Abstract
Purpose:
Risks of dehydration and cognitive decline increase with advancing age, yet the relation between dehydration, water intake, and cognitive performance among older adults remains understudied.
Methods:
Using data from the 2011–2014 cycles of the Nutrition and Health Examination Survey (NHANES), we tested if calculated serum osmolarity (Sosm) and adequate intake (AI) of water among women (n=1,271) and men (n=1,235) ≥60 years-old were associated with scores of immediate and delayed recall, verbal fluency, and attention/processing speed. Sosm was categorized as <285 (hyperhydrated), 285-289, 290-294, 295-300, or >300 (dehydrated) mmol/L. AI on water was defined as ≥2 L/day for women and ≥2.5 L/day for men.
Results:
Women with Sosm between 285-289 mmol/L scored 3.2-5.1 points higher on the Digit Symbol Substitution test (DSST) of attention/processing speed than women in other Sosm categories (P-values<0.05). There was evidence of a curvilinear relationship between DSST scores and Sosm among women and men (p-values for quadratic terms<0.02). Meeting an alternative AI on water intake of ≥1 mL/kcal and ≥1500 mL, but not the sex-specific AI, was associated with scoring one point higher on a verbal fluency test (P=0.02) and two points higher on the DSST (P=0.03) among women. Significant negative associations between dehydration or inadequate water intake and test scores were not observed among men.
Conclusion:
Hydration status and water intake were moderately associated with attention/processing speed among females. Future work should consider the effects of both dehydration and overhydration on cognitive function and investigate potential sex differences in cognitive responses to hydration status.
Keywords: dehydration, water intake, cognitive performance, older adults, serum osmolarity
INTRODUCTION
Researchers have long proposed that mild to moderate dehydration, particularly when surpassing 2% body mass loss, impairs cognitive function [1–4]. Yet the existing research has not consistently supported this hypothesis [5,6,4,7]. Moreover, much of what we know about the relation between hydration status and cognitive performance is based on trials that induce acute dehydration through exposure to heat and/or exercise, both of which may independently influence alertness, mood, and cognitive performance [5,8–12,6]. As it may be unlikely to reach >2% body water loss in the absence of heat or prolonged physical activity [6], it remains unclear if and how hydration status under typical daily living conditions relates to variations in cognitive performance.
This question is particularly important among older adults, who tend to be less likely to meet recommendations on water intake [13–15] and who are at greater risk for dehydration due to blunted sensitively to thirst signals, lower body water reserves due to reduced muscle mass, reduced ability to deal with heat stress, and use of medications or laxatives with diuretic effects [16–20,14]. Existing signs of cognitive impairment have been documented as a risk factor for inadequate water intake and dehydration among older adults living in long-term care facilities [21–24]. However, the literature scarcely examines water intake as a predictor of cognitive performance among free-living older adults, and the few studies that have assessed hydration status as a potential predictor of cognitive function among community dwelling older adults have been inconclusive. For instance, neither reported water intake nor urine specific gravity (USG), a biomarker of hydration status, were associated with immediate and delayed recall, visual processing speed and mental flexibility, or the Mini Mental State Examination (MMSE) screening for dementia in a sample 60 of community-living Polish adults between the ages of 60 to 93 [25]; but most of that sample was adequately hydrated (USG≤1.020 g/mL). In another study, the positive associations between total body water and declarative and working memory observed among a sample of 21 postmenopausal women were mediated by blood pressure [26], and it was unclear what proportion of that sample was actually dehydrated. Other studies reporting reduced reaction time in response to exercise-induced elevations in urinary osmolality [27] or lower scores on measures of psychomotor processing speed and attention/memory in relation to reduced bioelectric impedance measures of total body water following overnight water restriction [28] involved either multiple days of long durations of uphill walking [27] or caffeine restriction [28] that could bias the findings.
Given the dearth of information on hydration status, fluid intake, and cognitive performance among free-living elderly populations, the objective of this study was to assess how hydration status and water intake relate to cognitive tests scores among community-dwelling older adults in the United States using nationally-representative, cross-sectional survey data collected by the National Health and Nutrition Examination Survey (NHANES). Previous assessments of NHANES data have demonstrated that older adults in the United States tend to consume less fluid (both from beverages and moisture in food) compared with young and middle-aged adults [29,13,15] and have higher prevalence of elevated plasma tonicity [30]. However, those studies did not examine whether variation in water intake or the consequent effect on hydration status among older adults was related to measures of cognitive performance. Hence, this study specifically aimed to test how hydration status and total water intake relate to performance on cognitive tests of immediate and delayed recall, verbal fluency, processing speed, and attention.
METHODS
Study sample
NHANES, a program of the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC), uses a stratified, multistage probability design to obtain cross-sectional data from a nationally representative sample of the community-dwelling population in the United States. The NHANES program intentionally oversamples older adults, Hispanics, non-Hispanic (NH) blacks, low-income whites/other ethnicities and non-Hispanic Asians. This study was restricted to data from adults aged ≥60 years collected in the 2011–2012 and 2013–2014 NHANES cycles because cognitive tests were administered only to that age group and only in those two survey waves.
NHANES carries out both in-home interviews and physical examinations in the Mobile Examination Center (MEC). The examination response rate for older adults in these cycles was 58.1%. All protocols have received approval by the National Center for Health Statistics Research Ethics Review Board. More details on the study design, sampling scheme, response rates, and study procedures, as well as the publicly available data, can be found online [31].
Measures of cognitive performance
Three separate cognitive tests that measured 1) verbal learning and memory, 2) verbal fluency, and 3) processing speed and attention were conducted in participants’ preferred language during the 2011–2014 NHANES cycles [32–34]. The first test administered was the Consortium to Establish a Registry for Alzheimer’s Disease Word Learning module (CERAD W-L), a component of the neuropsychology battery [35] that tests immediate and delayed recall (verbal learning and memory) of a list of ten unrelated words. Participants read aloud a list of ten unrelated words that flashed on a computer screen and were immediately asked to recall as many words as possible. This was repeated three times, each time with the words presented in a different order. Following the three trials, there was an 8- to 10-minute delay, during which the other two cognitive tests were completed before being asked a final time to recall as many words as possible. One point was earned for each correct word recalled in each round. We used the sum of the first three rounds for a maximum score of 30 to assess immediate recall (CERAD-IR), and the maximum score for the single delayed recall trial (CERAD-DR) was 10. Neither this nor the other cognitive tests conducted by NHANES provide a clinical diagnosis of dementia [32]. However, previous research has suggested that, among older adults, CERAD-IR scores of <14 are associated with cognitive impairment [36,37]. CERAD-DR scores in the range of 6-7 appear to be common among non-impaired older adults [36,37] and younger adults [38].
The second cognitive test was the Animal Fluency test (AFT), another CERAD component of the neuropsychology battery used for evaluating categorical verbal fluency (a measure of executive function) [32–34]. For this test, participants were asked to name as many animals as possible in one minute, scoring one point per animal named. Only participants who were able to name three items of clothing on a practice test completed the AFT. Scores of less than 15 have been associated with a greater likelihood of dementia, though the scores of individuals without apparent cognitive impairment is variable [39,40].
The third cognitive test was the Digit Symbol Substitution test (DSST), which is a component of the Wechsler Adult Intelligence Scale III (WAIS-III) [41] that evaluates processing speed, sustained attention, and working memory [32–34]. For this test, participants were given a sheet of paper with a code table containing the numbers 1-9, each paired with a distinct symbol. Below the code table were 133 boxes containing numbers 1-9 in random order; participants were instructed to draw the symbol that corresponded with the number as paired in the code table. One point was earned for each correctly matching symbol drawn within a 2-minute timeframe. A brief practice test was given prior to the timed test to ensure that participants understood the instructions and could perform the task. Though there is no specific score that is considered diagnostic of cognitive impairment, an average of 28 symbol-number matchings per minute was observed among older adults without measurable cognitive impairment in a study that used a 90-second version of the DSST, while an average of 19 and 13 symbol-number matchings per minute were reported among older adults with subclinical and clinical cognitive impairment, respectively [42]. Another study reported average scores of approximately 35 to 40 points per minute for a broader population of younger adults between the ages of 20 and 54 and progressively lower scores, as well as increasing variability in scores, with age among older adults [43].
Measures of hydration and water intake
Calculated serum osmolarity
This study assessed hydration status using calculated serum osmolarity (Sosm), which is considered a more reliable biomarker of hydration status than urinary markers in older adults [44]. Venous blood draws were performed during the MEC visit in the morning, mid-day, or afternoon. Blood samples were processed, stored, and shipped to the Collaborative Laboratory Services, Ottumwa, Iowa for analysis where they were analyzed for a series of 22 analytes including blood urea nitrogen (BUN), glucose, sodium (Na+), and potassium (K+) on the DxC800 Modular chemistry analyzer [45]. With all analytes measured in mmol/L, Sosm was calculated using the following equation [46]:
This equation was demonstrated to provide good diagnostic accuracy of dehydration among older men and women with and without diabetes or poor renal function [47,48].
Dehydration and impending dehydration were defined as Sosm >300 and 295-300 mmol/L, respectively [47,44,23]. Hyperhydation was defined as Sosm <285 mmol/L[49]. The euhydrated Sosm range was divided into categories of 285-294 and 290-294 mmol/L.
Total water intake
Data on all foods and beverages consumed the day prior to the cognitive exams were collected by trained interviewers during the MEC visit using a computer-assisted multiple-pass 24-hour (midnight to midnight) dietary recall (24-HR). We assessed total water intake using the measure of total moisture content (mL) calculated from all foods, beverages, and plain water reported on the recall. The primary measure of adequate intake (AI) of water was based on the cut-points of ≥2 L/day for women and ≥2.5 L/day for men recommended by the European Food and Safety Authority recommendations [50]. These recommendations are more conservative than the 2.7 L/day and 3.7 L/day recommendations of the Institute of Medicine [51] and may be more appropriate for less active older adults who may be at greater risk not only for dehydration but also for water intoxication if they have low serum sodium levels (a common effect of salt-restricted diets and/or diuretic use) [52–55]. Acknowledging that water needs may vary depending on caloric intake and energy expenditure, an “alternative AI” defined as ≥1 mL/kcal and ≥1500 mL [56,57] was also used for a sensitivity analysis.
Covariates
Age (as an ordinal variable in 5-year categories), sex (male or female), self-reported race and Hispanic origin (NH white, NH black, Hispanic, or “Other Ethnicity”), and educational attainment (less than high school, high school graduate, some college, or college graduate) were accounted for in our study because of reports that younger adults, women, self-reported NH whites, and individuals with higher educational attainment tend to consume more moisture-dense diets and be more likely to meet recommendations on water intake [13,29]. Time of examination (morning, afternoon, or evening) was included because time of day can affect hydration measures [58] and alertness [59]. As some participants were asked to fast for ≥8.5 hours prior to their blood draw or reported voluntarily fasting for extended periods of time, we controlled for the self-reported number of hours (as a continuous, linear variable) since intake of food.
