Abstract
Electrical stimulation of circumscribed areas of the pontine and medullary reticular formation inhibits muscle tone in cats. In this report, we present an analysis of the anatomical distribution of atonia-inducing stimulation sites in the brain stem of the rat. Muscle atonia could be elicited by electrical stimulation of the nuclei reticularis pontis oralis and caudalis in the pons as well as the nuclei gigantocellularis, gigantocellularis alpha, gigantocellularis ventralis, and paragigantocellularis dorsalis in the medulla of decerebrate rats. This inhibitory effect on muscle tone was a function of the intensity and frequency of the electrical stimulation. Average latencies of muscle-tone suppressions elicited by electrical stimulation of the pontine reticular formation were 11.02 ± 2.54 and 20.49 ± 3.39 (SD) ms in the neck and in the hindlimb muscles, respectively. Following medullary stimulation, these latencies were 11.29 ± 2.44 ms in the neck and 18.87 ± 2.64 ms in the hindlimb muscles. Microinjection of N-methyl-d-aspartate (NMDA, 7 mM/0.1 μl) agonists into the pontine and medullary inhibitory sites produced muscle-tone facilitation, whereas quisqualate (10 mM/0.1 μl) injection induced an inhibition of muscle tone. NMDA-induced muscle tone change had a latency of 31.8 ± 35.3 s from the pons and 10.5 ± 0.7 s from the medulla and a duration of 146.7 ± 95.2 s from the pons and 55.5 ± 40.4 s from the medulla. The latency of quisqualate (QU)-induced reduction of neck muscle tone was 30.1 ± 37.9 s after pontine and 39.5 ± 21.8 s after medullary injection. The duration of muscle-tone suppression induced by QU injection into the pons and medulla was 111.5 ± 119.2 and 169.2 ± 145.3 s. Smaller rats (8 wk old) had a higher percentage of sites producing muscle-tone inhibition than larger rats (16 wk old), indicating an age-related change in the function of brain stem inhibitory systems. The anatomical distribution of atonia-related sites in the rat has both similarities and differences with the distribution found in the cat, which can be explained by the distinct anatomical organization of the brain stem in these two species.
INTRODUCTION
Rapid-eye-movement (REM) sleep is correlated with extensive suppression of skeletal muscle tone (Jouvet et al. 1959). Muscle-tone suppression requires the integrity of the pons and the medial medulla. Restricted lesions in the pons (Hendricks et al. 1982; Henley and Morrison 1974; Jouvet and Delorme 1965; Morrison 1983, 1988) and in the medulla (Holmes and Jones 1994; Schenkel and Siegel 1989) produce REM sleep without atonia. In unit recording studies, cell populations within these areas were found to be selectively active in REM sleep (REM-on cells) (Kanamori et al. 1980; Sakai 1980, 1988; Shiromani et al. 1987). Medullary REM-on cells are also active during cataplectic attacks in narcoleptic dogs (Siegel et al. 1991).
Magoun (1944) and Magoun and Rhines (1946) discovered that electrical stimulation of the medial medulla inhibits spinal reflexes and muscle tone in the cat. Our laboratory has found that stimulation in the mesopontine region and medial pons also suppresses muscle tone in decerebrate cat (Lai and Siegel 1988; Lai et al. 1987). Intracellular recordings have demonstrated that electrical stimulation of the pons and medulla of cats produces inhibitory postsynaptic potentials (IPSPs) in spinal and brain stem motoneurons (Chase et al. 1986; Fung et al. 1982; Jankowska et al. 1968; Llinas and Terzuolo 1964, 1965; Morales et al. 1987; Peterson et al. 1978).
The aim of the present study was to evaluate the effect of electrical and chemical stimulation in the pontomedullary reticular formation on muscle tone in the rat. Such studies would lay the foundation for further work using the more comprehensive knowledge of the anatomy, neurochemistry, and genetics of the rat to analyze the circuitry involved in the regulation of muscle tone. Furthermore, comparison of rat and cat anatomy with the results of physiological studies would provide further information as to the identity of the neural populations and processes responsible for the suppression of muscle tone in sleep.
METHODS
Surgical procedures
Experiments were performed on 54 male rats weighing between 170 and 510 g, corresponding to 8–16 wk of age. Animals were initially anesthetized with halothane (Fluothane) and ketamine HCl (Ketalar, 100 mg/kg im) for cannulation of the trachea. Both common carotid arteries were ligated to reduce bleeding. The animals’ heads were then fixed in a stereotaxic frame. After frontal and parietal craniotomy and immediately after removing the forebrain, anesthesia was discontinued. The brain stem was transected at the precollicular-postmammillary level with a spatula, and the forebrain was removed by aspiration. The rostral pole of the midbrain and the floor of the skull were covered with a small cotton ball soaked in hot saline solution. The exposed brain tissue was protected from dehydration with a thin layer of cotton gauze soaked with mineral oil. Multi-stranded stainless steel bipolar electrodes were implanted in the gastrocnemius muscle of the hindlimb, triceps brachii muscle of the forelimb and the splenius of the neck to record the electromyogram (EMG). Rectal temperature was continuously monitored with a probe connected to a thermoregulator and maintained between 36 and 37°C with an infrared lamp.
Stimulation and recording
Recording began 3 h after decerebration, when muscle tone had stabilized. All animals received electrical stimulation that was administered through stainless steel concentric bipolar electrodes (Rhodes, SNEX-100) that were moved stereotaxically in 0.3-mm steps from H2.8 to 0.0 between A0.7–P0.3 and L0.2–1.5 mm in the pons and from H2.0 to 20.7 between P2.0–P3.3 and L0.0–0.6 in the medulla according to Paxinos and Watson (1986). These regions correspond to the approximate anatomical levels of the pontine and medullary atonia eliciting regions of the cat (Lai and Siegel 1988; Lai et al. 1987). In all experiments, except in latency studies, electrical stimuli consisted of 500-ms trains of 0.2-ms cathodal rectangular pulses at 10–250 Hz and 5–250 μA. EMG signals were amplified and recorded on a Grass polygraph (Model 78E). The amplitude of muscle activity was integrated with a time constant of 1 s using a Grass polygraph integrator (Model 7P10F). Integrated EMG signals were used to measure the magnitude of muscle-tone suppression. In four experiments, the latencies of EMG changes were measured by a stimulus triggered averaging technique. At each point, during 20 consecutive trials, EMG signals were digitized by an A/D converter (CED 1401) over a 250-ms peristimulus period with a binwidth of 100 μs, rectified, and averaged by a computer. In these experiments, 50- to 100-ms trains at 150 Hz (8–15 pulses) and at an intensity of three times threshold (40–50 μA) for muscle-tone suppression were used.
Chemical microinjection
Glutamate agonists and antagonists were microinjected in 20 young adult rats, weighing 170–380 g. The pontomedullary inhibitory site was identified by electrical stimulation. Then, a Hamilton 1-μl microsyringe (25 sG, Model 7001) was inserted into that site according to the stereotaxic parameters. One-tenth microliter of glutamate agonists was microinjected over a period of 30 s. To test the effect of glutamate on muscle activity, 0.1 μl of the specific N-methyl-d-aspartate (NMDA) antagonist, dl – 2-amino-5-phosphonopentanoic acid (AP5) and non-NMDA receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were microinjected into the pontine site one minute before agonists were applied.
CHEMICALS.
All chemicals for the microinjection studies were acquired from Tocris Neuramin, Bristol, UK. The concentration of chemicals was as follows: NMDA (7 mM), quisqualate (QU, 10 mM), AP5 (20 μM), and CNQX (10 μM).
