Abstract
Background:
Pediatric patients with Crohn disease (CD) are frequently malnourished, yet how this affects surgical outcomes has not been evaluated. This study aims to determine the effects of malnourishment in children with CD on 30-day outcomes after surgery.
Study Design:
The ACS NSQIP-Pediatric database from 2012–2015 was used to select children aged 5–18 with CD who underwent bowel surgery. BMI-for-age Z-scores were calculated based on CDC growth charts and 2015 guidelines of pediatric malnutrition were applied to categorize severity of malnutrition into none, mild, moderate, or severe. Malnutrition’s effects on 30-day complications. Propensity weighted multivariable regression was used to determine the effect of malnutrition on complications.
Results:
516 patients were included: 349 (67.6%) without malnutrition, 97 (18.8%) with mild, 49 (9.5%) with moderate, and 21 (4.1%) with severe malnutrition. There were no differences in demographics, ASA class, or elective/urgent case type. Overall complication rate was 13.6% with malnutrition correlating to higher rates: none 9.7%, mild 18.6%, moderate 20.4%, and severe 28.6% (p<0.01). In propensity-matched, multivariable analysis, malnutrition corresponded with increased odds of complications in mild and severely malnourished patients (mild OR=2.1 [p=0.04], severe OR 3.26 [p=0.03]).
Conclusion:
Worsening degrees of malnutrition directly correlate with increasing risk of 30-day complications in children with CD undergoing major bowel surgery. These findings support BMI-for-age z scores as an important screening tool for preoperatively identifying pediatric CD patients at increased risk for post-operative complications. Moreover, these scores can guide nutritional optimization efforts prior to elective surgery.
Keywords: Pediatric Crohn Disease, Surgery Outcomes, Nutrition, Malnutrition, Inflammatory Bowel Disease
1. Introduction:
Patients with Crohn disease (CD) are frequently malnourished, especially those who require surgical resection [1–6]. CD can lead to growth failure in children and is often an indication for intestinal resection [2]. Fortunately, pediatric patients with CD often have improved growth after resection of diseased intestine [1,2]. In adults, numerous studies have demonstrated that preoperative malnourishment is associated with an increased risk of post-operative complications, especially in CD [4,7–10]. While malnourishment in pediatric CD is common, there are no dedicated pediatric studies evaluating its effects on the risk of post-operative surgical complications. Moreover, there are only a few studies evaluating the effect of malnutrition on surgical outcomes in pediatric patients with the majority focusing on congenital heart defects. Due to the near-absence of relevant pediatric data, current recommendations on the management of pediatric CD are based primarily on inferences from the adult literature and the few studies that do exist in pediatric patients [2,11–16].
Malnutrition in children is different from adults, especially in the setting of CD. First, children require adequate nutrition for normal development and adequate assessment must include nutritional measures. Second, CD can impair overall growth and development. Children with CD often have improved growth after surgical resection of their disease. In contrast, resection of diseased bowel in adult patients may not improve overall nutrition postoperatively. One challenge in studying pediatric malnutrition in the context of surgical disease is the vastly different set of nutritional requirements of pediatric patients [17–20]. Moreover, there is often debate about the best way to measure malnutrition in children because the clinical parameters used in adults often do not correlate well with pediatric malnutrition [17]. For these reasons, it is difficult to extrapolate the interactions between adult nutrition and outcomes after surgery in CD patients to children.
In 2015, the American Society for Parenteral and Enteral Nutrition (ASPEN) published guidelines for defining malnourishment in children [21]. Recommended assessments of nutrition by ASPEN have the following attributes: evidence-based and consensus-derived, universally applied and validated, diverse applications, inexpensive, require minimal training, reproducibly identify and quantify malnutrition (undernutrition), and can monitor changes in nutritional status [21]. While historically malnutrition or failure to thrive was determined by falling off standard growth curves, these assessments took at least two data points to determine [21]. Unfortunately, multiple data points may not be available when pediatric patients present, thus anthropometric growth indices (weight-for-height, length/height, BMI-for-age) z scores are now recommended to assess nutritional status [21]. Of note, the ASPEN guidelines do not include any biochemical markers because these measures often act as acute phase reactants in the setting of acute illness.
Since data on how malnourishment affects post-operative outcomes in pediatric patients with CD is lacking, we sought to determine the effect of malnutrition on postoperative complications using the National Surgical Quality Improvement Program Pediatric (NSQIP-P) database. NSQIP-P tracks 30 day outcomes of selected surgical procedures [22,23]. We hypothesized that worsening malnutrition in pediatric CD patients undergoing major intestinal surgery would be associated with an increasing risk of 30-day post-operative complications.
2. Methods:
2.1. Data source:
This study was exempt from review by the Johns Hopkins University School of Medicine Institutional Review Board. Data from the 2012 to 2015 American College of Surgeons National Surgical Quality Improvement Program Pediatric (NSQIP-P) participant use data file (PUF) was queried to select children with CD undergoing major bowel surgery. NSQIP-P collects over 90 data points from most pediatric surgical subspecialties with the goal of obtaining highly reliable clinical data to compare surgical outcomes across participant hospitals in the program. NSQIP-P samples and collects data in 8 day cycles with 35 procedures per cycle by dedicated clinical abstractors. Demographic information, comorbid diagnoses, laboratory values, operative variables, and 30-day outcomes are recorded. Operations are recorded by their primary procedure Current Procedural Terminology (CPT) codes [24].
2.2. Patient selection:
Patients between 5 and 18 years of age were selected by both a diagnosis code for Crohn disease and procedure code for major bowel surgery. Diagnosis codes were based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 555, 555.0, 555.1, 555.2, 555.9. When available, International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes were used: K50.0 to K50.9. Current Procedural Terminology (CPT) codes were used to capture patients undergoing small bowel resection, ileocecectomy, partial colectomy, total abdominal colectomy, proctectomy, ostomy creation, and ostomy takedown (Appendix 1). The authors felt it important to explore the effects of malnutrition across a broad swath of surgical procedures. Thus, we felt it important to include both ostomy takedowns and ostomy creations in the analysis. Patients with primary perineal disease were not included in the study. We limited our evaluation between the ages of 5–18 due to the lack of certainty in diagnosing CD prior to age 5 (due to the increased incidence of monogenic inflammatory bowel disease [IBD] in this age group) [25]. Thus, nine patients under five years of age were excluded.
2.3. Predictors of outcome
Patient with missing height or weight data were excluded from the study. In NSQIP-P, weight is recorded either preoperatively or if measured within 30 days of an operation. Given the importance weight-based dosing in the pediatric population, the authors presume it is most likely that the majority of patients were weighed preoperatively. Additionally, patients with BMI greater than 40 were excluded from the study because it was felt that extreme outliers were a result of error in data entry. This resulted in the exclusion of 6 patients. The lowest BMI was 11.1 and no patients were excluded based on a low BMI. This left a BMI range of 11.1 to 38.1 with a median 19.1 (interquartile range 17.0–21.2). For each patient, body-mass-index (BMI)-for-age Z-scores were calculated based on Center for Disease Control (CDC) growth charts. Z-scores are defined as the deviation of the value (BMI) for an individual from the mean value of the reference population, divided by the standard deviation for the reference population. The CDC BMI-for Age charts are applicable beginning at 2 years of age when an accurate stature can be obtained (https://www.cdc.gov/growthcharts/percentile_data_files.htm). The 2015 consensus of pediatric malnutrition was used to categorize BMI-for-age-Z-score for patients into levels of malnutrition: none: >−1, mild: −1 to −1.9, moderate: −2 to −2.9, severe: <−3 [21].
