, TACI = total arterial compliance index, PPA = pulse pressure amplification.
Figure 2.
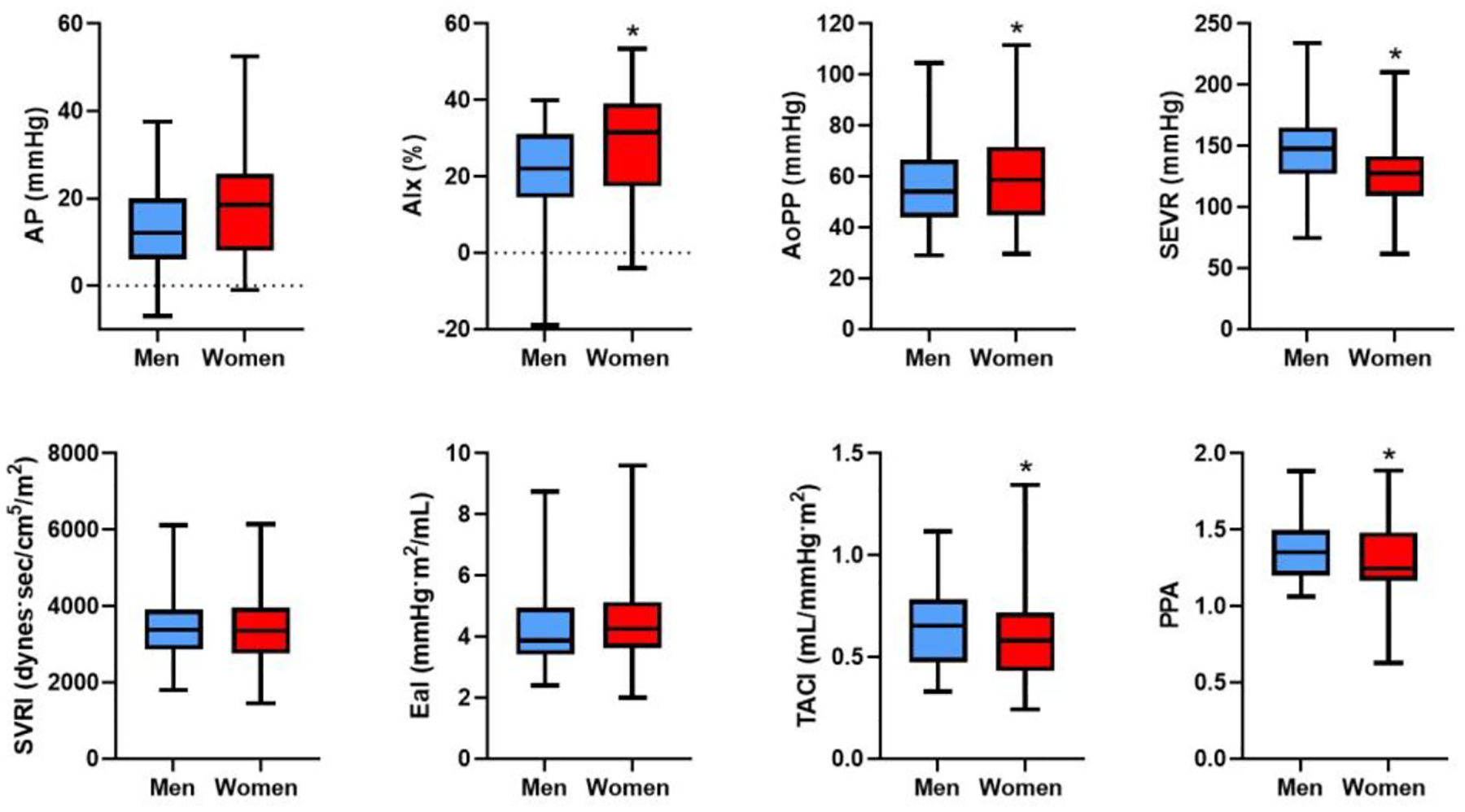
Arterial stiffness and load parameters at rest in men and women. Arterial stiffness measures (AP, AIx, and AoPP) adjusted for age and heart rate. Box and whisker plots indicate median and interquartile range. Error bars represent minimum and maximum.