Body mass index (BMI), which may modify the relationship between water intake and hydration status [60], was calculated (kg/m2) using weight and height measurements taken in the MEC visit. Physical activity, also associated with both hydration needs [61] and cognitive performance among older adults [62], was measured using the Global Physical Activity Questionnaire [63] and dichotomized by whether individuals were meeting the minimum national recommendations for older adults of ≥75 minutes of vigorous-intensity or ≥150 minutes of moderate-intensity physical activity/week, or an equivalent combination of moderate- and vigorous-intensity activity [64]. Alcohol, which may act as a diuretic at certain levels (e.g., >30 g for older men [65]) and is at similar amounts also associated with elevated risk of dementia when consumed regularly [66], was estimated in grams from the 24-HRs and categorized as 0, >0-30 g, or >30 g. Caffeine appears to increase urinary output only when consumed at more than “moderate” (e.g., >3-4 cups/day), at least among younger and health adults [67–71], but it can affect attention and alertness as lower amounts [67,72]. We therefore categorized caffeine estimates from the 24-HRs as 0 mg, >0-200 mg, >200-400 mg, and >400 mg.
Hours of nighttime sleep was assessed through the Computer-Assisted Personal Interviewing (CAPI) question, “How much sleep do you usually get at night on weekdays or workdays?” This variable was categorized as ≤6 hours, 7-8 hours and ≥9 hours, based on evidence that both short and long durations of sleep may be associated with hypohydration [73], and sleep deficits (≤6 hours) are associated with impaired cognitive performance [74].
An indicator for diabetes status was included because [20,30] diabetes can negatively impact kidney health [75], thereby affecting electrolyte and body water balance; furthermore, kidney disease, diabetes, insulin resistance, and elevated glycated hemoglobin (HbA1c) have all been associated with cognitive decline in epidemiological studies [76–81]. A participant was considered to have diabetes if their HbA1c at the MEC visit was ≥6.5% or if they reported a prior diagnosis from a doctor of diabetes [82,75].
Finally, we reported on the proportion of individuals in our sample with low (<135 mmol/L) or high (>145 mmol/L) levels of serum sodium or with signs of stage 3-5 kidney failure defined as estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 [83]. Estimations of eGFR were derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations specified for race, sex, and creatinine levels [84].
Statistical Analysis
To account for the complex, four-stage sample design of NHANES, all analyses used survey commands available through Stata 15.1 (College Station, TX; National Center for Health Statistics, 2015), which estimate standard errors using Taylor series linearization [85]. We used the NHANES day one dietary sampling weights to adjust for over-sampling, nonresponse, noncoverage, and day of the week. Weighted descriptive statistics were obtained for the combined 2011-2012 and 2013-2014 samples. Fractional polynomial scatterplots were generated to illustrate unadjusted relationships between Sosm (continuous) and each of the four test scores, and univariate t-statistics were used to compare pairwise differences in test scores across the categories of Sosm and water intake.
Multiple linear regression models were used to test the relationship between hydration status (Reference Sosm: 285-289 mmol/L) or meeting the EFSA AI of water and each of the four cognitive test scores adjusting for covariates: age, education, sex, race/Hispanic origin, time of exam, number of hours fasting, BMI, compliance with physical activity recommendations, caffeine intake, alcohol intake, nighttime sleep duration, and diabetes status. The models testing AI of water as the primary predictor also adjusted for caloric intake estimated from the 24-HRs. Because there was evidence of an interaction between sex and Sosm for the CERAD-IR, CERAD-DR, and DSST models, multivariate analyses were conducted separately for women and men.
Sensitivity analyses were performed to gain a better understand of whether the observed relationships held up if dichotomizing Sosm by dehydration status (>300 mmol/L) or using the alternative AI of water (≥1 mL/kcal and ≥1500 mL). Another sensitivity analysis tested for quadratic trends between test scores and Sosm. A final sensitivity analysis involved reanalyzing the models testing the relations between Sosm categories or the alternative AI and tests scores with further adjustment for blood glucose (modeled as a linear and quadratic covariate) and eGFR (<60 mL/min/1.73 m2) [83,84], while also excluding additional outliers: participants who reported fasting for >18 hours at the time of the blood draw; reported <500 kcal the day prior to their exam; had hypoglycemia (blood glucose <3 mmol/L); or presented with either low (<135 mmol/L) or high (>145 mmol/L) serum sodium levels.
RESULTS
Excluding older adults with missing data on any cognitive tests, Sosm, water intake, and covariate measures yielded an analytic sample of 2,506 adults (see Supplemental Figure 1 for participant flow chart): 1,271 women and 1,235 men (Table 1). Nearly two-thirds of the sample had at least some college education. Women had a mean BMI of 29.2 kg/m2, and men had a BMI of 28.9 kg/m2. Approximately 18.7% and 22.3% of women and men, respectively, had a known diagnosis of diabetes or HbA1c levels indicative of diabetes (≥6.5%) on the day of the MEC visit.
Table 1:
Demographic, lifestyle, and health characteristics of study sample, US adults ≥60 years old, NHANES 2011-2014
| Women (n=1,271) a | Men (n=1,235) a | |||
|---|---|---|---|---|
| Weighted Means | SE | Weighted Means | SE | |
| Age (years): | ||||
| 60-64 | 31.9% | 2.1% | 33.2% | 2.3% |
| 65-69 | 23.3% | 1.4% | 25.7% | 1.8% |
| 70-74 | 19.5% | 1.2% | 17.5% | 1.4% |
| 75-79 | 10.3% | 0.7% | 11.3% | 1.0% |
| ≥80 | 15.2% | 1.1% | 12.2% | 1.1% |
| Race/Ethnicity: | ||||
| NH white | 79.5% | 2.0% | 79.6% | 2.0% |
| NH Black | 8.6% | 1.4% | 7.4% | 1.1% |
| Hispanic | 7.0% | 1.3% | 7.5% | 1.1% |
| Other Ethnicity | 4.9% | 0.7% | 5.5% | 0.7% |
| Education level: | ||||
| <High school education | 15.3% | 1.6% | 16.0% | 1.8% |
| High school graduate | 24.0% | 1.8% | 19.9% | 1.9% |
| Some college | 35.4% | 2.0% | 27.7% | 2.0% |
| College graduate+ | 25.4% | 2.1% | 36.4% | 2.6% |
| BMI (kg/m2) | 29.2 | 0.2 | 28.9 | 0.3 |
| Moderate and vigorous activity (minutes/week) | 337 | 21 | 489 | 38 |
| Physical activity>150 minutes/week | 47.5% | 1.8% | 58.7% | 2.0% |
| Hours of sleep: | ||||
| Sleep ≤6 hours | 28.6% | 1.4% | 26.8% | 1.9% |
| Sleep 7-8 hours | 61.4% | 1.5% | 63.1% | 2.2% |
| Sleep ≤9 hours | 10.0% | 0.8% | 10.1% | 1.3% |
| Session Time: | ||||
| Morning | 50.4% | 1.8% | 50.3% | 2.1% |
| Midday | 41.1% | 1.7% | 38.5% | 1.8% |
| Afternoon | 8.6% | 1.2% | 11.2% | 1.4% |
| Kilocalories b | 1636 | 26 | 2167 | 28 |
| Caffeine (mg) b | 154 | 6 | 213 | 13 |
| Caffeine: 0 mg | 8.6% | 0.7% | 8.0% | 1.1% |
| Caffeine: >0 to 200 mg | 61.4% | 1.8% | 49.2% | 2.0% |
| Caffeine: >200 to 400 mg | 22.4% | 1.8% | 28.4% | 1.4% |
| Caffeine: >400 mg | 7.6% | 1.0% | 14.3% | 1.6% |
| Alcohol (g) b | 5 | 1 | 11 | 1 |
| Alcohol: 0 g | 79.8% | 1.7% | 69.2% | 1.7% |
| Alcohol: >0-30 g | 14.7% | 1.4% | 15.8% | 1.4% |
| Alcohol: >30 g | 5.5% | 1.0% | 15.0% | 1.4% |
| Total water intake moisture (gm) b | 2596 | 55 | 2850 | 63 |
| Meeting EFSA AI c | 66.2% | 2.2% | 54.3% | 2.2% |
| Meeting alternative AI d | 75.5% | 1.8% | 68.0% | 2.1% |
| Calculated Sosm (mmol/L) e | 293.5 | 0.2 | 294.2 | 0.4 |
| Sosm: <285 mmol/L | 6.9% | 1.0% | 5.1% | 1.1% |
| Sosm: 285-289 mmol/L | 16.4% | 1.7% | 14.2% | 1.3% |
| Sosm: 290-294 mmol/L | 36.9% | 1.9% | 34.7% | 1.9% |
| Sosm: 295-300 mmol/L | 29.6% | 1.9% | 33.3% | 1.9% |
| Sosm: >300 mmol/L | 10.2% | 1.0% | 12.6% | 1.5% |
| Blood Glucose (mmol/L) | 5.9 | 0.1 | 6.1 | 0.1 |
| Diabetes (Diagnosed or HbA1c ≥6.5%) | 18.7% | 1.3% | 22.3% | 1.6% |
| eGFR (mL/min/1.73 m2) f | 73.7 | 0.6 | 73.4 | 0.7 |
| eGFR <60 mL/min/1.73 m2 | 22.7% | 1.5% | 20.6% | 1.7% |
| Sodium (mmol/L) | 139.5 | 0.1 | 139.5 | 0.2 |
| Low sodium (<135 mmol/L) | 3.5% | 0.5% | 3.1% | 0.8% |
| Normal Sodium (135-145 mmol/L) | 95.9% | 0.6% | 96.6% | 0.7% |
| High sodium (>145 mmol/L) | 0.6% | 0.3% | 0.3% | 0.2% |
| Hours fasting at time of blood draw | 7.3 | 0.2 | 7.3 | 0.2 |
Unweighted sample size
Estimated from one 24 hour-recall on foods and beverages consumed from midnight to midnight the day prior to the exam.
EFSA AIs defined as ≥2 L/day for women and ≥2.5 L/day for men
Alternative AI defined as 1 mL/kcal and minimum 1500 mL total water intake
Estimated as 1.86×(Na++ K+)+1.15×glucose+BUN+14 (all analytes in mmol/L).
