Abstract
Background
Beyond sex, age, and various comorbidities, geographical origin and socioeconomic deprivation are associated with Coronavirus Disease (COVID-19) morbidity and mortality in the general population. We aimed to assess factors associated with severe forms of COVID-19 after a hospital emergency department visit, focusing on socioeconomic factors.
Methods
Patients with laboratory-confirmed COVID-19 attending the emergency department of Béclère Hospital (France) in March–April 2020 were included. Postal addresses were used to obtain two geographical deprivation indices at the neighborhood level. Factors associated with hospitalization and factors associated with adverse outcomes, i.e. mechanical ventilation or death, were studied using logistic and Cox analyses, respectively.
Results
Among 399 included patients, 321 were hospitalized. Neither geographical origin nor socioeconomic deprivation was associated with any of the outcomes. Being male, older, overweight or obese, diabetic, or having a neuropsychiatric disorder were independent risk factors for hospitalization. Among 296 patients hospitalized at Béclère Hospital, 91 experienced an adverse outcome. Older age, being overweight or obese, desaturation and extent of chest CT scan lesions > 25% at admission (aHR: 2.2 [95% CI: 1.3–3.5]) and higher peak CRP levels and acute kidney failure (aHR: 2.0 [1.2–3.3]) during follow-up were independently associated with adverse outcomes, whereas treatment with hydrocortisone reduced the risk of mechanical ventilation or death by half (aHR: 0.5 [0.3–0.8]).
Conclusion
No association between geographical origin or socioeconomic deprivation and the occurrence of a severe form of COVID-19 was observed in our population after arrival to the emergency department. Empirical corticosteroid use with hydrocortisone had a strong protective impact.
Keywords: COVID-19, Socioeconomic deprivation, Geographical origin, Corticosteroids
1. Introduction
The clinical course of COVID-19 is highly variable from one patient to another. Early prediction of severe SARS-CoV-2 infection is therefore of paramount importance, and numerous risk factors have been identified [1]. A study conducted in the United Kingdom also highlighted an association between socioeconomic deprivation or non-Caucasian ethnicity and the probability of dying from COVID-19 among the general population [2]. Other studies in the United States provided conflicting results concerning ethnicity, with a lower risk of hospitalization and death among non-Hispanic African Americans than among non-Hispanic white people [3]. In France, disparate excess mortality between geographical areas during the first outbreak of the pandemic suggested a link between socioeconomic factors and COVID-19 burden, with deprived areas being the most affected [4]. However, no data concerning the link between socioeconomic deprivation or ethnicity and hospital admission or mechanical ventilation and death after a visit to the emergency department for COVID-19 at an individual level have so far been published.
In the urgent context of the pandemic, some clinicians started using corticosteroids – a readily available, inexpensive, and well-known agent – in early March 2020, even before reliable evidence was available. There was indeed a coherent scientific rationale supporting the potential efficacy of corticosteroids by mitigating the inflammatory organ injury of severe COVID-19 [5]. Preliminary results of the Recovery trial of dexamethasone in hospitalized patients and of the meta-analysis of seven randomized trials in critically ill patients subsequently confirmed the association between corticosteroids and lower mortality [6], [7].
We therefore aimed to assess factors associated with severe forms of COVID-19, i.e. hospitalization of patients attending the emergency department with a polymerase chain reaction (PCR)-confirmed diagnosis on the one hand, and mechanical ventilation or death for hospitalized patients on the other hand, focusing on socioeconomic factors.
2. Material and methods
2.1. Study setting and participants
We conducted an observational cohort study in a French university hospital located in the South-West of Paris during the first wave of the COVID-19 pandemic in France. This university hospital is part of the public hospital system of Paris (French acronym AP–HP), which allows the use of routinely collected data for non-interventional research [8].
All adults (aged ≥ 18 years) who attended the emergency department of Béclère Hospital from March 2, 2020 to April 15, 2020, with a laboratory-confirmed COVID-19 (positive PCR assay from a nasopharyngeal swab) were included. Pregnant women were not included. All patients were informed of the study. The study ended when high-flow oxygen use (defined by using an Optiflow™ device at more than 15 L/min O2) became the standard of care to delay mechanical ventilation.
All patients benefitted from the current standard of care for COVID-19: antipyretic therapy, oxygen support, prevention of systematic thrombotic complications, and comorbidity management. Other treatments (antibiotics, corticosteroids, hydroxychloroquine) were provided at the discretion of the clinicians, but local recommendations were rapidly implemented to stop antibiotics and hydroxychloroquine. Hydrocortisone (200-mg loading dose followed by 50 mg four times a day for 8–10 days) was recommended when patients needed oxygen support.
2.2. Data collection
Data were collected upon arrival to the emergency department and during follow-up for hospitalized patients. Geographical origins were subdivided into four groups as follows: Western countries, North Africa, sub-Saharan Africa, and other. Postal addresses were geocoded by a local research assistant to match patients to their neighborhood of residence. IRIS (or regrouped statistical information blocks), which is the smallest geographical census unit as defined by the French National Institute for Statistics and Economic Studies (French acronym INSEE) [9], were used as a proxy for neighborhoods. Then, the correspondence was made between IRIS numbers and two freely available and validated deprivation indices, i.e. the French Deprivation Index (FDEP, last revision in 2009) and the French European Deprivation Index (French EDI, last revision in 2015), with the score increasing with socioeconomic deprivation [10], [11].