* denotes P<0.05 difference between men and women. Abbreviations: AP = augmentation pressure, AIx = augmentation index, AoPP = aortic pulse pressure, SEVR = subendocardial viability ratio, SVRI = systemic vascular resistance index, EaI = effective arterial elastance index, TACI = total arterial compliance index, PPA = pulse pressure amplification.
Table 3.
Sex as a predictor arterial stiffness and load measures
| ß* | SE | p-value | |
|---|---|---|---|
| Arterial Stiffness | |||
| AP, mmHgǂ | 0.72 | 0.12 | <0.001 |
| AIx, %ǂ | 0.67 | 0.12 | <0.001 |
| AoPP, mmHgǂ | 0.50 | 0.12 | <0.001 |
| Ischemic Predisposition | |||
| SEVR, mmHg | −0.57 | 0.13 | <0.001 |
| TTI, mmHg·sec / min | 0.48 | 0.13 | <0.001 |
| DPTI, mmHg·sec / min | −0.19 | 0.15 | 0.23 |
| Arterial Load | |||
| SVRI, dynes·sec/cmVm2 | 0.02 | 0.17 | 0.87 |
| Eal, mmHg·m2/mL | 0.16 | 0.12 | 0.22 |
| TACI, mL/mmHg·m2 | −0.44 | 0.11 | <0.001 |
| PPA | −0.50 | 0.11 | <0.001 |
ß-coefficient: regression coefficients represent difference between women (referent) and men for standardized and log-transformed continuous variables. Multivariable model adjusts for age, body mass index, HTN, diabetes status, current smoking, previous MI. Further adjusted for heart rate where noted by ǂ. Abbreviations: AP = augmentation pressure, AIx = augmentation index, AoPP = aortic pulse pressure, SEVR = subendocardial viability ratio, TTI = tension time index, DPTI = diastolic perfusion time index, SVRI = systemic vascular resistance index, EaI = effective arterial elastance index, TACI = total arterial compliance index, PPA = pulse pressure amplification.
Integrated measures of arterial load also demonstrated that women had worse arterial load compared with men as evidenced by lower TACI (TACI: ß −0.44, SE 0.11, p<0.0001) and PPA (PPA: ß −0.50, SE 0.11, p<0.001), although there were no differences in SVRi or EaI between men and women (P>0.05).
In exploratory analyses, we further adjusted arterial stiffness parameters for height and weight and found similar albeit attenuated findings (AP: ß 0.44, SE 0.18, p=0.01, AIx: ß 0.46, SE 0.19, p=0.02, AoPP: ß 0.16, SE 0.18, p=0.37) (Supplemental Table 4). Sensitivity analyses using an exPCWP cutoff of >25 mmHg to define HFpEF also showed similar and attenuated findings (Supplemental Table 5).
