Abstract
In the enteric nervous system, neurons make contact with smooth muscle cells to regulate gastrointestinal functions. Since neuronal cell alterations and intestinal motor dysfunctions are strictly linked, in vitro models based on the culture of neurons or smooth muscle cells are of great advantage to elucidate the functions of the enteric nervous system under physiological and pathological conditions. In this chapter, we provide the protocol for isolation of neurons and smooth muscle cells from the myenteric plexus of adult mice. The isolated cells are suitable for immunocytochemical applications or electrophysiological investigations and/or co-culturing experiments.
Keywords: Enteric nervous system, Longitudinal muscle/myenteric plexus, Glial cell line-derived neurotropic factor, Enzymatic digestion, Ganglia
1. Introduction
The enteric nervous system (ENS) is one of the divisions of the autonomic nervous system that consists of a network of enteric nerves and glia embedded in the wall of the gut and organized in the myenteric, submucosal, and mucosal plexuses. The ENS contains over 500 million neurons that closely communicate with intestinal smooth muscle cells to coordinate gastrointestinal functions. Indeed, the myenteric plexus neurons are flanked by the circular and longitudinal layers of the muscular digestive tract and regulate muscular tone and velocity and intensity of gut contractions [1]. Enteric neurons are composed of sensory neurons, interneurons, and motor neurons. Sensory neurons receive mechanical, thermal, osmotic, and chemical information from the mucosa and muscle. Upon integration of information in interneurons, motor neurons act directly on effector cells including smooth muscle, secretory cells, and gastrointestinal endocrine cells. Neural circuitries and the released neurotransmitters regulate peristalsis, fluid secretion and absorption, gastrointestinal motility, blood flow, inflammation, and pain perception [2].
The importance of the ENS is highlighted by the wide range of intestinal dysfunctions secondary to enteric neuropathies [3]. Many aspects of the normal organization and pathological functioning of the ENS have been resolved in recent years by the use of in vitro and ex vivo models mainly based on the culture of neurons, glia, and smooth muscle cells from the murine intestinal tract. Traditionally, neurons have been cultured from guinea pig [4]. However, cultures of enteric neurons from the intestine of mice have unique advantages of enabling the use of a variety of knockout models, large availability of species-specific reagents, and affordable housing and breeding costs.
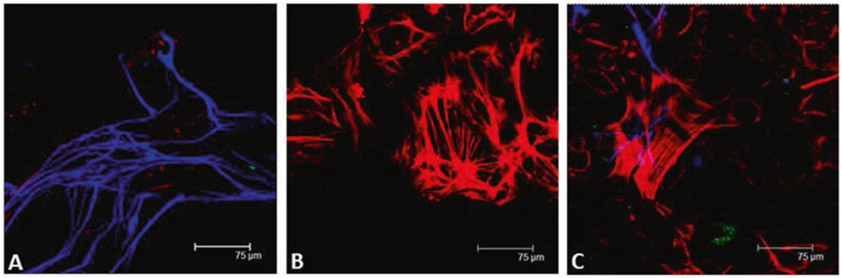
The protocol described in this chapter begins with the isolation of the longitudinal muscle layers with the attached myenteric plexus (LMMP, longitudinal muscle/myenteric plexus). By choosing the conditions of tissue digestion, the method results in a mixed culture of enteric neurons and glia cells or in a purified culture of smooth muscle cells. The presence of glial cells is highly advantageous since glia contributes to the survival of cultured neurons and maintains expression of neuronal cell surface receptors [5]. Phenotype of cultured cells can be assessed by immunostaining techniques using anti-βIII-tubulin or anti-peripherin antibodies for neuronal cells (Fig. 1, panel a), anti-S100β or anti-glial fibrillary acidic protein antibodies for glial cells, and anti-α-smooth muscle actin antibody for smooth muscle cells (Fig. 1, panel b) [6, 7]. Since neurons are isolated from the myenteric plexus, this protocol is ideal for studying cell integrity using immunocytochemical applications and for assessing neuronal cell function by electrophysiology. The two techniques, however, require different grades of cell isolation. In immunocytochemical studies, neurons ideally display growth in ganglia and connections by long neurites. The patch clamp technique requires that neurons grow separately or in small aggregates allowing for voltage control. Therefore, in this protocol, a gentle digestion (15 min at 37 °C) is indicated for culture, stimulation, and phenotype assessment of neurons, whereas LMMP are more extensively digested for electrophysiological studies [8] Overall, this procedure provides cultures of enteric neurons, glial cells, or smooth muscle cells for a wide range of studies and techniques. Moreover, the protocol is easy to master and describes a times-efficient and low-cost procedure.