Comorbidities included being overweight or obese, high blood pressure, type 2 diabetes, chronic cardiovascular disease including cerebrovascular events, chronic neuropsychiatric disorders, chronic respiratory disease, and cancer. Desaturation was defined by oxygen saturation < 95%. Chest computed tomography (CT) results were classified according to the extent of pulmonary lesions: none, minimal (< 10%), moderate (10–24%), extensive (25–49%), serious (50–75%), and critical (> 75%). Acute renal failure was defined according to the KDIGO criteria (increase in serum creatinine level by > 0.3 mg/dL within 2 days or by > 50% within 7 days) [12]. The effects of certain immunomodulatory drugs rely to some extent on genomic mechanisms [13]. Thus, a specific duration of treatment can be necessary to observe clinical effects. Corticosteroids and hydroxychloroquine delivered for ≥ 3 days were considered for the analysis.
2.3. Outcomes
Two levels of severe forms were studied. We first assessed factors associated with hospital admission (versus being discharged within 24 hours following emergency department attendance) for all patients. Then, we assessed factors associated with adverse clinical outcomes, defined as the use of mechanical ventilation or in-hospital death, for patients hospitalized at Béclère Hospital.
2.4. Statistical analysis
Categorical variables were compared using Chi2 tests (or Fisher's exact test when the expected numbers were small) and continuous variables using Student's t-tests. Multivariate analyses were performed using a logistic regression model for hospitalization as the outcome and using a Cox proportional hazards model for the risk of mechanical ventilation or death as the combined outcome in hospitalized patients. Follow-up ended at the date of discharge from hospital. CRP levels, occurrence of acute kidney failure, and corticosteroid and hydroxychloroquine administration for at least 3 days were used as time-dependent covariates.
For each multivariable model, the selection of covariates was based on a backward stepwise technique with a step-by-step exclusion of a single variable. Variables associated with the outcome of interest with P < 0.15 in the univariable analysis were investigated. The final models were those with the lowest AIC (Akaike information criterion). Analyses were performed using SAS software 9.4 (SAS Institute Inc.).
3. Results
3.1. Study population
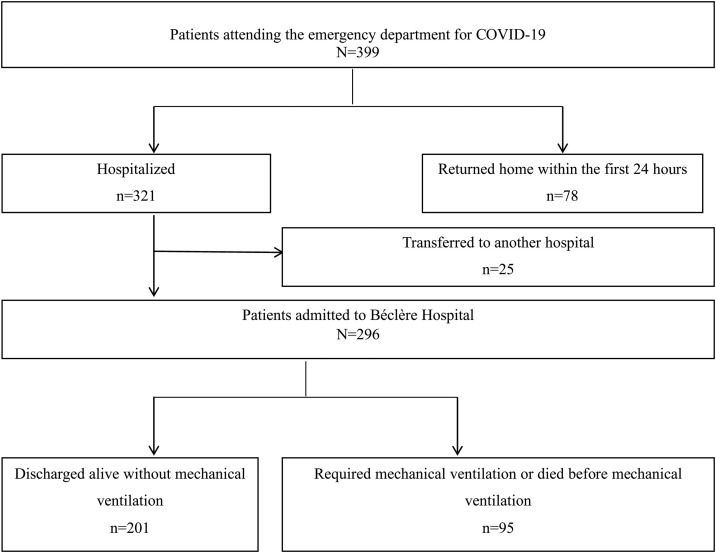
A total of 399 consecutive patients attending the emergency department of Béclère Hospital with a positive PCR for SARS-CoV-2 were included in the study (Fig. 1 ). The main baseline features are presented in Table 1 . The study included 226 men (56.6%) and the median age was 64 years (IQR: 50–79). The patients’ geographical origin was mainly Western countries (n = 227, 57%), followed by North Africa (n = 70, 18%) and sub-Saharan Africa (n = 67, 17%). Other patients were born in the French West Indies (n = 15, 4%), Asia (n = 10, 3%), and the Middle East region (n = 10, 3%). Overall, mean (SD, min, max) FDEP was −1.83 (1.84, −5.54, 3.30) and French EDI 1.99 (5.38, −6.71, 18.16).
Fig. 1.
Flow chart.
Table 1.
Characteristics of the overall study population according to the type of housing.