Patient factors and comorbidities included age, gender, race, ethnicity, American Society of Anesthesiologists (ASA) class (<3, ≥3), case status (elective vs. urgent/emergent), preexisting cardiac, respiratory, neurologic, or renal comorbidities, preoperative parenteral nutrition (PN), preoperative sepsis (defined as meeting systemic inflammatory response syndrome within 48 hours of surgery), preoperative blood transfusion, preoperative steroid use, preoperative immunosuppressive medications, or preoperative hypoalbuminemia. Cardiac comorbidities include any minor, major, and severe cardiac risk factors as defined by NSQIP-P. Respiratory comorbidities included the presence of asthma, cystic fibrosis, history of chronic lung disease, oxygen supplementation, preexisting tracheostomy, structural pulmonary abnormality, preexisting pneumonia, or ventilator requirement. Neurologic comorbidity included preexisting coma, cerebral vascular accident, impaired cognitive status, seizure, cerebral palsy, structural central nervous system abnormality, neuromuscular disorder, or intraventricular hemorrhage. Renal comorbidity included patients with preexisting renal failure or dialysis dependence. Immunosuppression included patients regularly taking immunosuppressant medications such as for chronic inflammatory conditions, chemotherapy, or transplant medications. Steroid use was defined as having oral or parenteral corticosteroid medication in the 30 days prior to surgery. Information on duration of treatment or strength of dose were not available.
2.4. Outcomes
The primary outcomes of interest were development of a post-operative complication, reoperation, or readmission within 30 days of surgery. Given the small number of individual complications, a composite outcome of post-operative complications was used which included any wound infection or dehiscence, neurological complication, respiratory complication (pneumonia, unplanned reintubation, pulmonary embolism, new oxygen requirement on discharge, prolonged ventilator requirement >24 hours), renal complication, septic shock, central line infection, urinary tract infection, or death. Post-operative and total hospital length of stay were also recorded.
2.5. Statistical analysis
The association of preoperative risk factors and postoperative outcomes across degrees of malnutrition were compared using Fisher’s exact test or Pearson’s Chi-squared test where appropriate for categorical variables and Kruskal-Wallis equality of populations rank test for continuous variables. Potential covariates for multivariable regression were identified by univariate statistical significance and biological plausibility for confounding (e.g., immunosuppression, albumin).
An a priori propensity score weight-adjusted analysis of primary data was then performed in R 3.4.1 (R Project for Statistical Computing, Vienna, Austria) to address potential differences in underlying covariates’ clustering across malnutrition severity. A previously reported multinomial propensity score weighting without matching methodology [26] was used via the mnps function of the twang package (RAND Corporation, Santa Monica, California) loaded into R Studio 1.01.153 (R Studio, Boston, MA) to assign each individual patient a propensity score relative to their individual representativeness of his or her respective malnutrition state. Propensity scores were estimated via a generalized boosted model – a 3,000-iteration tree-based regression model – with a balance rule based on minimizing the absolute standardized mean difference of all iterative pairwise comparisons. Propensity scores were trimmed to remove outlier cases with inverse probability scores greater than then 99th percentile as has been previously proposed [27].
Regression models were constructed using backwards stepwise logistic regression on potential covariates with an inclusion threshold of p < 0.20 and a significance definition of p < 0.05. Variables that a priori were identified by the authors to have a high biological likelihood for confounding (e.g., sex, age, operation type) were included into the model regardless of p value. All regression analyses were performed using Stata version 15 (StataCorp 2017, College Station, TX).
3. Results:
A total of 516 patients were included in the study, of which 97 (18.8%) had mild, 49 (9.5%) had moderate, and 21 (4.1%) had severe malnutrition. 349 (67.6%) were not malnourished by our criteria. Median age was 15.5 years and 218 (42.3%) were female. The majority of patients were white (82.4%). Elective cases were more common (87.8%) and ileocecectomy was the most common operation (45.5%). For the total cohort, 197 (38.2%) were on nonsteroidal immunosuppressive medication, 160 (31.0%) had preoperative steroid use 30 days prior to surgery, and 109 (21.1%) were on PN preoperatively. There were no differences in age, gender, ethnicity, ASA class, or case type (elective vs. urgent/emergent) across groups. The distribution of case types is also presented in Table 1. There was an increasing association of preoperative PN (p=0.01), preoperative sepsis (p=0.01), and preoperative blood transfusions (p=0.02) with worsening malnutrition, with the highest rates in the severely malnourished groups. Cardiac, respiratory, neurologic, and renal abnormalities were rare overall, with no difference seen across malnutrition groups. Preoperative hypoalbuminemia was the highest in the severely malnourished group (68.4%) compared to the moderate (41.9%), mild (45.2%), and non-malnourished (37.1%) groups although this trend was not statistically significant (p=0.06). Of note, preoperative albumin data was missing in 200 patients.
Table 1.
Patient Characteristics by Degree of Malnutrition
| Degree of Malnutrition | ||||||
|---|---|---|---|---|---|---|
| Total N=516 | None N=349 | Mild N=97 | Moderate N=49 | Severe N=21 | p-value | |
| Age, median (IQR) | 15.5 (13.7–16.7) | 15.6 (13.9–16.7) | 15.4 (13.6–16.9) | 15.0 (13.6–16.6) | 15.7 (14.5–16.7) | 0.71 |
| Female n(%) | 218 (42.3) | 153 (43.8) | 32 (33) | 25 (51.0) | 8 (38.1) | 0.14 |
| Race n(%) | 0.02 | |||||
| White | 425 (82.4) | 281 (80.5) | 85 (87.6) | 44 (89.8) | 15 (71.4) | |
| Black | 55 (10.7) | 47 (13.5) | 5 (5.2) | 1 (2.0) | 2 (9.5) | |
| Other | 4 (0.8) | 4 (1.1) | 0 (0) | 0 (0) | 0 (0) | |