Histology
At the end of the experiments, iron was deposited at the dorsal and ventral limits of the stimulation tracks by passing a 50-μA positive DC current for 20 s through the electrode. Rats were perfused with saline, followed by a buffered 10% formalin solution, and the brains were removed. Serial coronal sections (60 μm) were cut by a cryostat, stained with Neutral red, and counterstained with a potassium-ferro-cyanide solution to produce a Prussian-blue reaction with the deposited iron. Stimulation and injection sites were reconstructed according to the atlas of Paxinos and Watson (1986).
Data analysis
After perfusion of the four rats used for the measurement of response latencies, the length of motoneuron axons innervating splenius and gastrocnemius muscles was measured from the exit of ventral horn to the innervating muscles. Then, the brain stem with attached spinal cord was removed. The length of the spinal cord from C2 to L5 was measured to calculate the conduction velocity of the reticulospinal neurons. Student’s t-test was used for statistical analysis.
RESULTS
Localization of areas producing muscle-tone suppression
Areas producing bilateral muscle-tone suppression by electrical stimulation in the rostral pons (A0.7–A0.28) were located between L0.6–1.2 and H2.5–0.8, while in the caudal pons (A0.28–P0.3) effective areas were located between L0.4–0.8 and H2.6–1.0. These areas corresponded to the nuclei reticularis pontis oralis and caudalis. In the medulla, responsive sites were located from P2.0 to P3.3, L0.2 to L1.1, and H1.7 to H-0.7 and included nuclei reticularis gigantocellularis, paragigantocellularis dorsalis, gigantocellularis alpha, and gigantocellularis ventralis.
The effect of electrical stimulation on muscle activity varied as a function of stimulation site (Fig. 1). Bilateral inhibition or inhibition followed by rebound facilitation was restricted to a compact region in the medial pons and medulla. In contrast, stimulation sites inducing contralateral inhibition without effects on the ipsilateral muscle tone were located in a more scattered area. The percentage of sites producing bilateral inhibition was higher in smaller rats (Table 1). Statistical analysis revealed that the correlation between body size and site-induced muscle-tone suppression was significant in both the pons and medulla (pons: P < 0.01, df = 45, 2-tailed t-test; medulla: P < 0.05, df = 22, 2-tailed t-test).
FIG. 1.
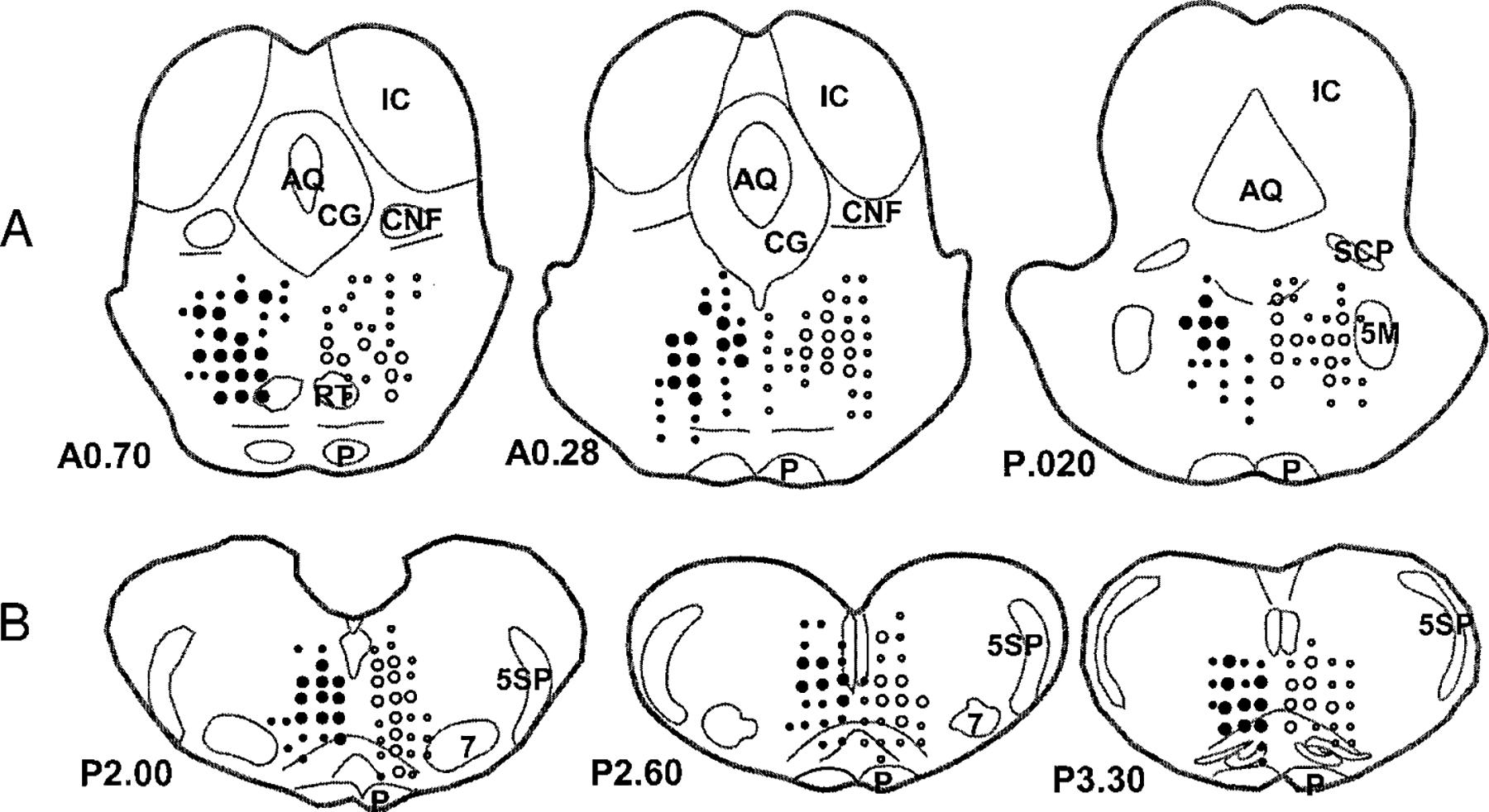
Schematic diagram showing the atonia-related regions in the pons (A) and medulla (B). Data are summarized from 28 rats and reconstructed according to Paxinos and Watson (1986). Stimulation was performed on both sides of the pons and medulla. ●, sites at which electrical stimulation produced bilateral inhibition of muscle tone. ○, sites at which stimulation produced contralateral inhibition with no effect on ipsilateral muscle tone. The size of symbols represents the potency of the effect. ○, ●, ≥50%; ○, ●: <50%. A and P indicate anterior and posterior to the interaural level, respectively. AQ, aqueduct; CG, central gray; CNF, nucleus cuneiformis; IC, inferior colliculus; P, pyramidal tract; RT, nucleus reticularis tegmenti; SCP, superior cerebellar peduncle; 5M, trigeminal motor nucleus; 5SP, spinal trigeminal tract; 7, facial nucleus.
TABLE 1.
Influence of age on the percentage of sites electrical stimulation produced bilateral muscle-tone suppression
| >300 g | <300 g | |||
|---|---|---|---|---|
| Pons | Medulla | Pons | Medulla | |
| Bilateral inhibition | 49.4 | 28.1 | 68.3 | 67.7 |
| Contralateral inhibition only | 50.6 | 71.9 | 31.7 | 32.3 |
Data were collected from 34 rats.