| All n = 399 |
Personal residence not deprived n = 244 |
Personal residence deprived n = 96 |
Nursing home n = 42 |
Home for the disabled n = 17 |
Pa | |
|---|---|---|---|---|---|---|
| Age (years) | 64 (50–79) | 61 (49–78) | 61 (49–73) | 86 (79–89) | 57 (53–69) | < 0.0001 |
| Gender | ||||||
| Female | 173 (43%) | 106 (43%) | 32 (33%) | 24 (57%) | 11 (65%) | |
| Male | 226 (57%) | 138 (57%) | 64 (67%) | 18 (43%) | 36 (35%) | 0.0158 |
| Continent of birth | ||||||
| Western countries | 227 (57%) | 146 (60%) | 28 (29%) | 38 (91%) | 15 (88%) | < 0.0001 |
| Northern Africa | 70 (18%) | 43 (18%) | 24 (25%) | 2 (5%) | 1 (6%) | |
| Sub-Saharan Africa | 67 (17%) | 34 (14%) | 32 (33%) | 1 (2%) | 0 | |
| Other | 35 (9%) | 21 (8%) | 12 (13%) | 1 (2%) | 1 (6%) | |
| Deprivation indexb | ||||||
| FDEP | −2.05 (−3.29; −0.47) |
−2.57 (−3.43; −1.63) |
0.65 (0.35; 1.16) |
−2.74 (−3.76; −2.43) |
−3.29 (−3.35; −2.91) |
< 0.0001 |
| French EDI | 0.39 (−2.21; 5.82) |
−0.39 (−3.12; 1.50) |
10.44 (8.03; 11.03) |
0.24 (−1.40; 0.86) |
−1.39 (−2.55; −1.39) |
< 0.0001 |
| Preexisting comorbidities | ||||||
| Overweight or obesity | 118 (30%) | 73 (30%) | 30 (31%) | 7 (17%) | 8 (47%) | 0.1041 |
| High blood pressure | 159 (40%) | 88 (36%) | 43 (45%) | 24 (57%) | 4 (24%) | 0.0242 |
| Type 2 diabetes | 86 (22%) | 46 (19%) | 29 (30%) | 9 (21%) | 2 (12%) | 0.1174 |
| Chronic cardiovascular disease | 86 (22%) | 44 (18%) | 21 (22%) | 20 (48%) | 1 (6%) | 0.003 |
| Neuropsychiatric disorder | 92 (23%) | 29 (12%) | 12 (13%) | 36 (86%) | 15 (88%) | < 0.0001 |
| Chronic respiratory disease | 69 (17%) | 43 (18%) | 16 (17%) | 5 (12%) | 5 (29%) | 0.4395 |
| Cancer | 37 (9%) | 26 (11%) | 6 (6%) | 3 (7%) | 2 (12%) | 0.5674 |
| Number of comorbidities other than being overweight or obese | ||||||
| No comorbidity | 113 (28%) | 77 (32%) | 34 (35%) | 1 (2%) | 1 (6%) | |
| One comorbidity | 120 (30%) | 88 (36%) | 19 (20%) | 8 (19%) | 5 (29%) | |
| At least two comorbidities | 166 (42%) | 79 (32%) | 43 (45%) | 33 (79%) | 11 (65%) | < 0.0001 |
Data are presented as counts (proportions) and medians (interquartile range).
FDEP: French Deprivation Index; French EDI: French European Deprivation index.
The Chi2 or Fisher's exact test and Wilcoxon tests were used for comparisons.
The lowest values correspond to the least deprived area.
Most patients were living in their personal residence (n = 340, 85%), 42 (11%) in a nursing home, i.e. retirement homes or facilities for dependent elderly people, and 17 (4%) in homes for disabled people. Individuals living in nursing homes or in homes for the disabled lived in less deprived areas. At least one comorbidity was reported in 310 (78%) patients, differentially according to their residence, i.e. 75% for those living in their personal residence, 98% for those living in nursing homes, and 94% for those living in homes for the disabled (P < 0.0001). The most frequent comorbidities were high blood pressure and being overweight or obese. High blood pressure and chronic cardiovascular diseases were more frequent in people living in nursing homes and neuropsychiatric disorders in those not living in their personal residence. Among people living in their personal residence, 96 (24%) were in the highest quintile of the FDEP index (≥ −0.53), defining the most deprived quintile. Living in the most deprived quintile was less frequent for people born in Western countries (16%) than for those born in sub-Saharan Africa (49%) or Northern Africa or another country (36%) (P < 0.0001). Diabetes mellitus was more frequent among people living in the most deprived quintile (30% vs. 19%, P = 0.03). Only six people lived in the most deprived quintile among people not living in their personal residence.
Median time between symptom onset and emergency department attendance was five days (IQR: 2–9). The most frequent symptoms were fever or chills (257, 64%), cough (233, 58%), and dyspnea (170, 43%). Other symptoms were tiredness (75, 19%), sore muscles (71, 18%), diarrhea (60, 15%), headache (31, 8%), vomiting (31, 8%), and anosmia or ageusia (25, 6%). Median (IQR) oxygen saturation was 94% (89–97) and 202 (51%) patients showed oxygen desaturation.
3.2. Factors associated with hospital admission
After medical evaluation, 78 patients (20%) returned home and 321 (80%) were hospitalized, either in the same hospital or in another hospital due to a lack of hospital beds (Table 2 ). There was no difference in time since symptom onset between people who returned home and people who were hospitalized (median [IQR] time 5 [2–9] and 5 [2–8] days, respectively; P = 0.33). People who returned home were not subsequently hospitalized.
Table 2.