| Unknown | 32 (6.2) | 17 (4.9) | 7 (7.2) | 4 (8.2) | 4 (19.1) | |
| Hispanic n(%) | 22 (4.3) | 12 (3.5) | 6 (6.3) | 3 (6.4) | 1 (4.8) | 0.41 |
| ASA class n(%) | 0.19 | |||||
| <3 | 303 (58.7) | 212 (60.7) | 58 (59.8) | 24 (49.0) | 9 (42.9) | |
| ≥ 3 | 213 (41.3) | 137 (39.3) | 39 (40.2) | 25 (51.0) | 12 (57.1) | |
| Case type n(%) | 0.42 | |||||
| Elective | 453 (87.8) | 308 (88.3) | 85 (87.6) | 44 (89.8) | 16 (76.2) | |
| Urgent/Emergent | 63 (12.2) | 41 (11.8) | 12 (12.4) | 5 (10.2) | 5 (23.8) | |
| Operation type n(%) | 0.24 | |||||
| Ostomy takedown | 33 (6.4) | 28 (8.0) | 2 (2.1) | 2 (4.1) | 1 (4.8) | |
| Ostomy only | 35 (6.8) | 21 (6.0) | 6 (6.2) | 5 (10.2) | 3 (14.3) | |
| Small bowel only | 79 (15.3) | 51 (14.6) | 14 (14.4) | 10 (20.4) | 4 (19.1) | |
| Partial colectomy | 87 (16.9) | 53 (15.2) | 22 (22.7) | 8 (16.3) | 4 (19.1) | |
| Total abdominal colectomy | 44 (8.5) | 26 (7.5) | 14 (14.4) | 4 (8.2) | 0 (0) | |
| Proctectomy | 3 (0.6) | 2 (0.6) | 0 (0) | 1 (0) | 0 (0) | |
| Ileocecectomy | 235 (45.5) | 168 (48.1) | 39 (40.2) | 19 (38.8) | 9 (42.9) | |
| Comorbidity n(%) | ||||||
| Cardiac | 4 (0.8) | 3 (0.9) | 1 (1.0) | 0 (0) | 0 (0) | 1.00 |
| Respiratory | 24 (4.7) | 21 (6.0) | 2 (2.1) | 0 (0) | 1 (4.8) | 0.14 |
| Neurologic | 21 (4.1) | 14 (4.0) | 3 (3.1) | 3 (6.1) | 1 (4.8) | 0.70 |
| Renal | 1 (0.2) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Immunosuppression n(%) | 197 (38.2) | 132 (37.8) | 34 (35.1) | 22 (44.9) | 9 (42.9) | 0.65 |
| Preoperative steroid use n(%) | 160 (31.0) | 104 (29.8) | 33 (34.0) | 13 (26.5) | 10 (47.6) | 0.29 |
| Preoperative PN n(%) | 109 (21.1) | 60 (17.2) | 27 (27.8) | 14 (28.6) | 8 (38.1) | 0.01 |
| Preoperative SIRS/sepsis n(%) | 29 (5.6) | 17 (4.9) | 6 (6.2) | 1 (2.0) | 5 (23.8) | 0.01 |
| Preoperative blood transfusion n(%) | 6 (1.2) | 2 (0.6) | 2 (2.1) | 0 (0) | 2 (9.5) | 0.02 |
| Preoperative Hypoalbuminemia* | 129 (41.1) | 75 (37.1) | 28 (45.2) | 13 (41.9) | 13 (68.4) | 0.06 |
IQR= interquartile range, ASA=American Society of Anesthesiologists, PN=parenteral nutrition, SIRS=systemic inflammatory response syndrome
For preoperative albumin, there are 200 missing values in the data, the total sample size for this row is 312
There was a total of 68 (13.2%) post-operative complications, 27 (5.2%) reoperations, and 36 (7.0%) readmissions (Table 2). Of note, there were no post-operative deaths in this cohort. Comparing by degrees of malnutrition, the rate of post-operative complications increased with each degree of worsening malnutrition (p=0.01). Similarly, worsening malnutrition was associated with an increasing rate of reoperations (p=0.04). There was no difference in readmission rates by malnutrition (p=0.37). Median total length of stay and post-operative length of stay were longer for the severely malnourished group compared to other degrees of malnutrition (p=<0.01).
Table 2.
30-day Outcomes by Degree of Malnutrition
| Degree of Malnutrition | ||||||
|---|---|---|---|---|---|---|
| Outcomes | All Patients | None | Mild | Moderate | Severe | p-value |
| Complication n(%) | 68 (13.2) | 34 (9.7) | 18 (18.6) | 10 (20.4) | 6 (28.6) | 0.01 |
| Reoperation n(%) | 27 (5.2) | 13 (3.7) | 6 (6.2) | 5 (10.2) | 3 (14.3) | 0.04 |
| Readmission n(%) | 36 (7.0) | 22 (6.3) | 6 (6.2) | 6 (12.2) | 2 (9.5) | 0.37 |
| Total LOS, days median (IQR) | 6 (4–10) | 6 (4–8) | 6 (4–13) | 6 (4–13) | 12 (6–19) | <0.01 |
| Post-operative LOS, days, median (IQR) | 5 (4–7) | 5 (4–7) | 5 (4–8) | 5 (4–8) | 7 (5–10) | <0.01 |
SSI=surgical site infection, DVT=deep venous thrombosis, IQR=interquartile range, LOS=length of stay (hospital)
Table 3 shows analysis for preoperative risk factors for post-operative complications, finding that ASA class 3 or greater, urgent/emergent case, preoperative sepsis, and preoperative hypoalbuminemia were associated with increased risk of complications. Diagnostic tests of post-propensity score balance are included in Appendix 3. Propensity-weighted unadjusted and adjusted odds ratios for complications are shown in Table 4. In our model, the overall trend is that with worsening levels of malnutrition, the odds of complication increase. This was statistically significant for mild and severely malnourished groups, but statistical significance was not obtained in the moderate group. In addition, having an urgent/emergent operation increased the odds of complication.
Table 3.
Risk Factors for 30-Day Post-Operative Complications
| Complication | ||||
|---|---|---|---|---|
| Variable | Yes | No | p-value | |
| Age, median (IQR) | 15.5 (14.2–16.8) | 15.5 (13.7–16.7) | 0.55 | |
| Sex n(%) | Female | 29 (13.3) | 189 (86.7) | 1.00 |
| Male | 39 (13.1) | 259 (86.9) | ||
| Race n(%) | White | 57 (13.4) | 368 (86.6) | 0.71 |
| Black | 7 (12.7) | 48 (87.3) | ||
| Other | 1 (25.0) | 3 (75.0) | ||
| Unknown | 3 (9.4) | 29 (90.6) | ||
| Hispanic n(%) | Yes | 3 (13.6) | 19 (86.4) | 1.00 |
| No | 64 (13.3) | 419 (86.8) | ||
| ASA class n(%) | <3 | 30 (9.9) | 273 (90.1) | 0.01 |
| ≥ 3 | 38 (17.8) | 175 (82.2) | ||
| Elective case n(%) | Yes | 52 (11.5) | 401 (88.5) | <0.01 |
| No | 16 (25.4) | 47 (74.6) | ||
| Comorbidities n(%) | ||||
| Cardiac | Yes | 0 (0) | 4 (100.0) | 1.00 |
| No | 68 (13.2) | 444 (86.8) | ||
| Respiratory | Yes | 4 (16.7) | 20 (83.3) | 0.54 |
| No | 64 (13.0) | 428 (87.0) | ||
| Neurologic | Yes | 3 (14.3) | 18 (85.7) | 0.75 |
| No | 65 (13.1) | 430 (86.9) | ||
| Renal | Yes | 0 (0) | 1 (100.0) | 1.00 |
| No | 68 (13.2) | 447 (86.8) | ||
| Immunosuppression n(%) | Yes | 23 (11.7) | 174 (88.3) | 0.50 |
| No | 45 (14.1) | 274 (85.9) | ||
| Preoperative steroid use n(%) | Yes | 28 (17.5) | 132 (82.5) | 0.07 |
| No | 40 (11.2) | 316 (88.8) | ||
| Preoperative PN n(%) | Yes | 19 (17.4) | 90 (82.6) | 0.15 |
| No | 49 (12.0) | 358 (88.0) | ||
| Preoperative SIRS/sepsis n(%) | Yes | 8 (27.6) | 21 (72.4) | 0.04 |
| No | 60 (12.3) | 427 (87.7) | ||
| Preoperative blood transfusion n(%) | Yes | 2 (33.3) | 4 (66.7) | 0.18 |
| No | 66 (12.9) | 444 (87.1) | ||
| Preoperative hypoalbuminemia* | Yes | 26 (20.2) | 103 (79.8) | <0.01 |
| No | 17 (9.2) | 168 (90.8) | ||
IQR=interquartile range, ASA=American Society of Anesthesiologists, PN=parenteral nutrition, SIRS=systemic inflammatory response syndrome
For preoperative albumin, there are 202 missing values in the data, the total sample size for this row is 314
Table 4.