Inhibition of muscle tone by brain stem stimulation
Two types of bilateral muscle-tone suppressions with long and short duration resulted from electrical stimulation of the pontomedullary reticular formation (Fig. 2). Short responses terminated when stimulation was discontinued, while long responses outlasted stimulation, often by several seconds (11.4 ± 2.6 s). The inhibitory effect was consistently more profound in the contralateral as opposed to the ipsilateral side (P < 0.001, df = 67, paired t-test; Fig. 3) for both short and long responses.
FIG. 2.
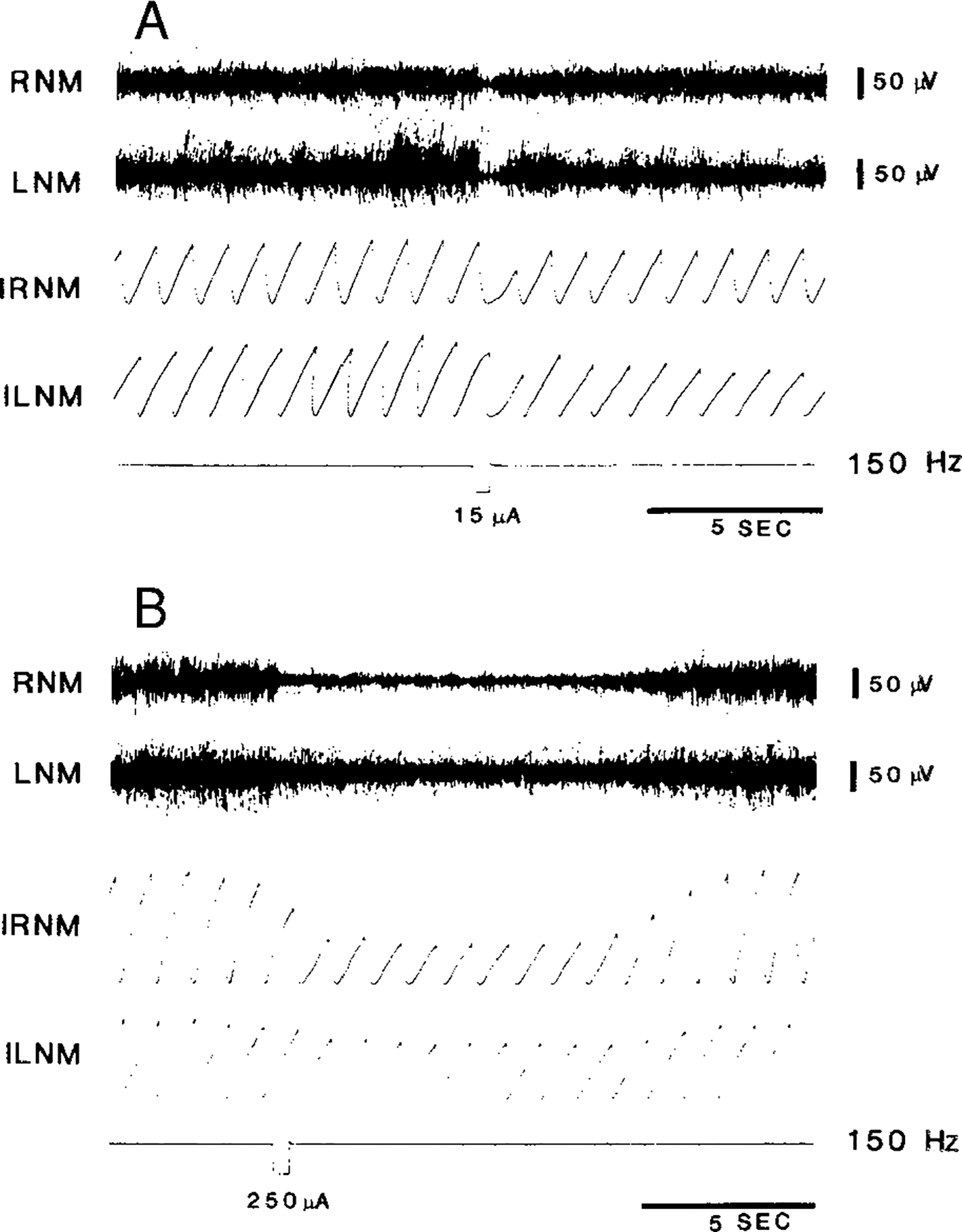
Two examples of muscle response to brain stem stimulation taken from different animals. A: short-duration bilateral atonia in the neck muscles following a 500-ms train applied to the medial pons. The muscle tone returned to the baseline level at the end of the stimulation. B: pontine stimulation produced very long duration (12 s) muscle-tone suppression. The stimulation induced more potent effect on the contralateral than the ipsilateral side. RNM and LNM, right and left neck muscles, respectively; IRNM and ILNM, integrated right and left neck muscle activities.
FIG. 3.
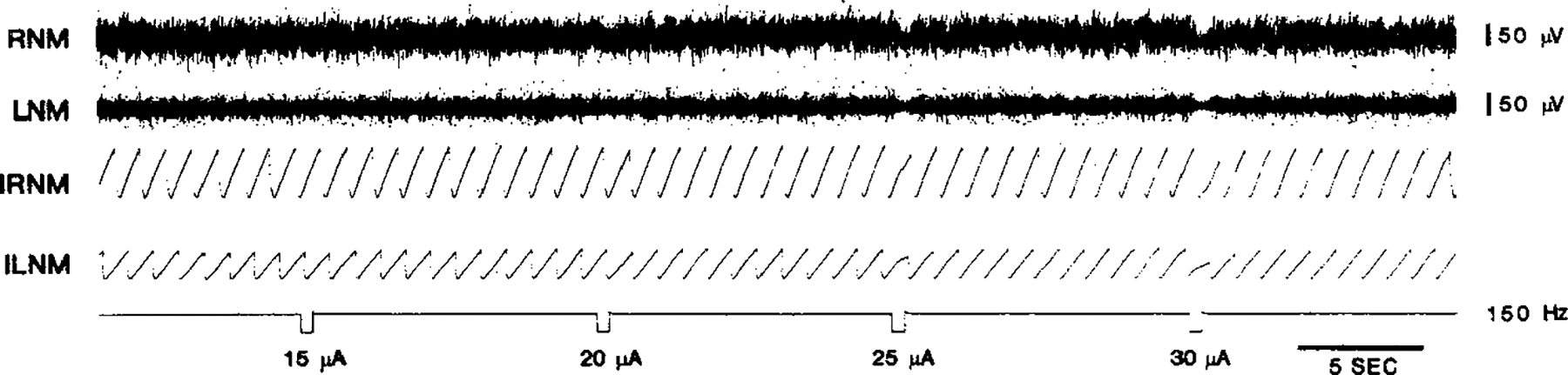
Effect of electrical stimulation on muscle tone. Five-hundred-millisecond trains with varying intensities were applied into the pons. Subthreshold stimulation at 15 μA caused no change in muscle tone. A very small muscle-tone suppression was induced by 20 μA. Higher intensity stimulation produced a profound decrease in neck muscle tone.
Muscle-tone suppressions induced by pontomedullary stimulation were a function of the intensity (Figs. 3 and 4) and frequency of the stimulation (Fig. 3). Current intensity thresholds that produced muscle-tone suppression varied from 5 to 100 μA across the animals. Therefore, current values were normalized with reference to the individual threshold intensities to express quantitatively the magnitude of tone reduction as a function of stimulation intensity. The relationship between the stimulation intensity and the muscle-tone suppression was biphasic, with the maximal effect at a current intensity of three times threshold (Fig. 3). As with the intensity, the effect of stimulus frequency on the muscle-tone suppression was also biphasic with a maximal effect at 150 Hz.