Estimated using the CKD+EPI equations specific for race, sex, and serum creatinine
Calculated Sosm ranged from 269 to 319 mmol/L (mean: 293±5.2). Approximately half the sample was within the euhydrated range of calculated Sosm between 285-294 mmol/L. Nonetheless, 29.6% and 33.3% of women and men had Sosm levels indicative of impending dehydration (295-300 mmol/L) while another 10.2% and 12.6% of women and men, respectively, had Sosm levels indicative of dehydration (>300 mmol/L). Individuals in the dehydrated category tended to have higher BMI and elevated blood glucose and BUN levels but lower eGFR levels; 49.1% had eGFR levels in the range associated with stage 3-5 kidney failure (Supplemental Table 1).
Mean total water intake was 2.6±1.0 L among women and 2.9±1.2 L among men, and an estimated 66.2% and 54.3% of women and men, respectively, were meeting the EFSA AI recommendations (Table 1). Of the overall sample that was falling short on their water intake, approximately 34.1% and 14.3% had Sosm levels in the 295-300 and >300 mmol/L range, respectively (data not shown). However, at least half of participants within each Sosm category, including those considered dehydrated, were estimated to be meeting EFSA AI recommendations (Supplemental Table 1).
Serum osmolarity and cognitive performance
CERAD-IR scores ranged from 0 to 30 with a mean of 19.8±4.0; CERAD-DR scores ranged from 0 to 10 with a mean of 6.3±2.0. In the bivariate comparisons among the full sample (women and men combined), scores for the CERAD-IR and CERAD-DR tests did not differ across categories of Sosm (Fig 1a and Fig 1b).
Fig 1.
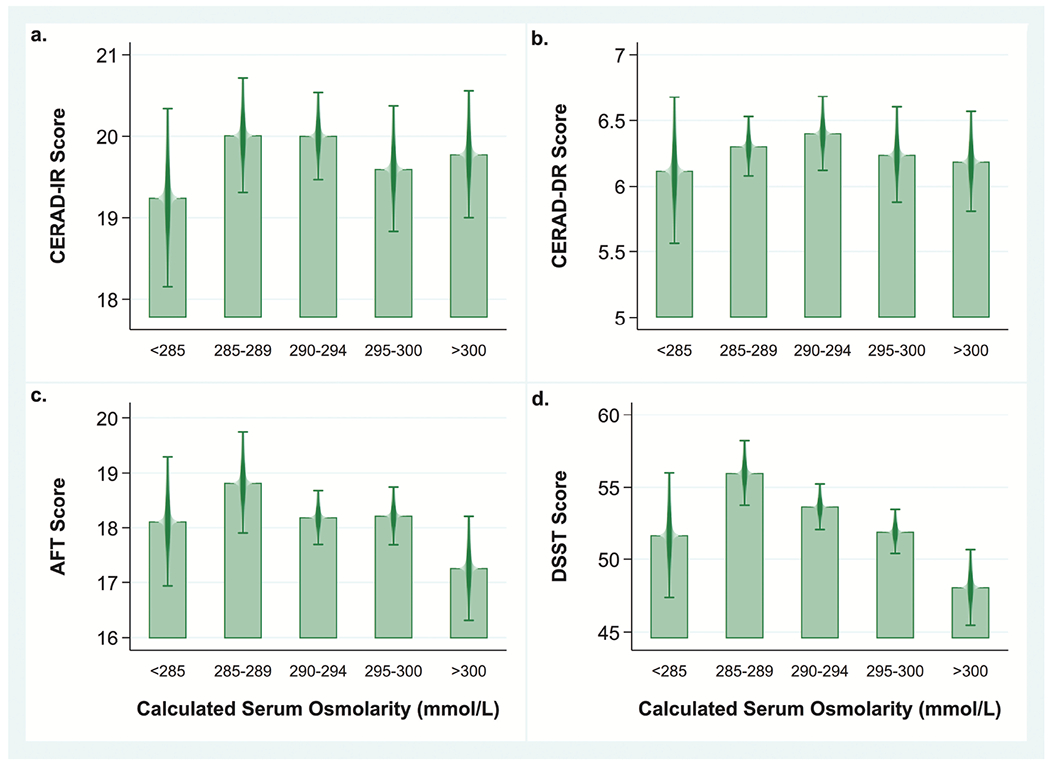
Mean cognitive tests scores by category of calculated serum osmolarity among US women and men ≥60 years old (n=2,506), NHANES 2011–2014.
Note: Bivariate analyses comparing mean cognitive test scores across the five categories of calculated serum osmolarity (Sosm) using a univariate t statistic at the P< 0.05 significance level not adjusted for covariates.
a. Mean CERAD IR scores for Sosm<285 mmol/L: 19.2±0.5; Sosm 285-289 mmol/L: 20.0±0.3; Sosm 290-294 mmol/L: 20.0±0.3; Sosm 295-300 mmol/L: 19.6±0.4; Sosm >300 mmol/L: 19.8±0.4. No significant differences across Sosm categories.
b. Mean CERAD IR scores for Sosm<285 mmol/L: 6.1±0.3; Sosm 285-289 mmol/L: 6.3±0.1; Sosm 290-294 mmol/L: 6.4±0.1; Sosm 2950-300 mmol/L: 6.2±0.2; Sosm >300 mmol/L:6.2±0.2. No significant differences across Sosm categories.
c. Mean AFT scores for Sosm<285 mmol/L: 18.1±0.6; Sosm 285-289 mmol/L: 18.8±0.5; Sosm 290-294 mmol/L: 18.2±0.2; Sosm 295-300 mmol/L: 18.2±0.3; Sosm >300 mmol/L: 17.3±0.5. AFT scores were significantly lower in the group with Sosm >300 mmol/L compared with the group with Sosm 285-289 mmol/L (−1.6±0.6, p=0.02) and the group with Sosm 295-300 mmol/L (−1.0±0.4, P=0.04).
d. Mean DSST scores Sosm<285 mmol/L: 51.7±2.1; Sosm 285-289 mmol/L: 56.0±1.1; Sosm 290-294 mmol/L: 53.6±0.8; Sosm 295-300 mmol/L: 51.9±0.8; Sosm >300 mmol/L: 48.0±1.3. DSST scores were significantly lower in the group with Sosm >300 mmol/L compared with the group with Sosm 285-289 mmol/L (−7.9±1.4, P<0.001), the group with Sosm 290-294 mmol/L (−5.6±1.3 p<0.001) and the group with Sosm 295-300 mmol/L (−3.8±1.3, p<0.005). They were also lower in the group with Sosm 295-300 compared with the group with Sosm 285-289 mmol/L (−4.1±1.2, P=0.002).
AFT scores ranged from 3 to 40 with a mean of 18.2±5.0. In the bivariate analysis, the AFT scores were, on average, 1.6±0.6 (P=0.02) and 0.9±0.4 (P=0.04) lower in the dehydrated group compared to those with Sosm between 285-289 and 295-300 mmol/L, respectively (Fig 1c).
The DSST scores ranged from 0 to 105 with a mean of 52.7±14.8, which translated into approximately 26.4 points/minute. The mean DSST score of 48.0±15.8 in the dehydrated group were 7.9±1.4, 5.6±1.3, and 3.8±1.3 points lower than those with Sosm between 285-289, 290-295, and 295-300 mmol/L, respectively, in the bivariate analysis (all P-values≤0.005) (Fig 1d).The 3.6-point difference between those with Sosm>300 versus <285 mmol/L was not significant (P=0.2)
Among women, the multivariate analyses provided no evidence for an association between Sosm and the CERAD-IR or CERAD-DR scores (Table 2). There was a trend toward lower scores on the AFT among women with high Sosm relative to the reference category (285-289 mmol/L), though the difference was only statistically significant for women with Sosm between 290-294 mmol/L. On the DSST, women in the reference group scored an average of 3.5±1.2 (P=0.007), 3.2±1.5 (P=0.04), and 4.6±1.8 (P=0.01) points higher than women in the 290-294, 295-300, and >300 mmol/L groups, respectively. They also scored an average of 5.1±2.3 (p=0.03) points higher on the DSST than women in the lowest Sosm group.