Propensity-Weighted Unadjusted and Adjusted Odds Ratios for Post-operative Complications.
| Variable | Unadjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Mild Malnutrition | 1.99 | 1.02–3.91 | 0.04 | 2.10 | 1.04–4.24 | 0.04 |
| Moderate Malnutrition | 1.32 | 0.54–3.21 | 0.54 | 1.38 | 0.56–3.42 | 0.49 |
| Severe Malnutrition | 3.57 | 1.16–10.99 | 0.03 | 3.26 | 1.11–9.58 | 0.03 |
| Gender | 0.85 | 0.45–1.60 | 0.61 | 0.75 | 0.39–1.42 | 0.37 |
| Age | 0.92 | 0.49–1.73 | 0.79 | 1.02 | 0.53–1.94 | 0.95 |
| Operation Type | 0.81 | 0.32–2.08 | 0.67 | 0.65 | 0.25–1.68 | 0.38 |
| Urgent/Emergent Case | 3.86 | 1.76–8.46 | <0.01 | 4.12 | 1.86–9.32 | <0.01 |
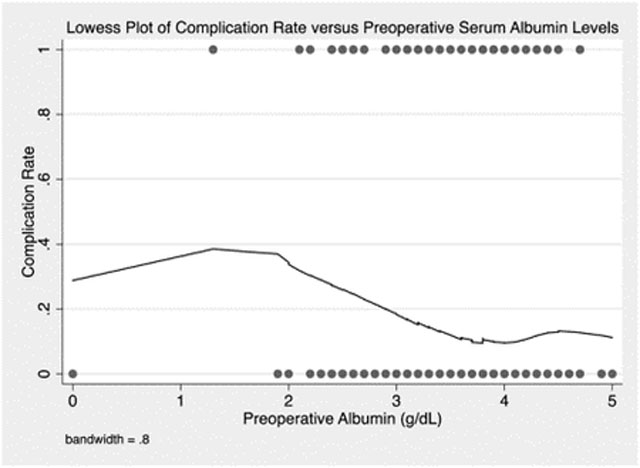
While performing analysis of the effects of albumin levels on complications we observed a large effect of low serum albumin on complications (Table 1). Knowing that albumin levels often overlap or correlate to nutrition status, especially in the adult population, we sought to determine the interactions of albumin and BMI-for-age z scores on complications. Unfortunately, albumin levels were not available for 200 (39.1%) of our patient cohort, but stratified subgroup analysis by albumin level was performed with the information available (Table 5). An albumin cutoff for hypoalbuminemia of 3.5 g/dL was selected based on analysis with a Lowess plot of post-operative complications vs. preoperative albumin levels (Appendix 2). This analysis demonstrated that in patients with hypoalbuminemia, degree of malnutrition was not associated with increased odds of complications. However, in patients with normal serum albumin, a trend towards increased odds of complications with worsening malnutrition was observed with severe malnutrition reaching statistical significance.
Table 5.
Propensity-Weighted Adjusted Odds Ratio of Complications in Patients with Hypoalbuminemia versus Normal Serum Albumin
| Albumin < 3.5 g/dL | |||
|---|---|---|---|
| Variable | OR | 95% CI | p-value |
| Mild Malnutrition | 1.68 | 0.55–5.14 | 0.36 |
| Moderate Malnutrition | 1.35 | 0.28–6.46 | 0.71 |
| Severe Malnutrition | 1.13 | 0.26–4.82 | 0.87 |
| Age | 0.84 | 0.27–2.67 | 0.77 |
| Operation Type | 1.86 | 0.35–10.05 | 0.47 |
| Urgent/Emergent | 3.86 | 1.36–10.98 | 0.01 |
| ASA Class | 4.25 | 1.29–13.97 | 0.02 |
| Albumin ≥ 3.5 g/dL | |||
| Variable | OR | 95% CI | p-value |
| Mild Malnutrition | 3.32 | 0.84–13.12 | 0.09 |
| Moderate Malnutrition | 1.50 | 0.31–7.14 | 0.61 |
| Severe Malnutrition | 24.22 | 2.33–251.95 | 0.01 |
| Age | 0.68 | 0.18–2.49 | 0.56 |
| Operation Type | 0.67 | 0.74–6.02 | 0.72 |
| Urgent/Emergent | 4.67 | 1.07–20.45 | 0.04 |
| ASA Class | 0.45 | 0.14–1.47 | 0.19 |
| Immunosuppressed | 0.35 | 0.09–1.42 | 0.14 |
4. Discussion:
Malnutrition has been known to increase the risk of post-operative complications for decades [3,4,8,17,28]. However, nearly all these studies were performed in adults. Few studies have closely investigated the effect of preoperative malnourishment on postoperative surgical complications in children [11–16]. In fact, we found only one study that evaluates the effects of preoperative malnutrition on pediatric general surgical procedures, but there are no specific studies for children with Crohn disease [11]. A recent review by Wessner and Burjonrappa highlight the paucity of data on nutrition in the pediatric surgical population [17]. To our knowledge, this is the first study to evaluate how nutrition affects surgical outcomes in pediatric patients with CD. Our findings suggest that BMI-for-age z score can serve as a useful tool for identifying pediatric CD patients at increased risk for a post-operative complication, thus allowing opportunity for preoperative optimization to mitigate this risk.
NSQIP-P provides a valuable opportunity to assess malnutrition on surgical outcomes in children with CD using a national clinical database. Using the guidelines for malnourishment from ASPEN [21], we found that severity of malnutrition does increase the risk of post-operative complications after major bowel surgery in this population. Since children with CD are prone to malnutrition and disease progression, this study provides important information for counseling pediatric CD patients about their surgical risk and potentially allowing earlier or more aggressive interventions to improve nutritional parameters. As malnutrition has known deleterious effects on hematopoiesis and the immune system [29–31], the increased rates of sepsis and the need for blood transfusion found in this study are not surprising. Moreover, while only associations between malnourishment, hematopoiesis, and immune function are presented here, given that malnutrition is a known attributable cause of global immunosuppression in surgical patients, it is biologically plausible that malnutrition influenced these effects directly [32–36].
The complication rate in this cohort was 13.6% which is lower than other reports in the literature (22–77%) [37–39]. Of interest, Hansen et al. recently demonstrated that patients with ileal or ileocecal resection only had lower complication rates (6% and 24% respectively) compared to children undergoing hemi- or total colectomy (42% and 52% respectively) [38]. A relatively high proportion of our cohort had small bowel resection only (15.3%) or ileocecectomy (45.5%) perhaps accounting for our lower overall complication rate. The majority of the observed complications were infectious in nature or post-operative blood transfusions.
The propensity-weighted, adjusted model demonstrated a trend of increased odds of post-operative complications with worsening malnutrition. The mildly malnourished and severely malnourished odds reached statistical significance, however, the moderately malnourished group did not. There are several potential explanations for this finding. First, the mildly malnourished group may not have been recognized as malnourished. Thus, they may have been less likely to be nutritionally optimized preoperatively which put them at risk for complications. On the other end of the spectrum, the severely malnourished group is likely to be chronically malnourished, have multiple risk factors for complications, and be more likely to require urgent operations. Therefore, the severely malnourished group would be at higher risk for complications. In contrast, the moderate group may have presented with signs of malnutrition which were recognized resulting in their nutritional optimization prior to surgery. Hence, the moderately malnourished patients may represent the only group that is both recognized as malnourished and has the benefit of time for the condition to be treated thereby resulting in fewer 30 day complications. This explanation is limited by the lack of existing optimization data and is an important area of future research.