FIG. 4.
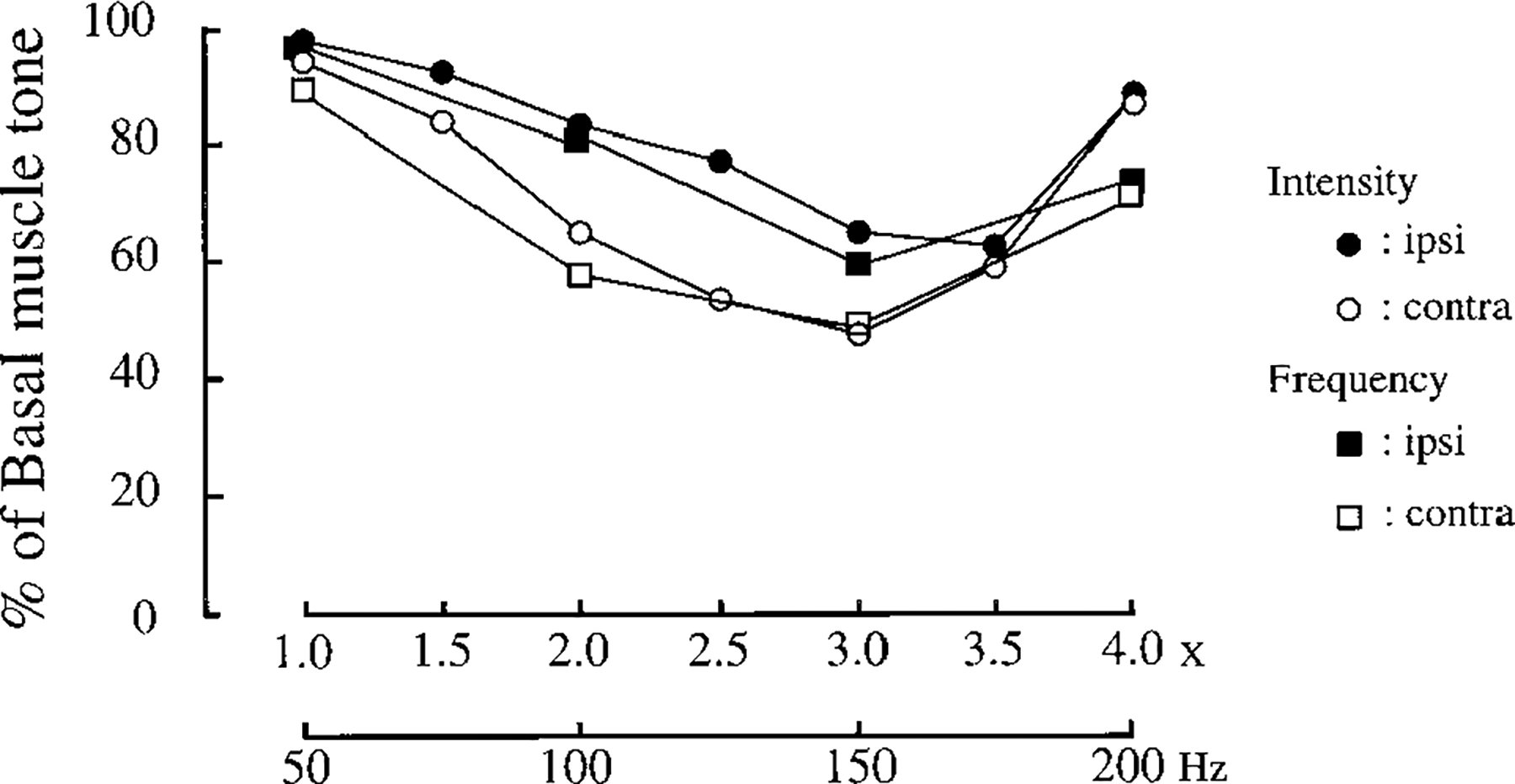
Intensity and frequency dependence of electrical stimulation on muscle-tone suppression. Intensity values (x) were calculated from the actual intensity divided by the threshold intensity. One hundred fifty hertz was used for intensity-dependent, whereas 3 times intensity was used for frequency-dependent studies. The effect of intensity and frequency on muscle-tone suppression was biphasic with the maximal effect at 3 times and 150 Hz, respectively. Contra, contralateral side to the stimulation; ipsi, ipsilateral side to the stimulation.
Response latency and conduction velocity
Response latencies were measured in 28 trials carried out in four rats. Latency was defined as the time from the start of the stimulation to the point when the rectified and averaged EMG signal fell below baseline by two times of the standard deviation (Fig. 5). Base line mean and standard deviation were calculated from the 50-ms prestimulus period. To measure latencies, 150-Hz stimulus trains at three times threshold intensity were delivered to the pontine and medullary reticular formations. The latency of muscle-tone suppression was 11.02 ± 2.54 ms in the neck muscles and 20.49 ± 3.39 ms in the hindlimb muscles when the pontine reticular formation was stimulated. Following medullary stimulation, latencies of 11.29 ± 2.44 ms in the neck and 18.87 ± 2.64 ms in the hindlimb were seen.
FIG. 5.
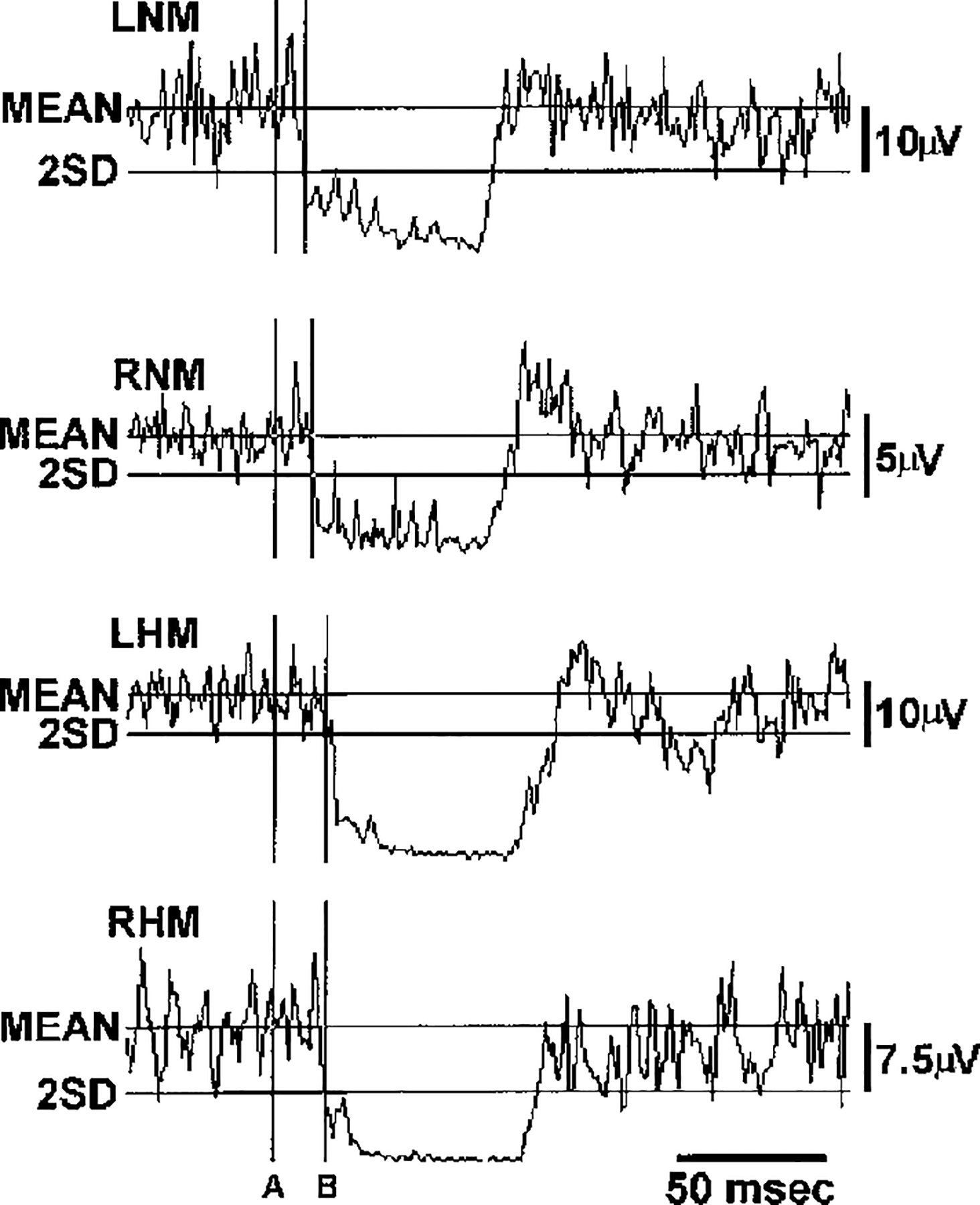
Computer average of electromyogram (EMG) change produced by 20 trials of medullary stimulation. Fifty-millisecond trains with 150-Hz pulses and 50-μA intensity were applied into the right side of medial medulla. Baseline mean and SD were calculated from the 50-ms prestimulation period. A, the stimulation onset; and B, the time when the depression of the EMG activity exceeded twice the baseline SD. The latencies of medullary-induced muscle-tone suppression were 8.8 ms in the left neck muscle, 12.1 ms in the right neck muscle, and 17.3 ms in both left and right hindlimb muscles. Mean, baseline mean EMG activity; 2SD, 2 times baseline SD; LHM and RHM, left and right hindlimb muscles, respectively.