Table 2:
Linear regression analyses estimating relationships between calculated serum osmolarity (Sosm) and cognitive performance test scores among women and men ≥60 years old, NHANES 2011-2014.a
| Women (n=1,271) b | Men (n=1,235) b | |||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLES | CERAD-IR | CERAD-DR | AFT | DSST | CERAD-IR | CERAD-DR | AFT | DSST |
| Calculated Sosm | ||||||||
| 285-289 mmol/L | REF | REF | REF | REF | REF | REF | REF | REF |
| <285 mmol/L | −0.5 (0.5) | −0.03 (0.2) | −0.8 (0.8) | −5.1** (2.3) | −0.7 (0.7) | −0.1 (0.4) | 0.2 (0.9) | −2.5 (2.5) |
| 290-294 mmol/L | −0.3 (0.3) | 0.1 (0.2) | −1.2** (0.5) | −3.5*** (1.2) | 0.8 (0.6) | 0.3 (0.2) | 0.6 (0.8) | 1.6 (1.0) |
| 295-300 mmol/L | −0.2 (0.5) | 0.04 (0.2) | −0.2 (0.6) | −3.2** (1.5) | 0.8* (0.5) | 0.5** (0.2) | 0.5 (0.8) | 2.4* (1.2) |
| >300 mmol/L | 0.5 (0.5) | 0.4 (0.3) | −1.0 (0.6) | −4.6** (1.8) | 1.9*** (0.7) | 0.9** (0.3) | 0.5 (0.9) | 1.6 (1.4) |
| Age (5 yrs) | −1.0*** (0.1) | −0.5*** (0.1) | −1.1*** (0.1) | −4.7*** (0.3) | −1.0*** (0.1) | −0.5*** (0.1) | −0.9*** (0.2) | −3.7*** (0.3) |
| < High school | REF | REF | REF | REF | REF | REF | REF | REF |
| High school graduate | 1.5*** (0.4) | 0.4* (0.3) | 1.7*** (0.4) | 8.3*** (1.2) | 0.4 (0.4) | −0.3 (0.3) | −0.3 (0.6) | 7.5*** (1.7) |
| Some college | 1.8*** (0.5) | 0.6** (0.2) | 2.6*** (0.3) | 12.2*** (1.3) | 1.9*** (0.5) | 0.6*** (0.2) | 2.4*** (0.6) | 11.6*** (1.2) |
| College graduate+ | 2.9*** (0.4) | 1.1*** (0.3) | 4.8*** (0.5) | 15.1*** (1.6) | 2.0*** (0.6) | 0.5* (0.3) | 3.8*** (0.5) | 16.5*** (1.4) |
| Physical activity (≥150 mins/week) | 0.5* (0.3) | 0.3** (0.1) | 1.1*** (0.3) | 3.1*** (0.8) | 0.3 (0.3) | 0.2 (0.2) | 1.1** (0.5) | 2.9*** (0.9) |
| Sleep, 7-8 hours | REF | REF | REF | REF | REF | REF | REF | REF |
| Sleep, ≥6 hours | −0.1 (0.3) | 0.3* (0.1) | −0.2 (0.4) | −0.1 (0.9) | −0.4 (0.4) | 0.01 (0.2) | −0.4 (0.4) | 0.3 (1.1) |
| Sleep, ≥9 hours | −1.1** (0.5) | −0.3 (0.3) | −1.2** (0.5) | −4.8*** (1.4) | −0.7 (0.5) | −1.0*** (0.3) | −0.7 (0.8) | −1.2 (1.1) |
| Diabetes (diagnosed or HbA1c≥6.5%) | −0.8** (0.4) | −0.7*** (0.2) | −0.5 (0.4) | −4.1*** (1.1) | −0.4 (0.3) | −0.3* (0.2) | −0.5 (0.6) | −3.3*** (1.1) |
| Caffeine, 0 mg c | REF | REF | REF | REF | REF | REF | REF | REF |
| Caffeine, >0-200 mg c | 0.9* (0.5) | 0.3 (0.2) | 0.5 (0.5) | 2.9** (1.1) | 0.2 (0.5) | 0.4 (0.3) | −0.8 (0.6) | 0.6 (1.2) |
| Caffeine, >200-400 mg c | 1.2** (0.5) | 0.4 (0.3) | 0.9 (0.7) | 5.5*** (1.4) | 0.7 (0.4) | 0.7** (0.3) | 0.2 (0.6) | 1.4 (1.2) |
| Caffeine, >400 mg c | 1.0 (0.6) | 0.4* (0.3) | −0.3 (0.8) | −0.1 (2.0) | 0.1 (0.6) | 0.2 (0.3) | −0.2 (0.7) | −0.8 (1.9) |
| Alcohol, 0 g c | REF | REF | REF | REF | REF | REF | REF | REF |
| Alcohol, >0-30 g c | −0.1 (0.5) | 0.1 (0.2) | 0.3 (0.5) | 3.1** (1.4) | −0.1 (0.4) | −0.1 (0.3) | 0.5 (0.5) | 1.0 (1.2) |
| Alcohol, >30 g c | −0.5 (0.7) | −0.5 (0.4) | −0.1 (0.6) | 2.7 (2.2) | −0.2 (0.4) | 0.2 (0.3) | 0.6 (0.7) | 2.6 (1.6) |
| R-squared | 0.20 | 0.19 | 0.31 | 0.48 | 0.19 | 0.19 | 0.23 | 0.46 |
Standard errors in parentheses
P<0.001,
P<0.05,
P<0.1
Notes: Constant not shown; REF=Reference category; CERAD-IR=Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning– Immediate Recall; CERAD-DR=CERAD Word Learning – Delayed Recall; AFT=Animal Fluency Test. DSST=Digit Symbol Substitution Test.
Adjusted for covariates shown and covariates not shown (race/Hispanic origin, time of exam, number of hours between last meal and blood draw, and BMI).
Unweighted sample size
Estimated from one 24 hour-recall on foods and beverages consumed from midnight to midnight the day prior to the exam.
In contrast, the multivariate analyses suggested that men in the reference group scored 1.8±0.7 (P =0.008) points lower on the CERAD-IR compared with men in the dehydrated group and 0.5±.02 (P=0.03) and 0.9±0.3 (P=0.01) points lower on the CERAD-DR tests than men in the 295-300 and >300 mmol/L groups, respectively. There were no significant differences in the AFT or DSST scores across distinct Sosm categories among men.
The covariates with significant negative associations with cognitive tests scores included older age, lower level of education, indication of diabetes, failure to meet physical activity recommendations, and reported sleep durations of ≥9 hours. There was also evidence that women who attended the MEC visit in the afternoon scored, on average, 3.1±1.4 (p0.04) points higher than women whose visits were in the morning, but time of day was otherwise not associated with the other test scores for women or with any of the test scores for men (data not shown).
Despite the evidence of lower DSST scores among women in the dehydrated when Sosm was categorized into 5 distinct levels, there was no evidence of such a relationship when Sosm was dichotomized as ≤300 or >300 mmol/L (Supplemental Table 2). This was likely due to a significant quadratic relationship between Sosm and DSST, suggesting that both high and low Sosm levels were associated with lower DSST scores among both women and men (Supplemental Table 3). Similar but non-significant curvilinear trends appeared with the other test scores as well (Fig 3a–3d).
Fig. 3.
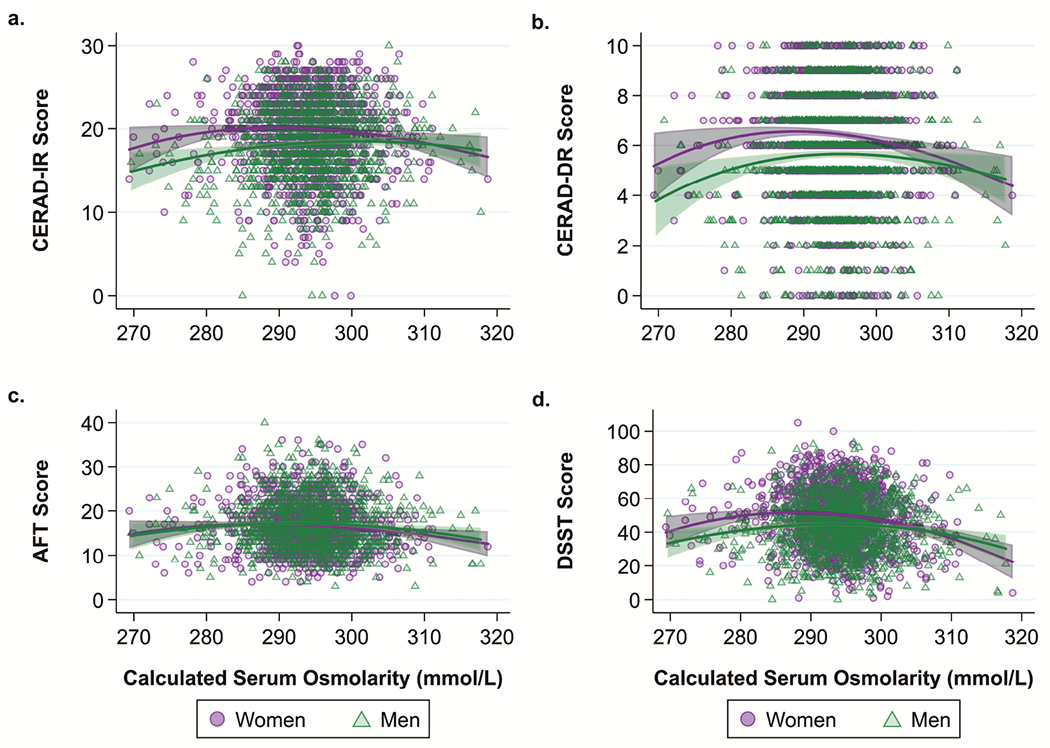
Fractional polynomial plots of continuous relationship between cognitive test scores and calculated serum osmolarity (Sosm) among US women (n=1,271) and men (n=1,235) ≥60 years old, NHANES 2011–2014.
Note: Not controlling for covariates
a. Fractional polynomial plot of the relationship between Consortium to Establish a Registry for Alzheimer’s Disease Word Learning–Immediate Recall (CERAD-IR) score (runs 1-3 combined) and Sosm.
b. Fractional polynomial plot of the relationship between Consortium to Establish a Registry for Alzheimer’s Disease Word Learning–Delayed Recall (CERAD-DR) score and Sosm.
c. Fractional polynomial plot of the relationship between Animal Fluency Test (AFT) score and Sosm.
d. Fractional polynomial plot of the relationship between Digit Symbol Substitution Test (DSST) score and Sosm.
In our final sensitivity analyses, adjustment for blood glucose and eGFR and exclusion of individuals with particularly low caloric intake, extended fasting prior to the test, hypoglycemia, or low or high serum sodium levels attenuated the relationship between Sosm categories and DSST among women (Supplemental Table 4). Notably, being in the low eGFR category (indicative of kidney problems) was associated with an estimated 2.3±1.0 (P=0.04) lower DSST score among women, and there was evidence of a significant quadratic relationship (p=0.02) between blood glucose and DSST even when controlling for known diabetes or elevated HbA1c. In contrast, the additional covariates and exclusions in these sensitivity analyses only made the positive relationships between higher Sosm categories and CERAD-IR, CERAD-DR, and DSST scores among men appear more prominent.
Water intake and cognitive performance
In the bivariate comparisons, scores for all four cognitive tests were significantly lower among adults who failed to meet EFSA recommendations on AI of water (all P-values for pairwise comparisons<0.001) (Fig 2a–2d). However, the multivariate analyses found no significant associations between AI of water as defined by EFSA and test scores among either women or men (Table 3). Using the alternative AI defined as ≥1 mL/kcal and ≥1500 mL/day, on the other hand, the data suggested that women not meeting recommendations scored on average 1.0±0.4 (P=0.02) and 2.2 ± 1.0 (P=0.03) lower score on the AFT and DSST, respectively; while men trended in that same direction (Supplemental Table 5). These findings were consistent in the sensitivity analyses that adjusted for glucose and eGFR and excluded outliers (Supplemental Table 6).
Fig. 2.