Arterial stiffness correlates with abnormal LV diastolic reserve in women with HFpEF
In order to interrogate the impact of arterial stiffness on LV hemodynamic response to exercise, we examined the association of arterial stiffness with an abnormally steep rise in PCWP relative to CO (defined as ΔPCWP/ΔCO slope >2.0 mmHg/L/min), a measure of LV diastolic function during exercise in men and women with HFpEF. We found that resting measures of arterial stiffness were associated with abnormal left ventricular diastolic response to exercise in women, whereas this association was less pronounced among men. Specifically, in age-adjusted analyses, resting AP and AoPP were associated with 3-fold increased odds and resting AIx was associated with 2-fold increased odds of having abnormal diastolic function during exercise in women (AP: OR 3.16, 95% CI 1.34 – 7.42, p=0.008 [women] vs OR 2.07, 95% CI 0.95 – 5.49 [men]; AIx: OR 2.11, 95% CI 1.03–4.36, p=0.04 [women] vs OR 1.93, 95% CI 0.78 – 4.77, p=0.16 [men]; AoPP: OR 2.96, 95% CI 1.25–7.00 [women] vs 1.86, 95% CI 0.78–4.41 [men], Figure 3, Table 4). Worse ischemic predisposition as measured by SEVR was also associated with greater odds of worse diastolic exercise response among women (OR 0.41, 95% CI 0.18–0.93, p=0.03), with similar directionality in men although this did not reach statistical significance among men (OR 0.66, 95% CI 0.33–1.32, p=0.24). These differences persisted after accounting for age and heart rate (Supplemental Table 6). By contrast, measures of arterial load including SVRi, EaI, and PPA were not associated with LV diastolic response to exercise. Findings were similar albeit attenuated when examining associations of arterial stiffness with PCWP/CO slope (Supplemental Table 7).
Figure 3.
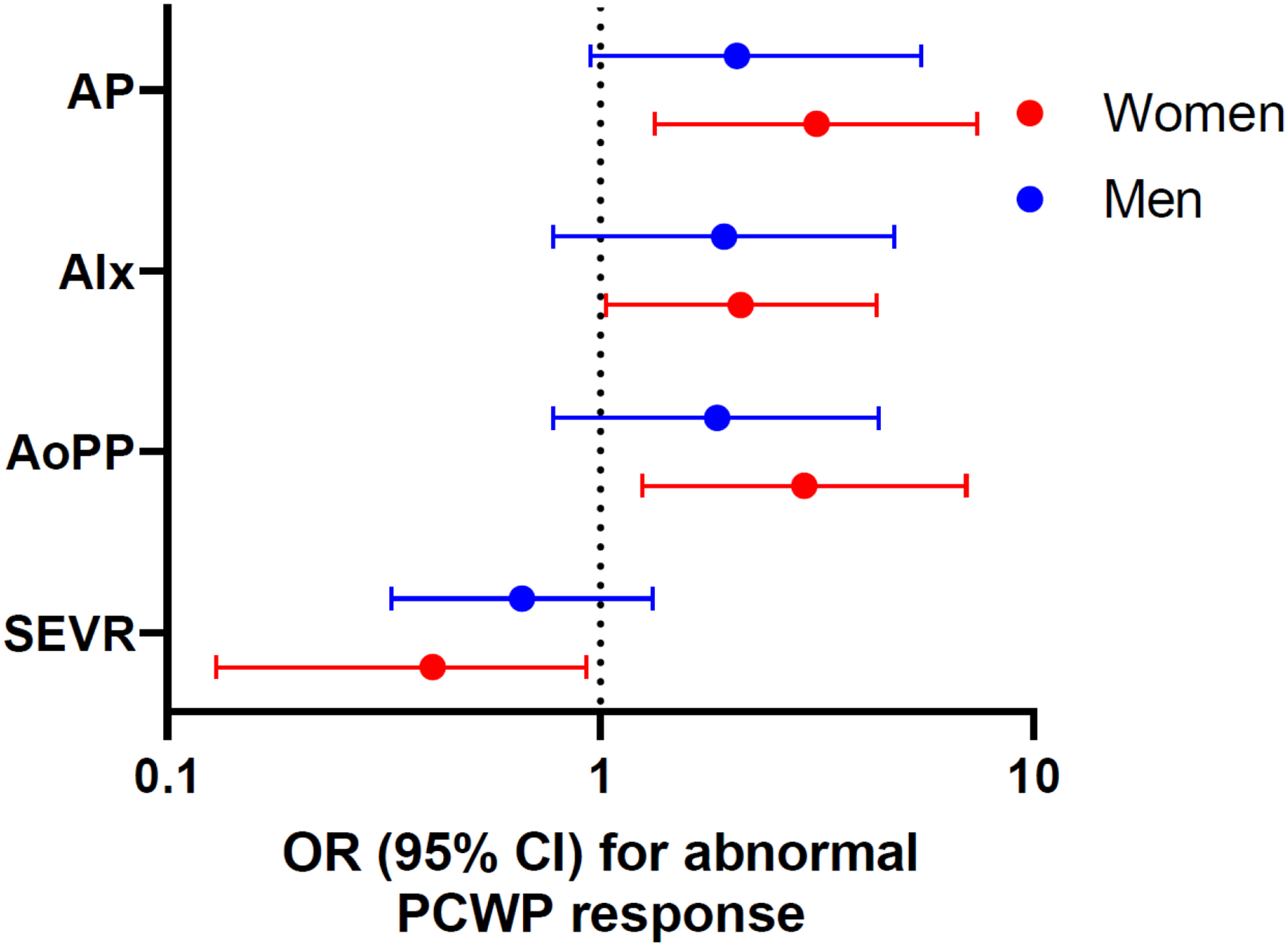
Cross-sectional association of arterial stiffness measures with abnormal PCWP/CO relationship in men and women: (A) augmentation pressure, (B) augmentation index, (C) aortic pulse pressure, and (D) SEVR.
Table 4.
Association of arterial stiffness and load with abnormal PCWP/CO slope in men and women with HFpEF
| Women | Men | ||||
|---|---|---|---|---|---|
| Arterial Stiffness | OR (95% CI) | p-value | OR (95% CI) | p-value | P int |