The conduction velocity of the reticulospinal inhibitory system was calculated from the latency differences of tone suppressions in ipsilateral neck and hindlimb muscles. Conduction velocity of rodent motoneurons was assumed to be 60 m/s (Bakels and Kernell 1993; Gardiner 1993). The length of motoneuron axons innervating neck and hindlimb muscles was taken as 5 and 67 mm, respectively. The time needed for action potentials to propagate from the axon of the motoneuron to the muscle (0.08 and 1.1 ms for the neck and hindlimb motoneurons, respectively) was deducted from the latencies. The remainder of the latency difference was caused by the greater distance the descending inhibitory impulses had to travel from C2 to L5. Thus the conduction velocity of the reticulospinal inhibitory system can be calculated by dividing the length of the spinal cord between C2 and L5 by the corrected latency difference between hindlimb and neck muscle-tone suppression. The length of the spinal cord between C2 and L5 was 47 ± 3 mm (range: 42–50 mm). The conduction velocity of the reticulospinal inhibitory system was calculated in this way as 6.79 ± 3.30 m/s (range: 3.12–26.40 m/s).
Changes in muscle activity produced by the microinjection of glutamate agonists
To determine if neuronal somas rather than axons of passage were responsible for the electrical stimulation effect, glutamate agonists and antagonists were microinjected into the brain stem. The concentration of glutamate agonists and antagonists used in this study was based on our previous work on the decerebrate cat (Lai and Siegel 1991). One-tenth microliter of quisqualate (10 mM) injected into the pons produced muscle-tone suppression, bilaterally (Fig. 6). The average latency of QU-induced muscle-tone suppression was 30.1 ± 37.9 and 39.5 ± 21.8 s after pontine and medullary injection, respectively. The duration of muscle-tone suppression was 111.5 ± 119.2 s when QU was injected into the pons and 169.2 ± 145.3 s when it was injected into the medulla. This QU-induced muscle-tone suppression could be blocked by prior injection of the specific non-NMDA antagonist, CNQX (10 μM/0.1 μl) into the sites (Fig. 6). In contrast, NMDA (7 mM/0.1 μl) injected into the same or nearby sites elicited muscle-tone facilitation (Fig. 7). The average latency of muscle-tone facilitation was 31.8 ± 35.3 s and duration was 146.7 ± 95.2 s when NMDA was microinjected into the pons. NMDA microinjection into the medulla produced facilitation of muscle tone with an average latency of 10.5 ± 0.7 s and duration of 55.5 ± 40.3 s. The facilitatory effect of NMDA injection on muscle tone was blocked by prior injection of the specific NMDA antagonist, AP5 (20 μM/0.1 μl; Fig. 7).
FIG. 6.
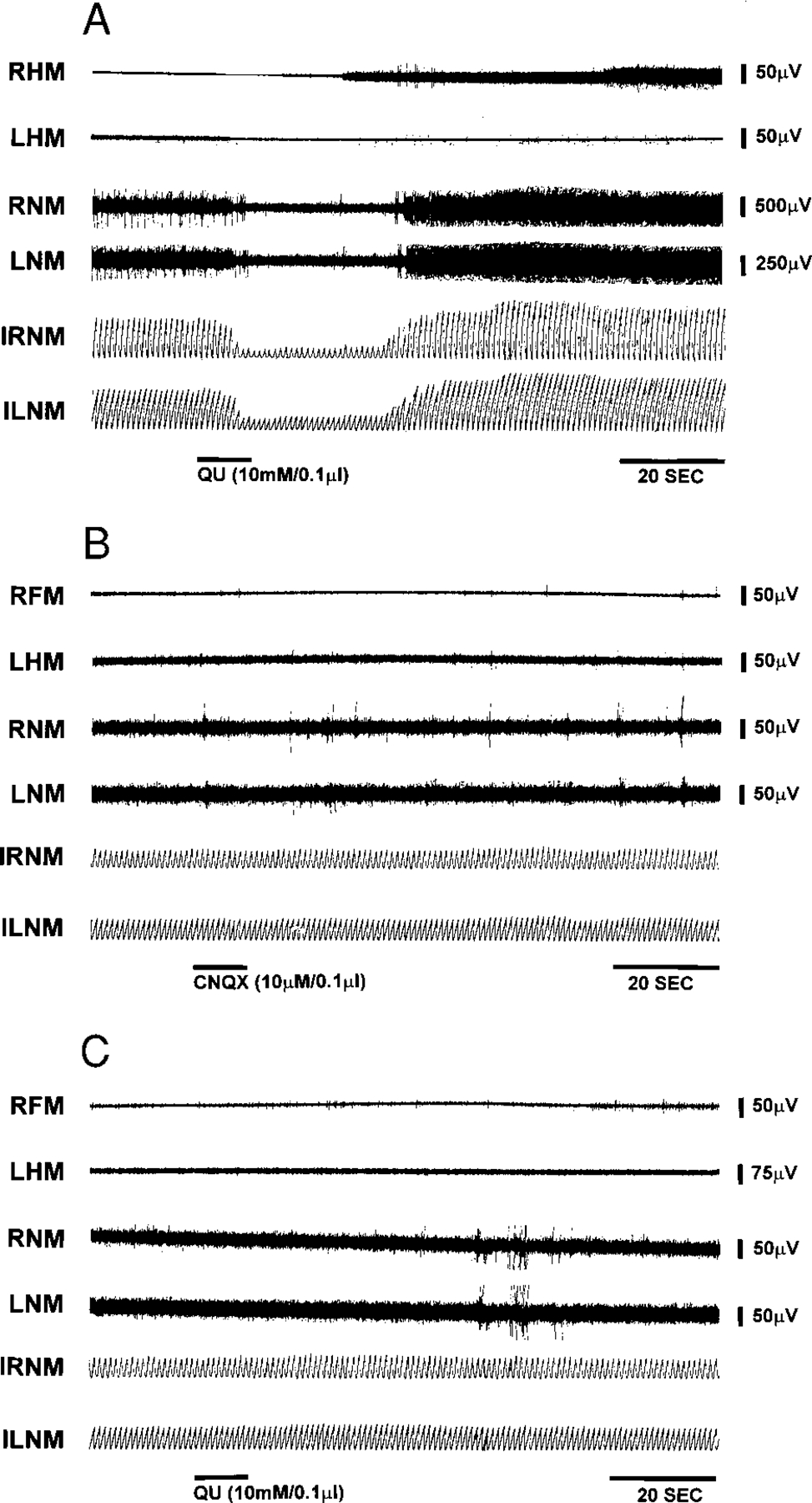
Effect of quisqualate (QU) injection into the pons on muscle activity. A: microinjection of QU (10 mM/0.1 μl) into pons produced suppression of muscle tone in the neck (RNM and LNM) and hindlimb (RHM and LHM). B: the specific non-N-methyl-d-aspartate (non-NMDA) antagonist, 6-cyano-7-nitroquin-oxaline-2,3-dione (CNQX, 10 μM/0.1 μl) microinjected into the same site does not have an effect on muscle activity. However, prior CNQX injection blocked the QA effect on muscle activity (C). RFM, right forelimb muscle.