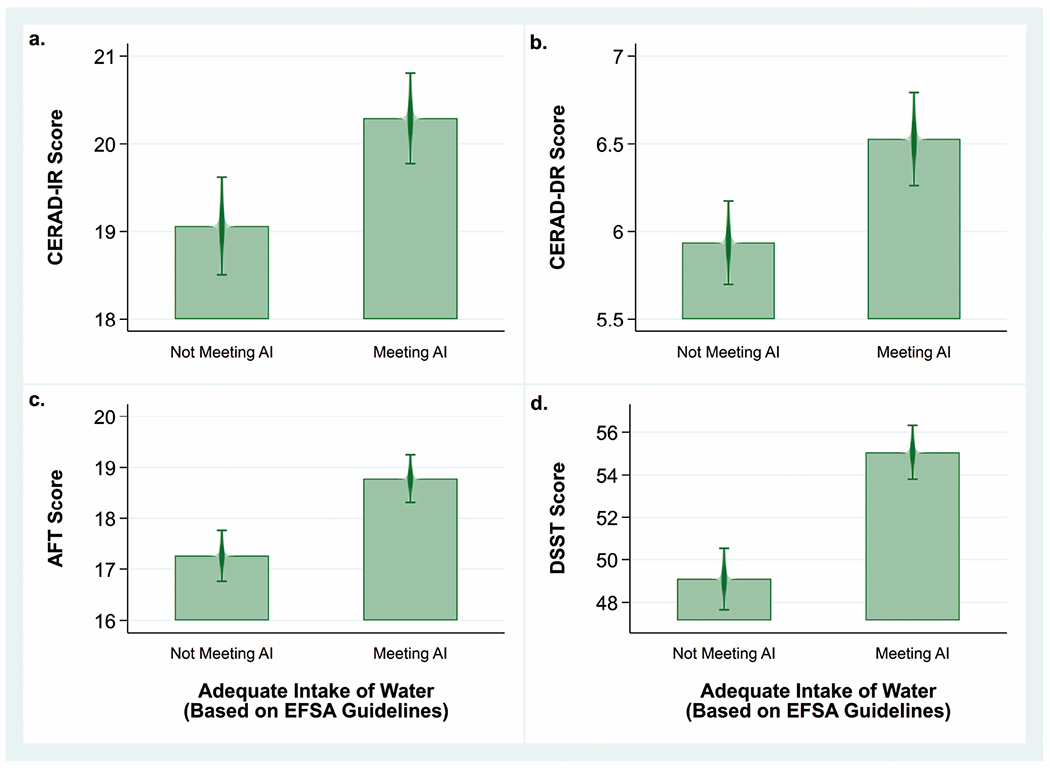
Mean cognitive test scores by category of water intake among US women and men ≥60 years old (n=2,506), NHANES 2011–2014.
Note: Bivariate analyses comparing mean cognitive test scores between participants meeting or not meeting EFSA recommendations for adequate intake (AI) of water (≥2 L/day for women and ≥2.5 L/day for men) using a univariate t statistic at the P< 0.05 significance level not adjusted for covariates.
a. Mean CERAD-IR scores among those not meeting AI (19.1±0.3) and meeting AI (20.3±0.3). Difference=1.2±0.2, P<0.001
b. Mean CERAD-DR scores among those not meeting AI (5.9±0.1) and meeting AI (6.5±0.1). Difference=0.6±0.1, P<0.001.
c. Mean AFT scores among those not meeting AI (17.3±0.2) and meeting AI (18.8±0.2). Difference=1.5±0.3, P<0.001.
d. Mean DSST scores among those not meeting AI (49.1±0.7) and meeting AI (55.1±0.6). Difference=6.0±0.7, P<0.001.
Table 3:
Linear regression analyses estimating relationships between EFSA guidelines on adequate water intake and cognitive performance test scores among women and men ≥60 years old, NHANES 2011-2014.a
| Women (n=1,271) b | Men (n=1,235) b | |||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLES | CERAD-IR | CERAD-DR | AFT | DSST | CERAD-IR | CERAD-DR | AFT | DSST |
| Meeting AI c | REF | REF | REF | REF | REF | REF | REF | REF |
| Not Meeting AI c | 0.1 (0.3) | −0.1 (0.2) | 0.2 (0.4) | 0.1 (0.7) | −0.2 (0.3) | 0.01 (0.2) | −0.5 (0.4) | −0.5 (0.9) |
| Age (5 yrs) | −1.0*** (0.1) | −0.5*** (0.1) | −1.1*** (0.1) | −4.8*** (0.3) | −0.9*** (0.1) | −0.5*** (0.1) | −0.9*** (0.2) | −3.5*** (0.2) |
| < High school | REF | REF | REF | REF | REF | REF | REF | REF |
| High school graduate | 1.4*** (0.5) | 0.4 (0.3) | 1.6*** (0.3) | 8.4*** (1.1) | 0.4 (0.4) | −0.2 (0.3) | −0.3 (0.6) | 7.4*** (1.8) |
| Some college | 1.8*** (0.5) | 0.6** (0.3) | 2.6*** (0.3) | 12.4*** (1.3) | 1.9*** (0.5) | 0.6*** (0.2) | 2.3*** (0.7) | 11.5*** (1.1) |
| College graduate+ | 2.8*** (0.5) | 1.0*** (0.3) | 4.7*** (0.5) | 15.2*** (1.6) | 2.1*** (0.7) | 0.5* (0.3) | 3.8*** (0.5) | 16.5*** (1.4) |
| Physical activity (≥150 mins/week) | 0.5* (0.3) | 0.3** (0.1) | 1.0*** (0.3) | 2.9*** (0.8) | 0.2 (0.3) | 0.1 (0.1) | 1.0* (0.5) | 2.8*** (0.9) |
| Sleep, 7-8 hours | REF | REF | REF | REF | REF | REF | REF | REF |
| Sleep, ≤6 hours | −0.1 (0.3) | 0.3** (0.1) | −0.2 (0.4) | 0.03 (0.9) | −0.4 (0.4) | −0.00 (0.2) | −0.4 (0.4) | 0.3 (1.2) |
| Sleep, ≥9 hours | −1.1** (0.5) | −0.3 (0.3) | −1.2** (0.5) | −4.9*** (1.4) | −0.7 (0.4) | −1.0*** (0.3) | −0.7 (0.8) | −1.5 (1.1) |
| Diabetes (diagnosed or HbA1c≥6.5%) | −0.6 (0.4) | −0.6*** (0.2) | −0.5 (0.4) | −4.4*** (1.1) | −0.2 (0.3) | −0.2 (0.2) | −0.5 (0.6) | −3.1*** (1.0) |
| Caffeine, 0 mg d | REF | REF | REF | REF | REF | REF | REF | REF |
| Caffeine, >0-200 mg d | 0.8* (0.5) | 0.2 (0.2) | 0.3 (0.5) | 2.5** (1.1) | 0.01 (0.5) | 0.3 (0.3) | −0.8 (0.6) | 0.3 (1.2) |
| Caffeine, >200-400 mg d | 1.1** (0.5) | 0.4 (0.3) | 0.8 (0.7) | 5.1*** (1.5) | 0.5 (0.4) | 0.7** (0.3) | 0.1 (0.5) | 1.1 (1.2) |
| Caffeine, >400 mg d | 0.8 (0.7) | 0.3 (0.3) | −0.4 (0.9) | −0.7 (2.0) | −0.3 (0.7) | 0.1 (0.3) | −0.4 (0.7) | −1.5 (1.8) |
| Alcohol, 0 g d | REF | REF | REF | REF | REF | REF | REF | REF |
| Alcohol, >0-30 g d | −0.2 (0.5) | 0.02 (0.2) | 0.1 (0.5) | 2.7* (1.4) | −0.02 (0.4) | −0.1 (0.3) | 0.5 (0.5) | 1.1 (1.3) |
| Alcohol, >30 g d | −0.6 (0.7) | −0.5 (0.4) | −0.3 (0.7) | 2.4 (2.3) | −0.3 (0.4) | 0.2 (0.3) | 0.5 (0.7) | 2.3 (1.6) |
| R-squared | 0.20 | 0.19 | 0.30 | 0.48 | 0.18 | 0.18 | 0.23 | 0.45 |
Standard errors in parentheses
P<0.001,
P<0.05,
P<0.1
Notes: Constant not shown. REF=Reference category; CERAD-IR=Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning– Immediate Recall; CERAD-DR=CERAD Word Learning – Delayed Recall; AFT=Animal Fluency Test. DSST=Digit Symbol Substitution Test.
Adjusted for covariates shown and covariates not shown (race/Hispanic origin, time of exam, number of hours between last meal and blood draw, BMI, and estimated calorie intake on previous day).
Unweighted sample size
EFSA AIs defined as ≥2 L/day for women and ≥2.5 L/day for men of total water from foods and beverages. Estimated from one 24 hour-recall on foods and beverages consumed from midnight to midnight the day prior to the exam.
Estimated from one 24 hour-recall on foods and beverages consumed from midnight to midnight the day prior to the exam.
DISCUSSION
Given the increased risks of dehydration and cognitive decline that accompany the aging process, this study aimed to test whether hydration status and water intake among a nationally representative sample of community-dwelling older adults was associated with measures of cognitive function. Despite bivariate analyses suggesting significantly lower average scores on the AFT and DSST among all individuals (women and men combined) with Sosm>300 mmol/L relative to those with Sosm 285-289 mmol/L, the magnitude of the association was diminished among women and was completely absent among men when we controlled for age, education, diabetes status, sleep duration, physical activity, and other confounding factors. Similarly, bivariate associations between meeting EFSA recommendations on water disappeared in the multivariate analyses. Nonetheless, the multivariate analyses suggested that women in the higher and the lowest Sosm categories tended to score approximately 3- to 5-points lower on the DSST than those with Sosm 285-289 mmol/L. Additionally, women failing to consume ≥1mL/kcal and ≥1500 mL water scored approximately two points lower on the DSST score and one point lower on the AFT. These performance differences in relation to Sosm and water intake were similar in magnitude to the difference in scores observed within the same models in relation to a 5-year increase in age.
The finding that the DSST was the cognitive test associated with hydration status and water intake among women is in line with one meta-analysis suggesting that attention is one of the cognitive domains most consistently shown to be negatively impacted by heat- and exercise-induced dehydration in experimental studies [4]. Although these findings were not supported in another meta-analysis restricted to only cross-over experimental studies [7], there is reason to believe that tasks that are more cognitively demanding or that require longer durations of sustained attention may be more affected by hydration levels than short, less complex tasks [86]. In contrast, the CERAD tests of verbal memory and AFT evaluation of verbal fluency may have been less cognitively demanding than the DSST, or perhaps there was simply greater mental fatigue by the time participants finished the CERAD-IR and AFT and moved on to the DSST.
It is difficult to explain the spurious results in the data suggesting higher scores on the CERAD test scores among men in the dehydrated group relative to those with Sosm between 285-289 mmol/L. Sex differences with dehydration appearing favorable for young men but detrimental for young women have been reported for tests of reaction time [87] and error rates on choice reaction tests [88] in experimental studies inducing dehydration through a 24-hour water restricted diet [87] or water-restricted exercise [88]. However, sex reportedly did not modify the negative effect of a 4-hour heat-induced dehydration experiment on immediate and delayed word-list recall exercises similar to the CERAD-IR and CERAD-DR [89], and few other studies have included both men and women much less explored differences by sex [6].