| AP, mmHgǂ | 3.16 (1.34–7.42) | 0.008 | 2.07 (0.95–5.49) | 0.15 | 0.27 |
| AIx, %ǂ | 2.11 (1.03–4.36) | 0.04 | 1.93 (0.78–4.77) | 0.16 | 0.21 |
| AoPP, mmHgǂ | 2.96 (1.25–7.00) | 0.01 | 1.86 (0.78–4.41) | 0.16 | 0.49 |
| Ischemic Predisposition | |||||
| SEVR, mmHgǂ | 0.41 (0.18–0.93) | 0.03 | 0.66 (0.33–1.32) | 0.24 | 0.96 |
| Arterial Load | |||||
| SVRI, dynes·sec/cm5/m2 | 1.58 (0.85–2.95) | 0.15 | 1.13 (0.62–2.07 | 0.68 | 0.64 |
| Eal, mmHg·m2/mL | 1.52 (0.77–3.00) | 0.22 | 0.92 (0.51–1.68) | 0.79 | 0.27 |
| TACI, mL/mmHg·m2 | 0.36 (0.16–0.81) | 0.01 | 0.58 (0.26–1.33) | 0.20 | 0.27 |
| PPA | 0.61 (0.35–1.06) | 0.08 | 0.59 (0.30–1.15) | 0.12 | 0.23 |
OR: odds ratio per 1-SD increase in standardized and log-transformed continuous variable. Model adjusts for age. Further adjusted for heart rate where noted by ǂ. Abbreviations: AP = augmentation pressure, AIx = augmentation index, AoPP = aortic pulse pressure, SEVR = subendocardial viability ratio, TTI = tension time index, DPTI = diastolic perfusion time index, SVRI = systemic vascular resistance index, EaI = effective arterial elastance index, TACI = total arterial compliance index, PPA = pulse pressure amplification.
Arterial stiffness and arterial load are correlated with age, SBP, and BMI in men and women with HFpEF
In exploratory analyses, we examined clinical correlates of arterial stiffness and load in men and women with HFpEF (Supplemental Table 8). Among both men and women with HFpEF, worse AP, AoPP, and EaI were positively correlated with older age (partial correlation coefficient, r range 0.33 to 0.55, p<0.01 for all), while TACI and PPA were negatively correlated with older age (partial correlation coefficient, r range −0.67 to −0.27, p<0.01 for all). In addition, after adjusting for age and heart rate, we found that measures of arterial stiffness (AP, AIx, and AoPP) and vascular load (EaI and SVRi) were positively associated with SBP (partial correlation coefficient, r range 0.23 to 0.76, p<0.03 for all) in both men and women with HFpEF. Conversely, arterial load measures TACI and PPA, and ischemic predisposition measured by SEVR were inversely correlated with SBP in both men and women (partial correlation coefficient, r range −0.2 to −0.52, p<0.03 for all).