FIG. 7.
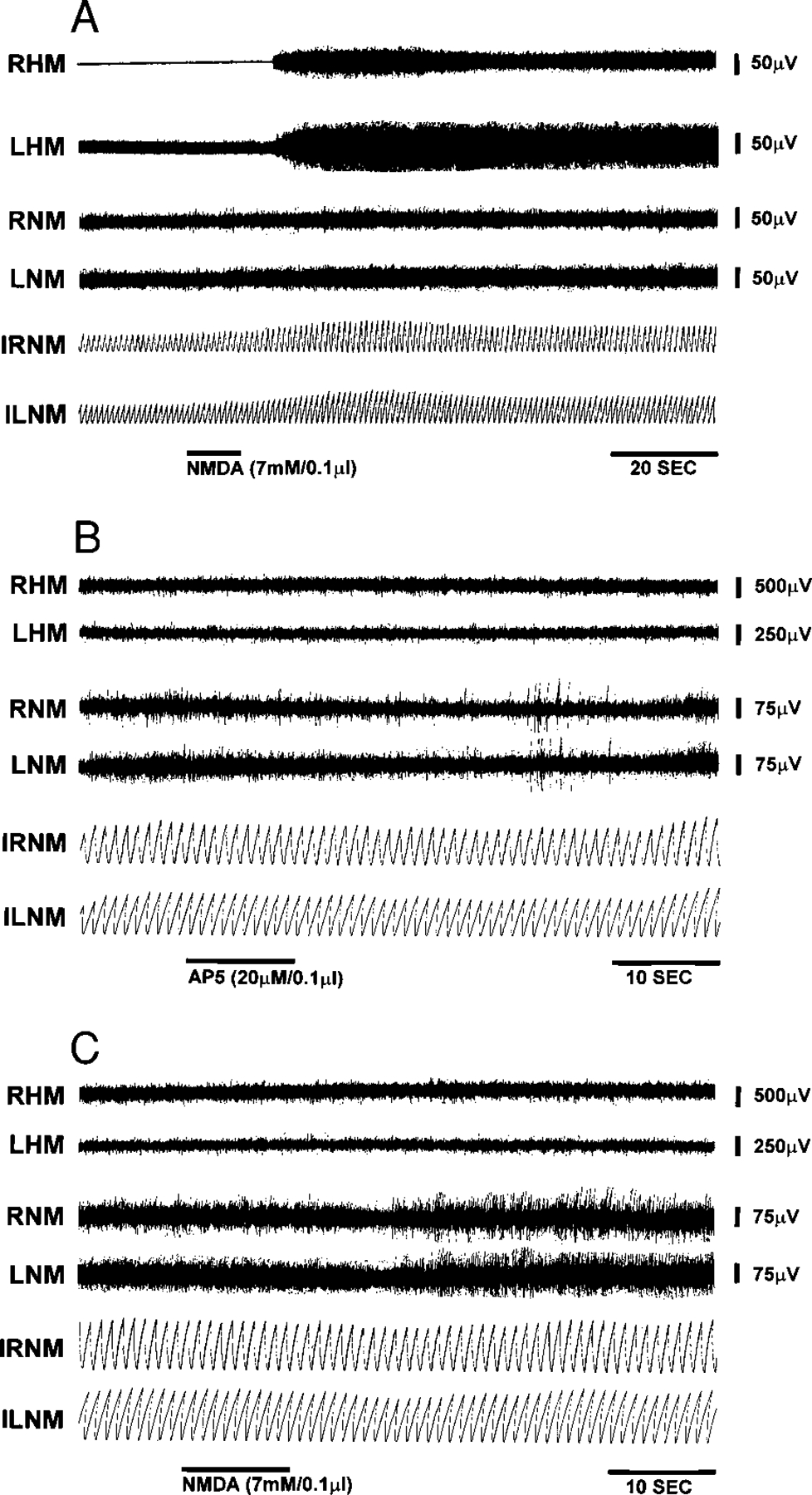
Effect of NMDA injection on muscle-tone activity. NMDA (7 mM/0.1 μl) microinjected into medulla produced muscle-tone facilitation (A). The specific NMDA antagonist, dl – 2-amino-5-phosphonopentanoic acid (AP5) injected into the same site has no effect (B) but blocked the NMDA effect on muscle activity (C).
DISCUSSION
Muscle-tone suppression could be induced in the decerebrated rat by electrical and chemical stimulation of the medial pons and medulla. As in the decerebrate cat, quisqualate microinjected into the pons induced muscle-tone suppression, whereas NMDA agonist injected into the same or nearby sites elicited muscle-tone facilitation. The anatomical distribution of atonia-related sites in the rat has both similarities and differences from the distribution found in the cat. In the decerebrate cat, electrical stimulation of the nuclei magnocellularis and paramedianus of the medulla and the medial pontine reticular formation was found to be effective (Lai and Siegel 1990b, 1991; Lai et al. 1987). These sites are generally thought to correspond to the nuclei reticularis gigantocellularis, paragigantocellularis dorsalis, gigantocellularis alpha, gigantocellularis ventralis, and reticularis pontis oralis and caudalis stimulated in the rat. Our present study found that stimulation sites inducing bilateral inhibition were located in a restricted area of the medial pons and medulla, whereas sites where stimulation that caused contralateral inhibition only were encountered in a wider area that included the inhibitory regions that included the bilateral inhibitory area. In contrast, these two areas were found to be separated in the cat (Lai et al. 1987), in which stimulation in the medial medulla produced bilateral inhibition, while stimulation in more lateral medulla produced contralateral inhibition and facilitated ipsilateral neck muscles. One possible explanation for this species difference is a greater anatomical overlap of the atonia regulating area with other areas involved in the facilitation of muscle tone in the rat. Alternatively, it can be explained by the relatively small size of the rat brain causing electrical stimulation to spread to a relative wider range of structures (Ranck 1975; Rattay 1999) than in the cat, thus co-activating anatomically segregated inhibitory and facilitatory neurons.