Though sex hormones can influence thirst and fluid regulation [90–92], they would not likely play as important of a role in elderly populations. Nevertheless, there could be variation in the thresholds at which women and men begin to sense the physical effects of inadequate hydration, and the point at which someone feels discomforted by thirst and dehydration, rather than a given level of dehydration itself, may be what disrupts attention, focus, and ability to perform complex tasks [93,94]. Likewise, different habits around water consumption may shape over a lifetime how well different individuals tolerate a given level of water restriction or begin to feel thirsty or fatigued in response to water deprivation. Our study and others suggest that men are generally less likely than women to meet their respective sex-specific water intake recommendations [13,25,95] and more likely to show signs of dehydration [23,30]. Hence, men may, on average, be accustomed to operating at lower levels of water relative to their body mass and higher levels of Sosm, potentially because they have higher muscle mass and, thereby, higher body water reserves than women. Further research would be needed to determine the degree to which the observed sex differences in response to dehydration in this study or others are real or simply a statistical problem.
The evidence of a curvilinear relationship between Sosm and DSST scores among both women and men was also a noteworthy finding in our study, and it is possible that a similar quadratic relationship went undetected in the models of the CERAD tests but could nonetheless contribute to the unexpected positive relationship between Sosm and CERAD test scores among men. Importantly, these findings raise questions about the appropriateness of defining Sosm levels between 275 and 294 mmol/L as “normal” hydration for older adults [47,44,23]. Our results suggest that mean DSST scores were not significantly different between those with Sosm levels <285 versus >300 mmol/L. Individuals in the lowest Sosm category may have been overhydrated, which can be a result of aging-related reductions in renal capacity to excrete free-water and a cause of hyponatremia [55,52,96], a condition of low serum sodium levels that may independently be associated with increased risk of attention deficits [97], cognitive impairment [98], and dementia [99]. Indeed, a substantial proportion (44.4%) of individuals with calculated Sosm<285 mmol/L had serum sodium levels indicative of hyponatremia (<135 mmol/L). It is telling that excluding those with low serum sodium eliminated the negative association between being in the Sosm<285 mmol/L and DSST scores among women.
Finally, our results suggest that there may be a point of diminishing returns on water intake. This was demonstrated by the findings that meeting EFSA recommendations on AI for water was not significantly associated with cognitive performance among women or men but that failure to meet the alternative AI of 1 mL/kcal and ≥1500 mL was negatively associated with AFT and DSST scores among women with a similar direction, but not statistically significant effect, for men. It is also possible that meeting the alternative AI was also associated with obtaining more calories and nutrients in general. However, these analyses controlled for caloric intake, and the association between meeting this alternative AI and cognitive tests remained robust after excluding individuals with <500 kcal and including adjustment for glucose and eGFR. Other scholars have also proposed that recommendations on AI of water may need to be adjusted downward for older adults who are at risk for overhydration [52].
This study adds to a scarce body of literature among older adults testing but not providing convincing evidence for associations between dehydration and cognitive performance, in part because of study samples not being particularly dehydrated [25], not controlling for confounders but identifying blood pressure as a mediator [26], or experimental designs that hinder the ability to isolate the effects of dehydration from the effects of prolonged uphill walking [27] or caffeine restriction [28]. Notwithstanding, this study has its own set of limitations. First, while many have argued that Sosm is a better measure of hydration status than urinary biomarkers among older adults given the reduced capacity to concentrate urine with age [14,44,100], research has demonstrated that plasma osmolality can be similar among both low and high water drinkers [101,51,102]. This suggests that strong physiological mechanisms are in place to maintain osmolality in the blood unless more extreme levels of dehydration occur [103,104]. For instance, two experimental studies demonstrated that even 24 hours of water deprivation did not necessarily result in Sosm values of >300 mmol/kg [87,105]. Conversely, in a sample of healthy adults (20-70 y) a 3 L/day increase in water intake for a week was shown to decrease plasma osmolality by only 2 mmol/kg while still remaining >290 mmol/kg [106]. Any acute dehydration or rehydration immediately prior to the cognitive testing, for example, would not necessarily be observed in the Sosm.
Second, it is possible that the hydration status of some individuals was misclassified because Sosm was calculated as opposed to being directly measured. The equation for Sosm developed by Khajuria and Krahn [46] was shown to predict directly measured serum osmolality well in older adult men and women with and without diabetes or renal problems [47,48]. Nonetheless, the BUN, glucose, and sodium levels in the serum that were used to calculate Sosm can vary independently from hydration status in relation to dietary factors, kidney function, or pathological conditions [94]. Furthermore, because blood samples were collected at different times of day and not consistently in the fasted state, it is possible that calculated Sosm measures could have also been affected by recent food consumption or other physiological and hormonal processes that fluctuate throughout the day. However, we did control for fasting status and time of day.
Third, the cognitive tests used in this study were chosen for their ease of administration [32], but they may not be as sensitive to variations in hydration status. The chosen language for test administration and cultural factors may also influence test scores [32]. Moreover, the tests did not include a measure or reaction time, which could provide a distinct signal of cognitive performance from the total score.
Fourth, this study used cross-sectional data, so we were unable to determine the directionality of the observed associations. Older adults in our sample with existing cognition impairment may also be less likely to meet hydration needs [23,14,22,24]. Indeed, approximately 46% of our sample scored ≤14 on the CERAD-IR (15.7%), ≤3 on the CERAD-DR (13.9%), <15 on the AFT (35.1%), or <13 points per minute on the DSST (11.7%), which are scores that may be associated with a greater risk of cognitive impairment [39,42,36]. However, only 2.2% of the sample scored below those respective levels on all tests, and average test scores were within the typical ranges reported for non-cognitively impaired older adults [39,40,42,37,36]. Additionally, with cross-sectional data we are making comparisons across individuals, when it may be deviations in one’s own baseline hydration status and resulting shift from one’s own baseline cognitive test score that may be more informative of the relationship between hydration and cognitive performance.
Fifth, we lacked information on the climate, temperature, and altitude of the study participants’ location of residency, which could be additional sources of variation in the degree to which individuals perceive or feel discomforted by thirst.
Finally, it is possible that the study conditions themselves may have disrupted certain factors that could have impacted test scores. For instance, coming to the MEC in a fasted state or an otherwise disrupted eating and drinking schedule on the day of the exam could have biased the study findings. Likewise, our measures of water and caffeine intake were from the previous day and may not accurately reflect the relative degree of water and caffeine intake on the day of the study. Any unmeasured deviations in consumption of these substances prior to the exam may have hindered our ability to detect associations between hydration status or water intake and cognitive performance.
In conclusion, this study is the largest, most comprehensive, well-controlled study known to examine how hydration status or water intake relate to cognitive performance in a nationally representative sample of community-dwelling older women and men. This study provides clues into how hydration status and water intake on a given day may relate to cognitive performance in the absence of major heat or exercise stressors, and it demonstrates that differences in age, education, physical activity, sleep patterns, and diabetes status can account for large portion of any bivariate associations between hydration or water intake and cognitive performance. Nonetheless, scores on the DSST, a measure of sustained attention, processing speed, and working memory, were significantly lower among older women that either had higher Sosm levels, low levels of Sosm, or were not consuming ≥1 mL/kcal and ≥1500 mL total water on the day prior to the exam. There was also evidence of a curvilinear relationship between Sosm and DSST scores among both women and men, suggesting that overhydration, particularly if it results in hyponatremia, may be just as much of a concern for cognitive function as dehydration. Further research is needed to better understand which cognitive tasks are most likely to be affected by water intake, hydration status, and electrolyte imbalance and to investigate potential differences across older women and men in their responses to dehydration.
Supplementary Material
Footnotes
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- 1.Wilson MM, Morley JE (2003) Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr 57 Suppl 2:S24–29. doi: 10.1038/sj.ejcn.1601898 [DOI] [PubMed] [Google Scholar]
- 2.Grandjean AC, Grandjean NR (2007) Dehydration and cognitive performance. J Am Coll Nutr 26 (sup5):549S–554S. doi: 10.1080/07315724.2007.10719657 [DOI] [PubMed] [Google Scholar]
- 3.Adan A (2012) Cognitive performance and dehydration. J Am Coll Nutr 31 (2):71–78. doi: 10.1080/07315724.2012.10720011 [DOI] [PubMed] [Google Scholar]
- 4.Wittbrodt MT, Millard-Stafford M (2018) Dehydration impairs cognitive performance: A meta-analysis. Med Sci Sports Exerc 50 (11):2360–2368. doi: 10.1249/mss.0000000000001682 [DOI] [PubMed] [Google Scholar]
- 5.Masento NA, Golightly M, Field DT, Butler LT, van Reekum CM (2014) Effects of hydration status on cognitive performance and mood. Br J Nutr 111 (10):1841–1852. doi: 10.1017/s0007114513004455 [DOI] [PubMed] [Google Scholar]
- 6.Benton D, Young HA (2015) Do small differences in hydration status affect mood and mental performance? Nutr Rev 73 (suppl_2):83–96. doi: 10.1093/nutrit/nuv045 [DOI] [PubMed] [Google Scholar]