Fig. 1.
(a) Neurons were isolated from myenteric plexus of adult mice and cultured as described in Subheading 3.3. Five days later, cells were fixed in 10% paraformaldehyde for 20 min and labeled with anti-peripherin antibody (blue). (b) Enteric smooth muscle cells were isolated and cultured as described in Subheading 3.5, fixed, and labeled with anti-α-smooth muscle actin antibody (red). (c) Enteric smooth muscle cells were isolated and seeded on pre-coated glass coverslips in complete smooth muscle cell culture medium. Two days later, freshly prepared enteric neurons were added, and co-cultures were incubated in complete neuron medium. Five days later, cultures were fixed and labeled with anti-peripherin antibody (blue) and anti-α-smooth muscle actin antibody (red)
2. Materials
2.1. Culture Media and Other Solutions
Dilute laminin stock solution to 5 μg/mL with double-distilled H2O (ddH2O). Aliquot laminin solution and store at −70 °C.
Prepare DNase type II (bovine pancreas) solution by dissolving 10 mg in 1 mL RPMI Medium 1640. Sterilize by filtration using 0.22 μm pore size filters. Aliquot and store at −20 °C.
Krebs solution. Add the following components and amounts: 13.79 g NaCl, 0.686 g KCl, 0.312 g NaH2PO4, 4.20 g NaHCO3, 3.96 g D-glucose, 0.289 g MgSO4, and 0.555 g CaCl2 in 2 L ddH2O. Store the solution at 4 °C.
Neuronal digestion solution. Prepare 13 mg collagenase type II and 3 mg bovine serum albumin in 10 mL RPMI Medium 1640. Sterilize by filtration using 0.22 μm pore size filters.
Complete neuron medium. Culture medium for enteric neurons consists of Neurobasal-A medium with B-27 supplement 1×, L-glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), sodium pyruvate (1 mM), 1% fetal bovine serum, and 10 ng/mL human recombinant glial cell line-derived neurotropic factor.
Trypsin solution. Prepare 0.05% trypsin solution by combining 1 mL warmed 0.25% trypsin and 4 mL warmed RPMI Medium 1640.
Smooth muscle cell digestion solution. Prepare 14 mg collagenase type II and 3 mg dispase II in 5 mL RPMI Medium 1640. Sterilize by filtration using 0.22 μm pore size filters. Add DNase type II solution to final concentration of 10 μg/mL.
Complete smooth muscle cell culture medium. Culture medium for enteric smooth muscle cells consists of Dulbecco’s Modified Eagle’s Medium with 2 mM L-glutamine, penicillin-streptomycin 1×, 1 mM sodium pyruvate, and 10% fetal bovine serum.
3. Methods
3.1. Preparation of Poly-L-Lysine- and Laminin-Coated Glass Coverslips
Perform all procedures under sterile conditions, working in a biological safety cabinet and using sterile reagents. Use sterile forceps to place coverslips into culture tissue plates.
Pipette poly-L-lysine (MW ≥300,000) stock (0.01% in ddH2O) on top of each glass coverslip. Use 150 μL of stock/cm2. Incubate at room temperature for 30 min.
Remove poly-L-lysine by aspiration and rinse coverslips once with ddH2O. Allow coverslips to dry for at least 30 min under a laminar flow biological cabinet.
Thaw aliquots of laminin. Use 100 μL of laminin solution/cm2. Incubate at room temperature for 1 h.
Aspirate the laminin solution and rinse coverslips once with ddH2O. Allow coverslips to dry for at least 1 h under the laminar flow cabinet (see Note 1).