Muscle-tone suppressions were either localized to the contralateral side or appeared bilaterally in the decerebrate rat. Those sites where stimulation induced bilateral muscle-tone suppression may be involved in the generalized regulation of muscle tone. In contrast, those sites where stimulation produced contralateral inhibition only may be involved in more specialized motor control, including acoustic startle response (Davis et al. 1982; Lee et al. 1996), lordosis (Cohen et al. 1987), and locomotion. Low-frequency (40 Hz), long-duration (10 s) stimulation in the ventromedial medulla has been shown to induce locomotion (Kinjo et al. 1990). Although high-frequency (>100 Hz) stimulation may cause depolarization block (Benazzouz et al. 1995, 1996), it is unlikely that the muscle-tone suppression seen in the present study is due to inactivation of neurons. We have found that muscle-tone suppression could be elicited by low-frequency (50 Hz) stimulation in the present study. Whereas, high-intensity stimulation is generally required for depolarization block (Rattay 1999). Our previous work in the decerebrate cat has also found that muscle-tone suppression could be elicited through a wide range of stimulation frequencies (10–200 Hz), and the inhibitory effect is the first alteration in muscle tone seen when stimulation intensity is gradually increased from subthreshold levels (Lai and Siegel 1990a). Furthermore, the QU-injection-induced muscle-tone suppression that we found in the present study at the effective electrical stimulation sites demonstrates that muscle-tone suppression is mediated by activation of pontine and medullary neurons rather than fibers of passage.
The percentage of sites where stimulation induced bilateral inhibition was higher in younger rats than in older ones as shown on Table 1. This suggests that aging is associated with a deterioration of the atonia-controlling system in humans. The reduction of stimulation sites with bilateral atonia induction seen in the older rats during our experiments may reflect a similar deterioration in the efficacy of the atonia systems. The rat may be a useful model for determining the neurological cause of this disorder.
The pontine sites where electrical stimulation induced bilateral muscle-tone suppression overlaps with the sites where carbachol injection has been shown to induce atonia in the decerebrated rat (Taguchi et al. 1992) or paradoxical sleep in the freely moving rat (Bourgin et al. 1995; Gnadt and Pegram 1986). This part of the pontine reticular formation contains cholinoceptive cells (Jones 1990; Kimura et al. 1981) and receives innervation from the mesopontine cholinergic neurons (Jones 1990; Lai et al. 1993; Shiromani et al. 1988a,b), consistent with a cholinergic contribution to REM sleep atonia. In addition, glutamatergic fibers also participate in these phenomena, since microinjection of glutamate into the pontine and medial medullary reticular formation produces bilateral inhibition of muscle tone and blockade of medullary glutamatergic sites partially reversed pontine elicited atonia in cats (Lai and Siegel 1988). As in our previous studies in the cat (Lai and Siegel 1991), we found non-NMDA agonists microinjected into the medial pons and medulla of the decerebrate rat induced muscle-tone suppression, whereas NMDA injection elicited muscle-tone facilitation. There are NMDA and non-NMDA glutamatergic receptors in the pontomedullary reticular formation in the rat and cat (Lai et al. 1995, 1996; Petralia et al. 1994a,b; Winsky et al. 1996) at the sites where we obtained these effects. Glutamatergic cells of the rostral brain stem project to the atonia induction sites in the pons (Lai at al 1993) and medulla (Lai et al. 1999).
Neuroanatomical studies using retrograde and anterograde transport tracers have demonstrated that the medioventral medulla, corresponding to the nuclei gigantocellularis alpha and ventralis, is a major source of reticulospinal projections. The medial pons also has a significant spinal projection (Kuypers and Maisky 1977; Matsuyama et al. 1988, 1993; Robbins et al. 1992; Suzuki 1985; Tohyama et al. 1979; Zemlan et al. 1984). Reticulospinal fibers from the pons and medulla descend both ipsi- and contralaterally. Anterograde and retrograde tracer studies have demonstrated that neurons in the feline pons (Matsuyama et al. 1999) and rodent medulla (Huisman et al. 1984; Martin et al. 1981) innervate both cervical and lumbosacral spinal cord by way of axonal collaterals. We calculate that the average conduction velocity of the pontine inhibitory reticulospinal system is 6.8 m/s. The conduction velocity of descending neurons mediating atonia may be somewhat higher than the conduction velocity that we calculate for the inhibitory system. If one assumes a synaptic delay of 0.5 ms (Lorente de No 1935; Renshaw 1940) and two synapses in the lumbar spinal cord (Peterson 1978), the conduction velocity of reticulospinal neurons mediating atonia would be 8.0 m/s. Our previous study demonstrated that glutamatergic neurons in the medial pons project to the nucleus magnocellularis of the medioventral medulla in the cat (Lai et al. 1999). These results suggest that inhibitory stimulations in the medial pons induced bilateral muscle-tone suppression either directly or indirectly through the medullary reticulospinal neurons.
Cataplexy, a sudden loss of muscle tone in waking, appears to represent a triggering of the mechanism responsible for the suppression of muscle tone in REM sleep. Studies in the narcoleptic dog have indicated that the pontomedullary inhibitory system is responsible for cataplexy (Siegel et al. 1991, 1992). The decerebrate rat may be useful in the analysis of the neural physiology of the atonia system and its modification in pathological conditions.