- 7.Goodman SPJ, Moreland AT, Marino FE (2019) The effect of active hypohydration on cognitive function: A systematic review and meta-analysis. Physiol Behav 204:297–308. doi: 10.1016/j.physbeh.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 8.Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, Sorrentino G (2018) Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front Psychol 9:509. doi: 10.3389/fpsyg.2018.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell AR, Burchfield J, Moyen NE, Tucker MA, Butts CL, Elbin RJ, Ganio MS (2018) Obesity, but not hypohydration, mediates changes in mental task load during passive heating in females. PeerJ 6:e5394. doi: 10.7717/peerj.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malcolm RA, Cooper S, Folland JP, Tyler CJ, Sunderland C (2018) Passive heat exposure alters perception and executive function. Front Physiol 9:585. doi: 10.3389/fphys.2018.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benton D (2011) Dehydration influences mood and cognition: A plausible hypothesis? Nutrients 3 (5):555–573. doi: 10.3390/nu3050555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman HR (2007) Hydration and cognition: A critical review and recommendations for future research. J Am Coll Nutr 26 (sup5):555S–561S. doi: 10.1080/07315724.2007.10719658 [DOI] [PubMed] [Google Scholar]
- 13.Drewnowski A, Rehm C, Constant F (2013) Water and beverage consumption among adults in the united states: Cross-sectional study using data from nhanes 2005-2010. BMC Public Health 13 (1):1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper L (2016) Why, oh why, are so many older adults not drinking enough fluid? J Acad Nutr Diet 116 (5):774–778. doi: 10.1016/j.jand.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 15.Rosinger A, Herrick K (2016) Daily water intake among u.S. Men and women, 2009-2012. NCHS data brief Hyattsville, MD: National Center for Health Statistics; 242. [PubMed] [Google Scholar]
- 16.Rolls B, Phillips P (1990) Aging and disturbances of thirst and fluid balance. Nutr Rev 48 (3):137–144 [DOI] [PubMed] [Google Scholar]
- 17.Kenney WL, Hodgson JL (1987) Heat tolerance, thermoregulation and ageing. Sports Med 4 (6):446–456. doi: 10.2165/00007256-198704060-00004 [DOI] [PubMed] [Google Scholar]
- 18.Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL (1990) Age and hypohydration independently influence the peripheral vascular response to heat stress. J Appl Physiol 68 (5):1902–1908. doi: 10.1152/jappl.1990.68.5.1902 [DOI] [PubMed] [Google Scholar]
- 19.Begg DP (2017) Disturbances of thirst and fluid balance associated with aging. Physiol Behav 178:28–34. doi: 10.1016/j.physbeh.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Hooper L, Bunn D, Jimoh FO, Fairweather-Tait SJ (2014) Water-loss dehydration and aging. Mech Ageing Dev 136-137:50–58. doi: 10.1016/j.mad.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong-Esther CA, Browne KD, Armstrong-Esther DC, Sander L (1996) The institutionalized elderly: Dry to the bone! Int J Nurs Stud 33 (6):619–628. doi: 10.1016/S0020-7489(96)00023-5 [DOI] [PubMed] [Google Scholar]
- 22.Marra MV, Simmons SF, Shotwell MS, Hudson A, Hollingsworth EK, Long E, Kuertz B, Silver HJ (2016) Elevated serum osmolality and total water deficit indicate impaired hydration status in residents of long-term care facilities regardless of low or high body mass index. J Acad Nutr Diet 116 (5):828–836.e822. doi: 10.1016/j.jand.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper L, Bunn DK, Downing A, Jimoh FO, Groves J, Free C, Cowap V, Potter JF, Hunter PR, Shepstone L (2016) Which frail older people are dehydrated? The uk drie study. The Journals of Gerontology: Series A 71 (10):1341–1347. doi: 10.1093/gerona/glv205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauriola M, Mangiacotti A, D’Onofrio G, Cascavilla L, Paris F, Paroni G, Seripa D, Greco A, Sancarlo D (2018) Neurocognitive disorders and dehydration in older patients: Clinical experience supports the hydromolecular hypothesis of dementia. Nutrients 10 (5):562. doi: 10.3390/nul0050562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bialecka-Dęek A, Pietruszka B (2018) The association between hydration status and cognitive function among free-living elderly volunteers. Aging Clin Exp Res. doi: 10.1007/s40520-018-1019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suhr JA, Patterson SM, Austin AW, Heffner KL (2010) The relation of hydration status to declarative memory and working memory in older adults. J Nutr Health Aging 14 (10):840–843 [DOI] [PubMed] [Google Scholar]
- 27.Ainslie PN, Campbell IT, Frayn KN, Humphreys SM, MacLaren DP, Reilly T, Westerterp KR (2002) Energy balance, metabolism, hydration, and performance during strenuous hill walking: The effect of age. J Appl Physiol (1985) 93 (2):714–723. doi: 10.1152/japplphysiol.01249.2001 [DOI] [PubMed] [Google Scholar]
- 28.Suhr JA, Hall J, Patterson SM, Niinistö RT (2004) The relation of hydration status to cognitive performance in healthy older adults. International Journal of Psychophysiology 53 (2):121–125. doi: 10.1016/j.ijpsycho.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Kant A, Graubard B, Atchison E (2009) Intakes of plain water, moisture in foods and beverages, and total water in the adult us population--nutritional, meal pattern, and body weight correlates: National health and nutrition examination surveys 1999-2006. Am J Clin Nutr 90 (3):655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stookey JD (2005) High prevalence of plasma hypertonicity among community-dwelling older adults: Results from NHANES III. J Am Diet Assoc 105 (8):1231–1239. doi: 10.1016/j.jada.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics About the National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed February 22 2019
- 32.Brody DJ, Kramarow EA, Taylor CA, McGuire LC (2019) Cognitive performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011–2014. vol no 126. National Center for Health Statistics, Hyattsville, MD: [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (2017) National Health and Nutrition Examination Survey. 2011-2012 data documentation, codebook, and frequencies. Cognitive functioning (cfq_g). https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CFQ_G.htm#Component_Description. Accessed February 2019
- 34.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (2017) National Health and Nutrition Examination Survey. 2013-2014 data documentation, codebook, and frequencies. Cognitive functioning (cfq_h). https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CFQ_H.htm#Component_Description. Accessed February 2019
- 35.Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC, Tariot PN, Silverman JM, Clark CM, Welsh-Bohmer KA, Heyman A (2008) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The first twenty years. Alzheimers Dement 4 (2):96–109. doi: 10.1016/j.jalz.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfsgruber S, Jessen F, Wiese B, Stein J, Bickel H, Mösch E, Weyerer S, Werle J, Pentzek M, Fuchs A, Köhler M, Bachmann C, Riedel-Heller SG, Scherer M, Maier W, Wagner M (2014) The CERAD neuropsychological assessment battery total score detects and predicts Alzheimer disease dementia with high diagnostic accuracy. The American Journal of Geriatric Psychiatry 22 (10):1017–1028. doi: 10.1016/j.jagp.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 37.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A (1994) The consortium to establish a registry for alzheimer’s disease (CERAD). Part v. A normative study of the neuropsychological battery. Neurology 44 (4):609–609. doi: 10.1212/wnl.44.4.609 [DOI] [PubMed] [Google Scholar]
- 38.Hankee LD, Preis SR, Piers RJ, Beiser AS, Devine SA, Liu Y, Seshadri S, Wolf PA, Au R (2016) Population normative data for the cerad word list and victoria stroop test in younger- and middle-aged adults: Cross-sectional analyses from the framingham heart study. Exp Aging Res 42 (4):315–328. doi: 10.1080/0361073X.2016.1191838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canning SJD, Leach L, Stuss D, Ngo L, Black SE (2004) Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology 62 (4):556–562. doi: 10.1212/wnl.62.4.556 [DOI] [PubMed] [Google Scholar]
- 40.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ (1992) Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol 49 (12):1253–1258. doi: 10.1001/archneur.l992.00530360051017 [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D (1997) WAIS-III: Administration and scoring manual. Psychological Corporation, San Antonio, TX [Google Scholar]
- 42.Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S (2016) Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing 45 (5):688–695. doi: 10.1093/ageing/afwll6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardila A (2007) Normal aging increases cognitive heterogeneity: Analysis of dispersion in wais-iii scores across age. Arch Clin Neuropsychol 22 (8):1003–1011. doi: 10.1016/j.acn.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 44.Hooper L, Bunn DK, Abdelhamid A, Gillings R, Jennings A, Maas K, Millar S, Twomlow E, Hunter PR, Shepstone L, Potter JF, Fairweather-Tait SJ (2016) Water-loss (intracellular) dehydration assessed using urinary tests: How well do they work? Diagnostic accuracy in older people. Am J Clin Nutr 104 (1):121–131. doi: 10.3945/ajcn,115.119925 [DOI] [PubMed] [Google Scholar]
- 45.National Health and Nutrition Examination Survey (2015) 2013-2014 data documentation, codebook, and frequencies. Standard biochemistry profile (biopro_h). https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/BIOPRO_H.htm.
- 46.Khajuria A, Krahn J (2005) Osmolality revisited—deriving and validating the best formula for calculated osmolality. Clin Biochem 38 (6):514–519. doi: 10.1016/j.clinbiochem.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 47.Hooper L, Abdelhamid A, Ali A, Bunn DK, Jennings A, John WG, Kerry S, Lindner G, Pfortmueller CA, Sjöstrand F, Walsh NP, Fairweather-Tait SJ, Potter JF, Hunter PR, Shepstone L (2015) Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: Adding value to pathology laboratory reports. BMJ Open 5 (10):e008846. doi: 10.1136/bmjopen-2015-008846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siervo M, Bunn D, Prado CM, Hooper L (2014) Accuracy of prediction equations for serum osmolarity in frail older people with and without diabetes. Am J Clin Nutr 100 (3):867–876. doi: 10.3945/ajcn.114.086769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheuvront SN, Ely BR, Kenefick RW, Sawka MN (2010) Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr 92 (3):565–573. doi: 10.3945/ajcn.2010.29490 [DOI] [PubMed] [Google Scholar]
- 50.European Food Safety Association (2010) EFSA panel on dietetic products, nutrition, and allergies (nda); scientific opinion on dietary reference values for water. EFSA Journal 8 (3):1459 [Google Scholar]
- 51.Institute of Medicine (2005) Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. The National Academies Press, Washington, DC. doi:doi: 10.17226/10925 [DOI] [Google Scholar]
- 52.Begum MN, Johnson CS (2010) A review of the literature on dehydration in the institutionalized elderly. E Spen Eur E J Clin Nutr Metab 5 (1):e47–e53. doi: 10.1016/j.eclnm.2009.10.007 [DOI] [Google Scholar]
- 53.Ferry M (2005) Strategies for ensuring good hydration in the elderly. Nutr Rev 63 (suppl_1):S22–S29. doi: 10.1111/j.1753-4887.2005.tb00151.x [DOI] [PubMed] [Google Scholar]
- 54.Thomas DR, Tariq SH, Makhdomm S, Haddad R, Moinuddin A (2003) Physician misdiagnosis of dehydration in older adults. J Am Med Dir Assoc 4 (5):251–254. doi: 10.1097/01.Jam.0000083444.46985.16 [DOI] [PubMed] [Google Scholar]