Given that albumin is a commonly used marker of malnutrition in adult patients, we thought it prudent to evaluate its associations with post-operative complications in pediatric CD patients. As already mentioned, ASPEN guidelines do not recommend using albumin to assess nutritional status in children because it is an acute phase reactant. This point is relevant and seems to be the predominant practice pattern given that we had a significant proportion of children that had no preoperative albumin levels available (200 patients were missing albumin values). Analysis did reveal that hypoalbuminemia is associated with increased complications, a well-established phenomenon [40]. Interestingly, in the subset of patients with hypoalbuminemia, the anthropometric BMI-for-age z score no longer predicted post-operative complications. However, in the subset with normal albumin, BMI-for-age z score values were important. These findings have several potential implications. First, these findings suggest that albumin may in fact be a useful marker in this population for worse surgical outcomes and that albumin may be linked to poor nutrition. Alternatively, it may, as has been shown in the literature, only be a marker of acute illness and metabolic response to injury that portends worse surgical outcome [40]. In other words, hypoalbuminemia may only directly indicate a malnourished state in the subset of CD patients without active disease that require resection since those with ongoing acute or chronic inflammation are likely to have alterations in their serum albumin levels as a result of the inflammation. Second, while low serum albumin correlates to worse surgical outcomes in this subset, it is insufficient for capturing all malnourished patients that may have worse outcomes. This is evident by the fact that those children with normal serum albumin and severe malnutrition by BMI-for-age z score have increased odds of complications. This finding highlights the inadequacy of albumin as a marker of nutrition as there are many reasons for malnourished patients to have normal serum albumin such as having received intravenous albumin, being severely dehydrated, or having recently received parenteral nutrition thereby increasing their albumin stores. While these findings are of great interest, they are not definitive and will require further study to fully elucidate their significance.
In children with Crohn disease, optimization of nutritional status is not always possible, especially in the acute setting where one would need an emergent operation. However, the majority of patients do not require emergent operations and attempts to nutritionally optimize these patients preoperatively should be carried out when possible, using the ASPEN recommendations as a goal. It is also important to note that some patients may be refractory to nutrition interventions due to growth failure from their disease. In these cases, deciding to perform an operation before developing severe malnutrition could provide a safer operation. The benefits of nutritional optimization have to be carefully weighed against the risk of treatment or treatment delay. For instance, prolonged use of parenteral nutrition is associated with an increased risk of sepsis, both through a central line associated blood stream infection and by inducing gut mucosal atrophy, predisposing to mucosal bacterial translocation [41]. Further prospective studies will be needed to determine if tracking changes in anthropometric measures (such as BMI-for-age, weight-for-age) is feasible and results in improved outcomes.
These findings must be interpreted in light of limitations within the study and NSQIP-P. Using BMI for age z-scores is a useful screening tool for malnutrition but does not provide the complete picture for a patient’s nutritional status which is influenced by active disease including inflammation, malabsorption, or effects from disease treatment including steroids and immunosuppressive agents. Important components that are not available in NSQIP-P include duration and dosage of immunosuppression, etiology of sepsis, details of PN administration, and quantitative measure of degree of tissue injury or inflammation [22,42]. In this study, while no overall difference was found between use of immunosuppressive agents and malnutrition, lack of data on duration and dosage limits our ability to account for these agents affecting malnutrition and other factors such as preoperative SIRS/sepsis. Also, weight measurements alone can be unreliable in relation to fluid status; a particularly important point for patients with active inflammation leading to significant third spacing. Measurements such as repeated weight measures over time and mid-upper arm circumference are useful adjuncts in these circumstances, but are not available in NSQIP-P. It should also be noted that weight is likely to be affected by nutritional status prior to height and so BMI-for-age z score may not be as sensitive as other measures of malnutrition that are made over time. NSQIP-P does not temporally track weight, height, nor BMI. Thus, the authors are not able to distinguish between catabolic cachexia of disease and malnutrition. In addition, growth failure or delay, another component of nutrition, is difficult to capture in a retrospective database. While NSQIP-P has a highly reliable process of data entry, there still remains variation in physician coding [43]. Although we have observed significant associations, retrospective data do not allow us to define causality. These potential confounding effects are addressed with binary categorical variables (e.g., with or without preoperative sepsis) to the extent possible. While it is known that there can be variations in complications from hospital to hospital or surgeon to surgeon, NSQIP-P does not provide hospital site specific nor surgeon-level data to study variation in care. Finally, while we did find that rates of reoperation and median length of stay were increased in malnourished patients, this study was not adequately powered to perform multivariable models.
5. Conclusion:
Worsening degrees of malnutrition directly correlate with increasing risk of 30-day complications in children with CD undergoing major bowel surgery. After adjustment, mild and severe malnutrition had increased odds of complications after surgery. Additionally, albumin was found to be a powerful predictor for post-operative complications in this population but more investigation is needed to determine its interaction with malnutrition. This is the first study evaluating the effects of nutritional status on surgical outcomes in pediatric patients with CD. These data suggest that BMI-for-age Z-scores could be used to identify pediatric CD patients at high risk of post-operative complications due to malnutrition. Further studies are needed to determine whether BMI-for-age z scores can be used to guide nutritional optimization before major surgery in this population. Regardless, this study suggests that malnourished pediatric CD patients should have their nutritional status optimized prior to surgery when possible.
6. Acknowledgements:
The work of Ira Leeds on this project was supported from the NCI T32 grant 5T32CA126607. Mitchell Ladd received salary support during his contribution to this study under a National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases T32 training grant (2T32DK007713-21).
Abbreviations:
- CD
Crohn Disease
- IBD
Inflammatory Bowel Disease
- ASPEN
American Society for Parenteral and Enteral Nutrition
- NSQIP-P
National Surgical Quality Improvement Program Pediatric
- BMI
body-mass-index
Appendix 1.
ICD-9 and ICD-10 codes for Crohn disease and CPT codes for operations evaluated.
| Codes | |
|---|---|
| ICD-9, ICD-10 | 555, 555.1, 555.2, 555.9, K50, K50.0, K50.00, K50.01, K50.011, K50.012, K50.013, K50.014, K50.018, K50.019, K50.1, K50.10, K50.11, K50.12, K50.13, K50.14, K50.18, K50.19, K50.111, K50.112, K50.113, K50.114, K50.118, K50.119, K50.8, K50.80, K50.81, K50.811, K50.812, K50.813, K50.814, K50.818, K50.819, K50.9, K50.90, K50.91, K50.911, K50.912, K50.913, K50.914, K50.918, K50.919 |
| Ostomy Takedown | 44620, 44625, 44626 |
| Ostomy Only | 44310, 44187, 44320, 44322, 44188 |
| Small Bowel | 44120, 44125, 44130, 44202, 44615 |
| Partial Colectomy | 44204, 44140, 44141, 44143, 44144, 44145, 44146, 44206, 44207, 44208 |
| Total Abdominal Colectomy | 44150, 44151, 44155, 44156, 44157, 44158, 44210, 44211, 44212 |
| Proctectomy | 45110, 45111, 45112, 45113, 45114, 45119, 45120, 45123, 45397, 45499 |
| Ileocecectomy | 44160, 44205 |
Appendix 2
Appendix 3
Figure A3.1.