We also found that measures of arterial stiffness were inversely correlated with BMI and weight, particularly among women with HFpEF. Specifically, AP and AIx were negatively correlated with BMI, with greater correlations observed in women with HFpEF compared with men (AP: r=−0.32, p=0.0009 in women vs r=−0.23, p=0.04 in men; AIx r=−0.37, p=0.0001 in women vs r=−0.2, p=0.07 in men). By contrast, measures of vascular load and ischemic predisposition were not correlated with BMI (P>0.05 for all).
Other clinical risk factors including HTN and diabetes as well as biomarkers NT-pro BNP and hsCRP were not correlated with arterial stiffness or load (P>0.05 for all).
DISCUSSION
Our study presents important differences in invasively measured arterial properties between men and women in a sample of patients with hemodynamic HFpEF by invasive CPET. Our key findings are threefold. First, resting measures of arterial stiffness and arterial load are significantly higher in women compared with men with HFpEF. Second, compared with men, ischemic predisposition as measured by SEVR is also worse in women. Finally, arterial stiffness is associated with abnormal LV diastolic responses to exercise particularly in womenTogether, our findings suggest that arterial stiffness may contribute to the pathophysiology of HFpEF more frequently in women compared with men, and may offer a window into potential therapeutic options for patients with HFpEF, particularly women.
One of the notable features of HFpEF is that the prevalence of HFpEF is greater among women than in men. Cardiovascular adaptation to cardiometabolic disease is known to differ between men and women, and may contribute to the sex differences in predisposition to HFpEF.4, 17 Increased arterial stiffness, a hallmark of aging, has been implicated in the development of HF.9 Sex differences in vascular stiffness and function in the general population are well known and have led investigators to propose that the tendency toward arterial stiffness in women may in part explain the female susceptibility to HFpEF development. Numerous studies have documented higher arterial stiffness, lower SEVR, and more impaired metrics of arterial load in healthy women vs men.18, 19 Moreover, age-related vascular stiffness and function appears to be accelerated and more pronounced among women, further contributing to the differences between men and women, particularly in the elderly population.20, 21
Clinical manifestations of overt HFpEF also dramatically differ in men and women. While women have improved survival in HFpEF compared with men, women have more signs and symptoms of congestion, greater functional limitations, and lower quality of life.22, 23 We previously reported that despite similar exercise capacity and equivalent efforts achieved during invasive cardiopulmonary testing, women with proven HFpEF in our CPET cohort demonstrated greater deficits in oxygen transport and utilization including worse biventricular systolic reserve, diastolic reserve, and peripheral oxygen extraction.10 Similar sex differences in diastolic reserve and peripheral oxygen kinetics were found in a cohort of 47 men and 114 women with HFpEF confirmed by hemodynamic assessment.7 We now extend prior findings and describe significantly higher resting measures of arterial stiffness and arterial load, and worse ischemic predisposition in women with HFpEF compared with men. Collectively, these differences warrant further mechanistic exploration and offer a potential therapeutic target in a patient population lacking effective therapies. As a possible demonstration, recent randomized data demonstrated greater reduction in HF hospitalizations with sacubitril/valsartan in women with HFpEF preferentially compared with men,24 leading to the hypothesis that neprilysin inhibition may improve arterial stiffness preferentially in women. Of note, reductions in blood pressure with sacubitril/valsartan were greater for women at 4 weeks,25 but similar for both men and women at 8 months.24 Nevertheless, treatment effect of sacubitril/valsartan was independent of change in SBP and blood pressure is a crude measure of arterial stiffness.