Baseline characteristics and risk factors for admission to the hospital for 399 patients attending the emergency department for confirmed COVID-19: logistic regression analyses.
|
n/N (%) a |
Univariate |
Multivariate |
|||
|---|---|---|---|---|---|
| 321/399 (80%) | Crude OR | P-value | Adjusted OR | P-value | |
| Age (years) | |||||
| [< 60[ | 102/166 (61%) | Ref | < 0.0001 | Ref | < 0.0001 |
| [60–80[ | 124/134 (93%) | 7.8 [3.8–15.9] | 6.5 [3.0–13.7] | ||
| [> 80] | 95/99 (96%) | 14.9 [5.2–42.5] | 15.9 [5.3–47.9] | ||
| Gender | |||||
| Female | 128/173 (74%) | Ref | < 0.0001 | Ref | 0.0010 |
| Male | 193/226 (85%) | 2.1 [1.3–3.4] | 2.7 [1.5–4.9] | ||
| Continent of birth | |||||
| Western countries | 188/227 (83%) | Ref | 0.2539 | – | |
| Northern Africa | 54/70 (77%) | 0.7 [0.4–1.4] | |||
| Sub-Saharan Africa | 49/67 (73%) | 0.6 [0.3–1.1] | |||
| Other | 30/35 (86%) | 1.3 [0.5–3.4] | |||
| Housing | |||||
| Own accommodation | 264/340 (78%) | Ref | < 0.0001 | NR | |
| Other | 57/59 (97%) | 8.3 [2.0–33.3] | |||
| Deprivation index | |||||
| FDEPb | – | 1.0 [0.8–1.1] | 0.5757 | – | |
| French EDIb | – | 1.0 [0.9–1.0] | 0.7691 | – | |
| Preexisting comorbidities | |||||
| Overweight or obesity | 105/118 (89%) | 2.4 [1.3–-4.6] | 0.0065 | 3.3 [1.6–6.6] | 0.0012 |
| High blood pressure | 142/159 (89%) | 2.8 [1.6–5.1] | 0.0004 | NR | |
| Type 2 diabetes | 80/86 (93%) | 4.0 [1.7–9.5] | 0.0019 | 2.9 [1.1–7.6] | 0.0264 |
| Cardiovascular disease | 82/86 (95%) | 6.3 [2.3–17.9] | 0.0005 | NR | |
| Neuropsychiatric disorder | 87/92 (95%) | 5.4 [2.1–13.9] | 0.0004 | 3.5 [1.3–9.5] | 0.0165 |
| Chronic respiratory disease | 60/69 (87%) | 1.8 [0.8–3.7] | 0.1382 | NR | |
| Cancer | 34/37 (92%) | 3.0 [0.9–9.9] | 0.0781 | NR | |
| Number of comorbidities other than being overweight or obese | |||||
| No comorbidity | 67/113 (59%) | Ref | < 0.0001 | – | |
| One comorbidity | 98/120 (82%) | 3.1 [1.7–5.6] | |||
| At least two comorbidities | 156/166 (94%) | 10.7 [5.1–22.5] | |||
| Clinical parameters | |||||
| Desaturation | 197/202 (98%) | 23.2 [9.1–59.0] | < 0.0001 | – | |
| Fever | 202/257 (79%) | 0.7 [0.4–1.2] | 0.2109 | – | |
Data are presented as medians (IQR) or n (%).
NR: not retained by the model; FDEP: French Deprivation Index; French EDI: French European Deprivation Index; OR: odds ratio.
n hospitalizations for N people at risk (proportion hospitalized within the modality).
Odds ratio is estimated for 1-point increments.
In the univariate analysis, men, people ˃ 60 years, and people not living in their personal residence were more frequently hospitalized. There was no association between hospitalization and socioeconomic deprivation (P = 0.58), even when the analysis was restricted to people living in their personal residence (P = 0.67), or between hospitalization and geographical origin (P = 0.25). Each comorbidity was, or tended to be, associated with a higher likelihood of hospitalization, although the association was not statistically significant for chronic respiratory diseases and cancer.
In the multivariable analysis, being male and older remained associated with an increased risk of hospitalization. Among comorbidities, people who were overweight or obese, diabetic, or with a neuropsychiatric disorder had a risk of hospitalization approximately three times higher than those without these comorbidities.
When we excluded from the analyses people who lived in nursing homes or in homes for the disabled, people who were living in deprived areas had similar risk for hospitalization than those not living in deprived areas (OR [95% CI], 1.0 [0.6–1.7], P = 0.99). Other associations were unchanged (data not shown).
3.3. Factors associated with mechanical ventilation or death
Among the 296 patients admitted to Béclère Hospital, 95 (32%) met the composite criteria of adverse outcome within a median duration of follow-up of 7 days (IQR: 3–12), of whom 40 required mechanical ventilation and 55 died without ventilatory support. Among patients requiring mechanical ventilation, 18 (45%) subsequently died before discharge, leading to an overall in-hospital death rate of 25%.
Median (IQR) FDEP was −2.06 (−3.29; −0.90) for patients who had a favorable outcome and −2.48 (−3.35; −0.59) for those who experienced an adverse outcome; median (IQR) French EDI were 0.39 (−1.59; 6.06) and 0.35 (−2.64; 3.10), respectively.
Median (IQR) saturation at arrival to the emergency department was 93% (88–96%) and desaturation was reported in 180 patients (61%). Overall, 252 (85%) required oxygen support, either at admission or during follow-up. Among them, 136 (46%) required a flow rate of at least 9 L/min. Comorbidities are described in Table 3 ; 43 patients (15%) had no comorbidities and 181 (61%) had at least two comorbidities other than being overweight or obese.
Table 3.