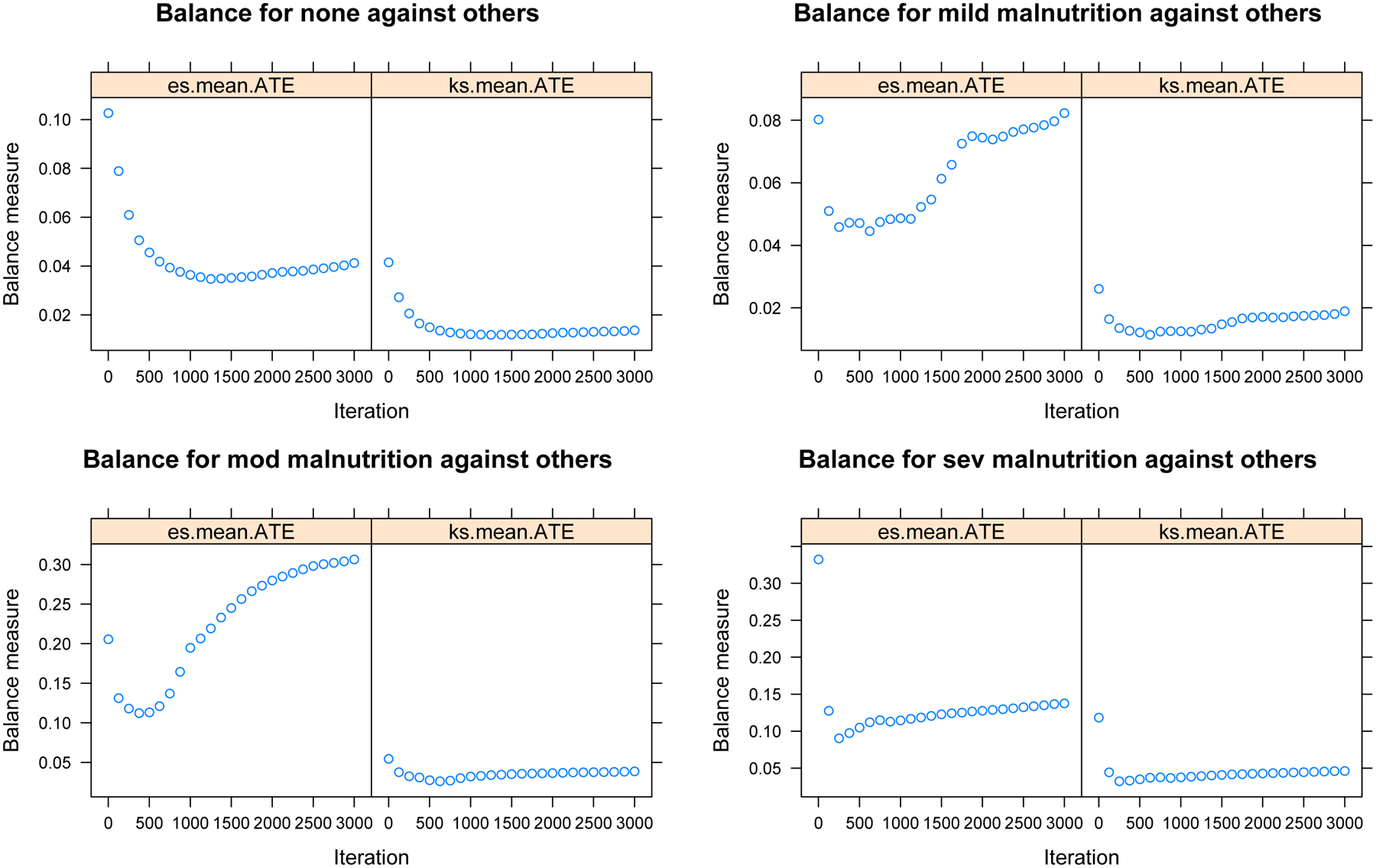
Plotted Balance Metrics for Absolute Standardized Mean Differences (“es.mean”) and Kolmogorov-Smirnov Statistic (“ks.mean”) Demonstrating Asymptotic Approach to Optimal Balance
Figure A3.2.
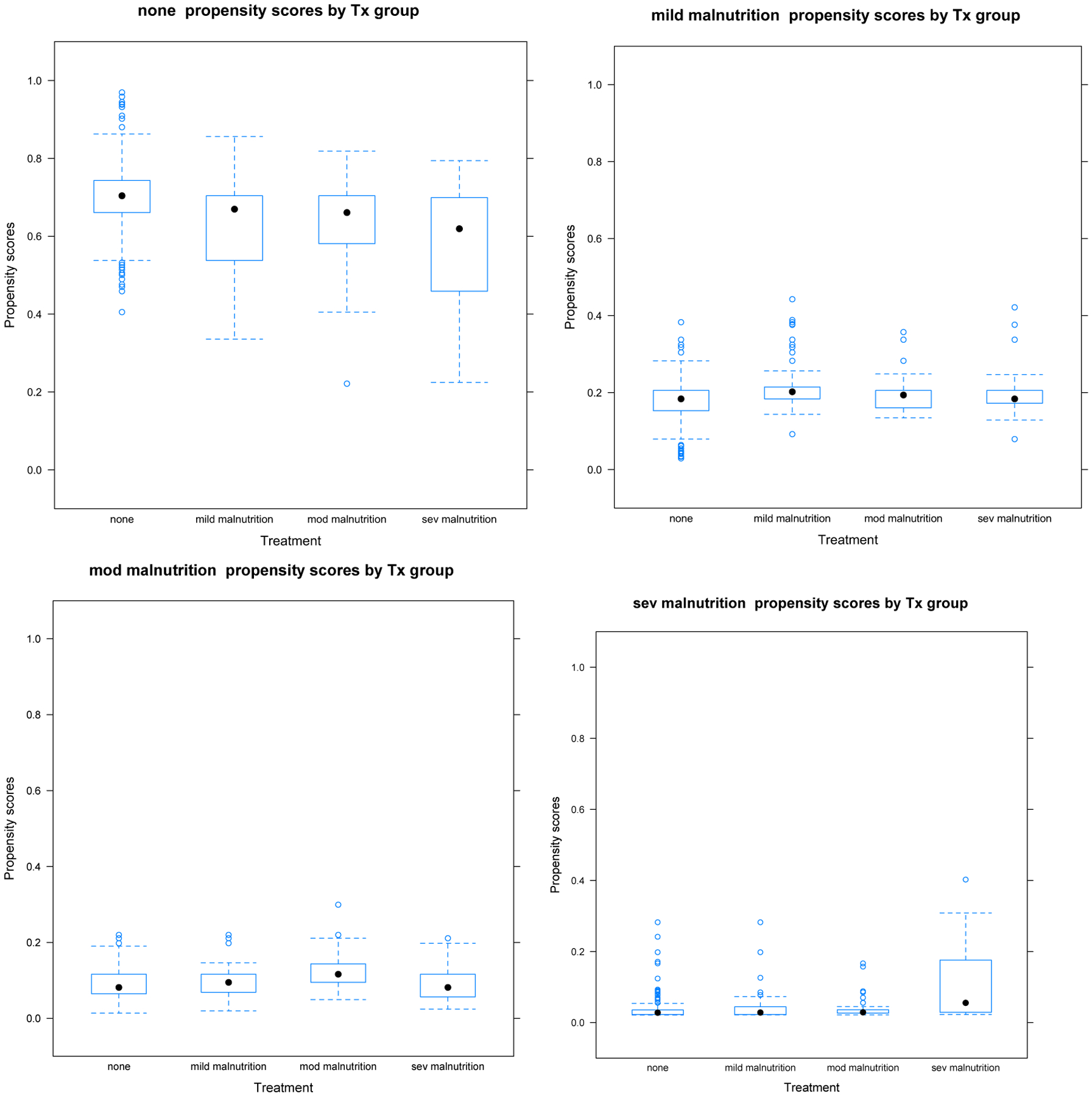
Propensity Score Distributions of Each Malnutrition Category on the Four Others Demonstrating Appropriate Non-Zero Overlap Between All Groups
Figure A3.3.