- 55.Filippatos TD, Makri A, Elisaf MS, Liamis G (2017) Hyponatremia in the elderly: Challenges and solutions. Clin Interv Aging 12:1957–1965. doi: 10.2147/CIA.S138535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgkinson B, Evans D, Wood J (2003) Maintaining oral hydration in older adults: A systematic review. Int J Nurs Pract 9 (3):S19–S28. doi:doi: 10.1046/j,1440-172X.2003.00425.x [DOI] [PubMed] [Google Scholar]
- 57.Mentes J (2006) Oral hydration in older adults: Greater awareness is needed in preventing, recognizing, and treating dehydration. Am J Nurs 106 (6):40–49 [DOI] [PubMed] [Google Scholar]
- 58.Perrier E, Demazières A, Girard N, Pross N, Osbild D, Metzger D, Guelinckx I, Klein A (2013) Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol 113 (8):2143–2151. doi: 10.1007/s00421-013-2649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight M, Mather M (2013) Look out-it’s your off-peak time of day! Time of day matters more for alerting than for orienting or executive attention. Exp Aging Res 39 (3):305–321. doi: 10.1080/0361073X.2013.779197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosinger AY, Lawman HG, Akinbami LJ, Ogden CL (2016) The role of obesity in the relation between total water intake and urine osmolality in us adults, 2009–2012. Am J Clin Nutr 104 (6):1554–1561. doi: 10.3945/ajcn,116.137414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sawka MN, Cheuvront SN, Carter R (2005) Human water needs. Nutr Rev 63 (suppl 1):S30–S39. doi: 10.1111/j.1753-4887.2005.tb00152.x [DOI] [PubMed] [Google Scholar]
- 62.Frith E, Loprinzi PD (2018) Physical activity is associated with higher cognitive function among adults at risk for alzheimer’s disease. Complement Ther Med 36:46–49. doi: 10.1016/j.ctim.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 63.Armstrong T, Bull F (2006) Development of the world health organization global physical activity questionnaire (gpaq). J Public Health 14 (2):66–70. doi: 10.1007/sl0389-006-0024-x [DOI] [Google Scholar]
- 64.US Department of Health and Human Services (2018) Physical activity guidelines for americans, 2nd edition. Washington, DC [Google Scholar]
- 65.Polhuis KCMM, Wijnen AHC, Sierksma A, Calame W, Tieland M (2017) The diuretic action of weak and strong alcoholic beverages in elderly men: A randomized diet-controlled crossover trial. Nutrients 9 (7):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, Yu J-T (2017) Alcohol consumption and dementia risk: A dose–response meta-analysis of prospective studies. Eur J Epidemiol 32 (1):31–42. doi: 10.1007/s10654-017-0225-3 [DOI] [PubMed] [Google Scholar]
- 67.Ruxton CHS (2008) The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutr Bull 33 (1):15–25. doi: 10.1111/j.1467-3010.2007.00665.x [DOI] [Google Scholar]
- 68.Armstrong LE, Pumerantz AC, Roti MW, Judelson DA, Watson G, Dias JC, Sökmen B, Casa DJ, Maresh CM, Lieberman H, Kellogg M (2005) Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption. 15 (3):252. doi: 10.1123/ijsnem,15.3.252 [DOI] [PubMed] [Google Scholar]
- 69.Killer SC, Blannin AK, Jeukendrup AE (2014) No evidence of dehydration with moderate daily coffee intake: A counterbalanced cross-over study in a free-living population. PLoS One 9 (1):e84154. doi: 10.1371/journal.pone.0084154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva AM, Júdice PB, Marias CN, Santos DA, Magalhães JP, St-Onge M-P, Gonçalves EM, Armada-da-Silva P, Sardinha LB (2013) Total body water and its compartments are not affected by ingesting a moderate dose of caffeine in healthy young adult males. Appl Physiol Nutr Metab 38 (6):626–632. doi: 10.1139/apnm-2012-0253 [DOI] [PubMed] [Google Scholar]
- 71.Neuhäuser-Berthold M, Beine S, Verwied SC, Lührmann PM (1997) Coffee consumption and total body water homeostasis as measured by fluid balance and bioelectrical impedance analysis. Ann Nutr Metab 41(1):29–36. doi: 10.1159/000177975 [DOI] [PubMed] [Google Scholar]
- 72.McLellan TM, Caldwell JA, Lieberman HR (2016) A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev 71:294–312. doi: 10.1016/j.neubiorev.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 73.Rosinger AY, Chang A-M, Buxton OM, Li J, Wu S, Gao X (2019) Short sleep duration is associated with inadequate hydration: Cross-cultural evidence from us and Chinese adults. Sleep 42 (2):1–10. doi: 10.1093/sleep/zsy210 [DOI] [PubMed] [Google Scholar]
- 74.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF (2003) The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26 (2):117–126. doi: 10.1093/sleep/26.2.117 [DOI] [PubMed] [Google Scholar]
- 75.Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, Hall YN, Hirsch IB, de Boer IH (2017) Diabetes and CKD in the united states population, 2009–2014. Clin J Am Soc Nephrol 12 (12):1984–1990. doi: 10.2215/cjn.03700417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH (2007) Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 18 (7):2205–2213. doi: 10.1681/asn.2006101165 [DOI] [PubMed] [Google Scholar]
- 77.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH (2003) Impact of diabetes on cognitive function among older latinos: A population-based cohort study. J Clin Epidemiol 56 (7):686–693. doi: 10.1016/S0895-4356(03)00077-5 [DOI] [PubMed] [Google Scholar]
- 78.Sherzai AZ, Shaheen M, Yu JJ, Talbot K, Sherzai D (2018) Insulin resistance and cognitive test performance in elderly adults: National Health and Nutrition Examination Survey (NHANES). J Neurol Sci 388:97–102. doi: 10.1016/j.jns.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 79.Christman AL, Matsushita K, Gottesman RF, Mosley T, Alonso A, Coresh J, Hill-Briggs F, Sharrett AR, Selvin E (2011) Glycated haemoglobin and cognitive decline: The Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 54 (7):1645–1652. doi: 10.1007/s00125-011-2095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nature Reviews Endocrinology 14 (10):591–604. doi: 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GEHM, Biessels GJ (2015) Cognitive function in patients with diabetes mellitus: Guidance for daily care. The Lancet Neurology 14 (3):329–340. doi: 10.1016/S1474-4422(T4)70249-2 [DOI] [PubMed] [Google Scholar]
- 82.The International Expert Committee (2009) International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32 (7):1327–1334. doi: 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Kidney Foundation (2018) Estimated glomerular filtration rate (EGFR). https://www.kidney.org/atoz/content/gfr. Accessed September 2019
- 84.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150 (9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korn EL, Graubard BI (2011) Analysis of health surveys, vol 323. John Wiley & Sons, [Google Scholar]
- 86.Kempton MJ, Ettinger U, Foster R, Williams SCR, Calvert GA, Hampshire A, Zelaya FO, O’Gorman RL, McMorris T, Owen AM, Smith MS (2011) Dehydration affects brain structure and function in healthy adolescents. Hum Brain Mapp 32 (1):71–79. doi:doi: 10.1002/hbm.20999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U (2005) Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol 289 (1):R275–R280. doi: 10.1152/ajpregu.00501.2004 [DOI] [PubMed] [Google Scholar]
- 88.D’Anci KE, Mahoney CR, Vibhakar A, Kanter JH, Taylor HA (2009) Voluntary dehydration and cognitive performance in trained college athletes. Percept Motor Skills 109 (1):251–269. doi: 10.2466/pms.109.1.251-269 [DOI] [PubMed] [Google Scholar]
- 89.Benton D, Jenkins KT, Watkins HT, Young HA (2016) Minor degree of hypohydration adversely influences cognition: A mediator analysis. Am J Clin Nutr 104 (3):603–612. doi: 10.3945/ajcn.116.132605 [DOI] [PubMed] [Google Scholar]
- 90.Stachenfeld NS (2014) Hormonal changes during menopause and the impact on fluid regulation. Reprod Sci 21 (5):555–561. doi: 10.1177/1933719113518992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vilhena-Franco T, Mecawi AS, Elias LLK, Antunes-Rodrigues J (2016) Oestradiol effects on neuroendocrine responses induced by water deprivation in rats. 231 (2):167. doi: 10.1530/joe-16-0311 [DOI] [PubMed] [Google Scholar]
- 92.Santollo J, Myers KE, Rainer IL, Edwards AA (2019) Gonadal hormones in female rats protect against dehydration-induced memory impairments in the novel object recognition paradigm. Horm Behav 114:104547. doi: 10.1016/j.yhbeh.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edmonds C, Crombie R, Gardner M (2013) Subjective thirst moderates changes in speed of responding associated with water consumption. Front Hum Neurosci 7 (363). doi: 10.3389/fnhum.2013.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheuvront SN, Kenefick RW (2014) Dehydration: Physiology, assessment, and performance effects. Comprehensive Physiology 4 (1):257–285. doi: 10.1002/cphy.c130017 [DOI] [PubMed] [Google Scholar]
- 95.Ferreira-Pêgo C, Guelinckx I, Moreno LA, Kavouras SA, Gandy J, Martinez H, Bardosono S, Abdollahi M, Nasseri E, Jarosz A, Babio N, Salas-Salvadó J (2015) Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur J Nutr 54 (2):35–43. doi: 10.1007/s00394-015-0943-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luckey AE, Parsa CJ (2003) Fluid and electrolytes in the aged. JAMA Surgery 138 (10):1055–1060. doi: 10.1001/archsurg.138.10.1055 [DOI] [PubMed] [Google Scholar]
- 97.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G (2006) Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 119 (1):71.e71–78. doi: 10.1016/j.amjmed.2005.09.026 [DOI] [PubMed] [Google Scholar]
- 98.Nowak KL, Yaffe K, Orwoll ES, Ix JH, You Z, Barrett-Connor E, Hoffman AR, Chonchol M (2018) Serum sodium and cognition in older community-dwelling men. Clin J Am Soc Nephrol 13 (3):366–374. doi: 10.2215/cjn.07400717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung M-C, Yu T-M, Shu K-H, Wu M-J, Chang C-H, Muo C-H, Chung C-J (2017) Hyponatremia and increased risk of dementia: A population-based retrospective cohort study. PLoS One 12 (6):e0178977. doi: 10.1371/journal.pone.0178977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fortes MB, Owen JA, Raymond-Barker P, Bishop C, Elghenzai S, Oliver SJ, Walsh NP (2015) Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc 16 (3):221–228. doi: 10.1016/j.jamda.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 101.Perrier E, Vergne S, Klein A, Poupin M, Rondeau P, Le Bellego L, Armstrong LE, Lang F, Stookey J, Tack I (2013) Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br J Nutr 109 (09):1678–1687. doi:doi: 10.1017/S0007114512003601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lemetais G, Melander O, Vecchio M, Bottin JH, Enhörning S, Perrier ET (2018) Effect of increased water intake on plasma copeptin in healthy adults. Eur J Nutr 57 (5):1883–1890. doi: 10.1007/s00394-017-1471-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Armstrong LE, Maughan RJ, Senay LC, Shirreffs SM (2013) Limitations to the use of plasma osmolality as a hydration biomarker. Am J Clin Nutr 98 (2):503–504. doi: 10.3945/ajcn.113.065466 [DOI] [PubMed] [Google Scholar]
- 104.Shirreffs SM (2003) Markers of hydration status. Eur J Clin Nutr 57:S6. doi: 10.1038/sj.ejcn.1601895 [DOI] [PubMed] [Google Scholar]
- 105.Shirreffs SM, Merson SJ, Fraser SM, Archer DT (2004) The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 91 (6):951–958. doi: 10.1079/bjn20041149 [DOI] [PubMed] [Google Scholar]
- 106.Enhörning S, Tasevska I, Roussel R, Bouby N, Persson M, Burri P, Bankir L, Melander O (2017) Effects of hydration on plasma copeptin, glycemia and gluco-regulatory hormones: A water intervention in humans. Eur J Nutr. doi: 10.1007/s00394-017-1595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