Factors associated with mechanical ventilation or death for 296 patients hospitalized for confirmed COVID-19: Cox proportional analyses.
|
n/N (%)a |
Univariate |
Multivariate |
|||
|---|---|---|---|---|---|
| 95/296 | Crude HR | P-value | Adjusted HR | P-value | |
| Age (years) | |||||
| [< 60[ | 18/94 (19%) | Ref | 0.0992 | Ref | 0.0563 |
| [60–80[ | 36/113 (32%) | 1.4 [0.8–2.5] | 1.5 [0.8–2.7] | ||
| [> 80] | 41/89 (46%) | 1.8 [1.1–3.2] | 2.2 [1.2–4.1] | ||
| Gender | |||||
| Female | 39/118 (33%) | Ref | 0.7645 | – | |
| Male | 56/178 (32%) | 0.9 [0.6–1.4] | |||
| Continent of birth | |||||
| Europe | 57/172 (33%) | Ref | 0.9529 | – | |
| Northwest Africa | 16/50 (32%) | 1.1 [0.6–2.0] | |||
| Sub-Saharan Africa | 12/44 (27%) | 0.9 [0.5–1.7] | |||
| Other | 10/30 (33%) | 1.1 [0.5–2.1] | |||
| Housing | |||||
| Own accommodation | 76/244 (31%) | Ref | 0.8421 | – | |
| Other | 19/52 (37%) | 1.1 [0.6–1.7] | |||
| Deprivation index | |||||
| FDEPb | – | 1.0 [0.9–1.1] | 0.9514 | – | |
| French EDIb | – | 1.0 [0.9–1.0] | 0.5063 | – | |
| Preexisting comorbidities | |||||
| Overweight or obesity | 40/100 (40%) | 1.7 [1.1–2.5] | 0.0168 | 2.1 [1.3–3.4] | 0.0042 |
| High blood pressure | 47/134 (35%) | 1.1 [0.7–1.6] | 0.6484 | – | |
| Type 2 diabetes | 26/75 (35%) | 1.0 [0.7–1.6] | 0.8982 | – | |
| Chronic cardiovascular disease | 33/74 (45%) | 1.7 [1.1–2.6] | 0.0141 | NR | |
| Neuropsychiatric disorder | 31/79 (39%) | 1.1 [0.7–1.7] | 0.7566 | – | |
| Chronic respiratory disease | 20/53 (38%) | 1.3 [0.8–2.1] | 0.3288 | – | |
| Cancer | 10/32 (31%) | 0.9 [0.5–1.7] | 0.7196 | – | |
| ≥ one comorbidity other than being overweight or obese | 85/233 (37%) | 2.2 [1.2–4.3] | 0.0163 | – | |
| Clinical | |||||
| Desaturation at admission | 75/180 (42%) | 2.5 [1.5–4.0] | < 0.0001 | 2.2 [1.3–3.8] | 0.0026 |
| Chest CT scan | |||||
| Not performed | 12/25 (48%) | 2.8 [1.4–5.4] | 0.0020 | 2.4 [1.2–4.8] | 0.0020 |
| Minor lesions | 35/150 (23%) | Ref | Ref | ||
| Major lesions | 48/121 (40%) | 1.8 [1.2–2.8] | 2.2 [1.3–3.5] | ||
| Biology | |||||
| Lymphocyte countc | |||||
| < 500/mm3 | 40/76 (53%) | 8.3 [2.0–33.3] | 0.0026 | NR | |
| ≥ 500/mm3 | 55/220 (25%) | Ref | |||
| Systemic inflammationd | |||||
| CRP peak < 100 | 15/120 (13%) | Ref | < 0.0001 | Ref | < 0.0001 |
| CRP peak [100; 200[ | 29/90 (32%) | 2.3 [1.2–4.2] | 2.5 [1.3–4.6] | ||
| CRP peak ≥ 200 | 22/39 (56%) | 4.3 [2.2–8.4] | 3.3 [1.7–6.4] | ||
| No measurement | 29/47 (62%) | 12.5 [6.6–23.8] | 9.3 [4.6–18.5] | ||
| Acute kidney failurec | 23/34 (68%) | 2.1 [1.3–3.3] | 0.0027 | 2.0 [1.2–3.3] | 0.0077 |
| Treatment | |||||
| Antibioticsc | 70/204 (34%) | 0.8 [0.5–1.3] | 0.3285 | – | |
| Corticosteroidsd | 19/82 (23%) | 0.4 [0.2–0.7] | 0.0003 | 0.5 [0.3–0.8] | 0.0075 |
| Hydroxychloroquined | 7/38 (18%) | 0.4 [0.2–1.0] | 0.0586 | 0.8 [0.3–1.8] | 0.5494 |
Data are presented as medians (IQR) or n (%).
FDEP: French Deprivation Index; French EDI: French European Deprivation Index; CT: computed tomography; CRP: C-reactive protein; HR: hazard ratio; Ref: reference; NR: not retained by the model.
n occurrences of mechanical ventilation or death within N people at risk (proportion mechanically ventilated or who died within the modality).
Hazard ratio is estimated for 1-point increments.
At admission.
During follow-up (time-dependent covariable).