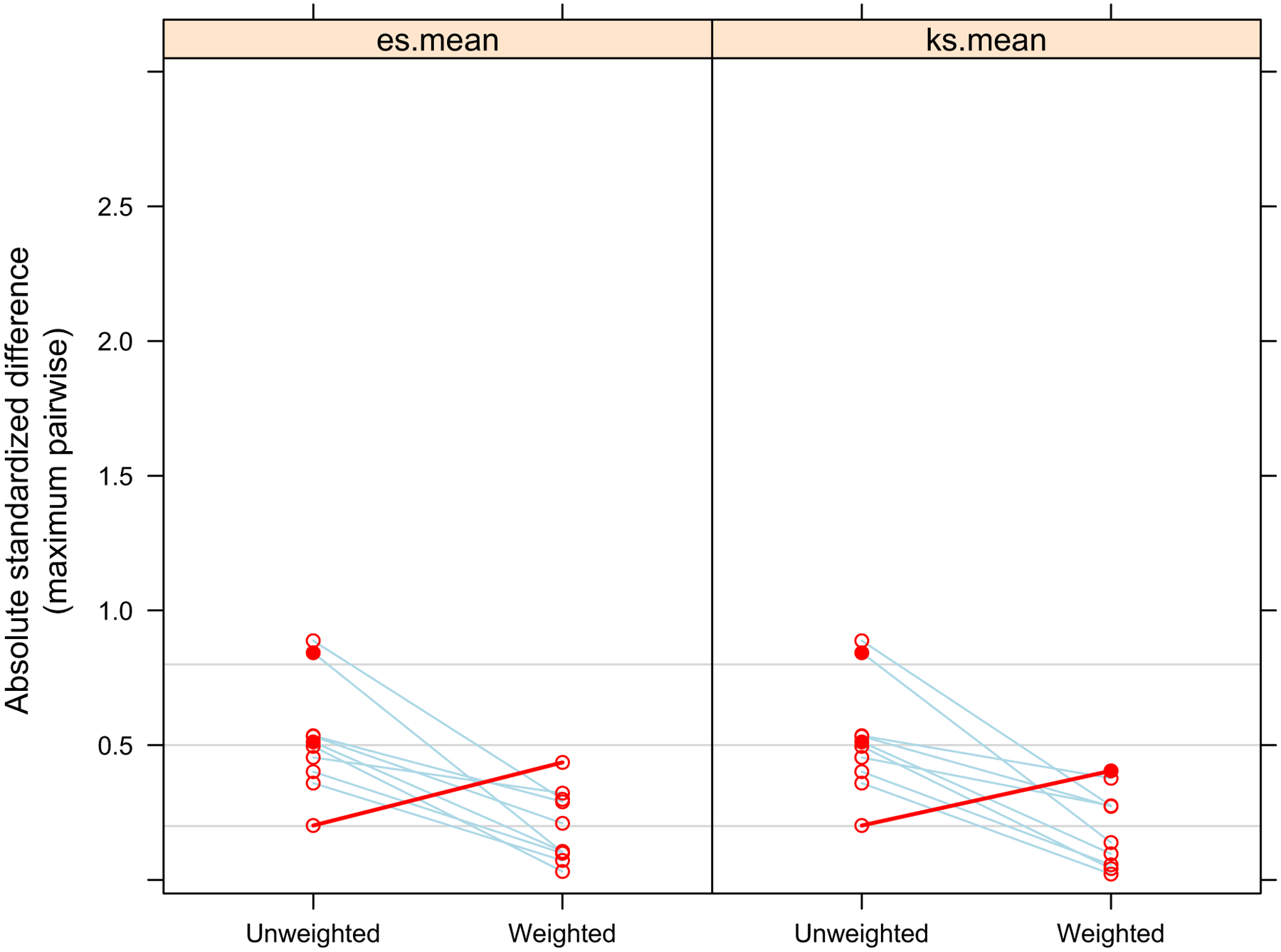
Comparison of Overall Absolute Standardized Mean Difference of Covariates Between Malnutrition Groups Before and After Propensity Score Weighting
Figure A3.4.
t- and Chi- square p-values for Differences in Covariates between Malnutrition Groups Before (solid dot) and After (hollow dot) Propensity Score Weighting
Footnotes
Presented at the American Academy of Pediatrics Section on Surgery and the American College of Surgeons
Level of Evidence: IV
References:
- [1].Diederen K, de Ridder L, van Rheenen P, Wolters VM, Mearin ML, Damen GM, et al. Complications and Disease Recurrence After Primary Ileocecal Resection in Pediatric Crohnʼs Disease. Inflamm Bowel Dis 2017;23:272–82. doi: 10.1097/MIB.0000000000000999. [DOI] [PubMed] [Google Scholar]
- [2].Amil-Dias J, Kolacek S, Turner D, Pærregaard A, Rintala R, Afzal NA, et al. Surgical Management of Crohn Disease in Children - Guidelines from the Paediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr 2017;64:1. doi: 10.1097/MPG.0000000000001562. [DOI] [PubMed] [Google Scholar]
- [3].Efron JE, Young-Fadok TM. Preoperative Optimization of Crohn Disease. Clin Colon Rectal Surg 2007;20:303–8. doi: 10.1055/s-0033-1348044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alves A, Panis Y, Bouhnik Y, Pocard M, Vicaut E, Valleur P. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn’s disease: A multivariate analysis in 161 consecutive patients. Dis Colon Rectum 2007;50:331–6. doi: 10.1007/s10350-006-0782-0. [DOI] [PubMed] [Google Scholar]
- [5].Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, et al. Natural History of Pediatric Crohn’s Disease: A Population-Based Cohort Study. Gastroenterology 2008;135:1106–13. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- [6].Malik S, Mason A, Bakhshi A, Young D, Bishop J, Garrick V, et al. Growth in children receiving contemporary disease specific therapy for Crohn’s disease. Arch Dis Child 2012;97:698–703. doi: 10.1136/archdischild-2011-300771. [DOI] [PubMed] [Google Scholar]
- [7].Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn’s disease. Dis Colon Rectum 2000;43:1141–5. doi: 10.1007/BF02236563. [DOI] [PubMed] [Google Scholar]
- [8].Crowell KT, Messaris E. Risk factors and implications of anastomotic complications after surgery for Crohn’s disease. World J Gastrointest Surg 2015;7:237–42. doi: 10.4240/wjgs.v7.i10.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yao GX, Wang XR, Jiang ZM, Zhang SY, Ni AP. Role of perioperative parenteral nutrition in severely malnourished patients with Crohn’s disease. World J Gastroenterol 2005;11:5732–4. doi: 10.3748/WJG.V11.I36.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang M, Gao X, Chen Y, Zhi M, Chen H, Tang J, et al. Body Mass Index Is a Marker of Nutrition Preparation Sufficiency Before Surgery for Crohn’s Disease From the Perspective of Intra-Abdominal Septic Complications: A Retrospective Cohort Study. Medicine (Baltimore) 2015;94:e1455. doi: 10.1097/MD.0000000000001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Secker DJ, Jeejeebhoy KN. Subjective Global Nutritional Assessment for children. Am J Clin Nutr 2007;85:1083–9. doi:85/4/1083 [pii]. [DOI] [PubMed] [Google Scholar]
- [12].Wakita M, Fukatsu A, Amagai T. Nutrition Assessment as a Predictor of Clinical Outcomes for Infants With Cardiac Surgery: Using the Prognostic Nutritional Index. Nutr Clin Pract 2011;26:192–8. [DOI] [PubMed] [Google Scholar]
- [13].Leite HP, Fisberg M, de Carvalho WB, de Camargo Carvalho AC. Serum albumin and clinical outcome in pediatric cardiac surgery. Nutrition 2005;21:553–8. doi: 10.1016/j.nut.2004.08.026. [DOI] [PubMed] [Google Scholar]
- [14].Radman M, Mack R, Barnoya J, Castaneda A, Rosales M, Azakie A, et al. The effect of preoperative nutritional status on postoperative outcomes in children undergoing surgery for congenital heart defects in San Francisco (UCSF) and Guatemala City (UNICAR). J Thorac Cardiovasc Surg 2014;147:442–50. doi: 10.1016/j.jtcvs.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vivanco-muñoz N, Buendía-hernández A, Osvaldo J, Piña T, Juanico-enríquez A, Peralta PC. Impact of nutritional support on length of hospitalization and mortality in children after open heart surgery. Bol Med Hosp Infant Mex 2010;67:430–8. [Google Scholar]
- [16].Toole BJ, Toole LE, Kyle UG, Cabrera AG, Orellana RA, Coss-bu JA. Perioperative Nutritional Support and Malnutrition in Infants and Children with Congenital Heart Disease. Congenit Heart Dis 2014;9:15–25. [DOI] [PubMed] [Google Scholar]
- [17].Wessner S, Burjonrappa S. Review of nutritional assessment and clinical outcomes in pediatric surgical patients: Does preoperative nutritional assessment impact clinical outcomes? J Pediatr Surg 2014;49:823–30. doi: 10.1016/j.jpedsurg.2014.01.006. [DOI] [PubMed] [Google Scholar]
- [18].Pierro A, Eaton S. Metabolism and nutrition in the surgical neonate. Semin Pediatr Surg 2008;17:276–84. doi: 10.1053/j.sempedsurg.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [19].Pierro A Perioperative Nutrition in Infants and Children. Nutrition 1999;15:962–4. [DOI] [PubMed] [Google Scholar]