3.2. Tissue Dissection
Enteric neurons and smooth muscle cells are routinely cultured from three adult C57BL/6 J mice weighing over 18 g (more than 4 weeks of age) (see Note 2). Following approval from ethics committee, euthanize mice by cervical dislocation.
Place the animal dorsal side down and clean skin by spraying 70% ethanol. Using scissors make an incision through the skin along the ventral line from the sternum to the pubis. Open the abdominal cavity and remove the ileum. Place the ileum in a plastic dish containing sterile Krebs solution.
Section each ileum in three pieces, and clean fecal matter by using a blunt needle attached to a 10 mL syringe containing sterile Krebs solution. Gently run Krebs through the gut section until all fecal matter is removed, and place the cleaned section into a new plastic dish with fresh Krebs solution.
To remove the LMMP, cut the ileum in pieces of approximately 2 cm in length. Insert a glass rod inside one intestinal segment, and using the thumb gently pin the tissue to the rod to prevent the segment from rotating (see Note 3). Using sharp forceps remove mesentery or fat attached to the intestinal segments. To separate the LMMP from the underlying circular muscle, first, gently rub a cotton swab along the line where the mesentery was attached, from top to bottom of the segment. Then, starting by the gap, gently tease away the longitudinal muscle all around the segment using the same cotton swab and applying light pressure. Use the forceps to collect the resulting thin strip of LMMP in a 50 mL tube containing 30 mL sterile Krebs solution. Repeat for all the segments.
After all the strips of LMMP have been collected from all mice. remove biological contaminants by centrifugation (350 × g, 5 min). Carefully remove the supernatant, fill with an additional 30 mL Krebs solution, and repeat twice.
3.3. Cultures of Enteric Neurons for Immunocytochemistry
Add neuronal cell digestion solution to the rinsed strips of LMMP. Transfer to a plastic dish and use scissors to snip the LMMP into tiny pieces.
Transfer to a new 50 mL tube, and digest the LMMP for 15 min at 37 °C in a water bath under continuous shaking (see Note 4).
Gather cells by centrifugation (350 × g, 8 min). Carefully remove the supernatant, and resuspend cell mixture in 3 mL complete neuron medium. To remove tissue fragments, filter the cell mixture through sterilized nylon mesh (100 μm pore size) placed on the top of a 15 mL tube.
Centrifuge (350 × g, 8 min), remove, and discard the supernatant. Resuspend cells in 3 mL complete neuron medium. Gently add 150 μL of cell suspension in the middle of a coated glass coverslip, and place in a humidified, water-jacketed culture incubator at 37 °C and 5% CO2/95% air for at least 2 h to let cells adhere. Add 1 mL of complete neuron medium. Change medium every 2 days (see Note 5).
3.4. Cultures of Enteric Neurons for Electrophysiological Use
For electrophysiological studies, LMMP are more extensively digested. Thus, strips of LMMP are incubated with neuronal digestion solution as described in Subheading 3.3 (step 1) for 1 h at 37 °C in water bath under continuous shaking.
After digestion is complete, collect cells by centrifugation (350 × g, 8 min, at 4 °C).
Carefully remove the supernatant and resuspend cell mixture in 5 mL of 0.05% trypsin solution.
Digest cells for 7 min at 37 °C in a water bath under continuous shaking (see Note 6).
Immediately after digestion is complete, neutralize trypsin by adding 10 mL of cold Krebs solution.
Centrifuge cells (350 × g, 8 min), and remove and discard the supernatant. Gently resuspend the cell mixture in 3 mL complete neuron medium (see Note 7).
To remove tissue fragments, filter cell mixture through sterilized nylon mesh (100 μm pore size) placed on the top of a 15 mL tube.
Centrifuge (350 × g, 8 min), remove, and discard the supernatant. Resuspend cells in 10 mL complete neuron medium. Gently add 850 μL of cell/cm2 coated glass coverslip. Change medium every 2 days. Neurons are ready for electrophysiological studies after 1–2 days in culture.
3.5. Cultures of Intestinal Smooth Muscle Cells
To obtain cultures of intestinal smooth muscle cells, prepare strips of LMMP as described in steps 1–5, Subheading 3.2.