Median (IQR) lymphocyte count was 730 (500–1020)/mm3 overall, 777 (560–1060) for patients with a favorable outcome, and 560 (390–870) for those who experienced an adverse outcome. During follow-up, median (IQR) peak CRP level was 102 mg/L (53–165) overall, 88 (36–141) and 148 (101–211) for patients with a favorable or adverse outcome, respectively. Median creatinine peak was 89 μM/L (68–117) overall, 82 (65–102) and 103 (78–154) according to the outcome, respectively. Acute kidney failure occurred in 34 patients. Chest CT was performed in 271 patients and major lesions (i.e. extent > 25%) were described in 121 patients (45%).
Antibiotics were administered at admission to 204 (69%) patients. Corticosteroids were administered to 127 patients within a median time of 1 day (IQR: 1–3) after admission, among whom 82 received it for ≥ 3 days. There was no difference in demographic characteristics or previous comorbidities between people who were treated with corticosteroids and those who were not, whereas desaturation at admission and severe CT scan lesions were more frequent in patients treated with corticosteroids (P < 0.0001 for both). Hydroxychloroquine (600 mg/day) was administered to 56 patients within a median time of 1 day (IQR: 1–2) after admission, among whom 38 received it for ≥ 3 days. Patients who were prescribed hydroxychloroquine were more frequently men, younger (median age 63 vs. 71), and living in their personal residence, less frequently had comorbidities, had more severe CT scan lesions, and had similar desaturation parameters at admission as patients who were not prescribed hydroxychloroquine.
In the univariate analysis, being older, being overweight or obese, chronic cardiovascular disease, desaturation at arrival to the emergency department, CT not performed or showing major lesions versus minor ones and lymphocyte count ≤ 500/mm3 at admission, peak CRP level ≥ 100 mg/L and acute kidney failure during follow-up, and no treatment with corticosteroids or hydroxychloroquine were associated with a significantly higher risk of mechanical ventilation or death (Table 3). There was no association between geographical origin or socioeconomic deprivation and mechanical ventilation or death.
In the multivariable analysis, being aged above 80 years (aHR: 2.2 [95% CI: 1.2–4.1] vs. below 60 years), being overweight or obese (aHR: 2.1 [1.3–3.4]), desaturation, and major chest CT lesions were associated with a significantly higher risk of mechanical ventilation or death. During hospitalization, higher peak CRP levels and occurrence of acute kidney failure as time-dependent variables were also associated with adverse outcomes, whereas treatment with hydrocortisone reduced the risk of mechanical ventilation or death by half (aHR: 0.5 [0.3–0.8]).
When we excluded from the analyses people who lived in nursing homes or in homes for the disabled, people who were living in deprived areas had a similar risk of adverse outcomes than those not living in deprived areas (HR [95% CI], 1.1 [0.6–1.7], P = 0.84). Other associations were similar (data not shown).
4. Discussion
During the first wave of the pandemic, patients referred to the emergency department of a French university hospital with laboratory-confirmed COVID-19 were more likely to be hospitalized if they were older, male, overweight or obese, or if they suffered from type 2 diabetes or neuropsychiatric disorders. Among hospitalized patients, being older, being overweight or obese, desaturation, major lesions on CT scan at admission, and higher peak CRP or onset of acute kidney failure during follow-up were associated with a higher risk of mechanical ventilation or death. Empirical corticosteroid use reduced the risk of this adverse outcome by half. Geographical origin and socioeconomic deprivation were not associated with either of the two outcomes in our population.
Contrary to what has been suggested by other studies in the general population [2], we found no association between geographical origin or socioeconomic deprivation and severity in our population infected with SARS-CoV-2 attending the emergency department. Black and ethnic minority people appear to be at higher risk of SARS-CoV-2 infection than white people [14], [15]. It must be interpreted carefully because these factors are difficult to disentangle from social factors [16]. However, findings concerning the association between the COVID-19 prognosis and ethnicity are inconsistent, showing either a poorer prognosis for the Black population or no impact in the general population or after hospital admission [17], [18], [19], [20]. No study assessed the risk of hospital admission after attendance to the emergency department or risk of mechanical ventilation or in-hospital death after hospital admission according to geographical origin or socioeconomic deprivation. Ethnicity is a complex factor that encompasses genetic background, behavioral patterns, and immune profiles. This may explain the heterogeneity of the results, with further studies being an urgent matter of public health [21]. Similarly, we found no association with socioeconomic deprivation indices. It could be expected that a higher prevalence of comorbidities (e.g. being overweight or obese) in deprived populations could lead to higher rates of severe forms [22]. Although poor living conditions led to an increased probability of infection, they were not associated with a more severe form of the disease in our population [23], [24].
We assessed two levels of severity in our study, hospitalization and the need for mechanical ventilation or death. After adjustment, our results show a strong association between comorbidities (such as being overweight or obese and type 2 diabetes) and hospitalization, consistent with literature data [1], [24], [25], [26]. Neuropsychiatric disorders have been less frequently highlighted [27], [28]. Being older and overweight or obese were once again significantly associated with unfavorable outcomes in the subgroup of hospitalized patients, underlining their strong and robust influence [2].
Laboratory biomarkers, in particular inflammation markers, can also help in predicting outcomes, as systemic inflammation plays a key role in SARS-CoV-2 physiopathology. Indeed, numerous studies suggest that CRP may predict subsequent respiratory deterioration in COVID-19 [29], [30]. We also show the importance of serum creatinine monitoring to enable early detection of acute kidney injury [31], [32].