Acknowledgments
This work was supported by the Medical Research Service of the Veterans Administration and National Institutes of Health Grants HL-41370, NS-14610, and NS-23724.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bakels R and Kernell DJ. Average but not continuous speed match between motoneurons and muscle units of rat tibialis anterior. Neurophysiology 70: 1300–1306, 1993. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Piallat B, Pollak P, and Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett 189: 77–80, 1995. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Boraud T, Feger J, Burbaud P, Bioulac B, and Gross C. Alleviation of experimental hemiparkinsonism by high-frequency stimulation of the subthalamic nucleus in primates: a comparison with the L-Dopa treatment. Movement Disorders 11: 627–632, 1996. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Escourrou CG, Gaultier C, and Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport 6: 532–536, 1995. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR, Boxer PA, Fung SJ, and Soja PJ. Effect of stimulation of the nucleus reticularis gigantocellularis on the membrane potential of cat lumbar motoneurons during sleep and wakefulness. Brain Res 386: 237–244, 1986. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Schwartz-Giblin S, and Pfaff DW. Brainstem reticular activation facilitates back muscle motoneuronal responses to pudendal nerve input. Brain Res 405: 155–158, 1987. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, and Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 2: 791–805, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Boxer PA, Morales FR, and Chase MH. Hyperpolarizing membrane responses induced in lumbar motoneurons by stimulation of the nucleus reticularis pontis oralis during active sleep. Brain Res 248: 267–273, 1982. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. Neurophysiology 69: 1160–1170, 1993. [DOI] [PubMed] [Google Scholar]
- Gnadt JW and Pegram GV. Cholinergic brainstem mechanisms of REM sleep in the rat. Brain Res 384: 29–41, 1986. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Morrison AR, and Mann GL. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res 239: 81–105, 1982. [DOI] [PubMed] [Google Scholar]
- Henley K and Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp 34: 215–232, 1974. [PubMed] [Google Scholar]
- Holmes CJ and Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62: 1179–1200, 1994. [DOI] [PubMed] [Google Scholar]
- Huisman AM, Ververs B, Cavada C, and Kuypers HGJM. Collateralization of brainstem pathways in the spinal ventral horn in rat as demonstrated with the retrograde fluorescent double-labeling technique. Brain Res 300: 362–367, 1984. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lundberg A, and Pompeiano O. Inhibitory effects evoked through ventral reticulospinal pathways. Arch Ital Biol 106: 124–140, 1968. [PubMed] [Google Scholar]
- Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol 295: 485–514, 1990. [DOI] [PubMed] [Google Scholar]
- Jouvet M and Delorme F. Locus coeruleus et sommeil paradoxal. C R Soc Biol 154: 895–899, 1965. [Google Scholar]
- Jouvet M, Michel F, and Courjon J. Sur un stade d’activite electrique cerebrale rapide au cours du sommeil physiologique. C R Séanc Soc Biol 153: 1024–1028, 1959. [Google Scholar]
- Kanamori N, Sakai K, and Jouvet M. Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res 189: 251–255, 1980. [DOI] [PubMed] [Google Scholar]
- Kimura H, Mcgeer PL, Peng JH, and Mcgeer EG. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol 200: 151–200, 1981. [DOI] [PubMed] [Google Scholar]
- Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, and Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res Bull 24: 509–516, 1990. [DOI] [PubMed] [Google Scholar]
- Kuypers HG and Maisky VA. Funicular trajectories of descending brain stem pathways in cat. Brain Res 136: 159–165, 1977. [DOI] [PubMed] [Google Scholar]
- Lai YY, Clements JR, and Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol 336: 321–330, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Wu XY, Shalita T, Wu J-P, Kuo JS, and Siegel JM. Brainstem projections to the ventromedial medulla in cat: retrograde transport horseradish peroxidase and immunohistochemical studies. J Comp Neurol 408: 419–436, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM. Medullary regions mediating atonia. J Neurosci 8: 4790–4796, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci 10: 2727–2734, 1990a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM. Cardiovascular and muscle tone changes produced by microinjection of cholinergic and glutamatergic agonists in dorsolateral pons and medial medulla. Brain Res 514: 27–36, 1990b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci 11: 2931–2937, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM, and Wilson WJ. Effect of blood pressure on medial medulla-induced muscle atonia. Am J Physiol Heart Circ Physiol 252: H1249–H1257, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Wu J-P, Kuo JS, and Siegel JM. Distribution of NMDA and non-NMDA receptors in the feline pons and medulla. Soc Neurosci Abstr 21: 838, 1995. [Google Scholar]
- Lai YY, Wu J-P, Kuo JS, and Siegel JM. Co-localization of NMDA and non-NMDA receptors in the neurons of rostral ventromedial medulla. Soc Neurosci Abstr 22: 1994, 1996. [Google Scholar]
- Lee Y, Lopez Z, Meloni EG, and Davis M. A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci 16: 3775–3789, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R and Terzuolo CA. Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons. J Neurophysiol 27: 579–591, 1964. [DOI] [PubMed] [Google Scholar]
- Llinas R and Terzuolo CA. Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons. J Neurophysiol 28: 413–422, 1965. [DOI] [PubMed] [Google Scholar]
- Lorente DE No R The synaptic delay of the motoneurons. Am J Physiol 111: 272–282, 1935. [Google Scholar]
- Magoun HW. Bulbar inhibition and facilitation of motor activity. Science 100: 549–550, 1944. [DOI] [PubMed] [Google Scholar]
- Magoun HW and Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol 9: 165–171, 1946. [DOI] [PubMed] [Google Scholar]
- Martin GF, Cabana T, and Humbertson AO Jr. Evidence for collateral innervation of the cervical and lumbar enlargements of the spinal cord by single reticular and raphe neurons. Studies using fluorescent markers in double-labeling experiments on the North American opossum. Neurosci Lett 24: 1–6, 1981. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi Y, Takakusaki K, Mori S, and Kimura H. Termination mode and branching patterns of reticuloreticular and reticulospinal fibers of the nucleus reticularis pontis oralis in the cat: an anterograde PHA-L tracing study. Neurosci Res 17: 9–21, 1993. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, and Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol 410: 413–430, 1999. [PubMed] [Google Scholar]
- Matsuyama K, Ohta Y, and Mori S. Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leukoagglutinin (PHA-L). Brain Res 460: 124–141, 1988. [DOI] [PubMed] [Google Scholar]
- Morales FR, Engelhardt JK, Soja PJ, and Pereda AE. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol 57: 1118–1129, 1987. [DOI] [PubMed] [Google Scholar]
- Morrison AR. A window on the sleeping brain. Sci Am 248: 94–102, 1983. [DOI] [PubMed] [Google Scholar]
- Morrison AR. Paradoxical sleep without atonia. Arch Ital Biol 126: 275–289, 1988. [PubMed] [Google Scholar]
- Paxinos G and Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1986. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K, and Mackel R. Reticulospinal excitation and inhibition of neck motoneurons. Exp Brain Res 32: 471–489, 1978. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, and Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol 349: 85–110, 1994a. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, and Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit, NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci 14: 667–696, 1994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975. [DOI] [PubMed] [Google Scholar]
- Rattay F The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 89: 335–346, 1999. [DOI] [PubMed] [Google Scholar]
- Renshaw B Activity in the simplest spinal reflex pathways. J Neurophysiol 3: 373–387, 1940. [Google Scholar]
- Robbins A, Schwartz-Giblin S, and Pfaff DW. Reticulospinal and reticuloreticular pathways for activating the lumbar muscles in the rat. Exp Brain Res 92: 46–58, 1992. [DOI] [PubMed] [Google Scholar]
- Sakai K Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to PGO waves and postural atonia during paradoxical sleep in the cat. In: The Reticular Formation Revisited, edited by Hobson JA and Brazier MAB. New York: Raven, 1980, p. 427–447. [Google Scholar]
- Sakai K Executive mechanisms of paradoxical sleep. Arch Ital Biol 126: 239–257, 1988. [PubMed] [Google Scholar]
- Schenkel E and Siegel JM. Rem sleep without atonia after lesions of the medial medulla. Neurosci Lett 98: 159–165, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, and Gillin JC. Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep 11: 1–16, 1988a. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Bruce G, Hersh LB, Groves PJ, and Gillin JC. Relation of pontine choline acetyltransferase immunoreactive neurons with cells which increase discharge during REM sleep. Brain Res Bull 18: 447–455, 1987. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, and Gillin JC. Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci Lett 95: 19–23, 1988b. [DOI] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, and Lufkin R. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J Neurosci 12: 1640–1646, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, and Chiu C. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science 262: 1315–1318, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K A HRP study in the cat of brainstem projections to the spinal cord, with particular reference to sacral afferents. Arch Ital Biol 123: 155–170, 1985. [PubMed] [Google Scholar]
- Taguchi O, Kubin L, and Pack AI. Evocation of postural atonia and respiratory depression by pontine carbachol in the decerebrate rat. Brain Res 595: 107–115, 1992. [DOI] [PubMed] [Google Scholar]
- Tohyama M, Sakai K, Salvert D, Touret M, and Jouvet M. Spinal projections from the lover brain stem in the cat as demonstrated by horseradish peroxidase technique. I. Origins of the reticulospinal tracts and their funicular trajectories. Brain Res 173: 383–403, 1979. [DOI] [PubMed] [Google Scholar]
- Winsky L, Isaacs KR, and Jacobowitz DM. Calretinin mRNA and immunoreactivity in the medullary reticular formation of the rat: colocalization with glutamate receptors. Brain Res 741: 123–133, 1996. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Behbehani MM, and Beckstead RM. Ascending and descending projections from nucleus reticularic magnocellularis and nucleus reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat. Brain Res 292: 207–220, 1984. [DOI] [PubMed] [Google Scholar]