Arterial stiffness has been associated with markers of HFpEF including diastolic dysfunction and ventricular-arterial coupling, particularly in women. In a study of 158 subjects (50% women) referred for diastolic stress echocardiography, arterial stiffness (as measured by PPA, AP, and AIx) was significantly associated with markers of diastolic dysfunction (E’ velocity, E/E’, and LA volume index) in women only.26 Similarly, a more recent investigation of 461 patients without heart failure found that aortic characteristic impedance (a measure of arterial stiffness) and TACI were associated with diastolic dysfunction and Ea/Ees (systolic ventricular-arterial coupling) in women but not men.5 Our findings show that arterial stiffness and load are intimately tied to invasive measures of hemodynamic responses to exercise in HFpEF, and that this association is particularly pronounced among women. Specifically, arterial stiffness and pulsatile load are correlated with abnormally steep rises in PCWP with exercise, reflecting impaired diastolic function during exercise. The underlying mechanisms are ill understood. The role of estrogen has been implicated in the pathogenesis of arterial stiffness preferentially in women as evidenced by fluctuating arterial stiffness throughout the menstrual cycle and dramatic decrease and increase in arterial stiffness during puberty and menopause, respectively.27 Sex differences in vascular smooth muscle-related stiffness and collagen deposition have also been postulated, but remain poorly characterized.28 While the mechanisms are yet to be elucidated, these findings are notable in light of recent data demonstrating improved arterial stiffness with exercise intervention and suggests that reducing arterial stiffness via exercise may be a potential therapeutic option for improving symptoms of exercise intolerance in patients with HFpEF, particularly women.29, 30
Finally, it is well established that cardiometabolic disease plays an important role in the development of arterial stiffness and future HFpEF in both men and women. In exploratory analyses, we examined the association of clinical risk factors with arterial stiffness among men and women with established HFpEF. As expected, we found that age and SBP were similarly correlated with arterial stiffness in men and women with HFpEF. Surprisingly, we found that BMI and weight were negatively associated with arterial stiffness and load, particularly among women with HFpEF. This is in contrast to previous data that demonstrate that obesity is associated with increased arterial stiffness among individuals without established CVD, although one study demonstrated a positive association between BMI and increased arterial stiffness in men only.17, 31, 32 Our findings may be confounded by lead time bias, whereby obese individuals present earlier in the disease course and have not yet developed significant arterial stiffness, akin to the well described obesity paradox in HF.33 This may be further explained by our unique study sample of physiologic HFpEF patients, who likely represent a patient population early in the HFpEF disease course. Alternatively, phenotypic heterogeneity in HFpEF is well recognized, and obesity-associated HFpEF may be characterized by less evidence of arterial stiffness in our sample. The association of obesity and other cardiometabolic risk factors with arterial stiffness in HFpEF warrants further investigation.
Our study has several limitations. First, our study sample included participants who underwent clinically-indicated invasive cardiopulmonary exercise testing and may have been subject to referral bias. Second, our study is an observational investigation, and as such, causality cannot be inferred. Third, differences in height between men and women may explain the observed differences in arterial stiffness parameters. Specifically, we found that the association between sex and SEVR (but not other arterial stiffness parameters) was no longer observed after adjusting for height and weight in lieu of BMI. Third, our sample may represent an exercise HFpEF phenotype reflective of earlier disease stage, and generalizability of our findings to other HFpEF samples with rest congestion may be limited. However, by applying a strict hemodynamic definition, we are able to capture a population of symptomatic HFpEF patients earlier in the disease course who exhibit abnormal filling pressures only during exercise. Moreover, we are able to overcome sex differences inherent to more commonly used HFpEF definitions that require natriuretic peptide or echocardiography measurements, which may inherently differ by sex.