- [20].Powis M, Smith K, Rennie M, Halliday D, Pierro A. Effect of Major Abdominal Operations on Energy and Protein Metabolism in Infants and Children. J Pediatr Surg 1998;33:49–53. [DOI] [PubMed] [Google Scholar]
- [21].Becker P, Carney LN, Corkins MR, Monczka J, Smith E, Smith SE, et al. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Indicators Recommended for the Identification and Documentation of Pediatric Malnutrition (Undernutrition). Nutr Clin Pract 2015;30:147–61. doi: 10.1177/0884533614557642. [DOI] [PubMed] [Google Scholar]
- [22].Raval MV, Dillon PW, Bruny JL, Ko CY, Hall BL, Moss RL, et al. American college of surgeons national surgical quality improvement program pediatric: A phase 1 report. J Am Coll Surg 2011;212:1–11. doi: 10.1016/j.jamcollsurg.2010.08.013. [DOI] [PubMed] [Google Scholar]
- [23].Dillon P, Hammermeister K, Morrato E, Kempe A, Oldham K, Moss L, et al. Developing a NSQIP module to measure outcomes in children’s surgical care: opportunity and challenge. Semin Pediatr Surg 2008;17:131–40. doi: 10.1053/j.sempedsurg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- [24].ACS National Surgical Quality Improvement Program. Https://www.facs.org/media n.d.
- [25].Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The Diagnostic Approach to Monogenic Very Early Onset. Gastroenterology 2014;147:990–1007. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on Propensity Score Estimation for Multiple Treatments Using Generalized Boosted Models. Stat Med 2013;32:3388–414. doi: 10.1002/sim.5753.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One 2011;6. doi: 10.1371/journal.pone.0018174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Patel KV, Darakhshan AA, Griffin N, Williams AB, Sanderson JD, Irving PM. Patient optimization for surgery relating to Crohn’s disease. Nat Rev Gastroenterol Hepatol 2016;13:707–19. doi: 10.1038/nrgastro.2016.158. [DOI] [PubMed] [Google Scholar]
- [29].Cohen S, Danzaki K, Maciver NJ. Nutritional effects on T-cell immunometabolism. Eur J Immunol 2017;47:225–35. doi: 10.1002/eji.201646423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carbone F, La C, Candia P De, Procaccini C, Colamatteo A, Micillo T, et al. Seminars in Immunology Metabolic control of immune tolerance in health and autoimmunity. Semin Immunol 2016;28:491–504. doi: 10.1016/j.smim.2016.09.006. [DOI] [PubMed] [Google Scholar]
- [31].Bird JK, Murphy RA, Ciappio ED, McBurney MI. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States 2017;9:1–20. doi: 10.3390/nu9070655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neumann C, Lawlor G. Immunologic children responses in malnourished children. Am J Clin Nutr 1975;28:89–104. [DOI] [PubMed] [Google Scholar]
- [33].Lewis RT, Klein H. Risk factors in postoperative sepsis: Significance of preoperative lymphocytopenia. J Surg Res 1979;26:365–71. doi: 10.1016/0022-4804(79)90021-0. [DOI] [PubMed] [Google Scholar]
- [34].Dempsey D, Mullen J, Buzby G. The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J … 1988:352–6. [DOI] [PubMed] [Google Scholar]
- [35].Mullen JL, Gertner MH, Buzby GP, Goodhart GL, Rosato EF. Implications of Malnutrition in the Surgical Patient. Arch Surg 1979;114:121–5. doi: 10.1055/s-0031-1295685. [DOI] [PubMed] [Google Scholar]
- [36].Isabel M, Correia T, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003;22:235–9. doi: 10.1016/S0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- [37].Blackburn SC, Wiskin a E, Barnes C, Dick K, Afzal N a, Griffiths DM, et al. Surgery for children with Crohn’s disease: indications, complications and outcome. Arch Dis Child 2014;99:420–6. doi: 10.1136/archdischild-2013-305214. [DOI] [PubMed] [Google Scholar]
- [38].Hansen LF, Jakobsen C, Paerregaard A, Qvist N, Wewer V. Surgery and postoperative recurrence in children with crohn disease. J Pediatr Gastroenterol Nutr 2015;60:347–51. doi: 10.1097/MPG.0000000000000616. [DOI] [PubMed] [Google Scholar]
- [39].Hojsak I, Kolacek S, Hansen LF olmer, Bronsky J, Piekkala M, Lionetti P, et al. Long-term outcomes after elective ileocecal resection in children with active localized Crohn’s disease--a multicenter European study. J Pediatr Surg 2015;50:1630–5. doi: 10.1016/j.jpedsurg.2015.03.054. [DOI] [PubMed] [Google Scholar]
- [40].Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: What is the mechanism behind the relationship? Am Surg 2017;83:1220–7. [DOI] [PubMed] [Google Scholar]
- [41].Pierre JF. Gastrointestinal immune and microbiome changes during parenteral nutrition. Am J Physiol - Gastrointest Liver Physiol 2017;312:G246–56. doi: 10.1152/ajpgi.00321.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Surgeons AC of. ACS NSQIP Pediatric. Https://www-Facs-Org.ezp.welch.jhmi.edu/quality-Programs/pediatric n.d.
- [43].Shiloach M, Frencher SK, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, et al. Toward Robust Information: Data Quality and Inter-Rater Reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]