The extent of radiological lesions on chest CT scans was associated with a poorer prognosis in our study. The diagnostic value of chest CT scan has been widely studied and has been shown to have high sensitivity, despite low specificity, for detecting COVID-19 [33]. The prognostic value has been less well described. A semi-quantitative CT severity score calculated by summing each of the five individual lobar scores according to the extent of anatomical involvement seems to be an independent predictor of death together with age [34].
Our findings are consistent with those of a randomized clinical trial on hospitalized patients requiring oxygen support and a meta-analysis in critically ill patients, both showing a reduction in death of 20% to 40% associated with corticosteroid use depending on the level of respiratory support [6], [7]. Dexamethasone use slightly reduced the risk of mechanical ventilation or death by 8% (95% CI: −1% to 16%) in people who were not receiving invasive mechanical ventilation at randomization [6]. Our study assessed all hospitalized patients, including those with neurocognitive impairment or psychiatric diseases, and confirms the strong reduction of adverse outcomes with the early prescription of corticosteroids in the context of ‘real life’ care according to the scientific rationale [35], [36], with a 50% reduction in the risk of mechanical ventilation or death. Other specific drug-based treatments, even if prescribed to younger patients with fewer comorbidities, were not associated with a positive outcome [37].
The monocentric design is the main limitation of our study. Our population may not be representative of the French metropolitan population. Our population seems slightly less disadvantaged than those assessed in previous publications focusing on the Paris area or France. However, heterogeneity seems good with representation of both disadvantaged and non-disadvantaged people [10], [11]. Thus, these results must be confirmed by wider studies including more regions and more people. We were unable to assess individual socioeconomic factors, such as education or professional activities. We thus used an ecological design for the socioeconomic element of the study. The very small geographical census allowed a gain in precision when assessing deprivation at an individual level. Nonetheless, in addition to socioeconomic deprivation indices, geographical origin was neither associated with hospitalization nor with adverse outcomes. This is the first study addressing the role of ethnicity and socioeconomic deprivation in the prognosis of COVID-19 in France, a country with universal access to health care [38]. The non-randomized design of the study may weaken our conclusions on the effect of treatments, but our models were largely adjusted for known prognostic factors. The study ended when high-flow oxygen became a standard of care in intensive care units. An assessment of the additional effect of such current oxygen support, either in intensive care units or in standard medical wards for patients who could not benefit from intensive care because of age or comorbidities, would be informative.
5. Conclusion
Our study does not show any link between geographical origin or socioeconomic deprivation and the occurrence of a severe form of COVID-19 in a French hospital after arrival to the emergency department. Public health strategies should facilitate timely access to healthcare resources in migrant groups in order to lower the overall COVID-19 burden in this population. Empirical corticosteroid use with hydrocortisone had a strong protective impact on the occurrence of mechanical ventilation or death.
Human and animal rights
The authors declare that the work described has not involved experimentation on humans or animals.
Informed consent and patient details
The authors declare that this report does not contain any personal information that could lead to the identification of the patient(s) and/or volunteers.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Conceptualization: Anne-Lise Beaumont, Jean-Paul Teglas, Sophie Abgrall.
Methodology: Laurence Meyer, Jean-Paul Teglas, Sophie Abgrall.
Software: Jean-Paul Teglas.
Validation: Anne-Lise Beaumont, Sophie Abgrall.
Formal analysis: Anne-Lise Beaumont, Sophie Abgrall.
Investigation: Dorothée Vignes, Raluca Sterpu, Guillaume Bussone, Imad Kansau, Carole Pignon, Raouia Ben Ismail, Marion Favier, Jean-Luc Molitor, Dorra Braham, Renato Fior, Sandrine Roy, Mathieu Mion, Marc Andronikof, Charles Damoisel, Pierre Chagué, Jean-Charles Aurégan, Nadège Bourgeois-Nicolaos, Christelle Guillet-Caruba, Sophie Abgrall.
Resources: Dorothée Vignes, Raluca Sterpu, Guillaume Bussone, Imad Kansau, Carole Pignon, Raouia Ben Ismail, Marion Favier, Jean-Luc Molitor, Dorra Braham, Renato Fior, Sandrine Roy, Mathieu Mion, Marc Andronikof, Charles Damoisel, Pierre Chagué, Jean-Charles Aurégan, Nadège Bourgeois-Nicolaos, Christelle Guillet-Caruba.
Data curation: Dorothée Vignes, Raluca Sterpu, Guillaume Bussone, Imad Kansau, Carole Pignon, Raouia Ben Ismail, Marion Favier, Jean-Luc Molitor, Dorra Braham, Renato Fior, Sandrine Roy, Mathieu Mion, Marc Andronikof, Charles Damoisel, Pierre Chagué, Jean-Charles Aurégan, Nadège Bourgeois-Nicolaos, Christelle Guillet-Caruba.
Writing – original draft: Anne-Lise Beaumont, Sophie Abgrall.
Writing – review and editing: all authors.
Visualization: Anne-Lise Beaumont, Sophie Abgrall.
Supervision: Sophie Abgrall.
Project administration: Jean-Paul Teglas, Sophie Abgrall.
Funding acquisition: no funding.
Acknowledgments
We would like to acknowledge all patients included in this research.
We gratefully thank Dr. Rey Grégoire for sharing the FDEP score with us and for his advice.