In sum, our study demonstrates marked differences in arterial stiffness and load between men and women with physiologically defined HFpEF. In particular, we show that despite similar blood pressures, women with HFpEF have significantly increased arterial stiffness and greater arterial load compared with men. Arterial stiffness is correlated with abnormal diastolic response to exercise in patients with HFpEF, particularly among women. Together, these findings suggest that abnormal arterial stiffness and load may play an important role in the pathogenesis of HFpEF, especially among women. Identifying mechanisms to improve arterial stiffness and load may ultimately provide potential therapeutic targets for the treatment of HFpEF, particularly in women.
Supplementary Material
FUNDING
This work was supported by grants from the NIH-5T32HL094301-07 (ESL), K23-HL138260 (MNayor), R01-HL131029 (GDL), R01-HL151841 (GDL), the American Heart Association 15GPSGC24800006 (GDL), NIH/NHLBI R01-HL134893 (JEH), R01-HL140224 (JEH), NIH/NHLBI K24-HL153669 (JEH), and a Gilead Sciences Research Scholar Award (JEH).
ABBREVIATIONS
- AP
augmentation pressure
- AIx
augmentation index
- BMI
body mass index
- CV
cardiovascular
- CPET
Cardiopulmonary exercise testing
- DPTI
diastolic perfusion time-index
- EaI
effective arterial elastance index
- HFpEF
heart failure with preserved ejection fraction
- hsCRP
high-sensitivity C-reactive protein
- HTN
hypertension
- LVEF
left ventricular ejection fraction
- NTproBNP
N-terminal pro-B type natriuretic peptide
- NYHA
New York Heart Association
- PCWP
pulmonary capillary wedge pressure
- PPA
pulse pressure amplification
- SEVR
subendocardial viability ratio
- SVRi
systemic vascular resistance index
- TACI
total arterial compliance index
- TTI
tension-time index
- VO2
oxygen consumption
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
TWITTER POST
Worse arterial stiffness and load in women vs men with HFpEF is associated with abnormal LV diastolic response to exercise.
CONFLICT OF INTEREST
No conflicts of interest to report.
REFERENCES
- 1.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 2016;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, of Foundation A, on Guidelines A. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62(16):239. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama 1996;275(20):1557–62. [PubMed] [Google Scholar]
- 4.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal Tracking of Left Ventricular Mass Over the Adult Life Course. Circulation 2009;119(24):3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex Differences in Arterial Stiffness and Ventricular-Arterial Interactions. Journal of the American College of Cardiology 2013;61(1):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy Y, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology 2017;70(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beale AL, Nanayakkara S, Segan L, Mariani JA, Maeder MT, van Empel V, Vizi D, Evans S, Lam CSP, Kaye DM. Sex Differences in Heart Failure With Preserved Ejection Fraction Pathophysiology. A Detailed Invasive Hemodynamic and Echocardiographic Analysis 2019;7(3):239–249. [DOI] [PubMed] [Google Scholar]
- 8.Zern EK, Ho JE, Panah LG, Lau ES, Liu E, Farrell R, Sbarbaro JA, Schoenike MW, Pappagianopoulos PP, Namasivayam M, Malhotra R, Nayor M, Lewis GD. Exercise intolerance in HFpEF: arterial stiffness and abnormal left ventricular hemodynamic responses during exercise. J Card Fail 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber T, Chirinos JA. Pulsatile arterial haemodynamics in heart failure. European Heart Journal 2018;39(43):3847–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau ES, Cunningham T, Hardin KM, Liu E, Malhotra R, Nayor M, Lewis GD, Ho JE. Sex Differences in Cardiometabolic Traits and Determinants of Exercise Capacity in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 2020;5(1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R, Lewis GD. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail 2018;11(5):e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 1985;131(5):700–8. [DOI] [PubMed] [Google Scholar]