Add smooth muscle cell digestion solution to the rinsed strips of LMMP. Transfer to a plastic dish and use scissors to snip the LMMP into tiny pieces.
Transfer to a new 50 mL tube, and digest LMMP for 10 min at 37 °C in a water bath under continuous shaking.
After digestion is complete, use a 1 mL pipette tip to triturate the cell suspension by mixing up and down.
Gather cells by centrifugation (350 × g, 8 min). Carefully remove the supernatant, and resuspend cell mixture in 4 mL complete smooth muscle cell culture medium. Gently add 1 mL of cell suspension to each 2.25 cm2 glass coverslip, or add the entire cell suspension to a 25 cm2 tissue culture flask. Incubate at 37 °C, 5% CO2. Change medium every 2 days (see Note 8).
4. Notes
Prepare poly-L-lysine- and laminin-coated glass coverslips at least 24 h before cell isolation. Let solution of poly-L-lysine completely dry before incubating with laminin. Once coated, store coverslips at 4 °C for up to 1 week. Avoid scraping the coated surface.
This protocol has been optimized for culturing neurons from the ileum of three adult C57BL/6 J mice. However, this method is adaptable to other strains of mice such as Swiss Webster mice [8]. Three adult C57BL/6 J mice are needed to prepare four glass coverslips of 2.25 cm2 for immunocytochemistry. In our experience, using more animals to increase cell yield is unadvisable. The use of a larger pool of animals can increase the time required in the first steps of the procedure thus greatly reducing cell survival. Moreover, low-density cell preparations are ideal to reduce the risk of contamination (by microbes or other cells) in the final cultures.
In this step, it is preferable to use glass Pasteur pipettes. Break the thinner edge of the pipettes and use the larger part as a rod. The intestinal segments should closely fit and be lightly stretched on the rod. Fire-polish the rough finish of the pipettes by exposing to a Bunsen burner flame to create a smooth and sealed end and avoid tissue damage.
Carefully monitor the time of digestion to avoid over-digesting the tissue. Check the digestion after 10 min and then every 2 min. Stop digestion when the medium becomes cloudy, and tissue fragments are still present but separate from each other.
After 24 h in culture, neurons will not be readily evident. Living, round cells of indistinct phenotype can be seen. Do not change the medium during the first day of culture. Neurons will begin to show neurite outgrowth starting by the second day. Cultures can be maintained for not longer than 6 days. After that, granulated enlarged cells usually contaminate the preparations.
Do not exceed 7 min of total trypsin treatment or neurons will perish.
While resuspending gently triturate the mixture using a 1 mL pipette tip and mix up and down. Avoid generation of air bubbles.
Smooth muscle cells do adhere to uncoated glass coverslips. Cultures can be maintained for over 20 days in culture, subculturing two or three times. However, for co-culture experiments (Fig. 1, panel c) with neuronal cell preparations, it is preferable to seed smooth muscle cells on glass coverslips pre-coated with poly-L-lysine and laminin 2 days before isolation of neurons.
References
- 1.Furness J (2008) The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil 20:32–38 [DOI] [PubMed] [Google Scholar]
- 2.Nezami BG, Srinivasan S (2010) Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep 12:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Giorgio R, Guerrini S, Barbara G et al. (2004) Inflammatory neuropathies of the enteric nervous system. Gastroenterology 126:1872–1883 [DOI] [PubMed] [Google Scholar]
- 4.Hirst GDS, McKirdy HC (1975) Synaptic potentials recorded from neurones of the sub-mucous plexus of Guinea-pig small intestine. J Physiol 249:369–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubé A-C, Cabarrocas J, Bauer J et al. (2006) Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55:630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brun P, Giron MC, Qesari M et al. (2013) Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 145:1323–1337 [DOI] [PubMed] [Google Scholar]
- 7.Brun P, Gobbo S, Caputi V et al. (2015) Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci 68:24–35 [DOI] [PubMed] [Google Scholar]
- 8.Smith TH, Ngwainmbi J, Grider JR et al. (2013) An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J Vis Exp 78:e50688. [DOI] [PMC free article] [PubMed] [Google Scholar]