We would also like to thank the Ligue Nationale Contre le Cancer for sharing the French EDI score via the MapInMed platform (https://www.canceropole-nordouest.org/plateforme-mapinmed/) and Ludivine Launay for her help and availability.
References
- 1.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20,133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:440. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:402. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goutte S., Péran T., Porcher T. The role of economic structural factors in determining pandemic mortality rates: evidence from the COVID-19 outbreak in France. Res Int Bus Financ. 2020;54:101281. doi: 10.1016/j.ribaf.2020.101281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P., Lim W., Emberson J., Group R.C. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:E1–E12. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assistance publique des Hôpitaux de Paris, Protection des données personnelles – Information https://www.aphp.fr/protection-des-donnees-personnelles [accessed 31 January 2022].
- 9.Institut national de la statistique et des études économiques. http://www.insee.fr/ [accessed 31 January 2022].
- 10.Rey G., Jougla E., Fouillet A., Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:1–12. doi: 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pornet C., Delpierre C., Dejardin O., Grosclaude P., Launay L., Guittet L., et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66:982–989. doi: 10.1136/jech-2011-200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey A. KDIGO Conference Report. Kidney Int. 2020;97:1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Strehl C., Ehlers L., Gaber T., Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P., et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raisi-Estabragh Z., McCracken C., Bethell M.S., Cooper J., Cooper C., Caulfield M.J., et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020;42:451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajos N. Les inégalités sociales au temps du COVID-19. Quest Sante Publique. 2020;40:65–79. doi: 10.4000/jda.9268. [DOI] [Google Scholar]
- 17.Rose T.C., Mason K., Pennington A., McHale P., Buchan I., Taylor-Robinson D., et al. Inequalities in COVID19 mortality related to ethnicity and socioeconomic deprivation. MedRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.04.25.20079491. [DOI] [Google Scholar]
- 18.Rentsch C.T., Kidwai-Khan F., Tate J.P., Park L.S., King J.T., Skanderson M., et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PloS Med. 2020;17(9):1–17. doi: 10.1371/journal.pmed.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sze S., Pan D., Nevill C.R., Gray L.J., Martin C.A., Nazareth J., et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yehia B.R., Winegar A., Fogel R., Fakih M., Ottenbacher A., Jesser C., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US Hospitals. JAMA Netw Open. 2020;3:e2018039. doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel P., Hiam L., Sowemimo A., Devakumar D., McKee M. Ethnicity and COVID-19. BMJ. 2020;369:1–2. doi: 10.1136/bmj.m2282. [DOI] [PubMed] [Google Scholar]
- 22.Raifman M.A., Raifman J.R. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. Am J Prev Med. 2020;59:137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand S., Montez-Rath M., Han J., Bozeman J., Kerschmann R., Beyer P., et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet (London, England) 2020;6736:1–10. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis N.M., Friedrichs M., Wagstaff S., Sage K., Lacross N., Bui D., et al. Disparities in COVID-19 incidence, hospitalizations, and testing, by area-level deprivation — Utah, March 3–July 9, 2020. MMWR. 2020;69:1369–1373. doi: 10.15585/mmwr.mm6938a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartoletti M., Giannella M., Scudeller L., Tedeschi S., Rinaldi M., Bussini L., et al. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: a multicentre cohort study (PREDI-CO study) Clin Microbiol Infect. 2020;26:1545–1553. doi: 10.1016/j.cmi.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czernichow Sébastien, Beeker Nathanael, Rives-Lange Claire, Guerot Emmanuel, Diehl Jean-Luc, Katsahian Sandrine, et al. AP-HP Covid CDR Initiative. AP-HP / Universities / Inserm COVID-19 research collaboration Obesity doubles mortality in patients hospitalized for SARS-CoV-2 in Paris hospitals, France: a cohort study on 5795 patients. Obesity. 2020:1–2. doi: 10.1002/oby.23014. [DOI] [Google Scholar]
- 27.Ko J.Y., Danielson M.L., Town M., Derado G., Greenlund K.J., Kirley P.D., et al. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2020;11:695–703. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menezes Soares R. de C., Mattos L.R., Raposo L.M. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103:1184–1190. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller A., Tamura T., Crowley C.P., De Grado J.R., Haider H., Jezmir J.L., et al. Inflammatory biomarker trends predict respiratory decline in COVID-19 patients. Cell Reports Med. 2020;100144:19–20. doi: 10.1016/j.xcrm.2020.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evidence-Based Med. 2020;0:1–12. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng Jia H., Hirsch J.S., Hazzan A., Wanchoo R., Shah H.H., Malieckal D.A., et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2020;77(2):204–215. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges do Nascimento I.J., von Groote T.C., O’Mathúna D.P., Abdulazeem H.M., Henderson C., Jayarajah U., et al. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: a systematic review and series of meta-analyses. PLoS One. 2020;15(9):e0239235. doi: 10.1371/journal.pone.0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toussie D., Voutsinas N., Finkelstein M., Cedillo M.A., Manna S., Maron S.Z., et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology. 2020;297:E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villar J., Confalonieri M., Pastores S.M., Meduri G.U. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2:e0111. doi: 10.1097/cce.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fadel R. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2018;6:1–32. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rki V., Destatis U. Social inequalities and COVID-19. J Heal Monit. 2020;7:1–120. [Google Scholar]