- 13.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 1972;30(1):67–81. [DOI] [PubMed] [Google Scholar]
- 15.Adji A, O’Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens 2011;24(1):5–17. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3(5):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gori M, Lam CSP, Gupta DK, Santos ABS, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJV, Solomon SD, Investigators P. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. European Journal of Heart Failure 2014;16(5):535–542. [DOI] [PubMed] [Google Scholar]
- 18.Kim J-Y, Park J, Kim D, Kim K, Jeong J, Park J, Oh B, Chung N, investigators K. Gender Difference in Arterial Stiffness in a Multicenter Cross-Sectional Study: The Korean Arterial Aging Study (KAAS). Pulse 2015;2(1–4):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieber A, Millasseau S, Bourhis L, Blacher J, Protogerou A, Levy BI, Safar ME. Aortic wave reflection in women and men. American journal of physiology. Heart and circulatory physiology 2010;299(1):42. [DOI] [PubMed] [Google Scholar]
- 20.Namasivayam M, McEniery CM, Wilkinson IB, Yasmin, Cockroft JR, McDonnell BJ, Adji A, O’Rourke MF. Different Effects of Vascular Aging on Ischemic Predisposition in Healthy Men and Women. Hypertension 2018;72(6):1294–1300. [DOI] [PubMed] [Google Scholar]
- 21.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension 2011;57(3):390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam CSP, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, McKelvie RS, McMurray JJ, Zile MR, Massie BM, Kitzman DW. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circulation. Heart failure 2012;5(5):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewan P, Rørth R, Raparelli V, Campbell RT, Shen L, Jhund PS, Petrie MC, Anand IS, Carson PE, Desai AS, Granger CB, Køber L, Komajda M, McKelvie RS, O’Meara E, Pfeffer MA, Pitt B, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Sex-Related Differences in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2019;12(12):e006539. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer N, Vardeny O, Claggett B, Jhund PS, Solomon SD. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 2020;141(5):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvaraj S, Claggett BL, Böhm M, Anker SD, Vaduganathan M, Zannad F, Pieske B, Lam CSP, Anand IS, Shi VC, Lefkowitz MP, McMurray JJV, Solomon SD. Systolic Blood Pressure in Heart Failure With Preserved Ejection Fraction Treated With Sacubitril/Valsartan. Journal of the American College of Cardiology 2020;75(14):1644–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim C, Park S, Choi D, Yang W-I, Cho I-J, Choi E-Y, Chung N, Ha J-W. Sex Differences in Central Hemodynamics and Their Relationship to Left Ventricular Diastolic Function. Journal of the American College of Cardiology 2011;57(10):1226–1233. [DOI] [PubMed] [Google Scholar]
- 27.Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab 2003;88(11):5375–80. [DOI] [PubMed] [Google Scholar]
- 28.DuPont JJ, Kenney RM, Patel AR, Jaffe IZ. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol 2019;176(21):4208–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkley TE, Leng I, Bailey MJ, Houston DK, Hugenschmidt CE, Nicklas BJ, Hundley WG. Effects of Exercise and Weight Loss on Proximal Aortic Stiffness in Older Adults With Obesity. Circulation;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhuva Anish N, D’Silva A, Torlasco C, Jones S, Nadarajan N, Van Zalen J, Chaturvedi N, Lloyd G, Sharma S, Moon James C, Hughes Alun D, Manisty Charlotte H. Training for a First-Time Marathon Reverses Age-Related Aortic Stiffening. Journal of the American College of Cardiology 2020;75(1):60–71. [DOI] [PubMed] [Google Scholar]
- 31.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 2005;23(10):1839–46. [DOI] [PubMed] [Google Scholar]
- 32.Safar ME, Czernichow S, Blacher J. Obesity, Arterial Stiffness, and Cardiovascular Risk. Journal of the American Society of Nephrology 2006;17(4 suppl 2):S109–S111. [DOI] [PubMed] [Google Scholar]
- 33.Hainer V, Aldhoon-Hainerová I. Obesity Paradox Does Exist. Diabetes Care 2013;36(Supplement 2):S276–S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


